Abstract
Epithelial cells have been suggested as potential drivers of lung fibrosis, although the epithelial-dependent pathways that promote fibrogenesis remain unknown. Extracellular matrix is increasingly recognized as an environment that can drive cellular responses in various pulmonary diseases. In this study, we demonstrate that transforming growth factor-β1 (TGF-β1)-stimulated mouse tracheal basal (MTB) cells produce provisional matrix proteins in vitro, which initiate mesenchymal changes in subsequently freshly plated MTB cells via Rho kinase- and c-Jun NH2-terminal kinase (JNK1)-dependent processes. Repopulation of decellularized lung scaffolds, derived from mice with bleomycin-induced fibrosis or from patients with idiopathic pulmonary fibrosis, with wild-type MTB cells resulted in a loss of epithelial gene expression and augmentation of mesenchymal gene expression compared with cells seeded into decellularized normal lungs. In contrast, Jnk1−/− basal cells seeded into fibrotic lung scaffolds retained a robust epithelial expression profile, failed to induce mesenchymal genes, and differentiated into club cell secretory protein-expressing cells. This new paradigm wherein TGF-β1-induced extracellular matrix derived from MTB cells activates a JNK1-dependent mesenchymal program, which impedes subsequent normal epithelial cell homeostasis, provides a plausible scenario of chronic aberrant epithelial repair, thought to be critical in lung fibrogenesis. This study identifies JNK1 as a possible target for inhibition in settings wherein reepithelialization is desired.
Keywords: ECM, epithelial, fibrosis, JNK
INTRODUCTION
Fibrotic airway remodeling is a manifestation of multiple chronic respiratory diseases that include idiopathic pulmonary fibrosis (IPF), asthma, chronic obstructive pulmonary disease (COPD), and bronchiolitis obliterans (34, 46). Fibrotic airway remodeling correlates with disease severity and is characterized by basement membrane thickening and excessive deposition of fibrotic extracellular matrix (ECM) proteins such as collagen, fibronectin, and laminin (21, 26, 46). Normal ECM provides mechanical and biochemical signals that direct multiple fundamental processes such as cell shape, cytoskeletal organization, differentiation, proliferation, migration, and production of ECM proteins (9–11, 57, 64). Growing research demonstrates that abnormal ECM can critically alter these processes and can contribute to fibrosis, tumorigenesis, and other pathologic processes (7). Most current knowledge addresses effects of abnormal ECM on behavior of fibroblasts. However, an increasing number of studies suggest pathophysiologic effects of abnormal ECM on other cell types including epithelial cells, endothelial cells, pericytes, mesenchymal stromal cells, and fibrocytes (20, 24, 25, 31, 36, 50, 70). Notably, injury-induced epithelial cell apoptosis (44, 53) and/or loss of reparative airway and alveolar epithelial progenitor function (38) with resultant lack of normal restitution of epithelial barriers (4) are increasingly recognized as critical events in the pathogenesis of both airway and also parenchymal fibrosis in diseases such as IPF. Some potential mechanisms of abnormal lung epithelial cell-ECM interactions have been elucidated. For example, epithelial-derived sonic hedgehog signals have been shown to actively repress proliferation of mesenchymal cells (43). Furthermore, genetic deletion of type I collagen within lung epithelial cells was shown to attenuate lung fibrosis (68). However, despite this, the full range of ECM-epithelial cell interactions in development of lung fibrosis, particularly airway fibrosis, remains unknown.
Basal cells consist of a heterogeneous multipotent stem cell population of the pseudostratified airway epithelium (35, 48). They help maintain the homeostasis of the normal airway epithelial layer by giving rise to differentiated airway epithelial cells and contribute to regeneration of epithelia in response to injury, based on their capacity to proliferate and differentiate into several different mature airway epithelial cell types including ciliated, nonciliated, and mucin-producing goblet cells (61). Airway basal cell dysfunction, particularly in response to cigarette smoke inhalation, is speculated to contribute to a number of pathologies, including COPD and lung cancers (47, 51). Upon damage to airway epithelia, basal cells show increased reactivity of p63 (29). Epithelial cells overlying fibroblastic foci in lungs from patients with IPF contain a layer of p63-positive basal cells, while lacking ciliated and goblet cells (29), suggesting a role for basal cells in epithelial dysregulation in IPF (66).
Epithelial-mesenchymal transition (EMT) of airway and/or epithelial cells, marked by loss of epithelial markers and acquisition of a mesenchymal phenotype, has been reported in epithelial cells from persons with asthma (17). In patients with COPD and interstitial lung disease, double staining of cells by both epithelial and mesenchymal markers in epithelium has been reported (19, 54), suggesting a role of EMT in these diseases. These findings, together with the results of lineage-tracing studies in mouse models (13, 33, 56), were the main reasons that EMT was considered to contribute to pulmonary fibrosis. However, reports questioned the contribution of EMT and indicate that few if any (myo)fibroblasts appearing during fibrogenesis are derived from epithelial cells (47, 67). Currently, it is believed that mesenchymal activation of epithelial cells (reflecting partial or reversible EMT events) may be particularly relevant in wound repair and fibrosis (8).
Transforming growth factor-β1 (TGF-β1) plays a dominant role in the pathogenesis of lung fibrosis. Previous work from our laboratory has demonstrated that c-Jun NH2-terminal kinase-1 (JNK1), a member of the family of mitogen-activated protein kinases, plays a critical role in TGF-β1- or β-catenin-induced EMT of basal cells (1, 58, 59) and in epithelial apoptosis associated with oxidative stress (39, 41, 52). Mice that globally lack the Jnk1 gene were protected from developing lung fibrosis and peribronchiolar fibrotic remodeling following administration of TGF-β1 or bleomycin or using the ovalbumin or house dust mite models of allergic airway disease (2, 58, 60). These data suggest a complex role of JNK1 in regulating epithelial cell plasticity and airway remodeling (40).
One of the highest JNK1-dependent genes induced by TGF-β1 in airway basal cells isolated from mouse tracheas (MTB cells) was the ECM protein fibronectin (1), suggesting a role for JNK1 in production of provisional matrix by epithelial cells. The goal of the present study was therefore to obtain further insights into the extent of provisional ECM production by TGF-β1-activated MTB cells and to determine the role of JNK1 in regulating the plasticity of epithelial cells residing on these epithelial-derived provisional matrices.
MATERIALS AND METHODS
All chemicals utilized were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Antibodies utilized were as follows: JNK and phospho-JNK antibodies were obtained from Cell Signaling Technology (Danvers, MA), actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), antibodies for keratin 5, pro-surfactant protein C (SP-C), p63, fibronectin, and laminin subunit-β3 (LamB3) were obtained from Abcam (Cambridge, MA), club cell 10-kDa secretory protein (CCSP) was a kind gift from Dr. B. Stripp (Cedars-Sinai Medical Center, Los Angeles, CA), and zonula occludens-1 (ZO-1) was obtained from Life Technologies (Grand Island, NY). Keratin 5-blocking antibody was obtained from MyBioSource (San Diego, CA), and p63-blocking peptide was obtained from Abcam. Rho kinase inhibitors Y27632 and fasudil and activin receptor-like kinase-5 (ALK5) inhibitor SB431542 were purchased from Abcam. Specific inhibitor of mothers against decapentaplegic homolog 3 (Smad3; SIS3) was purchased from Sigma-Aldrich.
Animals and induction of lung injury.
Bleomycin (5 U/kg body wt) was administered oropharyngeally in 2–4-mo-old C57BL/6 mice, as described previously (2, 3). PBS was administered as a respective control. Mice were housed with a 12:12-h light-dark cycle and allowed free access to standard laboratory chow and water. The Institutional Animal Care and Use Committee at the University of Vermont approved all animal studies.
Isolation and culture of basal cells from mouse tracheas.
MTB cells were isolated from the tracheas of wild-type (WT) or Jnk1−/− mice, cultured according to previously published methods (1, 65), and tested for mycoplasma contamination. In select experiments, human small airway epithelial cells (SAEC; 45) were grown and maintained in DMEM/F-12 media containing 10% FBS, with penicillin (50 U/ml), streptomycin (100 μg/ml), hydrocortisone (100 μg/ml), insulin (2.5 μg/ml), transferrin (2.5 μg/ml), and selenium (2.5 μg/ml).
Homogenization of cell and lung tissue and Western blot analysis.
Protein lysates were prepared by harvesting cells or mincing recellularized lung tissue in cold lysis buffer immediately followed by homogenization as previously described (2, 58). Lysates were incubated on ice for 30 min, followed by 30 min of centrifugation at 16,000 g. A portion of the supernatant was saved for protein determination, before the addition of Laemmli sample buffer. Total protein was assessed with the Bio-Rad DC Protein Assay kit (Bio-Rad). Total JNK1/2, phospho-JNK1/2, keratin 5, p63, α-smooth muscle actin (α-SMA), fibronectin, LamB3, and β-actin protein abundance were evaluated by Western blot analysis. Two dominant bands of JNK immunoreactivity are detected in Western blots, each of which contain both JNK1 and JNK2, which are not fully resolved using conventional Western blot analysis procedures. The top band dominantly contains JNK2 whereas the lower band dominantly contains JNK1, as is indicated in the figures. Antibody validation for species and application was performed within our laboratory or from the respective manufacturer’s website.
Cell seeding, confocal microscopy, and morphological imaging.
WT or Jnk1−/− MTB cells were seeded in eight-well glass chamber slides or onto decellularized normal and fibrotic murine tissue scaffolds (50,000 cells per well) and grown until confluent. At the conclusion of each study, the chamber slides or seeded lungs were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in 1% bovine serum albumin (BSA) in PBS for 20 min. Slides were washed two times with 1% BSA-PBS solution, blocked with 10% goat serum for 1 h, and then subjected to staining with commercially available antibodies against keratin 5, p63, ZO-1, CCSP, or α-SMA. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 10 μg/ml in 1% BSA/PBS). Images were taken on a Zeiss LSM 510 META laser scanning confocal microscope (Zeiss, Jena, Germany). Quantitation of CCSP and α-SMA signal intensities was done using MetaMorph version 5.0. Keratin 5 and p63 antibody specificity was evaluated with blocking peptides, and CCSP and α-SMA specificity was demonstrated by staining murine lung. Bright-field images were assessed by standard light microscopy.
Gene expression.
Total RNA was isolated from mouse lungs, reseeded MTB cells, and reseeded mouse and human lungs using the RNeasy Mini Kit (Qiagen, Valencia, CA) as directed by the manufacturer. Isolated RNA was subjected to reverse transcription and DNase treatment to produce cDNA for Taqman gene analysis using SYBR Green. PCR data were analyzed using the comparative cycle threshold (ΔΔCt) method of relative quantification. Primer sequences were taken from GenBank. All accession numbers are listed in Table 1.
Table 1.
Primer sequences used in real-time PCR analyses
| Gene | Accession No. | Direction | Sequences (5′ → 3′) | Amplicon, bp |
|---|---|---|---|---|
| Acta2 (α-SMA) | NM_007392.2 | Forward | CTGACAGAGGCACCACTGAA | 160 |
| Reverse | CATCTCCAGAGTCCAGCACA | |||
| S100a4 (S100 calcium-binding protein A4) | NM_011311.1 | Forward | CTGGGGAAAAGGACAGATGA | 109 |
| Reverse | TGCAGGACAGGAAGACACAG | |||
| Cdh1 (E-cadherin) | NM_009864.2 | Forward | AGCCATTGCCAAGTACATCC | 133 |
| Reverse | AAAGACCGGCTGGGTAAACT | |||
| Col1a1 [collagen-α1(I)] | NM_007742.3 | Forward | GAGCGGAGAGTACTGGATCG | 103 |
| Reverse | GTTCGGGCTGATGTACCAGT | |||
| Fn1 (fibronectin 1) | NM_010233 | Forward | GTGTAGCACAACTTCCAATTACGAA | 90 |
| Reverse | GGAATTTCCGCCTCGAGTCT | |||
| Vim (vimentin) | NM_011701.4 | Forward | TGAAGGAAGAGATGGCTCGT | 100 |
| Reverse | TCCAGCAGCTTCCTGTAGGT | |||
| Scgb1a1 (CCSP) | NM_011681.2 | Forward | ATCTGCCCAGGATTTCTTCA | 150 |
| Reverse | CTCTTGTGGGAGGGTATCCA | |||
| Tgfb1 (TGF-β1) | NM_011577 | Forward | TGCTTCAGCTCCACAGAGAA | 182 |
| Reverse | TGGTTGTAGAGGGCAAGGAC | |||
| Tjp1 (ZO-1) | NM_009386.2 | Forward | CCACCTCTGTCCAGCTCTTC | 249 |
| Reverse | CACCGGAGTGATGGTTTTCT | |||
| Sftpc (SP-C) | NM_011359.2 | Forward | CAGCTCCAGGAACCTACTGC | 121 |
| Reverse | AGCTTAGAGGTGGGTGTGGA |
CCSP, club cell 10-kDa secretory protein; SP-C, surfactant protein C; ZO-1, zonula occludens-1.
Preparation of decellularized conditional matrices and mass spectrometry.
MTB cells were plated on culture dishes and treated with vehicle or TGF-β1 (5 ng/ml; Roche, Indianapolis, IN) every 48 h, for a total duration of 1 wk. Cells were subsequently washed three times with PBS and incubated with 20 mM NH4OH for 3–5 min. Dishes were viewed under a microscope to ensure full cellular detachment. Dishes were washed three times with PBS and one time with double-distilled water before incubation with a collagenase-dispase solution (100 mg/ml; Roche) for 15 min at 37°C. The reaction was stopped, and samples were centrifuged for 5 min at 500 g. The supernatant was collected, and protein concentration was determined by bicinchoninic acid assay (Bio-Rad). For mass spectrometry analysis, 50 μg of protein were acetone precipitated, and the pellet was dried and solubilized in 20 mM HEPES and 2 M urea, pH 8.0. The samples were reduced using 4.5 mM DTT at 55°C for 30 min. Alkylation was done using 10 mM iodoacetamide for 15 min at room temperature in the dark. To digest the ECM proteins, trypsin at an enzyme-to-substrate ratio of 1:100 (Roche) was added, and the samples were incubated overnight (12–16 h) at 37°C. Trypsin activity was quenched with 1% trifluoroacetic acid. Samples were then centrifuged at 14,000 g, and 100 μl of supernatant were desalted using a C18 ZipTip (P10; Millipore) according to the manufacturer’s protocol. The ZipTip eluate was then dried again and then reconstituted in 20 μl 0.1% formic acid and 2% acetonitrile. Five microliters of each digest were loaded directly onto a 100 μm × 120 mm fused silica microcapillary column packed with Magic C18 (5-μm particle size, 20-nm pore size; Michrom Bioresources) at a flow rate of 500 nl/min, and peptides were separated by a gradient composed of 3–35% acetonitrile-0.1% formic acid in 45 min. The peptides were introduced into the LTQ mass spectrometer via a nanospray ionization source and a laser-pulled ~3-μm orifice with a spray voltage of 1.8 kV. Mass spectrometry data were acquired in a data-dependent “Top 10” acquisition mode, in which a survey scan from mass-to-charge ratio (m/z) 400 to 2,000 was paralleled by 10 collision-induced dissociation tandem mass spectrometry scans of the 10 most abundant ions in the LTQ. Tandem mass spectrometry scans were acquired with the following parameters: isolation width, 2 m/z; normalized collision energy, 35%; activation Q, 0.250; activation time, 30 ms. Dynamic exclusion was enabled (repeat count, 2; repeat duration, 30 s; exclusion list size, 500; exclusion duration, 60 s). The minimum threshold was 500.
Product ion spectra were searched against the mouse subset of the UniProt database (000000589; 53,289 entries; http://www.uniprot.org/proteomes/) containing sequences in forward and reverse orientations using the SEQUEST search engine embedded in Proteome Discoverer 1.4 (Thermo Fisher Scientific). Search parameters were as follows: fully enzymatic activity and two missed cleavage sites allowed for trypsin; peptide molecular mass of 350–5,000 Da; mass tolerance of 2 and 0.8 Da for precursor and fragment ions, respectively; four differential posttranslational modifications allowed per peptide; and dynamic modification on methionine (+15.9949 Da for oxidized methionine) and static modification on cysteine (+57.0215 Da for carbamidomethylated cysteine). Search results were further analyzed using Scaffold 4 (Proteome Software) to compare the distinct peptide counts with respect to specific protein isoforms/clusters. The following filtering criteria were used to achieve a 0% decoy false discovery rate at the peptide and protein level in the filtered data set: cross-correlation (XCorr) = 2.0 (1+), 2.7 (2+), 3.4 (3+), delta correlation (Delta Cn) >0.1, and “min # of peptide” = 2. The “total distinct peptide counts” of proteins identified from multiple replicates are presented in Supplemental Table S1 (Supplemental Material for this article is available online at the American Journal of Physiology-Lung Cellular and Molecular Physiology website).
Preparation of decellularized normal and fibrotic murine tissue scaffolds.
Normal (PBS-instilled) or fibrotic (bleomycin-instilled) WT mouse lungs were extracted at day 14 and decellularized as previously described (55). Following decellularization, the right lobes were ligated at the bronchus using thread, and 3% low-melting temperature SeaPrep Agarose (Cambrex) in PBS was instilled into the left lobe. The agarose-instilled left lobe was allowed to solidify at 4°C for 20 min and was subsequently sliced into ~1-mm-thickness slices before recellularization.
Preparation of decellularized normal and IPF human tissue scaffolds.
Normal cadaveric human lung lobes from two patients were obtained from the autopsy services at the University of Vermont Medical Center. Surgically explanted lungs obtained from patients with IPF were procured from Washington University in St. Louis, courtesy of David Hoganson (n = 2). Lungs were categorized as normal or diseased based on review of available clinical records, including known history of lung disease, smoking history, chest radiographs, lung function tests, and use of respiratory medications. Normal and IPF human lungs were decellularized as previously described (62), and IPF lung segments for this study underwent a subsequent decellularization step. We have previously described that using a protocol that is sufficient to decellularize both normal and COPD origin human lung lobes results in inhomogeneous decellularization of IPF lobes (62, 63). Therefore, following whole lobar perfusion decellularization [as described by Wagner et al. (62, 63)], we resected segments of ~3–5 cm3 from different regions of IPF lungs (subjectively mild and severe regions of fibrosis based on stiffness to palpation and morphological assessment) and individually exposed them to a second decellularization protocol consisting of the same sequence, timing, and duration (at least 4 segments per patient). All incubation steps took place under rocking conditions, and all deionized (DI) water rinse steps consisted of 5 × 5-min rinses in DI water containing 1X penicillin-streptomycin. Segments were first submerged in 0.1% Triton X-100 for 24 h and incubated at 4°C. Individual segments were then rinsed in DI water and incubated, submerged, overnight in 2% sodium deoxycholate solution at 4°C. After 24 h, samples were then washed in DI water and then incubated submerged in 1 M NaCl solution for 1 h at room temperature (~25°C). Segments were then rinsed with DI water followed by incubation with 30 μg/ml porcine pancreatic DNase (Sigma) containing 2 mM CaCl2 (Sigma) and 1.3 mM MgSO4 (Sigma) and 1X penicillin-streptomycin in DI water submerged for 1 h at room temperature. The segments were then rinsed with DI water and terminally sterilized using a 0.1% peracetic acid (Sigma) in 4% ethanol rinse for 60 min at room temperature. To remove the peracetic acid, all segments were then rinsed with a storage solution [1X PBS, 5X penicillin-streptomycin, 50 mg/l gentamicin (Cellgro), and 2.5 µg/ml amphotericin B (Cellgro)] 5 × 5 min and stored for up to 3 wk. Confirmation of decellularization was assessed histologically using hematoxylin and eosin staining of mounted 5-µm sections of 4% paraformaldehyde, paraffin-embedded decellularized lung samples. Prior to recellularization studies, individual thin slices were generated from both normal and IPF segments using a sterile razor blade. Slices of ~1-mm thickness were prepared from different regions of the same patient.
Statistical analysis.
Data were evaluated by one-way ANOVA using Tukey’s test to adjust for multiple comparisons. Results with a P value <0.05 or smaller were considered statistically significant. Heat maps and statistical analyses of mass spectrometry proteomics following in vitro decellularization of ECM produced with or without TGF-β1 stimulation in WT MTB cells were conducted as previously described (55, 62) using the nonparametric exact permutation test with P < 0.05 considered statistically significant. Heat maps utilized log-transformed data for visualization purposes, whereas in statistical analyses only individual samples containing two or more unique peptides for a given protein were used (i.e., samples containing only 1 unique peptide hit for a given protein were set to 0). The nonparametric test was used because of the anticipated nonnormality of the data and small sample sizes per group. Nonparametric exact permutation tests were conducted using SPSS version 19.0.
RESULTS
Characterization of WT and Jnk1−/− MTB cells.
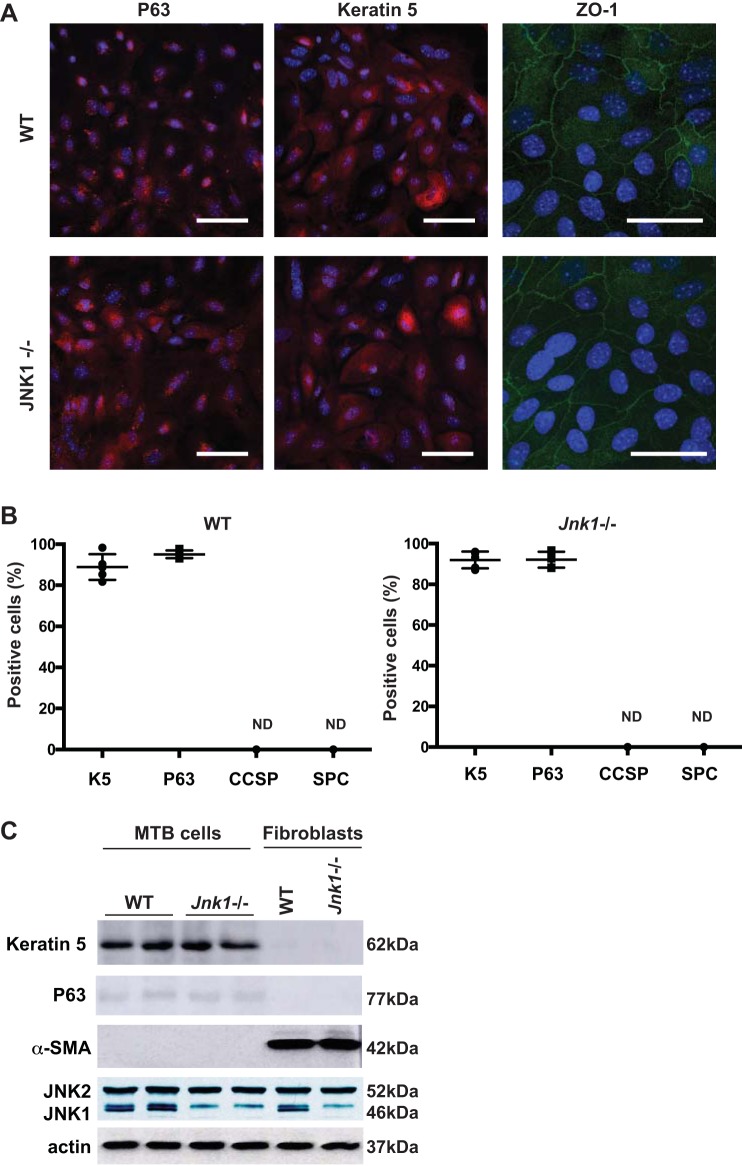
Classically, fibroblasts and myofibroblasts are considered the main producers of ECM components in diseased lung; however, little is known about the contribution and impact of epithelial cells in ECM production. We have previously demonstrated that epithelial JNK1-mediated cell signaling plays a significant role in TGF-β1- and canonical Wnt-induced mesenchymal changes in airway epithelial cells using MTB cells as an airway epithelial model (59). MTB cells from WT or Jnk1−/− mice robustly express the basal cell markers keratin 5 and p63 and the tight junction protein ZO-1. MTB cells were found to be negative for airway CCSP, SP-C, and α-SMA, in contrast to mouse lung fibroblasts, which did not express keratin 5 or p63 but robustly expressed α-SMA (Fig. 1, A–C).
Fig. 1.
Characterization of basal cells isolated from mouse tracheas (MTB). A: wild-type (WT) and Jnk1−/− MTB cells were isolated and propagated on rat tail collagen gels and passaged onto dishes coated with type I collagen for assessment of keratin 5, p63, club cell 10-kDa secretory protein (CCSP), surfactant protein C (SP-C), and zonula occludens-1 (ZO-1) via confocal microscopy (magnification, ×40 for keratin 5 and p63 and ×63 for ZO-1; scale bars: 50 μm). B: quantification of immunoreactivity of the indicated markers following counting of 300 cells from 5 independent slides. Scatterplot data represent each individual slide (means ± SE). K5, keratin 5; ND, not determined. C: Western blot analysis of keratin 5, p63, α-smooth muscle actin (α-SMA), JNK1/2, and actin as loading control in WT or Jnk1−/− MTB cells or in lung fibroblasts as a control. Blots are from one representative experiment of three independent experiments.
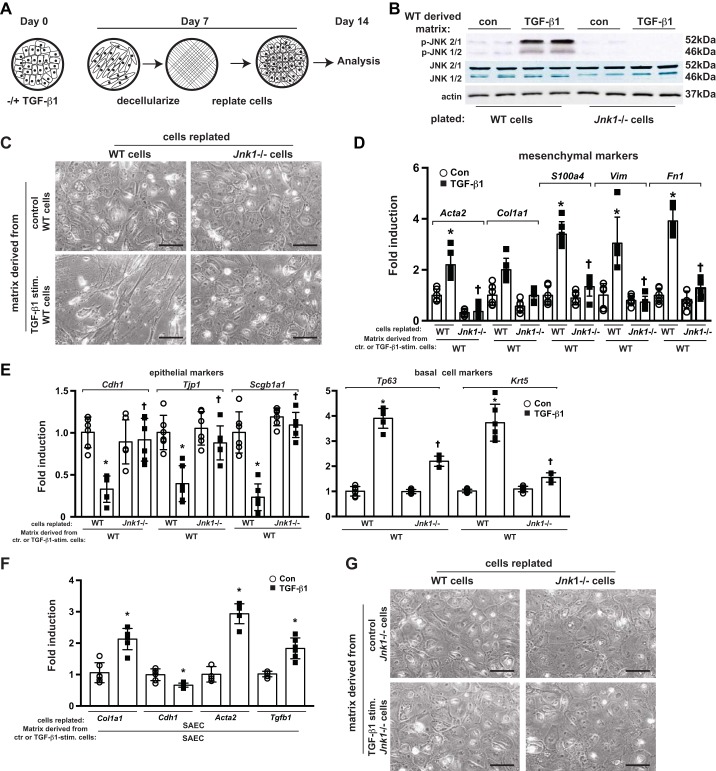
Provisional matrix deposition derived from TGF-β1-stimulated MTB cells promotes a mesenchymal phenotype in subsequently plated WT (but not Jnk1−/−) MTB cells.
To determine TGF-β1’s effects on MTB matrix production, WT MTB cells were cultured with TGF-β1 or vehicle. One-week poststimulation, in situ decellularization was performed leaving the putative secreted matrix on the tissue culture plates. WT or Jnk1−/− MTB cells were then plated onto the provisional ECM (Fig. 2A) and subsequently evaluated for morphologic appearance, JNK phosphorylation, and epithelial and mesenchymal gene expression profiles. WT or Jnk1−/− MTB cells plated onto ECM derived from unstimulated WT MTB cells did not display JNK activation and displayed normal epithelial morphology (Fig. 2, B and C). WT MTB cells plated onto the ECM produced by WT TGF-β1-stimulated MTB cells showed strong increases in phospho-JNK immunoreactivity (Fig. 2B) and displayed a spindlelike, fibroblastic phenotype (Fig. 2C), increased expression of mesenchymal and basal cell markers, and decreases in expression of Cdh1, Tjp1, and Scgb1a1 genes (Fig. 2, D–F). Absence of Jnk1 almost completely protected MTB cells plated onto ECM derived from TGF-β1-stimulated WT cells, based on retention of normal epithelial morphology, maintenance of robust expression of Cdh1, Tjp1, and Scgb1a1 genes, and attenuated expression of mesenchymal and basal cell genes (Fig. 2, D and E). The complete absence of JNK phosphorylation in Jnk1−/− MTB cells plated on an ECM from TGF-β1-stimulated WT MTB cells (Fig. 2B) demonstrates that the JNK phosphoimmunoreactivity observed in WT cells is largely attributable to JNK1, and not JNK2, and is consistent with our previous studies demonstrating that JNK2 does not play a role in TGF-β1-induced EMT (1). Similar changes were also observed with ECM produced by human SAEC exposed to TGF-β1, which induced mesenchymal genes in subsequently plated SAEC (Fig. 2F). In agreement with our previous observations that Jnk1−/− MTB cells are largely resistant to TGF-β1-induced EMT (10), the ECM derived from Jnk1−/− MTB cells stimulated with TGF-β1 failed to induce a mesenchymal phenotype in subsequently plated WT or Jnk1−/− MTB cells (Fig. 2G).
Fig. 2.
A provisional ECM deposited by mouse tracheal basal (MTB) cells stimulated with active TGF-β1 induces a mesenchymal phenotype in subsequently plated MTB cells, in a JNK1-dependent manner. A: schematic of the experimental design. B, C, and G: assessment of phospho-JNK (p-JNK) expression by Western blot analysis (B) and of morphology (C and G) of wild-type (WT) or Jnk1−/− cells plated onto a provisional matrix produced by WT (C) or Jnk1−/− (G) cells stimulated with TGF-β1 or vehicle control. D and E: assessment of mesenchymal (D) and epithelial (E) gene expression in WT or Jnk1−/− cells plated onto a provisional matrix produced by WT MTB cells stimulated with TGF-β1. F: mRNA expression of human small airway epithelial cells (SAEC) plated onto a provisional matrix produced by SAEC stimulated with TGF-β1 for 1 wk. Results were normalized to the housekeeping gene cyclophilin compared with WT MTB cells plated onto collagen I. For scatterplot data, experiments were repeated three times with an n of 3 for each experimental condition. Data are representative of two independent experiments containing three biological replicates each and are shown as means ± SE. Scale bars: 50 μm. Con, control; ctr., control; stim., stimulated. *P < 0.05 compared with control groups, †P < 0.05 compared with respective WT groups (ANOVA).
These results demonstrate that TGF-β1 stimulation of basal cells and SAEC results in a provisional ECM that promotes a JNK-dependent epithelial-to-mesenchymal-like switch based on cell morphology and alterations in epithelial and mesenchymal mRNA signatures in subsequently plated epithelial cells.
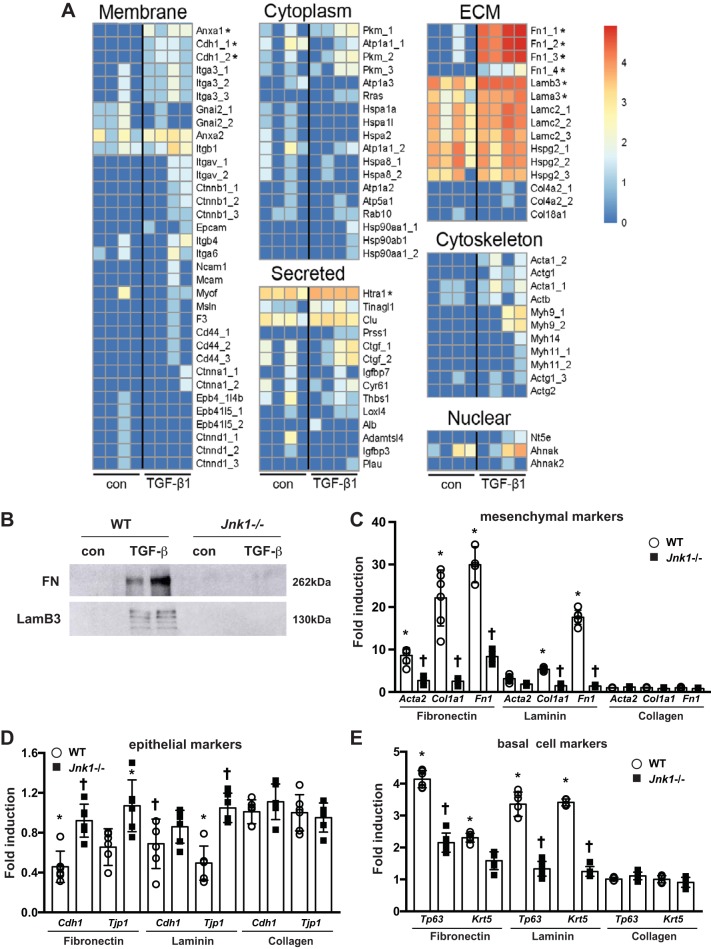
Characterization of provisional ECM from TGF-β1-stimulated MTB cells via mass spectrometry proteomics.
To further evaluate protein composition of the deposited matrix, mass spectrometry proteomics assessment demonstrated significant increases in the ECM proteins fibronectin and laminin (subunits-α3 and -β3), the membrane proteins annexin A1 and cadherin, and the secreted protease high-temperature requirement serine protease A1 (Htra1; Fig. 3A and Supplemental Table S1) from cells stimulated with TGF-β1 for 1 wk compared with vehicle controls. Western blot analysis of the deposited matrix following decellularization confirmed robust expression of both fibronectin and LamB3 in TGF-β1-induced ECM from WT MTB cells compared with control and showed that these increases were not observed in ECM from Jnk1−/− cells stimulated with TGF-β1 (Fig. 3B). Based on the observation that TGF-β1-stimulated MTB cells secreted significant amounts of fibronectin and laminin and to exclude potential effects of other residual proteins following in situ decellularization, WT MTB cells were directly plated onto fibronectin- or laminin-coated tissue culture plates. MTB cells plated on these ECM proteins showed marked decreases in expression of Cdh1 and Tjp1, and increases in mesenchymal and basal cell-specific markers, compared with cells plated on collagen I. MTB cells seeded onto collagen I showed preservation of epithelial markers and attenuated expression of basal cell-specific and mesenchymal genes. In contrast, Jnk1−/− MTB cells plated onto fibronectin or laminin preserved expression of Cdh1, and Tjp1, and largely failed to induce mesenchymal and basal cell-specific genes (Fig. 3, C–E).
Fig. 3.
Characterization of provisional ECM from TGF-β1-stimulated mouse tracheal basal (MTB) cells via mass spectrometry proteomics. A: heat maps of ECM from control wild-type (WT) MTB cells (con) or WT MTB cells stimulated with TGF-β1 for 1 wk. *P < 0.05 compared with control cells. B: Western blot analysis of fibronectin (FN) and laminin subunit-β3 (LamB3) in the ECM following in situ decellularization. C–E: assessment of mesenchymal (C), epithelial (D), and basal cell-specific (E) gene expression in WT or Jnk1−/− MTB cells plated onto fibronectin, laminin, or collagen I. Results were normalized to the housekeeping gene cyclophilin compared with WT MTB cells plated onto collagen I. Blots are from one representative experiment of three independent experiments. For scatterplot data, experiments were repeated three times with an n of 3 for each experimental condition. Data are representative of two independent experiments containing three biological replicates each and are shown as means ± SE. Act, actin; Adamtsl, a disintegrin and metalloproteinase with thrombospondin motifs-like protein; Ahnak, AHNAK nucleoprotein (desmoyokin); Alb, albumin; Anxa, annexin A; Atp, Na+/K+ ATPase; Cd44, CD44 antigen; Cdh, E-cadherin; Clu, clusterin; Col, collagen; Ctgf, connective tissue growth factor; Ctnna, Ctnnb, and Ctnnd, catenin-α, -β, and -δ, respectively; Cyr, cysteine-rich angiogenic inducer; Epb, erythrocyte membrane protein; Epcam, epithelial cell adhesion molecule; F3, tissue factor; Gnai, guanine nucleotide-binding protein; Hsp, heat shock protein; Hspa, heat shock 70-kDa protein; Hspg, basement membrane-specific heparan sulfate proteoglycan core protein; Htra1, high-temperature requirement serine protease A1; Igfbp, insulin-like growth factor-binding protein; Itga and Itgb, integrin-α and -β, respectively; Itgav, integrin-α-V; Loxl, lysyl oxidase homolog; Mcam, melanoma cell adhesion molecule; Myh, myosin; Myof, myoferlin; Msin, stress-activated MAPK-interacting protein; Ncam, neural cell adhesion molecule; Nt5e, 5′-nucleotidase; Pkm, pyruvate kinase PKM; Plau, urokinase-type plasminogen activator; Prss, trypsin; Rab, Ras-related protein Rab; Rras, Ras-related protein R-Ras; Thbs, thrombospondin; Tinagl, tubulointerstitial nephritis antigen-like. *P < 0.05 compared with control groups, †P < 0.05 compared with respective WT groups (ANOVA).
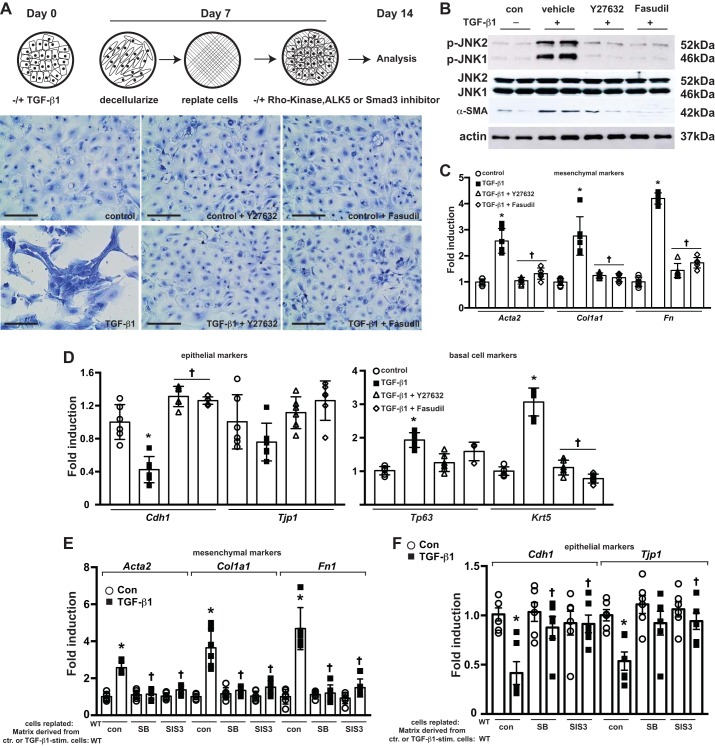
Contribution of Rho kinase and TGF-β1/Smad3 signaling pathway in EMT of WT MTB cells plated onto provisional ECM.
We next sought to determine potential upstream activators of JNK in this model. Rho kinase (ROCK) plays a prominent role in mechanotransduction and activation of myofibroblasts and lung fibrosis (71) and has been previously shown to regulate the activation of JNK (40). It remains unknown whether ROCK plays a role in the activation of epithelial JNK and contributes to the loss of an epithelial signature and acquisition of mesenchymal characteristics of epithelial cells. To address the role of ROCK, WT MTB cells were replated onto the provisional ECM derived from TGF-β1-stimulated WT MTB cells in the presence of the ROCK inhibitors Y27632 or fasudil. Both ROCK inhibitors attenuated phospho-JNK (Fig. 4B) and preserved cell morphology compared with cells treated with vehicle control (Fig. 4A). Inhibition of ROCK preserved expression of Cdh1 and Tjp1 and attenuated the expression of mesenchymal and basal cell-specific genes (Fig. 4, C and D) and α-SMA protein compared with the vehicle control group (Fig. 4B). These findings suggest that ROCK promotes the activation of JNK and the subsequent acquiescence of a mesenchymal signature in MTB cells plated onto a provisional matrix produced by TGF-β1-stimulated MTB cells. Because of the documented importance of the TGF-β1/Smad3 signaling pathway in EMT and the role of Smad3 in modulating RhoA activation in TGF-β1-induced EMT (37), we next evaluated the roles of TGF-β1 receptor kinase (ALK5) and Smad3 in gene expression changes in WT MTB cells plated onto the provisional ECM from TGF-β1-stimulated WT MTB cells. The ALK5 inhibitor SB431542 and the Smad3 inhibitor SIS3 both attenuated mesenchymal gene expression and preserved expression of Cdh1 and Tjp1 in WT MTB cells plated on TGF-β1-induced ECM (Fig. 4, E and F), suggesting a role of TGF-β1/Smad3 signaling axes in the observed EMT of WT MTB cells on a TGF-β1-induced provisional matrix.
Fig. 4.
Contribution of Rho kinase (ROCK) to JNK phosphorylation, the loss of epithelial morphology, and epithelial gene expression profiles in wild-type (WT) mouse tracheal basal (MTB) cells plated onto provisional ECM derived from TGF-β1-stimulated WT MTB cells. A, top: schematic of the experimental design. MTB cells seeded onto matrix derived from control or TGF-β-treated MTB cells were treated with ROCK inhibitors fasudil (10 μM) or Y27632 (10 μM) for 7 days before the assessment of cell morphology (A, bottom), phospho-JNK (p-JNK) and α-smooth muscle actin (α-SMA; B), and mesenchymal (C) and epithelial mRNA profiles (D). E and F: contribution of activin receptor-like kinase-5 (ALK5) and mothers against decapentaplegic homolog 3 (Smad3) to altered epithelial or mesenchymal gene expression profiles in MTB cells plated onto provisional ECM derived from TGF-β1-stimulated WT MTB cells. Reseeded MTB cells were treated with the ALK5 inhibitor SB431542 (SB; 10 μM) or the Smad3 inhibitor SIS3 (5 μM) for 7 days before examination of mesenchymal (E) and epithelial mRNA (F) profiles. For scatterplot data, experiments were repeated three times with an n of 3 for each experimental condition. Data are representative of two independent experiments containing three biological replicates each and are shown as means ± SE. Scale bars: 50 μm. Con, control; ctr., control; stim., stimulated. *P < 0.05 compared with control groups, †P < 0.05 compared with respective WT groups (ANOVA).
JNK1-dependent preservation of an epithelial signature in MTB cells plated on fibrotic lung scaffolds.
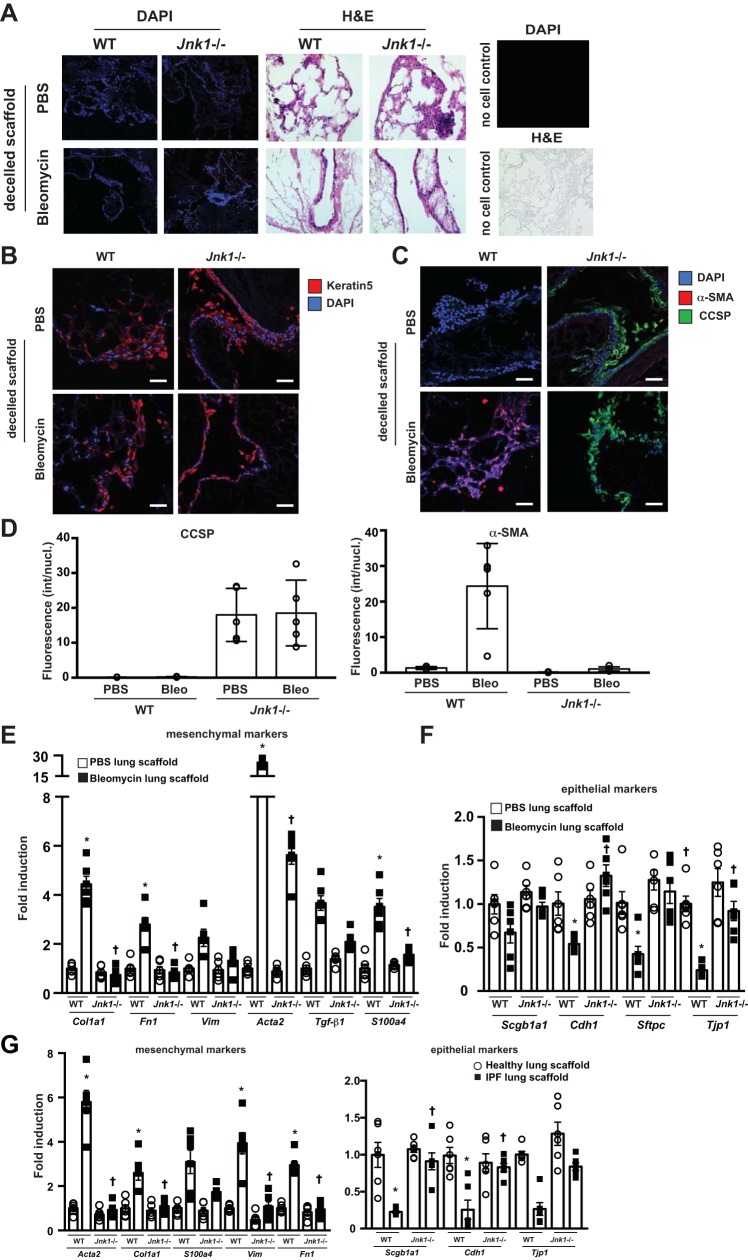
To better understand the impact of ablation of Jnk1 for the regeneration of epithelia in the setting of lung fibrosis, decellularized lung tissue scaffolds were prepared from mice with bleomycin-induced lung fibrosis and from corresponding PBS-treated controls and WT or Jnk1−/− MTB cells seeded onto these tissue scaffolds. Decellularized scaffolds produced from emphysematous or fibrotic lungs have been shown to retain disease phenotypes (5, 42, 55, 63) and promote increases of α-SMA and collagen expression in fibroblasts reseeded into acellular fibrotic human lung scaffolds (5). WT or Jnk1−/− MTB cells seeded onto decellularized scaffolds prepared from normal murine lungs demonstrated a marked and comparable reepithelialization with both cell types over a time frame of 28 days (Fig. 5A) with >95% of MTB cells retaining expression of keratin 5 over this time period (Fig. 5B). In accordance, WT cells seeded onto normal acellular lung scaffolds displayed robust epithelial and low mesenchymal mRNA expression profiles (Fig. 5E). In comparison, WT MTB cells seeded onto decellularized tissue scaffolds prepared from mice with bleomycin-induced fibrosis demonstrated increases in α-SMA (Fig. 5, C and D). Consistent with these observations, decreased epithelial and increased mesenchymal gene expression was observed (Fig. 5, E and F).
Fig. 5.
Genetic ablation of Jnk1 preserves expression of epithelial markers and epithelial differentiation of mouse tracheal basal (MTB) cells plated onto decellularized (decelled) fibrotic lung scaffolds. A, left and middle: recellularization of acellular lung tissue scaffolds prepared from control (PBS-treated) lungs or lungs with fibrotic lung disease induced by bleomycin. Wild-type (WT) and Jnk1−/− MTB cells were cultured for 28 days. Tissues were stained with 4′,6-diamidino-2-phenylindole (DAPI) or hematoxylin and eosin (H&E). A, right: confirmation of the lack of cells in tissue scaffolds. B and C: lung tissues from mice with bleomycin-induced fibrosis or control tissues were decellularized. Lung scaffolds were seeded with WT or Jnk1−/− MTB cells. Twenty-eight days thereafter, scaffolds were harvested for assessment of keratin 5 (B) and club cell 10-kDa secretory protein (CCSP) and α-smooth muscle actin (α-SMA; C), as assessed via confocal microscopy. D: quantification of CCSP and α-SMA fluorescence intensity using MetaMorph software (Molecular Devices, Sunnyvale, CA). Bleo, bleomycin; int/nucl, intensity per nucleus. E and F: impact of ablation of Jnk1 on mesenchymal (E) or epithelial (F) expression profiles in MTB cells plated onto decellularized matrix derived from lungs obtained from control mice or mice with bleomycin-induced fibrosis. Results were normalized to the housekeeping gene cyclophilin and compared with WT cells plated onto control lung scaffolds. G: impact of ablation of Jnk1 on mesenchymal or epithelial expression profiles in MTB cells plated onto decellularized matrix derived from healthy human lung tissue (n = 2 with 3 slices prepared from different regions of the same patient) or lung tissue derived from patients with idiopathic pulmonary fibrosis (IPF; n = 2 with 3 slices prepared from different regions of the same patient). Data are represented as described in E and F. For scatterplot data, experiments were repeated three times with an n of 3 for each experimental condition. Data are representative of two independent experiments containing three biological replicates each and are shown as means ± SE. Scale bars: 50 μm. *P < 0.05 compared with control scaffold groups, †P < 0.05 compared with respective WT groups (ANOVA).
In contrast, Jnk1−/− MTB cells inoculated onto either control or fibrotic decellularized tissue scaffolds remained α-SMA negative and instead showed expression of CCSP (Fig. 5, C and D). Accordingly, expression of epithelial genes remained high in Jnk1−/− cells plated onto fibrotic lung tissue scaffolds, whereas virtually no increases in mesenchymal gene expression were observed. These findings were similar to WT cells plated onto scaffolds prepared from nonfibrotic control lung tissue (Fig. 5, E and F).
To corroborate these findings, WT or Jnk1−/− MTB cells were inoculated onto decellularized lung tissue scaffolds derived from lungs of two patients with IPF, three segments from each patient. Notably, WT MTB cells demonstrated loss of epithelial gene signature and enhanced expression of mesenchymal genes compared with cells plated onto scaffolds derived from healthy human lungs (Fig. 5G). In contrast, Jnk1−/− MTB cells inoculated onto IPF lung scaffolds had preserved epithelial gene expression and attenuation of mesenchymal expression (Fig. 5G). All together, these findings demonstrate that basal cells respond to a fibrotic lung scaffold with an EMT-like response and that in the absence of JNK1, an epithelial signature is maintained.
DISCUSSION
Interstitial and subepithelial airway fibrosis are complex disease manifestations involving multiple cell types including airway and alveolar epithelial cells and the underlying lung stromal cells. Although still incompletely understood, a growing number of studies suggest that aberrant repair of epithelial cells represents a major pathophysiologic process driving fibrosis (4, 30, 50, 68). Findings from the present study demonstrate that epithelial cells stimulated with TGF-β1 are a source of provisional ECM, which negatively impacts an epithelial phenotype and which promotes a mesenchymal phenotype of subsequent epithelial cells that contact this matrix. This new paradigm, wherein aberrant epithelial ECM secretion initiates acquisition of mesenchymal markers by epithelial cells, which can impede subsequent normal reepithelialization, provides a plausible scenario of chronic aberrant repair of epithelia, thought to be critical in fibrogenesis. Furthermore, Jnk1 appears to play a necessary role in inhibiting normal reepithelialization.
The extent to which and mechanisms whereby EMT contributes to fibrotic lung remodeling remain highly debated. Multiple studies demonstrated that a substantial fraction of mouse lung fibroblasts in models of lung fibrosis and allergic airway disease were derived from epithelial cells, which had undergone EMT (13, 28, 33, 56). However, several reports have challenged these findings (47, 67). The presented data do not resolve this controversial topic but suggest a mechanism in which epithelial cell-derived provisional matrix, generated from TGF-β stimulation, decreases expression of epithelial genes and induces mesenchymal genes in epithelial cells. Our findings that epithelial cell-derived ECM alters plasticity of epithelial cells are intriguing, as ECM has emerged as a critical factor that regulates the behavior of many different cell types including epithelial cells and fibroblasts (42). Primary alveolar type II epithelial cells cultured on provisional matrix components, fibronectin, or fibrin undergo robust EMT via integrin-dependent activation of endogenous latent TGF-β1 (33), which can in turn lead to JNK1 activation (58). A stiff matrix has been shown to induce EMT via an integrin-TGF-β-dependent mechanism (6). Importantly, healthy fibroblasts plated on ECM derived from lung tissues from IPF patients demonstrated gene and protein expression reminiscent of activated myofibroblasts compared with fibroblasts plated on normal lung ECM (5, 43). Findings for the present study extend these prior observations, through the demonstration that the nature of the ECM dictates plasticity of basal airway epithelial cells and potentially governs their ability to repopulate and differentiate into other epithelial lineages.
Although the mechanisms whereby epithelial JNK1 is activated by “profibrotic” ECM will require additional studies, some preliminary clues have been suggested. Notably, in fibroblasts plated on stiff fibronectin-coated substrates, αvβ1-integrins have been shown to mediate activation of a RhoA-ROCK-myosin II pathway (49) and subsequently the upregulation of α-SMA (23). Given the observed increases in fibronectin associated with the ECM from TGF-β1-stimulated MTB cells, a similar integrin-linked mechanism of RhoA-ROCK activation may be operative. Rho and ROCK have been demonstrated to play a key role in bleomycin- or FITC-induced fibrosis in association with activation of myofibroblasts (71), and ROCK was shown to promote contraction of fibroblasts isolated from COPD lungs (18). Our present results in MTB cells plated on a provisional matrix produced by MTB cells stimulated with TGF-β1 demonstrate an involvement of ROCK not only in promoting JNK activation but also in enhancing mesenchymal expression while attenuating the epithelial gene expression signature in subsequently plated MTB cells. Therefore, in addition to the previously demonstrated role of ROCK in myofibroblasts, ROCK activation in epithelial cells also may contribute to fibrogenesis by limiting reepithelialization. Interestingly, the ROCK inhibitor Y27632 has been shown to promote basal cell proliferation and maturation, without affecting differentiation, and accelerates the localization of both E-cadherin and actin at the cell junction (22). Y27632 can be used to expand basal cell cultures, important when limited cell numbers are available. Our present findings demonstrate that Y27232 or fasudil decreased Krt5 and Tp63 and increased Cdh1 and Tjp1, while attenuating mesenchymal genes in MTB cells plated on a provisional matrix derived from TGF-β-stimulated WT MTB cells. These findings suggest a role of ROCK in the regulation of basal cell plasticity in the setting of a provisional TGF-β1-activated MTB cell-derived matrix.
Previous studies have demonstrated that JNK1 augments the mesenchymal response of epithelial cells to the profibrogenic mediators TGF-β1 or Wnt3a (58, 59). JNK1 promotes phosphorylation of Smad3 in the linker domain, which in turn increases mesenchymal expression and decreases epithelial mRNAs in epithelial cells stimulated with TGF-β1 (59). Cross talk between Rho/ROCK and the TGF-β1/Smad3 pathway has been suggested in lung fibroblast-to-myofibroblast differentiation (27). Smad3 has been shown to regulate Rho signaling in TGF-β1-induced EMT via NET1, a guanine nucleotide exchange factor of RhoA (37). We demonstrate herein that inhibition of ALK5 or Smad3 attenuated mesenchymal gene expression in WT MTB cells plated on a provisional matrix derived from TGF-β1-stimulated MTB cells. Further studies will be required to unravel the exact interplay between Smad3 and Rho/ROCK and their potential roles in limiting normal reepithelialization. Further studies also will be required to determine the exact nature of ALK5 activation. No TGF-β1 was observed in the ECM derived from TGF-β1-stimulated WT MTB cells, measured via mass spectrometry (Fig. 3A) or ELISA (data not shown). Although epithelial and mesenchymal marker expression was not analyzed, stimulation of Jnk1−/− MTB cells with TGF-β1 also failed to induce a mesenchymal phenotype in subsequently plated WT MTB cells (Fig. 2G). Collectively, these findings demonstrate that observed phenotypic changes in MTB cells plated onto provisional matrices from TGF-β1-stimulated WT MTB cells are not due to residual TGF-β1.
The overall importance of epithelial cell-derived ECM for pulmonary fibrosis also will require further study. Previous findings using laser capture microdissection have demonstrated that epithelial cells are a prominent source of increases in collagen-α1(I) mRNA and other profibrogenic mediators in rodent models of fibrosis (32). Specific ablation of collagen-α1(I) from epithelial cells, using an SP-C-directed Cre driver, strongly attenuated bleomycin-induced fibrosis, demonstrating the importance of epithelial-derived collagen in the development of pulmonary fibrosis (69). The same group also reported that TGF-β1-treated epithelial cells secrete soluble mediators that promote fibroblast activation (68). However, the precise functional contribution of altered lung epithelial cell plasticity in injury/repair responses during fibrosis, either through ECM deposition, mediator release, or other processes, will require additional studies. We demonstrated herein that TGF-β1 stimulation of airway basal cells resulted in deposition of an ECM that consisted of fibronectin and laminin, among other ECM and basement membrane components, and that this process required JNK1. However, we failed to detect collagen I in the ECM of control or TGF-β1-stimulated MTB cells. Therefore, the contribution of collagen-α1(I) from epithelial cells may be regionally or context specific in lung fibrosis. The inability to detect collagen-α1(I) in our ECM will require further study but may be linked to the use of collagenase and dispase for mass spectrometric analyses or the potential lack of cross-linking of collagen due to the absence of ascorbic acid in the cell culture media.
Decellularized lung tissues are currently being investigated as scaffolds for ex vivo lung regeneration and potential clinical use. In parallel, they are valuable as novel ex vivo models to investigate mechanisms that control lung repair and regeneration, as they retain ECM characteristics of the lung from which they were derived (63). Efforts to repopulate damaged lungs using a variety of cells, including induced pluripotent stem cells, are ongoing (12, 14–16). Results herein demonstrate robust reepithelialization of mouse tissue scaffolds derived from either WT or bleomycin-injured mice using MTB cells obtained from either WT or Jnk1−/− mice. With both types of cells the majority express keratin 5 and p63, and as expected, WT cells did not express CCSP in either WT or bleomycin-injured lungs. Of interest is that a subfraction of Jnk1−/− basal cells express CCSP when seeded onto control or fibrotic tissue scaffolds, findings that suggest that absence of JNK1 might facilitate differentiation of basal cells into CCSP-expressing club cells. Recently, p63/keratin 5-positive distal airway stem cells were found to be essential for lung regeneration (61, 72). Additional characterization of the identity of the cell types or subpopulations permissive of reepithelialization and the molecular signals that govern their subsequent transdifferentiation also will require further experimentation. Similarly, the mechanism whereby ablation of JNK1 augments the process of reepithelialization on fibrotic tissue scaffolds, and subsequent transdifferentiation, also will require additional studies. Nonetheless, the findings presented herein are strongly supportive of a rationale to inhibit JNK1 within lung epithelial cells in settings wherein reepithelialization is desired. The current preclinical development of JNK inhibitors, some of which have already been tested in clinical trials, coupled to targeted delivery to lung epithelia therefore holds considerable promise for the treatment of fibrotic lung disease.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute (NHLBI) Grants R01-HL-079331, R01-HL-060014, and R35-HL-135828 (Y. M. W. Janssen-Heininger). Decellularization of mouse and human lung scaffolds was funded by NHLBI Grants R01-HL-106625 and R01-HL-108689 (D. J. Weiss). The Vermont Genetics Network Proteomics Facility is supported through National Institute of General Medical Sciences Grant P20-GM-103449 from the Idea Networks of Biomedical Research Excellence Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.v.d.V. and Y.M.W.J.-H. conceived and designed research; J.L.v.d.V., D.E.W., K.G.L., S.T.A., and Y.-W.L. performed experiments; J.L.v.d.V., D.E.W., S.T.A., and Y.-W.L. analyzed data; J.L.v.d.V., D.E.W., and D.J.W. interpreted results of experiments; J.L.v.d.V. and D.E.W. prepared figures; J.L.v.d.V. and Y.M.W.J.-H. drafted manuscript; J.L.v.d.V., D.E.W., D.J.W., and Y.M.W.J.-H. edited and revised manuscript; J.L.v.d.V., D.E.W., K.G.L., S.T.A., Y.-W.L., D.J.W., and Y.M.W.J.-H. approved final version of manuscript.
REFERENCES
- 1.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci 121: 1036–1045, 2008. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YM. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol 40: 422–432, 2009. doi: 10.1165/rcmb.2008-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, Janssen-Heininger YM. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol Cell Biol 32: 3464–3478, 2012. doi: 10.1128/MCB.00125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol 306: C987–C996, 2014. doi: 10.1152/ajpcell.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876, 2012. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol 229: 25–35, 2013. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol 240: 397–409, 2016. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman HA. Epithelial responses to lung injury: role of the extracellular matrix. Proc Am Thorac Soc 9: 89–95, 2012. doi: 10.1513/pats.201112-053AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 306: 42–48, 1993. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci 108: 1251–1261, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A 18: 1–16, 2012. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299: L442–L452, 2010. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123: 4950–4962, 2013. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilpin SE, Charest JM, Ren X, Tapias LF, Wu T, Evangelista-Leite D, Mathisen DJ, Ott HC. Regenerative potential of human airway stem cells in lung epithelial engineering. Biomaterials 108: 111–119, 2016. doi: 10.1016/j.biomaterials.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilpin SE, Ren X, Okamoto T, Guyette JP, Mou H, Rajagopal J, Mathisen DJ, Vacanti JP, Ott HC. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann Thorac Surg 98: 1721–1729, 2014. doi: 10.1016/j.athoracsur.2014.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med 180: 122–133, 2009. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 18.Hallgren O, Rolandsson S, Andersson-Sjöland A, Nihlberg K, Wieslander E, Kvist-Reimer M, Dahlbäck M, Eriksson L, Bjermer L, Erjefält JS, Löfdahl CG, Westergren-Thorsson G. Enhanced ROCK1 dependent contractility in fibroblast from chronic obstructive pulmonary disease patients. J Transl Med 10: 171, 2012. doi: 10.1186/1479-5876-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada T, Nabeshima K, Hamasaki M, Uesugi N, Watanabe K, Iwasaki H. Epithelial-mesenchymal transition in human lungs with usual interstitial pneumonia: quantitative immunohistochemistry. Pathol Int 60: 14–21, 2010. doi: 10.1111/j.1440-1827.2009.02469.x. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 43: 161–172, 2010. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 29, Suppl 2: 90–95, 1999. doi: 10.1046/j.1365-2222.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 22.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol 49: 341–347, 2013. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, Fitzpatrick AM, Gaston BM, Israel E, Hastie A, Hoffman EA, Holguin F, Levy BD, Meyers DA, Moore WC, Peters SP, Sorkness RL, Teague WG, Wenzel SE, Busse WW; NHLBI Severe Asthma Research Program (SARP) . Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med 185: 356–362, 2012. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep 2: 787–792, 2014. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JR, Roos A, Berg T, Nord M, Fuxe J. Chronic respiratory aeroallergen exposure in mice induces epithelial-mesenchymal transition in the large airways. PLoS One 6: e16175, 2011. doi: 10.1371/journal.pone.0016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsdottir HR, Arason AJ, Palsson R, Franzdottir SR, Gudbjartsson T, Isaksson HJ, Gudmundsson G, Gudjonsson T, Magnusson MK. Basal cells of the human airways acquire mesenchymal traits in idiopathic pulmonary fibrosis and in culture. Lab Invest 95: 1418–1428, 2015. doi: 10.1038/labinvest.2015.114. [DOI] [PubMed] [Google Scholar]
- 30.Kage H, Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med 18: 517–523, 2012. doi: 10.1097/MCP.0b013e3283566721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol 32: 99–107, 2005. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 33.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King TE., Jr Bronchiolitis obliterans. Lung 167: 69–93, 1989. doi: 10.1007/BF02714935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538, 2011. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Moon HJ, Lee JM, Joo CK. Smad3 regulates Rho signaling via NET1 in the transforming growth factor-beta-induced epithelial-mesenchymal transition of human retinal pigment epithelial cells. J Biol Chem 285: 26618–26627, 2010. doi: 10.1074/jbc.M109.073155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, Kurkciyan A, Mena JM, Stripp BR, Jiang D, Noble PW. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med 22: 1285–1293, 2016. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McElhinney B, Poynter ME, Shrivastava P, Hazen SL, Janssen-Heininger YM. Eosinophil peroxidase catalyzes JNK-mediated membrane blebbing in a Rho kinase-dependent manner. J Leukoc Biol 74: 897–907, 2003. doi: 10.1189/jlb.0103028. [DOI] [PubMed] [Google Scholar]
- 40.Ongusaha PP, Qi HH, Raj L, Kim YB, Aaronson SA, Davis RJ, Shi Y, Liao JK, Lee SW. Identification of ROCK1 as an upstream activator of the JIP-3 to JNK signaling axis in response to UVB damage. Sci Signal 1: ra14, 2008. doi: 10.1126/scisignal.1161938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantano C, Anathy V, Ranjan P, Heintz NH, Janssen-Heininger YM. Nonphagocytic oxidase 1 causes death in lung epithelial cells via a TNF-RI-JNK signaling axis. Am J Respir Cell Mol Biol 36: 473–479, 2007. doi: 10.1165/rcmb.2006-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635, 2014. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, Zhou S, Cheng L, Lu MM, Morrisey EE. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526: 578–582, 2015. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perl AK, Riethmacher D, Whitsett JA. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am J Respir Crit Care Med 183: 511–521, 2011. doi: 10.1164/rccm.201005-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piao CQ, Liu L, Zhao YL, Balajee AS, Suzuki M, Hei TK. Immortalization of human small airway epithelial cells by ectopic expression of telomerase. Carcinogenesis 26: 725–731, 2005. doi: 10.1093/carcin/bgi016. [DOI] [PubMed] [Google Scholar]
- 46.Roche WR, Williams JH, Beasley R, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 333: 520–524, 1989. doi: 10.1016/S0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 47.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 108: E1475–E1483, 2011. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3: 545–556, 2010. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Théry M, Mann M, Fässler R. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 15: 625–636, 2013. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 50.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 51.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc 11, Suppl 5: S252–S258, 2014. doi: 10.1513/AnnalsATS.201402-049AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrivastava P, Pantano C, Watkin R, McElhinney B, Guala A, Poynter ML, Persinger RL, Budd R, Janssen-Heininger Y. Reactive nitrogen species-induced cell death requires Fas-dependent activation of c-Jun N-terminal kinase. Mol Cell Biol 24: 6763–6772, 2004. doi: 10.1128/MCB.24.15.6763-6772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohal SS, Reid D, Soltani A, Ward C, Weston S, Muller HK, Wood-Baker R, Walters EH. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary disease. Respir Res 12: 130, 2011. doi: 10.1186/1465-9921-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokocevic D, Bonenfant NR, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, Deng B, Desarno MJ, Ashikaga T, Loi R, Hoffman AM, Weiss DJ. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 34: 3256–3269, 2013. doi: 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 180: 657–665, 2009. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction. Ann Am Thorac Soc 12, Suppl 1: S24–S29, 2015. doi: 10.1513/AnnalsATS.201407-320MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Velden JL, Alcorn JF, Guala AS, Badura EC, Janssen-Heininger YM. c-Jun N-terminal kinase 1 promotes transforming growth factor-β1-induced epithelial-to-mesenchymal transition via control of linker phosphorylation and transcriptional activity of Smad3. Am J Respir Cell Mol Biol 44: 571–581, 2011. doi: 10.1165/rcmb.2009-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Velden JL, Guala AS, Leggett SE, Sluimer J, Badura EC, Janssen-Heininger YM. Induction of a mesenchymal expression program in lung epithelial cells by wingless protein (Wnt)/β-catenin requires the presence of c-Jun N-terminal kinase-1 (JNK1). Am J Respir Cell Mol Biol 47: 306–314, 2012. doi: 10.1165/rcmb.2011-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Velden JL, Hoffman SM, Alcorn JF, Tully JE, Chapman DG, Lahue KG, Guala AS, Lundblad LK, Aliyeva M, Daphtary N, Irvin CG, Janssen-Heininger YM. Absence of c-Jun NH2-terminal kinase 1 protects against house dust mite-induced pulmonary remodeling but not airway hyperresponsiveness and inflammation. Am J Physiol Lung Cell Mol Physiol 306: L866–L875, 2014. doi: 10.1152/ajplung.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625, 2015. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, Lathrop MJ, Wallis JD, Daly AB, Lam YW, Deng B, DeSarno MJ, Ashikaga T, Loi R, Weiss DJ. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35: 3281–3297, 2014. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner DE, Bonenfant NR, Sokocevic D, DeSarno MJ, Borg ZD, Parsons CS, Brooks EM, Platz JJ, Khalpey ZI, Hoganson DM, Deng B, Lam YW, Oldinski RA, Ashikaga T, Weiss DJ. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 35: 2664–2679, 2014. doi: 10.1016/j.biomaterials.2013.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White ES. Lung extracellular matrix and fibroblast function. Ann Am Thorac Soc 12, Suppl 1: S30–S33, 2015. doi: 10.1513/AnnalsATS.201406-240MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 18: 800–812, 1982. doi: 10.1007/BF02796504. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada M, Kuwano K, Maeyama T, Hamada N, Yoshimi M, Nakanishi Y, Kasper M. Dual-immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis. Histochem Cell Biol 129: 453–462, 2008. doi: 10.1007/s00418-008-0388-9. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. Am J Physiol Lung Cell Mol Physiol 306: L786–L796, 2014. doi: 10.1152/ajplung.00243.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, LaFemina MJ, Frank JA, Chapman HA, Christensen PJ, Kim KK. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol 183: 1559–1570, 2013. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108, 2013. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature 517: 616–620, 2015. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]