Abstract
Background and Purpose
The optic nerve sheath diameter (ONSD) is an indirect marker of the intracranial pressure, but the normal range of ONSD as measured using magnetic resonance imaging (MRI) and its associations with clinical parameters and the eyeball transverse diameter (ETD) remain unclear.
Methods
We included 314 healthy adults who underwent brain MRI examinations for health screening between June 2014 and September 2017. The ONSD and ETD of each eye were calculated using time-of-flight magnetic resonance angiography. Linear regression analyses were performed to assess the relationships between ONSD and variables including age, sex, height, weight, body mass index (BMI), mean arterial blood pressure (MABP), intraocular pressure (IOP), and ETD. We further investigated a normative value for the ONSD/ETD ratio and its associated factors.
Results
The mean ONSD and ETD were 4.71 mm [95% confidence interval (CI), 4.66–4.75 mm] and 21.24 mm (95% CI, 21.13–21.35 mm), respectively. Multiple linear regression analysis showed that ONSD was only associated with ETD (p<0.001), with it being independent of age, sex, height, weight, BMI, MABP, and IOP. The ONSD/ETD ratio had a mean value of 0.22 (95% CI, 0.22–0.22), and was not correlated with age, sex, height, weight, BMI, MABP, or IOP.
Conclusions
This study determined the normative value of MRI-based ONSD in healthy Korean adults. There was a strong correlation between the ETD and ONSD, which can be presented as the ONSD/ETD ratio. This parameter needs to be investigated further in disease populations.
Keywords: magnetic resonance imaging, optic nerve sheath diameter, eyeball transverse diameter, healthy, Korean
INTRODUCTION
Identifying increased intracranial pressure (ICP) is crucial in the management of various neurological disorders because this condition is associated with a poor prognosis, including the risk of death due to brain herniation.1,2,3 Although invasive methods including intraventricular catheterization and intraparenchymal probes remain the gold standard for ICP estimation,4 these procedures cannot be conducted in many cases owing to 1) non-availability of neurosurgeons or intensive-care units, 2) the risk of complications such as hemorrhage and infection, and 3) contraindications such as severe platelet disorders or coagulopathy.5,6 These factors have led to an emphasis on noninvasive measurements of ICP. There is increasing evidence that the optic nerve sheath diameter (ONSD) is an indirect marker of ICP,7 with a linear relationship having been found between these two parameters.8,9
Magnetic resonance imaging (MRI) is now routinely performed in patients with suspected brain lesions. Considering that the anatomical structures and borders of the optic nerve and nerve sheaths are clearly defined in MRI,10 the measurement of ONSD using MRI can provide reliable information regarding ICP without requiring additional procedures. However, the optimal cutoff value for an abnormal ONSD indicating increased ICP in MRI has been unclear because most of the investigating studies have included only a small number of healthy subjects.10,11,12,13,14 In addition, the clinical and anatomical parameters associated with ONSD are still inconclusive. Although our recent prospective study that included 585 healthy volunteers revealed that ONSD measured by ultrasound is associated with the eyeball transverse diameter (ETD) but not clinical variables including sex, height, weight, body mass index (BMI), and head circumference,15 these observations need to be further validated using an objective method. The present study therefore aimed to establish normative values for ONSD using MRI in a large number of healthy adults and assessed its associations with various clinical parameters and ETD.
METHODS
Patients
We reviewed 332 consecutive participants who underwent brain MRI examinations as part of comprehensive health screening at the Aerospace Medical Center of the Republic of Korea Air Force (ASMC-ROKAF) between June 13, 2014 and September 11, 2017. We excluded individuals who had any of the following conditions: a history of ophthalmological or neurological disorders (n=3), poor imaging quality due to severe motion or metallic artifacts (n=4), and incidentally detected intracranial pathologies on the written reports from the screening MRI that have the potential to influence ICP [intracranial neoplasms (n=7) and intracranial vascular malformations (n=4)]. In total, 314 individuals were finally included in this study (Fig. 1). The study protocol was approved by the ASMC-ROKAF Institutional Review Board (IRB No. ASMC-17-IRB-011) and conformed to the principles of the Declaration of Helsinki. Informed consent was not required from patients due to the retrospective nature of the study.
Fig. 1. Flow diagram of subject selection.
Magnetic resonance imaging
Patients underwent MRI scanning at 1.5 Tesla (Achieva, Philips Medical Systems, Best, the Netherlands). In our institution, time-of-flight (TOF) magnetic resonance angiography (MRA) is routinely applied to individuals who undergo brain MRI for health screening. Thus, we measured the ONSD using this protocol with the following imaging parameters: TR=25 ms, TE=6.91 ms, flip angle=20°, one signal average, section thickness=1 mm, field of view=18.9×18.9 cm2, and acquisition matrix=468×223 pixels. All MRI scans were conducted on the same day that the clinical data were obtained.
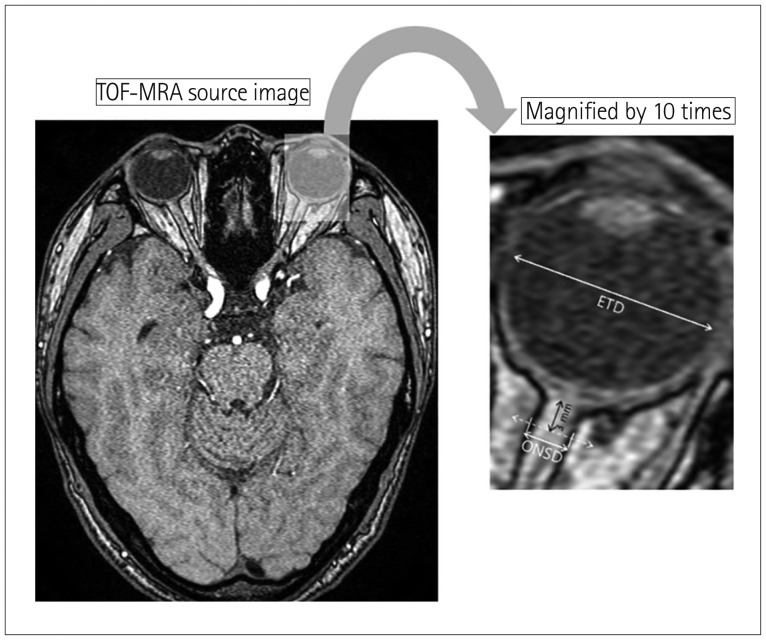
MRI data were independently evaluated with a picture archiving and communication system (Centricity RA1000, GE Healthcare, Barrington, IL, USA) by an experienced board-certified radiologist (D.H.K.) who was not involved in participant selection. The reviewer was blind to the clinical information of the participants. Axial TOF-MRA source images were used to measure the ONSD and ETD of each eye (Fig. 2). The retrobulbar area was magnified 10 times (zoom factor, 10.0), and then ONSD was measured along an axis perpendicular to the optic nerve at 3 mm behind the eyeball using electronic calipers. ONSD and ETD (retina to retina) were defined as the distances between the external margins of the thick sheath layers covering the optic nerve and the transverse diameter of the eyeball, respectively. Among the axial images, four images individually showing the maximum ONSD and ETD of each eye were selected to perform the measurement. To limit intraobserver variation, each measurement was made three times and its mean value was obtained.
Fig. 2. Sample image showing how the ONSD and ETD are estimated. ETD: eyeball transverse diameter, MRA: magnetic resonance angiography, ONSD: optic nerve sheath diameter, TOF: time-of-flight.
Clinical data collection
Clinical data were obtained by one neurologist (R.K.) based on standardized self-administered questionnaires and electronic medical records. The following demographic and physiological data were recorded for each individual: age, sex, weight, height, systolic blood pressure, diastolic blood pressure, and bilateral intraocular pressure (IOP). Hospital regulations dictated that all measurements were made in the morning. Mean arterial blood pressure (MABP) was calculated as 1/3×systolic blood pressure+2/3×diastolic blood pressure. IOP was measured in each participant bilaterally by an experienced nurse using a noncontact tonometer and automatic air-puff controller (TX-20P, Canon, Tokyo, Japan), which does not require topical anesthesia of the cornea. The tonometer was calibrated according to the manufacturer's guidelines before each measurement.
Statistical analysis
Categorical variables are reported as frequencies and percentages, and continuous variables are reported as mean±standard-deviation values or medians interquartile range (IQR) values. Continuous variables were compared between the left and right eyes using paired Student's t-tests. Linear regression models were used to determine radiological, demographic, or physiological factors related to ONSD. The potential influencing variables of sex, age, weight, height, BMI, MABP, IOP, and ETD were selected for inclusion in the models based on previously reported literature and clinical knowledge. We used the mean value for both eyes for variables including ONSD, ETD, and IOP. All variables for which p was less than 0.2 in the simple linear regression models were included in a multiple linear regression model. To clarify the relationship between ONSD and IOP, we further investigated their correlation for each eye using the Pearson correlation test. All statistical tests were two-tailed, and differences were considered significant for a probability of <0.05. Calculations were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The total of 314 healthy adults included 267 (85%) men. The age, height, weight, BMI, MABP, and IOP of the subjects were 47.8±7.3 years, 172.6±4.2 cm, 74.6±6.7 kg, 25.0±1.9 kg/m2, 93.3±7.3 mm Hg, and 13.7±2.0 mm Hg (right, 13.8±2.7 mm Hg; left, 13.7±2.7 mm Hg), respectively.
The mean [95% confidence interval (CI)] values of ONSD and ETD were 4.71 mm (4.66–4.75 mm) and 21.24 mm (21.13–21.35 mm), respectively (Table 1). The ONSD ranged from 3.75 to 6.20 mm, with a median of 4.70 mm and an IQR of 4.45–4.95 mm. The ONSD did not differ significantly between the right and left eyes (p=0.106). The results of the regression analyses are presented in Table 2. Although simple linear regression models showed that sex, weight, and ETD had a p value of less than 0.2, the ONSD was the only variable that was independently associated with ETD (p<0.001) in the subsequent multiple linear regression model. IOP was also not correlated with ONSD in either the left eye (r=−0.085, p=0.434) or the right eye (r=−0.031, p=0.638).
Table 1. ONSD, ETD, and ONSD/ETD ratio measured using magnetic resonance imaging.
| Mean±SD (95% CI) | Median (IQR) | Minimum | Maximum | |
|---|---|---|---|---|
| ONSD (mm) | ||||
| Right | 4.73±0.36 (4.67–4.78) | 4.70 (4.40–5.00) | 3.60 | 6.20 |
| Left | 4.69±0.33 (4.64–4.73) | 4.70 (4.40–4.90) | 3.50 | 6.30 |
| Overall | 4.71±0.31 (4.66–4.75) | 4.70 (4.45–4.95) | 3.75 | 6.20 |
| ETD (mm) | ||||
| Right | 21.29±0.87 (21.16–21.41) | 21.20 (20.60–22.00) | 18.60 | 25.00 |
| Left | 21.19±0.83 (21.07–21.32) | 21.10 (20.50–21.80) | 17.60 | 25.10 |
| Overall | 21.24±0.79 (21.13–21.35) | 21.18 (20.60–21.86) | 18.10 | 25.05 |
| ONSD/ETD ratio | ||||
| Right | 0.22±0.01 (0.22–0.22) | 0.22 (0.21–0.23) | 0.17 | 0.28 |
| Left | 0.22±0.01 (0.22–0.22) | 0.22 (0.21–0.23) | 0.18 | 0.28 |
| Overall | 0.22±0.01 (0.22–0.22) | 0.22 (0.21–0.23) | 0.18 | 0.27 |
The overall value is the value for the left and right eyes combined.
CI: confidence interval, ETD: eyeball transverse diameter, IQR: interquartile range, ONSD: optic nerve sheath diameter, SD: standard deviation.
Table 2. Results of linear regression analyses of the relationships between optic nerve sheath diameter and other variables.
| Variable | Simple linear regression analysis | Multiple linear regression analysis | ||||
|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | |
| Sex | −0.131 | 0.063 | 0.039 | 0.152 | 0.160 | 0.341 |
| Age | −0.002 | 0.002 | 0.320 | - | - | - |
| Height | 0.004 | 0.005 | 0.367 | - | - | - |
| Weight | 0.004 | 0.003 | 0.193 | 0.003 | 0.002 | 0.169 |
| BMI | 0.009 | 0.010 | 0.363 | - | - | - |
| MABP | 0.000 | 0.002 | 0.872 | - | - | - |
| IOP | −0.013 | 0.011 | 0.238 | - | - | - |
| ETD | 0.267 | 0.016 | <0.001 | 0.259 | 0.018 | <0.001 |
B: unstandardized regression coefficient, BMI: body mass index, ETD: eyeball trans verse diameter, IOP: intraocular pressure, MABP: mean arterial blood pressure, SE: standard error.
We further assessed normative values for the MRI-based ONSD/ETD ratio. The ONSD/ETD ratio ranged from 0.18 to 0.27, and 95% of individuals had a mean ONSD/ETD ratio of 0.22 (Table 1). The ONSD/ETD ratio did not differ significantly between the right and left eyes (p=0.238). In simple linear regression analyses, the ONSD/ETD ratio was not significantly associated with age (p=0.548) sex (p=0.201), height (p=0.971), weight (p=0.399), BMI (p=0.398), MABP (p=0.104), or IOP (p=0.364).
DISCUSSION
We evaluated normal values for MRI-based ONSD and its associated factors in 314 healthy Korean individuals. Our results showed that the mean values of the ONSD and ONSD/ETD ratio were 4.71±0.31 mm (95% CI, 4.66–4.75 mm) and 0.22±0.01 (95% CI, 0.22–0.22), respectively, and that ONSD was correlated with ETD but not with sex, age, weight, height, BMI, MABP, or IOP. MRI yields images of the eyeball with a high spatial resolution that allows the clear delineation of orbital structures and thereby avoids the disadvantages of ultrasound such as poor penetration and artifacts resulting from the tissues under observation exhibiting different indices of refraction for sound waves; these features facilitate objective and reproducible measurements of ONSD.12,16,17 Thus, we believe that ONSD measured using MRI is a reliable marker for ICP, and this method may be optimal for detecting increased ICP in specific settings. To our knowledge the current study included the largest number of healthy individuals for estimating MRI-based ONSD to date. In addition, this is the first study to use MRI to assess ONSD in healthy Asian individuals.
Several studies have determined the normal value of MRI-based ONSD measured 3 to 5 mm behind the eyeball in healthy subjects (Table 3).10,11,12,13,14,18 Their mean values ranged from 5.08 to 5.72 mm,10,11,12,13,14 which are larger than for ours. However, previous studies used conventional T2-weighted images with slice thicknesses from 2 to 5 mm to evaluate the ONSD, which may be unsuitable considering that the vertical size of the optic nerve is 3–4 mm. In the present study, the ONSD was measured using TOF-MRA source images with a 1-mm slice thickness and sufficient anatomical contrast. Ko19 measured the ONSD using the same MRI protocol in 301 Korean adults, and found a mean value of 4.37 mm, although they were patients with neurological disorders. Alternatively, variations in ONSD may be influenced by race, but this needs to be investigated further due to inconsistencies in the previous results.15,20,21,22,23
Table 3. Previously published studies of ONSD measured using MRI in healthy individuals.
| First author (reference) | Number* (mean age) | Nation | MRI sequence | Slice thickness (mm) | ONSD, mean±SD (95% CI, mm) |
|---|---|---|---|---|---|
| Mashima11 | 15 (N/A) | Japan | T2 FSE | 3 | 5.1±0.4 |
| Weigel12 | 32 (25 years) | Germany | T2 TSE / T2 HASTE | 3–5 | 5.7±0.6 |
| Lagrèze13 | 33 (25 years) | Germany | T2 HASTE | 3 | 5.72 (5.51–5.93) |
| Geeraerts10 | 36 (32 years) | UK | Proton density/ T2 TSE | 4 | 5.08±0.52 |
| Shoft0†18 | 86 (8.1 years) | Israel | Basic T2 sequence | 2.5–3.5 | 3.10–3.56 |
| Bäuerle14 | 15 (24.5 years) | Germany | T2 TSE / T2 HASTE | 2–3 | 5.69±0.77 (range, 4.7–7.9) |
| Current study | 314 (47.8 years) | South Korea | TOF-MRA source image | 1 | 4.71±0.40 (4.67–4.75) |
*Number of healthy individuals, †The ONSD was measured at 10 mm anterior to the optic foramina.
CI: confidence interval, FSE: fast spin-echo, HASTE: half-Fourier acquisition single-shot turbo spin-echo, MRA: magnetic resonance angiography, MRI: magnetic resonance imaging, N/A: not applicable, ONSD: optic nerve sheath diameter, SD: standard-deviation, T2: T2-weighted, TOF: time-of-flight, TSE: turbo spin-echo.
We found a significant association between the ETD and ONSD, which is in line with our previous results obtained using ultrasound.15 Vaiman et al.24 reported a strong correlation between ETD and ONSD for computed tomography measurements. This relationship led to the ONSD/ETD ratio being proposed as a more-useful parameter for increased ICP than ONSD alone, especially for individuals who normally have large ONSD.15,25,26,27 Although the ONSD/ETD ratio in the current study was slightly higher than the ultrasound- based mean value of 0.18 in our previous study,15 this may be attributed to differences between the imaging modalities. Two previous studies found that the ONSD measured using MRI was larger than when using ultrasound in the same healthy subjects, with mean differences between the two modalities of 0.25 and 0.19 mm.14,16 Ultrasound-based measurements of ONSD can be adversely affected by examiner inexperience, an incorrect cutting plane, or poor spatial resolution, which makes it difficult to measure the maximum ONSD using ultrasound and may explain the difference in ONSD values measured using ultrasound and MRI.
While previous studies have proposed that ONSD is associated with sex and BMI,28,29 the current study found that these factors were not related to ONSD, which is further supported by our previous results.15 The relationship between IOP and ICP seems controversial in individuals with a normal ICP. A previous study found a significant correlation between ICP and IOP in 50 nonophthalmological patients, about half of whom had normal ICP.30 However, no such significant correlation was observed in another study31 or in the current study. These findings might not be surprising given the anatomical and physiological relationship between the eye and the cerebrospinal fluid.31
Some limitations of the current study need to be addressed. First, the MRI measurements were made by a single experienced board-certified radiologist, and so interobserver reproducibility could not be assessed. However, each measurement was repeated in order to reduce the likelihood of errors and biases. Second, TOF-MRA source images were used to obtain thin slices and a small interslice spacing. However, since the TOF sequence was originally developed for assessing intracranial vessels, the clinical relevance of our results may be reduced. Third, eyeball positioning during an MRI examination may affect the measured ONSD, and the retrospective nature of this study meant that we could not control this.
In conclusion, this study found that the mean ONSD values and ONSD/ETD ratios determined using MRI in healthy Korean adults were 4.71 mm (95% CI, 4.66–4.75 mm; IQR, 4.45–4.95 mm) and 0.22 (95% CI, 0.22–0.22; IQR, 0.21–0.23), respectively. The ONSD was strongly associated with ETD while being independent of age, sex, height, weight, BMI, MABP, and IOP. Our findings suggest that the ONSD/ETD ratio is a more-reliable indicator of ICP than ONSD itself; this possibility needs to be investigated further in disease populations.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i23–i27. doi: 10.1136/jnnp.73.suppl_1.i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care. 2006;4:8–13. doi: 10.1385/NCC:4:1:008. [DOI] [PubMed] [Google Scholar]
- 3.Stevens RD, Shoykhet M, Cadena R. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2015;23(Suppl 2):S76–S82. doi: 10.1007/s12028-015-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raboel PH, Bartek J, Jr, Andresen M, Bellander BM, Romner B. Intracranial pressure monitoring: invasive versus non-invasive methods-a review. Crit Care Res Pract. 2012;2012:950393. doi: 10.1155/2012/950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–821. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristiansson H, Nissborg E, Bartek J, Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25:372–385. doi: 10.1097/ANA.0b013e31829795ce. [DOI] [PubMed] [Google Scholar]
- 7.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–1068. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 8.Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87:34–40. doi: 10.3171/jns.1997.87.1.0034. [DOI] [PubMed] [Google Scholar]
- 9.Wang LJ, Yao Y, Feng LS, Wang YZ, Zheng NN, Feng JC, et al. Noninvasive and quantitative intracranial pressure estimation using ultrasonographic measurement of optic nerve sheath diameter. Sci Rep. 2017;7:42063. doi: 10.1038/srep42063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geeraerts T, Newcombe VF, Coles JP, Abate MG, Perkes IE, Hutchinson PJ, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care. 2008;12:R114. doi: 10.1186/cc7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mashima Y, Oshitari K, Imamura Y, Momoshima S, Shiga H, Oguchi Y. High-resolution magnetic resonance imaging of the intraorbital optic nerve and subarachnoid space in patients with papilledema and optic atrophy. Arch Ophthalmol. 1996;114:1197–1203. doi: 10.1001/archopht.1996.01100140397006. [DOI] [PubMed] [Google Scholar]
- 12.Weigel M, Lagrèze WA, Lazzaro A, Hennig J, Bley TA. Fast and quantitative high-resolution magnetic resonance imaging of the optic nerve at 3.0 tesla. Invest Radiol. 2006;41:83–86. doi: 10.1097/01.rli.0000195820.98062.c5. [DOI] [PubMed] [Google Scholar]
- 13.Lagrèze WA, Lazzaro A, Weigel M, Hansen HC, Hennig J, Bley TA. Morphometry of the retrobulbar human optic nerve: comparison between conventional sonography and ultrafast magnetic resonance sequences. Invest Ophthalmol Vis Sci. 2007;48:1913–1917. doi: 10.1167/iovs.06-1075. [DOI] [PubMed] [Google Scholar]
- 14.Bäuerle J, Schuchardt F, Schroeder L, Egger K, Weigel M, Harloff A. Reproducibility and accuracy of optic nerve sheath diameter assessment using ultrasound compared to magnetic resonance imaging. BMC Neurol. 2013;13:187. doi: 10.1186/1471-2377-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Jun JS, Kim R. Ultrasonographic measurement of the optic nerve sheath diameter and its association with eyeball transverse diameter in 585 healthy volunteers. Sci Rep. 2017;7:15906. doi: 10.1038/s41598-017-16173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirodkar CG, Munta K, Rao SM, Mahesh MU. Correlation of measurement of optic nerve sheath diameter using ultrasound with magnetic resonance imaging. Indian J Crit Care Med. 2015;19:466–470. doi: 10.4103/0972-5229.162465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawfik EA, Walker FO, Cartwright MS. Neuromuscular ultrasound of cranial nerves. J Clin Neurol. 2015;11:109–121. doi: 10.3988/jcn.2015.11.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shofty B, Ben-Sira L, Constantini S, Freedman S, Kesler A. Optic nerve sheath diameter on MR imaging: establishment of norms and comparison of pediatric patients with idiopathic intracranial hypertension with healthy controls. AJNR Am J Neuroradiol. 2012;33:366–369. doi: 10.3174/ajnr.A2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko SB. Optic nerve sheath diameter on brain magnetic resonance imaging: a single center study. J Neurocrit Care. 2015;8:16–24. [Google Scholar]
- 20.Chen H, Ding GS, Zhao YC, Yu RG, Zhou JX. Ultrasound measurement of optic nerve diameter and optic nerve sheath diameter in healthy Chinese adults. BMC Neurol. 2015;15:106. doi: 10.1186/s12883-015-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehman H, Khan MS, Nafees M, Rehman AU, Habib A. Optic nerve sheath diameter on sonography in idiopathic intracranial hypertension versus normal. J Coll Physicians Surg Pak. 2016;26:758–760. [PubMed] [Google Scholar]
- 22.Wang L, Feng L, Yao Y, Wang Y, Chen Y, Feng J, et al. Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PLoS One. 2015;10:e0117939. doi: 10.1371/journal.pone.0117939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SU, Jeon JP, Lee H, Han JH, Seo M, Byoun HS, et al. Optic nerve sheath diameter threshold by ocular ultrasonography for detection of increased intracranial pressure in Korean adult patients with brain lesions. Medicine (Baltimore) 2016;95:e5061. doi: 10.1097/MD.0000000000005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaiman M, Gottlieb P, Bekerman I. Quantitative relations between the eyeball, the optic nerve, and the optic canal important for intracranial pressure monitoring. Head Face Med. 2014;10:32. doi: 10.1186/1746-160X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaiman M, Sigal T, Kimiagar I, Bekerman I. Intracranial pressure assessment in traumatic head injury with hemorrhage via optic nerve sheath diameter. J Neurotrauma. 2016;33:2147–2153. doi: 10.1089/neu.2015.4293. [DOI] [PubMed] [Google Scholar]
- 26.Vaiman M, Sigal T, Kimiagar I, Bekerman I. Noninvasive assessment of the intracranial pressure in non-traumatic intracranial hemorrhage. J Clin Neurosci. 2016;34:177–181. doi: 10.1016/j.jocn.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Bekerman I, Sigal T, Kimiagar I, Ben Ely A, Vaiman M. The quantitative evaluation of intracranial pressure by optic nerve sheath diameter/eye diameter CT measurement. Am J Emerg Med. 2016;34:2336–2342. doi: 10.1016/j.ajem.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Feng L, Yao Y, Deng F, Wang Y, Feng J, et al. Ultrasonographic evaluation of optic nerve sheath diameter among healthy Chinese adults. Ultrasound Med Biol. 2016;42:683–688. doi: 10.1016/j.ultrasmedbio.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Goeres P, Zeiler FA, Unger B, Karakitsos D, Gillman LM. Ultrasound assessment of optic nerve sheath diameter in healthy volunteers. J Crit Care. 2016;31:168–171. doi: 10.1016/j.jcrc.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Sajjadi SA, Harirchian MH, Sheikhbahaei N, Mohebbi MR, Malekmadani MH, Saberi H. The relation between intracranial and intraocular pressures: study of 50 patients. Ann Neurol. 2006;59:867–870. doi: 10.1002/ana.20856. [DOI] [PubMed] [Google Scholar]
- 31.Kirk T, Jones K, Miller S, Corbett J. Measurement of intraocular and intracranial pressure: is there a relationship. Ann Neurol. 2011;70:323–326. doi: 10.1002/ana.22414. [DOI] [PubMed] [Google Scholar]