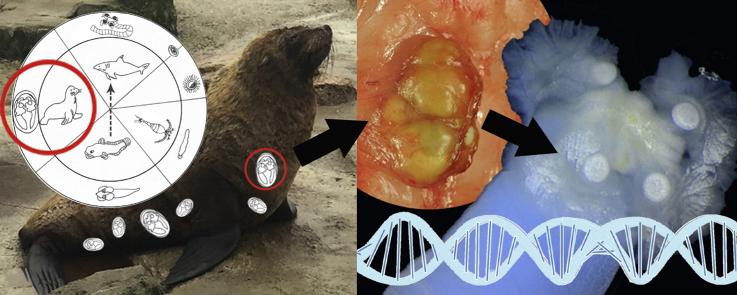
Abstract
Fur seals represent intermediate hosts of the cestode Clistobothrium. Large sharks are definitive hosts for these parasites. Two female, 25– and 27-year-old fur seals, caught in the 1980s at the South African coast, were examined pathomorphologically. Both animals showed multifocal, up to 1 cm in diameter large cavities of the thoracic and abdominal subcutaneous adipose tissue containing intraluminal metacestodes of tapeworms, which were surrounded by a locally extensive, pyogranulomatous panniculitis. The metacestodes (merocercoids) of one fur seal were isolated from the subcutaneous adipose tissue and characterized morphologically and for the first time from this host by molecular techniques. The morphometric data corresponded with ‘delphini'-morphotype merocercoids, but the sequence of the partial 28S ribosomal RNA gene identified them as conspecific with merocercoids of the morphotype ‘grimaldii’. These merocercoid types are morphologically Type XV metacestodes of marine tapeworms and represent different species of Clistobothrium. Sequence data were generated for 18S, ITS1, 5.8S, ITS2, partial 28S ribosomal DNA and partial mitochondrial cox1 gene and phylogenetic analysis of 18S rRNA and partial 28S rRNA genes identified the fur seal merocercoids as Clistobothrium species. However, it cannot yet be assigned to species level because of limited molecular data from adult stages. Most likely, both fur seals were infected as juveniles in their original habitat, the coastal regions of South Africa. The metacestode infection is probably an incidental finding, however, there is a chronic inflammatory reaction next to the subcutaneous merocercoids. It is noteworthy, that the merocercoids remain in a potentially infective stage even after more than 20 years.
Keywords: Arctocephalus pusillus pusillus, Clistobothrium sp., Fur seal, Cestode, Subcutaneous parasites, Monorygma grimaldii
Graphical abstract
Highlights
-
•
Subcutaneous metacestodes in fur seals cause asymptomatic chronic panniculitis.
-
•
Metacestodes remain potentially infectious for more than 20 years.
-
•
First molecular characterization of merocercoids from seals.
-
•
Merocercoids of marine tapeworm (metacestode Type XV): delphini-morphotype but grimaldii-genotype.
-
•
18S and 28S phylogeny verified Clistobothrium sp. as adult tapeworm.
1. Introduction
Newly discovered tapeworm species and poorly understood phylogenetic relationships within the Phyllobothriidea have resulted in numerous changes in the taxonomy of these parasites supported by increasing molecular data (Olson et al., 1999; Caira et al., 2014). Traditionally, the Phyllobothriidae represented a family of the Tetraphyllidea (Olson et al., 1999; Ruhnke, 2011). Caira and coworkers dismantled the polyphyletic order Tetraphyllidea and elevated the Phyllobothriidae to ordinal status (Caira and Jensen, 2014; Caira et al., 2014). The Phyllobothriidea include with a few exceptions most genera of the former Phyllobothridae, characterized by non–hooked scoleces bearing four simple, undivided bothridia each with an anterior accessory sucker; most are parasites of carcharhiniform sharks (Caira et al., 2014). Phyllobothriid metacestodes surrounded by a bladder with inverted or everted scoleces, so-called merocercoids (Chervy, 2002), have historically been referred to as ‘Phyllobothrium delphini’ (Bosc, 1802) and ‘Monorygma grimaldii’ (Moniez, 1889) and have been detected in several offshore epipelagic, deep feeding marine mammals (Aznar et al., 2007). Molecular analyses showed that these merocercoids are not related to the genera Phyllobothrium and Monorygma which have similar bothridial structures; consequently the genus combinations are invalid (Ruhnke, 2011 and Caira et al., 2014, 2017). Therefore, we will refer to them hereinafter as delphini- and grimaldii-morphotype merocercoids. According to the key of marine tapeworm larvae established by Jensen and Bullard (2010), both merocercoids represent larval type XV. These merocercoids have a wide geographic distribution and have been reported in numerous cetacean species worldwide (Norman, 1997; Abollo et al., 1998; Cornaglia et al., 2000; Failla Siquier and Le Bas, 2003; Beron-Vera et al., 2008; Colon-Llavina et al., 2009; Carvalho et al., 2010; Oliveira et al., 2011), but have also been reported from pinnipeds (Rennie and Reid, 1912; Southwell and Walker, 1936; Bester, 1989; Pansegrouw, 1990; Stewardson and Fourie, 1998; McFarlane et al., 2009). In captive fur seals, two cases of phyllobothriidian merocercoids were reported (Cordes and O'Hara, 1979; Mendonca, 1984). These animals most likely were infected in their natural environment — South Africa and New Zealand — before being transported to zoological gardens. The merocercoids found in pinnipeds morphologically resembled those of the delphini-type from cetaceans, only two infections with a grimaldii-type — one in the mesentery of an elephant seal and one incidental in testis of a fur seal— were described (Morgan et al., 1978; Bester, 1989).
In cetaceans, four types of phyllobothriidean metacestodes have been described, these include two types of plerocercoids, which were differentiated by their size and called “small plerocercoids” (SP) and “large plerocercoids” (LP) and the two morphotypes of merocercoids, delphini- and grimaldii-type (Aznar et al., 2007). Both merocercoid-types can be distinguished by morphological criteria: The scolex of the delphini–type is large, has folded bothridia and a thick, short connected invagination filament, whereas the scolex of the grimaldii–type is small, has bothridia with simple margins and a thin, very long connected invagination filament (Agusti et al., 2005a). Merocercoids of the delphini-type are frequently found in the subcutaneous blubber of the ventral abdominal wall concentrating in the perigenital region, whereas the grimaldii-type are encysted in the mesentery and located retroperitoneal parallel to the rectum, at the caudal pole of the kidneys, in the lateral ligaments of the urinary bladder, in the ligamentum latum of the uterus and close to the testis. LP plerocercoids are predominantly located inside the anal sac and free in the lumen of the intestine, hepatic and pancreatic ducts and SP plerocercoids free in the lumen and buried in the mucosa of the main and pyloric stomach and the intestine with concentrations in the terminal colon and rectum mucosa (Norman, 1997; Agusti et al., 2005a, 2005b; Oliveira et al., 2011).
As mentioned before, the historical names ‘P. delphini’ and ‘M. grimaldii’ — still used by some authors — are misleading as the adult cestodes of these merocercoids are not known and their assignment to the genera Phyllobothrium and Monorygma with the type species P. lactuca van Beneden, 1850 and M. perfectum van Beneden, 1853 (Diesing, 1863), respectively is invalid. The identification of these forms is complicated by the extensive variability of delphini-morphotypes (Testa and Dailey, 1977), which might represent stages developing with time spent in a host (Failla Siquier and Le Bas, 2003). Sequence analyses of the two merocercoid-types and LP- and SP-forms of plerocercoids suggested that they are congeneric and different species of the genus Clistobothrium (Agusti et al., 2005a).
Marine mammals represent intermediate hosts of Clistobothrium tapeworm species as shown by detecting plero- and meroceroids in cetaceans and pinnipeds (Aznar et al., 2007). Sequence data from adult Clistobothrium species in GenBank are limited to C. montaukensis and C. carcharodoni, the latter, which was confirmed by scolex morphology only. Trophic interaction between large sharks and cetaceans has been shown by identical sequence of Clistobothrium carcharodoni from the great white shark (Carcharodon carcharias; HM856632-33) and SP plerocercoids in striped dolphin (Stenella coeruleoalba; DQ839588) and Risso's dolphin (Grampus griseus; DQ839587) (Randhawa, 2011). Furthermore, sequence identity of more than 99.8% was also found between plerocercoids from the squid Doryteuthis gahi and Clistobothrium cf. montaukensis from porbeagle sharks (Randhawa and Brickle, 2011), plerocercoids from the squid Illex coindetii (KT148970), deep sea oarfish Regalecus glesne (KM272991) and C. montaukensis from shortfin mako sharks (AF286957; Kuris et al., 2015) suggesting transmission of tapeworms between these species. Both cetaceans and pinnipeds represent a preferred prey of large sharks (Long and Jones, 1996; Heithaus, 2001).
Here, two cases of subcutaneous merocercoids of Clistobothrium sp. in cape fur seals (Arctocephalus pusillus pusillus) are described and for the first time were molecularly characterized.
2. Materials and methods
Two female, 25– (case No. 1) and 27– (case No. 2) year–old fur seals caught in the 1980s at the South African coast were examined pathomorphologically. Both animals lived more than 20 years in the zoological garden of Bremerhaven, Germany. Case No. 1, a 25-year–old, female fur seal died after mating activity with suspected cardiovascular failure and fracture of both mandibular rami in May 2013. Case No. 2 was euthanized in October 2015 due to multiple geriatric diseases including blindness and reduced mobility and activity.
Both fur seals were necropsied and tissue samples were fixed in 10% neutral buffered formalin before being embedded in paraffin wax. For histological examination, 2–3 μm thick sections were cut and stained with hematoxylin and eosin. Additionally, a staining with the “von Kossa silver nitrate” –method for detection of calcium deposits was performed (Riedelsheimer and Büchl-Zimmermann, 2010).
Merocercoids from case No. 2 were isolated from the subcutaneous adipose tissue and examined morphologically using stereo and light microscopy, SC30 digital camera and CellSens Dimension software (Olympus, Germany).
Following morphological analysis, DNA was isolated from the merocercoid of case No. 2 using the DNeasy Blood & Tissue kit according to manufacturer's instructions (Qiagen, Hilden, Germany). The rDNA region including 18S, ITS1, 5.8S, ITS2 and partial 28S was amplified by polymerase chain reaction (PCR) in three overlapping fragments using the following primer pairs: WormA. 5′-GCGAATGGCTCATTAAATCAG-3′ and WormB. 5′-CTTGTTACGACTTTTACTTCC-3′ (Littlewood and Olson, 2001), NF1: 5′-GGTGGTGCATGGCCGTTCTTAGTT-3′ (Porazinska et al., 2009) and D3A: 5′-TCCGTGTTTCAAGACGGGTC-3′ (Nunn, 1992), Tph28S-f900: 5′-GTCTGATTGTCGTGTCGCCTG-3′ (new design) and L2230 5′-AGACCTGCTGCGGATATGGGT-3′ (Lockyer et al., 2003). PCR was performed in 50 μl reaction volume using HOT FIREPol Blend Master Mix 7.5 mM MgCl2 (Solis BioDyne, Tartu, Estonia) under the following conditions: 15 min 95 °C initial denaturation, 35 cycles 20 s 95 °C, 30 s 54 °C, 2 min 72 °C and 5 min 72 °C final extension. A partial cytochrome oxidase subunit 1 sequence (cox1) was amplified with primers Dice1F 5′-attaaccctcactaaaTTWCNTTRGATCATAAG-3′ and Dice11R 5′-taatacgactcactataGCWGWACHAAATTTHCGATC3′ using a touchdown-PCR protocol; lower case denote anchored primers T3s and T7s used for direct sequencing (Van Steenkiste et al., 2015). Amplicons were purified from agarose gels and sequenced through an external service provider (LGC Genomics GmbH, Berlin, Germany). Obtained sequences were analyzed by BLAST search against the GenBank database (https://www.ncbi.nlm.nih.gov/).
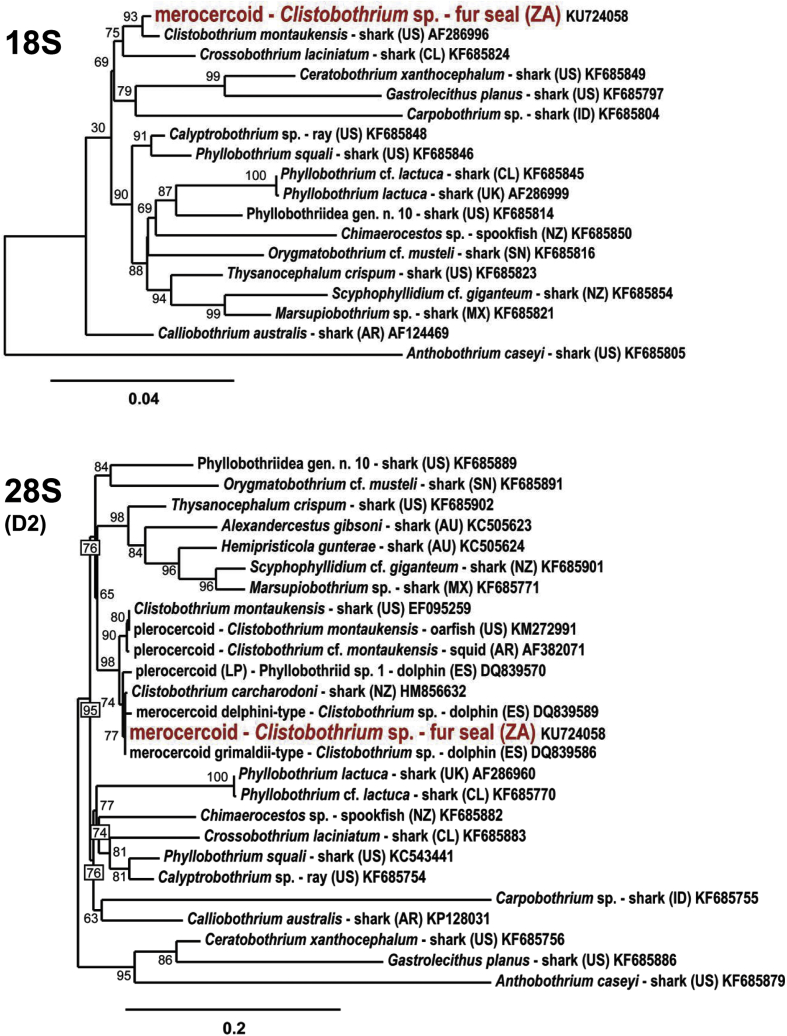
For phylogenetic analysis, a BLAST search in GenBank database was conducted and a dataset of Clistobothrium spp., Phyllobothrium spp and high-scoring taxa derived from BLAST search were chosen (Supplementary Table 1). Sequences were end-trimmed by manual inspection and aligned by MAFFT 7 (Katoh and Standley, 2013); for pairwise genetic distance see Supplementary Table 2. For the 18S rDNA phylogeny, the aligned sequences corresponded to nucleotide (nt) 8–1877 of the Clistobothrium merocercoid sequence KU724058, for the 28S D2 region nt 3459–3975. Phylogenetic trees were constructed using maximum likelihood software (PhyML 3.1 aLRT) and TreeDyn from the Phylogeny.fr website (Dereeper et al., 2008). Sequences are available under the GenBank accession numbers KU724058 (rDNA) and KU987913 (cox1).
3. Results
3.1. Pathological description
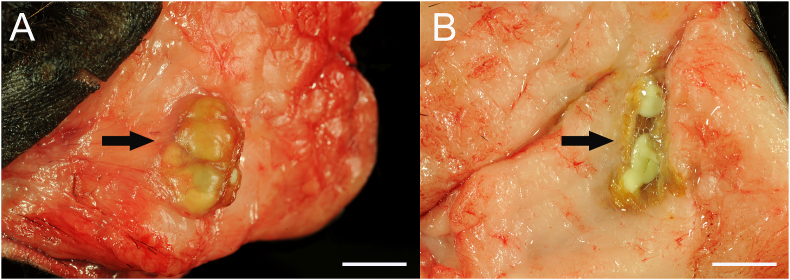
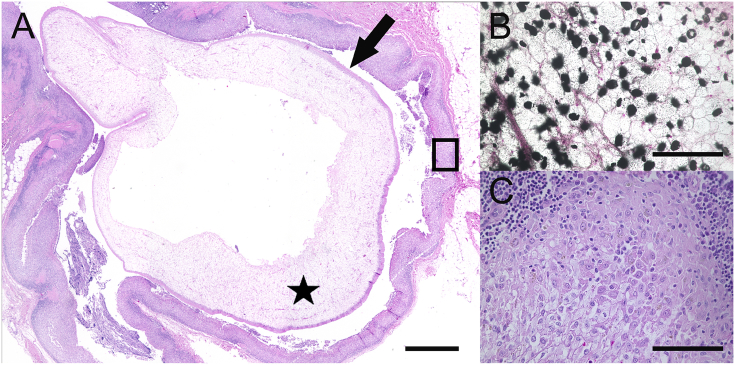
Besides the pathomorphological findings that were responsible for death or euthanasia of the animals (Case No. 1: multiple fractures and hemorrhages due to trauma; Case No. 2: multiple geriatric processes such as spondylosis, retinal atrophy and benign tumors) both animals showed multifocally approximately 15, up to 1 cm in diameter large cavities within the subcutaneous adipose tissue in the ventral thoracic and abdominal wall. In these cavities intraluminal parasites were detected (Fig. 1). Histological examination revealed larval stages of cestodes (merocercoids) with an approx. 15 μm thick, eosinophilic tegument, a loosely packed parenchyma, approx. 20 μm in diameter large, strongly “von Kossa”–positive “calcareous corpuscles” (Fig. 2 A) and a perifocal, pyogranulomatous panniculitis (Fig. 2 B) with multinucleated cells within the blubber.
Fig. 1.
Subcutaneous adipose tissue of a 27-year–old, female fur seal (case No. 2). Up to 1 cm in diameter large cavities (A, arrow) containing one or more parasites (B, arrow) as detected in the cross section. Bars = 1 cm.
Fig. 2.
Histological section of subcutaneous adipose tissue of a 25-year–old, female fur seal (case No. 1) containing metacestode tapeworms with associated inflammation (box). The parasitic structures are characterized by a tegument (arrow) and centrally a parenchymatous matrix (asterisks) is present (A, bar = 1000 μm). Within the parenchymatous matrix of the parasite, numerous calcareous corpuscles stained with the “von Kossa” –method are present (B, bar = 100 μm). The parasite is surrounded by an inflammatory reaction composed of lymphocytes, plasma cells, macrophages and neutrophils (C, bar = 100 μm). A, C = hematoxylin and eosin.
3.2. Morphological description, molecular characterization and analysis of the merocercoid
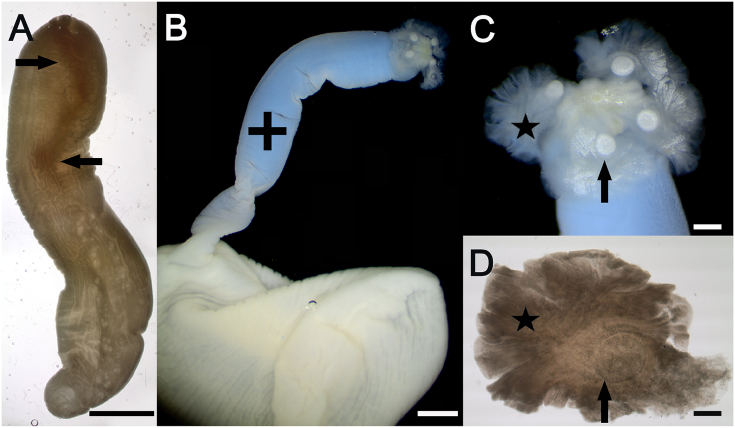
The parasitological examination revealed phyllobothriidean metacestodes (merocercoids) with a scolex with an anterior glandular apical organ (reduced sucker) and four undivided bothridia, each with a prominent anterior accessory sucker and single loculus (Fig. 3). The scolex was at the end of an 18 mm long and 2 mm wide invagination filament of the cystic wall. The bothridia were thin, foliose, fragile, with curled margins and the anterior sucker was large and slightly oval, 500 μm long by 400 μm wide. When compared with discriminating features of the two general types of merocercoids of marine mammals – delphini- and grimaldii-type – the merocercoids from the fur seal were unambiguously classified as delphini-type (Table 1). The most decisive features were the site of infection, the length of the invagination filament and the ratio of bladder length to invagination filament length. The features described above identified the two merocercoids as larvae Type XV according to the key of marine cestode larvae (Jensen and Bullard, 2010).
Fig. 3.
Light micrographs of isolated subcutaneous Clistobothrium sp. merocercoids of a fur seal (case No. 2). (A) merocercoid with invaginated scolex, (B) merocercoid with evaginated scolex on a long filament (cross), (C) scolex with terminal apical organ and four large bothridia (asterisk) each with anterior sucker (arrow), (D) bothridium with folded margin (asterisk) and large oval anterior sucker (arrow) with well–developed musculature.
Table 1.
Comparison of merocercoids from fur seals of the present study and from literature with the two common morphotypes of cetaceans (striped dolphins, sample I, Agusti et al., 2005a,b).
| Feature [mm] | Merocercoid |
||||||
|---|---|---|---|---|---|---|---|
| Present study protruded invaginated | Mendonca 1984 | Southwell 1936 | ‘Phyllobothrium delphini' | ‘Monorygma grimaldii' | |||
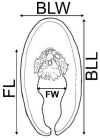 |
host | fur seal | striped dolphin | ||||
| Location in hosts | blubber | blubber | mesentery | ||||
| Bladder length (BLL) | 35.00 | 37.50 | 16.7 | 15.00 | 10.30 | 13.70 | |
| width (BLW) | 7.00 | 7.50 | 5.9 | 8.00 | 5.90 | 7.70 | |
| Filament length (FL) | 18.20 | 13.60 | 8.8 | 13.00 | 7.40 | 151.80 | |
| width (FW) | 2.45 | 1.50 | 1.78 | 3.00 | 1.63 | 0.27 | |
| BLL/FL | 1.92 | 2.76 | 1.87 | 1.15 | 1.39 | 0.09 | |
| Bothridium length | 1.50 | n.d. | 0.88 | 1.57 | 1.47 | 0.47 | |
| Bothridial sucker [μm] | 400 × 500 | 350 × 400 | 442 | 275 | 274 × 288 | 148 × 172 | |
| Bothridial margin | loculated | loculated | smooth | ||||
The most dicriminative features between the two merocercoid morpho-types (delphini, grimaldii) are highlighted in bold.
DNA of the merocercoid from case No. 2 was isolated and sequences of 5543 bp of the ribosomal DNA (18S rRNA, ITS1, 5.8S, ITS2 and partial 28S rRNA genes) and 585 bp of the mitochondrial COI gene (cox1) were obtained by amplification with universal primers.
The 28S rDNA sequence was 100% identical to all twelve partial (longest 653 bp) 28S rDNA sequences of grimaldii-type merocercoid isolates in GenBank followed by 99.7% identity — with only two nucleotide transitions — to adult Clistobothrium carcharodoni (HM856632 725/727 nt) and 99.6% identity to all fourteen 28S rDNA delphini-type merocercoid isolates (e.g. DQ839589 690/693 nt). The identity to the second Clistobothrium species in GenBank – C. montaukensis - was slightly lower (99.3%) and showed three nucleotide transversions (EF095259 2502/2524). The homology of the 28S rDNA sequence to adult Phyllobothrium species was lower than 96% (P. squali KF685897: 1413/1477 nt, 95.5%; P. lactuca KF685770: 2352/2491 nt, 94.4%). Sequences from adult Monorygma species and 18S rDNA sequences from the delphini- and grimaldii-type merocercoids are not present in GenBank.
The 18S rDNA sequence is less discriminative because of the higher conservation in comparison to the 28S D1-D3 rDNA region. The best matches are sequences from C. montaukensis with a homology of 99.1–99.4% identity (AF126069: 1467/1481 nt; AF286996: 1923/1934 nt) followed by Crossobothrium sp. with 98.2% (JX845132: 1921/1957 nt). Phyllobothrium species have less than 98% identity with the present fur seal merocercoid sequence (P. squali KF685846: 1904/1944 nt, 97.9%; P. lactuca AF286999: 1878/1943 nt, 96.7%).
3.3. Phylogenetic analyses confirm assignment to Clistobothrium
The new 18S rDNA sequence and the D2 region of the 28S rDNA — identical to the grimaldii-type merocercoid sequence — were used for phylogenetic analyses using a dataset of 17 and 25 sequences covering 15 phyllobothriidean genera (Fig. 4, Supplementary Table 1). In the 28S D2 rDNA analysis, the merocercoid from the fur seal, the delphini- and grimaldii-type merocercoids and the LP plerocercoids group in one clade with adults of the two Clistobothrium species C. montaukensis and C. carcharodoni as shown previously by several authors (Agusti et al., 2005a; Aznar et al., 2007; Jensen and Bullard, 2010; Randhawa, 2011; Randhawa and Brickle, 2011). This assignment to Clistobothrium was also verified using the near complete 18S rRNA gene for analysis, although with a smaller number of available taxa.
Fig. 4.
Phylogenetic trees of Clistobothrium sp. merocercoids from the Cape fur seal and related phyllobothriid species based on the 18S and 28S D2 rDNA regions using maximum-likelihood method. Nodal support is indicated by bootstrap values in percent; scale: number of substitutions per site; country and accession no. after the species name.
4. Discussion
In the present study, subcutaneous tapeworms of two adult captive fur seals were identified as merocercoids of the genus Clistobothrium. Their sequences were 100% identical with the sequence of grimaldii-type merocerocoids from dolphins and more than 99% identical with other members of the Clistobothrium clade including C. montaukensis and C carcharodoni. Morphological criteria and molecular analyses underline the result of studies dealing with cetaceans, that delphini- and grimaldii-type merocercoids and the LP-plerocercoid belong to different, molecularly uncharacterized adult Clistobothrium species (Agusti et al., 2005a; Randhawa, 2011). The bothridia of the scolex of grimaldii-type merocercoids from dolphins are smooth like those of Monorygma species. In contrast, the merocercoids from the seals have foliose bothridia and morphologically resemble those of delphini-type merocercoids from dolphins. We therefore conclude that this Clistobothrium species develops different in the two intermediate hosts leading to less developed grimaldii-type merocercoids in dolphins and further-developed delphini-type merocercoids in seals. Due to the highly similar scolex morphology it is likely that the tapeworm metacestodes, isolated from the adipose tissue of the two captive fur seals, display merocercoids of the adult C. tumidum (Syn: Phyllobothrium tumidum; Linton, 1922; Ruhnke, 1993). Future molecular analysis of adult specimens of C. tumidum should clarify this hypothesis. The apex predator of the marine food web, the great white shark (Carcharodon carcharias) is known to be one definitive host for this Clistobothrium species (Linton, 1922). In addition, C. tumidum was also described from mackerel sharks: Riser (1955) found specimens in the salmon shark Lamna ditropis from California and Euzet (1959) in the shortfin mako shark Isurus oxyrinchus from Europe at the Mediterranean and the Brittany coast.
One problem in discriminating the different members of the Clistobothrium clade is the low sequence diversity in 18S and 28S regions. The ITS and the cox1 gene sequences determined here from the fur seal merocercoids could not be used for species discrimination in the Clistobothrium clade due to the lack of data. From the current 451 Phyllobothriidea sequences in GenBank only 26 are cox1 (23 belonging to Anindobothrium spp.) and 24 are ITS sequences (23 belonging to Anindobothrium spp.). Importantly, the cox1 sequence of the fur seal Clistobothrium species has only 85% nucleotide identity to the sequence from C. montaukensis (JQ268541: 497/584 nt). Therefore, the cox1 and ITS sequences might be better biomarkers for barcoding of closely related species of phyllobothriidean genera as have been shown recently for the genus Anindobothrium (Trevisan et al., 2017).
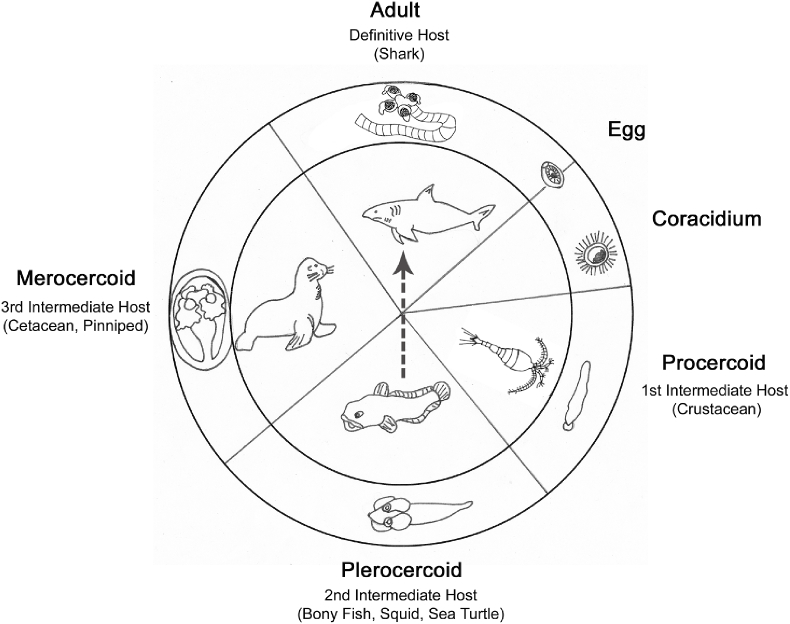
The phylogenetic analyses also verified that the historical names of the two merocercoid Clistobothrium types — ‘Phyllobothrium delphini’ and ‘Monorygma grimaldii’ — are invalid genus combinations. The two genera Phyllobothrium and Chimaerocestos, — the latter which is close to Monorygma (Caira et al., 1999) — were both in a clade clearly separated from Clistobothrium. The complete life cycle for all species of Clistobothrium has yet to be elucidated. However, data available for other elasmobranch-hosted tapeworms support the following general life cycle (Fig. 5; Caira and Reyda, 2005): The definitive hosts for Phyllobothriidea, sharks, shed embryonated eggs (Dick et al., 2006). Within the water, from the eggs a floatable coracidium emerges, which is taken up by invertebrates such as crustaceans. In the body cavity of a copepod crustacean (Copepoda) — the first intermediate host — the development to a procercoid takes place (Caira and Reyda, 2005; Cortés and Muñoz, 2008). Teleost fish and squid, which ingest the procercoid–containing invertebrate are second intermediate hosts of Phyllobothriidea (Dick et al., 2006). The development from procercoids to plerocercoids is suggested to occur in the muscles or the liver as it was described for other tapeworms (Zissler, 1999; Caira and Reyda, 2005). Phyllobothriidean plerocercoids are also sometimes described from sea turtles (Innis et al., 2009). In marine mammals, plerocercoids and merocercoids can be detected at the same time indicating that they are third intermediate or paratenic hosts for Phyllobothriidea. Presumably, Phyllobothriidea were transported as plerocercoids via the lymphatic system of the marine mammal into the body cavity or the subcutaneous adipose tissue, where they develop to merocercoids (Aznar et al., 2007). Predatory sharks get infected by ingestion of marine mammal tissue containing merocercoids (Aznar et al., 2007; Randhawa, 2011) and the adult tapeworms develop in the spiral intestine of elasmobranch (Caira and Reyda, 2005). Consequently, accumulation of metacestodes in mammalian hosts increases the chance for the parasite to complete its life cycle, but infection of large sharks through squids was also suggested (Randhawa and Brickle, 2011).
Fig. 5.
Suggested life cycle of Phyllobothriidea and the potential way of infection in the present cases.
Infection of the two fur seals examined here occurred more than 20 years ago in the original habitat (South Africa) of the animals before they were transported to Germany. This theory is supported by the fact, that only these animals and none of the other captive marine mammals of the same zoological garden showed phyllobothriid parasites in spite of the same fish food. In addition, studies on Cape fur seals from southern Africa demonstrated a high infection rate with Clistobothrium merocercoids of the delphini-type. Pansegrouw (1990) reported 75% infections of 90 examined seals from Namibia and Stewardson and Fourie (1998) 25% of 53 seals collected along the Eastern Cape coast of South Africa. Based on the well preserved morphology and a lack of degenerative lesions, it is suggested that the detected metacestodes were fully infectious. Although both animals showed an inflammatory reaction in the adipose tissue adjacent to the parasites, a clinical relevance of these parasites is probably lacking and therefore, these tapeworm metacestodes represent an incidental finding.
5. Conclusion
This is the first molecular characterization of merocercoids from the blubber of seals. The sequence of the fur seal meroceroids is identical with the sequence of grimaldii-type merocercoids from dolphins and bothridial morphology resembles those of Clistobothrium tumidum. The molecular and phylogenetic analysis support previous assumptions that the two merocercoid types — grimaldii- and delphini-type — are congeneric and distinctive species of the genus Clistobothrium. Most likely, both fur seals were infected as juveniles in their original habitat, the coastal regions of southern Africa by ingestion of squid or teleosts containing metacestodes of this Clistobothrium tapeworm. Pinnipeds in addition to cetaceans serve as intermediate hosts in the life cycle of Clistobothrium in geographical regions where they represent the preferred prey of large adult lamniform sharks. A clinical relevance of this infestation for the fur seals as intermediate hosts is unlikely, but even after 20 years these long–living metacestode stages seem to be potentially infectious for their definitive host.
Funding
The author(s) received no financial support for the research and/or authorship of this article. This publication was supported by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation within the funding program Open Access Publishing.
Authors' note
The content of this article was presented as poster at the ‘‘59. Jahrestagung der Fachgruppe Pathologie der Deutschen Veterinärmedizinischen Gesellschaft’’ (4–6 March 2016 Fulda, Germany) and at the ‘‘Jahrestagung der Deutschen Veterinärmedizinischen Gesellschaft der Fachgruppe Parasitologie’’ (2–4 May 2016 in Berlin, Germany). The abstracts were published in Tierärztliche Praxis 2016 and in the Tagungsband Parasitologische und parasitäre Krankheiten 2016, respectively.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Bettina Buck, Petra Grünig, Christine Henrich, Claudia Hermann und Caroline Schütz for excellent technical assistance.
Footnotes
‘Nucleotide sequence data reported in this paper are available in the GenBank™, EMBL and DDBJ databases under the accession numbers KU724058 and KU987913’.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.02.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Data sets of 18S und 28S sequences for phylogenetic analysis.
Estimates of evolutionary divergence between sequences.
References
- Abollo E., Lopez A., Gestal C., Benavente P., Pascual S. Macroparasites in cetaceans stranded on the northwestern Spanish Atlantic coast. Dis. Aquat. Org. 1998;32:227–231. doi: 10.3354/dao032227. [DOI] [PubMed] [Google Scholar]
- Agusti C., Aznar F.J., Olson P.D., Littlewood D.T., Kostadinova A., Raga J.A. Morphological and molecular characterization of tetraphyllidean merocercoids (Platyhelminthes: Cestoda) of striped dolphins (Stenella coeruleoalba) from the Western Mediterranean. Parasitology. 2005;130:461–474. doi: 10.1017/s0031182004006754. [DOI] [PubMed] [Google Scholar]
- Agusti C., Aznar F.J., Raga J.A. Tetraphyllidean plerocercoids from Western Mediterranean cetaceans and other marine mammals around the world: a comprehensive morphological analysis. J. Parasitol. 2005;91:83–92. doi: 10.1645/GE-372R. [DOI] [PubMed] [Google Scholar]
- Aznar F.J., Agusti C., Littlewood D.T., Raga J.A., Olson P.D. Insight into the role of cetaceans in the life cycle of the tetraphyllideans (Platyhelminthes: Cestoda) Int. J. Parasitol. 2007;37:243–255. doi: 10.1016/j.ijpara.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Beron-Vera B., Crespo E.A., Raga J.A. Parasites in stranded cetaceans of Patagonia. J. Parasitol. 2008;94:946–948. doi: 10.1645/GE-1296.1. [DOI] [PubMed] [Google Scholar]
- Bester M.N. Endoparasites of the subantarctic fur seal Arctocephalus tropicalis from Gough Island. S. Afr. J. Zool. 1989;24:363–365. [Google Scholar]
- Caira J.N., Jensen K. A digest of elasmobranch tapeworms. J. Parasitol. 2014;100:373–391. doi: 10.1645/14-516.1. [DOI] [PubMed] [Google Scholar]
- Caira J.N., Jensen K., Healy C.J. On the phylogenetic relationships among tetraphyllidean, lecanicephalidean and diphyllidean tapeworm genera. Syst. Parasitol. 1999;42:77–151. doi: 10.1023/a:1006192603349. [DOI] [PubMed] [Google Scholar]
- Caira J.N., Jensen K., Ruhnke T.R. “Tetraphyllidea” van Beneden, 1850 relics. In: Caira J.N., Jensen K., editors. Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth. University of Kansas, Natural History Museum; Lawrence, KS, USA: 2017. pp. 371–400. Special Publication No. 25. [Google Scholar]
- Caira J.N., Jensen K., Waeschenbach A., Olson P.D., Littlewood D.T. Orders out of chaos — molecular phylogenetics reveals the complexity of shark and stingray tapeworm relationships. Int. J. Parasitol. 2014;44:55–73. doi: 10.1016/j.ijpara.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caira J.N., Reyda F.B. Eucestoda (true tapeworms) In: Rohde K., editor. Marine Parasitology. CSIRO Publishing; Collingwood, Australia: 2005. pp. 92–104. [Google Scholar]
- Carvalho V.L., Bevilaqua C.M., Iniguez A.M., Mathews-Cascon H., Ribeiro F.B., Pessoa L.M., de Meirelles A.C., Borges J.C., Marigo J., Soares L., de Lima Silva F.J. Metazoan parasites of cetaceans off the northeastern coast of Brazil. Vet. Parasitol. 2010;173:116–122. doi: 10.1016/j.vetpar.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Chervy L. The terminology of larval cestodes or metacestodes. Syst. Parasitol. 2002;52:1–33. doi: 10.1023/a:1015086301717. [DOI] [PubMed] [Google Scholar]
- Colon-Llavina M.M., Mignucci-Giannoni A.A., Mattiucci S., Paoletti M., Nascetti G., Williams E.H., Jr. Additional records of metazoan parasites from Caribbean marine mammals, including genetically identified anisakid nematodes. Parasitol. Res. 2009;105:1239–1252. doi: 10.1007/s00436-009-1544-4. [DOI] [PubMed] [Google Scholar]
- Cordes D.O., O'Hara P.J. Diseases of captive marine mammals. N. Z. Vet. J. 1979;27:147–150. doi: 10.1080/00480169.1979.34630. [DOI] [PubMed] [Google Scholar]
- Cornaglia E., Rebora L., Gili C., Di Guardo G. Histopathological and immunohistochemical studies on cetaceans found stranded on the coast of Italy between 1990 and 1997. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2000;47:129–142. doi: 10.1046/j.1439-0442.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- Cortés Y., Muñoz G. Eumetazoan parasite infracommunities of the toadfish Aphos porosus (Valenciennes, 1837) (Actinopterygii: Batrachoidiformes) from central Chile. Rev. Biol. Mar. Oceanogr. 2008;43:255–263. [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M., Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick T.A., Chambers C., Isinguzo I. Cestoidea (Pylum Platyhelmintes) In: Woo P.T.K., editor. Fish Diseases and Disorders: Protozoan and Metazoan Infections. CAB International; Canada: 2006. [Google Scholar]
- Euzet L. Causse, Graille & Castelnau; Montpellier: 1959. Recherches sur les Cestodes Tétraphyllides des Sélaciens des côtes de France; p. 263. Thèses a Ia Faculté dcs Scicnccs de Montpelier. [Google Scholar]
- Failla Siquier G., Le Bas A.E. Morphometrical categorization of Phyllobothrium delphini (Cestoidea, Tetraphyllidea) cysts from Fraser's Dolphin, Lagenodelphis hosei (Cetacea, Delphinidae) LAJAM. 2003;2:95–100. [Google Scholar]
- Heithaus M.R. Predator-prey and competitive interactions between sharks (order Selachii) and dolphins (suborder Odontoceti): a review. J. Zool. 2001;253:53. [Google Scholar]
- Innis C., Nyaoke A.C., Williams C.R., 3rd, Dunnigan B., Merigo C., Woodward D.L., Weber E.S., Frasca S., Jr. Pathologic and parasitologic findings of cold-stunned Kemp's ridley sea turtles (Lepidochelys kempii) stranded on Cape Cod, Massachusetts, 2001-2006. J. Wildl. Dis. 2009;45:594–610. doi: 10.7589/0090-3558-45.3.594. [DOI] [PubMed] [Google Scholar]
- Jensen K., Bullard S.A. Characterization of a diversity of tetraphyllidean and rhinebothriidean cestode larval types, with comments on host associations and life-cycles. Int. J. Parasitol. 2010;40:889–910. doi: 10.1016/j.ijpara.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuris A.M., Jaramillo A.G., McLaughlin J.P., Weinstein S.B., Garcia-Vedrenne A.E., Poinar G.O., Jr., Pickering M., Steinauer M.L., Espinoza M., Ashford J.E., Dunn G.L. Monsters of the sea serpent: parasites of an oarfish, Regalecus russellii. J. Parasitol. 2015;101:41–44. doi: 10.1645/14-581.1. [DOI] [PubMed] [Google Scholar]
- Linton E. A new cestode from the maneater and mackerel sharks. Proc. U. S. Natl. Mus. 1922;61:1–19. [Google Scholar]
- Littlewood D.T.J., Olson P.D. Small subunit rDNA and the phylum Platyhelminthes: signal, noise, conflict and compromise. In: Littlewood D.T.J., Bray R.A., editors. Interrelationships of the Platyhelminthes. Taylor and Francis; London: 2001. pp. 262–278. [Google Scholar]
- Lockyer A.E., Olson P.D., Littlewood D.T.J. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biol. J. Linn. Soc. 2003;78:155–171. [Google Scholar]
- Long D.J., Jones R.E. White shark predation and scavenging on cetaceans in the Eastern North pacific Ocean. In: Klimley A.P., Ainley D.G., editors. Great White Sharks: the Biology of Carcharodon carcharias. Academic Press; San Diego: 1996. [Google Scholar]
- McFarlane R.A., Norman R.J., Jones H.I. Springer-Verlag; Berlin Heidelberg: 2009. Chapter 3: Diseases and Parasites of Antarctic and Sub Antarctic Seals. [Google Scholar]
- Mendonca M.M. Phyllobohtrium delphini (Bosc. 1802) (cestoda, Tetraphyllidea) from Arctocephalus pusillus (Schreber 1778) (carnivora, Otariidae) in captivity. Rev. Iber. Parasitol. 1984;44:39–44. [Google Scholar]
- Morgan I.R., Caple I.W., Westbury H.A., Campell J. Disease investigations of penguins and elephant seals on Macquarie Island (Australia) Res. Project Ser. Depart. Agricult. Victoria (Australia) 1978;47 [Google Scholar]
- Norman R.J. Tetraphyllidean cysticerci in the peritoneal cavity of the common dolphin. J. Wildl. Dis. 1997;33:891–895. doi: 10.7589/0090-3558-33.4.891. [DOI] [PubMed] [Google Scholar]
- Nunn G. University of Nottingham; United Kingdom: 1992. Nematode Molecular Evolution. An Investigation of Evolutionary Patterns Among Nematodes Based upon DNA Sequences. [Google Scholar]
- Oliveira J.B., Morales J.A., Gonzalez-Barrientos R.C., Hernandez-Gamboa J., Hernandez-Mora G. Parasites of cetaceans stranded on the Pacific coast of Costa Rica. Vet. Parasitol. 2011;182:319–328. doi: 10.1016/j.vetpar.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Olson P.D., Ruhnke T.R., Sanney J., Hudson T. Evidence for host-specific clades of tetraphyllidean tapeworms (Platyhelminthes: Eucestoda) revealed by analysis of 18S ssrDNA. Int. J. Parasitol. 1999;29:1465–1476. doi: 10.1016/s0020-7519(99)00106-x. [DOI] [PubMed] [Google Scholar]
- Pansegrouw H.M. University of Stellenbosch; South Africa: 1990. A Taxonomic and Quantitative Study of the Endoparasites of the South African Fur Seal Arctocephalus pusillus pusillus Schreber, with Comments on Their Life-histories and Veterinary and Medical Importance; p. 105. MSc. thesis. [Google Scholar]
- Porazinska D.L., Giblin-Davis R.M., Faller L., Farmerie W., Kanzaki N., Morris K., Powers T.O., Tucker A.E., Sung W., Thomas W.K. Evaluating high-throughput sequencing as a method for metagenomic analysis of nematode diversity. Mol. Ecol. Resour. 2009;9:1439–1450. doi: 10.1111/j.1755-0998.2009.02611.x. [DOI] [PubMed] [Google Scholar]
- Randhawa H.S. Insights using a molecular approach into the life cycle of a tapeworm infecting great white sharks. J. Parasitol. 2011;97:275–280. doi: 10.1645/GE-2530.1. [DOI] [PubMed] [Google Scholar]
- Randhawa H.S., Brickle P. Larval parasite gene sequence data reveal cryptic trophic links in life cycles of porbeagle shark tapeworms. Mar. Ecol. Prog. Ser. 2011;431:215–222. [Google Scholar]
- Rennie J., Reid A. XXII.-The Cestoda of the Scottish National Antarctic Expedition. Earth Environ. Sci. Trans. R. Soc. Edinb. 1912;48:441–453. [Google Scholar]
- Riedelsheimer B., Büchl-Zimmermann S. Färbungen. In: Mulisch M., Welsch U., editors. Romeis Mikroskopische Technik. eighteenth ed. Springer Spektrum Akademischer Verlag; Berlin, Germany: 2010. [Google Scholar]
- Riser N.W. Studies on cestode parasites of sharks and skates. J. Tenn. Acad. Sci. 1955;30:265–312. [Google Scholar]
- Ruhnke T.R. A new species of Clistobothrium (Cestoda: Tetraphyllidea), with an evaluation of the systematic status of the genus. J. Parasitol. 1993:37–43. [Google Scholar]
- Ruhnke T.R. Bull. Univ. Nebr. State Mus.; Lincoln, Nebraska: 2011. A Monograph on the Phyllobothriidae (Platyhelminthes, Cestoda): Tapeworms of Elasmobranchs. Part III; pp. 1–208. [Google Scholar]
- Southwell T., Walker A.J. Notes on a larval Cestode from a Fur-Seal. Ann. Trop. Med. Parasitol. 1936;30:91–100. [Google Scholar]
- Stewardson C.L., Fourie H.J. Endoparasites of the cape fur seal Arctocephalus pusillus pusillus from the eastern Cape Coast of South Africa. Trans. Roy. Soc. S. Afr. 1998;53:33–51. [Google Scholar]
- Testa J., Dailey M.D. Five new morphotypes of Phyllobothrium delphini (Cestoda: Tetraphyllidea), their relationship to existing morphotypes, and their zoogeography. Bull. South Calif. Acad. Sci. 1977;76:99–110. [Google Scholar]
- Trevisan B., Primon J.F., Marques F.P. Systematics and diversification of Anindobothrium Marques, Brooks & Lasso, 2001 (Eucestoda: Rhinebothriidea) PLoS One. 2017;12 doi: 10.1371/journal.pone.0184632. e0184632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steenkiste N., Locke S.A., Castelin M., Marcogliese D.J., Abbott C.L. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes) Mol. Ecol. Resour. 2015;15:945–952. doi: 10.1111/1755-0998.12358. [DOI] [PubMed] [Google Scholar]
- Zissler D. Spektrum Akademischer Verlag; Heidelberg, Germany: 1999. Diphyllobothrium, Lexikon der Biologie. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data sets of 18S und 28S sequences for phylogenetic analysis.
Estimates of evolutionary divergence between sequences.