Abstract
Lung function is inherently mechanical in nature and depends on the capacity to conduct air and blood to and from the gas exchange regions. Variations in the elastic properties of the human lung across anatomical compartments and with aging are likely important determinants of lung function but remain relatively poorly characterized. Here we applied atomic force microscopy microindentation to characterize human lung tissue from subjects ranging in age from 11 to 60 yr old. We observed striking anatomical variations in elastic modulus, with the airways (200- to 350-µm diameter) the stiffest and the parenchymal regions the most compliant. Vessels (diameter < 100 µm) represented an intermediate mechanical environment and displayed diameter-dependent trends in elastic modulus. Binning our samples into younger (11–30 yr old) and older (41–60 yr old) groups, we observed significant age-related increases in stiffness in parenchymal and vessel compartments, with the most pronounced changes in the vessels. To investigate cellular mechanisms that might contribute to vascular stiffening with aging, we studied primary human pulmonary artery smooth muscle cells from subjects ranging in age from 11 to 60 yr old. While we observed no change in the mechanical properties of the cells themselves, we did observe trends toward increases in traction forces and extracellular matrix deposition with aging. These results demonstrate age-related changes in tissue mechanical properties that likely contribute to impaired lung function with aging and underscore the potential to identify mechanisms that contribute to mechanical tissue remodeling through the study of human cells and tissues from across the aging spectrum.
Keywords: AFM, elastic modulus, extracellular matrix, pulmonary
INTRODUCTION
The lung’s function is inextricably linked with its ability to deform (7), and aging is strongly associated with gradual changes in respiratory system mechanics and declining lung function (14, 28). Chronic respiratory diseases such as asthma, fibrosis, and pulmonary hypertension also result in progressive architectural and mechanical remodeling of the lung (2, 13, 29) that may feedback to influence cell function (5, 21, 30). Despite the importance of tissue mechanical properties to lung and cellular function, very little is known about microscale changes in lung mechanics across anatomical compartments and normal human aging.
Atomic force microscopy (AFM) permits micrometer-scale spatial measurement of lung tissue mechanical properties, allowing anatomical compartments to be studied in intact tissue slices (31). In this study we applied AFM to characterize the variations in tissue stiffness across three anatomical compartments (airways, vessels, and parenchyma) in human lung tissue from subjects ranging in age from 11 to 60 yr old. We observe age-dependent stiffening in vessels and parenchymal tissue, with the most pronounced changes in the vascular compartment. To gain insight into potential cellular mechanisms that may account for such changes, we also characterized primary pulmonary artery smooth muscle cells (PASMCs) from human subjects spanning an age range from 11 to 60 yr old. While we observed no age-related changes in the cellular stiffness of PASMCs, we report evidence suggesting age-dependent increases in basal PASMC traction forces and extracellular matrix deposition, both of which may contribute to alterations in pulmonary vascular stiffness with aging. Together these observations provide new insights into anatomical and age-related changes in lung tissue mechanics that likely influence lung function and suggest phenotypic shifts in cell function with aging that may contribute to alterations in tissue mechanics.
MATERIALS AND METHODS
Tissue samples.
Human lung tissues were obtained from the Pulmonary Hypertension Breakthrough Initiative (PHBI) Research Network under a protocol approved by the Partners Human Research Committee. Lung tissues were collected from organ donors whose lungs were not suitable for transplantation. In total, our study involved 13 subjects aged between 11 and 60 yr old (Table 1). The demographic information including sex, race, ethnicity, and the clinical characteristics (cause of death and reason for not being selected for lung transplantation) of our donor group are indicated in Table 1. All human tissue samples were embedded in Tissue-Plus Optimal Cutting Temperature (O.C.T.) Compound (Fisher HealthCare) and then frozen in dry ice-cooled 2-methylbutane and stored at −80°C.
Table 1.
List of human lung tissue donors including demographics and clinical information
| Group/Subject Name | Age, yr | Sex | Race | Ethnicity | Type of Lethal Injury | Reason for No Organ Transplantation |
|---|---|---|---|---|---|---|
| 11–30 y/o subject group | ||||||
| Lung-1 | 11 | M | White | NH | Anoxia | Low |
| Lung-2 | 20 | M | / | H or L | Head trauma | Lung trauma |
| Lung-3 | 24 | M | White | NH | Intracranial hemorrhage | Inadequate lung function |
| Lung-4 | 25 | M | White | NH | Intracranial hemorrhage | No recipient |
| Lung-5 | 26 | M | White | NH | Head trauma | Poor organ quality |
| Lung-6 | 30 | M | White | NH | Head trauma | Inadequate lung function |
| 41–60 y/o subject group | ||||||
| Lung-7 | 41 | M | White | NH | Head trauma | No recipient |
| Lung-8 | 49 | W | White | NH | Intracranial hemorrhage | Poor organ function |
| Lung-9 | 50 | W | White | NH | Stroke | Poor organ quality |
| Lung-10 | 52 | M | White | NH | Stroke | Poor organ quality |
| Lung-11 | 56 | W | White | NH | Stroke | Poor organ quality |
| Lung-12 | 58 | M | White | NH | Cardiogenic cerebral anoxia/brain death | No recipient |
| Lung-13 | 60 | W | White | NH | Intracranial trauma | Age of donor |
NH, non-Hispanic; H, Hispanic; L, Latino; M, man; W, woman.
AFM experiments on tissue samples.
To perform AFM microindentation on lung tissue, 10-µm thickness tissue slices were cut from lung tissue samples by cryosection (CM1860 UV; Leica) and then mounted on poly-l-lysine-coated glass slides. To avoid drying of samples, PBS solution was added to each slice and adequate hydration was maintained throughout AFM characterization. The preparation and AFM analysis of each sample were done on the same day.
An optical microscope with a bright-field channel (×200; Olympus) was used to identify airways, pulmonary vessels, and parenchymal areas within lung tissue slice, as shown in Fig. 1, A–C. AFM measurements were performed in PBS solution at room temperature using a Catalyst Bioscope atomic force microscope (Bruker). Force curves were acquired at different locations on the parenchymal tissue and on airway and vessel walls, as shown in Fig. 1, A–C, insets, using MIRO 2.0 software (NanoScope 9.1; Bruker). A 2.5-µm radius borosilicate sphere AFM tip (Novascan) was used with a spring constant k estimated at 100 pN/nm by thermal tune (34). As described in Sicard et al. (31), force curve acquisition was parameterized to operate indentation with a 10-µm ramp size, a rate of 20.6 µm/s, and an applied force between 20 and 30 nN. Thirty force curves were performed per airway and two airways were analyzed per donor (Table 1). For vessels, five points of measurement were completed on the pulmonary vessel wall, and between two and five vessels were identified per donor. Finally, three parenchymal areas were analyzed per donor with 30 force curves acquired per area. Airways, vessels, and parenchymal areas were identified from each donor included in Table 1.
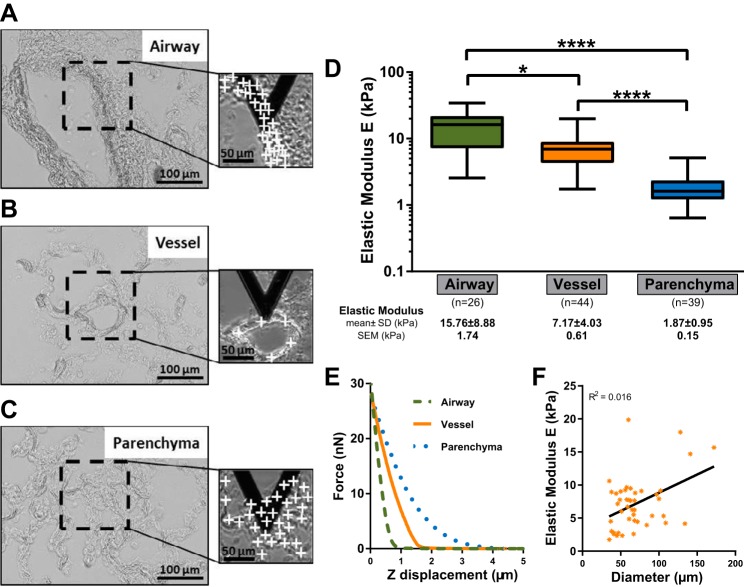
Fig. 1.
Bright-field images (×200) of human lung tissue: airway (A), vessel (B), and parenchymal tissue (C). Scale bar = 100 µm. A, B, and C, insets: optical images of a triangular atomic force microscopy (AFM) tip indenting lung tissue. White crosses represent the points of measurement of the airway and vessel wall and on the parenchymal tissue. Scale bar = 50 µm. D: elastic modulus of airway, vessel, and parenchyma area from human lung tissue samples. *P < 0.05 and ****P < 0.0001. E: representative force curves (force vs. Z displacement) performed on airway and vessel wall and on parenchymal tissue. F: elastic modulus as a function of vessel diameter. Black line is the linear regression model showing the increase in elasticity with the increase in vessel diameter. R2 is the coefficient of determination.
The Young’s modulus (elastic modulus E) of airway, pulmonary vessel, and parenchymal tissue was estimated by fitting force curves with NanoScope Analysis (Bruker) using the Hertz spherical indentation model (6) and assuming a Poisson’s ratio of 0.4 for lung tissue (3).
Cell culture.
Primary human PASMCs from nondiseased lung tissue were obtained from the PHBI (10) under a protocol approved by the Partners Human Research Committee. Informed consent was obtained by the PHBI from the legal guardians of the donors before they were enrolled in the study. Cell isolation, characterization, and maintenance were performed under the PHBI as previously described in (10). Briefly, PASMCs were isolated from distal pulmonary arteries (<1-mm diameter) from control donor lungs not suitable for transplantation. PASMCs were characterized by fluorescence-activated cell-sorting analysis of α-smooth muscle actin and by immunohistochemistry to confirm expression of α-smooth muscle actin, smooth muscle myosin heavy chain, and smooth muscle protein 22α.
Cells were cultured in smooth muscle basal medium supplemented with 5% FBS, SmGM-2 SingleQuots (Lonza), penicillin (100 IU/ml), and streptomycin (100 μg/ml) in a humidified incubator (21% O2-5% CO2) at 37°C. Experiments were performed at passages 6 or less. For our study, we considered PASMCs from eight donors spanning an age range of 11 to 60 yr, as detailed in Table 2 with demographic information (sex, race, and ethnicity).
Table 2.
List of primary human pulmonary artery smooth muscle cells from lung donors with demographic data
| Group/Subject Name | Age, yr | Sex | Race | Ethnicity |
|---|---|---|---|---|
| 11–30 y/o subject group | ||||
| Cell-1 | 11 | M | White | NH |
| Cell-2 | 13 | M | Unknown | NH |
| Cell-3 | 25 | M | White | NH |
| Cell-4 | 25 | M | White | NH |
| 41–60 y/o subject group | ||||
| Cell-5 | 50 | W | White | NH |
| Cell-6 | 51 | M | White | NH |
| Cell-7 | 57 | W | White | NH |
| Cell-8 | 60 | W | White | NH |
NH, non-Hispanic; H, Hispanic; L, Latino; M, man; W, woman.
AFM experiments on PASMCs.
PASMCs were grown and analyzed in glass-bottom tissue culture dishes (GWST-5040; WillCo-Dish) at ~80% confluence. AFM microindentation was performed at room temperature in cell culture media with parameters described above for lung tissue AFM experiments. For each subject, we studied one to two independent replicate cultures. In each replicate culture, three different areas were analyzed per dish. Multiple force curves were performed across the cell membrane, with special care taken to avoid the cell’s nucleus (see Fig. 3A, inset).
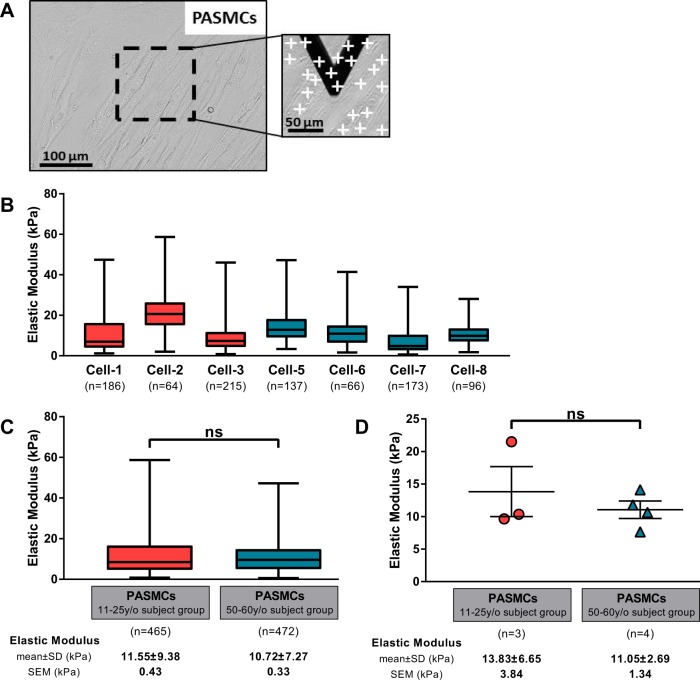
Fig. 3.
A: a representative area of interest in cells is shown (bright-field image, ×200) with example of points of measurement (white crosses) in the inset. B–D: triangular atomic force microscopy (AFM) microindention was performed on human pulmonary artery smooth muscle cells (PASMCs) to determinate the stiffness of cells (B) and compare the elasticity between the 2 age groups (C and D). In C, all force curves are treated as independent measures, while in D a single average elastic modulus value is shown for each subject. Bars show means ± SE.
Traction force microscopy on PASMCs.
Polyacrylamide hydrogels with shear moduli of 6.4 kPa were prepared as previously described in Marinković et al. (25). Briefly, the glass area of glass-bottom six-well plates (In Vitro Scientific) was silanized to allow adherence of polyacrylamide by covering the glass with acetone containing 0.4% 3-methacryloxypropyltrimethoxysilane (Acros Organics) and incubating until the acetone solution was completely evaporated in air. A hydrophobic coverslip treated by SurfaSil (Thermo Scientific) was used to make the top surface of hydrogel. Twenty-five microliters of each prepolymerization mixture [%acrylamide:%bisacrylamide (Young's modulus, 20 kPa); 7.5:0.34] was sandwiched with SurfaSil (Thermo Scientific)-treated, hydrophobic glass coverslips (18 mm in diameter) until the acrylamide:bisacrylamide mixture was polymerized. Fluorescent sulfate-modified latex microspheres (0.2 μm, 505/515 excitation/emission; FluoSpheres; Life Technologies) were conjugated to the gel surfaces after treatment with 1 mg/ml of dopamine hydrochloride (Sigma-Aldrich) in 50 mM HEPES solution (pH 8.5) for 20 min. The gels were functionalized by incubation for 30 min with 0.05 mg/ml sterile collagen I (PureCol; Advanced BioMatrix) in PBS. Cells (4,000 cells/well) were plated on the gels and incubated for 3 days before traction force measurements. Images of gel surface-conjugated fluorescent beads were acquired for each cell before and after trypsinization using an Eclipe Ti microscope (Nikon) at ×100 magnification. Tractions forces were estimated by measuring bead displacement fields and computing corresponding traction fields using TractionsForAll (http://www.mayo.edu/research/labs/tissue-repair-mechanobiology/software) (4, 25).
Immunoextracellular matrix assays on PASMCs.
Adapting from previously published methods developed by Jones et al. (15), we plated the PASMCs to confluence in clear-bottomed 96-well plates. After cells attached, they were starved in media containing 0.1% FBS and incubated for 72 h. Cells were then lysed and lifted from the plate with 0.016 N NH4OH leaving behind extracellular matrix proteins. Wells were washed with TBS and blocked with Odyssey Blocking Buffer (Li-Cor) for 60 min before overnight incubation in a polyclonal rabbit antibody for fibronectin (sc-9068; Sigma) or collagen I (Novus NB600-408) diluted 1:100 in blocking buffer. Wells were washed with TBS-Tween before a 45-min incubation with IR-dye-conjugated secondary antibody (No. 926–32211; Li-Cor) diluted 1:400. Plates were imaged via an Odyssey CLx system (Li-Cor) with quantification performed via densitometry.
Statistical analysis.
Data are presented as scatter plots showing means ± SE, in box and whiskers plots with the box indicating the 25th and 75th percentiles of the values, the line inside the box the median value, and the whiskers the smallest and largest values. Statistical analysis was performed using Prism software (GraphPad Software, Version 7). Data groups were compared using nonparametric one-way ANOVA, Tukey’s post hoc test, and Mann-Whitney test with an accepted statistical significance at P < 0.05.
RESULTS
Anatomical variations in the elastic modulus of lung tissue.
To assess the elastic modulus of human lung tissue and determine the anatomical variations in the mechanical properties of airways, pulmonary vessels, and parenchymal tissue, optical microscopy was used to choose areas of interest (airway, vessel, and parenchymal areas). Airways and pulmonary vessels were identified by their characteristic shape, wall thickness, and morphology. We focused on airways of diameter between 200 and 350 µm and vessels with a diameter <100 µm. Figure 1 shows representative optical images of an airway (A), pulmonary vessel (B), and parenchymal tissue region (C). Multiple measurements were taken in each area of interest: 30 force curves for each airway and parenchyma region, and 5 force curves on the wall of each vessel, as shown in Fig. 1, A–C, insets. Representative force Z-displacement curves are presented in Fig. 1E, showing the extent of indentation (δ > 500 nm) into the tissue. We previously demonstrated that the extent of such indentations exerts minimal effects on the resulting elastic moduli (31). After averaging all measurements within a region to produce a single value per airway or vessel or parenchymal region, we observed that the elasticity of human lung tissue exhibited substantial anatomical variations across compartments (Fig. 1D). On average, airways were significantly stiffer than pulmonary vessels and parenchymal tissue with Young’s moduli estimated at 15.76 ± 8.88 kPa (with a range of 2.57–34.44 kPa), 7.17 ± 4.03 kPa (with a range of 1.74–19.86 kPa), and 1.87 ± 0.95 kPa (with a range of 0.64–5.13 kPa) respectively. The elastic modulus of vessels was also significantly higher than for parenchymal tissue. These elastic modulus values represent the means ± SD determined from 26 airways, 44 vessels, and 39 parenchyma areas taken from across 13 human lung samples. Because the 44 vessels we examined spanned a range of sizes, we also plotted vessel wall elastic modulus as a function of vessel diameter (Fig. 1F) and observed a clear trend toward increasing elastic modulus with increasing vessel size, consistent with systemic variations within the stiffness of the vessel compartment as a function of location within the vascular tree.
Aging-related increases in lung tissue elastic modulus.
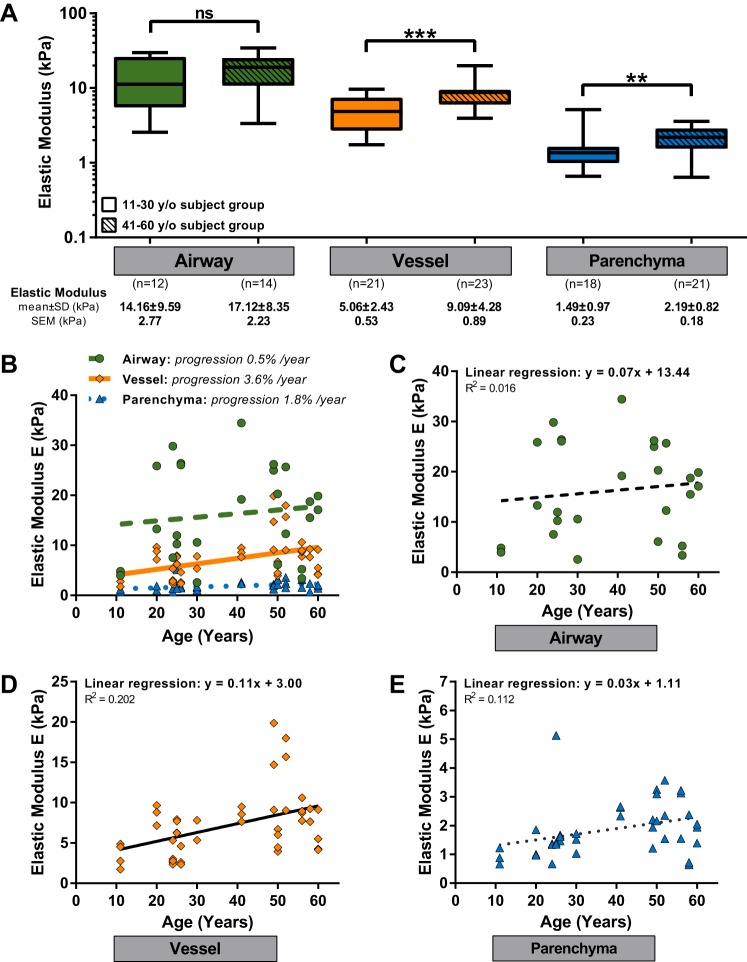
To understand the influence of aging on lung tissue elastic modulus, the initial donor group (Table 1) was divided into two age groups: one younger group termed the 11–30 y/o subject group including the six lung tissue donors from subjects between 11 and 30 yr old (lung-1 to lung-6) and one older group termed the 41–60 y/o subject group with the seven subjects aged between 41 and 60 yr old (lung-7 to lung-13). These two groups have the same age range (19 yr) and the respective median ages in the two groups are 24.5 and 52 yr old.
Our prior measurements of Young’s modulus for airways, pulmonary arteries, and parenchymal tissue were reanalyzed for each of these two groups. As shown clearly in Fig. 2A, lung tissues from the 41–60 y/o subject group exhibited significantly higher elastic moduli than from the 11–30 y/o subject group across two anatomical compartments: vessel and parenchymal tissue. For the older group, the elastic values were 9.09 ± 4.28 kPa for vessels and 2.19 ± 0.82 kPa for parenchymal tissue. For the younger group, the elastic moduli were 5.06 ± 2.43 kPa for vessels and 1.49 ± 0.97 kPa for parenchyma. Although similar age-dependent trends were observed for airways, with mean values at 17.12 ± 8.35 kPa for the 41–60 y/o subject group and 14.16 ± 9.59 kPa for the 11–30 y/o subject group, no significant difference was observed.
Fig. 2.
A: elastic modulus of lung tissue (airway, vessel, and parenchymal tissue) for 2 aging groups: 11–30 y/o subject group and 41–60 y/o subject group. Elastic results were compared using the Mann-Whitney test with **P < 0.01 and ***P < 0.001. B: graph combines all the results presented in C–E. C–E: elastic modulus for airways (C), pulmonary arteries (D), and parenchymal region (E) as a function of lung donor age. Each point is the mean elastic value of all the measurements performed on one area. Each line represents the fitting of the data by a linear regression model. R2 is the coefficient of determination.
To examine the mechanical properties of each anatomical compartment as continuous functions of subject age, we plotted the elastic modulus values per airway/vessel/parenchymal region as functions of donor age for airways (Fig. 2C), vessels (Fig. 2D), and parenchymal areas (Fig. 2E), and for direct comparisons across anatomical compartments, we plotted results from all three compartments in a single graph (Fig. 2B). As a first order approximation of changes in elastic modulus with aging, each relationship was fit by linear regression. The slopes extracted from such linear equations give us a rough approximation of trends in the evolution of the elastic modulus. For each compartment, the slope was positive with the highest slope observed for vessels (slope of 0.11). In Fig. 2B, we also approximated the average percent change in elastic modulus extrapolated from the theoretical y-intercept (age = 0) and observed an increase in elastic modulus for vessels of 3.6%/yr, with lower values for airways (0.5%/yr) and parenchyma (1.8%/yr).
Aging effect on PASMCs.
Based on our observation that age-related effects on lung tissue stiffness were most pronounced in the vascular compartment, we focused our further investigation on pulmonary vascular cells and tested whether aging was associated with alterations in extracellular matrix production, contractile function, and cellular elastic properties of primary human PASMCs. We analyzed primary human PASMCs from eight donors aged between 11 and 60 yr old. As in lung tissue, to compare two age groups we separated samples into an 11–25 y/o subject group composed of cells from four different donors aged between 11 and 25 yr old and a 50–60 y/o subject group with cells from four donors aged between 50 and 60 yr old (Table 2).
We first directly assessed the elastic modulus of PASMCs by AFM microindentation to evaluate whether age influences the cellular mechanical properties of PASMCs. Cells were grown in glass-bottom tissue culture dishes until 80% confluent (Fig. 3A). We analyzed between three and six different regions per subject. Multiple force curves were performed on several cells per area, as presented in the inset of Fig. 3A, inset. All force curves were first treated as independent measures. The elastic modulus of PASMCs for each subject is shown in Fig. 3B. There was substantial variability in individual force curve measurements of elastic modulus within each sample, as is typical for cellular AFM measurements (16). However, there was no systematic trend in Young’s modulus with subject age, and we observed no significant difference between the distribution of stiffness values in the 11–25 y/o subject group (11.6 ± 9.4 kPa) and the 50–60 y/o subject group (10.7 ± 7.3 kPa) when considering all force curves independently (Fig. 3C). After averaging to generate a single elastic modulus value per subject, we again identified no significant difference between the two age groups (Fig. 3D).
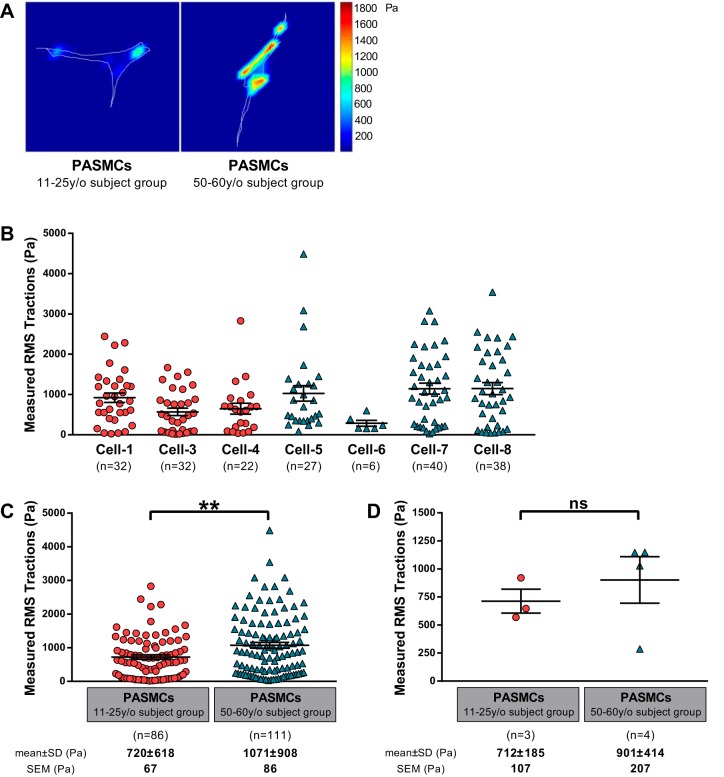
We next assessed the baseline “tone” of PASMCs in cell culture using traction force microscopy. For these experiments, PASMCs were grown on 6.4-kPa shear modulus (~18 kPa Young’s modulus) hydrogels. Based on the availability of low passage PASMCs, we were able to perform between one and three independent experiments per subject, with multiple cells (6–20) analyzed per experiment. Representative images of cell tractions generated within the 11–25 y/o subject group and 50–60 y/o subject group are shown in Fig. 4A. There was substantial variability in the baseline tractions generated across individual cells, as is typical for this technique (25). With the exception of cells from cell-6 subject, for which only a single experiment was possible due to limited cell replication, there appeared to be a modest relationship between increasing age and cellular tractions. When all of the traction data were binned into the two age groups (Fig. 4C), we did observe that traction forces generated by cells from the 50–60 y/o subject group (1,071 ± 908 Pa) were modestly, but significantly, higher than for PASMCs from the 11–25 y/o subject group (720 ± 618 Pa). In Fig. 4D, we present a single traction average per subject from all measurements. In this analysis, we observed no significant difference between the two age groups.
Fig. 4.
The contractile function of pulmonary artery smooth muscle cells (PASMCs) was assessed by traction force microscopy on polyacrylamide gels of 6.4-kPa shear modulus. A: example of cell traction images for PASMCs from the 11–25 y/o subject group and 50–60 y/o subject group. B–D: the root mean square (RMS) traction was computed for individual cells (B) and analyzed for the 2 age groups considering all the measurements on a per cell basis (C) or by averaging results by subject (D). Bars show means ± SE with **P < 0.01.
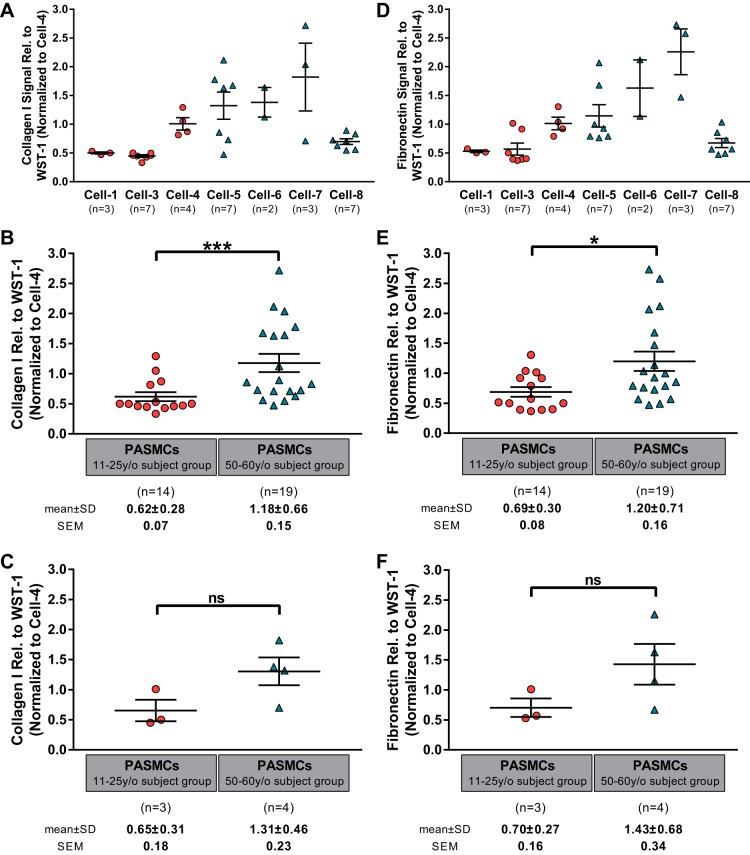
As a final metric of cell function that might translate aging effects into tissue stiffness changes, we measured deposition of type I collagen and fibronectin protein by cultured PASMCs over 72 h by immunoassay (Fig. 5, A and D, respectively). Based on the varying replicative potential of low passage PASMCs, we were able to perform assays on two to seven replicate cultures per subject (with 2 or 3 technical replicate wells averaged within each experiment). Once again there was substantial intersubject variability, with a trend toward increased collagen I and fibronectin deposition by cells from the older group, although with the notable exception of the cells from cell-8 subject, the oldest in our cohort. When we treated each biological replicate as an independent outcome and binned the results into two age groups as above, we observed that cells from the 50–60 y/o subject group deposited significantly more collagen I and fibronectin than do cells from the 11–25 y/o subject group (Fig. 5, B and E). However, when representing our results as a single average per subject, the difference between younger and older subject groups does not reach statistical significance.
Fig. 5.
Pulmonary artery smooth muscle cells (PASMCs) were assessed using immunoextracellular matrix assay to measure the production of collagen I (A) and fibronectin (D). Comparisons of the 2 age groups for collagen I and fibronectin production were made considering all biological replicate measurements (B and E, respectively) and after averaging all biological replicates into a single mean per subject (C and F, respectively). Bars show means ± SE with *P < 0.05 and ***P < 0.001.
DISCUSSION
This study contributes new findings to our understanding of how human lung mechanics vary across anatomical compartments and change with normal aging and how age-related changes in cellular phenotype may influence tissue mechanics. Our results demonstrate that the elastic modulus varies significantly across anatomical compartments of the lung, with stiffness being highest in the airways and lowest in parenchymal regions. Vessels represent an intermediate state, and our results also demonstrate size-dependent variations in vessel stiffness that scale with vessel diameter. Aging significantly influences the mechanical properties of the vessels and parenchymal tissue, with the greatest rate of increase seen in the vascular compartment. Focusing on potential cellular mechanisms that might explain age-related vascular stiffening, we find no evidence that PASMCs themselves are stiffer with aging. However, we observed that baseline PASMC tone and extracellular matrix deposition may increase with aging, although expansion of these preliminary results to include much larger subject populations will be necessary given the substantial subject to subject variability seen in our cellular studies.
At first glance, the observation that the elastic modulus varies across anatomical compartments seems counter to the observed relative homogeneity of lung deformation with inflation and deflation. However, it is known that preferential paths of stress transmission occur within the lung (8). The anatomical variations in mechanical properties that we observed likely serve the functional needs of these compartments and serve to match the distribution of respiratory stresses, resulting in the efficient distribution of gases and blood during the respiratory cycle. An important aspect of our results is that the lung does not represent a single homogeneous mechanical environment for cells. Given the broad influence that mechanical cues play in determining cell fate in health and disease (35, 36), our results indicate that anatomical variations in mechanical properties may provide important cues that shape local cellular phenotype and function. As a corollary to this idea, we note that diseases that alter the mechanical environment (7, 20, 21) may therefore provide aberrant mechanical cues to tissue resident cells, potentially impeding normal tissue repair and regeneration.
Aging is well known to be accompanied by changes in respiratory system mechanics and function (14, 28). Our data are the first to examine changes in microscale mechanical properties within specific anatomical compartments across human aging. In general we observed that aging is associated with an increase in the elastic modulus within vascular and parenchymal compartments we examined. For the vasculature, which we observed to be the most dramatically altered with aging, our results build on prior work that demonstrate increases in proximal pulmonary vessel stiffness with aging (1, 11, 12, 23) but now extend these findings to smaller intrapulmonary vessels. Notably, recent work has demonstrated that remodeling of such small vessels in models of pulmonary hypertension may propagate proximally and precede alterations in hemodynamics and right ventricular function (20). In the systemic circulation, aging-related vessel stiffening predisposes to cardiovascular dysfunction (33); hence, the changes we observe in pulmonary vascular stiffness may set the stage for cardiopulmonary dysfunction and declining aerobic capacity with aging (18).
Our observation that parenchymal regions exhibit increased stiffness with aging is potentially counterintuitive, as many studies have demonstrated that the lungs and respiratory system become more compliant with aging (14, 28). Nevertheless, our results agree with prior observations of increasing parenchymal tissue stiffness with aging (17). These observations can be reconciled by noting the anatomical loss of alveolar walls in aging (9, 37), with the resulting changes in architecture, alveolar radii, and surface forces likely playing a dominant role in the age-related increase in organ level compliance (7). However our measurements and those of Lai-Fook and Hyatt (17) both demonstrate that the elastic modulus of alveolar tissue itself actually increases modestly with aging. Combined with other observations that lung fibroblasts are acutely sensitive to profibrotic activation by stiffness in the range observed here (35), these results suggest that age-associated changes in the distal lung may shift the mechanical environment in a direction conducive for fibrogenesis as a consequence of aging.
The mechanisms by which cells generate mechanical environments, and how aging alters such environments, remain incompletely understood (35). Based on the dramatic age-related changes in vascular stiffness that we observed, and the availability of vascular cells from across a similar age spectrum, we focused on PASMCs as a major constituent of vessel walls that possess the capacity to both contract and deposit extracellular matrix. Recent work from systemic vascular cells has suggested that vascular smooth muscle cells themselves can alter their mechanical properties and that such changes may play an important role in hypertension-related changes in vascular stiffness (26, 27). Using AFM microindentation, we found no evidence that aging produced significant changes in cellular mechanical properties of cultured PASMCs. As a caveat, we note that our measurements were made on tissue culture plates rather than compliant hydrogels and substrate stiffness itself can alter cell mechanical properties (32); hence, future work could attempt to evaluate PASMC stiffness on hydrogels approximating the range of normal vessel stiffness observed during human aging. While we found little evidence of age-related changes in PASMC stiffness, we did observe trends toward age-related increases in basal tractions and collagen I and fibronectin deposition. These trends were observed only when biological replicates were treated as independent observations and binned into two age groups, a necessity due to the limited availability of human samples across the aging spectrum and the limited proliferative capacity of these cells. Thus while our in vitro results are inherently limited by the small sample size and emphasize the need for larger populations in assessing aging cell function, they do suggest that aging impacts the phenotype and function of vascular smooth muscle cells in an important fashion that likely contributes to the age-related evolution of vascular wall stiffness. While these studies are only a starting point in defining the mechanisms of age-related mechanical remodeling in the lung, they do suggest that efforts aimed at studying primary human cells from across the aging spectrum may provide novel insights and allow generation of hypotheses regarding cellular and molecular targets that coordinate changes in tissue mechanics.
There are several limitations to our approach and unanswered questions that our study generates. Notably, AFM mechanical characterization is inherently invasive and requires removal of the lung from its normal prestressed environment, sectioning, and examination in the absence of perfusion and inflation. Encouragingly, noninvasive approaches to measure lung mechanics generate results largely in line with AFM methodology (17, 24), supporting the potential physiologic relevance of our findings. Our study was also limited to human tissue previously frozen for storage. While carefully managed freeze-thaw cycling largely preserves tissue mechanical properties and we have observed similar lung tissue mechanics in fresh and previously frozen specimens (20–22), there may be some modest deterioration in tissue mechanical properties with freezing and thawing and associated with use of O.C.T. as an embedding medium. Such limitations were unavoidable in the present study based on the availability of archived frozen lung tissue specimens. The selection of AFM indentation parameters can also influence the resulting mechanical properties that are observed (31), although with consistent methodology these will largely alter the absolute reported values and not the differences with aging or anatomical location. The absence of perfusion and inflation is inherent to this technique, and the lung is inherently a nonlinear mechanical tissue. Hence, to the extent that aging alters both inflation and perfusion pressures, our results may fail to account fully for age-related changes in tissue mechanical properties. However, we would expect such changes to exaggerate the age-related increases in stiffness we observed, as pulmonary pressures generally trend upward with aging (19). Finally, our analysis of tissues and cells was limited to a relatively small number of samples and a range of 11- to 60-yr-old subjects. It would be interesting to know the further changes in lung tissue mechanics and cellular phenotypes beyond 60 yr of age, as progressive changes in lung function continue in this age range (14, 28). At the other end of the age spectrum, it would be extremely helpful to study tissue and cells from human lungs during development and maturation, as it is widely appreciated that the morphogenetic processes that play out on this time scale can dramatically influence the trajectory of lung function. Finally, we note that our analysis did not consider possible sex differences, as the number of samples was too few to independently assess the relationship among aging, lung stiffness, and donor sex. Access to a larger population of samples from both sexes would allow future consideration of this important biological variable.
In conclusion, our results demonstrate that the elastic modulus of human lung tissue varies across anatomical compartments and increases with aging. Conceptually, our work builds on the notion that the local compartment-specific mechanical environments within the lung are likely optimized to support lung function and that age-related changes in these mechanical compartments contribute to progressive declines in lung function. Although multiple cell types cooperate in the generation, maintenance, and alteration of tissue mechanical environments, our work also points to age-related changes in PASMC phenotype as one likely contributor to alterations in pulmonary vascular compliance and lung function with aging. Future efforts to preserve or restore aging- and disease-related loss of lung function will need to take such tissue mechanical and cellular phenotypic changes into account.
GRANTS
This work supported by National Heart, Lung, and Blood Institute (NHLBI) HL-114839, HL-115106, HL-137366, HL-092961, and HL-133320. Funding for the Pulmonary Hypertension Breakthrough Initiative is provided under NHLBI Grant R24-HL-123767 and the Cardiovascular Medical Research and Education Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., L.E.F. and D.J.T conceived and designed research; D.S., A.J.H., K.M.C. and A.R.C performed experiments; D.S., A.J.H., K.M.C. and A.R.C analyzed data; D.S, A.J.H and D.J.T. interpreted results of experiments; D.S. prepared figures; D.S and D.J.T. drafted manuscripts; D.S., A.J.H., K.M.C., A.R.C., L.E.F. and D.J.T. edited, revised, and approved final version of manuscript.
ACKNOWLEDGMENTS
Data and tissue samples were provided under the Pulmonary Hypertension Breakthrough Initiative.
REFERENCES
- 1.Banks J, Booth FV, MacKay EH, Rajagopalan B, Lee GD. The physcial properties of human pulmonary arteries and veins Clin Sci Mol Med 55: 477–484, 1978. [DOI] [PubMed] [Google Scholar]
- 2.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol 240: 397–409, 2016. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JP, Nakamura M, Sasaki H, Sasaki T, Takishima T. Poissons’ ratio of lung parenchyma and parenchymal interaction with bronchi Jpn J Physiol 36: 91–106, 1986. doi: 10.2170/jjphysiol.36.91. [DOI] [PubMed] [Google Scholar]
- 4.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 5.Dieffenbach PB, Mallarino Haeger C, Coronata AMF, Choi KM, Varelas X, Tschumperlin DJ, Fredenburgh LE. Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol 313: L628–L647, 2017. doi: 10.1152/ajplung.00173.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J 82: 2798–2810, 2002. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev 89: 759–775, 2009. doi: 10.1152/physrev.00019.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredberg JJ, Kamm RD. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol 68: 507–541, 2006. doi: 10.1146/annurev.physiol.68.072304.114110. [DOI] [PubMed] [Google Scholar]
- 9.Gillooly M, Lamb D. Airspace size in lungs of lifelong non-smokers: effect of age and sex Thorax 48: 39–43, 1993. doi: 10.1136/thx.48.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129: 864–874, 2014. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gozna ER, Marble AE, Shaw A, Holland JG. Age-related changes in the mechanics of the aorta and pulmonary artery of man J Appl Physiol 36: 407–411, 1974. doi: 10.1152/jappl.1974.36.4.407. [DOI] [PubMed] [Google Scholar]
- 12.Harris P, Heath D, Apostolopoulos A. Extensibility of the human pulmonary trunk Br Heart J 27: 651–659, 1965. doi: 10.1136/hrt.27.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 144: 1026–1032, 2013. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 14.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing Eur Respir J 13: 197–205, 1999. doi: 10.1183/09031936.99.14614549. [DOI] [PubMed] [Google Scholar]
- 15.Jones B, Bucks C, Wilkinson P, Pratta M, Farrell F, Sivakumar P. Development of cell-based immunoassays to measure type I collagen in cultured fibroblasts. Int J Biochem Cell Biol 42: 1808–1815, 2010. doi: 10.1016/j.biocel.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsova TG, Starodubtseva MN, Yegorenkov NI, Chizhik SA, Zhdanov RI. Atomic force microscopy probing of cell elasticity. Micron 38: 824–833, 2007. doi: 10.1016/j.micron.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Lai-Fook SJ, Hyatt RE. Effects of age on elastic moduli of human lungs J Appl Physiol (1985) 89: 163–168, 2000. doi: 10.1152/jappl.2000.89.1.163. [DOI] [PubMed] [Google Scholar]
- 18.Lalande S, Yerly P, Faoro V, Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 590: 4279–4288, 2012. doi: 10.1113/jphysiol.2012.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119: 2663–2670, 2009. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, Suarez Velandia MM, Vitali S, Colas RA, Norris PC, Marinkovic A, Liu X, Ma J, Rose CD, Lee SJ, Comhair SA, Erzurum SC, Mcdonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 1: e86987, 2016. 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp 54: 2911, 2011. doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age Thorax 33: 335–344, 1978. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marinelli JP, Levin DL, Vassallo R, Carter RE, Hubmayr RD, Ehman RL, McGee KP. Quantitative assessment of lung stiffness in patients with interstitial lung disease using MR elastography. J Magn Reson Imaging 46: 365–374, 2017. doi: 10.1002/jmri.25579. [DOI] [PubMed] [Google Scholar]
- 25.Marinković A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 303: L169–L180, 2012. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol 305: H1281–H1287, 2013. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology Clin Interv Aging 1: 253–260, 2006. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 91: 297–309, 2013. doi: 10.1007/s00109-013-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 308: L1125–L1135, 2015. doi: 10.1152/ajplung.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sicard D, Fredenburgh LE, Tschumperlin DJ. Measured pulmonary arterial tissue stiffness is highly sensitive to AFM indenter dimensions. J Mech Behav Biomed Mater 74: 118–127, 2017. doi: 10.1016/j.jmbbm.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453–4461, 2007. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension 65: 252–256, 2015. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thundat T, Allison DP, Warmack RJ. Stretched DNA structures observed with atomic force microscopy Nucleic Acids Res 22: 4224–4228, 1994. doi: 10.1093/nar/22.20.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction. Ann Am Thorac Soc 12, Suppl 1: S24–S29, 2015. doi: 10.1513/AnnalsATS.201407-320MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Curr Opin Rheumatol 25: 92–100, 2013. doi: 10.1097/BOR.0b013e32835b13cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects Chest 101: 793–799, 1992. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]