Abstract
This mini-review summarizes recent new developments in visceral analgesics. This promising field is important, as a new approach to address abdominal pain with peripheral visceral analgesics is considered a key approach to addressing the current opioid crisis. Some of the novel compounds address peripheral pain mechanisms through modulation of opioid receptors via biased ligands, nociceptin/orphanin FQ opioid peptide (NOP) receptor, or dual action on NOP and μ-opioid receptor, buprenorphine and morphiceptin analogs. Other compounds target nonopioid mechanisms, including cannabinoid (CB2), N-methyl-d-aspartate, calcitonin gene-related peptide, estrogen, and adenosine A2B receptors and transient receptor potential (TRP) channels (TRPV1, TRPV4, and TRPM8). Although current evidence is based predominantly on animal models of visceral pain, early human studies also support the evidence from the basic and animal research. This augurs well for the development of nonaddictive, visceral analgesics for treatment of chronic abdominal pain, an unmet clinical need.
Keywords: cannabinoid, estrogen, receptors, transient receptor potential
INTRODUCTION: ABDOMINAL PAIN AND APPROACHES TO ADDRESS THE OPIOID EPIDEMIC
Chronic abdominal pain may result from diverse conditions, ranging from chronic pancreatitis and Crohn's disease to chronic abdominal pain, and its treatment remains a challenge in clinical practice. While interventions such as celiac plexus blocks and nonopioid pain medications provide some relief, they are typically less efficacious and limited by adverse effects. Thus, opioid medications are commonly used to manage chronic pain syndromes refractory to other pain management interventions (70). From 1997 to 2008, opioid prescriptions for chronic abdominal pain more than doubled in US outpatient clinics (27). The adjusted prevalence of visits for which an opioid was prescribed increased from 5.9% (95% CI, 3.5–8.3%) in 1997–1999 to 12.2% (95% CI, 7.5–17.0%) in 2006–2008 (P = 0.03 for trend), and opioid prescriptions were most common among patients aged 25 to 40 yr (odds ratio 4.6; 95% CI, 1.2–18.4). Among patients with Crohn’s disease, it has been reported that concurrent functional gastrointestinal disorders constitute a risk factor for opioid misuse, and 20% of patients with Crohn’s disease at a referral gastroenterology clinic were using chronic narcotics (22). Concurrent irritable bowel syndrome (IBS) was also a risk factor for the initiation of opioid therapy in hospitalized patients with Crohn’s disease (45). In a population-based, epidemiological study from Olmsted County, Minnesota, 12% of 3,515 people were using narcotics, and 18–21% of patients with dyspepsia, IBS, or bloating reported using narcotics (20).
A report from the National Academies of Sciences, Engineering, and Medicine (50) documented that, as of 2015, at least 2 million people in the United States have an opioid-use disorder involving prescription opioids, and an average of ~90 Americans die every day from overdoses that involve illicit opioids, including heroin and synthetic opioids such as fentanyl; this number has nearly tripled in the 5 yr leading up to 2015. In 2010, Dr. Susan Okie, a family physician, alerted the medical profession to the flood of opioids, the rising tide of deaths, the plans of the US Food and Drug Administration to require opioid makers to provide training for physicians and patient-education materials on the appropriate prescribing and use of extended-release and long-acting opioids, and the need for action (49). It is clear that, for a growing number of people, opioid-use disorder began with prescription opioids. This has led to recommendations for fundamental shifts in analgesic-prescribing practices, a mandate for pain-related education for all health professionals who provide care to people with pain, and education to the general public on risks and benefits of opioids. This report from the National Academies and the 138-page report The President’s Commission on Combating Drug Addiction and the Opioid Crisis (65) detailed societal approaches to addressing the opioid epidemic and pharmacological approaches to structured withdrawal; examples of these approaches are summarized in Table 1. The two reports also urged the US Congress and the Administration to block-grant federal funding for opioid-related and substance use disorder-related activities to the states.
Table 1.
Examples of approaches to address the opioid epidemic
| Societal approaches |
| Reducing restrictions on lawful access to prescription opioids, because such restrictions may be driving some people toward the illegal market |
| Treatment for the millions who have opioid-use disorder |
| Removal of impediments to full coverage of medications approved by the US Food and Drug Administration for treatment of opioid-use disorder |
| Partnerships between public and private payers, including insurance companies, to develop reimbursement models that support evidence-based and cost-effective comprehensive pain management, including drug and nondrug treatments for pain |
| Pharmacological approaches |
| Access to methadone and buprenorphine (substitute µ-opioid agonists) in a structured withdrawal program |
| Access to naloxone (a µ-opioid antagonist prescribed to rescue people dying as a result of respiratory depression from an opioid) |
Approaches to address the opioid epidemic are from Refs. 50 and 65.
The report from the President’s Commission also mentioned that the National Institutes of Health have been partnering with pharmaceutical companies to develop nonaddictive painkillers and new treatments for addiction and overdose. Volkow and Collins (69) outline the role of science in addressing the opioid crisis and identify short-, intermediate-, and long-term strategies to address overdose prevention and reversal, treatment of opioid-use disorders, and treatment of chronic pain.
Given the clinical unmet need represented by the paucity of approved analgesics specifically for visceral pain syndromes and the societal imperative to develop alternative pharmacological approaches to treat abdominal pain, this review aims to document the current repertoire of visceral pharmacological (rather than behavioral) analgesics and to explore novel medications in development for treatment of abdominal pain to expand on the approaches mentioned by Volkow and Collins (69).
CURRENT VISCERAL ANALGESICS
A recent review on the treatment of abdominal pain in IBS (15) provided details on pharmacological approaches such as antispasmodics, peppermint oil, antidepressants (tricyclic agents and selective serotonin reuptake inhibitors), 5-HT3 receptor antagonists (alosetron, ondansetron, and ramosetron), nonabsorbed antibiotic (rifaximin), secretagogues (lubiprostone and linaclotide), a combination μ- and κ-opioid receptor (OR) agonist and δ-OR antagonist (eluxadoline), histamine H1 receptor antagonist (ebastine), neurokinin (NK)-2 receptor antagonist (ibodutant), and GABAergic agents (gabapentin and pregabalin). Some of these medications are approved for treatment of IBS, but their predominant effects are on the diarrhea or constipation associated with IBS. Other medications (ebastine, ibodutant, and the GABAergic agents) are not approved for IBS or abdominal pain, act centrally, and may affect other cerebral functions, including induction of somnolence. In addition, some of the approved medications may have adverse effects in the gastrointestinal tract, such as sphincter of Oddi dysfunction and pancreatitis (eluxadoline) or ischemic colitis (alosetron), and are associated with warnings restricting their use.
Earlier attempts to develop κ-OR agonists as visceral analgesics were abandoned after initial trials with asimadoline (46) and fedotozine (23, 54) were promising in IBS or functional dyspepsia, and another κ-OR agonist, JNJ-38488502, was ineffective at reducing sensation after colorectal distension, although it exerted opioid-related effects, specifically increasing colonic compliance in male, healthy volunteers (32).
A nonsedating H1 receptor antagonist, ebastine, is a novel and attractive mechanism based on inhibition of the transient receptor potential (TRP) channel TRPV1 receptors on afferent mechanisms; it reduced abdominal pain in patients with evidence of rectal hypersensitivity (72). The NK2 antagonist ibodutant is promising and, in a phase 2B trial, was not associated with adverse central or peripheral effects (63). Further clinical trials with these two promising drug classes are awaited.
There is still an unmet need in the treatment of chronic functional abdominal pain and the pain component of IBS. This overview addresses several classes of classical modulators of afferent function (Table 2) that are particularly attractive, because they represent pharmacological moieties with peripheral selectivity.
Table 2.
Classes of novel peripheral visceral analgesics
| Class of Peripheral Visceral Analgesic | Examples | Summary of Mechanism of Action |
|---|---|---|
| Novel µ-opioid agents | ||
| Novel biased ligand of the μ-opioid receptor | TRV130 and PZM21 | Activates G protein without β-arrestin pathway |
| Targeting μ-opioid receptor under acidic conditions | NFEPP | A fluorinated derivative of fentanyl exclusively activated at acidic sites, e.g., inflammation |
| NOP receptor modulation | SCH 221510 | NOP ligand may reverse a possible deficiency of endogenous nociceptin in IBS |
| Dual action on NOP and μ-opioid receptor | Cebranopadol and BU08070 | Peripherally active mixed μ-opioid peptide-NOP receptor agonist |
| Buprenorphine analogs | ORP-101 | Combines analgesia from μ-opioid receptor agonism and the antiaddictive effects of a κ-opioid receptor antagonist |
| Morphiceptin analog | P-317 | Cyclic pentapeptide derivative of morphiceptin with indirect evidence of peripheral action |
| Novel CB2 agonists | LY3038404, PF-03550096, and APD371 | Selective CB2 agonists; may have superior analgesic effects devoid of the centrally mediated CB1 effects |
| N-methyl-d-aspartate receptor antagonists | Ketamine | Prevent central sensitization to opioid analgesics, improving effectiveness of opioids |
| CGRP-related peptide receptor antagonists | CGRP-(8–37) | Blocks endosomal signaling of the CLR to pain transmission |
| Drugs targeting TRP channels | ||
| TRPV1 | JYL1421 | TRPV1 receptor antagonist reduces mechanical, but not chemical, hyperalgesia |
| TRPV4 | RN1734 | Selective TRPV4 antagonist reduces chemically induced hyperalgesia |
| TRPM8 | Peppermint oil and caraway oil | Agonists that reduce colonic hypersensitivity to mechanical stimuli |
| GPER and ER ligands | G-1 and estradiol | Nonselective GPER agonist and ER ligand inhibit muscle contractility and chemically induced colonic hyperalgesia |
| Adenosine A2B receptor antagonist | Aminophylline | Antagonizes A2B receptors that are involved in control of intestinal secretion, motility, and sensation |
NOP, nociceptin/orphanin FQ opioid peptide; IBS, irritable bowel syndrome; CB2, cannabinoid receptor type 2; CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like receptor; TRP, transient receptor potential; GPER, G protein-coupled estrogen receptor; ER, estrogen receptor.
Novel µ-Opioid Agents
Opioids are effective for the treatment of moderate-to-severe pain but are associated with opioid-related adverse events such as addiction, respiratory depression, gastrointestinal effects (e.g., nausea, vomiting, and constipation), sedation, and the “rising tide of deaths” noted in recent years (49). These adverse effects are directly the result of actions on ORs and may result in limiting the dose to below that required for analgesic efficacy or lead to deleterious, perhaps even fatal, effects to achieve relief. One of the main objectives is to provide analgesia or antinociception with reduced dependency, reduced likelihood of addiction, or reduced respiratory depression. Several novel approaches are in development to obviate these risks.
Novel Biased Ligand of the μ-OR
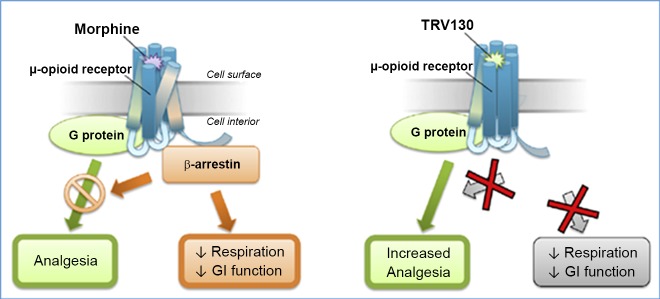
Conventional opioids bind to the μ-OR and induce analgesia through activation of G protein-mediated pathways; μ-OR activation also recruits β-arrestin, which mediates receptor desensitization and internalization and also induces respiratory depression and inhibits gastrointestinal motility. Biased ligands produce differentiated pharmacology in preclinical studies compared with unbiased ligands; thus, novel compounds that activate the G protein pathway without activating β-arrestin are in development (Fig. 1). The expected effect of such an approach would be analogous to the effects of β-arrestin-2 knockout in mice, that is, enhanced analgesia with reduced gastrointestinal and respiratory effects (12, 52).
Fig. 1.
Hypothesis for increased analgesia and reduced adverse events for a biased G protein μ-opioid receptor ligand. Morphine binding to the μ-opioid receptor (left) engages analgesic signaling through G protein coupling to inhibit nociception by neuronal hyperpolarization and also engages β-arrestins to the same receptor, which inhibits G protein coupling and promotes respiratory depression and constipation. TRV130 is a G protein-biased ligand that engages G protein coupling similarly to morphine, but with less β-arrestin recruitment. [Reproduced from Ref. 68.]
One relatively selective μ-OR agonist that is predominantly a biased G protein ligand and produces G protein activation with little β-arrestin recruitment is TRV130 (also called oliceridine). It is presumed that TRV130 changes the conformation of the μ-OR when it binds, resulting in less β-arrestin recruitment. In preclinical models, TRV130 produced potent analgesia with less constipation and respiratory depression at doses that were equianalgesic to morphine (25). In phase I studies conducted in healthy volunteers, TRV130 demonstrated dose-related pupil constriction (indicative of central nervous system efficacy) that correlated with plasma concentration and an effect that was equianalgesic to morphine (10 mg) during the cold pressor test, with less reduction in respiratory drive and less severe nausea (60). The effects of this medication have been tested in phase II, randomized, placebo- and active-controlled studies of the efficacy and tolerability of TRV130 in acute pain after bunionectomy. After the first dose, TRV130 at 2 and 3 mg (dosed every 3 h) produced significantly greater categorical pain relief than morphine (dosed at 4 mg every 4 h), with meaningful pain relief in <5 min. TRV130 produced no serious adverse events, with tolerability similar to that of morphine. However, the proportion of patients who developed nausea was higher in the group treated with TRV130 at 3 mg every 3 h than in the group treated with morphine at 4 mg every 4 h, and constipation was more prevalent in all TRV130- than morphine-treated groups (68). This experience was also demonstrated in rodents (3), in which repeated TRV130 treatment failed to produce tolerance to antinociception or gastrointestinal inhibition and enhanced abuse-related effects, despite its bias for G protein signaling. Several phase I and II trials are recorded in ClinicalTrials.gov, but no other efficacy or safety data are available. Thus, while biased μ-OR activation is a promising and attractive approach to produce antinociception, more specific ligands will be needed to avoid the adverse effects of this class of agonists on respiratory depression and gastrointestinal inhibition.
Another novel, biased G protein μ-OR ligand was identified from a structure-based discovery approach that computationally docked >3 × 106 molecules against the structure of the μ-OR and, thereby, identified new scaffolds unrelated to known opioids (47). This approach led to the discovery of PZM21, a potent gastrointestinal activator with exceptional selectivity for μ-OR and minimal β-arrestin-2 recruitment. In mice, PZM21 was more efficacious than morphine for analgesia, particularly for the affective component of analgesia, produced less constipation, and was devoid of both respiratory depression and morphine-like reinforcing activity in mice at equianalgesic doses. In fact, direct comparisons in these experiments in mice suggest that TRV130 significantly depresses respiration at 15 min, correlating with its peak analgesic response, whereas PZM21 is not associated with respiratory depression.
Targeting μ-OR Under Acidic Conditions
Low pH is a hallmark of injured tissue, as occurs in inflammation. Spahn et al. (61) developed a pH-sensitive opioid that, because of its low pKa, selectively activates peripheral μ-ORs at the source of pain generation. Acidosis can augment the function of heterotrimeric G protein-coupled receptors and affects the protonation of ligands, which is essential for binding and activation of ORs. The prototype molecule, (±)-N-(3-fluoro-1-phenethylpiperidin-4-yl)-N-phenylpropionamide, is a fluorinated derivative of fentanyl with a pKa of 6.8; the fluorine serves to attract protons. This approach produced injury-restricted analgesia in rats with different types of inflammatory pain without respiratory depression, sedation, constipation, or addiction potential. This approach may therefore have advantages to target visceral pain associated with pathophysiological conditions, particularly inflammation.
Nociceptin/Orphanin FQ Opioid Peptide Receptor Modulation
Nociceptin/orphanin FQ opioid peptide (NOP) receptor and its endogenous ligand N/OFQ make up the fourth members of the OR and opioid peptide family (66). NOP modulates μ-OR-mediated actions, thereby affecting opioid analgesia, tolerance development, physiological processes, and reward. NOP receptors are highly expressed in brain and spinal cord; however, there are also NOP receptors on a large number of peripheral systems, including respiratory, gastrointestinal, genitourinary, cardiovascular, and renal systems. N/OFQ and NOP receptor activate inwardly rectifying K+ conductance and inhibit Ca2+ channels. N/OFQ produces inhibitory effects on pain transmission at the peripheral (41) and spinal (42) levels in monkeys. NOP receptors are widely expressed in the gastrointestinal tract on muscle cell membranes and neurons. In rat models of visceral hypersensitivity induced by inflammation [2,4,6-trinitrobenzene sulfonic acid (TNBS)] or stress, UFP-101 (a selective NOP receptor antagonist) blocked the reduction of visceral hypersensitivity induced by N/OFQ (2).
In postmortem control human dorsal root ganglia (DRG), 75–80% of small/medium (≤50-μm-diameter) neurons in the lumbar and sacral DRG were positive for NOP; moreover, NOP-positive nerve fibers within the bladder suburothelium revealed a severalfold increase in specimens from patients with detrusor overactivity and painful bladder syndrome compared with controls. These data suggest that NOP agonists have potential to alter urinary bladder overactivity and pain syndromes (5), and, given the distribution of NOP receptors in lumbar and sacral DRG, it is conceivable that there may be analgesic effects in painful gastrointestinal conditions.
The NOP-selective ligand SCH 221510 was tested for its inhibitory effects on intestinal motility and visceral pain in preclinical animal models of diarrhea-predominant IBS (IBS-D). In the same study, expression of the endogenous nociception system (NOP mRNA expression) was lower in colon biopsies from six patients with IBS-D than healthy controls, and plasma nociceptin levels were also lower in patients with IBS-D than controls. These data suggest that altered expression occurs in the tissues, rather than the extrinsic afferents. The assumption is that the NOP ligand may be able to reverse the deficiency of endogenous nociceptin and replicate the antimotility and antinociception (after oral or intraperitoneal. administration) reported in mouse models of IBS-D. Together, these data support the hypothesis that NOP receptors may become a novel pharmacological target for IBS-D treatment (31). However, the effects of SCH 221510 may be mediated centrally, since it has also shown anxiolytic actions, and NOP receptors are documented in many centers in the brain; hence, new ligands that are more selective or peripherally restricted may be required to optimize this antinociceptive approach.
Dual Action on NOP and μ-OR
Cebranopadol is a novel new agent that combines dual, full agonist action at ORs and NOP receptors (53). It has demonstrated good efficacy and safety in a variety of preclinical models of acute pain and particularly potent efficacy in preclinical models of neuropathic pain (44). It is centrally acting, although its adverse-effect profile has been reported to be superior to that of morphine at equianalgesic doses. It is also analgesic when injected locally, as in inhibiting the nociceptive effect of formalin injected into the hindpaw of mice (55). A novel orvinol analog, BU08028, which is a buprenorphine analog and mixed μ-opioid peptide-NOP receptor agonist, appears to be a safe opioid analgesic without abuse liability in primates (26).
A dual-action agonist, BU08070, has antinociceptive effects in a mouse model of IBS-D (59), as well as anti-inflammatory effects in a preclinical model of inflammatory colitis; this was associated with concentration-dependent inhibition of inflammatory markers and with antinociceptive action (76). These combined effects would suggest that BU08070 acts through peripheral mechanisms. Further studies of the potential combination effects would be of considerable interest in IBS and inflammatory bowel disease.
Buprenorphine Analogs
Buprenorphine is an opioid partial agonist that produces effects, such as euphoria or respiratory depression, that are weaker than those of full opioid agonists such as heroin and methadone. Buprenorphine can block effects of exogenous opioids, thus reducing illicit opioid use. In addition, it does not produce the “rush” sought by addicted individuals; the most effective approach to treatment of opioid withdrawal is use of a long-acting oral opioid (usually methadone or buprenorphine) to relieve symptoms and then gradual reduction of the dose to allow the patient to adjust to the absence of an opioid (57).
κ-OR agonists result in analgesia accompanied by a feeling of dysphoria. On the other hand, antagonists of κ-ORs were found to block depression, anxiety, and drug-seeking behaviors in animal models. Recently, selective κ-OR antagonists have been developed as an addiction treatment that does not cause dependence (38). One novel approach in experimental therapeutics for visceral pain combines analgesia from μ-OR agonism and the antiaddictive effects of a κ-OR antagonist. ORP-101 is a buprenorphine dimer that is an agonist at μ-ORs and an antagonist at κ-ORs. In animal studies, Pasricha et al. found that ORP-101 was highly effective in reducing stool volume and acute abdominal pain in the mustard oil model, relaxed the sphincter of Oddi, and did not retard gastric emptying. In the IBS mouse model, ORP-101 (50 mg/kg) had a significant antihyperalgesic effect. These results contrasted with parallel studies of the effects of eluxadoline (a combination μ- and κ-OR agonist and δ-OR antagonist), which slowed gastric emptying, induced sphincter of Oddi contraction, and did not have antihyperalgesic effects (51). It is unclear whether ORP-101 targets heteromeric receptors.
Morphiceptin Analog
Morphiceptin, a tetrapeptide (NH4-tyr-pro-phe-pro-COHN2) amide of a fragment of the milk protein β-casein, is a selective μ-OR agonist (19). It is derived from β-casomorphin and has >1,000 times more selectivity for μ- than δ-ORs. P-317, a novel cyclic pentapeptide derivative of morphiceptin, has been shown in mouse models to inhibit colonic and ileal smooth muscle contractions in a concentration-dependent way in vitro, to inhibit gastrointestinal transit when administered intraperitoneally or orally in vivo, and to display potent antinociceptive action in abdominal pain tests without influencing locomotor activity and grip strength, which were used as surrogates to check for effects mediated by the central nervous system (77). More direct measurements that prove peripheral restriction, reduced susceptibility to tolerance, and respiratory depression are awaited.
NOVEL CANNABINOID AGENTS
The endocannabinoid system is a widely distributed transmitter system that controls gut functions peripherally and centrally and is involved in the control of nausea and vomiting and visceral sensation. Cannabinoid receptor (CB) type 1 (CB1) localizes to the cell bodies of DRG neurons and controls visceral sensation, and transcription of CNR1 is modified through epigenetic processes under conditions of chronic stress. In addition, a population of small-diameter nociceptive neurons expresses CB1 along with TRPV1 and contains peptides such as substance P and calcitonin gene-related peptide. Activation of CB1 inhibits pain perception (nociception), whereas activation of TRPV1 increases pain perception. Reviewing this information and published data, Sharkey and Wiley proposed that peripherally restricted cannabinoids and modulators of endocannabinoid synthesis and/or degradation might be used for short-term treatment of symptoms of IBS and other functional bowel disorders (58).
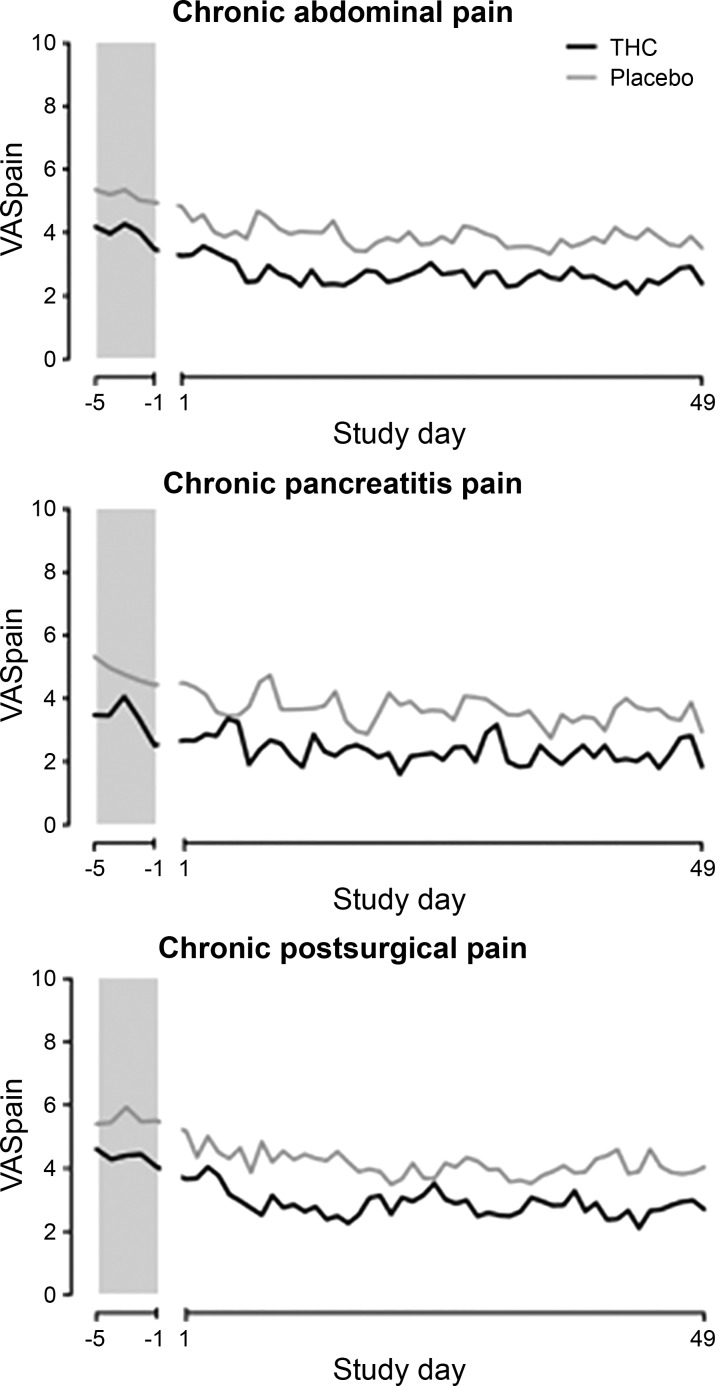
In humans, the nonselective CB agonist dronabinol was shown to increase colonic compliance, inhibit postprandial tone, and, paradoxically, increase the sensory rating for pain during random phasic distensions at all pressures tested and in both sexes (28). Moreover, in a study of 65 patients with chronic abdominal pain for ≥3 mo after surgery or because of chronic pancreatitis, Δ9-tetrahydrocannabinol (THC) did not reduce pain measures compared with placebo control; THC, administered three times daily, was safe and well tolerated during a 50- to 52-day treatment period (Fig. 2) (24). Similarly, THC did not reduce postoperative pain compared with placebo (14).
Fig. 2.
Mean visual analog scale (VAS) pain scores at baseline and during study treatment show randomized controlled trial of Δ9-tetrahydrocannabinol (THC) and placebo in 3 forms of chronic pain. There was no significant effect of treatment with THC. [Reproduced from Ref. 24.]
CB type 2 (CB2) is a class A G protein-coupled receptor that has an amino acid sequence similar to that of CB1 and mediates some of the effects of cannabinoids on the immune system. In addition to expression in the immune system, CB2 has widespread tissue expression in the brain, peripheral nervous system, and gastrointestinal tract. Several “mixed” cannabinoid agonists are in clinical use primarily for controlling pain, and it is believed that selective CB2 agonism may afford a superior analgesic agent devoid of the centrally mediated effects of CB1. Thus, selective CB2 agonists may be candidates for treating pain and other disease states. There are predominantly preclinical data in support of this concept. For example, LY3038404 HCl, a potent CB2 agonist, possesses tissue-protective and analgesic properties without effects on higher brain function, and it attenuated pain in a rat model of pancreatitis (74). Orally administered PF-03550096 (3 and 10 mg/kg) inhibited the TNBS-induced decrease in colonic pain threshold in a rat model of visceral hypersensitivity (40).
Thus, activation of CB2 is suggested as a potential therapeutic target for visceral inflammation and pain management. APD371, an orally available, peripherally restricted, highly selective, full agonist of CB2, is being tested for efficacy in patients. It was designed to provide pain relief without psychotropic effects and without the potential for dependence or abuse. It is listed in ClinicalTrials.gov (NCT03155945) as a treatment being tested in patients with Crohn’s disease (21), although no results are available.
N-METHYL-d-ASPARTATE RECEPTOR ANTAGONISTS
The rationale for use of N-methyl-d-aspartate (NMDA) antagonists for visceral pain is the observation that development and maintenance of human visceral pain hypersensitivity are dependent on the NMDA receptor. This was demonstrated using acid exposure in the esophagus (71). NMDA receptor antagonists (e.g., ketamine, dextromethorphan, and magnesium) have also been utilized as adjuncts for acute and chronic pain management. Ketamine is a unique parenteral anesthetic with analgesic-like properties. Subhypnotic doses of ketamine and its more potent S(+) isomer may prevent central sensitization to opioid analgesics and, thereby, improve their effectiveness. Because of the well-known central nervous system side effects of anesthetic doses of ketamine (e.g., hallucinations, excessive sedation, bad dreams, dizziness, blurred vision, nausea, and vomiting), only subhypnotic doses (<150 μg/kg) should be administered as an analgesic adjuvant. For noxious visceral stimulation, low-dose ketamine produced significant attenuation of both pain intensity and unpleasantness (62). S-ketamine did not reduce pain sensation with gastric distensions in healthy subjects (43). In 10 patients with postpancreatitis pain, S-ketamine infusion was more effective than placebo in increasing pressure thresholds in somatic pain (suggesting an analgesic effect) immediately after infusion; however, this effect did not outlast the infusion (13).
Adverse effects of recreational use of ketamine include acute neurobehavioral abnormalities (such as agitation, hallucinations, anxiety, and psychosis), cardiovascular features (hypertension and tachycardia), and, with long-term use, psychological dependence and tolerance, gastrointestinal toxicity, particularly abdominal pain and abnormal liver function tests, and hemorrhagic cystitis (39).
Overall, these data suggest the need for more research to appraise utility and benefit-to-risk ratio, given the potentially significant adverse effects.
CALCITONIN GENE-RELATED PEPTIDE RECEPTOR ANTAGONISTS
Recent evidence has shown that endosomal signaling of the calcitonin-like receptor (CLR) contributes to pain transmission and that a cholestanol-conjugated antagonist, calcitonin gene-related peptide [CGRP-(8–37)], accumulated in CLR-containing endosomes and selectively inhibited CLR signaling in endosomes. CGRP caused sustained excitation of neurons in slices of rat spinal cord (73). These data suggest that CGRP receptors function not only at the plasma membrane, but also in endosomes, to control complex processes in vivo, and the data suggest that development of CLR antagonists may be efficacious in a variety of pain syndromes. A number of candidate molecular antagonists of CGRP are being tested (reviewed in Ref. 56).
TARGETING TYROSINE KINASES IN PROTEASE-ACTIVATED RECEPTOR 2-MEDIATED RECEPTOR-OPERATED GATING OF TRPV4 CHANNELS
TRP channels constitute a large group of ion channels prevalent in mammalian central and peripheral nervous systems and in nonneuronal cells; they function in sensing temperature, noxious substances, and pain. TRP channels are classified into two major groups according to their amino acid sequence, rather than by selectivity or ligand-binding affinity: group 1 consists of TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPN (no mechanoreceptor potential C), and TRPA (ankyrin) channels; group 2 is composed of TRPP (polycystic) and TRPML (mucolipin) channels (reviewed in Ref. 79). The TRPs with greatest evidence for a role in nociception are TRPV1, TRPV4, and TRPM8.
First, TRPV1, also referred to as the capsaicin receptor, is a ligand-gated, nonselective cation channel with high permeability for Ca2+. TRPV1 is activated by several ligands and physiochemical stimuli, including noxious heat (>43°C), pH (pH 5–6), vanilloids (e.g., capsaicin), ethanol, and lipid mediators derived from arachidonic acid, such as anandamide. TRPV1 is widely expressed on DRG neurons, innervating the colon, bladder, pancreas, stomach, and duodenum. TRPV1-expressing afferents participate in the regulation of gastrointestinal blood flow, secretion, mucosal homeostasis, motility, and nociception. In patients with rectal hypersensitivity, the increase in TRPV1 expression was positively correlated with the level of hypersensitivity (18). Additional studies have assessed TRPV1 activity in subtypes of IBS and demonstrated TRPV1 upregulation and sensitization of submucosal neurons from rectal biopsies in IBS patients with visceral hypersensitivity (67, 72). This suggests that treatment may target expression of TRPV1, rather than sensitization of the capsaicin receptor itself. The upregulation of such visceral sensory mechanisms involving TRPV1 has been recently demonstrated through studies of supernatants generated from tissue biopsy of patients with postinfectious IBS, which were tested in murine DRG (visceral afferent) neurons (11).
Second, the TRPV1 receptor antagonist JYL1421 prevented the visceral hypersensitivity to mechanical and chemical stimuli and the increase in TRPV1 immunoreactivity in the DRG. Moreover, TRPV1 antagonist, injected 1 wk after TNBS instillation, decreased the visceral hypersensitivity to mechanical distension of the colon, but not to chemical stimulation (48). In the intestine, TRPV4 is a nonselective cation channel that is sensitized by G protein-coupled receptors. TRPV4 expression is enhanced by inflammatory mediators, including histamine and serotonin (16). A selective TRPV4 antagonist, RN1734, reduced abdominal pain associated with intestinal inflammation based on a mouse model of intracolonic administration of mustard oil and measurement of the number of postures defined as spontaneous pain-related behaviors (30).
The relevance of TRPV4 to gastrointestinal or colonic pain is supported by the observation of colonic biopsies in 40 patients with IBS that demonstrated levels of the polyunsaturated fatty acid metabolite 5,6-epoxyeicosatrienoic acid (a TRPV4 agonist), but not levels of TRPV1 or TRPA1 agonists, which were increased in IBS biopsies compared with controls, or increases in levels of 5,6-epoxyeicosatrienoic acid, which correlate with pain and bloating scores (17). The same study also showed that, in human DRG, TPV4 was expressed by 35% of neurons. These data suggest that visceral hypersensitivity involves activation of TRPV4. Protease-activated receptor 2 sensitizes TRPV4 channels to cause hyperalgesia in mice (35), and the tyrosine kinase inhibitor bafetinib inhibits the protease-activated receptor 2-induced activation of TRPV4 (34). Although bafetinib is a second-generation dual BCR-ABL/Lyn tyrosine kinase inhibitor in development for glioma and hematological malignancies, it is conceivable that other agents in this class might be developed for visceral pain.
Third, TRPM8 is also expressed by peripheral sensory neurons of visceral organs (37) and may be involved in the reduction of visceral hypersensitivity by coupling of TRPM8 to both TRPV1 and TRPA1 (37). TNBS-induced colonic hypersensitivity to mechanical stimuli was significantly reduced by the TRPM8 agonists peppermint oil and caraway oil, administered together (1). In addition, small clinical trials with enteric-coated peppermint oil showed decreased abdominal pain and increased quality-of-life scores in patients with IBS (reviewed in Ref. 10).
G PROTEIN-COUPLED ESTROGEN RECEPTOR AND ESTROGEN RECEPTOR LIGANDS
Estrogen receptor (ER)-α and ERβ have been implicated in the regulation of visceral pain by estrogens (33). ERα is predominantly expressed in the superficial dorsal horn of the spinal cord, which receives information from nociceptive sensory neurons, while ERβ is found in a deeper layer in the gut (4). In addition, ERα, ERβ, and G protein-coupled ER (GPER) are expressed in the membrane and cytosolic protein fractions of spinal cord dorsal horn in both sexes, supporting a crucial role of all ERs in pain signaling (75). In addition to the sex differences noted in humans with IBS (64), numerous animal studies have shown that visceral and somatic sensitivity vary during the rat estrous cycle and that high ovarian hormone levels are associated with visceral hypersensitivity (36). All these factors provide the rationale for considering the development of antinociceptive agents through modulation of ERs.
GPER is expressed in the human colon and in the mouse colon and ileum. G-1 (a nonselective GPER agonist) and estradiol (an ER ligand) inhibited muscle contractility in vitro in human and mouse colon, prolonged the time to bead expulsion in female mice, and reduced pain sensation in vivo, as shown by the number of behaviors observed in mice in response to intracolonic instillation of 1% mustard oil (78).
ADENOSINE A2B RECEPTOR AGONIST
Adenosine is an extracellular purine nucleoside-signaling molecule responsible for diverse actions in the nervous, cardiopulmonary, renal, and gastrointestinal systems. The biological actions of adenosine are mediated by binding to four subtypes of G protein-coupled receptors: A1, A2A, A2B, and A3. The adenosine A2B receptors are activated by micromolar levels (10–100 μM) of adenosine, which are achieved in pathological conditions; the A2B receptors are involved in the control of intestinal secretion, motility, and sensation (8). The adenosine A2B receptors are mainly expressed in the peripheral tissues, such as the large intestine (29). Adenosine’s role in diverse enteric functions and disease processes are extensively reviewed elsewhere (6, 7). Recent data show that, in rat models of IBS (based on stress such as maternal separation or wrap-restraint stress), the A2B receptor antagonist aminophylline reduced visceral pain as well as colonic propulsion (9).
While there are no human studies assessing such adenosine A2B receptors as targets for visceral analgesia in humans, this class of medications, which has been used for obstructive airway disease for several decades, may be available for formal testing in patients with abdominal pain.
CONCLUSIONS
Although unproven for efficacy in patients, these novel approaches for the treatment of visceral pain with peripherally restricted or targeted mechanisms augur well for the future hope of developing peripheral visceral analgesics for IBS and chronic abdominal pain.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-115950.
DISCLOSURES
M. Camilleri reports research support from Allergan for a study involving eluxadoline.
AUTHOR CONTRIBUTIONS
M.C. conceived and designed research; M.C. drafted manuscript; M.C. edited and revised manuscript; M.C. approved final version of manuscript.
REFERENCES
- 1.Adam B, Liebregts T, Best J, Bechmann L, Lackner C, Neumann J, Koehler S, Holtmann G. A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. Scand J Gastroenterol 41: 155–160, 2006. doi: 10.1080/00365520500206442. [DOI] [PubMed] [Google Scholar]
- 2.Agostini S, Eutamene H, Broccardo M, Improta G, Petrella C, Theodorou V, Bueno L. Peripheral anti-nociceptive effect of nociceptin/orphanin FQ in inflammation and stress-induced colonic hyperalgesia in rats. Pain 141: 292–299, 2009. doi: 10.1016/j.pain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. Effects of acute and repeated treatment with the biased μ-opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31: 730–739, 2017. doi: 10.1177/0269881116689257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amandusson A, Blomqvist A. Estrogen receptor-α expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. Eur J Pain 14: 245–248, 2010. doi: 10.1016/j.ejpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Anand P, Yiangou Y, Anand U, Mukerji G, Sinisi M, Fox M, McQuillan A, Quick T, Korchev YE, Hein P. Nociceptin/orphanin FQ receptor expression in clinical pain disorders and functional effects in cultured neurons. Pain 157: 1960–1969, 2016. doi: 10.1097/j.pain.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 6.Antonioli L, Colucci R, Pellegrini C, Giustarini G, Tuccori M, Blandizzi C, Fornai M. The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther 139: 157–188, 2013. doi: 10.1016/j.pharmthera.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther 120: 233–253, 2008. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Asano T, Takenaga M. Adenosine A2B receptors: an optional target for the management of irritable bowel syndrome with diarrhea? J Clin Med 6: E104, 2017. doi: 10.3390/jcm6110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asano T, Tanaka KI, Tada A, Shimamura H, Tanaka R, Maruoka H, Takenaga M, Mizushima T. Aminophylline suppresses stress-induced visceral hypersensitivity and defecation in irritable bowel syndrome. Sci Rep 7: 40214, 2017. doi: 10.1038/srep40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balemans D, Boeckxstaens GE, Talavera K, Wouters MM. Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 312: G635–G648, 2017. doi: 10.1152/ajpgi.00401.2016. [DOI] [PubMed] [Google Scholar]
- 11.Balemans D, Mondelaers SU, Cibert-Goton V, Stakenborg N, Aguilera-Lizarraga J, Dooley J, Liston A, Bulmer DC, Vanden Berghe P, Boeckxstaens GE, Wouters MM. Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Sci Rep 7: 13606, 2017. doi: 10.1038/s41598-017-12618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 286: 2495–2498, 1999. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 13.Bouwense SA, Buscher HC, van Goor H, Wilder-Smith OH. S-ketamine modulates hyperalgesia in patients with chronic pancreatitis pain. Reg Anesth Pain Med 36: 303–307, 2011. doi: 10.1097/AAP.0b013e3182177022. [DOI] [PubMed] [Google Scholar]
- 14.Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral Δ9-tetrahydrocannabinol in postoperative pain. Pain 106: 169–172, 2003. doi: 10.1016/S0304-3959(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 66: 966–974, 2017. doi: 10.1136/gutjnl-2016-313425. [DOI] [PubMed] [Google Scholar]
- 16.Cenac N, Altier C, Motta JP, d’Aldebert E, Galeano S, Zamponi GW, Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 59: 481–488, 2010. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- 17.Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, Bertrand J, Liedtke W, Dubourdeau M, Bertrand-Michel J, Zecchi L, Stanghellini V, Bunnett NW, Barbara G, Vergnolle N. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 149: 433–44.e7, 2015. doi: 10.1053/j.gastro.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 361: 385–391, 2003. doi: 10.1016/S0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- 19.Chang KJ, Lillian A, Hazum E, Cuatrecasas P, Chang JK. Morphiceptin (NH4-tyr-pro-phe-pro-COHN2): a potent and specific agonist for morphine (μ) receptors. Science 212: 75–77, 1981. doi: 10.1126/science.6259732. [DOI] [PubMed] [Google Scholar]
- 20.Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Associations between medication use and functional gastrointestinal disorders: a population-based study. Neurogastroenterol Motil 25: 413–419, e298, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov A Randomized, Open-Label, Parallel, Phase 2a Study to Determine the Tolerability, Pharmacokinetics, and Efficacy of APD371 in Subjects with Crohn's Disease Experiencing Abdominal Pain (Online). Bethesda, MD: US National Library of Medicine, 2018, NCT03155945. [Google Scholar]
- 22.Crocker JA, Yu H, Conaway M, Tuskey AG, Behm BW. Narcotic use and misuse in Crohn’s disease. Inflamm Bowel Dis 20: 2234–2238, 2014. doi: 10.1097/MIB.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 23.Dapoigny M, Abitbol JL, Fraitag B. Efficacy of peripheral κ-agonist fedotozine versus placebo in treatment of irritable bowel syndrome. A multicenter dose-response study. Dig Dis Sci 40: 2244–2249, 1995. doi: 10.1007/BF02209014. [DOI] [PubMed] [Google Scholar]
- 24.de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H; Pain and Nociception Neuroscience Research Group . Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol 15: 1079–1086.e4, 2017. doi: 10.1016/j.cgh.2016.09.147. [DOI] [PubMed] [Google Scholar]
- 25.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JDA. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344: 708–717, 2013. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 26.Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko MC. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci USA 113: E5511–E5518, 2016. doi: 10.1073/pnas.1605295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorn SD, Meek PD, Shah ND. Increasing frequency of opioid prescriptions for chronic abdominal pain in US outpatient clinics. Clin Gastroenterol Hepatol 9: 1078–85.e1, 2011. doi: 10.1016/j.cgh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol 293: G137–G145, 2007. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- 29.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev 49: 381–402, 1997. [PubMed] [Google Scholar]
- 30.Fichna J, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Małecka-Panas E, Janecka A, Krajewska WM, Storr MA. Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment? Neurogastroenterol Motil 24: e557–e560, 2012. doi: 10.1111/j.1365-2982.2012.01999.x. [DOI] [PubMed] [Google Scholar]
- 31.Fichna J, Sobczak M, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Cenac N, Sałaga M, Timmermans JP, Vergnolle N, Małecka-Panas E, Krajewska WM, Storr M. Activation of the endogenous nociceptin system by selective nociceptin receptor agonist SCH 221510 produces antitransit and antinociceptive effect: a novel strategy for treatment of diarrhea-predominant IBS. Neurogastroenterol Motil 26: 1539–1550, 2014. doi: 10.1111/nmo.12390. [DOI] [PubMed] [Google Scholar]
- 32.Floyd BN, Camilleri M, Busciglio I, Sweetser S, Burton D, Wong GY, Kell S, Khanna S, Hwang S, Zinsmeister AR. Effect of a κ-opioid agonist, i.v. JNJ-38488502, on sensation of colonic distensions in healthy male volunteers. Neurogastroenterol Motil 21: 281–290, 2009. doi: 10.1111/j.1365-2982.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- 33.Gintzler AR, Liu NJ. Importance of sex to pain and its amelioration; relevance of spinal estrogens and its membrane receptors. Front Neuroendocrinol 33: 412–424, 2012. doi: 10.1016/j.yfrne.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grace MS, Lieu T, Darby B, Abogadie FC, Veldhuis N, Bunnett NW, McIntyre P. The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 channels in vitro and pain in vivo. Br J Pharmacol 171: 3881–3894, 2014. doi: 10.1111/bph.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578: 715–733, 2007. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafsson JK, Greenwood-Van Meerveld B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am J Physiol Gastrointest Liver Physiol 300: G1080–G1085, 2011. doi: 10.1152/ajpgi.00349.2010. [DOI] [PubMed] [Google Scholar]
- 37.Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw LA, Brierley SM. A novel role for TRPM8 in visceral afferent function. Pain 152: 1459–1468, 2011. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Helal MA, Habib ES, Chittiboyina AG. Selective κ-opioid antagonists for treatment of addiction, are we there yet? Eur J Med Chem 141: 632–647, 2017. doi: 10.1016/j.ejmech.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J 4: 7107, 2011. doi: 10.3402/ehtj.v4i0.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuchi A, Ohashi K, Sugie Y, Sugimoto H, Omura H. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci 106: 219–224, 2008. doi: 10.1254/jphs.FP0071599. [DOI] [PubMed] [Google Scholar]
- 41.Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther 301: 698–704, 2002. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- 42.Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther 318: 1257–1264, 2006. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- 43.Kuiken SD, van den Berg SJ, Tytgat GN, Boeckxstaens GE. Oral S(+)-ketamine does not change visceral perception in health. Dig Dis Sci 49: 1745–1751, 2004. doi: 10.1007/s10620-004-9563-6. [DOI] [PubMed] [Google Scholar]
- 44.Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schröder W, Kögel BY, Beier H, Englberger W, Schunk S, De Vry J, Jahnel U, Frosch S. Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349: 535–548, 2014. doi: 10.1124/jpet.114.213694. [DOI] [PubMed] [Google Scholar]
- 45.Long MD, Barnes EL, Herfarth HH, Drossman DA. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis 18: 869–876, 2012. doi: 10.1002/ibd.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, Urso D. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther 28: 239–249, 2008. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 47.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang XP, Sassano MF, Giguère PM, Löber S, Da Duan, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537: 185–190, 2016. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 148: 1021–1032, 2007. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med 363: 1981–1985, 2010. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 50.Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use Released 13 July 2017. http://www.nationalacademies.org/opioidstudy. [3 January 2018]. [PubMed]
- 51.Pasricha PJ, Li Q, Liu L, Sanders V, Singh N. A novel partial µ agonist κ antagonist for the treatment of IBS-D without the risk of pancreatitis/sphincter of Oddi spasm. Gastroenterology 152: S1306–S1307, 2017. doi: 10.1016/S0016-5085(17)34353-6. [DOI] [Google Scholar]
- 52.Raehal KM, Walker JK, Bohn LM. Morphine side effects in β-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201, 2005. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 53.Raffa RB, Burdge G, Gambrah J, Kinecki HE, Lin F, Lu B, Nguyen JT, Phan V, Ruan A, Sesay MA, Watkins TN. Cebranopadol: novel dual opioid/NOP receptor agonist analgesic. J Clin Pharm Ther 42: 8–17, 2017. doi: 10.1111/jcpt.12461. [DOI] [PubMed] [Google Scholar]
- 54.Read NW, Abitbol JL, Bardhan KD, Whorwell PJ, Fraitag B. Efficacy and safety of the peripheral κ-agonist fedotozine versus placebo in the treatment of functional dyspepsia. Gut 41: 664–668, 1997. doi: 10.1136/gut.41.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizzi A, Cerlesi MC, Ruzza C, Malfacini D, Ferrari F, Bianco S, Costa T, Guerrini R, Trapella C, Calo’ G. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol Res Perspect 4: e00247, 2016. doi: 10.1002/prp2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94: 1099–1142, 2014. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med 375: 357–368, 2016. doi: 10.1056/NEJMra1604339. [DOI] [PubMed] [Google Scholar]
- 58.Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology 151: 252–266, 2016. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobczak M, Cami-Kobeci G, Sałaga M, Husbands SM, Fichna J. Novel mixed NOP/MOP agonist BU08070 alleviates pain and inhibits gastrointestinal motility in mouse models mimicking diarrhea-predominant irritable bowel syndrome symptoms. Eur J Pharmacol 736: 63–69, 2014. doi: 10.1016/j.ejphar.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, Violin JD, Lark MW. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol 54: 351–357, 2014. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]
- 61.Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 355: 966–969, 2017. doi: 10.1126/science.aai8636. [DOI] [PubMed] [Google Scholar]
- 62.Strigo IA, Duncan GH, Bushnell MC, Boivin M, Wainer I, Rodriguez Rosas ME, Persson J. The effects of racemic ketamine on painful stimulation of skin and viscera in human subjects. Pain 113: 255–264, 2005. doi: 10.1016/j.pain.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Tack J, Schumacher K, Tonini G, Scartoni S, Capriati A, Maggi CA; Iris-2 Investigators . The neurokinin-2 receptor antagonist ibodutant improves overall symptoms, abdominal pain and stool pattern in female patients in a phase II study of diarrhoea-predominant IBS. Gut 66: 1403–1413, 2017. doi: 10.1136/gutjnl-2015-310683. [DOI] [PubMed] [Google Scholar]
- 64.Thakur ER, Gurtman MB, Keefer L, Brenner DM, Lackner JM; Representing the IBS Outcome Study Research Group . Gender differences in irritable bowel syndrome: the interpersonal connection. Neurogastroenterol Motil 27: 1478–1486, 2015. doi: 10.1111/nmo.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The President’s Commission on Combating Drug Addiction and the Opioid Crisis (Online) https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Final_Report_Draft_11-1-2017.pdf [3 January 2018]. [DOI] [PubMed]
- 66.Toll L, Bruchas MR, Calo’ G, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 68: 419–457, 2016. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Wanrooij SJ, Wouters MM, Van Oudenhove L, Vanbrabant W, Mondelaers S, Kollmann P, Kreutz F, Schemann M, Boeckxstaens GE. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? Am J Gastroenterol 109: 99–109, 2014. doi: 10.1038/ajg.2013.371. [DOI] [PubMed] [Google Scholar]
- 68.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain 157: 264–272, 2016. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 69.Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med 377: 391–394, 2017. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 70.Wang D. Opioid medications in the management of chronic abdominal pain. Curr Pain Headache Rep 21: 40, 2017. doi: 10.1007/s11916-017-0640-x. [DOI] [PubMed] [Google Scholar]
- 71.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-d-aspartate receptor. Gastroenterology 126: 683–692, 2004. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 72.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, Van der Merwe S, Mols R, Ghesquière B, Cirillo C, Kortekaas I, Carmeliet P, Peetermans WE, Vermeire S, Rutgeerts P, Augustijns P, Hellings PW, Belmans A, Vanner S, Bulmer DC, Talavera K, Vanden Berghe P, Liston A, Boeckxstaens GE. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150: 875–87.e9, 2016. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 73.Yarwood RE, Imlach WL, Lieu T, Veldhuis NA, Jensen DD, Klein Herenbrink C, Aurelio L, Cai Z, Christie MJ, Poole DP, Porter CJH, McLean P, Hicks GA, Geppetti P, Halls ML, Canals M, Bunnett NW. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci USA 114: 12309–12314, 2017. doi: 10.1073/pnas.1706656114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, Kline RH IV, McNearney TA, Johnson MP, Westlund KN. Cannabinoid receptor 2 agonist attenuates pain related behavior in rats with chronic alcohol/high fat diet induced pancreatitis. Mol Pain 10: 66, 2014. doi: 10.1186/1744-8069-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Lü N, Zhao ZQ, Zhang YQ. Involvement of estrogen in rapid pain modulation in the rat spinal cord. Neurochem Res 37: 2697–2705, 2012. doi: 10.1007/s11064-012-0859-1. [DOI] [PubMed] [Google Scholar]
- 76.Zielińska M, Ben Haddou T, Cami-Kobeci G, Sałaga M, Jarmuż A, Padysz M, Kordek R, Spetea M, Husbands SM, Fichna J. Anti-inflammatory effect of dual nociceptin and opioid receptor agonist, BU08070, in experimental colitis in mice. Eur J Pharmacol 765: 582–590, 2015. doi: 10.1016/j.ejphar.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zielińska M, Chen C, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Sałaga M, Małecka-Panas E, Wlaź P, Krajewska WM, Fichna J. Orally administered novel cyclic pentapeptide P-317 alleviates symptoms of diarrhoea-predominant irritable bowel syndrome. J Pharm Pharmacol 67: 244–254, 2015. doi: 10.1111/jphp.12335. [DOI] [PubMed] [Google Scholar]
- 78.Zielińska M, Fichna J, Bashashati M, Habibi S, Sibaev A, Timmermans JP, Storr M. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol Motil 29: e13025, 2017. doi: 10.1111/nmo.13025. [DOI] [PubMed] [Google Scholar]
- 79.Zielińska M, Jarmuż A, Wasilewski A, Sałaga M, Fichna J. Role of transient receptor potential channels in intestinal inflammation and visceral pain: novel targets in inflammatory bowel diseases. Inflamm Bowel Dis 21: 419–427, 2015. doi: 10.1097/MIB.0000000000000234. [DOI] [PubMed] [Google Scholar]