Abstract
Poaching of both black (Diceros bicornis) and white (Ceratotherium simum) rhinoceros in Africa has increased significantly in recent years. In an effort to ensure the survival of these critically endangered species, breeding programs were established in the 1990s in Australia, where a similar climate and habitat is available. In this study we examined blood samples from two C. simum, including a 16 yr old female (Aluka) who died in captivity, and a 17 yr old asymptomatic male (Umfana). Bloods from seven healthy D. bicornis housed at the zoo were also collected. All samples were tested for the presence of piroplasms via blood smear and PCR. A generic PCR for the 18S rRNA gene of the Piroplasmida revealed the presence of piroplasm infection in both dead and asymptomatic C. simum. Subsequent sequencing of these amplicons revealed the presence of Theileria bicornis. Blood smear indicated that this organism was present at low abundance in both affected and asymptomatic individuals and was not linked to the C. simum mortality. T. bicornis was also detected in the D. bicornis population (n = 7) housed at Taronga Western Plains Zoo using PCR and blood film examination; however only animals imported from Africa (n = 1) tested T. bicornis positive, while captive-born animals bred within Australia (n = 6) tested negative suggesting that transmission within the herd was unlikely. Phylogenetic analysis of the full length T. bicornis 18S rRNA genes classified this organism outside the clade of the transforming and non-transforming Theileria with a new haplotype, H4, identified from D. bicornis. This study revealed the presence of Theileria bicornis in Australian captive populations of both C. simum and D. bicornis and a new haplotype of the parasite was identified.
Keywords: Piroplasm, Theileria bicornis, 18S rRNA, Rhinoceros, Translocation
Graphical abstract
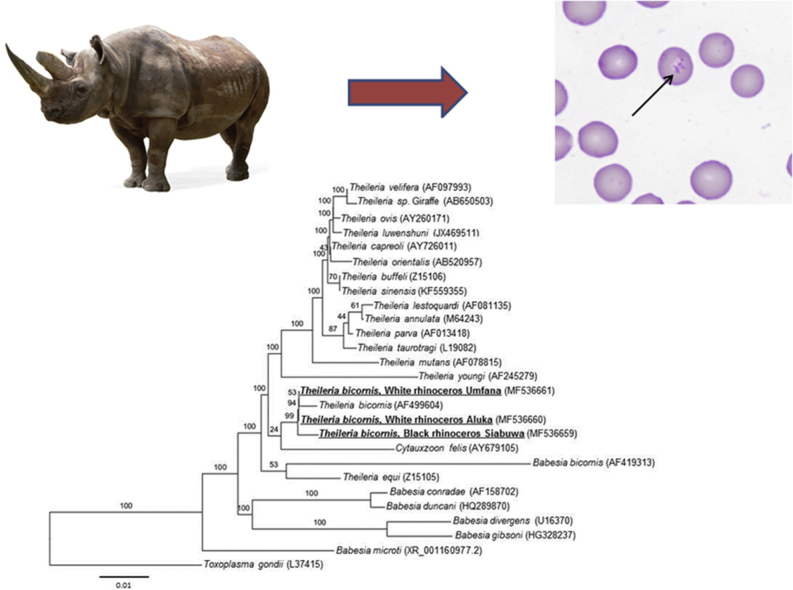
Highlights
-
•
Australian captive rhinoceros were screened for haemoparasites following mortalities.
-
•
Theileria bicornis was identified in three rhinoceroses imported from Africa.
-
•
Theileria bicornis was present in low numbers and was not considered to be related to the mortalities.
-
•
Molecular analysis revealed a new T. bicornis haplotype H4.
1. Introduction
Parasites and their hosts have co-evolved over the centuries. Benign infections enable the parasite to establish a symbiotic relationship with the host and potentially persist for life. However, factors such as translocation, stress, and suppression of host immunity can allow parasites to proliferate and cause clinical disease. A variety of haemoparasites have been identified in the rhinoceros (Penzhorn et al., 1994) in particular protozoan parasites such as, trypanosomes (McCulloch and Achard, 1969, Mihok et al., 1992a, Mihok et al., 1992b) and the recently described piroplasms, Babesia bicornis and Theileria bicornis (Nijhof et al., 2003). Trypanosomes are commonly transmitted by tsetse flies (Glossina spp.) and also cause nagana in livestock (Mihok et al., 1992a), while the genera Babesia and Theileria are haemoparasites transmitted by arthropod vectors, usually ticks. Babesia species such as B. bigemina (Figueroa et al., 1992), B. gibsoni (Conrad et al., 1991), B. divergens (Telford et al., 1993), and B. bovis (Adjou Moumouni et al., 2015) has been identified in many wildlife and domestic animals (Penzhorn, 2006, Uilenberg, 2006) and it causes Babesiosis (tick fever) in cattle (Figueroa et al., 1992, Telford et al., 1993, Adjou Moumouni et al., 2015) and haemolytic anaemia in dogs (Conrad et al., 1991). The symptoms of babesiosis include high fever, anorexia, haemolysis, and even death in severe clinical cases (Conrad et al., 1991, Colly and Nesbit, 1992). B. bicornis was shown to be fatal in small isolated populations of rhinoceros in Tanzania and South Africa (Nijhof et al., 2003, Otiende et al., 2015).
Theileria species could be classified into two groups based on the pathogenesis and lifecycle (Mans et al., 2015). The transforming Theileria species such as T. annulata and T. parva causes tropical theileriosis (TT) and East Coast fever (ECF) respectively and the diseases have been proven to be fatal in bovines (Uilenberg, 1981, Latif et al., 2002, Bishop et al., 2004). The non-transforming Theileria species such as T. orientalis causes oriental theileriosis in cattle (Sugimoto and Fujisaki, 2002, Izzo et al., 2010, Sivakumar et al., 2014); symptoms include muscle weakness, ataxia and even death due to the haemolytic properties of the parasite (Izzo et al., 2010). T. orientalis has caused disease outbreaks in cattle in Australia and New Zealand in recent years due to introduction of a pathogenic genotype of the parasite (Kamau et al., 2011, McFadden et al., 2011, Eamens et al., 2013a, Eamens et al., 2013b, Eamens et al., 2013c). T. bicornis was first identified in the black rhinoceros D. bicornis, in Africa (Nijhof et al., 2003). Since then, there have been reports of T. bicornis in wild rhinoceros (Otiende et al., 2015, Otiende et al., 2016), cattle (Muhanguzi et al., 2010) and other wildlife (Oosthuizen et al., 2009), indicating that the parasite has a broad host range. Epidemiological studies in Kenya showed that T. bicornis is more prevalent in C. simum (66%) as compared to D. bicornis (43%), while factors such as age, sex, location, and population mix did not have any significant impact on prevalence for either species (Otiende et al., 2015). The pathogenic potential of T. bicornis is currently unclear. This parasite is generally considered to be benign, with evidence of endemic stability in some rhinoceros populations (Otiende et al., 2015). However, there have been relatively few studies on T. bicornis and many Theileria species are known to be capable of inducing disease in the host under stress caused by translocation, pregnancy, lactation and general immunosuppression (Sugimoto and Fujisaki, 2002, Eamens et al., 2013b, Hammer et al., 2016). Furthermore, T. bicornis is thought to be related to T. equi which causes equine piroplasmosis, a disease known to be induced by stress (Nijhof et al., 2003, Laus et al., 2015, Otiende et al., 2016). In this study, we identified a new haplotype of T. bicornis in Australian captive rhinoceros housed at Taronga Western Plains Zoo.
2. Materials and methods
2.1. Animals
Nine rhinoceros, C. simum (n = 2), and D. bicornis minor (n = 7) between the ages of one and 24 were included in this study and consisted of both wild-caught animals from Africa and captive bred animals (Table 1).
Table 1.
Summary of the nine animals used in this study.
| Rhinoceros name | Year of birth | Origin (year of introduction to Australia) | Species | Gender | Date sampled |
|---|---|---|---|---|---|
| Aluka | 1996 | Ex-wild, Africa (2002) | Ceratotherium simum | Female | 18/3/2012 |
| Umfana | 1995 | Ex-wild, Africa (2002) | Ceratotherium simum | Male | 20/3/2012 |
| Bakhita | 2002 | Captive-born, Australia | Diceros bicornis minor | Female | 16/5/2016 |
| Chikundo | 2000 | Captive-born, Australia | Diceros bicornis minor | Male | 16/5/2016 |
| Dafari | 2015 | Captive-born, Australia | Diceros bicornis minor | Male | 16/5/2016 |
| Kufara | 2010 | Captive-born, Australia | Diceros bicornis minor | Female | 16/5/2016 |
| Kwanzaa | 1992 | Captive-born, Australia | Diceros bicornis minor | Male | 16/5/2016 |
| Mpenzi | 2005 | Captive-born, Australia | Diceros bicornis minor | Male | 16/5/2016 |
| Siabuwa | 1992 | Ex-wild, Africa (1993) | Diceros bicornis minor | Male | 17/5/2016 |
2.2. Blood smear examination
Blood smears were stained with Giemsa or Diff-Quik and viewed under an Olympus BX50 microscope at 100 × magnification and microscopic image was captured with an Olympus DP70 camera.
2.3. Piroplasmida PCRs
DNA extractions were conducted using the DNeasy blood and tissue DNA extraction kit according to manufacturer's protocol (Qiagen). T. orientalis qPCR was conducted according to a previously described multiplex hydrolysis probe qPCR assay (Bogema et al., 2015). A generic PCR assay targeting the 18S rRNA gene of the Piroplasmida was also conducted using previously described primers Piroplasmid-F: 5′-CCAGCAGCCGCGGTAATT-3′ and Piroplasmid-R: 5′-CTTTCGCAGTAGTTYGTCTTTAACAAATCT-3’ (Tabar et al., 2008, Baneth et al., 2013). Amplification was carried out with the BIOTAQ™ DNA Polymerase kit (Bioline) to make up a 25 μL PCR cocktail containing: 1 X BIOTAQ buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 μM of each primer, 1 unit of BIOTAQ DNA polymerase and molecular grade water. PCR was carried out with the following thermal cycling parameters: Initial denaturation 94 °C for 3 min followed by 35 cycles at 94 °C for 30 s, 64 °C for 45 s, 72 °C for 30 s with a final extension of 72 °C for 7 min. Full length Theileria bicornis 18S rRNA gene was amplified with primers 18SAN, 18SBN (Nijhof et al., 2003) and another pair of internal primers designed for this study, 18S-F1: 5′-GATCCTGCCAGTAGTCATATG-3′ and 18S-R1: 5′-TACTCCCCCCAGAACCCA-3’. PCR products were viewed on a 1.5% agarose 0.5 × TBE gel stained with GelRed (Biotium, USA), purified with QIAquick PCR purification kit (Qiagen) and subjected to Sanger sequencing with the primers described above.
2.4. Molecular phylogeny
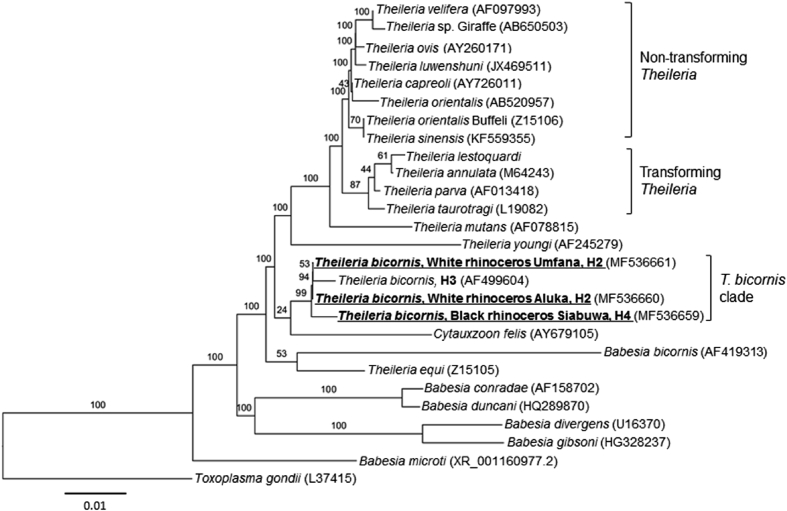
T. bicornis 18S rRNA sequences were aligned using Geneious version (7.1.9) (Kearse et al., 2012) and subjected to a nucleotide BLAST comparison with the Genbank database. Phylogenetic analysis was conducted using DNAdist within the PHYLIP package (Felsenstein, 2005) and a neighbour-joining tree was generated with 1000 bootstrap replicates to estimate phylogenies (Fig. 2). The analysis included 19 Theileria spp., six Babesia spp., one Cytauxzoon sp. and Toxoplasma gondii as the outgroup.
Fig. 2.
Molecular phylogenetic analysis of the piroplasm 18S rRNA gene, including the three rhinoceros samples (Aluka, Umfana and Siabuwa) used in this study. The 18S rRNA sequences were extracted from Genbank during BLAST analysis. The rooted phylogenetic tree was constructed using the neighbour-joining method with T. gondii forming the outgroup. Bootstrap percentages are represented on each node based on 1000 replicates. Phylogenetic analyses were conducted using the PHYLIP packages (Felsenstein, 2005). The haplotypes of the three T. bicornis sequences from this study are indicated at the end of the sequence label.
3. Results
3.1. Blood smear examinations
Piroplasms were observed on blood smears from Aluka, but in Umfana, there were observations of red cell inclusions reflected by dot forms, signet ring forms and also rod forms on Giemsa-stained smears, but not Diff-Quik-stained smears. Given that Umfana was an asymptomatic animal, the clinical significance of these inclusions was doubtful.
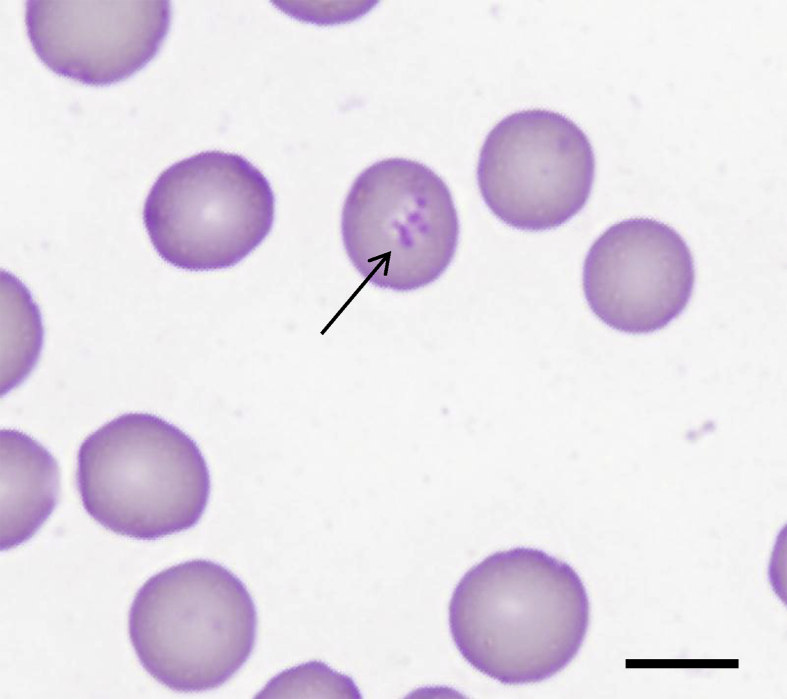
Serum biochemistry results from the D. bicornis cohort were generally unremarkable, with minor elevations in alkaline phosphatase and aspartate aminotransferase (AST) in some animals. Siabuwa additionally displayed elevated creatine kinase (CK; 1154 U/L, reference range 142–742 U/L) and blood urea nitrogen (BUN; 9.0 mmol/L, reference range 2.5–8.1 mmol/L). Siabuwa was positive for piroplasms on blood smear (Fig. 1), while all other D. bicornis tested negative. Piroplasm morphology resembled and was in the size range (1.5 μm) of Theileria spp (Izzo et al., 2010). rather than Babesia spp. and was generally of the comma-shaped form (Fig. 1), although ring forms were also occasionally observed.
Fig. 1.
Photomicrograph of Diff-Quik stained blood smear from black rhinoceros, Siabuwa infected with T. bicornis. The morphology of T. bicornis is not well-described in the literature. We observed comma-shaped piroplasms approximately 1.5 μm in length closely resembling T. orientalis. Ring forms were also occasionally observed (not shown). Bar = 5 μm.
3.2. PCR amplification and sequencing
All animals tested negative for Theileria orientalis. Despite a negative blood smear, Aluka tested positive for Piroplasmida 18S rRNA using a generic piroplasmid PCR. Umfana, the asymptomatic male C. simum which returned a positive blood smear, also tested positive for piroplasmids. Of the seven D. bicornis tested, only one (Siabuwa) returned a positive PCR result for Piroplasmida, which was also consistent with the blood smear results. Sequencing of the 18S rRNA amplicons revealed the presence of T. bicornis in all three samples. Additional PCR amplification of the 18S rRNA gene resulted in 1729 bp, 1691 bp and 1616 bp of sequence from the samples of white rhinoceros Umfana (MF536661), Aluka (MF536660) and black rhinoceros Siabuwa (MF536659) respectively.
3.3. Molecular phylogeny
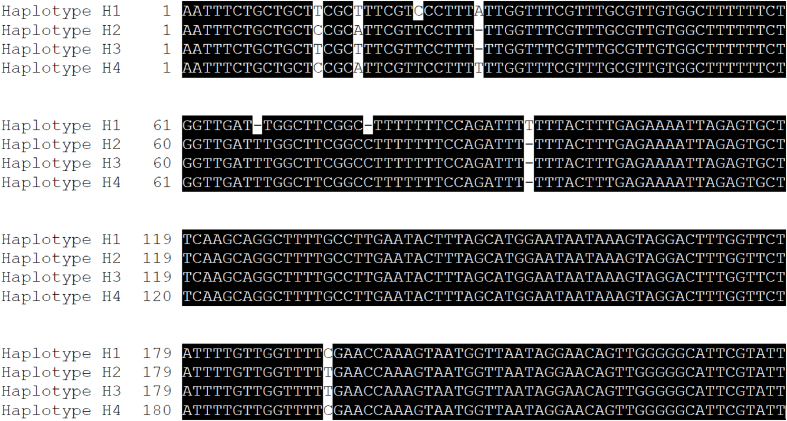
Phylogenetic analysis of 1474 bp of the 18S rRNA gene of the C. simum and D. bicornis piroplasmid strains demonstrates the close relationship to T. bicornis (Fig. 2). Furthermore, the T. bicornis cluster falls outside the non-transforming and transforming Theileria groups along with Cytauxzoon felis and Theileria equi (Fig. 2), which is consistent with prior studies (Nijhof et al., 2003, Schreeg et al., 2016). Alignment of the 18S rRNA genes also revealed the presence of two haplotypes of T. bicornis H2, which was previously described (Otiende et al., 2016) and alignment of the 396 bp T. bicornis haplotypes revealed a new T. bicornis haplotype, H4, identified in this study (see Fig. 2, Fig. 3). The T. bicornis haplotype in the two infected white rhinoceros, Aluka and Umfana was 100% homologous to haplotype H2; however, a new haplotype, H4, was identified in D. bicornis. H4 (accession number MF567493) in the infected black rhinoceros (Siabuwa) is 99% homologous to the H2 (accession number KC771141).
Fig. 3.
Truncated alignment of the 18S rRNA T. bicornis haplotypes including the new haplotype H4 identified in this study. Geneious version (7.1.9) (Kearse et al., 2012) was used to generate alignments to highlight the differences between the four T. bicornis haplotypes. 18S rRNA sequences of the three previously described T. bicornis haplotypes H1 to H3 (accession numbers KC771140 to KC771142 respectively) were extracted from Genbank for this analysis. The new T. bicornis haplotype H4 was submitted to Genbank and assigned accession number MF567493.
4. Discussion
In this study, we examined blood samples from captive C. simum and D. bicornis housed at Taronga Western Plains Zoo, Australia for piroplasmid parasites. Haemoparasites such as trypanosomes (McCulloch and Achard, 1969, Mihok et al., 1992a, Mihok et al., 1992b) and Babesia bicornis (Nijhof et al., 2003) have been associated with rhinoceros mortalities in Africa and were considered in the differential diagnosis of a 16 yr old female C. simum; however, neither of these parasites was detected. Theileria bicornis was detected in blood samples from both dead (Aluka) and asymptomatic (Umfana) C. simum via PCR; however, piroplasms were only observed in blood films from Umfana, suggesting that the T. bicornis infection in Aluka was of a low intensity. The pathogenic potential of T. bicornis is not fully understood as this organism has been poorly studied; however asymptomatic infections are common in both black and white rhinoceroses in Africa (Otiende et al., 2015). Only a single case of T. bicornis infection has been recorded in association with rhinoceros deaths although it presented as a coinfection with B. bicornis (Nijhof et al., 2003, Otiende et al., 2015). Thus, the presence of T. bicornis was considered an incidental finding unlikely to be involved in the C. simum mortality.
PCR screening of the captive population of D. bicornis for piroplasmids revealed only a single T. bicornis-positive animal (Siabuwa), which was also confirmed by blood smear. This animal had elevated AST and CK levels that were potentially linked to tissue damage, but blood parameters were otherwise normal. The blood profile of Siabuwa was similar to a piroplasm prevalence study in white rhinoceroses by Govender et al. (2011) where blood parameters of the animals had no significant changes as well. Siabuwa was translocated from Africa as were the two T. bicornis-positive C. simum, while the remaining 6 captive-bred D. bicornis were T. bicornis negative. This suggests that T. bicornis was introduced to Australia with wild-caught rhinoceros and that transmission amongst the Australian captive population did not occur. Currently, the vectors identified to be capable of transmitting T. bicornis are Dermacentor rhinocerinus and Amblyomma rhinocerotis (Knapp et al., 1997, Otiende et al., 2016). These ticks are present in Africa, have not been identified in Australia and are characterized by long, sturdy mouthparts capable of penetrating the thick rhinoceros hide (Horak et al., 2017). Whether endemic Australian tick species are competent vectors of T. bicornis and other rhinoceros blood parasites is unclear, but they may lack the necessary mouthparts to achieve transmission. Transplacental transmission has been demonstrated for several piroplasmid species (Phipps and Otter, 2004, Fukumoto et al., 2005, Mierzejewska et al., 2014, Zakian et al., 2014, Sudan et al., 2015, Swilks et al., 2017) but tends to occur only at low frequencies and there was no evidence from this study that T. bicornis was transmitted via this route within the captive population.
Sequence alignments of H2 and H4 revealed a difference of a single nucleotide substitution and a single thymine nucleotide insertion. Current literature of T. bicornis haplotype indicates H1 and H3 to only occur in black rhinoceros and H2 in white rhinoceros (Table 1 in Otiende et al., 2016). Whether haplotype H4 only occurs in black rhinoceros or any of these haplotypes contributes to disease remains unknown, but further studies could be done to determine haplotype specificity for a particular host or if the haplotypes play a pathogenic role in infected animals. Phylogenetic analysis placed the T. bicornis clade in this study outside the transforming and non-transforming clades of the Theileria group (Fig. 2). T. bicornis appears to be close relatives of C. felis, T. youngi, B. bicornis and T. equi which are consistent to previous studies (Nijhof et al., 2003, Otiende et al., 2016). The complete lifecycle of T. bicornis has not been established but it has similar characteristics to the non-transforming Theileria group suggesting it may be a largely benign parasite (Nijhof et al., 2003). However as both horses and rhinoceros are odd-toed ungulates classified under the order Perissodactyla, it is worth to noting that T. equi that causes clinical equine piroplasmosis (Nijhof et al., 2003, Laus et al., 2015, Otiende et al., 2015, Otiende et al., 2016), was recently detected in rhinoceros (Govender et al., 2011).
Piroplasmids have coevolved with their hosts (Otiende et al., 2015). Benign infections are common in non-transforming Theileria species, for example T. orientalis genotype Buffeli in cattle (Kamau et al., 2011) and T. velifera (Uilenberg, 1981, Mans et al., 2015). Some of these haemoparasites can persist as lifelong infections in the host, only causing clinical signs when the animals are immunosuppressed or undergo stress from translocation, rearing conditions or pregnancy (Sugimoto and Fujisaki, 2002). Translocation of the rhinoceros in Africa to suitable and safe environments is integral for the conservation of these magnificent animals. However, translocation stress has been reported to decrease PCV levels (Kock et al., 1999) and is also linked to immune suppression in the animals which can lead to morbidity and/or fatality (Glaser and Kiecolt-Glaser, 2005, Martin, 2009, Otiende et al., 2015). Thus screening of animals for haemoparasites prior to translocation would be prudent for future breeding programs.
5. Conclusion
We revealed for the first time in Australia the presence of T. bicornis in both white and black rhinoceros. Evidence from this study suggests that the parasite was acquired in Africa and was not transmitted within the captive rhinoceros population within Australia. A new T. bicornis haplotype, H4, has been identified. T. bicornis infection intensity was low and haematological parameters within infected rhinoceros were unremarkable suggesting that infection with this parasite was likely incidental rather than the cause of the 2012 white rhinoceros mortality event. However, given that translocation-induced stress is a major trigger factor for theileriosis; future screening of translocated rhinoceros would be prudent to ensure successful breeding programs.
Accession numbers
The three 18S rRNA sequences and new T. bicornis haplotype H4 sequence of the rhinoceroses have been deposited in Genbank and were assigned accession numbers: MF536659 (Siabuwa), MF536660 (Aluka), MF536661 (Umfana) and MF567493 (T. bicornis haplotype H4).
Author's contributions
CJ and DB conceived the study and together with JY designed the study. JY performed the molecular analysis, interpretation of data and drafted the manuscript. SG performed the microscopic analysis and imaging. BB and MC collected the samples and performed the biochemical analysis. CJ assisted with data interpretation and provided critical comments on the manuscript. All authors read and approved the final manuscript.
Declaration of interest
Declarations of interest: none.
Funding
This study was funded by the NSW Department of Primary Industries.
Acknowledgements
JY is a grateful recipient of the International Research Scholarship (UTS-IRS) and i3 Institute Postgraduate Research Scholarship through the University of Technology Sydney.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2017.12.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adjou Moumouni P.F., Aboge G.O., Terkawi M.A., Masatani T., Cao S., Kamyingkird K., Jirapattharasate C., Zhou M., Wang G., Liu M., Iguchi A., Vudriko P., Ybanez A.P., Inokuma H., Shirafuji-Umemiya R., Suzuki H., Xuan X. Molecular detection and characterization of Babesia bovis, Babesia bigemina, Theileria species and Anaplasma marginale isolated from cattle in Kenya. Parasit. Vector. 2015;8:496. doi: 10.1186/s13071-015-1106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneth G., Sheiner A., Eyal O., Hahn S., Beaufils J.P., Anug Y., Talmi-Frank D. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasites Vectors. 2013;6:102. doi: 10.1186/1756-3305-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R., Musoke A., Morzaria S., Gardner M., Nene V. Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitol. 2004;129:S271–S283. doi: 10.1017/s0031182003004748. [DOI] [PubMed] [Google Scholar]
- Bogema D.R., Deutscher A.T., Fell S., Collins D., Eamens G.J., Jenkins C. Development and validation of a quantitative PCR assay using multiplexed hydrolysis probes for detection and quantification of Theileria orientalis isolates and differentiation of clinically relevant subtypes. J. Clin. Microbiol. 2015;53:941–950. doi: 10.1128/JCM.03387-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colly L., Nesbit J. Fatal acute babesiosis in a juvenile wild dog (Lycaon pictus) J. S. Afr. Vet. Assoc. 1992;63:36–38. [PubMed] [Google Scholar]
- Conrad P., Thomford J., Yamane I., Whiting J., Bosma L., Uno T., Holshuh H., Shelly S. Hemolytic anemia caused by Babesia gibsoni infection in dogs. J. Am. Vet. Med. Assoc. 1991;199:601–605. [PubMed] [Google Scholar]
- Eamens G.J., Bailey G., Gonsalves J.R., Jenkins C. Distribution and temporal prevalence of Theileria orientalis major piroplasm surface protein types in eastern Australian cattle herds. Aust. Vet. J. 2013;91:332–340. doi: 10.1111/avj.12078. [DOI] [PubMed] [Google Scholar]
- Eamens G.J., Bailey G., Jenkins C., Gonsalves J.R. Significance of Theileria orientalis types in individual affected beef herds in New South Wales based on clinical, smear and PCR findings. Vet. Parasitol. 2013;196:96–105. doi: 10.1016/j.vetpar.2012.12.059. [DOI] [PubMed] [Google Scholar]
- Eamens G.J., Gonsalves J.R., Jenkins C., Collins D., Bailey G. Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet. Parasitol. 2013;191:209–217. doi: 10.1016/j.vetpar.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Department of Genome Sciences, University of Washington; Seattle: 2005. PHYLIP (Phylogeny Inference Package) Version 3.6. Distributed by the author. [Google Scholar]
- Figueroa J., Chieves L., Johnson G., Buening G. Detection of Babesia bigemina-infected carriers by polymerase chain reaction amplification. J. Clin. Microbiol. 1992;30:2576–2582. doi: 10.1128/jcm.30.10.2576-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S., Suzuki H., Igarashi I., Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. Int. J. Parasitol. 2005;35:1031–1035. doi: 10.1016/j.ijpara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Govender D., Oosthuisen M.C., Penzhorn B.L. Piroplasm parasites of white rhinoceroses (Ceratotherium simum) in the Kruger National Park, and their relation to anaemia. J. S. Afr. Vet. Assoc. 2011;82:36–40. [PubMed] [Google Scholar]
- Hammer J.F., Jenkins C., Bogema D., Emery D. Mechanical transfer of Theileria orientalis: possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasites Vectors. 2016;9:34. doi: 10.1186/s13071-016-1323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak I., Boshoff C., Cooper D., Foggin C., Govender D., Harrison A., Hausler G., Hofmeyr M., Macfadyen D., Nel P., Peinke D., Squarre D., Zimmermann D., Horak I., Boshoff C., Cooper D., Foggin C., Govender D., Harrison A., Hausler G., Hofmeyr M., Macfadyen D., Nel P., Peinke D., Squarre D., Zimmermann D. Parasites of domestic and wild animals in South Africa. XLIX. Ticks (Acari: Ixodidae) infesting white and black rhinoceroses in southern Africa. Onderstepoort J. Vet. Res. 2017;84:1–11. doi: 10.4102/ojvr.v84i1.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo M.M., Poe I., Horadagoda N., De Vos A.J., House J.K. Haemolytic anaemia in cattle in NSW associated with Theileria infections. Aust. Vet. J. 2010;88:45–51. doi: 10.1111/j.1751-0813.2009.00540.x. [DOI] [PubMed] [Google Scholar]
- Kamau J., de Vos A.J., Playford M., Salim B., Kinyanjui P., Sugimoto C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasites Vectors. 2011;4:22. doi: 10.1186/1756-3305-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S.E., Krecek R.C., Horak I.G., Penzhorn B.L. Helminths and arthropods of black and white rhinoceroses in southern Africa. J. Wildl. Dis. 1997;33:492–502. doi: 10.7589/0090-3558-33.3.492. [DOI] [PubMed] [Google Scholar]
- Kock R.A., Mihok S.R., Wambua J., Mwanzia J., Saigawa K. Effects of translocation on hematologic parameters of free-ranging black rhinoceros (Diceros bicornis michaeli) in Kenya. J. Zoo Wildl. Med. 1999;30:389–396. [PubMed] [Google Scholar]
- Latif A., Hove T., Kanhai G., Masaka S. Buffalo-associated Theileria parva: the risk to cattle of buffalo translocation into the Highveld of Zimbabwe. Ann. N. Y. Acad. Sci. 2002;969:275–279. doi: 10.1111/j.1749-6632.2002.tb04392.x. [DOI] [PubMed] [Google Scholar]
- Laus F., Spaterna A., Faillace V., Veronesi F., Ravagnan S., Beribé F., Cerquetella M., Meligrana M., Tesei B. Clinical investigation on Theileria equi and Babesia caballi infections in Italian donkeys. BMC Vet. Res. 2015;11:100. doi: 10.1186/s12917-015-0411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans B.J., Pienaar R., Latif A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites. Wildl. 2015;4:104–118. doi: 10.1016/j.ijppaw.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.B. Stress and immunity in wild vertebrates: timing is everything. Gen. Comp. Endocrinol. 2009;163:70–76. doi: 10.1016/j.ygcen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- McCulloch B., Achard P.L. Mortalities associated with the capture, translocation, trade and exhibition of black rhinoceroses Diceros bicornis. Int. Zoo Yearbk. 1969;9:184–191. [Google Scholar]
- McFadden A.M.J., Rawdon T.G., Meyer J., Makin J., Morley C.M., Clough R.R., Tham K., Müllner P., Geysen D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naïve cattle. N. Z. Vet. J. 2011;59:79. doi: 10.1080/00480169.2011.552857. [DOI] [PubMed] [Google Scholar]
- Mierzejewska E.J., Welc-Faleciak R., Bednarska M., Rodo A., Bajer A. The first evidence for vertical transmission of Babesia canis in a litter of Central Asian Shepherd dogs. Ann. Agric. Environ. Med. 2014;21:500–503. doi: 10.5604/12321966.1120590. [DOI] [PubMed] [Google Scholar]
- Mihok S., Munyoki E.L.I., Brett R.A., Jonyo J.F., RÖTtcher D., Majiwa P.A.O., Kang'Ethe E.K., Kaburia H.F.A., Zweygarth E. Trypanosomiasis and the conservation of black rhinoceros (Diceros bicornis) at the ngulia rhino sanctuary, tsavo west national park, Kenya. Afr. J. Ecol. 1992;30:103–115. [Google Scholar]
- Mihok S., Olubayo R.O., Moloo S.K. Trypanosomiasis in the black rhinoceros (Diceros bicornis Linnaeus, 1758) Revue scientifique et technique (International Office of Epizootics) 1992;11:1169–1173. doi: 10.20506/rst.11.4.651. [DOI] [PubMed] [Google Scholar]
- Muhanguzi D., Matovu E., Waiswa C. Prevalence and characterization of Theileria and Babesia Species in cattle under different husbandry systems in western Uganda. Int. J. Anim. Vet. Adv. 2010;2:51–58. [Google Scholar]
- Nijhof A.M., Penzhorn B.L., Lynen G., Mollel J.O., Morkel P., Bekker C.P.J., Jongejan F. Babesia bicornis sp. nov. and Theileria bicornis sp. nov.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis) J. Clin. Microbiol. 2003;41:2249–2254. doi: 10.1128/JCM.41.5.2249-2254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen M.C., Allsopp B.A., Troskie M., Collins N.E., Penzhorn B.L. Identification of novel Babesia and Theileria species in South african giraffe (Giraffa camelopardalis, linnaeus, 1758) and roan antelope (Hippotragus equinus, desmarest 1804) Vet. Parasitol. 2009;163:39–46. doi: 10.1016/j.vetpar.2009.03.045. [DOI] [PubMed] [Google Scholar]
- Otiende M.Y., Kivata M.W., Jowers M.J., Makumi J.N., Runo S., Obanda V., Gakuya F., Mutinda M., Kariuki L., Alasaad S. Three novel haplotypes of Theileria bicornis in black and white rhinoceros in Kenya. Transbound. Emerg. Dis. 2016;63:e144–150. doi: 10.1111/tbed.12242. [DOI] [PubMed] [Google Scholar]
- Otiende M.Y., Kivata M.W., Makumi J.N., Mutinda M.N., Okun D., Kariuki L., Obanda V., Gakuya F., Mijele D., Soriguer R.C., Alasaad S. Epidemiology of Theileria bicornis among black and white rhinoceros metapopulation in Kenya. BMC Vet. Res. 2015;11:4. doi: 10.1186/s12917-014-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzhorn B., Krecek R., Horak I., Verster A., Walker J., Boomker J., Knapp S., Quandt S. vols. 1–4. 1994. Parasites of African rhinos: a documentation; pp. 1–242. (Proceedings of a Symposium on Rhinos as Game Ranch Animals). [Google Scholar]
- Penzhorn B.L. Babesiosis of wild carnivores and ungulates. Vet. Parasitol. 2006;138:11–21. doi: 10.1016/j.vetpar.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Phipps L.P., Otter A. Transplacental transmission of Theileria equi in two foals born and reared in the United Kingdom. Vet. Rec. 2004;154:406–408. doi: 10.1136/vr.154.13.406. [DOI] [PubMed] [Google Scholar]
- Schreeg M.E., Marr H.S., Tarigo J.L., Cohn L.A., Bird D.M., Scholl E.H., Levy M.G., Wiegmann B.M., Birkenheuer A.J. Mitochondrial genome sequences and structures aid in the resolution of piroplasmida phylogeny. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar T., Hayashida K., Sugimoto C., Yokoyama N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014;27:250–263. doi: 10.1016/j.meegid.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Sudan V., Singh S.K., Jaiswal A.K., Parashar R., Shanker D. First molecular evidence of the transplacental transmission of Theileria annulata. Trop. Anim. Health Prod. 2015;47:1213–1215. doi: 10.1007/s11250-015-0835-2. [DOI] [PubMed] [Google Scholar]
- Sugimoto C., Fujisaki K. Non-transforming Theileria parasite of ruminants. In: McKeever D., Dobbelaere D., editors. Theileria. Kluwer Academic Publishers; Boston, London: 2002. pp. 93–106. [Google Scholar]
- Swilks E., Fell S.A., Hammer J.F., Sales N., Krebs G.L., Jenkins C. Transplacental transmission of Theileria orientalis occurs at a low rate in field-affected cattle: infection in utero does not appear to be a major cause of abortion. Parasites Vectors. 2017;10:227. doi: 10.1186/s13071-017-2166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar M.D., Altet L., Francino O., Sánchez A., Ferrer L., Roura X. Vector-borne infections in cats: molecular study in Barcelona area (Spain) Vet. Parasitol. 2008;151:332–336. doi: 10.1016/j.vetpar.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Telford S.R., Gorenflot A., Brasseur P., Spielman A. Babesial infections in humans and wildlife. Parasitic protozoa. 1993;5:1–47. [Google Scholar]
- Uilenberg G. Theilerial species of domestic livestock. In: Irvin A.D., Cunningham M.P., Young A.S., editors. Advances in the Control of Theileriosis: Proceedings of an International Conference Held at the International Laboratory for Research on Animal Diseases in Nairobi, 9–13th February, 1981. Springer; Netherlands, Dordrecht: 1981. pp. 4–37. [Google Scholar]
- Uilenberg G. Babesia—a historical overview. Vet. Parasitol. 2006;138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Zakian A., Nouri M., Barati F., Kahroba H., Jolodar A., Rashidi F. Vertical transmission of Theileria lestoquardi in sheep. Vet. Parasitol. 2014;203:322–325. doi: 10.1016/j.vetpar.2014.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.