Abstract
The toads Rhinella spp. are in constant contact with humans and domestic animals and are commonly parasitized by ticks, which are also potential vectors of pathogenic microorganisms, such as apicomplexans and rickettsia. However, little is known about microorganisms associated with toad ticks. In this work, we molecularly evaluated the presence of Rickettsia spp. and hemogregarines in ticks of Rhinella horribilis and R. humboldti in the Colombian Caribbean, finding two different species of Rickettsia: the colombianensi strain and one close to R. bellii. In the case of hemogregarines, since only 18S gene sequences are available, it is difficult to define species and place them correctly in a phylogeny, but most of our samples show a 99% identity with Hemolivia stellata, while others identical to each other seem to form another clade within this genre. All collected ticks were identified as Amblyomma dissimile, representing the first time that H. stellata was recorded in this tick. The prevalence of both microorganisms was very high, which makes it necessary to generate robust phylogenies to clarify their taxonomic diversity and to correctly define their ecological role and pathogenicity, which should be taken into account in amphibian conservation plans and veterinary medicine.
Keywords: Amblyomma dissimile, Rhinella spp., Hemolivia spp., Amphibian host
Graphical abstract
Highlights
-
•
Amblyomma dissimile ticks are reported in Rhinella humboldti and R. horribillis.
-
•
Rickettsia was not detected in toad tissues but it was detected in ticks with high prevalence.
-
•
Hemogregarins were detected in all types of tissue analyzed.
-
•
Two Rickettsia species were detected, the Colombianensi strain and a R. bellii-like.
-
•
Two possible Hemolivia species were detected, mostly H. stellata.
1. Introduction
There are several types of ectoparasites that transmit pathogens to anurans, among which are leeches, mosquitoes and ticks. Within the latter, nine species of the family Ixodidae and one of Argasidae are known; they parasitize toads of the genus Rhinella (Burridge, 2011; Bermúdez et al., 2013), and Amblyomma dissimile and Amblyomma rotundatum are the most common. Both are tri-host ticks that generally use amphibians and reptiles as hosts during their life cycle, although they can accidentally parasitize birds and mammals, including humans (Lampo et al., 1997; Guglielmone et al., 2006); Amblyomma dissimile has a greater diversity of hosts, but within anurans, it is only recorded in toads of the Bufonidae family (Guglielmone and Nava, 2010).
The parasitism of these ticks leaves clear cutaneous lesions in the toads, weakening the animal and directly increasing the risk of subsequent infections, whether primary infections, starting from cutaneous lesions, or secondary infections through vector transmission of microorganisms, which is considered a mechanism of population regulation for the hosts (Lampo and Bayliss, 1996; Smith et al., 2008). Although these infections often show no symptoms in wild vertebrates, there is evidence of pathologies, such as anemia, erythrocytic hypertrophy and loss of hemoglobin, along with the possibility of causing phenotypic and phenological changes in the vectors (Desser et al., 1995; Wozniak et al., 1996; Ferguson et al., 2013), especially in infections by hemogregarines (Gupta et al., 2012; Rodrigues-Calil et al., 2017).
This artificial group is composed of six Apicomplexa genera (Barta et al., 2012), of which only Hepatozoon and Hemolivia parasitize the anurans (Maia et al., 2016). In fact, the latter was described in its natural hosts Rhinella marina (s.l.) and the tick Amblyomma rotundatum, with the species Hemolivia stellata (Petit et al., 1990). Like this one, its congeners use heterothermic vertebrates as intermediate hosts and ticks as definitive hosts (Karadjian et al., 2015) and can also be transmitted through the consumption of other infected vertebrates (Davies and Johnston, 2000).
At the same time, ticks are important vectors of bacteria to multiple vertebrate groups. The genus Rickettsia (Rickettsiales: Rickettsiaceae) has been reported more frequently in Latin America, given its importance in public and veterinary health (Witter et al., 2016); despite this incidence, in the country, the knowledge of transmission to amphibians is scarce. Studies focused on vectors have resulted in the discovery of several species of unknown pathogenicity, thanks to the progressive improvement of detection techniques. Such is the case of the Rickettsia sp. Colombianensi strain in reptile ticks (Miranda et al., 2012; Santodomingo et al., 2018); this species is phylogenetically related to R. monacensis, which was considered nonpathogenic when it was discovered but is currently known to cause disease in humans, as has occurred with other species, such as R. aeschlimannii, R. massiliae and R. slovaca in the old world (Parola et al., 2005; Jado et al., 2007).
Despite the great advances in the study of these bacteria and other microorganisms associated with ticks, very little has been reported about the roles played by amphibians and their ticks in the epidemiology of human and animal diseases. In Colombia, there has been no report of pathogens associated with amphibian ticks, with Bufonidae being one of the most abundant and diverse anuran families in the country, where species such as Rhinella marina and R. horribillis are present in urban, rural and wild lowland environments, very close to humans and domestic animals (Acevedo-Rincón et al., 2016; Acosta-Galvis, 2017). Therefore, the objective of this work was to evaluate the presence of Rickettsia spp., Hepatozoon spp. and Hemolivia spp. in ticks and some tissues of toads in the department of Magdalena (Colombia).
2. Materials and methods
2.1. Samples studied
The ticks were taken directly from toads from seven localities located in the department of Magdalena in northern Colombia. The toads were captured under the visual encounter search method and through pitfall traps at two locations. Samples taken from each individual were placed in 1.5-ml vials with 96% ethanol and were maintained at −20 °C until their identification. For the taxonomic identification of ticks, the keys from Voltzit (2007), Osorno-Mesa (1940) and Jones et al. (1972) were used for adult specimens, and the keys from Martins et al. (2010) were used for the nymphs. Larvae were identified using the key from Osorno-Mesa (1940) and were corroborated using molecular methods (the COI gene). Additionally, six individuals of Rhinella horribillis collected at the Universidad del Magdalena were sacrificed, from which ticks, blood, liver, heart and spleen samples were also extracted. Permission for trapping and manipulating the animals as well as collecting the ectoparasites for this study was given by ANLA (Autoridad Nacional de Licencias Ambientales) under the permit no. 1293, and was approved by Universidad del Magdalena Ethical Committee (Acta 001–15).
2.2. DNA extraction
For the adult ticks, the anterior half was used to extract the DNA; for the larvae and nymphs, the complete individual was used. The DNA was extracted individually or in pools, depending on the number of ticks of each stage found in each toad, using the MasterPure ™ DNA Purification Kit (Epicenter, USA). Each pool included up to 5 individuals. DNA extraction of the liver, heart and spleen samples was performed following the instructions of the ISOLATE II Genomic DNA kit, and the MasterPure™ DNA Purification Kit for Blood was used for the blood samples. DNA extraction and its quality were confirmed by means of electrophoresis in agarose gel and with GelRed (Biotium) staining.
2.3. Amplification of COI
Amplification of COI was performed through conventional PCR in an Eppendorf Mastercycler® Pro thermocycler, using the universal primers for invertebrates that amplify the gene: LCO1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) and HCO2198 (5′TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) (Folmer et al., 1994). Amplifications were designed with a volume of 25 μL, containing 3 μL of extracted DNA, 0.5 μL of Taq Polymerase (5 U/μL, Bioline), 1 μL of MgCl (50 mM), 2.5 μL of PCR Buffer (10X), 0.5 μL of dNTPs (10 mM) and 1 μL of each primer (10 pmol). The conditions of the amplifications were as follows: an initial denaturation of 95 °C for 1.5 min, followed by 35 cycles of 94 °C for 30 s, annealing at 45 °C for 1 min, extension at 72 °C for 1 min and a final extension at 72 °C for 7 min.
2.4. Amplification of GltA/16S rRNA/Omp A//from Rickettsia
Amplification was performed by conventional PCR in an Eppendorf Mastercycler® Pro thermal cycler, using the primers with the following sequences: CS-78 (GCAAGTATCGGTGAGGATGTAAT) and CS-323(GCTTCCTTAAAATTCAATAAATCAGGAT) (Labruna et al., 2004). For the PCR, 4 μL of extracted DNA was used, and the final reaction volume was 25 μL, containing 0.5 μL of Taq Polymerase (BIOLASE TM, Bioline), 1 μL of MgCl (50 mM), 2.5 μL of PCR Buffer (10X), 0.5 μL of dNTPs (10 mM) and 1 μL of each primer (10 pmol). The conditions of the amplifications were as follows: an initial denaturation of 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s, annealing at 48 °C for 30 s, extension at 72 °C for 30 s and a final extension at 72 °C for 7 min.
The samples positive for gltA from Rickettsia were further amplified by conventional PCR with the 16S rRNA primer genes (Rick-16S— F3 5′-ATCAGTACGGAATAACTTTTA-3′, Rick-16S- R4 5′-TGCCTCTTGCGTTAGCTCAC- 3′) (Anstead and Chilton, 2013) and ompA primers (OMPA-F 5′- CAC YAC CTC AAC CGC AGC-3′, OMPA-R 5′- AAA GTT ATA TTTCCT AAA CCY GTA TAA KTA TCR GC -3′) (Phan et al., 2011). The reaction was performed in a total volume of 25 μL with the following conditions: initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C (16S rRNA)/52 °C (ompA) for 1 min, extension at 72 °C for 2 min and a final extension at 72 °C for 7 min.
2.5. Amplification of 18S gene of Hepatozoon
The amplification was performed by conventional PCR in an Eppendorf Mastercycler® Pro thermocycler, using primers HepF300 (GTTTCTGACCTATCAGCTTTCGACG) and Hep900 (CAAATCTAAGAATTTCACCTCTGAC) (Ujvari et al., 2004). For the PCR, 4 μL of extracted DNA was used, and the final reaction volume was 25 μL, which contained 0.5 μL of Taq Polymerase (5 U/μL, Bioline), 1 μL of MgCl (50 mM), 2.5 μL of PCR Buffer (10X), 0.5 μL of dNTPs (10 mM) and 1 μL of each primer (10 pmol). The conditions of the amplifications were as follows: an initial denaturation at 94 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, annealing at 60 °C for 30 s, extension at 72 °C for 1 min and a final extension at 72 °C for 10 min. The products obtained from the amplification of COI, gltA, 16S, ompA and 18S were purified with SureClean Plus (Bioline, USA) following the supplier's instructions. These products were sequenced in both directions.
2.6. Analysis of the sequences
The sequences were checked using the NCBI BLAST tool (www.ncbi.nlm.nih.gov) and were then edited with ProSeq V3 software (Filatov, 2009). Using the program MEGA 7.0 (Kumar et al., 2016) with the ClustalW algorithm (Thompson et al., 1994), the sequences obtained in this study and others available in GenBank were aligned.
2.7. Phylogenetic analyses
For phylogenetic reconstruction, Bayesian inference and maximum likelihood were used in the MrBayes 3.2.2 (Ronquist et al., 2012) and RAxML 8.0.24 (Stamatakis, 2006) programs, respectively. The best nucleotide substitution model for each of the datasets was selected using the Partition Finder program (Lanfear et al., 2012), with the Bayesian Information Criterion (BIC; Schwarz, 1978). The GTR + G model was implemented for the 1st and 2nd codon positions of the gltA gene and for 2nd and 3rd codon positions of OmpA; the GTR model was used for the 3rd codon position of gltA, and the GTR + I + G model was used for the 16S gene (Rickettsia spp.). The 18S (hemogregarines) was analyzed using the GTR + G model.
Two independent runs of 10, 000, 000 generations were used, sampling the trees every 100 generations, discarding 25% of the trees. The standard deviation of the independent sequences (<0.01) was used to validate the convergence, grouping the likelihood values over time and using the SumP command in MrBayes. The posterior probability of each clade was measured based on the percentage of trees that recovered that particular clade (Huelsenbeck and Ronquist, 2001). For the maximum likelihood analyses, the fast-scaling algorithm was used with 1000 Bootstrap (BP) pseudoreplicas, taking BP values greater than 70% as a high statistical support (Hillis and Bull, 1993).
3. Results
A total of 208 ticks were collected (16 adults, 90 nymphs and 102 larvae), which were identified as Amblyomma dissimile and Amblyomma cf. dissimile. 4 nymphs and 6 larvae were extracted from 5 R. humboldti toads, whereas the rest were collected from 44 R. horribilis individuals. Specifically, 1 to 3 ticks were collected per R. humboldti toad, whereas we found an average of 4.5 ticks per R. horribilis toad. We analyzed 9 adults, 41 pools of nymphs and 32 pools of larvae using molecular methods, including 4 pools of nymphs and 4 pools of larvae from R. humboldti and 5 females, 4 males, 37 pools of nymphs and 28 pools of larvae from R. horribilis.
Rickettsia DNA was detected in 5/9 (55%) adults, 24/41 (57%) pools of nymphs and 28/32 (88%) pools of larvae, including 2 females, 3 males, 22 pools of nymphs and 24 pools of larvae from R. horribilis, and 2 pools of nymphs and 4 pools of larvae from R. humboldti. None of the tissues was positive for Rickettsia. The percentage of positivity for both Rickettsia and for hemogregarines was expressed as a minimum infection rate (MIR), assuming that one tick was positive in each positive pool.
Hemogregarine DNA was detected in 6/9 adults (66%), 33/41 (80%) pools of nymphs and 18/32 (56%) pools of larvae including 3 females, 3 males, 31 pools of nymphs and 16 pools of larvae from R. horribilis and 2 pools of nymphs and 2 pools of larvae from R. humboldti. Tissues from the six toads were also found positive for hemogregarine DNA; however, we successfully sequenced tissues from only two. The first was a blood sample (cod HSS3) of an individual who also had a positive female tick (cod NH114). The second individual was found positive for liver, blood and spleen tissues (cod HHS7, HSS7, HBS7 respectively). Among the infected ticks, 5 adults, 13 pools of nymphs and 18 pools of larvae from R. horribilis and 2 pools of nymphs and 4 pools of larvae from R. humboldti were coinfected with both Rickettsia and hemogregarine DNA.
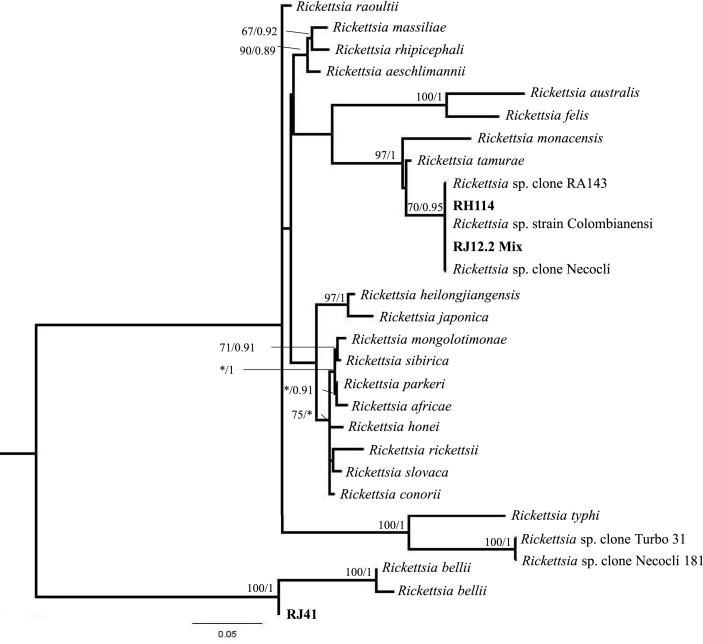
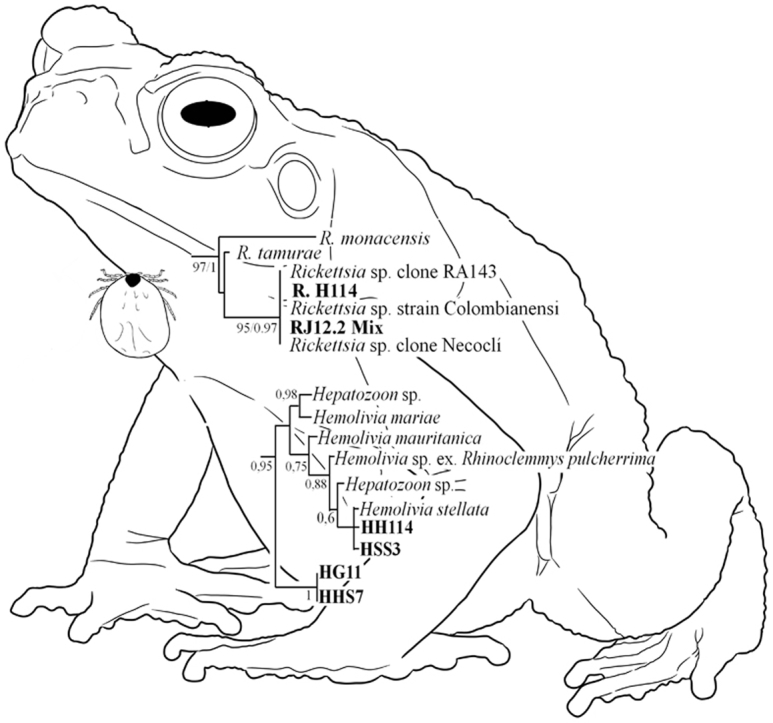
All COI sequences showed 99% identity with A. dissimile. The sequences of the genes gltA and ompA showed identities of 100% with Rickettsia sp. strain Colombianensi (e.g., JF905456.1/MF034497.1) and 99% with R. tamurae sequences (e.g., KT753265.1) and R. monacensis (e.g., KU586332.1). GltA sequence corresponding to the J41 sample (pool of larvae from R. horribilis) had 99% identity with R. bellii (e.g., NR_074484.2). The phylogenetic analyses also showed the proximity between our sequences with the group conformed by R. monacensis, Rickettsia strain Colombianensi and R. tamurae, and the sample J41 with R. belli (Fig. 1, Table 1).
Fig. 1.
Tree topology of phylogenetic analyses by Maximum Likelihood and Bayesian Inference including concatenated sequences of the genes gltA, ompA and 16S obtained in this work and others from GenBank (Table 1). The numbers correspond to the values of Bootstrap/posterior probabilities.
Table 1.
Sequences of Rickettsia downloaded from GenBank and generated in this study (in bold) that were included in the phylogenetic analyses shown in Fig. 1.
| Species | Genes | GenBank accesion numbers |
|---|---|---|
| Rickettsia sp. clone RA143 | gltA | KY996395 |
| Rickettsia sp. Strain Colombianensi | gltA/ompA/16S | JF905456/KF691749/KF691750 |
| Rickettsia sp. clone Necocli 190 | gltA | JX519583 |
| Rickettsia tamurae strain AT-1 | gltA/ompA/16S | AF394896/DQ103259/NR_042727 |
| Rickettsia monacensis | gltA/ompA/16S | DQ100163/LN794217/LN794217 |
| Rickettsia sibirica sibirica | gltA/ompA/16S | KM288711/KM288712 |
| Rickettsia mongolotimonae | gltA/ompA | DQ097081/DQ097082 |
| Rickettsia slovaca | gltA/ompA | U59725/CP002428 |
| Rickettsia conorii | gltA | U59728 |
| Rickettsia parkeri | gltA/ompA | KJ158742/KJ158741 |
| Rickettsia africae | gltA/ompA | U59733/CP001612 |
| Rickettsia japonica | gltA/ompA/16S | AY743327/AP011533/AP011533 |
| Rickettsia heilongjiangensis | gltA/ompA/16S | AY285776/AH012829/NR_041770 |
| Rickettsia honei | gltA | AF018074 |
| Rickettsia raoultii | gltA/16S | KU310590/KR608783 |
| Rickettsia aeschlimannii | gltA/ompA/16S | AY259084/AY259083/KT318741 |
| Rickettsia massiliae | gltA/ompA/16S | HM050293/DQ494551/L36106 |
| Rickettsia_rhipicephali | gltA/ompA | DQ865206/DQ865208 |
| Rickettsia australis | gltA/16S | U59718/U12459 |
| Rickettsia sp. clone Turbo 31 | gltA | JX519576 |
| Rickettsia sp. clone Necocli 181 | gltA | JX519577 |
| Rickettsia typhi | gltA/16S | U59714/NR_074394 |
| Rickettsia bellii | gltA/16S | JQ519684/U11014 |
| Rickettsia rickettsii | gltA/ompA/16S | CP006009 |
| Rickettsia bellii | gltA/16S | CP015010 |
| Rickettsia felis | gltA/16S | CP000053 |
| Rickettsia sp. strain Colombianensi (RJ12.2 MIX) | gltA/ompA/16S | MH196484/MH196501/MH196503 |
| Rickettsia sp. strain Colombianensi (RH114) | gltA | MH196496 |
| Rickettsia bellii-like (RJ41) | gltA | MH196501 |
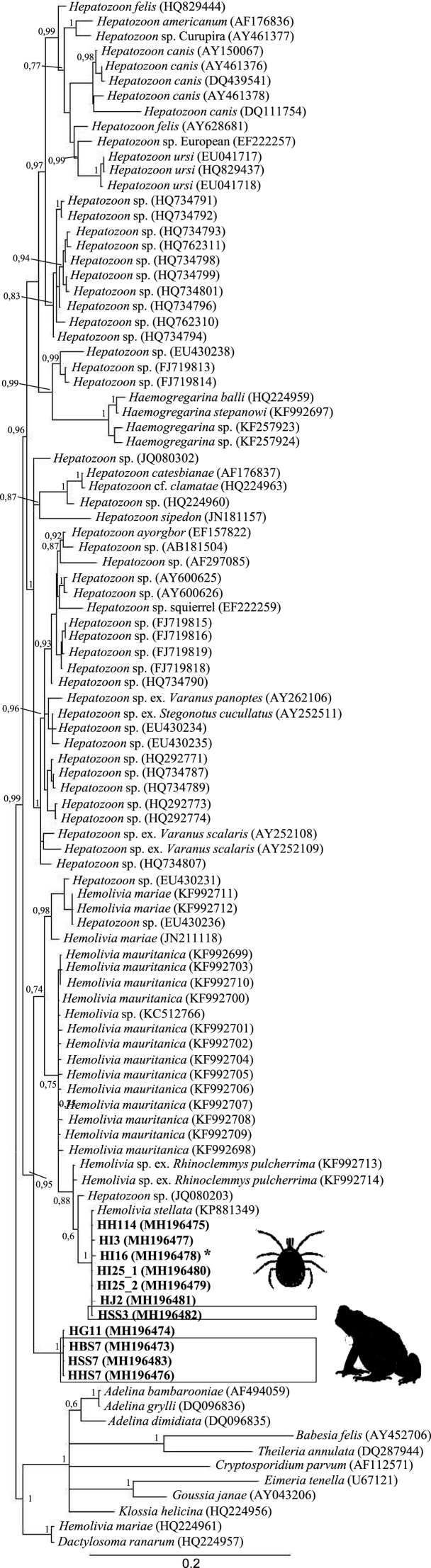
The sequences of the fragments amplified with the Hepatozoon primers showed identities of 99% with Hemolivia stellata sequences (e.g., KP881349.1) and 98% with Hemolivia sp. (e.g., KX347435.1). Bayesian Inference and Maximum Likelihood analyses grouped 6/7 of the samples obtained from ticks with H. stellata and one taken from sample blood. By Bayesian Inference, the remaining sample taken from ticks and the others obtained from the tissues appeared within the clade of Hemolivia as sisters to all of the others (Fig. 2); by Maximum Likelihood, they appeared to be sisters to all of the others except to the group of Hemolivia marie. All our sequences were deposited in GenBank under accession nos. MH196473-MH196505.
Fig. 2.
Tree topology of phylogenetic analysis by Bayesian inference, including sequences of the 18S gene obtained in this work and others from GenBank. The numbers correspond to values of posterior probabilities. Our samples come from blood (HHSS3, HHSS7), spleen (HBS7) and liver (HHS7) from Rhinella horribilis. The rest come from Amblyomma dissimile larvae (HJ2), nymphs (HI3, HI25_1, HI25_2), a male (HH114) and a female (HG11) collected in Rhinella horribilis, and one nymph (HI16) collected in Rhinella humboldti*.
4. Discussion
This is the first report of parasitism by Amblyomma dissimile in Rhinella humboldti; a previous report described A. dissimile using R. horribillis and R. marina as hosts (Osorno-Mesa, 1940; Guglielmone and Nava, 2010). Likewise, this report represents the first time that Hemolivia stellata has been detected in Amblyomma dissimile and the third time that Rickettsia spp. has been reported (Miranda et al., 2012; Santodomingo et al., 2018), with two possible different species detected: Rickettsia sp. strain Colombianensi and another close to Rickettsia bellii. The latter was detected in only one pool of larvae, in contrast, R. bellii had been found in A. rotundatum taken from Rhinella spp. from Brazil and Panama, with high infestation rates (Labruna et al., 2004; Andoh et al., 2015; Horta et al., 2015; Silva et al., 2016).
Despite the high prevalence of Rickettsia spp. in the tick samples, none of the toad tissue samples analyzed here were positive for these bacteria, as shown by Horta et al. (2015), which, although the finding must be corroborated with a larger number of samples, may be due to Rickettsia spp. being transmitted transovarially and interstadially maintained in the vector (Horta et al., 2006; Zemtsova et al., 2010; Sakai et al., 2014), which may mean that ticks had been previously infected and that horizontal transmission does not occur with these hosts, given that Rickettsia spp. most commonly infect homeothermic vertebrates (Parola et al., 2013).
However, De Sousa et al. (2012) found Rickettsia monacencis and Rickettsia helvetica in muscle tissue of the lizard Teira dugesii (Lacertidae) and its Ixodes ricinus ticks. Therefore, it cannot be ruled out that heterothermic hosts such as amphibians and reptiles are sentinels or reservoirs of Rickettsia spp., since there are some studies that demonstrate the presence of Rickettsia in amphibians, though they are not very precise in their identification (Desser and Barta, 1984, 1989; Desser, 1987; Bataille et al., 2018). It is possible that the molecular detection of Rickettsia spp. is not sensitive enough, though, as proposed by Levin et al. (2016), this may vary depending on the stage in which the infection is found.
In contrast, infection by hemogregarines was found in all types of tissue analyzed, and there was also a high prevalence in ticks. Our phylogenetic analyses showed that the samples analyzed corresponded to the genus Hemolivia, with the majority being grouped with H. stellata and the remaining ones forming an independent clade within this genus, using the phylogenetic framework suggested by Kvičerová et al. (2014) and including the only sequence of H. stellata available in GenBank (KP881349). This species of hemogregarine has only been described in Amblyomma rotundatum as the definitive host, R. marina as an intermediate host and, more recently, in Ameiva ameiva (Petit et al., 1990; Lainson et al., 2007).
Although not previously reported in Amblyomma dissimile, Amblyomma rotundatum and Amblyomma dissimile share much of their geographic distribution and hosts, including the toads Rhinella spp. (Guglielmone and Nava, 2010; Nava et al., 2017); therefore, it is possible that H. stellata is also associated with these ticks. However, many authors agree that the identification and phylogenetic positions of these hemogregarines, especially with respect to the genus Hepatozoon, are very imprecise given the lack of biogeographic, morphological, and especially molecular information (Barta et al., 2012; Kvičerová et al., 2014; Karadjian et al., 2015; Maia et al., 2016; O'Donoghue, 2017). Sequences other than those for the 18S gene are necessary, given that 18S sequences are insufficient to resolve the relationship between these groups; in our study, the differences were evidenced by obtaining different topologies with the Maximum Likelihood and Bayesian Inference methods.
In this study, tissue histopathological examinations were not performed, but the tissues studied did not present detectable macroscopic lesions. Shutler et al. (2009) suggest that infection by Hepatozoon sp. in Lithobates clamitans (Ranidae) is benign or undetectable to the immune system. In contrast, Sailasuta et al. (2011) show an infection by two morphotypes of Hepatozoon sp. caused pathological lesions with subacute to chronic inflammation in the liver of Hoplobatrachus rugulosus (Dicroglossidae). These reports illustrate the need to develop reliable phylogenies given their importance in areas such as conservation biology, especially in a group such as amphibians, which have suffered considerable population declines for several decades (Stuart et al., 2004). In addition, it has been shown that R. marina, in areas such as Australia where it is considered invasive, has propitiated the expansion of its natural pathogens, which entails a risk for native amphibians that were previously not exposed (Selechnik et al., 2017).
5. Conclusions
In this study, it is shown that the prevalence of Rickettsia spp. associated with toads is quite high; however, Rickettsia spp. were either not present or not detectable in some tissues of these hosts. Therefore, future studies should focus on elucidating whether or not the toads may be infected with these bacteria to understand the ecological relationship that exists between these bacteria and the toads. In addition, it is imperative that sequences from regions other than the 18S gene be obtained for the phylogenetic identification and location of hemogregarines and to clarify the true vectors and intermediate hosts for these species, their transmission dynamics and their life cycles, to clarify the true diversity in these taxa and their role in the population dynamics of their hosts, which are fundamental for the conservation of a group that is particularly sensitive to climate change, habitat fragmentation and habitat loss.
Conflicts of interest
The authors declare no conflicts of interest.
Financing
This study has been funded by the patrimonial fund for research (Fonciencias) of Universidad del Magdalena [VIN2016104] and the scholarship of young researchers and innovators 706–2015 of the administrative department of science, technology and innovation (COLCIENCIAS) [FP44842-561-2015].
Acknowledgements
We thank Gustavo López Valencia for guiding us in the taxonomic identification of the ticks. To Jorge Eguis, Jefferson Villalba, Sintana Rojas, Harold Cuello, Juan Carlos Dib, Cristhian Cotes, Sebastian Contreras and Juan David Jiménez for their collaboration in the collection of samples. To German Blanco and his students for help in taking tissue samples.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.06.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Acevedo-Rincón A.A., Lampo M., Cipriani R. The cane or marine toad, Rhinella marina (Anura, Bufonidae): two genetically and morphologically distinct species. Zootaxa. 2016;4103:574–586. doi: 10.11646/zootaxa.4103.6.7. [DOI] [PubMed] [Google Scholar]
- Acosta Galvis A.R. 2017. Lista de los Anfibios de Colombia: Referencia en línea V.07.2017.0 (07/01/2018). Página web accesible en.http://www.batrachia.com (Batrachia, Villa de Leyva, Boyacá, Colombia) [Google Scholar]
- Andoh M., Sakata A., Takano A., Kawabata H., Fujita H., Une Y. Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and Amphibians imported into Japan. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstead C., Chilton N. A novel Rickettsia species detected in Vole Ticks (Ixodes angustus) from Western Canada. Appl. Environ. Microbiol. 2013;79(24):7583–7589. doi: 10.1128/AEM.02286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta J.R., Ogedengbe J.D., Martin D.S., Smith T.D. Phylogenetic position of the Adeleorinid coccidia (myzozoa, Apicomplexa, coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Eukaryot. Microbiol. 2012;59(2):171–180. doi: 10.1111/j.1550-7408.2011.00607.x. [DOI] [PubMed] [Google Scholar]
- Bataille A., Lee-Cruz L., Tripathi B., Waldman B. Skin bacterial community reorganization following metamorphosis of the fire-bellied toad (Bombina orientalis) Microb. Ecol. 2018;75(2):505–514. doi: 10.1007/s00248-017-1034-7. [DOI] [PubMed] [Google Scholar]
- Bermúdez S.E., Miranda R.J., Kadoch N. Reporte de larvas de Ornithodoros puertoricensis Fox 1947 (Ixodida: Argasidae) parasitando a Rhinella marina (L. 1758) (Anura: Bufonidae) en David, Chiriquí, Panamá. Puente Biológico. 2013;5:81–85. [Google Scholar]
- Burridge M.J. University Press of Florida; 2011. Non-native and Invasive Ticks: Threats to Human and Animal Health in the United States; p. 320. [Google Scholar]
- Davies A.J., Johnston M.R.L. The biology of some intraerythrocytic parasites of fishes, amphibia and reptiles. Adv. Parasitol. 2000;45:1–107. doi: 10.1016/s0065-308x(00)45003-7. [DOI] [PubMed] [Google Scholar]
- De Sousa R., de Carvalho I.L., Santos A.S., Bernardes C., Milhano N., Jesus J., Núncio M.S. Role of the lizard Teira dugesii as a potential host for Ixodes ricinus tick-borne pathogens. Appl. Environ. Microbiol. 2012;78:3767–3769. doi: 10.1128/AEM.07945-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desser S., Barta J. An intraerythrocytic virus and rickettsia of frogs from Algonquin Park, Ontario. Can. J. Zool. 1984;62:1521–1524. [Google Scholar]
- Desser S.S. Aegyptianella ranarum sp. N. (Rickettsiales, Anaplasmataceae): ultrastructure and prevalence in frogs from Ontario. J. Wildl. Dis. 1987;23:52–59. doi: 10.7589/0090-3558-23.1.52. [DOI] [PubMed] [Google Scholar]
- Desser S., Barta J.R. The morphological features of Aegyptianella bacterifera: an intraerythrocytic rickettsia of frogs from Corsica. J. Wildl. Dis. 1989;25:313–318. doi: 10.7589/0090-3558-25.3.313. [DOI] [PubMed] [Google Scholar]
- Desser S.S., Hong H., Martin D.S. The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbeiana and the mosquito, Culex territans in Algonquin Park, Ontario. J. Parasitol. 1995;81:212–222. [PubMed] [Google Scholar]
- Ferguson L.V., Hillier N.K., Smith T.G. Influence of Hepatozoon parasites on host-seeking and host-choice behaviour of the mosquitoes Culex territans and Culex pipiens. Int. J. Parasitol. Parasites Wildl. 2013;2:69–76. doi: 10.1016/j.ijppaw.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov D.A. Processing and population genetic analysis of multigenic datasets with ProSeq3 software. Bioinformatics. 2009;25:3189–3190. doi: 10.1093/bioinformatics/btp572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Guglielmone A.A., Beati L., Barros-Battesti D.M., Labruna M.B., Nava S., Venzal J.M., Mangold A.J., Szabó M.P.J., Martins J.R., González Acuña D., Estrada-Peña A. Ticks (ixodoidea) on humans in South America. Exp. Appl. Acarol. 2006;40:83–100. doi: 10.1007/s10493-006-9027-0. [DOI] [PubMed] [Google Scholar]
- Guglielmone A.A., Nava S. Hosts of Amblyomma dissimile Koch, 1844 and Amblyomma rotundatum Koch, 1844 (Acari: Ixodidae) Zootaxa. 2010;2541:27–49. [Google Scholar]
- Gupta D.K., Gupta N., Gangwar R. Infectivity of Bufo melanostictus (Amphibia: Bufonidae) to two new species of haematozoan parasites from rohilkhand, India. Proc. Zool. Soc. 2012;65:22–32. [Google Scholar]
- Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993;42:182–192. [Google Scholar]
- Horta M.C., Pinter A., Schumaker T.T.S., Labruna M.B. Natural infection, transovarial transmission, and transstadial survival of Rickettsia bellii in the tick Ixodes loricatus (Acari: Ixodidae) from Brazil. Ann. N. Y. Acad. Sci. 2006;1078:285–290. doi: 10.1196/annals.1374.053. [DOI] [PubMed] [Google Scholar]
- Horta M.C., Saraiva D.G., Oliveira G.M.B., Martins T.F., Labruna M.B. Rickettsia bellii in Amblyomma rotundatum ticks parasitizing Rhinella jimi from northeastern Brazil. Microb. Infect. 2015;17:856–858. doi: 10.1016/j.micinf.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jado I., Oteo J.A., Aldámiz M., Gil H., Escudero R., Ibarra V., Portu J., Portillo A., Lezaun M.J., García-Amil C., Rodríguez-Moreno I., Anda P. Rickettsia monacensis and human disease, Spain. Emerg. Infect. Dis. 2007;13:1405–1407. doi: 10.3201/eid1309.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.K., Clifford C.M., Keirans J.E., Kohls G.M. The ticks of Venezuela (Acarina: ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigh. Young Univ. Stud. 1972;17:1–40. [Google Scholar]
- Karadjian G., Chavatte J.M., Landau I. Systematic revision of the adeleid haemogregarines, with creation of Bartazoon n. g., reassignment of Hepatozoon argantis Garnham, 1954 to Hemolivia, and molecular data on Hemolivia stellata. Parasite. 2015;22:1–15. doi: 10.1051/parasite/2015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K., Stecher G. MEGA7: molecular evolutionary genetics analysis Version 7.0 using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvičerová J., Hypsa V., Dvoráková N., Mikulícek P., Jandzik D., Gardner M.G., Javanbakht H., Tiar G., Siroky P. Hemolivia and Hepatozoon: haemogregarines with tangled evolutionary relationships. Protist. 2014;165:688–700. doi: 10.1016/j.protis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Labruna M.B., Whitworth T., Bouyer D.H., McBride J., Camargo L.M.A., Camargo E.P., Popov V., Walker D.H. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of rondônia, Western Amazon. Brazil. J. Med. Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- Lampo M., Bayliss P. The impact of ticks on Bufo marinus from native habitats. Parasitology. 1996;113:199–206. [Google Scholar]
- Lampo M., Rangel Y., Mata A. Genetic markers for the identification of two tick species, Amblyomma dissimile and Amblyomma rotundatum. J. Parasitol. 1997;83:382–386. [PubMed] [Google Scholar]
- Lanfear R., Calcott B., Ho S.Y.W., Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Lainson R., De Souza M.C., Franco C.M. Natural and experimental infection of the lizard Ameiva ameiva with Hemolivia stellata (Adeleina: haemogregarinidae) of the toad Bufo marinus. Parasite. 2007;14:323–328. doi: 10.1051/parasite/2007144323. [DOI] [PubMed] [Google Scholar]
- Levin M.L., Snellgrove A.N., Zemtsova G.E. Comparative value of blood and skin samples for diagnosis of spotted fever group rickettsial infection in model animals. Ticks Tick Borne Dis. 2016;7:1029–1034. doi: 10.1016/j.ttbdis.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia J.P., Carranza S., Harris D.J. Comments on the systematic revision of adeleid haemogregarines: are more data needed? J. Parasitol. 2016;102:549–552. doi: 10.1645/15-930. [DOI] [PubMed] [Google Scholar]
- Martins T.F., Onofrio V.C., Barros-Battesti D.M., Labruna M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis. 2010;1:75–99. doi: 10.1016/j.ttbdis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Miranda J., Portillo A., Oteo J.A., Mattar S. Rickettsia sp. Strain Colombianensi (Rickettsiales: Rickettsiaceae): a New Proposed Rickettsia Detected in Amblyomma dissimile (Acari: Ixodidae) from Iguanas and Free-Living Larvae Ticks from Vegetation. J. Med. Entomol. 2012;49:960–965. doi: 10.1603/me11195. [DOI] [PubMed] [Google Scholar]
- Nava S., Venzal J.M., González-Acuña D., Martins T.F., Guglielmone A.A. Academic Press; 2017. Chapter 2-Genera and Species of Ixodidae Ticks of the Southern Cone of America (Pp. 25–267. [Google Scholar]
- O'Donoghue P. Haemoprotozoa: making biological sense of molecular phylogenies. Int. J. Parasitol. Parasites Wildl. 2017;6:241–256. doi: 10.1016/j.ijppaw.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorno-Mesa E. Las Garrapatas de la República de Colombia. Revista Acad. Colomb. Ci. Exact. 1940;4:6–24. [PubMed] [Google Scholar]
- Parola P., Davoust B., Raoult D. Tick and flea-borne rickettsial emerging zoonoses. Vet. Res. (Paris) 2005;36:469–492. doi: 10.1051/vetres:2005004. [DOI] [PubMed] [Google Scholar]
- Parola P., Paddock C.D., Socolovschi C., Labruna M.B., Mediannikov O., Kernif T., Abdad M.Y., Stenos J., Bitam I., Fournier P.E., Raoult D. Update on tick-borne rickettsioses around the world: a geographic approach Clin. Microbiol. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G., Landau I., Baccam D., Lainson R. Description et cycle biologique d'Hemolivia stellata, n.g., n.sp., hemogrégarine de Crapauds brésiliens. Ann. Parasitol. Hum. Comp. 1990;65:3–15. [Google Scholar]
- Phan J.N., Lu C.R., Bender W.G., Smoak R.M., Zhong J. Molecular detection and identification of Rickettsia species in Ixodes pacificusin California. Vector Borne Zoonotic Dis. 2011;11(7):957–961. doi: 10.1089/vbz.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Calil P., Lima-Gonzalez I.H., Borges-Salgado P.A., Batista da Cruz J., Locosque-Ramos P., Fernandes-Chagas C.F. Hemogregarine parasites in wild captive animals, a broad study in São Paulo. ZooHemogregarine parasites in wild captive animals, a broad study in São Paulo Zoo. J. Entomol. Zool. Stud. 2017;5:1378–1387. [Google Scholar]
- Ronquist F., Teslenko M., Van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta A., Satetasit J., Chutmongkonkul M. Pathological study of blood parasites in rice field frogs, Hoplobatrachus rugulosus (Wiegmann, 1834) Vet. Med. Int. 2011:1–5. doi: 10.4061/2011/850568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R.K., Costa F.B., Ueno T.E.H., Ramirez D.G., Soares J.F., Fonseca A.H., Labruna M.B., Barros-Battesti D.M. Experimental infection with Rickettsia rickettsii in an Amblyomma dubitatum tick colony, naturally infected by Rickettsia bellii. Ticks Tick Borne Dis. 2014;5:917–923. doi: 10.1016/j.ttbdis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Santodomingo A., Cotes-Perdomo A., Foley J., Castro L.R. Rickettsial infection in ticks (Acari: Ixodidae) from reptiles in the colombian caribbean. Ticks Tick Borne Dis. 2018;9:623–628. doi: 10.1016/j.ttbdis.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–464. [Google Scholar]
- Selechnik D., Rollind L.A., Brown G.P., Kelehear C., Shine R. The things they carried: the pathogenic effects of old and new parasites following the intercontinental invasion of the Australian cane toad (Rhinella marina) Int. J. Parasitol. Parasites Wildl. 2017;6:375–385. doi: 10.1016/j.ijppaw.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutler D., Smith T.G., Robinson S.R. Relationships between leukocytes and Hepatozoon spp. in green frogs, Rana clamitans. J. Wildl. Dis. 2009;45:67–72. doi: 10.7589/0090-3558-45.1.67. [DOI] [PubMed] [Google Scholar]
- Silva T.K.S., Moreira Blanco C., Sampaio de Lemos E.R., Ogrzewalska M. Notes on parasitism and screening for microorganism of ticks Amblyomma (Acari: Ixodidae), Amazon. Brazil. Virus Rev. Res. 2016;21:41–44. [Google Scholar]
- Smith R.L.S.,J.A., Schaeferf E.F., Kehrf A.I. Ticks, Amblyomma rotundatum (Acari: Ixodidae), on toads, Chaunus schneideri and Chaunus granulosus (Anura: Bufonidae), in northern Argentina. J. Parasitol. 2008;94:560–562. doi: 10.1645/GE-1271.1. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stuart S.N., Chanson J.S., Cox N.A., Young B.E., Rodrigues A.S., Fischman D.L. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Thompson J., Higgins D., Gibson T. Clustal W: improving the sensitivity of the progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujvari B., Madsen T., Olsson M. High prevalence of Hepatozoon spp. (Apicomplexa, hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 2004;90:670–672. doi: 10.1645/GE-204R. [DOI] [PubMed] [Google Scholar]
- Voltzit O.V. A review of neotropical Amblyomma species (Acari: Ixodidae) Acarina. 2007;15:3–134. [Google Scholar]
- Witter R., Martins T.F., Campos A.K., Melo L.T., Corrêa S.H., Morgado T.O., Wolf R.W., May-Júnior J.A., Sinkoc A.L., Strüssmann C., Aguiar D.M., Rossi R.V., Semedo T.B., Campos Z., Desbiez A.L., Labruna M.B., Pacheco R.C. Rickettsial infection in ticks (Acari: Ixodidae) of wild animals inmidwestern Brazil. Ticks Tick Borne Dis. 2016;7:415–423. doi: 10.1016/j.ttbdis.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Wozniak E.J., Kazacos K.R., Telford S.R., Telford S.R., Jr., McLaughlin G.L. Characterization of the clinical and anatomical pathological changes associated with Hepatozoon mocassini infections in unnatural reptilian hosts. Int. J. Parasitol. Parasites Wildl. 1996;26:141–146. doi: 10.1016/0020-7519(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Zemtsova G., Killmaster L.F., Mumcuoglu K.Y., Levin M.L. Co-feeding as a route for transmission of Rickettsia conorii israelensis between Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 2010;52:383–392. doi: 10.1007/s10493-010-9375-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.