Abstract
Background and Purpose
Perampanel is the first α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-receptor antagonist developed to treat epilepsy. The effects of either rapid or slow dose titration on adverse events remain to be elucidated.
Methods
Eighty-five patients received perampanel between March 2016 and August 2016. Patients were divided into two groups according to their dosing schedule: rapid dose titration (2-mg increments at intervals of 1 to 2 weeks) and slow dose titration (2-mg increments at intervals of at least 3 weeks). Seizure frequency and adverse events were analyzed over 3 months.
Results
Adverse events were reported by 47 (58%) of the 81 patients analyzed, with 12 (15%) patients discontinuing perampanel due to adverse events. Common adverse events included dizziness (n=30, 37%), aggressive mood and behavior (n=19, 24%), gait disturbance (n=16, 20%), and sleep problems (n=10, 12.4%). The overall adverse events were similar in the slow-titration group (38 of 61 patients) and the rapid-titration group (8 of 20 patients, p=0.081). However, none of the 20 patients in the slow-titration group experienced gait disturbance, compared with 16 of the 61 patients in the rapid-titration group (p=0.009), while appetite change was experienced by 4 patients in the slow-titration group but only 1 in the rapid-titration group (p=0.003). No relationship was noted between adverse events and the maximum dose of perampanel (p=0.116). Sex differences were observed, with the response to perampanel being better and the rate of adverse events being higher in females (p=0.015 and p=0.046, respectively).
Conclusions
Slow titration of perampanel may reduce perampanel-related adverse events.
Keywords: perampanel, drug-resistant epilepsy, antiepileptic drug, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
INTRODUCTION
The new antiepileptic drug (AED) perampanel is an antagonist to the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-type glutamate receptor. Perampanel inhibits the generation and spread of epileptiform activity at postsynaptic excitatory synapses by blocking AMPA receptors.1 Several prospective and retrospective studies have investigated the efficacy of perampanel as an adjunctive AED for epilepsy.2,3,4,5,6,7,8,9,10 Overall 42–50% of the patients in these previous studies with drug-resistant epilepsy found that perampanel decreased the frequency of seizures by at least 50%.5,6,7,8,9,10 However, unexpected adverse events such as gait disturbance and aggressive behavior have been reported. Several experts have recommended applying slow dose titration of perampanel in order to reduce adverse events, but there are few data supporting this recommendation.2,8,9,11
In the present study we investigated the effects of rapid and slow dose titration on the frequency of adverse events when taking perampanel.
METHODS
We reviewed patients who started taking perampanel as an adjunctive therapy from March 1 to August 31, 2016 at Severance Children's Hospital in Korea. All patients were aged 12 years or older and experienced focal seizures classified according to the 2017 International League Against Epilepsy Classification of Epileptic Seizures.12
Patients included in the study had drug-resistant epilepsy, meaning uncontrolled seizures despite taking more than two appropriate AEDs, and a minimum (min) follow-up period of 3 months. Patients who had incomplete data were excluded, as were those with coexisting undistinguishable nonepileptic events from seizures. This study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2016-0684).
For the analysis, patients who underwent titration of 2-mg increments at intervals of 1 to 2 weeks were categorized into the rapid-titration group, while those who underwent titration at intervals of 3 weeks or longer were categorized into the slow-titration group. Perampanel was titrated from a daily dose of 2 mg at bedtime. For some patients who took enzyme-inducing AEDs, including phenytoin, carbamazepine, or oxcarbazepine, perampanel was started at 4 mg and then increased in increments of 2 mg daily at intervals of 1 week. The perampanel dose or titration was scheduled on an individual basis for all patients at the discretion of the physician in charge. The number of concomitant medications, characteristics of the patients and their caregivers, tolerability, and adverse events were considered. Dose titration was stopped if either seizure freedom was achieved or further dose titration was not tolerated.
Adverse events and the discontinuation rate during the initial 3 months of perampanel treatment were collected. Physicians specifically asked the patients about the presence of known perampanel-related adverse events, including gait disturbance, ataxia, dizziness, lethargy, appetite change, weight change, sleep change, mood change, aggressive behavior, slurred speech, and confusion.
To assess efficacy, the monthly seizure frequency measured immediately before the initiation of perampanel was compared to that during the third month of perampanel treatment. Patients or caregivers counted seizures at home, and reported their seizure frequency at each visit. Seizure intensity was not measured. Before the initiation of perampanel, seizure types and baseline seizure frequency were reviewed and clarified with caregivers and patients. Responders were defined as persons exhibiting a reduction in monthly seizure frequency of at least 50% compared to the baseline frequency.
Data were also collected by reviewing medical charts. The age at seizure onset, epilepsy syndrome, etiology, seizure type, medical history, past AED history, level of cognition, surgery history, and ketogenic diet history were reviewed. Intellectual disability was defined as significant limitations in both intellectual functioning and adaptive behavior.13 Limitation of intellectual functioning was defined as an intelligence quotient of less than 70. Brain MRI and electroencephalography results were also reviewed. The perampanel use was quantified by recording the dose at each visit, the titration schedule, the seizure frequency at each visit, the dose at the time of adverse events, and the maximum (max) dose. Reasons for the discontinuation of perampanel and the time when this happened were collected.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics (version 23.0, IBM Corp., Armonk, NY, USA). Normally distributed continuous variables were summarized as mean±standard-deviation values and analyzed using Student's t-test. Nonparametric continuous variables are expressed as median: max, min, interquartile range (IQR) values, and were analyzed using either the Mann-Whitney U test or the Kruskal-Wallis test. The chi-square test and Fisher's exact test were applied to categorical variables. Differences in seizure frequency over 3 months were analyzed using the Wilcoxon signed-rank test. Ordinal variables were analyzed using either the Mantel-Haenszel chi-square test or a mixed linear model. A probability value of p<0.05 was considered statistically significant.
RESULTS
Demographics
Four of 85 patients who received perampanel with a min follow-up of 3 months were excluded due to insufficient seizure data, and so 81 patients were finally included in the assessment.
Males comprised 44 of the 81 patients. The median age of the patients was 17 years (max=32 years, min=12 years, IQR=14–20 years). The median follow-up duration was 3 months (max=6 months, min=3 months, IQR=3–4 months). MRI revealed lesions related to epilepsy in 45 (55.6%) patients: common etiologies included malformations of cortical development (n=17, 21.0%), hypoxic ischemic encephalopathy (n=11, 13.6%), and infection (n=10, 12.4%), followed by genetic or metabolic etiologies (n=9, 11.1%), and trauma (n=3, 3.7%). All patients had drug-resistant epilepsy, and the median number of concomitant AEDs was 3 (max=6, min=1, IQR=3–4). Many patients had consumed a ketogenic diet (n=33, 40.7%) or received epilepsy surgery (n=36, 44.4%) including vagal nerve stimulation (n=19, 23.5%), highlighting the intractability of their seizures. Twenty (24.7%) of the 81 patients used carbamazepine, oxcarbazepine, and/or phenytoin concomitantly, while 57 (85.1%) of the 67 patients with available data had intellectual disability (Table 1)
Table 1. Demographic variables of the 81 patients.
| Variable | Value |
|---|---|
| Sex, male | 44 (54.3) |
| Adolescents* | 53 (65.4) |
| Age, years | 17 (32, 12, 14–20) |
| Body weight, kg | 54.3±19.5 |
| Age at onset of seizures, years (n=79) | 4 (15, 0, 1–8) |
| Presence of lesion on MRI | 45 (55.6) |
| Previous ketogenic diet | 33 (40.7) |
| Previous epilepsy surgery | 36 (44.4) |
| Previous vagal nerve stimulation surgery | 19 (23.5) |
| Intellectual disability (n=67) | 57 (85.1) |
| Type of seizures | |
| Focal seizures | 81 (100) |
| Focal to bilateral tonic-clonic seizures | 48 (59.3) |
| Others† | 44 (54.3) |
| History of IS or LGS | 37 (45.7) |
| Number of concomitant AEDs | 3 (6, 1, 3–4) |
| Concomitant use of CBZ, PHT, or OXC`20 (24.7) | |
| Initial EEG‡ | |
| Normal | 3 (3.7) |
| Abnormal background only | 7 (8.6) |
| Focal slowing or epileptiform discharges | 42 (51.9) |
| Multifocal epileptiform discharges | 29 (35.8) |
Data are median (maximum, minimum, interquartile range), mean±standard-deviation, or n (%) values.
*Up to 18-years-old, †Epileptic spasm (n=11), atypical absence (n=10), drop attacks (n=8), eyelid myoclonus (n=4), or tonic seizure (n=24); 15 patients had multiple seizure types, ‡EEG immediately before administering perampanel.
AED: antiepileptic drug, CBZ: carbamazepine, IS: infantile spasms, LGS: Lennox-Gastaut syndrome, OXC: oxcarbazepine, PHT: phenytoin.
Adverse events and related factors
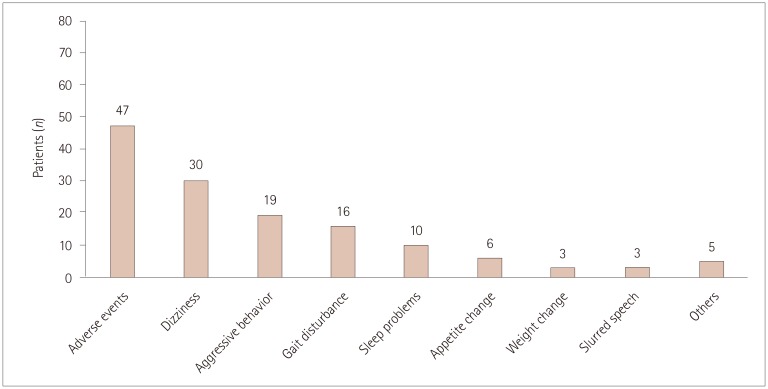
Overall, 47 (58.0%) patients reported adverse events, with 30 (37.0%) experiencing more than two adverse events. The most-common adverse events were dizziness and somnolence (n=30, 37.0%), followed by aggressive mood and behavior (n=19, 23.5%) and gait disturbance (n=16, 19.8%). Sleep problems (n=10, 12.4%), appetite change (n=6, 7.4%), weight change (n=3, 3.7%), and confused or slurred speech (n=3, 3.7%) were also common (Fig. 1). Other adverse events included excessive sputum production, drooling, dysphagia, nausea, memory impairment, and bizarre feeling.
Fig. 1. Numbers of patients who experienced adverse events. The most-common adverse events were dizziness and somnolence, followed by aggressive mood and behavior. Other adverse events included excessive sputum production, drooling, dysphagia, nausea, memory impairment, and bizarre feeling.
There were no significant differences between rapid titration and slow titration in the overall occurrence of adverse events events (38 of 61 patients vs. 8 of 20 patients, p=0.081), withdrawal rates of perampanel (16 of 61 patients vs. 4 of 20 patients, p=0.575), and reasons for early withdrawal (p=0.155). However, gait disturbance was reported more frequently in the rapid-titration group than in the slow-titration group (16 of 61 patients vs. 0 of 20 patients, p=0.009), while appetite change occurred less in the former group (1 of 61 patients vs. 4 of 20 patients, p=0.003). The occurrence of dizziness, weight change, sleep change, behavior change, and slurred speech did not differ between the two groups (Table 2).
Table 2. Comparison of fast titration (2-mg increments at intervals of 1 to 2 weeks) and slow titration (2-mg increments at intervals of 3 weeks or longer).
| Fast titration (n=61) | Slow titration (n=20) | p | |
|---|---|---|---|
| Adverse events | 38 (62.3) | 8 (40.0) | 0.081 |
| Early withdrawal of perampanel | 16 (26.2) | 4 (20.0) | 0.575 |
| Reason for withdrawal | |||
| Adverse events | 11 (18.0) | 1 (5.0) | 0.155 |
| Ineffectiveness | 5 (8.2) | 3 (15.0) | |
| Adverse events | |||
| Gait disturbance | 16 (26.2) | 0 (20.0) | 0.009 |
| Dizziness | 23 (37.7) | 4 (20.0) | 0.145 |
| Appetite change | 1 (1.6) | 4 (20.0) | 0.003 |
| Weight change | 3 (4.9) | 0 | 0.571 |
| Sleep disturbance | 6 (9.8) | 4 (20.0) | 0.210 |
| Aggressiveness or mood change | 15 (24.6) | 2 (10.0) | 0.164 |
| Slurred or confused speech | 2 (3.3) | 1 (5.0) | 0.724 |
| Others* | 4 (6.6) | 2 (10.0) | 0.610 |
Data are n (%) values.
*Others include hypersalivation, dysphagia, nausea, memory impairment, and bizarre feeling.
Experiencing or not experiencing adverse events did not affect the use of enzyme-inducing AEDs (13 of 47 patients vs. 7 of 34 patients, p=0.603), max dose of perampanel [8 (max=12, min=2, IQR=8–10) vs. 10 (max=12, min=6, IQR=8–12), p=0.116], or number of concomitant AEDs [4 (max=5, min=1, IQR=3–4) vs. 3 (max=6, min=2, IQR=3–5), p=0.502]. However, more than half of the patients who experienced adverse events were female, while only 32.4% of the patients who did not experience adverse events were female, indicating a significant sex difference (26 of 47 patients vs. 11 of 34 patients, p=0.046) (Table 3).
Table 3. Comparison between patients who experienced and did not experience adverse events.
| Adverse events (n=47) | No adverse events (n=34) | p | |
|---|---|---|---|
| Sex, female | 26 (55.3) | 11 (32.4) | 0.046 |
| Age at seizure onset, years | 4 (15, 0, 1–8) | 5 (13, 0, 1–8) | 0.988 |
| Adolescents* | 29 (61.7) | 24 (70.6) | 0.482 |
| Age, years | 17 (32, 12, 14–22) | 17 (24, 12, 14–20) | 0.818 |
| CBZ, PHT, or OXC use | 13 (27.7) | 7 (20.6) | 0.603 |
| Titration every week | 6 (12.8) | 5 (14.7) | 0.653 |
| Titration every 2 weeks | 31 (66.0) | 19 (55.9) | |
| Titration every 3 weeks or longer | 10 (21.3) | 10 (29.4) | |
| Maximum dose of perampanel, mg | 8 (12, 2, 8–10) | 10 (12, 6, 8–12) | 0.116 |
| Number of concomitant AEDs | 4 (5, 1, 3–4) | 3 (6, 2, 3–5) | 0.502 |
Data are median (maximum, minimum, interquartile range) or n (%) values.
*Up to 18 years old.
AED: antiepileptic drug, CBZ: carbamazepine, OXC: oxcarbazepine, PHT: phenytoin.
Adverse events subsided in 89.4% (n=42) of the patients. Of 47 who reported adverse events, 25 patients received an intervention and 23 experienced resolution. The interventions included discontinuation of perampanel (n=15), reduction of perampanel (n=9), and reduction of other drugs (n=1). Overall, 20 patients (24.7%) discontinued perampanel within 3 months: 12 (14.8%) withdrew early due to adverse events including gait disturbance (n=5, 6.2%), dizziness (n=5, 6.2%), and aggressive mood and behavior (n=2, 2.5%), and 8 (9.9%) withdrew early due to ineffectiveness.
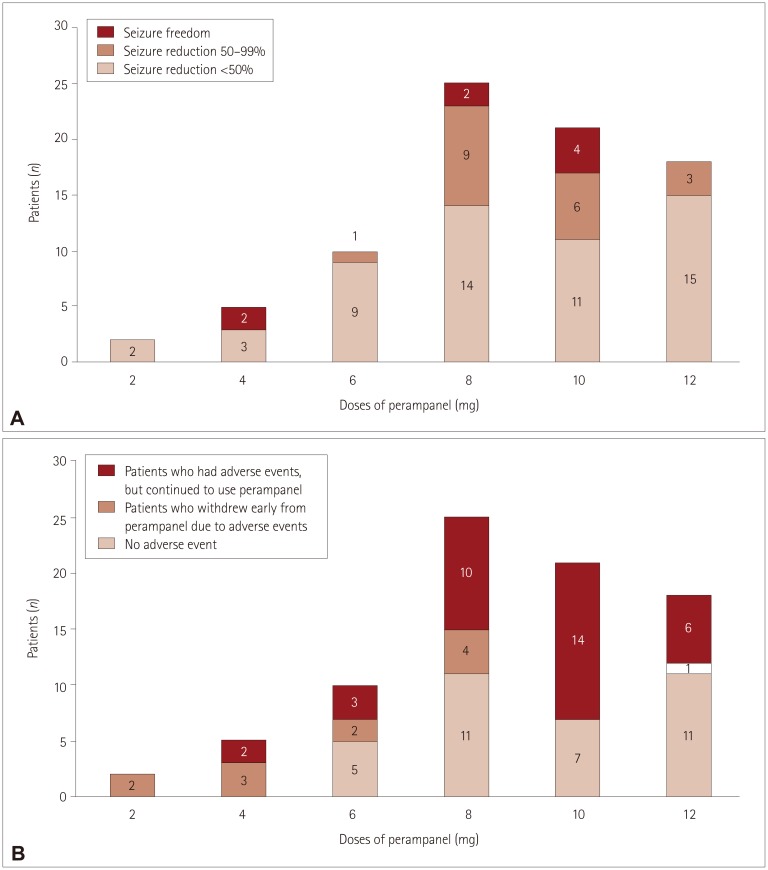
Five patients unexpectedly stopped taking perampanel at low doses (e.g., 2 or 4 mg) due to adverse events. Only one patient stopped perampanel at 12 mg. Two patients who stopped at 2 mg experienced severe dizziness, and three patients who stopped at 4 mg reported experiencing multiple side effects including gait disturbance, dizziness, and aggressive behavior (Fig. 2).
Fig. 2. Three-month seizure outcomes and occurrence of adverse events for different maximum doses of perampanel. A: The maximum doses were 2 and 4 mg in 7 patients. Two patients achieved seizure freedom when taking perampanel at the relatively low dose of 4 mg. The maximum doses were 6, 8, and 10 mg in more than two-thirds (56 of 81 patients) of patients. The rates of responders and seizure freedom were high for doses between 6 and 10 mg. The perampanel dose was increased to 12 mg when their seizures persisted, and no serious adverse events occurred. However, the rates of responders and seizure freedom were significantly low at a dose of 12 mg. B: Five patients stopped taking perampanel at low doses (e.g., 2 or 4 mg) due to adverse events. Only one patient stopped taking perampanel at 12 mg. Two patients who stopped at a dose of 2 mg experienced severe dizziness, and three patients who stopped at a dose of 4 mg reported experiencing multiple side effects including gait disturbance, dizziness, and aggressive behavior.
Efficacy
The median baseline monthly seizure frequency was 13 (max=900, min=1, IQR=5–90), and this reduced to 8 after 3 months of perampanel use (max=300, min=0, IQR=2–70) (p<0.001). Eight (9.9%) of the 81 patients became seizure-free, while an additional 20 (24.7%) patients experienced a reduction in seizure frequency of at least 50%. The overall responder rate was 34.6% (28 of 81 patients).
More patients in the responder group were female (18 of 28 patients vs. 19 of 53 patients, p=0.015). The responder group had a younger age at seizure onset [2 years (max=11 years, min=0 years, IQR=1–4 years) vs. 5 years (max=15 years, min=0 years, IQR=2–10 years), p=0.002] and fewer concomitant AEDs [3 (max=5, min=1, IQR=2–4) vs. 4 (max=6, min=2, IQR=3–5), p=0.005]. Age, body weight, type of seizures, baseline seizure frequency, and max dose of perampanel did not differ between the responder and nonresponder groups. The number of patients who took enzyme-inducing AEDs did not differ between responders and nonresponders (Table 4).
Table 4. Comparison of responders (reduction in seizure frequency of ≥50%) and nonresponders.
| Responders (n=28) | Nonresponders (n=53) | p | |
|---|---|---|---|
| Sex, female | 18 (64.3) | 19 (35.8) | 0.015 |
| Age, years | 17 (27, 12, 14–20) | 17 (32, 12, 14–20) | 0.226 |
| Age at onset of seizures, years | 2 (11, 0, 1–4) | 5 (15, 0, 2–10) | 0.002 |
| Adolescents* (n=53) | 21 (75.0) | 32 (60.4) | 0.188 |
| Body weight, kg | 52.3±15.5 | 55.2±21.2 | 0.517 |
| Type of seizures | |||
| Focal to bilateral tonic-clonic seizures | 16 (57.1) | 32 (60.4) | 0.778 |
| Others | 5 (17.9) | 7 (13.2) | 0.743 |
| Cognitive impairment (n=67) | 23/24 (95.8) | 34/43 (79.1) | 0.082 |
| Concomitant AEDs | 3 (5, 1, 2–4) | 4 (6, 2, 3–5) | 0.005 |
| Concomitant use of CBZ, PHT, or OXC | 7 (25.0) | 13 (24.5) | 0.963 |
| Seizure frequency per month | |||
| Baseline | 10 (900, 1, 5–70) | 30 (300, 1, 5–101) | 0.618 |
| After 3 months | 1 (300, 0, 0–10) | 27 (300, 0, 7–90) | <0.001 |
| Maximum dose of perampanel, mg | 9 (12, 4, 8–10) | 8 (12, 2, 6–12) | 0.945 |
Data are median (maximum, minimum, interquartile range), mean±standard-deviation, or n (%) values.
*Up to 18 years old.
AED: antiepileptic drug, CBZ: carbamazepine, OXC: oxcarbazepine, PHT: phenytoin.
The responder rates were high for doses between 6 and 10 mg and low for a dose of 12 mg (Fig. 2).
DISCUSSION
Several experts have suggested associations between adverse events and rapid dose titration of perampanel,2,8,9,11 although the supporting data have been sparse. The present study shows that gait instability-one of the common perampanel-related adverse events-may occur less often if the dose of perampanel is slowly escalated at intervals of 3 weeks or longer. This finding is supported by previous findings of similar reductions in the occurrence of dizziness in the slow-titration group (>2 mg/2 weeks)8 and reduced falls among elderly patients with slower titration.14 It is particularly interestingly that the serum level of perampanel takes 2 weeks to reach a steady state due to its long half-life.15
Reportedly 31% to 87% of patients experience adverse events when taking perampanel.2,3,4,5,6,7,8,9,10 Such a high frequency of adverse events might be due to AMPA receptors being widely distributed in the brain. In our study, 60% of the patients reported adverse events, but most of them resolved spontaneously or immediately after interventions, such as reducing the perampanel dose. Only about 15% of our patients withdrew early due to adverse events. The slow dose titration that was used in our patients may have been responsible for the low withdrawal rates; in contrast, most previous clinical trials and other real-world studies titrated perampanel rapidly and increased doses in 2-mg increments every 1 to 2 weeks.2,3,4,5,6,7,8,9,10
One-third of our patients with drug-resistant epilepsy experienced benefits from perampanel, while one-tenth achieved seizure freedom. The patients who responded favorably to perampanel had a few characteristics that were similar: First, the responders comprised a higher percentage of female patients. Sex differences regarding drug responses have been reported for other AEDs, and lower glomerular filtration rates and lower body weight in females have been hypothesized as underlying mechanisms.16 The clearance rate of perampanel is reportedly lower in females than in males,11 possibly resulting in a higher serum concentration of perampanel in females for the same dose. A previous pooled-data study found that the response to perampanel was better in females.17 Second, the age at seizure onset was younger for responders than for nonresponders. Since this result has not been reported previously, it needs to be confirmed in future research. There may be differences in the nature of focal epilepsies between those with early and later onset. Although the efficacy of perampanel in children still needs to be investigated, this finding suggests that perampanel can play a special role in tackling childhood epilepsies.
With its short postmarketing period, the optimal administration conditions of perampanel remain to be determined. In our study, similarly high rates of responders and seizure freedom were achieved with perampanel at doses of 6, 8, and 10 mg. Doses of 2, 4, and 12 mg were less effective at reducing the rate of seizures. Most previous studies applied perampanel at doses of 7–8 mg.5,6,7,8,9 The additional gain from administering perampanel at the high dose of 12 mg remains controversial. Previous clinical trials found that responder rates were similar at doses of 12 and 8 mg, while some advocated that the efficacy was higher at 12 mg.2,3,4
Some of the present patients seemed to respond dramatically to low doses of perampanel, with two of them discontinuing perampanel at 2 mg due to adverse events, while another two patients achieved seizure freedom at 4 mg, which is inconsistent with the well-known dose dependency of perampanel.15 Similar phenomena have been reported previously,6 and they might be due to the high variability in the serum concentration of perampanel between subjects.18 The clinical characteristics in this patient group therefore need to be investigated further.
This study was subject to some limitations. First, the 3-month follow-up period was relatively short. However, since our patients experienced severe drug-resistant epilepsy and frequent seizures, we thought that 3 months was sufficient for determining the efficacy and tolerability of a newly added drug in most of our patients. Second, the serum perampanel concentration was not measured. There is a linear relationship between the administered dose and plasma concentration of perampanel, and also between the plasma concentration of perampanel and its efficacy,15 although there may be interindividual variability.
In conclusion, perampanel is a novel AED that is effective and safe for epilepsy. Although adverse events are common, some of them may be prevented by the slow titration of perampanel.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11:56–63. doi: 10.5698/1535-7511-11.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79:589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 4.Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 5.De Liso P, Vigevano F, Specchio N, De Palma L, Bonanni P, Osanni E, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: an Italian observational multicenter study. Epilepsy Res. 2016;127:93–100. doi: 10.1016/j.eplepsyres.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Steinhoff BJ, Hamer H, Trinka E, Schulze-Bonhage A, Bien C, Mayer T, et al. A multicenter survey of clinical experiences with perampanel in real life in Germany and Austria. Epilepsy Res. 2014;108:986–988. doi: 10.1016/j.eplepsyres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Steinhoff BJ, Bacher M, Bast T, Kornmeier R, Kurth C, Scholly J, et al. First clinical experiences with perampanel--the Kork experience in 74 patients. Epilepsia. 2014;55(Suppl 1):16–18. doi: 10.1111/epi.12492. [DOI] [PubMed] [Google Scholar]
- 8.Shah E, Reuber M, Goulding P, Flynn C, Delanty N, Kemp S. Clinical experience with adjunctive perampanel in adult patients with uncontrolled epilepsy: a UK and Ireland multicentre study. Seizure. 2016;34:1–5. doi: 10.1016/j.seizure.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Heyman E, Lahat E, Levin N, Epstein O, Lazinger M, Berkovitch M, et al. Tolerability and efficacy of perampanel in children with refractory epilepsy. Dev Med Child Neurol. 2017;59:441–444. doi: 10.1111/dmcn.13362. [DOI] [PubMed] [Google Scholar]
- 10.Biró A, Stephani U, Tarallo T, Bast T, Schlachter K, Fleger M, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics. 2015;46:110–116. doi: 10.1055/s-0035-1546276. [DOI] [PubMed] [Google Scholar]
- 11.Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56:12–27. doi: 10.1111/epi.12865. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RS. The new classification of seizures by the international league against epilepsy 2017. Curr Neurol Neurosci Rep. 2017;17:48. doi: 10.1007/s11910-017-0758-6. [DOI] [PubMed] [Google Scholar]
- 13.Schalock RL, Borthwick-Duffy SA, Buntinx WHE, Coulter DL, Craig EM. Intellectual disability: definition, classification, and systems of supports. 11th ed. Washington, D.C.: American Association on Intellectual and Developmental Disabilities; 2010. [Google Scholar]
- 14.Trinka E, Steinhoff BJ, Nikanorova M, Brodie MJ. Perampanel for focal epilepsy: insights from early clinical experience. Acta Neurol Scand. 2016;133:160–172. doi: 10.1111/ane.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gidal BE, Ferry J, Majid O, Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013;54:1490–1497. doi: 10.1111/epi.12240. [DOI] [PubMed] [Google Scholar]
- 16.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez B, Yang H, Williams B, Zhou S, Laurenza A. Perampanel efficacy and safety by gender: subanalysis of phase III randomized clinical studies in subjects with partial seizures. Epilepsia. 2015;56:e90–e94. doi: 10.1111/epi.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villanueva V, Majid O, Nabangchang C, Yang H, Laurenza A, Ferry J, et al. Pharmacokinetics, exposure-cognition, and exposure-efficacy relationships of perampanel in adolescents with inadequately controlled partial-onset seizures. Epilepsy Res. 2016;127:126–134. doi: 10.1016/j.eplepsyres.2016.08.025. [DOI] [PubMed] [Google Scholar]