Abstract
Lewisite (2-chlorovinyldichloroarsine) is an organic arsenical chemical warfare agent that was developed and weaponized during World Wars I/II. Stockpiles of lewisite still exist in many parts of the world and pose potential environmental and human health threat. Exposure to lewisite and similar chemicals causes intense cutaneous inflammatory response. However, morbidity and mortality in the exposed population is not only the result of cutaneous damage but is also a result of systemic injury. Here, we provide data delineating the pathogenesis of acute kidney injury (AKI) following cutaneous exposure to lewisite and its analog phenylarsine oxide (PAO) in a murine model. Both agents caused renal tubular injury, characterized by loss of brush border in proximal tubules and tubular cell apoptosis accompanied by increases in serum creatinine, neutrophil gelatinase-associated lipocalin, and kidney injury molecule-1. Interestingly, lewisite exposure enhanced production of reactive oxygen species (ROS) in the kidney and resulted in the activation of autophagic and DNA damage response (DDR) signaling pathways with increased expression of beclin-1, autophagy-related gene 7, and LC-3A/B-II and increased phosphorylation of γ-H2A.X and checkpoint kinase 1/2, respectively. Terminal deoxyribonucleotide-transferase-mediated dUTP nick-end labeling-positive cells were detected in renal tubules along with enhanced proapoptotic BAX/cleaved caspase-3 and reduced antiapoptotic BCL2. Scavenging ROS by cutaneous postexposure application of the antioxidant N-acetyl-l-cysteine reduced lewisite-induced autophagy and DNA damage. In summary, we provide evidence that topical exposure to lewisite causes AKI. The molecular mechanism underlying these changes involves ROS-dependent activation of autophagy and DDR pathway associated with the induction of apoptosis.
Keywords: apoptosis, deoxyribonucleic acid damage, kidney damage, reactive oxygen species, systemic toxicity
INTRODUCTION
Lewisite, an organic arsenical and a chemical warfare agent, was developed and weaponized during World Wars I and II (18). Fortunately, because of war accords, it had not been used in the battlefield, and its shipment was known to be destroyed at sea (19). Later, with the efforts of the Organization for the Prohibition of Chemical Weapons, the stockpiles of lewisite and other chemical weapons were destroyed in a timely manner. Unfortunately, large amounts of lewisite and other similar arsenicals are known to exist in Russia, China, Japan, Germany, Italy, and the United States (47). Therefore, their accidental exposure during excavation/road construction or because of decay of buried shells containing these chemicals or the unfortunate access to terrorists could pose a significant threat as is evident from past experience. For example, in 2002, several hundred beer bottles containing lewisite were discovered at a road construction site in Samukawa, Kanagawa, where the Sagami Naval Arsenal was formally situated. Laborers working in that area were also reported to be exposed to lewisite (20). Similarly, in 2004, as a result of the consumption of well water contaminated with the degradation products of arsenic-based chemical weapons, perhaps Clark-I and Clark-II, the residents of Kamisu, Japan, developed some complex syndrome with symptoms of nervous system damage (23).
Lewisite is known to cause rapid and severe vesicant action (28, 29). Because of its oily and lipophilic nature, its rapid absorption through the skin is the main route of its systemic exposure (37) although other routes of exposure can also occur (39a). Skin exposure to lewisite is known to produce intense pain, inflammation, and blistering (33). Lewisite also damages the eye and airways (21, 46). We have recently demonstrated the molecular pathogenesis of cutaneous lesions following lewisite and phenylarsine oxide (PAO) exposure in a murine model. These studies demonstrated that endoplasmic reticulum (ER) stress-regulated pathways are involved in the skin blistering and inflammatory responses, since early treatment with a chemical chaperone that reduces ER stress also diminishes inflammation and blistering (29, 42).
The systemic toxicity of cutaneous exposure to lewisite is also known, although it has not been investigated in-depth (22, 28). The organs damaged because of systemic injury by lewisite include the liver, kidneys, lungs, gastrointestinal system, and heart as well as others (1). High concentrations of lewisite cause a condition termed as “lewisite shock,” which contributes to the enhanced capillary permeability associated with intravascular fluid loss and subsequently death (9). It is estimated that 37.6 mg/kg lewisite applied on the skin of an adult man could be deadly within several hours (35a). However, nonlethal toxicity of lewisite liquid on human skin requires doses of 3.5–14 μg to produce erythema and doses of 22–40 μg to produce vesication (17). Being an organic derivative of arsenic, some effects of lewisite are considered to be the result of arsenic poisoning. Interestingly, fluid from human lewisite blisters was found to accumulate arsenic at 0.8–1.3 mg/ml (9a). A corollary to this are the partial protective effects of British anti-Lewisite (BAL) and other similar metal chelators against skin lesions caused by lewisite exposure (34). These reports support the earlier belief that the toxic effects of arsenical vesicants are the result of arsenic. In addition, arsenic is mainly excreted by the kidneys in arsenic-exposed populations (8, 39). Thus, direct interaction of arsenic with the kidney in mediating tissue damage is also possible. Overall, these effects together may be involved in acute kidney injury (AKI) and systemic damage to other vital organs. However, the molecular pathogenesis of systemic damage is not clearly described.
In a sensitive hairless murine model of dermal lewisite toxicity, we confirm the occurrence of AKI. We demonstrate that an increased reactive oxygen species (ROS) production is associated with dysregulation of autophagic and DNA damage pathways. Blocking ROS by N-acetyl-l-cysteine (NAC) attenuates autophagy and DNA damage response (DDR) signaling pathways, which reduces apoptosis in renal tubular epithelial cells. We also confirmed these results using the surrogate arsenical PAO (42), a known analog of lewisite (12), in a C57BL/6 mouse model. In summary, these data confirm that cutaneous exposure to lewisite is associated with AKI and provide a novel molecular mechanism underlying renal injury.
MATERIALS AND METHODS
Animals and exposure.
Before cutaneous lewisite challenge, male and female mice were anesthetized with ketamine and xylazine (100 mg/kg ketamine and 5 mg/kg xylazine) by intraperitoneal injection. The anesthetized animals were topically treated with ethanol-diluted lewisite (30 μl applied on the dorsal skin over the area of 2 cm2 at the dose of 1.54 mg/kg). The dose of lewisite employed here is 1/10th of LD50 (41), which is relevant to a lewisite dose of ~32–40 µg to produce vesications on human skin (17). For testing the role of ROS in mediating tissue injury, NAC (5 mg/mouse) was applied topically at 5 and 30 min after lewisite exposure. In this efficacy experiment, a 2 mg/kg dose of lewisite was administered. All lewisite exposure-related studies were performed employing Ptch1+/−/SKH-1 mice at MRIGlobal (Kansas City, MO), since access or synthesis of these dangerous chemicals in the United States is only allowed to agencies that have adequate facilities for safe storage, handling, use, and decontamination of these chemicals. However, identical experiments were performed in our laboratory to demonstrate the effects of surrogate arsenical PAO on AKI employing C57BL/6 mice. For this, shaved C57BL/6 mice were administered PAO topically (150 µg/mouse diluted in 30 µl ethanol and applied over 2.56 cm2 skin area). Dose selection of PAO was based on previously published studies (12, 42). Control mice were also similarly challenged with vehicle alone (ethanol). All mice (control and experimental) were euthanized 24 h after treatment except for NAC study where animals were euthanized at 8 h. Selection of an 8 h time point for this study was based on our hypothesis that blocking of an early inflammatory cytokine surge in the murine skin following cutaneous lewisite exposure (29) could diminish AKI. All animal protocols were approved by our Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Cell culture.
Human embryonic kidney (HEK 293) cells were procured from ATCC (CRL-1573). HEK 293 cells were maintained in DMEM (Hyclone, Logan, UT) containing 10% fetal bovine serum (Sigma, St. Louis, MO) and 1% penicillin-streptomycin solution (Mediatechm, Manassas, VA) at 37°C in a 5% CO2 incubator.
PAO preparation and cell culture treatments.
Stock solution (1 M) of PAO (Sigma) was prepared freshly by dissolving in 100% DMSO at 37°C. PAO was handled following all chemical safety rules and regulations approved by the University of Alabama at Birmingham. Treatment of cells was done either with vehicle (control) or with various concentrations of PAO (100 and 200 nM) diluted in culture medium. Selection of concentrations of PAO is based on our previously published study (42). NAC (10 mM) was used in some experiments. In these experiments, cells were pretreated with NAC for 1 h before PAO challenge, and then these treatments were continued for 24 h.
Histopathological examination.
Hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain was performed as described earlier (3). Briefly, kidneys were fixed in 10% buffered formalin and embedded in paraffin. Tissues were cut into 5 μm sections using a microtome (HM 325; ThermoFisher, Grand Island, NY). Sections were deparaffinized in xylene and then rehydrated. At least three independent tissue sections from each group were stained with H&E or PAS reagent and examined for histological alterations using an Olympus BX51 microscope with an Olympus DP71 digital camera.
Determination of serum creatinine.
Serum creatinine levels were measured by liquid chromatography-tandem mass spectrometry at the University of Alabama-University of California San Diego (UAB-UCSD) O'Brien Center Bioanalytical Core facility as described earlier (45).
Determination of urinary albumin.
Urinary albumin was quantified using the Mouse Albumin ELISA kit (Bethyl Laboratories, Montgomery, TX), and data are presented as an albumin-to-creatinine ratio. The kit components were reconstituted, and the assay was performed according to the manufacturer’s instruction.
Arsenic measurement.
Blood samples from vehicle or lewisite-treated mice were shipped to the Trace Element Analysis Laboratory at Chemical Solutions (Harrisburg, PA) for analysis via inductively coupled plasma mass spectrometry (ICP-MS). Results were quantified as nanogram per gram of whole blood.
Blood pressure measurements by radiotelemetry.
Blood pressure was recorded using a radiotelemetry system previously described by Feng et al. (14, 15). Mice were anesthetized using 2% isoflurane, and the left common carotid artery was isolated. The catheter of a PA-C10 telemetry implant (DSI, St. Paul, MN) was introduced in the carotid artery and advanced until the tip was just inside the thoracic aorta. The implant was positioned along the left flank, close to the hindlimb. Mice were allowed to recover for 7 days. Baseline measurements were taken over the last 24 h of the 7-day recovery period and before exposure. Mice were removed from the receiver for PAO exposure and returned for monitoring 3 h postexposure. Blood pressure was continuously monitored for 48 h. The implantation of devices and monitoring were performed by the UAB-UCSD O’Brien Center Core Facility.
Determination of ROS.
Freshly cut optimum cutting temperature-embedded kidney cryosections (5 μm) were incubated with CellROX Deep Red Reagent (Invitrogen, Grand Island, NY) followed by Lotus tetragonolobus lectin (LTL) dissolved in ACAS buffer (in mM: 127 NaCl, 0.8 MgCl2, 3.8 KCl, 1.2 KH2PO4, 1.2 CaCl2, 5 glucose, and 10 HEPES, pH 7.4) for 1.5 h at room temperature as described earlier (30). Slides were protected from light, washed three times, mounted with mounting medium containing DAPI, and visualized under an Olympus BX51 microscope. In vitro ROS assessment was done using a CM-H2DCFDA probe (Invitrogen). ROS analysis was also performed on the frozen kidney tissue lysates using an OxiSelect in vitro ROS/reactive nitrogen species assay kit (Cell Biolabs, San Diego, CA) per the manufacturer’s protocol.
Immunohistochemical staining.
For immunohistochemical (IHC) staining, tissue sections were deparaffinized. Following rehydration, they were incubated in antigen unmasking solution according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). To avoid nonspecific binding of antibodies, a blocking buffer containing 2% BSA in PBS for 30 min at 37°C was used. These sections were incubated with a primary antibody for heme oxygenase-1 (HO-1, 1:250; Enzo Life Sciences) and p-H2A.X (1:100; Cell Signaling) followed by a universal peroxidase-coupled secondary antibody and visualized with diaminobenzidine substrate as described earlier (29). Mounted sections were visualized using an Olympus BX51 microscope with an Olympus DP71 digital camera.
Immunofluorescence staining.
Following various treatments, HEK 293 were washed with PBS, fixed in 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100. Cells were then blocked for 1 h with 1% bovine serum albumin followed by incubation with the primary antibody against p-H2A.X (1:100; Cell Signaling) overnight at 4°C. After PBS washes, cells were incubated with fluorescein-conjugated secondary antibodies for 1–1.5 h at room temperature. Labeled cells were visualized by fluorescence microscopy.
Real-time PCR.
Levels of inflammatory cytokines were measured by qRT-PCR analysis. Total RNA was prepared from kidney tissues using TRIzol followed by cDNA preparation using a cDNA synthesis kit (Bio-Rad, Hercules, CA) as per the manufacturer’s instructions. qRT-PCR reactions were carried out in a 7500 fast Real-Time PCR system (Applied Biosystem, Foster City, CA) using Taqman Fast Advanced PCR master mix and Taqman primers (Thermo-Scientific, Grand Island, NY). The mRNA expression levels of IL-6 and interferon (IFN)-α are presented as fold change after normalization to endogenous control GAPDH.
Protein quantification and Western blot analysis.
Protein quantification and Western blot analysis were performed as described earlier (17). Protein assay was done by a DC kit (Bio-Rad). Briefly, protein lysates following various treatments were electrophoretically resolved on SDS-PAGE and transferred to polyvinylidene difluoride membrane. Nonspecific sites were blocked with 5% nonfat dry milk in Tris-buffered saline-Tween 20 (TBST) for 1 h at room temperature. The membrane was probed with various primary antibodies [kidney injury molecule-1 (KIM-1, 1:1,000; Abcam), BAX (1:1,000; Cell Signaling), BCL2 (1:1,000; Cell Signaling), cleaved caspase-3 (1:1,000; Cell Signaling), phosphorylated (p)- checkpoint kinase (CHK)-1 (1:1,000; Cell Signaling), p-CHK-2 (1:1,000; Cell Signaling), total CHK1 (1:1,000; Cell Signaling), p-γH2A.X (1:1,000; Cell Signaling), beclin-1 (1:1,000; Cell Signaling), autophagy-related gene (ATG) 7 (1:1,000; Cell Signaling), ATG5 (1:1,000; Cell Signaling), LC3A/B (1:1,000; Cell Signaling), or β-actin (1:5,000; Sigma)] overnight at 4°C or for 2 h at room temperature. After being washed three times, the membranes were treated with horseradish peroxidase-conjugated secondary antibody for 2 h. The blots were developed with enhanced chemiluminescence according to the manufacturer’s instructions (Thermo-Scientific).
Terminal deoxyribonucleotide-transferase-mediated dUTP nick-end labeling assay.
Terminal deoxyribonucleotide-transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed using a commercial apoptosis detection kit (Roche Diagnostics, Indianapolis, IN) following the manufacturer’s instruction. The TUNEL-stained slide sections were mounted with VECTASHIELD mounting substance-containing DAPI and were visualized using an Olympus BX51 microscope with an Olympus DP71 digital camera.
Transmission electron microscopy.
Transmission electron microscopy (TEM) analysis was performed as described earlier (17). Briefly, kidney tissues were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in a solution of 0.1 M cacodylate buffer. Kidneys were then placed in ice-cold solution of 1% osmium tetraoxide (EMS, Hatfield, PA), 0.8% potassium tetraoxide, and 3 mM calcium chloride. Ultrathin sections of kidneys were prepared using a Reichert-Ultracut E ultramicrotome and supported on copper grids/75 mesh, followed by Sato lead stain. Stained sections were observed and imaged for ultrastructural changes using a Tecnai Spirit Twin 20–120 kV electron microscope (FEI, Hillsboro, OR).
Cytokine antibody arrays.
Whole blood was collected from vehicle- and PAO-exposed mice in BD Microtainer tubes with serum separator additive (BD, Franklin Lakes, NJ), and serum was obtained by centrifugation. Serum samples (100 μl) were incubated with RayBio Mouse Cytokine Antibody Array C1 membrane (CODE: AAM-CYT-1-2) according to the manufacturer’s protocol (RayBiotech, Norcross, GA). The Mouse Cytokine Antibody Array C1 membrane was used to evaluate granulocyte colony-stimulating factor (GCSF), granulocyte macrophage colony-stimulating factor (GM-CSF), IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 p40/p70, IL-12 p70, IL-13, IL-17A, IFN-γ, monocyte chemoattractant protein (MCP)-1 (CCL2), MCP-5, regulated on activation normal T cell expressed and secreted (CCL5), stem cell factor, tumor necrosis factor receptor I (TNFRSF1A), tumor necrosis factor (TNF)-α, thrombopoietin (TPO), and vascular endothelial growth factor-A expression patterns according to the user manual. X-ray films were analyzed by Excel-based analysis software tools (RayBiotech) for the automatic computation of the extracted numerical data obtained from the array images.
Statistical analysis.
The data reported here are expressed as means ± SE. Statistical analysis between two groups was performed using the two-tailed Student’s t-test, and a P value <0.05 was considered to be statistically significant.
RESULTS
Cutaneous lewisite and PAO treatment induces AKI.
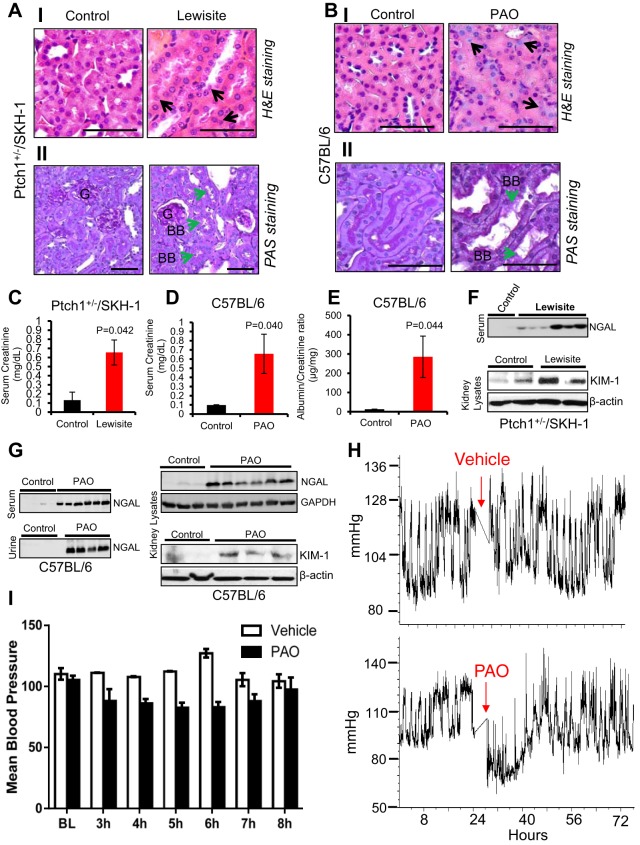
H&E-stained kidney sections from mice challenged topically with either lewisite or PAO revealed tubular injury with degenerative nuclei and infiltration of inflammatory cells (Fig. 1, AI and BI). PAS staining of tubules displayed loss of brush border and detachment from basement membranes (Fig. 1, AII and BII). Histological alterations in the kidney were associated with significant increase in biomarkers of AKI such as serum creatinine, neutrophil gelatinase-associated lipocalin (NGAL), and KIM-1 in both Ptch1+/−/SKH-1 and C57BL/6 mice exposed to lewisite or PAO for 24 h, respectively. Compared with vehicle-treated controls, mice from lewisite- and PAO-treated groups exhibited higher serum creatinine levels (Fig. 1, C and D). The albumin-to-creatinine ratio was also increased among the PAO-treated animals (Fig. 1E). Urinary and serum NGAL, early biomarkers of AKI (11), showed increased expression in both lewisite- and PAO-treated animals (Fig. 1, F and G). Similarly, kidney tissue lysates revealed enhanced expression of KIM-1 and NGAL in both lewisite- and PAO-treated animals (Fig. 1, F and G). Interestingly, using ICP-MS, we found a significant (P < 0.05) increase in blood levels of arsenic (range between 127 ± 0.004 and 336 ± 0.08 ng/g blood) between 6 and 24 h in lewisite-treated animals compared with the control (7 ± 0.001 ng/g blood) vehicle-treated animals. Blood pressure was measured by telemetry and showed a transient drop in mean arterial pressure within a few hours following PAO exposure but was not statistically significant (Fig. 1, H and I). These data provide the basis for assessing AKI following cutaneous exposure to vesicants and validate the use of biomarkers (NGAL and KIM-1) in arsenical-mediated renal injury.
Fig. 1.
Effects of topical lewisite and phenylarsine oxide (PAO) exposure on acute kidney injury (AKI). Ptch1+/−/SKH-1 mice were exposed to lewisite, whereas C57BL/6 mice were exposed to PAO as described in materials and methods. Hematoxylin and eosin (H&E) staining showing representative photomicrographs of kidney tissue sections (5 µm) from control vs. lewisite/PAO-treated animals for 24 h. A–I: kidney sections of vehicle control and lewisite-treated Ptch1+/−/SKH-1 mice. B–I: kidney sections of vehicle and PAO-treated C57BL/6 mice. Vehicle-treated control mice show normal histological structure of tubules with little interstitial space, whereas lewisite- and PAO-treated animals show tubular injury with degenerative nuclei (black arrows). Bars = 25 µm. AII and BII: representative images of periodic acid–Schiff (PAS) staining showing the altered structural architecture and loss of brush border (green arrows) in proximal tubules of lewisite (AII)- and PAO (BII)-treated mice kidneys. Bars = 25 µm. BB, brush-border membrane; G, glomeruli. Photomicrographs were captured using an Olympus BX51 microscope with an Olympus DP71 digital camera. C and D: effects of cutaneous exposure of lewisite in Ptch1+/−/SKH-1 (C) or PAO in C57BL/6 (D) mice on serum creatinine (mg/dl) relative to their vehicle-treated control animals. E: albumin-to-creatinine ratio for control vs. PAO-exposed mice. P value represents significance level (n = 5/group). F: representative Western blot analysis of neutrophil gelatinase-associated lipocalin (NGAL) in serum and kidney injury molecule-1 (KIM-1) in kidney lysates of vehicle controls and lewisite-exposed Ptch1+/−/SKH-1 mice. G: Western blot analysis of NGAL in serum, urine, and kidney lysates and KIM-1 in kidney lysates of vehicle control and PAO-exposed C57BL/6 mice. H: blood pressure was recorded using a radiotelemetry system. Baseline measurements were taken over the 24 h before exposure. C57BL/6 mice were then exposed to either vehicle or PAO and returned for monitoring 3 h postexposure. Blood pressure was continuously monitored for 48 h. I: mean arterial pressure following PAO exposure. BL, baseline. Each lane represents band intensity from individual animal samples except for F (bottom), which represents pooled kidney lysates for each group (n = 5, control vs. lewisite).
Cutaneous lewisite and PAO administration induces oxidative stress and inflammatory cytokines in the kidney.
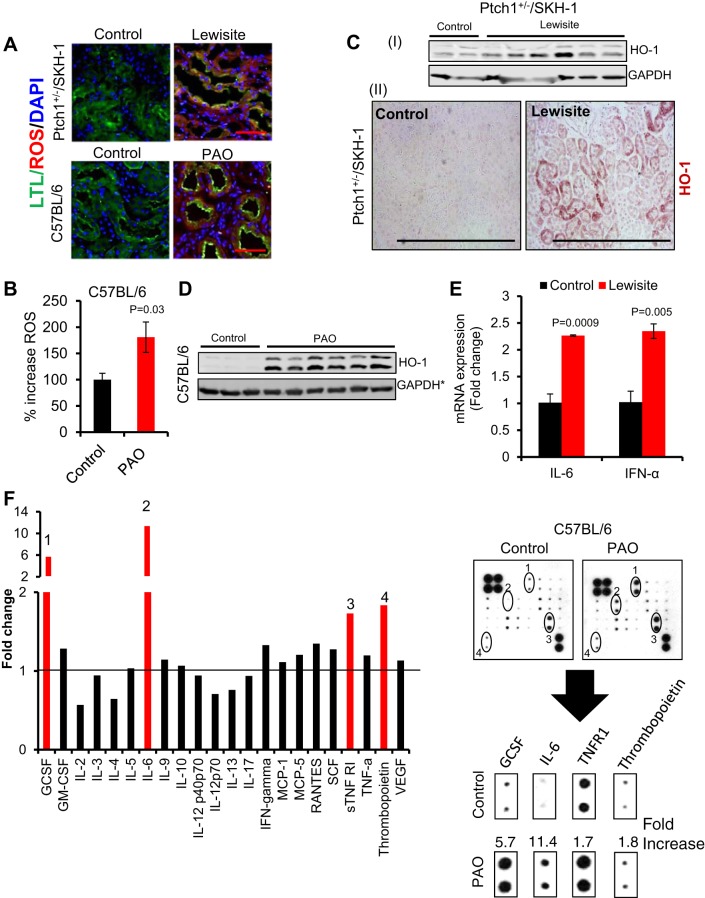
Oxidative stress and inflammatory responses have been implicated as the major underlying mechanisms involved in the pathogenesis of AKI (5, 16). In this regard, heme oxygenase-1 (HO-1), an important biomarker of oxidative stress, has been widely studied in earlier reports (reviewed in Ref. 35). Employing CellROX Deep Red and LTL costaining, we observed an accumulation of ROS mainly in renal proximal tubular cells of lewisite- and PAO-treated mice (Fig. 2A). LTL, a fluorescent-coupled specific marker of proximal convoluted tubules (3), was used to demonstrate generation of ROS in proximal tubules. This was confirmed in kidney lysates of PAO-treated mice (Fig. 2B). Western blot data demonstrated high induction of HO-1 levels in kidney lysates of lewisite-exposed animals compared with vehicle controls (Fig. 2, CI). This increase in HO-1 was also confirmed in the kidney sections, specifically in renal proximal tubules (Fig. 2, CII). Similar changes were also observed through Western analysis in the kidneys of PAO-exposed C57BL/6 mice (Fig. 2D). Interestingly, we observed higher levels of a lower-molecular-weight band of HO-1 (~28 kDa) in addition to the upper ~32-kDa band in the kidneys of mice exposed to lewisite or PAO. Previously, we have demonstrated that topically administered arsenicals induce ROS in the skin (29, 42). However, this is the first demonstration of arsenical-mediated renal oxidative stress following cutaneous exposure.
Fig. 2.
Cutaneous exposure of lewisite and PAO induces oxidative stress and inflammatory cytokines in the kidney. A: fluorescence-based photomicrograph analysis of reactive oxygen species (ROS) generation in the proximal tubules of kidneys of lewisite-treated Ptch1+/−/SKH-1 or PAO-treated C57BL/6 mice as assessed using CellROX Deep Red Reagent (red intensity). Proximal tubules were stained using fluorescence-conjugated Lotus tetragonolobus lectin (LTL) (green intensity). Bars = 25 µm. B: histogram showing plate reader-based quantification of ROS generation in the kidney lysates of vehicle- and PAO-treated C57BL/6 mice. n = 5. C: representative Western blot analysis of heme oxygenase-1 (HO-1) in kidney tissue lysates (CI) and IHC staining (CII). Bars = 50 µm in kidney tissue sections of vehicle- and lewisite-treated Ptch1+/−/SKH-1 mice. D: representative Western blot analysis of HO-1 in kidney tissue lysates of vehicle- and PAO-treated C57BL/6 mice. The lower band is ~28 kDa. *GAPDH loading control represent stripping of same blot as shown in Fig. 1G. E: RT-PCR analysis of mRNA expression of IL-6 and interferon (IFN)-α in kidney tissue of vehicle control and lewisite-exposed Ptch1+/−/SKH-1 mice. F: mouse cytokine antibody array expression patterns of different cytokines in serum samples of vehicle control and PAO-exposed C57BL/6 animals. Cytokines showing >1.5-fold induction [granulocyte colony-stimulating factor (GCSF) (1), IL-6 (2), tumor nerosis factor-α receptor (TNFR) 1 (3), and thrombopoietin (4)] are presented separately from the array images. In this experiment, serum samples of 3 mice from each group (control vs. PAO) were pooled and incubated with mouse cytokine antibody array membrane. Densitometry data obtained from the array images were analyzed by Excel-based analysis software tools (Ray Biotech). GM-CSF, granulocyte macrophage colony-stimulating factor; MCP, monocyte chemoattractant protein; RANTES, regulated on activation normal T cell expressed and secreted; SCF, stem cell factor; STNF RI, soluble tumor necrosis factor receptor I; VEGF, vascular endothelial growth factor.
Oxidative stress induces inflammation, and both processes are interrelated in the pathogenesis of many kidney diseases (reviewed in Ref. 43). RT-PCR analysis revealed that renal oxidative stress is associated with the induction of inflammatory cytokines IL-6, IFN-α (Fig. 2E) and neutrophil marker myeloperoxidase (MPO) (data not shown) in lewisite-treated animals. Furthermore, mouse cytokine antibody array analysis demonstrated significant induction of GCSF, IL-6, tumor necrosis factor receptor 1, and thrombopoietin (TPO) in serum samples of PAO-exposed mice compared with vehicle-treated mice (Fig. 2F). Previous studies have shown that IL6−/− and TNF-α−/− mice are resistant to mercuric chloride (HgCl2)-induced AKI (36). Similarly, association of higher level of GCSF and thrombopoietin are also reported with kidney injury (32, 50). Our findings suggest the importance of GCSF-, TPO-, IL-6-, and IFN-α-mediated inflammatory response in arsenicals-induced AKI. Together, these data suggest that the cytokine surge caused by cutaneous exposure to vesicants may be a key mediator of systemic injury in this setting.
Cutaneous exposure of lewisite and PAO enhances autophagy and DNA damage signaling pathways in the kidney.
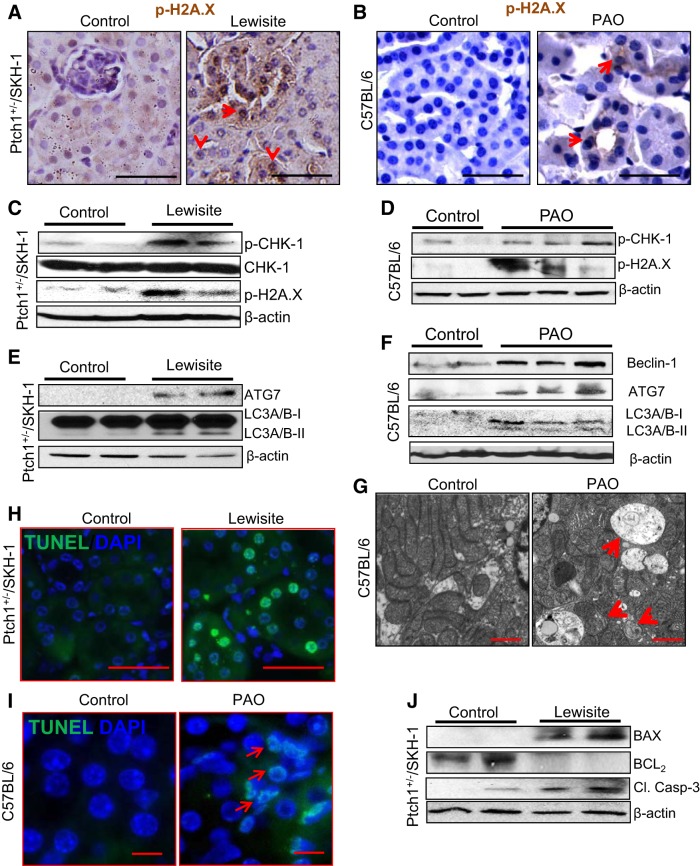
Because lewisite- and PAO-mediated tissue damage accompanies enhanced ROS production, which is known to cause DNA damage (38) and autophagy (43), we determined whether AKI in these animals is associated with an augmented DDR and a dysregulated autophagic signaling pathway. Immunohistochemical analysis of kidney sections showed an enhanced nuclear staining for p-H2A.X in both the glomeruli and renal proximal tubules of lewisite- and PAO-treated mice (Fig. 3, A and B). DNA damage is known to induce the DDR signaling pathway, which is initiated or identified by the phosphorylation-dependent activation of the DNA damage-sensing protein kinase ataxia telangiectasia mutated (ATM). Phosphorylated ATM then triggers a downstream cascade of signals by activating phosphorylation of ataxia telangiectasia and Rad3-related kinase and ultimately activating CHK1/2 kinase by their phosphorylation. Consistently, we observed enhanced p-CHK1 and p-H2A.X in kidney lysates of lewisite-treated Ptch1+/−/SKH-1 mice (Fig. 3C). Identical changes were also observed in the kidneys of PAO-exposed C57BL/6 mice (Fig. 3D). Consistent with these observations, earlier studies demonstrated a role for the DDR pathway in cisplatin-induced AKI (10, 40).
Fig. 3.
Cutaneous exposure to lewisite or PAO induces autophagy, DNA damage, and apoptosis responses in the kidney. A and B: immunohistochemical analysis showing enhanced nuclear staining of phosphorylated (p)-H2A.X (red arrows) in the tubules of the kidney tissue of lewisite-treated Ptch1+/−/SKH-1 mice or PAO-treated C57BL/6 mice compared with vehicle-treated controls. Bars = 25 µm. C and D: representative Western blot analysis shows enhanced phosphorylation-dependent activation of checkpoint kinase (CHK) 1 and H2A.X in the kidney tissue of lewisite (C)- and PAO (D)-treated mice compared with their respective control tissues. E and F: representative Western blot analysis of autophagy-related gene (ATG) 7 and LC3A/B-II in the kidney tissue lysates of lewisite-treated Ptch1+/−/SKH-1 mice (E) and beclin-1, ATG7, and LC3A/B-II in PAO-treated C57BL/6 mice (F) compared with their respective controls. G: transmission electron microscopy (TEM) analysis of kidney tissues of vehicle- and PAO-treated C57BL/6 mice (n = 3/group). Bars = 150 nm. Arrows indicate autophagosomes with damaged organelles and mitochondrial cristae loss. H and I: terminal deoxyribonucleotide-transferase-mediated dUTP nick-end labeling (TUNEL) staining of the kidney sections showing frequent presence of green TUNEL-positive cells in lewisite-treated (H, bars = 25 µm) and PAO-treated (I, bars = 12.5 µm) animals compared with vehicle-treated control animals. Apoptotic cells (green positive nuclei) were present in the tubules of the kidney. J: representative Western blot analysis showing induction of proapoptotic proteins, BAX and cleaved caspase-3, and reduced expression of anti-apoptotic BCL2 in the kidneys of lewisite-treated mice. β-Actin was used as a loading control. Western blot data shown in C, E, and J represent pooled kidney lysates for each group (n = 5, control vs. lewisite).
It has been shown that dysregulation of autophagy is important in the pathogenesis of AKI (reviewed in Ref. 26). Therefore, we further investigated whether AKI is associated with autophagy in mice exposed cutaneously to arsenicals. Indeed, our results demonstrate that both lewisite and PAO induce autophagic marker proteins beclin-1 and ATG7 and conversion of LC-3A/B-I to LC-3A/B-II in kidney tissue lysates (Fig. 3, E and F). Autophagic vacuoles, including autophagosomes and autophagolysosomes, were markedly increased in the tubular cells of PAO-exposed animals compared with vehicle-exposed animals as ascertained by TEM analysis. These vacuoles were double membrane in structure, having damaged cytoplasmic organelles, electron-dense material, and mitochondria with cristae loss (Fig. 3G). These findings are consistent with the detection of autophagosomes in response to renal ischemia-reperfusion injury (44) and cisplatin-induced AKI (4). Furthermore, we observed frequent appearance of TUNEL-positive tubular cells in the kidney sections of both lewisite- and PAO-exposed animals at 24 h (Fig. 3, H and I). This induction of apoptosis was confirmed by increased BAX and cleaved caspase-3 and decreased BCL2 in kidney lysates of lewisite-treated mice (Fig. 3J).
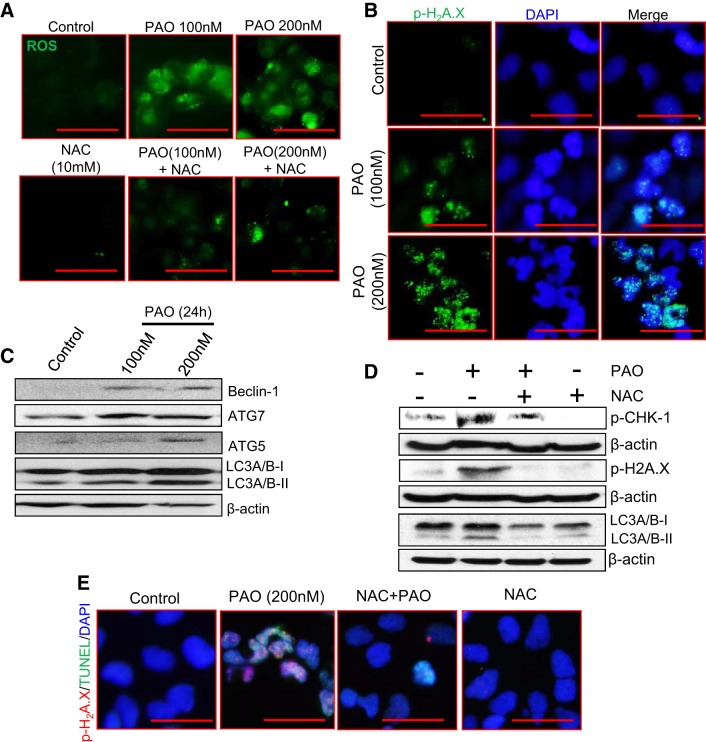
PAO-mediated ROS generation enhances DNA damage and autophagic responses in HEK 293 cells.
To probe the molecular mechanism underlying lewisite- and PAO-induced AKI, we employed human embryonic kidney cells (HEK 293). Similar to our observations with cutaneous exposure of lewisite and PAO in mice kidneys, we demonstrate a dose-dependent enhancement of ROS accumulation in PAO-treated HEK 293 cells (Fig. 4A), which accompany enhanced numbers of p-H2A.X-positive cells (Fig. 4B). Western blot analysis showed a dose-dependent increase in autophagy marker proteins beclin-1, ATG7, ATG5, and LC-3A/B-II in PAO-treated HEK 293 cells (Fig. 4C). Co-treatment with the antioxidant NAC reduced PAO-induced production of ROS (Fig. 4A) and subsequently expression of p-CHK-1, p-H2A.X, and LC-3A/B-II (Fig. 4D) as well as apoptosis as evident by reduced TUNEL and p-H2A.X in costained HEK 293 cells (Fig. 4E). These data demonstrate the ROS-dependent activation of DNA damage and autophagic signaling pathways are associated with the induction of apoptosis following treatment with arsenicals.
Fig. 4.
PAO-induced ROS generation is involved in DNA damage and autophagic responses in HEK 293 cells. A: ROS generation was analyzed using CM-H2DCFDA fluorescent probe in PAO-treated HEK 293 cells with or without N-acetyl-l-cysteine (NAC, 10 mM). Bars = 25 µm. B: immunofluorescence microscopy showing dose-dependent enhancement of nuclear p-H2A.X in PAO-treated HEK 293 cells. Bars = 25 µm. C: Western blot analysis of beclin-1, ATG7, ATG5, and LC-3A/B-II in vehicle- vs. PAO-treated HEK 293 cells. D: Western blot analysis of p-CHK-1, p-H2A.X, and LC-3A/B-II in PAO-treated HEK 293 cells with or without NAC. β-Actin was used as a loading control. E: coimmunofluorescence staining for p-H2A.X and TUNEL-positive HEK 293 cells. Bars = 25 µm. Photomicrographs were captured using an Olympus BX51 microscope with an Olympus DP71 digital camera.
Lewisite-mediated ROS generation is involved in AKI.
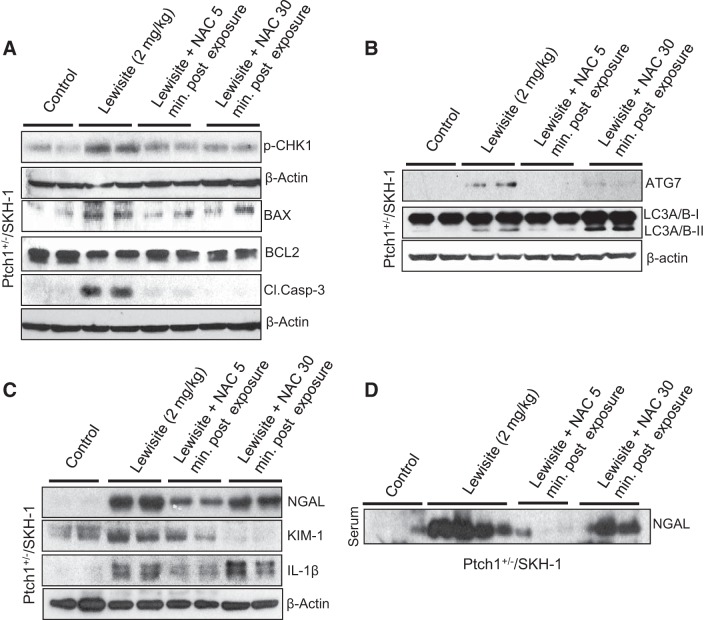
Since we observed an enhancement in inflammatory cytokines at early time points in the skin of lewisite-exposed mice (29), we tested the efficacy of an antioxidant, NAC, at the 8-h time point to access protection on an early moleculer biomarker of AKI following lewisite exposure. Topical treatment of Ptch1+/−/SKH-1 mice with the antioxidant NAC [5 and 30 min after lewisite (2 mg/kg) treatment] resulted in significant reduction of lewisite-induced renal expression of p-CHK-1, BAX, and cleaved caspase-3 and an increase in BCL2 (Fig. 5A). We also observed that NAC not only inhibited DNA damage- and apoptosis-regulating signaling pathways but also reduced the autophagic response as ascertained by the reduced expression of ATG7 and LC-3A/B-II (Fig. 5B). This effect was more prominent when NAC was administered within 5 min following lewisite exposure compared with 30 min posttreatment with NAC. These effects were also associated with reduced expression of biomarkers of kidney injury such as NGAL and KIM-1 in both kidney lysates and serum (Fig. 5, C and D). We also found reduced expression of inflammatory cytokine IL-1β when NAC was applied at 5 min after lewisite exposure (Fig. 5C). However, at the 8-h time point, we did not find significant changes in serum creatinine levels, suggesting that serum creatinine is not a good early biomarker for depicting molecular changes associated with AKI. This is consistent with earlier published reports where serum creatinine increases are not evident until later time points, for example, with ischemia-reperfusion injury (IRI) (48).
Fig. 5.
NAC treatment protects against lewisite-induced AKI. Ptch1+/−/SKH-1 mice were exposed to lewisite (2 mg/kg, 8 h). NAC was applied 5 or 30 min after lewisite treatment. Kidney and serum samples were harvested and analyzed for protein expression analysis. A: Western blot analysis of p-CHK-1, BAX, BCL2, and cleaved caspase-3 in kidney lysates of lewisite-exposed animals with or without NAC treatment. B: Western blot analysis of ATG7, LC-3-IIA/B-1, and LC-3A/B-II in kidney lysates of lewisite-exposed animals with or without NAC treatment. C: Western blot analysis of NGAL, KIM-1, and IL-1β in kidney tissue lysates of the indicated groups. β-Actin was used as a loading control. D: Western blot analysis of NGAL in serum samples of lewisite-exposed mice with or without NAC treatment. Western blot data shown in A–C represent pooled samples for each group (n = 5).
DISCUSSION
Lewisite is a fatal cutaneous vesicant and systemic poison. Lewisite may be lethal following its ingestion, inhalation, or dermal exposure (39a). Being lipophilic in nature, its cutaneous absorption is rapid and is associated with systemic toxicity, which could be characterized by multi-organ damage. In this study, we demonstrated AKI following cutaneous exposure to lewisite using the Ptch1+/−/SKH-1 mouse model and investigated the molecular mechanism for the systemic effects of arsenicals. Our data showing tubular injury, loss of proximal tubule brush border, and infiltration of inflammatory cells in the kidney of lewisite-treated animals confirm some of the earlier reported data (1). These data also provide novel insights into the molecular mechanism of systemic toxicity of these chemicals following their skin exposure.
Although this study focused on ROS-mediated autophagy and DNA damage-related AKI, we realize that multifactorial mechanisms may underlie the pathogenesis of cutaneous lewisite-induced AKI. For example, the cytokine surge and hemodynamic effects produced by cutaneous exposure to lewisite may be an important mediator and contributor to systemic pathobiology (49). This was further confirmed by the fact that reduction in cutaneous injury caused by these arsenicals manifested reduced renal damage. However, our data showing that augmented ROS generation increased inflammatory cytokines and associated apoptotic cell death in the kidney point to the involvement of oxidative injury as a major underlying mechanism by which these chemicals elaborate systemic damage. The transient drop in blood pressure observed by telemetry in the early hours following exposure to arsenicals may also contribute to the AKI in this model. It is known that exposure to heavy metals such as cadmium, lead, and mercury besides arsenic causes AKI and chronic kidney disease by generation of ROS (24). Human exposure to one or a mixture of these toxicants is associated with tubulointerstitial nephritis, acute tubular necrosis, albuminuria, reduced glomerular filtration rate, polyuria, and generalized tubular dysfunction (24). However, this is the first demonstration that single cutaneous exposure to arsenic-containing chemicals causes renal injury.
Furthermore, an increase in HO-1, an important marker of oxidative stress, was also found to be associated with lewisite/PAO-mediated kidney injury. Cytoprotective roles for HO-1 have been demonstrated in many animal models. Nevertheless, all of the by-products of the HO reaction, despite being potentially cytoprotective, are also cytotoxic. For example, the HO reaction releases iron, which could interact with cellular oxidants to generate the hydroxyl radical (27). Interestingly, we observed two HO-1 immunoreactive bands on Western analysis in the kidney lysates of lewisite- or PAO-treated animal samples, with one band migrating at 28 kDa and the other migrating at 32 kDa, suggesting the cleavage of HO-1 protein. Earlier in vitro experiments documented that, under hypoxic conditions, a faster migrating HO-1 immunoreactive band was enriched in nuclear extracts, suggesting that HO-1 was cleaved to allow nuclear entry (31), which was associated with truncation of the COOH-terminus of HO-1. Recent studies have demonstrated the presence of this lower-molecular-weight band of HO-1 in mice kidneys in cisplatin-induced AKI (2). We are currently pursuing additional studies to clarify the functional significance of this cleaved form of HO-1.
Autophagy and DNA damage-associated DDR signaling are probably the initial key events contributing to the molecular pathogenesis of these lesions. However, we are expanding these observations to correlate with other parallel cytotoxic signals that orchestrate the lethality in exposed animals. In this regard, we have recently demonstrated the involvement of the unfolded protein response (UPR) pathway in the production of cytokines and inflammatory responses in the skin of lewisite-treated animals (29). We found that blocking the UPR signaling pathway by a chemical chaperone and the UPR signaling inhibitor 4-phenylbutyric acid and the antioxidant NAC affords protection against lewisite-induced skin damage. However, it remains to be shown whether similar mechanisms are involved in the activation of inflammatory signaling pathways in the kidney. Earlier we have demonstrated the involvement of ATF4 in arsenic-mediated ER-mitochondrial cross talk (17). Here, we speculate that ATF4 may also be involved in the pathogenesis of AKI but need further investigation.
In summary, we demonstrate that cutaneous chemical injury in a sensitive animal model leads to systemic organ injury, particularly AKI. These data also reveal that ROS-mediated apoptotic cell death contributes to AKI where autophagic and the DDR signaling pathway play a critical role. In addition, arsenical-induced renal injury could be predicated by the biomarkers of AKI.
GRANTS
This work was supported by the Countermeasures Against Chemical Threats Program, Office of the Director of the National Institutes of Health (NIH), and NIH Grant UO1-NS-095678 to M. Athar and R01-DK-59600 and P30-DK-079337 to A. Agarwal.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. The study sponsors had no involvement in the study design, collection, analysis and interpretation of data, the writing of the manuscript and the decision to publish the manuscript.
AUTHOR CONTRIBUTIONS
R.K.S. and M.A. conceived and designed research; R.K.S., A.M.T., and C.L. performed experiments; R.K.S., A.M.T., C.L., L.G., T.R.S., and A.A. analyzed data; R.K.S., A.M.T., W.F., L.G., T.R.S., A.A., and M.A. interpreted results of experiments; R.K.S., A.M.T., W.F., L.G., and A.A. prepared figures; R.K.S., A.A., and M.A. drafted manuscript; R.K.S., A.M.T., C.L., V.B.A., A.A., and M.A. edited and revised manuscript; R.K.S., A.M.T., C.L., W.F., L.G., V.B.A., T.R.S., A.A., and M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical expertise of Reny Joseph in performing the albumin ELISA.
REFERENCES
- 1.Blister Agents Lewisite (L) (C2 H2 AsCl3) CAS 541–25–3, UN 1556; and Mustard-Lewisite Mixture (HL). Available at https://www.atsdr.cdc.gov/mhmi/mmg163.pdf.
- 2.Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol 313: F740–F755, 2017. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolisetty S, Traylor A, Joseph R, Zarjou A, Agarwal A. Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 310: F385–F394, 2016. doi: 10.1152/ajprenal.00335.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 8.Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect 107: 663–667, 1999. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullumbine H, Box GE. Sodium salt solutions for lewisite shock. Br Med J 1: 607–608, 1946. doi: 10.1136/bmj.1.4450.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Davis MI. Clinical and Laboratory Evidence of the Nontoxic Effect of Lewisite Vesicle Fluid on the Skin. Memorandum, Report 82. Edgewood, MD: Edgewood Arsenal, 1943. [Google Scholar]
- 10.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther 314: 1052–1058, 2005. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis 15: 222–234, 2008. doi: 10.1053/j.ackd.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGwon EL, van Ravenswaay T, Damlao CR, O’Connor RJ, Black KE. Histologic Changes Caused by Application of Lewisite Analogs to Mouse Skin and Human Skin Xenografts. San Francisco, CA: Letterman Army Institute of Research, 1985. [Google Scholar]
- 14.Feng W, Chen B, Xing D, Li X, Fatima H, Jaimes EA, Sanders PW. Haploinsufficiency of the transcription factor Ets-1 is renoprotective in Dahl salt-sensitive rats. J Am Soc Nephrol 28: 3239–3250, 2017. doi: 10.1681/ASN.2017010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng W, Chumley P, Hua P, Rezonzew G, Jaimes D, Duckworth MW, Xing D, Jaimes EA. Role of the transcription factor erythroblastosis virus E26 oncogen homolog-1 (ETS-1) as mediator of the renal proinflammatory and profibrotic effects of angiotensin II. Hypertension 60: 1226–1233, 2012. doi: 10.1161/HYPERTENSIONAHA.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest 124: 4989–5001, 2014. doi: 10.1172/JCI76042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiling EMK CR, Bloom W. Toxicity of Chemical Warfare Agents. Washington, DC: National Defense Research Committee, 1944. [Google Scholar]
- 18.Goldman M, Dacre JC. Lewisite: its chemistry, toxicology, and biological effects. Rev Environ Contam Toxicol 110: 75–115, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg MI, Sexton KJ, Vearrier D. Sea-dumped chemical weapons: environmental risk, occupational hazard. Clin Toxicol (Phila) 54: 79–91, 2016. doi: 10.3109/15563650.2015.1121272. [DOI] [PubMed] [Google Scholar]
- 20.Hanaoka S, Nomura K, Wada T. Determination of mustard and lewisite related compounds in abandoned chemical weapons (Yellow shells) from sources in China and Japan. J Chromatogr A 1101: 268–277, 2006. doi: 10.1016/j.chroma.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Harrison HE, Ordway NK. Poisoning from inhalation of the vapors of lewisite and phenyldichlorarsine; its pathology in the dog and treatment with 2,3-dimercaptopropanol (BAL). J Pharmacol Exp Ther Suppl 87: 76–80, 1946. [PubMed] [Google Scholar]
- 22.Inns RH, Rice P. Efficacy of dimercapto chelating agents for the treatment of poisoning by percutaneously applied dichloro(2-chlorovinyl)arsine in rabbits. Hum Exp Toxicol 12: 241–246, 1993. doi: 10.1177/096032719301200307. [DOI] [PubMed] [Google Scholar]
- 23.Ishii K, Tamaoka A, Otsuka F, Iwasaki N, Shin K, Matsui A, Endo G, Kumagai Y, Ishii T, Shoji S, Ogata T, Ishizaki M, Doi M, Shimojo N. Diphenylarsinic acid poisoning from chemical weapons in Kamisu, Japan. Ann Neurol 56: 741–745, 2004. doi: 10.1002/ana.20290. [DOI] [PubMed] [Google Scholar]
- 24.Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16: 29592–29630, 2015. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int 89: 779–791, 2016. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA 86: 99–103, 1989. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Srivastava RK, Athar M. Biological and environmental hazards associated with exposure to chemical warfare agents: arsenicals. Ann N Y Acad Sci 1378: 143–157, 2016. doi: 10.1111/nyas.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Srivastava RK, Weng Z, Croutch CR, Agarwal A, Elmets CA, Afaq F, Athar M. Molecular mechanism underlying pathogenesis of lewisite-induced cutaneous blistering and inflammation: chemical chaperones as potential novel antidotes. Am J Pathol 186: 2637–2649, 2016. doi: 10.1016/j.ajpath.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Xu J, Li F, Chaudhary SC, Weng Z, Wen J, Elmets CA, Ahsan H, Athar M. Unfolded protein response signaling and MAP kinase pathways underlie pathogenesis of arsenic-induced cutaneous inflammation. Cancer Prev Res (Phila) 4: 2101–2109, 2011. doi: 10.1158/1940-6207.CAPR-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282: 20621–20633, 2007. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 32.Linthorst GE, Folman CC, van Olden RW, von dem Borne AE. Plasma thrombopoietin levels in patients with chronic renal failure. Hematol J 3: 38–42, 2002. doi: 10.1038/sj.thj.6200153. [DOI] [PubMed] [Google Scholar]
- 33.McManus J, Huebner K. Vesicants. Crit Care Clin 21: 707–718, 2005. doi: 10.1016/j.ccc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Mouret S, Wartelle J, Emorine S, Bertoni M, Nguon N, Cléry-Barraud C, Dorandeu F, Boudry I. Topical efficacy of dimercapto-chelating agents against lewisite-induced skin lesions in SKH-1 hairless mice. Toxicol Appl Pharmacol 272: 291–298, 2013. doi: 10.1016/j.taap.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.National Research Council (US) Subcommittee on Chronic Reference Doses for Selected Chemical Warfare Agents Review of the US Army's Health Risk Assessments For Oral Exposure to Six Chemical-Warfare Agents. Washington, DC: National Academies Press, 1999. [PubMed] [Google Scholar]
- 36.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguon N, Clery-Barraud C, Vallet V, Elbakdouri N, Wartelle J, Mouret S, Bertoni M, Dorandeu F, Boudry I. Time course of lewisite-induced skin lesions and inflammatory response in the SKH-1 hairless mouse model. Wound Repair Regen 22: 272–280, 2014. doi: 10.1111/wrr.12147. [DOI] [PubMed] [Google Scholar]
- 38.Peres LA, da Cunha AD Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol 35: 332–340, 2013. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- 39.Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J 79: 391–396, 2003. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.Review of the U.S. Army’s health risk assessments for oral exposure to six chemical-warfare agents. Introduction. J Toxicol Environ Health A 59: 281–526, 2000. [PubMed] [Google Scholar]
- 40.Sahu BD, Kuncha M, Sindhura GJ, Sistla R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 20: 453–460, 2013. doi: 10.1016/j.phymed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Sang Ho Lee GR. Wildlife toxicity assessment for lewisite. In: Wildlife Toxicity Assessments for Chemicals of Military Concern, edited by Reddy G, Williams M, Quinn M, Johnson MS. New York, NY: Elsevier, p. 637–649. [Google Scholar]
- 42.Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, Athar M. Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype. Sci Rep 6: 34865, 2016. doi: 10.1038/srep34865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 4: 208–214, 2015. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, Uchiyama Y, Takahara S, Imai E. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun 368: 100–106, 2008. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 46.Tewari-Singh N, Croutch CR, Tuttle R, Goswami DG, Kant R, Peters E, Culley T, Ammar DA, Enzenauer RW, Petrash JM, Casillas RP, Agarwal R. Clinical progression of ocular injury following arsenical vesicant lewisite exposure. Cutan Ocul Toxicol 35: 319–328, 2016. doi: 10.3109/15569527.2015.1127255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environ Health Perspect 98: 259–280, 1992. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods 37: 1–7, 1997. doi: 10.1016/S1056-8719(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 45: 27–37, 2007. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Woodward VK, Shelton JM, Richardson JA, Zhou XJ, Link D, Kielar ML, Jeyarajah DR, Lu CY. Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro. Am J Physiol Renal Physiol 286: F1193–F1201, 2004. doi: 10.1152/ajprenal.00379.2002. [DOI] [PubMed] [Google Scholar]