Abstract
The pentose phosphate pathway (PPP) is widely assumed to play a key role in both reductive biosynthesis and protection from oxidative stress because it is the major source of NADPH. However, little is known about the activity of the PPP in fatty liver, which is characterized by both oxidative stress and lipogenesis. This study was designed to test whether the PPP is active in parallel with lipogenesis and antioxidant processes in the fatty liver of whole animals. Eight- and 16-wk-old obese Zucker diabetic fatty rats and their lean littermates received [U-13C3]glycerol, and 13C labeling patterns of glucose and triglycerides were analyzed for the assessment of hepatic PPP activity and the potentially related processes simultaneously. Oxidative stress, antioxidant activity, and NADPH-producing enzymes in the liver were further examined. Both PPP activity and lipogenesis increased in the fatty liver of young obese Zucker rats but decreased together in older obese Zucker rats. As expected, lipid peroxidation measured by malondialdehyde increased in the fatty liver of obese Zucker rats at both ages. However, evidence for antioxidant processes such as [glutathione] or activities of glutathione reductase, glutathione peroxidase, and catalase was not altered. Hepatic PPP activity paralleled lipogenesis but was dissociated from biomarkers of oxidative stress or antioxidant processes. In summary, NADPH from the PPP was presumably consumed for reductive biosynthesis rather than antioxidant defense in the fatty liver.
Keywords: gluconeogenesis, glutathione, glycerol, lipid peroxidation, NADPH
INTRODUCTION
When carbohydrate intake exceeds the capacity for storage and oxidation, the extra energy is converted to triglycerides (TG) in the liver. Although hepatic lipogenesis may benefit short-term glucose homeostasis, the process may have the adverse effect of increasing TG and contributing to hepatic steatosis. TG accumulation may lead to oxidative stress and liver injury. Even in healthy liver, superoxide radicals are generated at a low rate by the electron transport chain (2, 4). However, excessive reactive oxygen species (ROS) in hepatic steatosis attack unsaturated fatty acids, generating lipid peroxides plus fatty acid radicals that can react with other fatty acids. As this cycle continues, initiated by ROS, a cascade of lipid peroxides and reactive end products are generated. These results of oxidative stress are associated with profound consequences, including insulin resistance, mitochondrial dysfunctions, and inflammation (5, 22, 26).
In the process of fat accumulation and oxidative stress development, NADPH plays a central role in both reductive biosynthesis and protection from oxidative damage. NADPH is required for both fatty acid synthesis from acetyl-CoA and the regeneration of glutathione catalyzed by glutathione reductase. The pentose phosphate pathway (PPP) is thought to be the major source of NADPH produced at the levels of glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase. Thus, the PPP is often assumed to be essential for both de novo lipogenesis and glutathione reduction. This concept suggests that activity of the PPP would be maximal when de novo lipogenesis is active and oxidative stress is present. However, little is known about the activity of the PPP in the evolution of fatty liver.
Here, we studied the PPP and NADPH-requiring processes in the fatty liver of whole animals to determine if the PPP was active in parallel with both lipogenesis and antioxidant process. We administered [U-13C3]glycerol to obese Zucker diabetic fatty (ZDF, fa/fa) rats, rodent models of fatty liver, and lean littermates (+/?) at ages of 8 and 16 wk. Obese ZDF rats of these ages were chosen because they represent prediabetic and overtly diabetic stages of fatty liver, respectively (7, 24). After phosphorylation in the liver, [U-13C3]glycerol undergoes various metabolic processes that produce unique 13C labeling patterns in metabolic products such as glucose and TG (Fig. 1). The glucose and TG labeling patterns are sensitive to PPP activity, TG synthesis, and mitochondrial biosynthetic functions (12, 13). We found that PPP activity was increased in parallel with TG synthesis in young animals, consistent with the assumption that NADPH from the PPP is used in hepatic lipogenesis. However, prolonged hepatic steatosis was associated with reduced PPP activity even in the presence of hepatocellular injury and oxidative stress. These studies indicate that NADPH from the PPP was used in reductive biosynthesis but not for protection against oxidative stress.
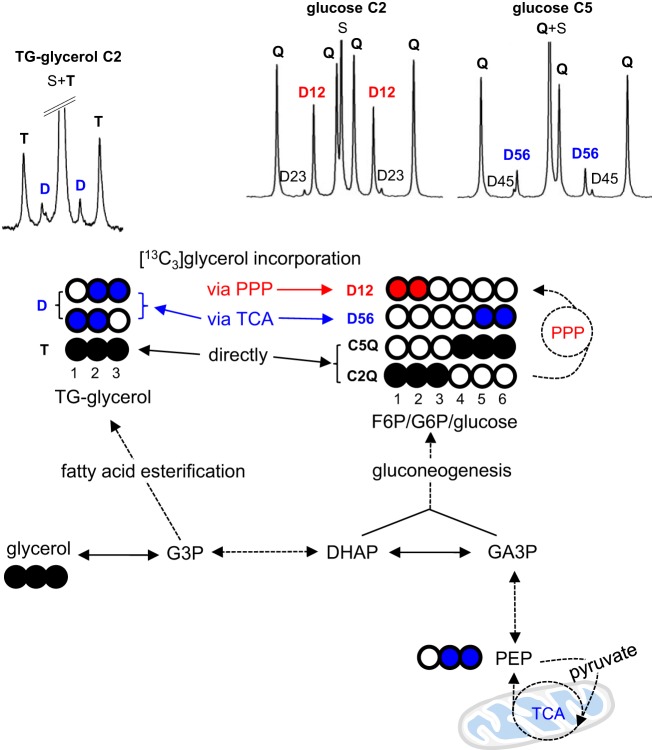
Fig. 1.
[U-13C3]glycerol incorporation into triglycerides (TG) and glucose. Direct [U-13C3]glycerol incorporation into fatty acid esterification and gluconeogenesis produces triple-labeled glycerol backbones of TG and glucose, respectively (“direct” contribution). A fraction of [U-13C3]glycerol is metabolized to [U-13C3]pyruvate, entering the TCA cycle prior to incorporation into TG or glucose, producing double-labeled glycerol backbones or glucose (“indirect” contribution via the TCA cycle). [1,2,3-13C3]glusose 6-phosphate (G6P), produced through [U-13C3]glycerol-gluconeogenesis, may enter the full cycle of the PPP, producing [1,2-13C2]fructose 6-phosphate (F6P) and consequently [1,2-13C2]glucose. These processes can be detected by 13C NMR analysis of the metabolic products such as TG-glycerol and glucose. G3P, glycerol 3-phosphate; PEP, phosphoenolpyruvate; PPP, pentose phosphate pathway; TCA, tricarboxylic acid. Open circle, 12C; black circle, 13C; blue circle, =13C after metabolism through the TCA cycle; red circle, 13C after metabolism through the PPP.
MATERIALS AND METHODS
Research design.
The study was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. Male obese ZDF (GmiCrl fa/fa) rats and their lean littermates (GmiCrl +/?) were purchased from Charles River Laboratories (Wilmington, MA). Four groups of rats were studied: 8- and 16-wk-old fa/fa rats (n = 7 in each group) and their lean littermates (+/? rats; n = 6 in 8-wk group and n = 7 in 16-wk group). All rats were placed on a 12:12-h day-night cycle and had free access to ad libitum feed and water. Rats were fasted beginning at 4:00 PM for an overnight fast with free access to water before the study day. Both 8- and 16-wk fa/fa rats were heavier than lean littermates the following morning after the fast (Table 1). At ~10:00 AM, rats received [U-13C3]glycerol (50%, 100 mg/kg; Cambridge Isotopes, Andover, MA) dissolved in 2 ml of water intraperitoneally under isoflurane anesthesia. After the injection, rats awakened immediately and rested for 60 min before being euthanized under isoflurane anesthesia. During the great majority of the time probed in this experiment, the animals were awake and active. In our previous study, an isotopic steady state for the measurement of hepatic PPP activity was demonstrated in rats under these experimental conditions (12). Blood was drawn from the inferior vena cava, and liver tissues were freeze-clamped using liquid nitrogen and kept at −80°C.
Table 1.
General features of ZDF rats and biochemical analysis of plasma
| 8-wk |
16-wk |
|||||
|---|---|---|---|---|---|---|
| +/? (n = 7) | fa/fa (n = 7) | P | +/? (n = 6) | fa/fa (n = 7) | P | |
| General feature | ||||||
| Body weight, g | 216 ± 7 | 287 ± 6 | <0.001 | 335 ± 4 | 367 ± 11 | 0.022 |
| Liver weight, g | 6.6 ± 0.3 | 12.0 ± 0.5 | <0.001 | 8.7 ± 0.2 | 16.4 ± 0.6 | <0.001 |
| Liver/body, % | 3.0 ± 0.0 | 4.2 ± 0.0 | <0.001 | 2.6 ± 0.0 | 4.5 ± 0.0 | <0.001 |
| Plasma | ||||||
| Glucose, mM | 6.65 ± 0.20 | 9.25 ± 0.50 | <0.001 | 8.52 ± 0.28 | 24.27 ± 1.62 | <0.001 |
| Insulin, ng/ml | ND | 1.42 ± 0.21 | <0.001 | 0.16 ± 0.04 | 1.18 ± 0.26 | 0.003 |
| Albumin, g/dl | 2.76 ± 0.05 | 3.16 ± 0.02 | <0.001 | 3.03 ± 0.07 | 3.10 ± 0.10 | 0.604 |
Values are expressed as means ± SE. ZDF, Zucker diabetic fatty; ND, not detected.
Sample processing for nuclear magnetic resonance analysis.
For glutathione and other water-soluble metabolite extraction, ground liver tissue (3 g) was treated using perchloric acid (10%, 15 ml), the mixture was vortexed for 1 min and centrifuged, and the supernatant was transferred to a new tube. The extraction was repeated, and supernatant was neutralized with KOH, centrifuged, and lyophilized. The dried residue was dissolved in 2H2O (300 μl for 8-wk rats and 16-wk +/? rats; 500 μl for 16-wk fa/fa rats) containing 4,4-dimethyl-4-silapentane-1-sulfonic acid (5 mM) as a nuclear magnetic resonance (NMR) reference and centrifuged, and supernatant was transferred to a NMR tube.
For lipid extraction, ground liver tissue (2 g) was treated with a chloroform-methanol mixture as described previously (13), and the dried lipids were dissolved in deuterated chloroform (CDCl3, 170 μl, Cambridge Isotopes) for NMR acquisition. Plasma glucose was extracted, purified, and derivatized to monoacetone glucose (MAG) for NMR acquisition as reported previously (12).
NMR spectroscopy.
NMR spectra were collected using a Varian INOVA 14.1 T spectrometer (Agilent, Santa Clara, CA) equipped with a 3-mm broadband probe with the observe coil tuned to 13C (150 MHz). 13C NMR spectra of lipids were collected using a 60° pulse, a sweep width of 36,765 Hz, 110,294 data points, and a 1.5-s acquisition time with 1.5-s interpulse delay at 25°C. Proton decoupling was performed using a standard WALTZ-16 pulse sequence. Spectra averaged ~23,000 scans requiring 20 h. NMR spectra of perchloric acid extracts and MAG were collected as described previously (12). Spectra were analyzed using ACD/Laboratories NMR spectral analysis program (Advanced Chemistry Development, Toronto, ON, Canada).
Assessment of hepatic TG synthesis and the PPP by NMR.
After administration of [U-13C3]glycerol, the 13C labeling patterns in the glycerol backbones of TG and glucose are informative about multiple metabolic processes in the liver (13). Briefly, as illustrated in Fig. 1, direct [U-13C3]glycerol incorporation into fatty acid esterification and gluconeogenesis produces triple-labeled glycerol backbones of TG and triple-labeled glucose, respectively. A small fraction of [U-13C3]glycerol may undergo the downstream of glycolysis, the tricarboxylic acid (TCA) cycle, and then gluconeogenic pathway before incorporation into TG or glucose. This indirect incorporation via the TCA cycle produces double-labeled trioses, and consequently, double-labeled glycerol backbones of TG or glucose (i.e., [5,6-13C2]glucose), informing mitochondrial biosynthetic functions. [1,2,3-13C3]hexose, produced through gluconeogenesis directly from [U-13C3]glycerol, may experience the PPP producing [1,2-13C2]glucose. These metabolic products with 13C in specific positions were quantified by 13C NMR analysis (Fig. 1).
Immunoblot assay.
Liver tissues were prepared wth RIPA buffer supplemented with a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Proteins were mixed with sodium dodecyl sulfate sample buffer and denatured by boiling for 5 min. Samples were separated by 8–15% SDS-PAGE gels and transferred onto a nitrocellulose membrane (Millipore, Billerica, MA). Membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (TBS/T) for 1 h. After blocking, blots were incubated with primary antibodies in diluted blocking buffer overnight at 4°C, washed in TBS/T, and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) for 1 h at room temperature. Blots were developed using an Immobilon Western Chemiluminescent HRP substrate (Millipore). Relative densities of bands were quantified with UN-SCAN-IT Gel 6.1 software. The results were normalized by α-tubulin intensity of the same sample. The following antibodies were used: glutathione peroxidase-1, glutathione reductase, catalase, isocitrate dehydrogenase (IDH)2 (Abcam, Cambridge, MA), G6PDH, IDH1 (Cell Signaling, Beverly, MA), malic enzyme (ME)1 (Santa Cruz Biotechnology, Dallas, TX), rabbit IgG HRP-linked whole antibody, and mouse IgG HRP-linked whole antibody (Sigma, St. Louis, MO).
Malondialdehyde, glutathione, enzyme activity and other assays.
Plasma insulin was measured using a rodent insulin ELISA kit (ALPCO). Plasma glucose was assayed using glucose oxidase (YSI 2300 Glucose Analyzer; GMI). Plasma aspartate aminotransferase, alanine aminotransferase, and albumin were measured using the Vitros 250 analyzers (Johnson & Johnson). Malondialdehyde from liver tissues was measured using a thiobarbituric acid reactive substances assay kit (Caymen Chemical, Ann Arbor, MI). Lipids were extracted and TG were measured using a commercial kit (Thermo Fisher Scientific). The level of glutathione in the liver was measured by 13C NMR analysis of perchloric acid extracts. The signal from the glycine moiety of glutathione at 44.4 ppm chemical shift was selected for [glutathione] measurement. 13C enrichments in glucose and the glycerol moiety of TG were estimated as described previously (13). The activities of liver glutathione reductase, catalase, and glutathione peroxidase were measured using commercial kits (Cayman Chemical) according to the manufacturer's instructions. The activity was measured by Synergy H1 Hybrid reader at an absorbance of 340 or 540 nm.
Statistical analysis.
Data are expressed as means ± SE. Comparisons between two groups were made using Student's two-tailed t-test, where P < 0.05 was considered significant.
RESULTS
Hepatic TG synthesis increased in 8-wk fa/fa rats but decreased in 16-wk fa/fa rats.
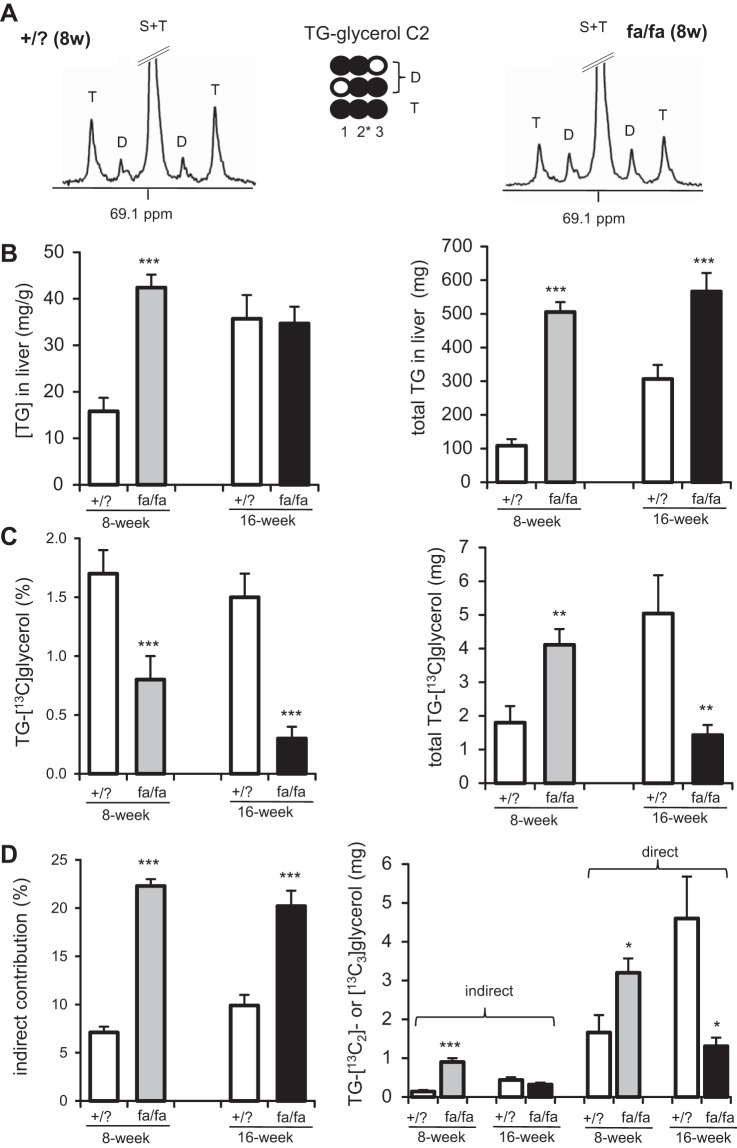
The liver was larger in both 8- and 16-wk fa/fa rats compared with lean littermates (Table 1). Hepatic [TG] increased in 8-wk fa/fa rats, but not in 16-wk fa/fa rats. Thus, the size of TG pool in the liver was increased in fa/fa rats at both ages. The multiplets due to 13C-13C spin-spin coupling in the glycerol backbones of TG were readily detected in all animals, demonstrating [U-13C3]glycerol incorporation into fatty acid esterification (Fig. 2, A and B). The fraction of TG containing [13C]glycerol was lower in fa/fa rats at both ages compared with lean littermates. The total amount of TG-[13C]glycerol in the liver was higher in 8-wk fa/fa rats but lower in 16-wk fa/fa rats than lean littermates (Fig. 2C). These values reflect TG synthesis that occurred for 60 min after the administration of [13C3]glycerol. Together, these results demonstrate that the rate of fatty acid esterification increased in 8-wk fa/fa animals. However, although TG mass was higher in 16-wk fa/fa rats, the rate of fatty acid esterification in these animals was reduced compared with lean littermates.
Fig. 2.
TG synthesis in liver after [U-13C3]glycerol administration. Overnight-fasted rats received [U-13C3]glycerol, and liver was harvested after 60 min. A: 13C NMR of lipid extracts show signals of the glycerol backbones of TG from an 8-wk fa/fa rat and a lean littermate (+/?). Doublet (D) reflects double-labeled ([1,2-13C2]- and [2,3-13C2]-) glycerol, and triplet (T) arises exclusively from [U-13C3]glycerol moiety of TG. B: hepatic [TG] was higher in 8-wk fa/fa rats than in lean littermates but was not altered in 16-wk fa/fa rats. Total amounts of TG in liver increased in both 8- and 16-wk fa/fa rats than in lean littermates. C: fractions of TG-[13C]glycerol were lower in fa/fa rats at both ages than in lean littermates. Total amounts of TG-[13C]glycerol in liver increased in 8-wk fa/fa rats but decreased in 16-wk fa/fa rats. D: fraction of indirect contribution of [U-13C3]glycerol to TG was higher in fa/fa rats at both ages than in lean littermates. Amounts of TG-[13C2]glycerol (“indirect” contribution) increased in 8-wk fa/fa rats but not in 16-wk fa/fa rats. Amounts of TG-[13C3]glycerol (“direct” contribution) increased in 8-wk fa/fa rats but decreased in 16-wk fa/fa rats. D, doublet from coupling of C1 with C2 or from coupling of C2 with C3; T, triplet arising from coupling of C2 with both C1 and C3; S, singlet; open circle, 12C; black circle, 13C; *P < 0.05, **P < 0.01, ***P < 0.001; n = 5–7 in each group.
Double-labeled glycerol backbones of TG (“D” in Fig. 2A) reflect [U-13C3]glycerol metabolism via the TCA cycle before resynthesis to glycerol and incorporation into TG. The fraction of this indirect contribution increased in fa/fa rats at both ages compared with lean littermates. The total amount of TG containing double-labeled glycerol (TG-[13C2]glycerol; indirect incorporation) increased in 8-wk fa/fa rats only, and not in 16-wk fa/fa rats. The total amount of TG-[13C3]glycerol (direct incorporation) was higher in 8-wk fa/fa rats but lower in 16-wk fa/fa rats compared with lean littermates (Fig. 2D).
Hepatic PPP activity increased in 8-wk fa/fa rats but decreased in 16-wk fa/fa rats.
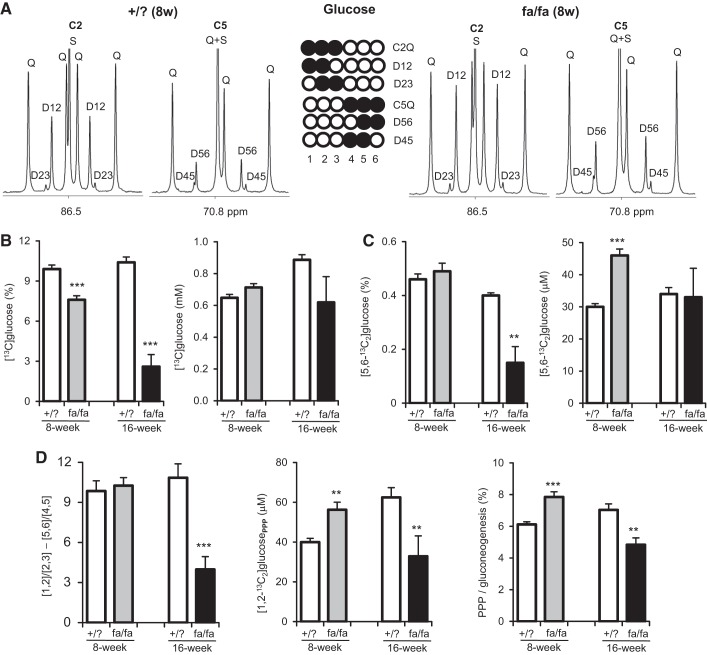
Plasma [glucose] slightly increased in 8-wk fa/fa rats and substantially increased in 16-wk fa/fa rats compared with lean littermates. Plasma [insulin] increased in fa/fa rats at both ages (Table 1). Direct gluconeogenesis from [U-13C3]glycerol produces triple-labeled ([1,2,3-13C3]- and [4,5,6-13C3]) glucose, which dominated the spectra from all ZDF rats (Fig. 3A). The fraction of [13C]glucose in plasma was lower in fa/fa rats at both ages than in lean littermates due to dilution by larger glucose pools. The levels of [13C]glucose were the same between fa/fa rats and +/? rats at both ages, suggesting similar gluconeogenesis from glycerol (Fig. 3B). Interestingly, the level of [5,6-13C2]glucose was higher in 8-wk fa/fa rats only, and not altered in 16-wk fa/fa rats (Fig. 3C), indicating that metabolism of [U-13C3]glycerol through the TCA cycle before entering gluconeogenesis was increased in 8-wk fa/fa rats only.
Fig. 3.
Hepatic PPP activity and gluconeogenesis from [U-13C3]glycerol. Plasma glucose was derivatized for NMR analysis to estimate gluconeogenesis and hepatic PPP activity. A: spectra show signals of glucose carbons 2 and 5 from an 8-wk fa/fa rat and a lean littermate (+/?). Triple-labeled ([1,2,3-13C3] and [4,5,6-13C3]) glucose reflects gluconeogenesis directly from [U-13C3]glycerol. Double-labeled (especially [5,6-13C2]) glucose reflects [U-13C3]glycerol metabolism via the TCA cycle. Hepatic PPP interruption in gluconeogenesis from [U-13C3]glycerol produces additional [1,2-13C2]glucose, causing the ratio difference between [1,2-13C2]/[2,3-13C2] and [5,6-13C2]/[4,5-13C2] in glucose. B: fractions of 13C-labeled glucose in both 8-wk and 16-wk fa/fa rats were lower vs. lean littermates. Levels of 13C-labeled glucose were similar between +/? and fa/fa rats at both ages. C: the fraction of [5,6-13C2]glucose was not altered in 8-wk fa/fa rats but decreased in 16-wk fa/fa rats. The level of [5,6-13C2]glucose increased in 8-wk fa/fa rats but was not changed in 16-wk fa/fa rats vs. lean littermates. D: hepatic PPP activity increased in 8-wk fa/fa rats but decreased in 16-wk fa/fa rats vs. lean littermates based on 1) the ratio difference between [1,2-13C2]/[2,3-13C2] and [5,6-13C2]/[4,5-13C2] in glucose, 2) [1,2-13C2]glucose produced through the PPP, and 3) PPP flux relative to gluconeogenesis. D12, doublet from coupling of C1 with C2; D23, doublet from coupling of C2 with C3; Q, doublet of doublets, or quartet, arising from coupling of C2 with both C1 and C3 or from coupling of C5 with both C4 and C6; D45, doublet from coupling of C4 with C5; D56, doublet from coupling of C5 with C6; S, singlet; open circle, 12C; black circle, 13C. *P < 0.05; **P < 0.01; ***P < 0.001; n = 6–7 in each group.
Assessment of hepatic PPP relies on the measured ratio difference between [1,2-13C2]/[2,3-13C2] and [5,6-13C2]/[4,5-13C2] in glucose (12). The ratio difference between D12/D23 and D56/D45 was not altered in 8-wk fa/fa rats but dramatically decreased in 16-wk rats (Fig. 3D). With this information, activity of the PPP was represented in two ways: 1) [1,2-13C2]glucose produced through the PPP, and 2) the ratio of PPP to gluconeogenesis from [U-13C3]glycerol (i.e., PPP flux relative to gluconeogenesis). The level of [1,2-13C2]glucose produced through PPP was higher in 8-wk fa/fa rats but lower in 16-wk fa/fa rats than in lean littermates. The ratio PPP/gluconeogenesis was also higher in 8-wk fa/fa rats, but lower in 16-wk fa/fa rats than in lean littermates (Fig. 3D). Together, these data demonstrate that the PPP is more active in young fa/fa animals and that simultaneously gluconeogenesis from the TCA cycle plays a more important role in these young animals compared with older rats.
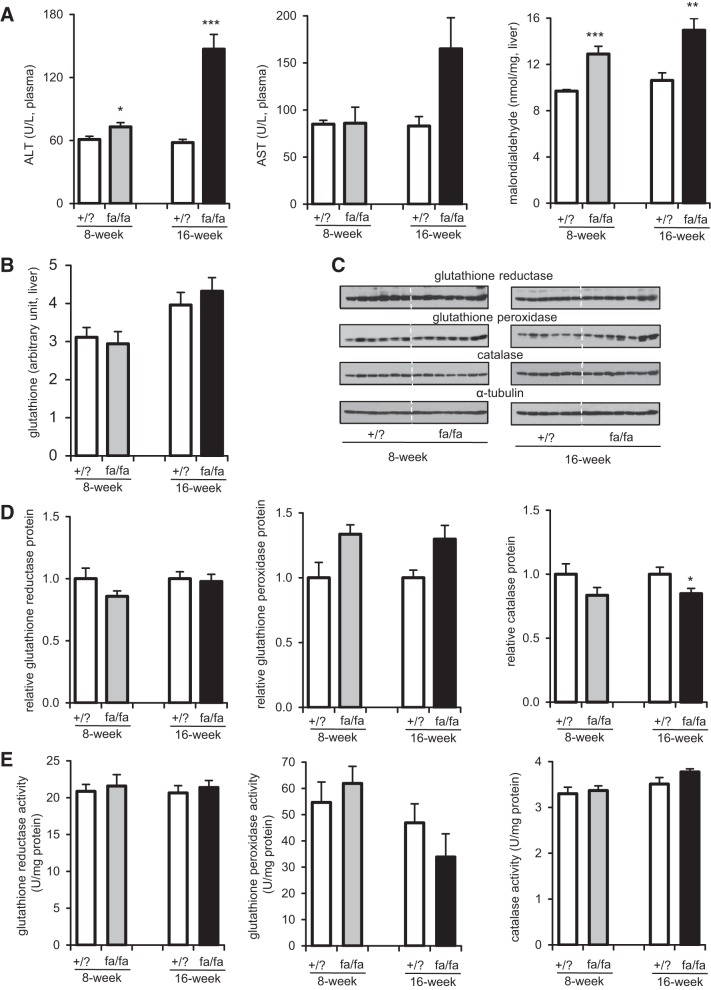
Glutathione and antioxidant enzymes were not altered in the liver of fa/fa rats.
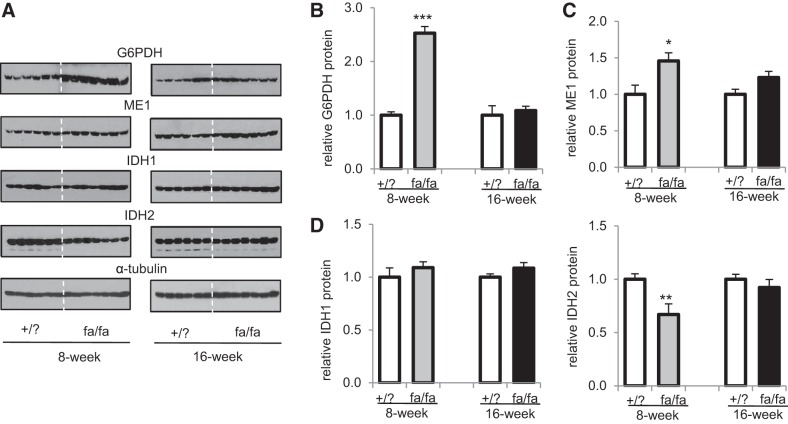
Hepatic steatosis was associated with evidence of hepatic injury and oxidative stress, especially in older fa/fa animals. Plasma alanine and aspartate aminotransferases were similar in 8-wk fa/fa rats but higher in older fa/fa rats compared with lean littermates. [Malondialdehyde], a marker of lipid peroxidation, was also higher in the liver of fa/fa rats at both ages (Fig. 4A). However, the level of glutathione was not altered in the liver of fa/fa rats compared with lean littermates (Fig. 4B). In addition, both activities and protein levels of antioxidant enzymes (i.e., glutathione reductase, glutathione peroxidase, and catalase) were not noticeably different between +/? rats and fa/fa rats at both ages (Fig. 4, C–E). As noted, glutathione reductase activity requires NADPH, and we further examined NADPH-producing enzymes. G6PDH, the rate-determining enzyme of the PPP, substantially increased in 8-wk fa/fa rats but was not altered in 16-wk fa/fa rats (Fig. 5, A and B). Upregulated G6PDH is consistent with NMR analysis of glucose reporting enhanced PPP activity in 8-wk fa/fa rats. Cytosolic malic enzyme (ME1) also increased in 8-wk fa/fa rats only (Fig. 5C). Cytosolic isocitrate dehydrogenase (IDH1) remained the same in both fa/fa rat groups, whereas mitochondrial isocitrate dehydrogenase (IDH2) decreased in 8-wk fa/fa rats only (Fig. 5D).
Fig. 4.
Liver injury/oxidative stress/antioxidant. A: plasma alanine aminotransferase (ALT) increased in fa/fa rats at both ages than lean littermates, whereas aspartine aminotransferase (AST) tended to increase in 16-wk fa/fa rats only without statistical significance. Malondialdehyde, an indicator of lipid peroxidation, was higher in fa/fa rats at both ages than in lean littermates. B: glutathione measured by NMR analysis of liver tissue extracts was not altered in fa/fa rats at both ages. C and D: in immunoblot assays, protein levels of antioxidant enzymes (i.e., glutathione reductase, glutathione peroxidase, and catalase) are similar between +/? rats and fa/fa rats at both ages. E: enzyme activities of glutathione reductase, glutathione peroxidase, and catalase are also similar between +/? and fa/fa rats at both ages. *P < 0.05; **P < 0.01; ***P < 0.001; n = 6–7 in each group.
Fig. 5.
NADPH-producing enzymes in the livers. A: Western blot analysis was performed to measure protein levels of NADPH-producing enzymes in the liver. B: G6PDH, the rate-limiting step of the PPP, substantially increased in 8-wk fa/fa rats but was not altered in 16-wk fa/fa rats vs. lean littermates. C: ME1 slightly increased in 8-wk fa/fa rats but was not altered in 16-wk fa/fa rats. D: IDH1 was not altered in fa/fa rats at both ages, but IDH2 decreased in 8-week fa/fa rats compared to lean littermates. G6PDH, glucose-6-phosphate dehydrogenase; IDH1, cytosolic NADP+-dependent isocitrate dehydrogenase; IDH2, mitochondrial NADP+-dependent isocitrate dehydrogenase; ME1, cytosolic NADP+-dependent malic enzyme. *P < 0.05; **P < 0.01; ***P < 0.001; n = 6–7 in each group.
DISCUSSION
Interactions among hepatic lipogenesis, oxidative stress, and glucose metabolism are complex. This study demonstrates that hepatic PPP activity paralleled lipogenesis in the liver of whole animals, but it was dissociated from antioxidant process, lipid peroxidation, or clinical markers of hepatic injury. This conclusion is strengthened by the internal consistency of results from NMR analysis, direct measurement of enzymes in the PPP, and antioxidant processes and markers of lipid peroxidation and liver injury. Together, these findings indicate that NADPH from the PPP was utilized primarily for lipogenesis rather than for protection against oxidative stress. Failure of the PPP to maintain NADPH production may play a causal role in late consequences of hepatic steatosis. This study further demonstrated that mitochondrial anabolic functions declined with prolonged hepatic steatosis based on biosynthesis of glucose and TG through the TCA cycle.
In many respects, the results of the current work are consistent with prior studies. It is generally accepted that the PPP provides NADPH for fatty acid biosynthesis (11). This study found that activity of the PPP indeed paralleled fatty acid esterification in the liver of ZDF rats. Earlier reports found that glycerol contributes to glucose production and supplies the backbones of TG through a direct pathway and that only a small fraction is metabolized through the TCA cycle before gluconeogenesis (13), consistent with the current results. It is also generally accepted that the PPP plays a central role in antioxidant defense because of NADPH production (8, 23, 25). The current study, in contrast, found that PPP activity was dissociated from antioxidant processes in the liver. This suggested that NADPH from the PPP was utilized for reductive biosynthesis in the liver of ZDF rats but not for antioxidant processes. In erythrocytes, the PPP is the only source of NADPH and is important for protection from oxidative damage (9, 10). However, it may not be the case for other organs such as liver with multiple sources of NADPH. In addition to G6PDH and 6-phosphogluconate dehydrogenase of the PPP, ME and IDH are also NADPH-producing enzymes. IDH is considered to have a role in combatting oxidative damage in cells (14, 16, 18). The decrease of IDH2 in young fa/fa rats in the current work could be related to impaired mitochondrial redox balance or oxidative stress. ME, in contrast, was reported to correlate with lipogenesis (1, 15), which is consistent with the current observation of enhanced ME1 in 8-wk fa/fa rats with most active lipogenesis.
Mitochondrial dysfunctions play a role in the pathophysiology of high-impact liver diseases (3). This study further demonstrated that mitochondrial biosynthesis in the fatty liver declined in extended steatosis. It is interesting to note that the biosynthesis in the fatty liver of young rats was enhanced, suggesting that anaplerotic functions of mitochondria are increased at an early stage of hepatic steatosis. These observations were based on the production of double-labeled TG-glycerol and glucose (i.e., TG-[13C2]glycerol and [5,6-13C2]glucose). The consistency between results from the TG backbone and glucose labeling is expected, because phosphoenolpyruvate generated from the TCA cycle must pass through a common pool of trioses. Glucose and TG can be obtained from either liver or blood, since they are synthesized in the liver and released into the circulation. Thus, the detection of double-labeled TG-glycerol and glucose from blood could serve as biomarkers of mitochondrial anabolic functions in the liver with the use of [U-13C3]glycerol.
Although both young and older obese ZDF rats had fatty liver in common, they were quite different in many aspects, including biochemical processes in the liver. As discussed, hepatic PPP activity, TG synthesis, and mitochondrial biosynthetic functions were all enhanced in young rats but declined in older rats. Plasma glucose and aminotransferases were higher in older obese ZDF rats compared with young rats or lean littermates. Independently of lipid peroxidation, hyperglycemia itself could aggravate oxidative stress, hepatic injury, or inflammation in the liver of these older rats (19). Aminotransferase elevations also suggest hepatic inflammation or injury in older obese ZDF rats. Thus, whreas the young rats are considered to have simple hepatic steatosis, the older rats could be in an advanced stage of fatty liver, possibly in emerging nonalcoholic steatohepatitis.
In the experimental procedures of the present work, animals were briefly exposed to isoflurane anesthesia to minimize the discomfort associated with intraperitoneal injection of [U- 13C3]glycerol. Isoflurane is generally acceptable in rat experiments without causing noticeable alterations in liver metabolism (6, 20, 21). However, continuously inhaled isoflurane with the use of bolus propofol anesthesia was reported to cause hepatic insulin resistance in dogs (17). Thus, we cannot completely eliminate potential alteration in insulin action due to isoflurane use in the present work. Nonetheless, the alteration might not be severe, because we observed that rats quickly awakened immediately, usually within 1 min, after the injection under isoflurane. In addition, overall plasma insulin levels were low in rats after an overnight fast. If isoflurane caused hepatic insulin resistance in the current work, it is most likely to obese ZDF rats only, with relatively higher insulin levels compared with lean rats potentially contributing to hyperglycemia.
In summary, we demonstrated that hepatic PPP activity increased in parallel with the demand for reductive biosynthesis of TG in the liver, but it did not parallel evidence for oxidative stress or antioxidant processes. The assumption that the PPP plays a key role in antioxidant defense may not be applicable to all tissues. We also demonstrated that anabolic functions in fatty liver increased initially but declined with prolonged steatosis. Previously, we suggested that the [U-13C3]glycerol method could be useful in monitoring liver diseases by detecting multiple functions of liver metabolism (13). In the current work using rodent models of hepatic steatosis in both prediabetic and overtly diabetic stages, we indeed demonstrated dysregulations in hepatic PPP activity, TG synthesis, and mitochondrial biosynthesis in these animals. Since many high-impact liver diseases have dysregulations in these processes, the current work supports the idea of applying [U-13C3]glycerol for monitoring patients with liver diseases.
GRANTS
This study was supported by the National Institutes of Health Grants DK-099289, EB-015908, and DK-058398.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.S.J. and C.R.M. conceived and designed research; E.S.J., M.H.L., and R.E.M. analyzed data; E.S.J., M.H.L., R.E.M., and C.R.M. interpreted results of experiments; E.S.J. prepared figures; E.S.J. drafted manuscript; E.S.J. and C.R.M. edited and revised manuscript; E.S.J., M.H.L., R.E.M., and C.R.M. approved final version of manuscript; M.H.L. and R.E.M. performed experiments.
ACKNOWLEDGMENTS
We thank Xiaodong Wen and Thomas Hever for excellent technical support.
REFERENCES
- 1.Al-Dwairi A, Brown AR, Pabona JM, Van TH, Hamdan H, Mercado CP, Quick CM, Wight PA, Simmen RC, Simmen FA. Enhanced gastrointestinal expression of cytosolic malic enzyme (ME1) induces intestinal and liver lipogenic gene expression and intestinal cell proliferation in mice. PLoS One 9: e113058, 2014. doi: 10.1371/journal.pone.0113058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA. Mitochondrial ROS metabolism: 10 years later. Biochemistry (Mosc) 80: 517–531, 2015. doi: 10.1134/S0006297915050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion 6: 1–28, 2006. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 6.Davis AT. The use of isoflurane anaesthesia in studies of carnitine metabolism in the laboratory rat. Lab Anim 30: 377–382, 1996. doi: 10.1258/002367796780739934. [DOI] [PubMed] [Google Scholar]
- 7.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism 49: 684–688, 2000. doi: 10.1016/S0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 8.Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, Abrescia P, Bautista JM, Martini G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J 370: 935–943, 2003. doi: 10.1042/bj20021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaetani GD, Parker JC, Kirkman HN. Intracellular restraint: a new basis for the limitation in response to oxidative stress in human erythrocytes containing low-activity variants of glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci USA 71: 3584–3587, 1974. doi: 10.1073/pnas.71.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho HY, Cheng ML, Chiu DT. Glucose-6-phosphate dehydrogenase–from oxidative stress to cellular functions and degenerative diseases. Redox Rep 12: 109–118, 2007. doi: 10.1179/135100007X200209. [DOI] [PubMed] [Google Scholar]
- 11.Garrett RH, Grisham CM. Biochemistry (3rd ed.). Pacific Grove, CA: Thomson Brooks/Cole, 2005, p. 764. [Google Scholar]
- 12.Jin ES, Sherry AD, Malloy CR. Interaction between the pentose phosphate pathway and gluconeogenesis from glycerol in the liver. J Biol Chem 289: 32593–32603, 2014. doi: 10.1074/jbc.M114.577692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin ES, Sherry AD, Malloy CR. An oral load of [13C3]glycerol and blood NMR analysis detect fatty acid esterification, pentose phosphate pathway, and glycerol metabolism through the tricarboxylic acid cycle in human liver. J Biol Chem 291: 19031–19041, 2016. doi: 10.1074/jbc.M116.742262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, Song BJ, Huh TL. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem 276: 16168–16176, 2001. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 15.Kazumi T, Odaka H, Hozumi T, Ishida Y, Amano N, Yoshino G. Effects of dietary fructose or glucose on triglyceride production and lipogenic enzyme activities in the liver of Wistar fatty rats, an animal model of NIDDM. Endocr J 44: 239–245, 1997. doi: 10.1507/endocrj.44.239. [DOI] [PubMed] [Google Scholar]
- 16.Kil IS, Park JW. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem 280: 10846–10854, 2005. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 17.Kim SP, Broussard JL, Kolka CM. Isoflurane and sevoflurane induce severe hepatic insulin resistance in a canine model. PLoS One 11: e0163275, 2016. doi: 10.1371/journal.pone.0163275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32: 1185–1196, 2002. doi: 10.1016/S0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 19.Ling PR, Mueller C, Smith RJ, Bistrian BR. Hyperglycemia induced by glucose infusion causes hepatic oxidative stress and systemic inflammation, but not STAT3 or MAP kinase activation in liver in rats. Metabolism 52: 868–874, 2003. doi: 10.1016/S0026-0495(03)00057-X. [DOI] [PubMed] [Google Scholar]
- 20.Mets B, Janicki PK, James MF, Hickman R. Hepatic energy charge and adenine nucleotide status in rats anesthetized with halothane, isoflurane or enflurane. Acta Anaesthesiol Scand 41: 252–255, 1997. doi: 10.1111/j.1399-6576.1997.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsu N, Igarashi Y, Aoshi T, Hamaguchi I, Saito M, Mizukami T, Momose H, Ishii KJ, Yamada H. Isoflurane is a suitable alternative to ether for anesthetizing rats prior to euthanasia for gene expression analysis. J Toxicol Sci 42: 491–497, 2017. doi: 10.2131/jts.42.491. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract 77, Suppl 1: S161–S164, 2007. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 14: 5209–5215, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson RG, Shaw WN, Neel M-A, Little LA, Eichberg J. Zucker diabetic fatty as a model for non-insulin-dependent diabetes mellitus. Ilar News 32: 16–19, 1990. doi: 10.1093/ilar.32.3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulusu NN, Sahilli M, Avci A, Canbolat O, Ozansoy G, Ari N, Bali M, Stefek M, Stolc S, Gajdosik A, Karasu C. Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: effects of stobadine and vitamin E. Neurochem Res 28: 815–823, 2003. doi: 10.1023/A:1023202805255. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet 43: 95–118, 2009. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]