Abstract
The roles of Matrix MetalloProteinases (MMPs), such as MMP-9, in tumor metastasis are well studied, and this in turns stimulates the development of MMP inhibitors as antitumor agents. Previously, Salmonella accumulation was observed in the metastatic nodules of the lungs after systemic administration. Salmonella significantly enhanced the survival of the pulmonary metastatic tumor-bearing mice. Based on our previous observation, we hypothesized that Salmonella could affect metastasis-related protein expression. The treatment of Salmonella clearly reduced the expression of MMP-9. Meanwhile, the MMP-9 related signaling pathways, including Phosph-Protein Kinase B (P-AKT) and Phosph-mammalian Targets Of Rapamycin (P-mTOR) were decreased after a Salmonella treatment. The Salmonella inhibited tumor cell migration by wound-healing and Transwell assay. The anti-metastatic effects of Salmonella were evaluated in mice bearing experimental metastasis tumor models. Consequently, Salmonella inhibited the expression of MMP-9 by reducing the AKT/mTOR pathway and metastatic nodules in vivo.
Keywords: matrix metalloproteinases, metastasis, tumor-targeting, Salmonella
1. Introduction
Solid tumors frequently develop the microenvironment due to physiological stresses [1]. The expression of Matrix MetalloProteinase 9 (MMP-9) in tumors, which may be induced by a tumor microenvironment, contributes to a poor prognosis [2]. Much evidence has demonstrated that MMP-9 plays an important role in tumor growth and metastasis [3]. The MMP-9 as gelatinase is involved in the degradation of the extracellular matrix, angiogenesis, and the progression of tumors. The specific MMP-9 inhibitor is an active area of translational research for tumor treatment [4].
Salmonella is a Gram-negative facultative anaerobic rod that affects the gastrointestinal tract. Salmonella has been demonstrated as a tumor-targeting agent and has antitumor potential [5]. Salmonella exerts a variety of biological effects in antitumor responses, such as tumor-targeting, stimulating host immunity, chemosensitivity, and anti-angiogenesis [6,7,8]. However, the mechanism of Salmonella in the regulation of tumor metastasis is still unclear. Depending on our previous observation, Salmonella can reduce metastatic nodules and prolong the survival of mice in metastatic tumor models [9]. The aim of the present study was to characterize the mechanism of action of Salmonella on metastasis. The present study revealed that Salmonella inhibited cell migration of tumor cells via the Phosph-Protein Kinase B (P-AKT) and Phosph-mammalian Targets Of Rapamycin (P-mTOR) signaling pathways.
2. Results
2.1. Salmonella Inhibition of Tumor Cell Migration
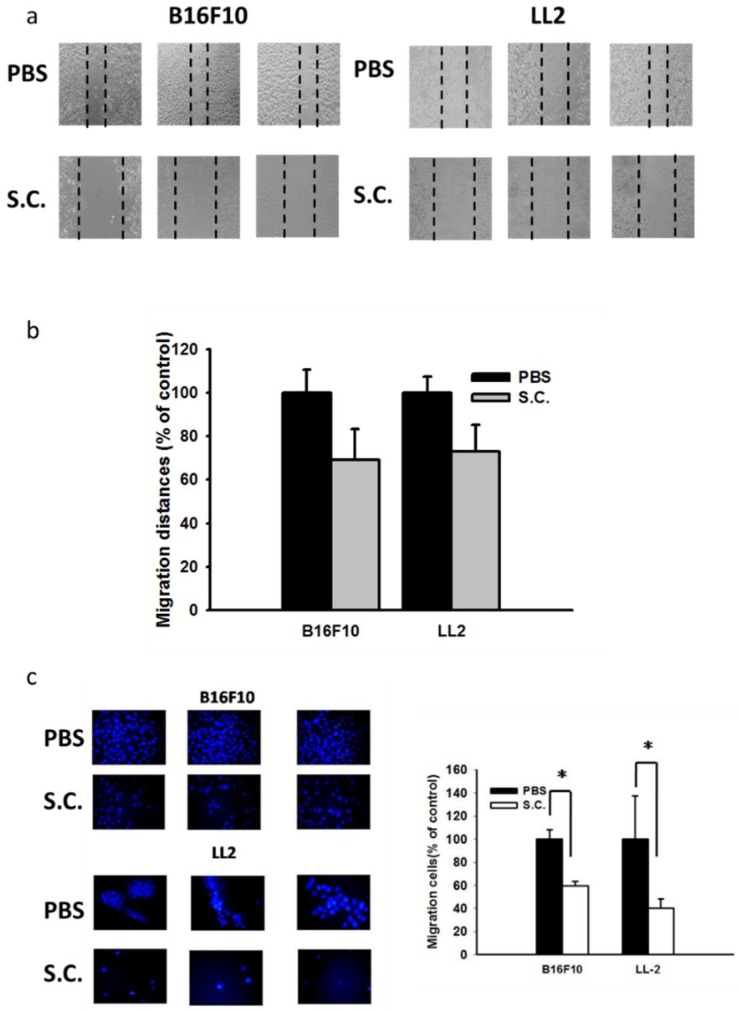
The B16F10 mouse melanoma and LL2 mouse lung tumor cells, both treated with Salmonella, were tested for their ability to inhibit the migration of tumor cells. By using the wound–healing assay, the migration of B16F10 cells was significantly decreased upon the addition of Salmonella compared with that of phosphate buffered saline (PBS) groups. Meanwhile, Salmonella reduced the migration of LL2 cells (Figure 1a,b). Although the migration of tumor cells treated with Salmonella was observed by using the wound-healing assay, it did not preclude the possibility of cellular proliferation. Indeed, the migration of the tumor cells, including B16F10 and LL2 cells was significantly reduced after a Salmonella treatment by using the Transwell assay (Figure 1c). The results suggested that Salmonella reduced the migration of tumor cells.
Figure 1.
Effects of Salmonella (S.C.) on cellular migration in B16F10 and LL2 cells. B16F10 cells and LL2 cells (2 × 105 cells/well) were placed onto plates and incubated at 37 °C for 24 h. The cells cocultured with Salmonella (Multiplicity Of Infection (MOI) = 10) for 4 h. The migration distances of different groups of B16F10 and LL2 cells were shown (a) and measured (b) (×400); (c) Tumor cells were placed on the upper layer of a cell culture insert with permeable membrane and then treated with Salmonella (MOI = 10) for 4 h. Following an incubation 24 h, the cells migrated through the membrane were stained with 4′,6-Diamidino-2-Phenylindole (DAPI) and counted under a fluorescence microscope (×400).
2.2. The Phospho-Protein Kinase B (P-AKT)/Phospho-Mammalian Targets of the Rapamycin (P-mTOR) Pathway as Requirement for Salmonella-Mediated MMP-9 Expression
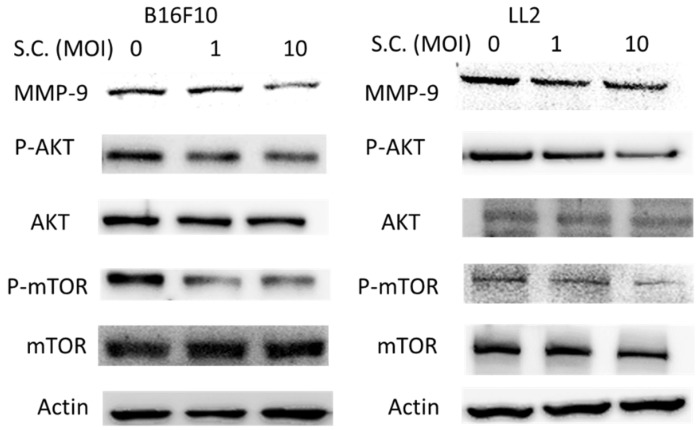
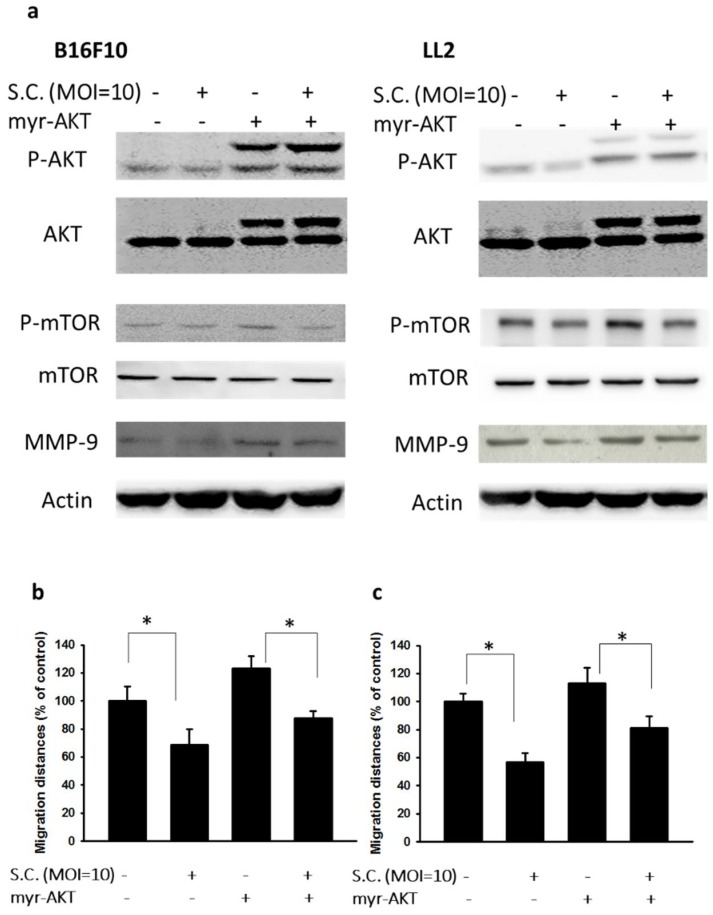
Salmonella can regulate the migration of tumor cells. As expected, Salmonella may affect the enzyme responsible for tumor migration, such as MMP-9. B16F10 cells and LL2 cells were treated with Salmonella (Multiplicity Of Infection (MOI) = 0–10) and the expression of MMP-9 in tumor cells was measured. The AKT/mTOR axis was involved in the expression of MMP-9 in tumors. The Phosphatidylinositol 3-Kinase (PI3K) inhibitors suppressed MMP-9 expression, suggesting that the PI3K/AKT pathways are involved in MMP-9 regulation [10]. The protein expressions of phospho-AKT/mTOR were examined in two cell lines treated with Salmonella (Figure 2). We found that Salmonella did in fact reduce the expression of MMP-9 in a dose-dependent manner (Figure 2). Meanwhile, the expression of phospho-AKT/phospho-mTOR was reduced after the Salmonella infection. Regarding the involvement of the AKT/mTOR axis in the Salmonella-mediated suppression of MMP-9, we were surprised to learn that cells were transfected with constitutively active AKT plasmids. Determination of the Western blotting showed that transfection with constitutively active AKT plasmid in tumor cells resulted in an inversion of Salmonella-regulated MMP-9 expression in tumor cells (Figure 3a). The migration distance of tumor cells was suppressed after Salmonella treatment, as previous described. The migration distances of B16F10 and LL2 cells treated with Salmonella could be reversed after constitutively active AKT plasmid transfection (Figure 3b,c). The results indicated that AKT/mTOR signaling pathway might play the role in the Salmonella-regulated decrease of MMP-9 and tumor cell migrated behavior.
Figure 2.
Effects of Salmonella (S.C.) on Matrix MetalloProteinase 9 (MMP-9) expression in B16F10 and LL2 cells. B16F10 cells and LL2 cells (2 × 105 cells/well) were placed into plates and incubated at 37 °C for 24 h. The cells cocultured with Salmonella (MOI = 1–10) for 4 h. The protein expression of B16F10 and LL2 cells was measured.
Figure 3.
Constitutively active- Phosph-Protein Kinase B (P-AKT) reduced Salmonella (S.C.)-induced decrease of MMP-9. The B16F10 and LL2 cells were transfected with constitutively active AKT plasmids for 16 h prior to 4 h Salmonella treatment. (a) The expression of phosphorylation AKT, AKT, phosphorylation Phosph-mammalian Targets Of Rapamycin (P-mTOR), mTOR, and MMP-9 protein in B16F10 and 4T1 cells was determined. The immunoblotting assay was repeated three times with similar results. The migration distances of B16F10 (b) and LL2 (c) cells were measured. (n = 3, data are mean ± SD. * p < 0.05).
2.3. Salmonella Supresssion of Matrix MetalloProteinase 9 Expression In Vivo
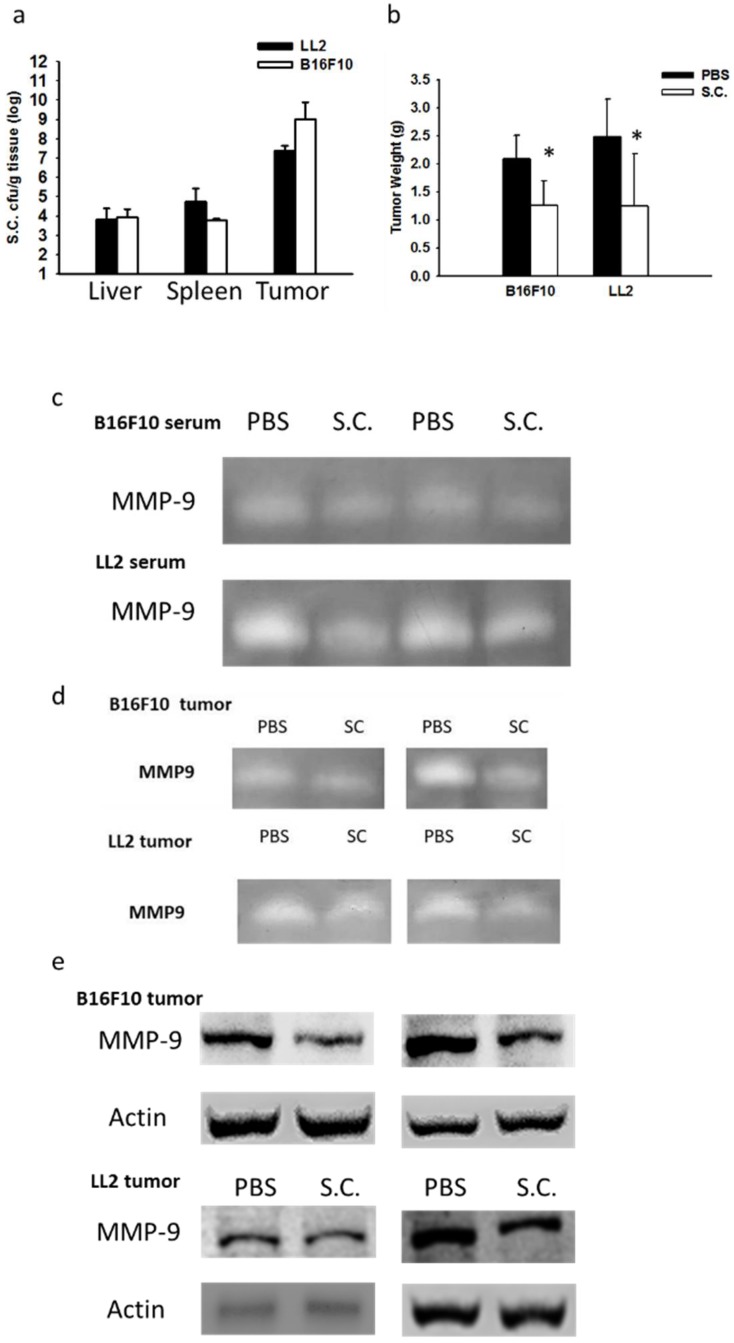
We obtained similar results demonstrating that the tumor-targeting potential of Salmonella and anti-tumor activity of Salmonella in two tumor models (Figure 4a,b). Tumors can digest extracellular matrixes to facilitate their migration to secondary sites by releasing MMP-9 themselves [11]. Using gelatin zymography to detect MMP-9 activity is required. To verify the study in vivo, tumor-bearing mice were treated with Salmonella (106 colony-forming units (cfu)) and sacrificed after 3 days. Serum and tumor tissues were collected and analyzed by gelatin zymography and Western blotting (Figure 4c–e). Salmonella reduced the function of MMP-9 in serum and tumors from tumor-bearing mice (Figure 4c,d). By using Western blotting analysis, the reduced protein expressions of MMP-9 in two tumor models were observed in Salmonella-treated group (Figure 4e) These findings suggested that Salmonella inhibited the expression and activity of MMP-9 in vivo.
Figure 4.
Salmonella (S.C.) reduced the function and expression of MMP-9 in vivo. The C57BL/6 mice had been subcutaneously inoculated with B16F10 and LL2 cells at day 0, and the tumor bearing mice were intraperitoneal injected with Salmonella (106 cfu) at day 7. At day 10, the mice were sacrificed. The serum, tumors, livers, and spleen were collected. (a) The amounts of accumulated Salmonella in organs was determined; (b) The antitumor activity of Salmonella was determined by tumor weight. (n = 4, data are mean ± SD. * p < 0.05); The enzyme activity of MMP-9 in serum (c) and tumors (d) was determined by gelatin zymography; (e) The protein expression of MMP-9 in tumors derived from Salmonella-treated mice or control mice was determined by Western blotting.
2.4. Salmonella Inhibition of Tumor Metastasis In Vivo
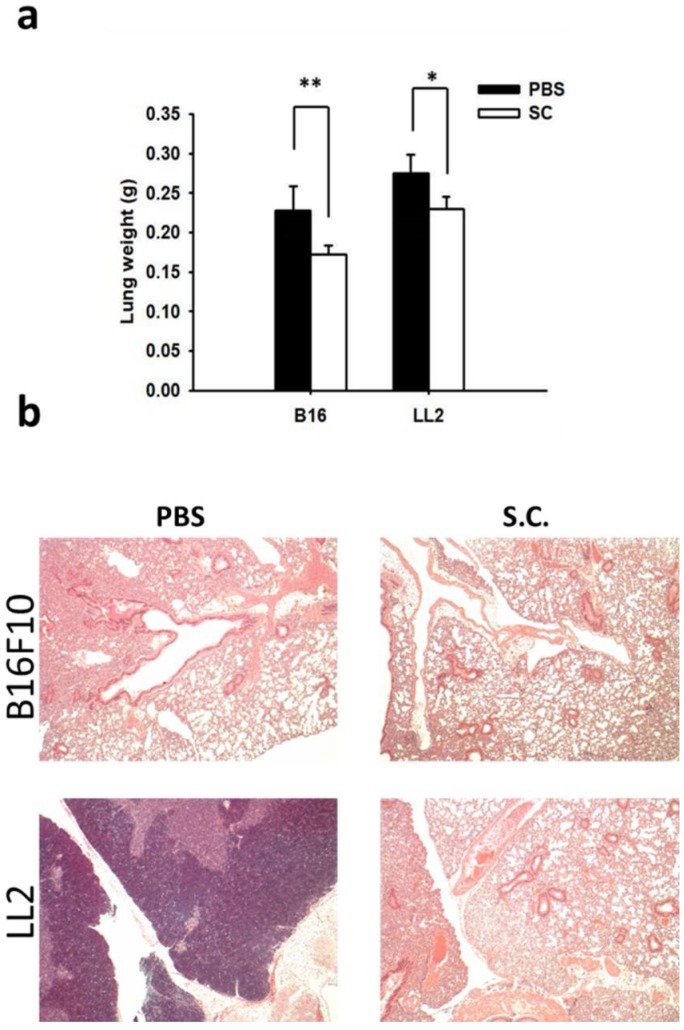
On our previous study, we established that mice injected with tumor cells admixed with metastatic inducers or metastatic inhibitors to identify the activity of anti-metastatic molecules [12]. We now want to know whether Salmonella could inhibit metastasis: the tumor cells either pre-incubated with Salmonella or not were injected into mice via tail vein. Salmonella affected the tumor mass in lungs (Figure 5a). There are more B16F10 tumor nodules in the mice injected with cells than those in the mice injected cells admixed with Salmonella (Figure 5b). The similar results were obtained in LL2 tumor models. Taken together, these results demonstrated that Salmonella affects tumor metastasis in vivo.
Figure 5.
Salmonella (S.C.) inhibited metastasis in vivo. The C57BL/6 mice were injected with B16F10 and LL2 cells (105) pre-incubated with or without Salmonella (MOI = 10) for 4 h via the tail vein at day 0. (a) At day 20, the wet lung weight of the lungs was measured. (n = 3, data are mean ± SD. * p < 0.05; ** p < 0.01). (b) The metastatic pulmonary nodules were observed after the intravenous injection of cells (×400).
3. Discussion
Tumor-target therapy of Salmonella is attracting increasingly more attention for researchers and is considered to be a new strategy against solid tumors [8,13,14,15]. When Salmonella is used as an antitumor agent, it has many features for inhibiting tumor growth. Previously, we found that Salmonella not only targeted primary tumors, but also small metastatic tumors [16]. The results imply that Salmonella could reduce tumor metastasis. Observing the expression of MMP-9 protein in both tumor cells treated with Salmonella were significantly decreased, which was consistent with the result of the cell migration ability detected by wound-healing and Transwell assay. In animal studies, the less metastatic nodules and lung weights were observed in Salmonella-administrated groups.
Increasingly more evidence has suggested that cancer stem cells contribute to metastasis [17]. The MMP-9 is one of the cancer stem cell markers [18]. In this study, Salmonella reduced the expression of MMP-9 via the AKT/mTOR pathway. Salmonella had been demonstrated to inhibit tumor growth by targeting the cancer stem cell niche [19]. The hypoxic regions of the tumor are a privileged site in which the Salmonella as well as the cancer stem cells reside; therefore, the Salmonella is able to aim at the at the cancer stem cell [19]. The results are consistent with previous reports. The accumulation of Salmonella in tumor sites has been confirmed in various solid tumors, including breast cancer, melanoma, bladder cancer, liver cancer, lung cancer, and colon cancer [9,16,19,20]. Although Salmonella has enormous potential for targeting solid tumors, the mechanisms are largely unknown. Salmonella may use enhanced permeability and retention effect to specifically target to tumor sites [21]. In addition, host immune cells in healthy organs cleared Salmonella more rapidly than those in a tumor microenvironment, where immune sites are privileged [22,23]. Moreover, the nutrients and hypoxia regions in tumors may attract Salmonella to target the tumor sites [9].
This study helps to better understand how the interaction between Salmonella and tumor microenvironment. Salmonella may reduce the modification of a second organ microenvironment by inhibiting the secretion and function of MMP-9 from a primary tumor. Salmonella influences oncoproteins—such as hypoxia-inducible factors, indoleamine 2,3-dioxygenase, and p-glycoprotein—through the AKT/mTOR signaling pathways in tumor cells [6,24,25]. The cellular AKT/mTOR axis plays an important role in cellular physiology and homeostasis [26]. Many small molecular drugs target this axis and contribute to control the tumor growth [27]. Autophagy is initiated in response to cellular stress, including Salmonella infection. Salmonella triggered cell autophagy through Salmonella-induced amino acid starvation [25,26]. Autophagy can regulate protein synthesis [28]. The AKT/mTOR pathway is involved in a negative regulator of autophagy [29]. A Salmonella infection could influence the AKT/mTOR signaling pathways in cells [30]. Salmonella induces autophagy by decreasing AKT/mTOR signaling pathway [29,30]. In our system, the MMP-9 is downregulated through Salmonella, which decreases AKT/mTOR signaling pathway. Salmonella has a tumor-targeting potential and inhibits the AKT activity, implying that Salmonella suppresses tumor growth through inhibiting the AKT/mTOR signal pathway.
4. Materials and Methods
4.1. Cells, Reagents, Animal, Bacteria, and Plasmids
Murine melanoma cells (B16F10) and murine lung carcinoma (LL2) cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), containing 10% of fetal bovine serum and 50 μg/mL gentamicin at 37 °C in 5% CO2. The 4′,6-Diamidino-2-Phenylindole (DAPI) and gelatin were purchased from Sigma-Aldrich (Sigma Aldrich, St. Louis, MO, USA). C57BL/6 mice were purchased from the National Laboratory Animal Center of Taiwan. The experimental protocol was approved by the Laboratory Animal Care and Use Committee of the National Sun Yat-sen-University (permit number: 10635, 20 December 2017). The Salmonella and the constitutively active AKT plasmid were previously described [9,24].
4.2. Wound-Healing and Transwell Assay
The culture inserts (IBIDI, Martinsried, Germany) plated on 24 well plates were used to measure the wound-healing according to the manufacturer’s instruction. The migration distance was measured after 24 h using a microscope. The migration distances of untreated tumor cells were set to 100% and were compared with cells treated with Salmonella for 4 h. The Transwell cultures (ThermoFisher Scientific, Waltham, MA, USA) plated on 24 well plates were used to observe the cell migration according to the manufacturer’s instruction. The migration cells were stained with DAPI and counted under fluorescence microscope. The number of migration cells of untreated tumor cells were set to 100% and were compared with cells treated with Salmonella for 4 h.
4.3. Western Blotting, Gelatin Zymography, and Transfection
The Bicinchoninic Acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL, USA) was used to determine the protein contents. The SDS-PAGE was used to fractionate the protein samples. Then, protein samples were transferred onto hybond-enhanced chemiluminescence nitrocellulose membranes (Pall Life Science, Glen Cove, NY, USA). The membranes were probed with various antibodies, such as MMP-9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phosphorylation-AKT (Santa Cruz Biotechnology), AKT (Santa Cruz Biotechnology), phosphorylation-mTOR (Cell Signaling, Danvers, MA, USA), mTOR (Cell Signaling), or β-actin (Sigma-Aldrich). The appropriate horseradish peroxidase-conjugated antibodies were used as secondary antibodies. The protein-antibody complexes were visualized by enhanced chemiluminescence system (T-Pro Biotechnology, New Taipei City, Taiwan). A 7.5% acrylamide gel containing gelatin was used to separate protein. Then, the gel was stained with a staining solution for 1 h and was washed with destaining solution until bands could clearly be seen. Tumor cells were transfected with the constitutively active AKT plasmid, using Lipofectamine 2000. At post-transfection, cells were treated with Salmonella for 4 h or not. The cell lysates were then harvested.
4.4. Mouse Experiments
The C57BL/6 mice was subcutaneously inoculated with B16F10 (106) and LL2 (106) cells at day 0, and the tumor bearing mice were intraperitoneal injected with Salmonella (106 cfu) at day 7. At day 10, the mice were sacrificed. The serum, tumors, livers, and spleen were collected and weighed, and the number of Salmonella was counted on Lysogeny broth plates. MMP-9 in serum and tumors was determined by gelatin zymography and Western blotting. In a parallel experiment, mice were injected with B16F10 (106) and LL2 cells (105) pre-incubated with or without Salmonella (MOI = 10) for 4 h via the tail vein at day 0. At day 20, tumor-bearing mice were sacrificed, and the lungs were removed, weighed, and histologically examined.
4.5. Statistical Analysis
The significant differences between groups were determined by Analysis of variance (ANOVA). Any p value less than 0.05 is considered statistically significant.
5. Conclusions
Salmonella has an advantage over other antitumor agents because Salmonella could target to tumor sites [31]. Salmonella preferentially accumulated not only in primary tumors but also in metastatic nodules. In this study, Salmonella reduced tumor migration in vitro and the formation of tumor nodules in vivo by inhibiting MMP-9 expression. Thus, Salmonella-mediated tumor therapy should attract more attention in the future.
Acknowledgments
Chiau-Yuang Tsai (Department of Molecular Immunology, Osaka University, Japan) provided the constitutively active AKT plasmid.
Abbreviations
| MMP | Matrix metalloproteinases |
| AKT | Protein kinase B |
| mTOR | Mammalian targets of rapamycin |
| PI3K | Phosphatidylinositol 3-kinase |
| S.C. | Salmonella |
| MOI | The multiplicity of infection |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| cfu | Colony-forming units |
Author Contributions
Y.-T.T. and C.-Y.K. conceived and designed the experiments. Y.-T.T. and C.-Y.K. performed the experiments. S.-P.C. and C.-H.L. wrote the manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 104-2320-B039-042-MY3) and NSYSU-KMU joint research project, (#NSYSUKMU 107-P004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lv Y., Zhao X., Zhu L., Li S., Xiao Q., He W., Yin L. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics. 2018;8:2830–2845. doi: 10.7150/thno.23209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh E., Kim Y.J., An H., Sung D., Cho T.M., Farrand L., Jang S., Seo J.H., Kim J.Y. Flubendazole elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Int. J. Cancer. 2018 doi: 10.1002/ijc.31585. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y., Lan M., Chen X., Dai Y., Zhao X., Wang L., Zhao T., Li Y., Zhu J., Zhang X., et al. Anti-invasion and anti-metastasis effects of Valjatrate E via reduction of matrix metalloproteinases expression and suppression of MAPK/ERK signaling pathway. Biomed. Pharmacother. 2018 doi: 10.1016/j.biopha.2018.04.136. [DOI] [PubMed] [Google Scholar]
- 4.Asghar M.Y., Kemppainen K., Lassila T., Törnquist K. Sphingosine 1-phosphate attenuates MMP2 and MMP9 in human anaplastic thyroid cancer C643 cells: Importance of S1P2. PLoS ONE. 2018;13:e0196992. doi: 10.1371/journal.pone.0196992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaimala S., Al-Sbiei A., Cabral-Marques O., Fernandez-Cabezudo M.J., Al-Ramadi B.K. Attenuated bacteria as immunotherapeutic tools for cancer treatment. Front. Oncol. 2018;8:136. doi: 10.3389/fonc.2018.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C.J., Chang W.W., Lin S.T., Chen M.C., Lee C.H. Salmonella overcomes drug resistance in tumor through p-glycoprotein downregulation. Int. J. Med. Sci. 2018;15:574–579. doi: 10.7150/ijms.23285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi K., Kawaguchi K., Kiyuna T., Miyake K., Miyake M., Singh A.S., Eckardt M.A., Nelson S.D., Russell T.A., Dry S.M., et al. Tumor-targeting Salmonella typhimurium A1-R is a highly effective general therapeutic for undifferentiated soft tissue sarcoma patient-derived orthotopic xenograft nude-mouse models. Biochem. Biophys. Res. Commun. 2018;497:1055–1061. doi: 10.1016/j.bbrc.2018.02.174. [DOI] [PubMed] [Google Scholar]
- 8.Kramer M.G., Masner M., Ferreira F.A., Hoffman R.M. Bacterial therapy of cancer: Promises, limitations, and insights for future directions. Front. Microbiol. 2018;9:16. doi: 10.3389/fmicb.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C.H., Wu C.L., Tai Y.S., Shiau A.L. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Mol. Ther. 2005;11:707–716. doi: 10.1016/j.ymthe.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Wan D., Zhou R., Zhong W., Lu S., Chai Y. Geraniin inhibits migration and invasion of human osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2 signaling pathways. Anticancer Drugs. 2017;28:959–966. doi: 10.1097/CAD.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 11.Miao F., Zhang X., Cao Y., Wang Y., Zhang X. Effect of siRNA-silencing of SALL2 gene on growth, migration and invasion of human ovarian carcinoma A2780 cells. BMC Cancer. 2017;17:838. doi: 10.1186/s12885-017-3843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M.C., Chang W.W., Kuan Y.D., Lin S.T., Hsu H.C., Lee C.H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012;18:685–693. doi: 10.1177/1753425912436589. [DOI] [PubMed] [Google Scholar]
- 13.Lee C.H. Engineering bacteria toward tumor targeting for cancer treatment: Current state and perspectives. Appl. Microbiol. Biotechnol. 2012;93:517–523. doi: 10.1007/s00253-011-3695-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang W.W., Lee C.H. Salmonella as an innovative therapeutic antitumor agent. Int. J. Mol. Sci. 2014;15:14546–14554. doi: 10.3390/ijms150814546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felgner S., Kocijancic D., Frahm M., Heise U., Rohde M., Zimmermann K., Falk C., Erhardt M., Weiss S. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 2017;7:e1382791. doi: 10.1080/2162402X.2017.1382791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C.H., Wu C.L., Shiau A.L. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther. 2005;12:175–184. doi: 10.1038/sj.cgt.7700777. [DOI] [PubMed] [Google Scholar]
- 17.Lee C.H., Yu C.C., Wang B.Y., Chang W.W. Tumorsphere as an effective in vitro platform for screening anticancer stem cell drugs. Oncotarget. 2016;7:1215–1226. doi: 10.18632/oncotarget.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S., Yan Y., Cheng Z., Hu Y., Liu T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018;4:26. doi: 10.1038/s41420-018-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang W.W., Kuan Y.D., Chen M.C., Lin S.T., Lee C.H. Tracking of mouse breast cancer stem-like cells with Salmonella. Exp. Biol. Med. 2012;237:1189–1196. doi: 10.1258/ebm.2012.012063. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.H., Wu C.L., Shiau A.L. Salmonella choleraesuis as an anticancer agent in a syngeneic model of orthotopic hepatocellular carcinoma. Int. J. Cancer. 2008;122:930–935. doi: 10.1002/ijc.23047. [DOI] [PubMed] [Google Scholar]
- 21.Lee C.H., Wu C.L., Shiau A.L. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J. Gene Med. 2004;6:1382–1393. doi: 10.1002/jgm.626. [DOI] [PubMed] [Google Scholar]
- 22.Lee C.H., Hsieh J.L., Wu C.L., Hsu P.Y., Shiau A.L. T cell augments the antitumor activity of tumor-targeting Salmonella. Appl. Microbiol. Biotechnol. 2011;90:1381–1388. doi: 10.1007/s00253-011-3180-z. [DOI] [PubMed] [Google Scholar]
- 23.Lee C.H., Hsieh J.L., Wu C.L., Hsu H.C., Shiau A.L. B cells are required for tumor-targeting Salmonella in host. Appl. Microbiol. Biotechnol. 2011;92:1251–1260. doi: 10.1007/s00253-011-3386-0. [DOI] [PubMed] [Google Scholar]
- 24.Tu D.G., Chang W.W., Lin S.T., Kuo C.Y., Tsao Y.T., Lee C.H. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget. 2016;7:37513–37523. doi: 10.18632/oncotarget.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuan Y.D., Lee C.H. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2, 3-dioxygenase 1 expression. Oncotarget. 2016;7:374–385. doi: 10.18632/oncotarget.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C.H., Lin S.T., Liu J.J., Chang W.W., Hsieh J.L., Wang W.K. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014;21:309–316. doi: 10.1038/gt.2013.86. [DOI] [PubMed] [Google Scholar]
- 27.Sun X., Ma X., Li Q., Yang Y., Xu X., Sun J., Yu M., Cao K., Yang L., Yang G., et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018;42:811–820. doi: 10.3892/ijmm.2018.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J., Wang Z.Y., Yang H.Z., Liu H.Z., Mi S., Lv X.X., Fu X.M., Yan H.M., Zhang X.W., Zhan Q.M., et al. Timing is critical for an effective anti-metastatic immunotherapy: The decisive role of IFNγ/STAT1-mediated activation of autophagy. PLoS ONE. 2011;6:e24705. doi: 10.1371/journal.pone.0024705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tattoli I., Philpott D.J., Girardin S.E. The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole. Biol. Open. 2012;1:1215–1225. doi: 10.1242/bio.20122840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budak G., Eren Ozsoy O., Aydin Son Y., Can T., Tuncbag N. Reconstruction of the temporal signaling network in Salmonella-infected human cells. Front. Microbiol. 2015;20:730. doi: 10.3389/fmicb.2015.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C.H., Nishikawa T., Kaneda Y. Salmonella mediated the hemagglutinating virus of Japan-envelope transfer suppresses tumor growth. Oncotarget. 2017;8:35048–35060. doi: 10.18632/oncotarget.17037. [DOI] [PMC free article] [PubMed] [Google Scholar]