Abstract
Expression of Tamm-Horsfall protein (THP or uromodulin) is highly restricted to the kidney thick ascending limb (TAL) of loop of Henle. Despite the unique location and recent association of THP gene mutations with hereditary uromodulin-associated kidney disease and THP single nucleotide polymorphisms with chronic kidney disease and hypertension, the physiological function(s) of THP and its pathological involvement remain incompletely understood. By studying age-dependent changes of THP knockout (KO) mice, we show here that young KO mice had significant salt and water wasting but were partially responsive to furosemide, due to decreased luminal translocation of Na-K-Cl cotransporter 2 (NKCC2) in the TAL. Aged THP KO mice were, however, markedly oliguric and unresponsive to furosemide, and their NKCC2 was localized primarily in the cytoplasm as evidenced by lipid raft floatation assay, cell fractionation, and confocal and immunoelectron microscopy. These aged KO mice responded to metolazone and acetazolamide, known to target distal and proximal tubules, respectively. They also had marked upregulation of renin in juxtaglomerular apparatus and serum, and they were hypertensive. Finally, the aged THP KO mice had significant upregulation of Na-coupled urate transporters Slc5a8 and Slc22a12 as well as sodium-hydrogen exchanger 3 (NHE3) in the proximal tubule and elevated serum uric acid and allantoin. Collectively, our results suggest that THP deficiency can cause progressive disturbances in renal functions via initially NKCC2 dysfunction and later compensatory responses, resulting in prolonged activation of the renin-angiotensin-aldosterone axis and hyperuricemia.
Keywords: hyperuricemia, lipid rafts, NKCC2, Tamm-Horsfall protein, thick ascending limb, uromodulin
INTRODUCTION
Tamm-Horsfall protein (THP), also named uromodulin, is the most abundant protein in normal human urine (17, 27, 46). It is made by the tubular epithelial cells comprising the functionally specialized thick ascending limb (TAL) of loop of Henle in the kidney. Initially synthesized in the rough endoplasmic reticulum (rER) and modified with secondary modifications in the Golgi complex, the protein is later transported to the apical surface of TAL and anchored in the luminal leaflet of the lipid bilayer via a glycophosphatidylinositol (GPI) linkage (54). It has been recently demonstrated, however, that the protein portion of THP is cleaved and released into the urine by the serine protease hepsin (9) but not by GPI-specific phospholipases as previously thought (48). Whether the GPI linkage of THP plays a role in targeting of THP to the apical surface of TAL and whether the lack of it would lead to cytoplasmic localization of THP and/or its release from the basolateral surface into renal interstitium to regulate interstitial inflammation and systemic neutrophil homeostasis (15, 16, 18, 31, 33) require experimental analysis. Nonetheless, there is a fairly large body of evidence indicating that GPI-linked proteins in general preferentially partition with the lipid microdomains or lipid rafts on the cell surface membrane (8, 39, 49), although whether THP behaves in a similar manner at the apical surface of TAL and if, by doing so, THP affects the targeting of other surface membrane proteins such as ion transporters remain incompletely understood.
Since its first description THP has been assigned a wide range of physiological functions and pathological involvements, including effects on immunosuppression, water impermeability of TAL, renal cast formation, urolithiasis, bacterial trapping, and tubulointerstitial nephritis (24, 27, 46, 54). However, its exact function(s) could not be experimentally verified or refuted until relatively recently, thanks to disease association studies and technological advancements. In particular, landmark genetic linkage analysis discovered THP gene mutations in familial juvenile hyperuricemic nephropathy and in what was formerly known as type II medullary cystic kidney disease (6, 7, 10, 11, 28, 51). Transmitted in an autosomal dominant fashion, THP mutations, which often involve the cysteine residues (i.e., loss or gain), can cause protein misfolding, aggregation, and retention in the rER, leading to rER overload and cytotoxicity (28). Patients with these mutations can develop gout at an early age and chronic kidney disease that progresses to end-stage renal kidney disease. In addition to the inherited mutations, single nucleotide polymorphisms (SNPs) have been identified in the THP gene by genome-wide association studies (25, 52). Some of the SNPs located in the noncoding regions have been shown in patients and animal models to alter THP expression and affect glomerular and tubular functions and risks of developing chronic kidney disease (CKD) and hypertension (21, 58). However, divergent results exist regarding the urinary levels of THP and the risk of CKD, some showing that high levels of THP are associated with increased risks of CKD while others show that high levels of THP predict a lower risk of CKD (14, 19, 58). Another recent study by Steubl et al. (56) concluded that plasma THP is a robust marker for renal function and it may allow the identification of early stage CKD. The authors also suggested that, as a marker of tubular secretion, THP might be a more accurate reflection of the remaining nephron mass and hence the overall renal function than that of the glomerular filtration during early stages of CKD.
Another recent advancement that has significantly improved our understanding of THP’s biological functions relates to the development of genetically engineered mice. For instance, knockout (KO) mice lacking THP exhibit reduced ability to clear, and increased bladder retention of, type 1-fimbriated Escherichia coli inoculated via the transurethral route (4, 13, 35, 38). THP KO mice also are not as efficient as their wild-type (WT) counterparts to eliminate circulatory cytokines, due in part to a significantly decreased glomerular filtration rate (GFR) (29). Contrary to a previously suggested role for THP to promote acute kidney injury, THP is protective from it, because THP KO mice are significantly more susceptible to experimentally induced acute kidney injury than normal controls (15–18). Notwithstanding this progress, the role of THP in chronic kidney disease, either as a promoter or a protector, remains unsettled. Thus far, most studies of THP KO mice have focused on relatively young animals (3, 34, 36, 44), probably due to the prohibitively high cost of generating, maintaining and analyzing sufficient number of aged animals. The longer term effects of THP deficiency on renal physiology have not been assessed, and consequently, the THP KO models have not been as informative as they can be in helping elucidate the molecular mechanisms of kidney diseases. The present study was designed to address some of these critical questions.
MATERIALS AND METHODS
Experimental animals.
KO mice lacking expression of THP and WT control mice that were generated previously (34, 35) were both maintained in a 129/SvEv inbred background in a specific pathogen-free facility. Breeding age siblings (2–3 mo old) of either genotype were crossed to produce a sufficient number of offspring that were carefully genotyped before experiments and regenotyped upon death. Young and aged mice were operationally defined as 1- and 12-mo old mice, respectively, for the great majority of our studies. An intermediate time point (5-mo-old KO and WT mice) was included for some of the analyses (see below). Data from the male mice were presented here. All animal-related experiments were carried out in accordance with the regulatory policies and after the approval of a protocol by the Institutional Animal Care and Use Committee of New York University School of Medicine.
Twenty-four-hour urine measurement.
Mice were placed individually in single-mouse metabolic cages (Nalgene) in a procedure room with 12-h light-dark cycles. Collection vials were preloaded with 20 μl of 10% thymol and 10 μl of a Halt protease inhibitor cocktail (100 mM AEBSF, 80 μM aprotinin, 5 mM bestatin, 1.5 mM E64, 2 mM leupeptin, and 1 mM pepstatin A; ThermoScientific) and 10 μl of 0.5 M EDTA. At the end of the 24-h collection, urine volumes were recorded and urine samples spun at 500 g for 5 min, and the supernatants were stored at −80°C until further use. Urine output was also measured after mice were pair fed. Briefly, WT and THP KO mice (both 12 mo of age; 8/genotype) were housed individually in metabolic cages and fed ad libitum with standard diet and water for a week, with food intake measured daily. The daily food intake of the THP KO mice was then averaged and used to feed all the individually housed mice (both WT and THP KO) for 1 wk. The 24-h urine, water intake, and body weight of each mouse were measured and recorded.
Diuretic treatment.
Separate groups of THP KO and WT control mice were administered intraperitoneally vehicle only (0.9% NaCl) or the vehicle containing diuretics (all purchased from Sigma-Aldrich, Saint Louis, MO): furosemide (15 mg/kg), metolazone (50 μg/kg), or acetazolamide (200 mg/kg). Twenty-four-hour urine collection commenced immediately after one injection of furosemide or metolazone, whereas acetazolamide was injected once daily for 3 consecutive days and the 24-h urine collection was started immediately after the third injection.
Lipid raft floatation assay.
Freshly dissected kidneys from THP KO and WT mice were homogenized in a Dounce homogenizer followed by sonication on ice in a neutral pH buffer containing 250 mM sucrose, 10 mM triethanolamine, 1% Triton X-100, and a protease inhibitor cocktail (see above). The homogenates were centrifuged at 300 g for 10 min at 4°C. The nuclei-free, protein concentrations of the homogenates were determined using a BCA assay kit (Pierce), and 1 mg of each of the nuclei-free, Triton X-100-insoluble protein extract was loaded atop a stepwise sucrose gradient comprising 5, 10, 15, 20, 25, 30, 35, and 40% sucrose made in 50 mM HEPES (pH 7.4), 1% Triton X-100 and 10 mM NaCl. After centrifugation at 200,000 g for 24 h at 4°C, 1-ml fractions were collected from each gradient interphase and 20 μl of each fraction were mixed with 5 μl of 20% SDS and 20 μl 2× SDS-PAGE loading buffer. After SDS-PAGE, the proteins were electrophoretically transferred onto PVDF membrane and subjected to Western blotting using an anti-NKCC2 antibody (Developmental Studies Hybridoma Bank; 1:5,000 dilution) followed by a peroxidase-conjugated secondary antibody. The membrane was stripped and reblotted consecutively with an anti-flotillin antibody (Santa Cruz Biotechnology; 1:500 dilution) and an anti-THP (R&D Systems; dilution 1:1,000). The membranes were developed using a chemiluminescent method and exposed to X-ray film.
Cell fractionation.
Cell fractionation was done with a Plasma Membrane Protein Extraction Kit (Abcam). Briefly, kidneys from 12-mo-old WT and THP KO mice were homogenized and the homogenates were centrifuged at 700 g at 4°C for 10 min to separate into soluble and insoluble fractions, the latter of which were resuspended, centrifuged, extracted, pelleted, and used as plasma membrane fractions. The cytosol-enriched and plasma membrane-enriched proteins (20 μg/lane) were resolved on an 8% SDS-PAGE, transferred to PVDF membrane; and blotted with primary antibodies followed by peroxidase-conjugated secondary antibodies. The primary antibodies were against NKCC2 (Developmental Studies Hybridoma Bank; 1:5,000 dilution); phospho-NKCC2 (Thr96 and Thr101) (Millipore; 1:2,000 dilution); flotillin (Santa Cruz Biotechnology; 1:500 dilution); and β-actin (Sigma-Aldrich; 1:8,000 dilution). For quantitative analysis, integrated band densities were measured using ImageJ software (National Institutes of Health, Bethesda, MD) and normalized to flotillin and β-actin for plasma membrane-enriched fractions and cytosol-enriched fractions, respectively.
Western blotting analysis of urate transporters and sodium-hydrogen exchanger 3.
Kidneys from 12-mo-old WT and THP KO Mice were homogenized in a lysis buffer containing 20 mM Tris·HCl (pH 7.5), 50 mM NaCl, 10% SDS, and a mixture of protease inhibitor cocktail (ThermoScientific). After centrifugation at 17,000 g at 4°C for 20 min, the supernatants were collected and their protein concentrations determined by the BCA reagent. Western blotting was carried using rabbit anti-slc5a8 antibody (Novus; 1:2,000 dilution) or rabbit anti-slc22a12 (URAT1) antibody (Abbiotec; 1:2,000 dilution) or rabbit anti-sodium-hydrogen exchanger 3 (NHE3; Novus; dilution 1:1,000). β-Actin was used as a loading control (Sigma-Aldrich; 1:8,000 dilution), and densitometry was performed as described above.
Confocal immunofluorescence and immunoelectron microscopy.
Kidneys were freshly dissected out and routinely fixed and paraffin-embedded. Four-micrometer thick sections were deparaffinized, hydrated, and microwaved in a citrate buffer (pH 6.0) to unmark the antigens. The sections were then incubated first with 5% BSA in PBS to block nonspecific binding sites. For single-staining, the sections were incubated with the primary antibody (rabbit anti-NKCC2 antibody from Alpha Diagnostic International (No. NKCC21-S; dilution 1:1,000) or rabbit anti-Slc22a12 (URAT1) (Abbiotec; dilution 1:200); or rabbit anti-Slc5a8 (Novus; dilution 1:200); or rabbit anti-NHE3 (Novus; dilution 1:100), followed by a secondary antibody conjugated with Alexa-Fluor 488 or 594. For double staining, the sections were incubated with primary antibodies (rabbit anti-NKCC2 antibody from Alpha Diagnostic International; No. NKCC21-S; dilution 1:1,000; and goat anti-flotillin; Santa Cruz Biotechnology; dilution 1:200), followed by secondary antibodies (Alexa Fluor 594-conjuated donkey anti-mouse IgG and Alexa Fluor 488-conjuated donkey anti-goat IgG). For triple staining, the sections that had been preincubated with 5% BSA in PBS were incubated with the following primary antibodies: sheep anti-THP (R&D Systems; dilution 1:200); rabbit anti-NKCC2 antibody (Alpha Diagnostic International; No. NKCC21-S; dilution 1:1,000); and goat anti-renin (Santa Cruz Biotechnology; 1:50), followed by corresponding secondary antibodies conjugated with Alexa-Fluor 594, 488, and 647. After DAPI counterstaining, the sections were subject to confocal microscopy.
For immunoelectron microscopy, inner medullary zones of mouse kidneys were excised and processed using a postembedding method. Briefly, the tissue slices were fixed in a freshly prepared fixative (pH 7.4) containing 3% paraformaldehyde in 0.1 M sodium cacodylate buffer, 0.1% glutaraldehyde, and 4% sucrose. They were then washed, dehydrated, and embedded in Lowicryl K4M (Polysciences, Warrington, PA) and polymerized with an ultraviolet lamp (360 nm). Ultrathin sections (80 nm) were cut and mounted on Formvar-carbon-coated nickel grids. After incubation with the anti-NKCC2 antibody at 4°C overnight, protein A-conjugated 10-nm gold particles were added, and the sections were stained with uranyl acetate and lead citrate, before being examined with a Philips CM-12 electron microscope. The specificity of the anti-NKCC2 antibody for immunoelectron microscopy has not been tested using NKCC2 KO mice. Semiquantification of the immunogold labeling of the anti-NKCC2 antibody in WT and THP KO mice was performed on ultrastructural micrographs. Gold particles on the apical membrane and cytoplasm were counted from five micrographs from each of the two WT and the two KO mice examined (total of 10 micrographs per genotype). One TAL cell per micrograph was randomly selected to count the particles, and three independent areas for each TAL cell were counted. “Apical membrane” was defined as a length of 1.5 μm and a depth of 0.01 μm from both sides of the outer and inner membrane leaflet. “Cytoplasm” was defined a cytoplasmic area (1.5 × 1.5 μm) immediately below and within 1.5 μm of apical membrane counted. Collectively, a total of 30 apical membrane and cytoplasmic areas were counted for each genotype, and a total number of 934 particles for WT and 1,037 particles for KO mice were counted. The total number of gold particles for “apical membrane” or “cytoplasm” was divided by 30 (total counted areas), and compared between WT and KO mice.
Real-time quantitative PCR.
Total RNAs were extracted from THP KO and WT mouse kidneys using a TRIzol RNA extraction kit (Invitrogen). Double-stranded cDNAs were synthesized and used as templates for real-time PCR using a QuantiTect SYBR Green PCR kit (Qiagen). The primers were as follows: Slc5a8/forward, 5′-CTCCGGGTCCCAGGGTGTCA-3′ and Slc5a8/reverse, 5′-GAGTGCACGGTGGTGGCAGG-3′; Slc5a12/forward, 5′-GAGCACAGTGGCTGCCAGCA-3′ and Slc5a12/reverse, 5′-ATCCCAGGCAGCCCACAGCA-3′; and Slc22a12/forward, 5′-GGTTTACGACCACAGCACCT-3′ and Slc22a12/reverse, 5′-GGACACGGACACCAGAAGAT-3′. The PCR products were quantified by measuring the incorporation of SYBR Green I into newly synthesized DNA. The values were calculated using ΔΔCt formula and expressed as ratios vs. the simultaneously amplified β-actin using specific primers (forward, 5′-TGTTACCAACTGGGACGACA-3′ and reverse, 5′-TCTCAGCTGTGG TGGTGAAG-3′). PCR conditions were 95°C for 15 min for the first cycle; 95°C for 10 s, 60°C for 15 s, and 72°C for 15 s for 45 cycles.
Measurement of serum uric acid and allantoin.
Whole blood was collected by retro-orbital bleeding with mice placed under anesthesia with ketamine and xylazine. Sera were separated out by spinning clotted blood at 2,000 g for 10 min at 4°C, and kept at −80°C until analysis. Uric acid was measured with uric acid assay kit (BioVision, Milpitas, CA) according to the manufacturer’s instructions. For allantoin assay, 100 μl of serum from each mouse were incubated with a working solution of allantoinase for 15 min at 37°C and supplemented with allantoate amidohydrolase and incubated for 10 min at 37°C. The reactions were read by spectrometry at 340 nm, and allantoin levels were calculated by plotting in a standard curve prepared using allantoin standards.
Serum renin, angiotensin II, and aldosterone assays.
Sera were diluted 20 times in mouse renin assay buffer (AnaSpec, Fremont, CA), and 50 μl of the diluted sera were transferred into the ELISA plates and assayed following the manufacturer’s instructions. Angiotensin II was measured similarly using an Angiotensin II Enzyme Immunoassay Kit (RayBiotech, Norcross, GA), and aldosterone was determined using a kit from Assay Designs (Ann Arbor, MI).
Measurement of serum Na, Cl, and K.
Mouse sera were diluted 50-fold in an assay buffer after which 10 µl of the diluted sera were transferred into ELISA plates and assayed using a sodium assay kit (Biovision, Milpitas, CA), a chloride assay kit (Abcam, Cambridge, MA), or a potassium assay kit (Diazyme Laboratories, Poway, CA), following manufacturers’ instructions.
Urine chemistry.
Key urine chemical indexes were determined using a commercial service (Preclinical Research Services, IDEXX Laboratories, Westbrook, Maine).
Blood pressure measurement.
Blood pressure was measured using a CODA 8-Channel High Throughput Non-Invasive Blood Pressure System (Kent Scientific, Torrington, CT). The setup utilizes an occlusion tail-cuff system to measure blood pressure by determining the tail blood volume using a differential pressure transducer. The mice were acclimated to the measurement procedure for 5 days before the measurements were taken. Briefly, the individual mice were placed in medium-size mouse holders on a warm platform and acclimated for 10 min. After pilot experiments, small occlusion cuffs were determined to be the most appropriate, and they were placed close to the base of the tails and securely connected to the mouse holder. The VPR cuffs were then placed within 2 mm of the occlusion cuffs and connected to the CODA controller. The settings were the following: maximum occlusion pressure: 250 mmHg; deflation time: 20 s; minimum volume: 15 μl; acclimation cycles: 5; time between sets: 30 s; cycles: 20; and time between cycles: 5′. Systolic and diastolic values were recorded.
Statistical analysis.
Student's t-test (two-tailed) was used to assess the statistical significance between two experimental groups using a web-based SPSS software. Values are expressed as averages and SDs for all the assays. P < 0.05 was considered statistically significant.
RESULTS
THP deficiency in young KO mice led to NKCC2 dysfunction and salt wasting.
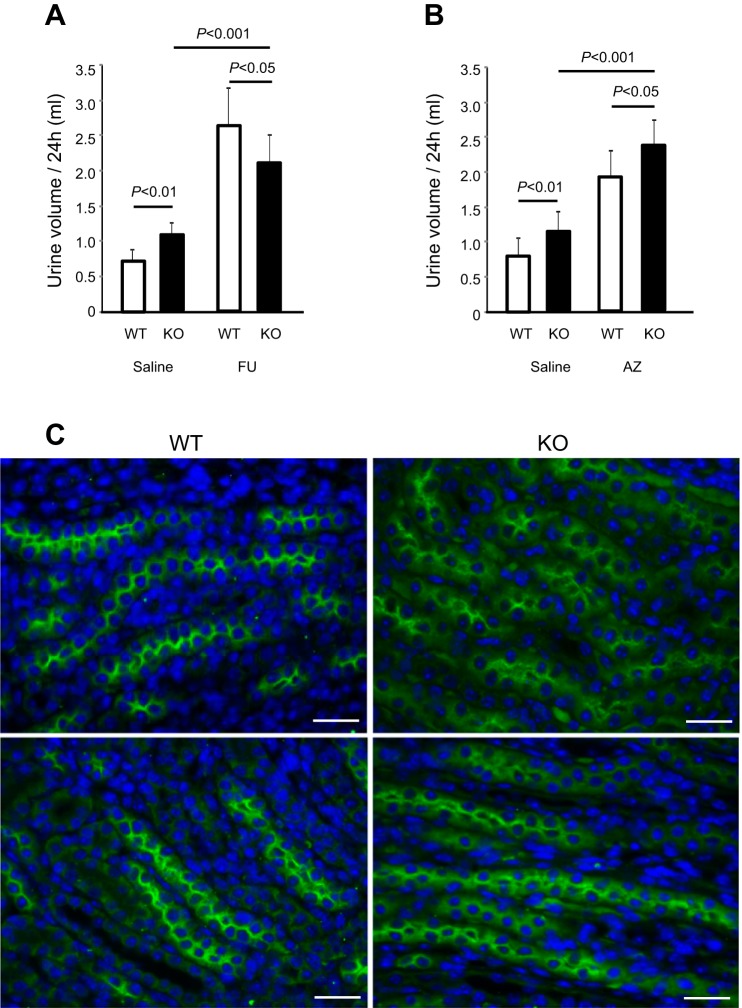
To examine the initial effects of THP deficiency on TAL in relation to its salt and water homeostasis, we compared the 24-h urine volume of 1-mo-old THP KO mice with that of the age-matched WT mice. Of the two independent cohorts, the KO group had ~70% more urine than the WT group (P < 0.01; Fig. 1, A and B, left. Urine chemistry showed that the KO mice excreted significantly greater amounts of Na, Cl, and K over a 24-h period (Table 1). Administration of furosemide, a diuretic that inhibits NKCC2 in the TAL (22), more than tripled the urine output in WT mice but only doubled the urine output in THP KO mice (Fig. 1A). Administration of another diuretic, acetazolamide, which inhibits carbonic anhydrase in the proximal tubule (53), increased the urine volume of THP KO mice significantly more than untreated KO mice (P < 0.001; Fig. 1B) as well as treated WT mice (P < 0.05; Fig. 1B). Immunofluorescent staining of NKCC2 in the kidneys showed that, whereas the protein was located primarily at the apical surface of TAL in WT mice, the apical staining was weaker and the cytoplasmic staining stronger in TAL of THP KO mice (Fig. 1C). Together, these data suggest that THP deficiency in young mice partially compromised the apical translocation and transport function of NKCC2, leading to salt wasting from the TAL.
Fig. 1.
Urine output, response to diuretics and localization of Na-K-Cl cotransporter 2 (NKCC2) in young Tamm-Horsfall protein (THP) knockout (KO) mice. A and B: 1-mo-old THP KO mice and age-matched wild-type (WT) mice were subject to 24-h urine measurement using single-mouse metabolic cages, after they were intraperitoneally administered with either normal saline as control or normal saline containing furosemide (FU; A) or acetazolamide (AZ; B); n = 8/diuretic/genotype for both (A and B). Note that, at the steady state, young THP KO mice excreted significantly more urine than age-matched WT mice (P < 0.01) in both cohorts (A and B); that young THP KO mice responded sensitively to furosemide by producing more urine than without furosemide treatment (P < 0.001), albeit less than the WT mice treated with furosemide (P < 0.05); and that young THP KO mice responded more robustly to acetazolamide than WT mice (P < 0.05). C: immunofluorescent staining of NKCC2 in the kidneys of 2 individual 1-mo-old WT and THP KO mice, representing 8 mice per genotype analyzed and showing that NKCC2 was predominantly located at the apical surface of thick ascending limb (TAL) in WT mice, but it was reduced at apical surface and increased in the cytoplasm in THP KO Mice. Bars equal = 30 μm.
Table 1.
Urine chemistry of young and aged WT and THP KO mice (24-h value normalized by body weight)
| Young Mice (1-mo-old) Regularly Fed |
Aged Mice (12-mo-old) Regularly Fed |
Aged Mice (12-mo-old) Pair Fed |
||||
|---|---|---|---|---|---|---|
| WT (n = 16) | KO (n = 16) | WT (n = 19) | KO (n = 18) | WT (n = 8) | KO (n = 8) | |
| Na, µEq/g | 5.873 (2.180) | 8.017 (2.446)* | 4.862 (1.615) | 3.087 (1.257)* | 6.917 (1.480) | 4.514 (0.900)* |
| Cl, µEq/g | 8.076 (3.426) | 14.400 (3.649)* | 6.597 (2.632) | 4.786 (2.151)* | 9.892 (3.753) | 11.848 (8.695) |
| K, µEq/g | 10.216 (4.251) | 22.786 (4.966)* | 7.891 (3.617) | 6.521 (2.654) | 10.785 (5.547) | 11.337 (3.036) |
| Ca, µg/g | 6.601 (3.189) | 14.731 (4.497)* | 2.790 (2.381) | 1.990 (1.301) | 5.604 (1.381) | 4.975 (2.175) |
| Mg, µg/g | 14.411 (6.926) | 42.813 (8.823)* | 9.750 (6.732) | 11.584 (4.712) | 12.510 (3.634) | 10.680 (6.530) |
| P, µg/g | 122.370 (50.793) | 242.955 (65.253)* | 81.612 (31.974) | 67.123 (19.396) | 102.6 (25.663) | 103.995 (63.639) |
| UA, µg/g | 7.762 (4.050) | 9.270 (3.518) | 5.751 (4.090) | 3.405 (2.045)* | 3.658 (1.492) | 2.875 (1.567) |
| Cr, µg/g | 19.269 (8.093) | 46.411 (8.421)* | 13.356 (3.354) | 9.836 (3.567)* | 15.506 (4.029) | 12.979 (2.282) |
Values shown are averages (SD). Urine chemistry of young (1-mo-old) and aged (12-mo-old) Tamm-Horsfall protein (THP) knockout (KO) and wild-type (WT) mice (24-h value normalized by body weight). For aged mice, measurements were performed under either regular diet or pair-feeding condition (duration: 1 wk).
Group comparisons with significant differences in statistical analysis between THP KO and WT mice.
THP deficiency in aged mice led to oliguria and differential responses to diuretics.
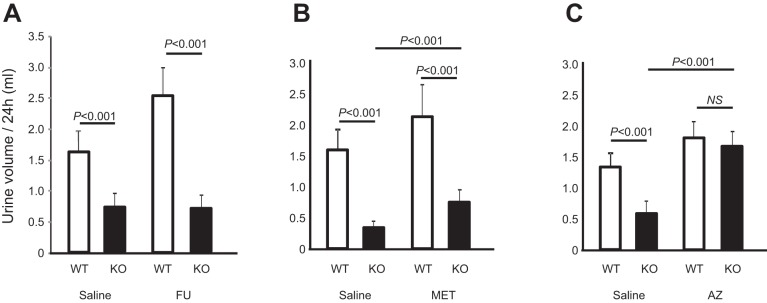
As opposed to the young THP KO mice which were polyuric, aged (12-mo old) THP KO mice had much less 24-h urine output, e.g., only about 30–40% of their age-matched WT controls in all four independent cohorts (P < 0.01; Fig. 2A; P < 0.001; Fig. 3, A–C). As we previously reported, the urine of aged THP KO mice was highly concentrated (30). The total 24-h excretion of Na and Cl as we determined here in regularly fed mice was, however, considerably lower than age-matched WT controls (Table 1), likely due to marked volume contraction. The uric acid excretion was significantly lower (see later), as was the total 24-h creatinine excretion (Table 1, middle 2 columns). In pair-fed mice (Table 1, right 2 columns), the differences of these parameters moderated, with the exception of Na, which remained significantly lower in THP KO mice than in WT controls. Simultaneous measurement of water consumption and body weight of an aged mouse cohort that underwent 24-h urine collection showed that the aged THP KO mice consumed significantly less water (P < 0.01; Fig. 2B) and weighed significantly less (P < 0.01; Fig. 2C) than the aged THP WT mice. On an individual mouse basis, urine output (Fig. 2A) had a fairly good correlation with water consumption (Fig. 2B and see later).
Fig. 2.
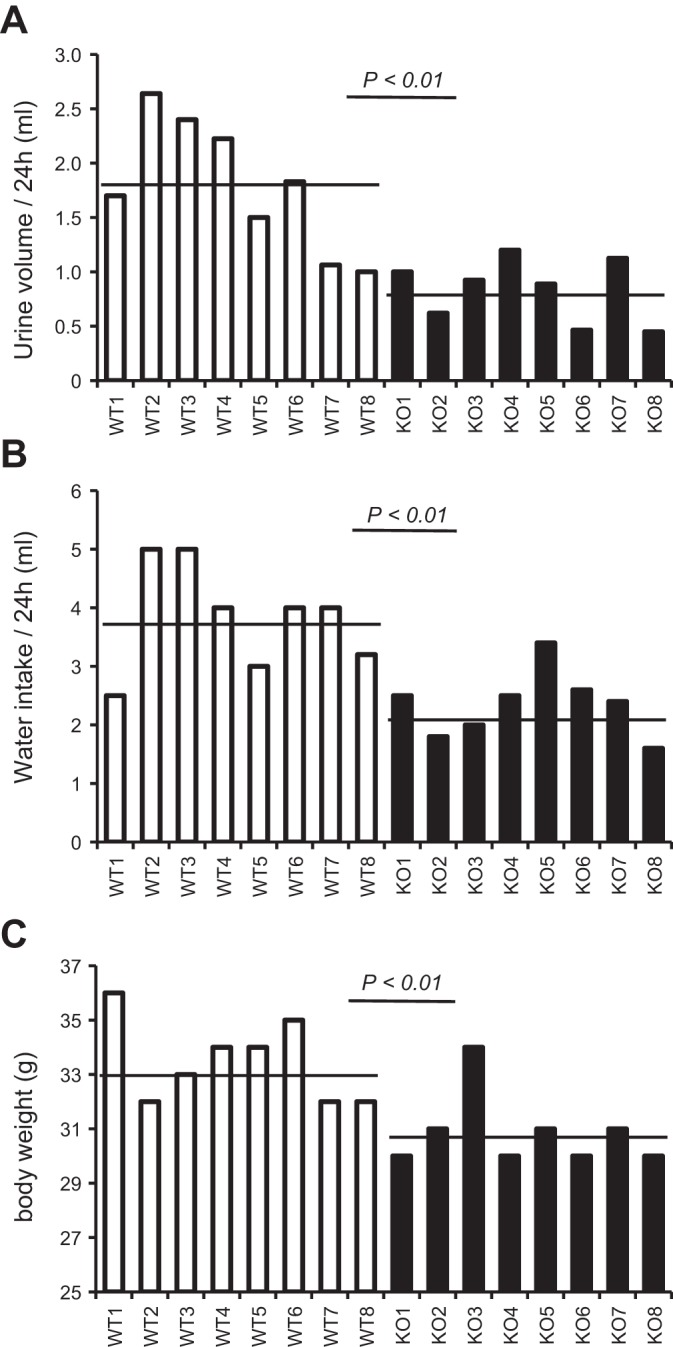
Urine output, water consumption, and body weight of aged THP KO mice. A: 12-mo-old THP KO mice and age-matched WT control mice (n = 8 each genotype) were subjected to 24-h urine measurement using metabolic cages. B: the water supply of the same individual mice was measured at the beginning and end of the 24-h period, and the difference was calculated to determine the water consumption. C: the body weight was measured immediately before the 24-h urine collection. Each column represents the reading from the same mouse for the aforementioned 3 parameters aligned vertically, and the horizontal bars denote the averages. Note the significantly lower 24-h urine volume in aged THP KO mice, compared with the WT controls, and that this corresponded with reduced water intake and body weight.
Fig. 3.
Response to diuretics in aged THP KO mice. A–C: 12-mo-old THP KO mice and age-matched WT mice were subject to 24-h urine measurement, after they were intraperitoneally administered with normal-saline as control or normal saline containing furosemide (FU; A); metolazone (MET; B); or acetazolamide (AZ; C). n = 8/group. Note that, at the steady state, aged THP KO mice excreted considerably less urine than age-matched WT mice (P < 0.001) in all 3 cohorts (A–C), that aged THP KO mice were completely unresponsive to furosemide (A), and that aged THP KO mice responded moderately to metolazone (B) and robustly to acetazolamide (C). NS, no statistical significance.
Upon diuretic treatment, the aged THP KO mice were remarkably unresponsive to furosemide, producing no extra urine (Fig. 3A). They were, however, moderately responsive to metolazone, a thiazide-like diuretic that inhibits the sodium-chloride symporter NCC in the distal tubule (42) (Fig. 3B). Interestingly, aged THP KO mice were highly sensitive to acetazolamide, which nearly tripled the urine output in aged THP KO mice, to a level comparable to that in acetazolamide-treated WT mice (Fig. 3C).
THP deficiency impaired the apical membrane translocation of NKCC2.
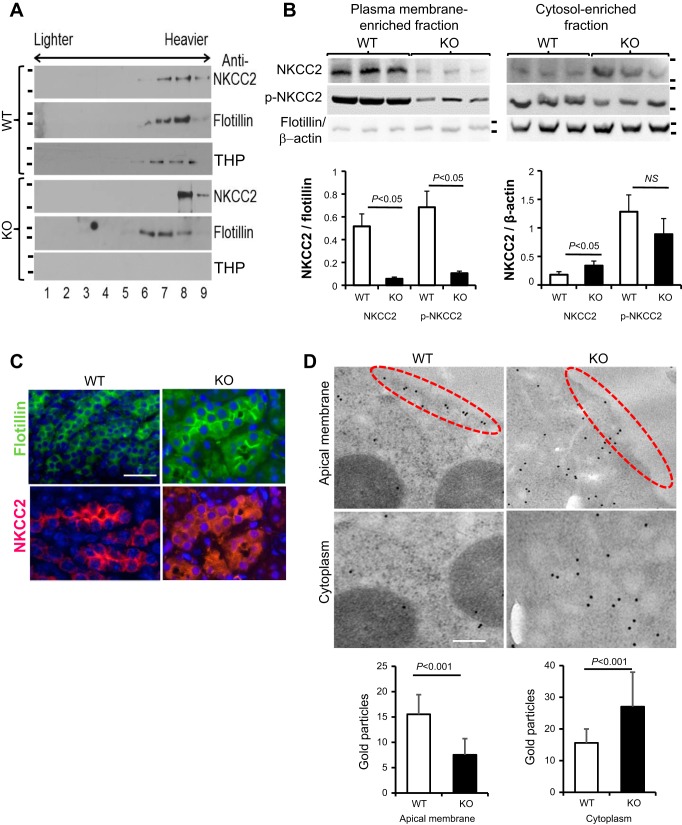
Because THP belongs to a family of GPI-linked proteins that tend to partition with the lipid rafts of the surface membrane (49), we next assessed the status of NKCC2 in relation to the lipid raft association in the presence or absence of THP, using lipid raft floatation assay. Upon loading Triton X-100-insoluble fractions (operationally defined as lipid raft-associated fractions) of the kidney extracts from WT mice on a sucrose gradient, NKCC2 copartitioned precisely with a lipid-raft marker, flotillin, in lipid-rich, “lighter” fractions 6 and 7 (Fig. 4A), which are representative of the apical membrane. In contrast, in the absence of THP in aged THP KO mice, NKCC2 was absent in the “lighter” fractions 6 and 7 (Fig. 4A). To corroborate these results, we carried out cell fractionation into plasma membrane-associated and cytosol-associated fractions using kidneys of aged THP WT and KO mice. We then detected NKCC2 and phosphorylated NKCC2 (p-NKCC2) by Western blotting and semiquantified the proteins using densitometry using flotillin as a reference for plasma membrane-associated fraction and β-actin as a reference for cytosol-associated fraction. We found a marked reduction of NKCC2 and p-NKCC2 in the plasma membrane-associated fraction and an increase of NKCC2 in the cytosol-associated fraction in THP KO mice (Fig. 4B). Confocal immunofluorescent microscopy using anti-NKCC2 revealed considerably less apical membrane labeling and more cytoplasmic labeling of NKCC2 in aged THP KO mice than in WT mice (Fig. 4C). In comparison, the apical labeling of flotillin, an apical membrane marker, did not significantly differ between THP KO and THP WT mice. Immunoelectron microscopy/gold-colloid staining revealed that NKCC2 was closely associated with the TAL’s luminal membrane in WT mice but was largely absent in the same structure in aged THP KO mice (Fig. 4D, dashed ovals). Significantly more gold particles were found in the cytoplasm of THP KO mice than in that of WT mice, a result confirmed by semiquantification (Fig. 4D, bottom).
Fig. 4.
Effects of THP loss on the apical translocation of NKCC2. A: lipid raft flotation assay of the partitioning of NKCC2 in the lipid rafts. Triton X-100-insoluble protein extracts from 12-mo-old THP KO mice and age-matched WT mice were centrifuged on a stepwise sucrose density gradient (5–40% in 5% increments), and equal volumes from different fractions (1–9: lighter density fractions to the left and heavier density fractions to the right) were resolved on an SDS-PAGE and immunoblotted with anti-NKCC2 and reblotted with anti-flotillin, a lipid raft marker, and anti-THP. The results are representative of 3 similar experiments. Note the lack of partitioning of NKCC2 in the lighter fractions (6, 7) in THP KO mice, as opposed to the situation in WT mice and the coexistence of NKCC2 and THP in the same fractions in WT mice. Short bars on the left denote positions of molecular mass standards (from top to bottom): 170 and 135 kDa; 55 and 40 kDa; 135 and 100 kDa; 170 and 135-kDa; 55 and 40 kDa; 135 and 100 kDa. B, top: cellular fractionation and Western blotting. Renal cells from aged WT and THP KO mice (n = 3 per genotype) were fractionated into plasma membrane-enriched and cytosol-enriched fractions. The resultant fractions were dissolved in SDS-PAGE loading buffer and analyzed by Western blotting using anti-NKCC2 and anti-p-NKCC2 antibodies. Anti-flotillin and anti-β-actin served as loading controls for membrane and cytosol, respectively. Short bars on the right denote positions of molecular mass standards (from top to bottom): 170 and 135 kDa; 170 and 135 kDa; 55 and 40 kDa. B, bottom: densitometry of the Western blotting results shown is top, expressed as ratio to flotillin or -β−actin. Note the remarked reduction of NKCC2 and p-NKCC2 in the membrane fraction and the significant increase of NKCC2 in the cytosol. NS, no statistical significance. C: confocal immunofluorescent microcopy showing colocalization of NKCC2 with membrane marker flotillin at the apical surface of TAL of aged WT mice and, in contrast, the reduced apical labeling and increased cytoplasmic labeling of NKCC2 in the TAL of aged THP KO mice. All panels are of the same magnification; bar = 30 μm: ×400. D: immunoelectron microscopy of NKCC2 of 12-mo old THP KO mice and age-matched WT mice showing apical association of NKCC2 in WT mice and the cytoplasmic staining of NKCC2 in THP KO mice. Please refer to materials and methods for the operationally defined “Apical membrane” and “Cytoplasm”, and procedures of enumeration and tabulation of the gold particles in these two compartments. Bar = 200 nm. Bottom: average number of gold particles and SD per counted area in the apical membrane and cytoplasm.
Marked upregulation of renin in juxtaglomerular apparatus in aged THP KO mice but not in young THP KO mice.
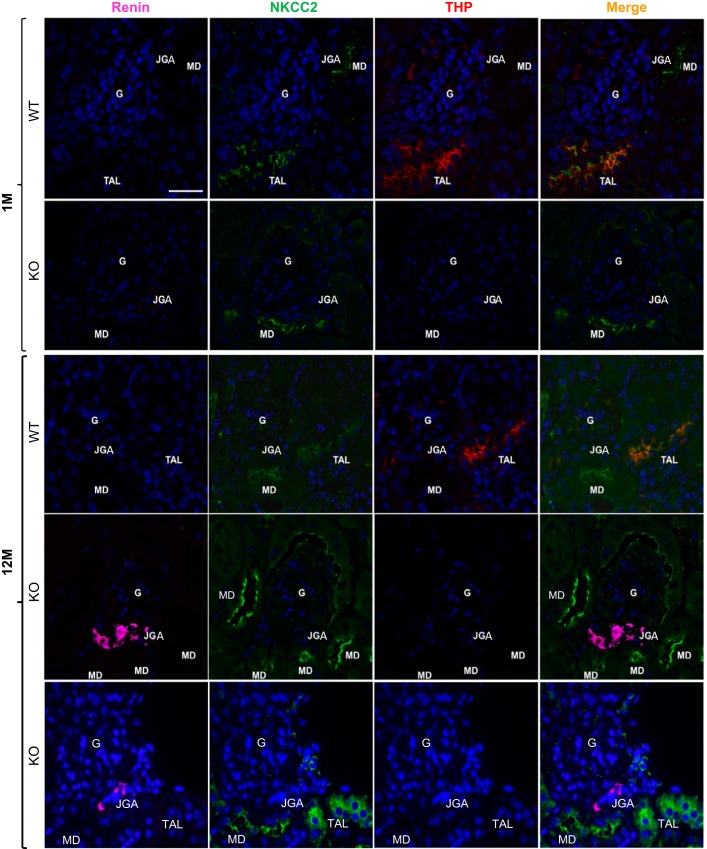
To determine whether renal compensatory responses underlie some of the age-dependent changes during THP deficiency, we compared the expression of NKCC2, renin, and THP in both young and aged THP KO mice with their age-matched WT mice as controls by triple immunofluorescent confocal microscopy. In young WT mice, THP was coexpressed with NKCC2 in the TAL (orange color in merged image) but was absent from macula densa (MD) cells (Fig. 5, 1st row), the latter consistent with the previous observations (2, 41). Renin was undetectable at juxtaglomerular apparatus (JGA). In young THP KO mice, THP was absent and NKCC2 had reduced apical staining in TAL (also see Fig. 1C). However, the apical localization of NKCC2 at MD was unaffected (Fig. 5, the 2nd row). Only a slight upregulation of renin at JGA was observed after image zoom-in in young THP KO mice (Fig. 5, the 2nd row). In aged WT mice, the expression of NKCC2, renin, and THP was similar to that in the young WT mice (Fig. 5, the 3rd row). In stark contrast, in aged THP KO mice, there was an increased expression of NKCC2 at the apical surface of MD and a marked upregulation of renin at JGA (Fig. 5, 4th and 5th rows), as compared with aged WT mice (Fig. 5, 3rd row) and young THP KO mice (Fig. 5, 2nd row). In contrast, NKCC2 at the TAL of aged THP KO mice was primarily cytoplasmic (Fig. 5, 5th row).
Fig. 5.
Triple immunofluorescent detection and localization of renin (color purple), NKCC2 (green), and THP (red) in young (1-mo-old) and aged (12-mo-old) THP KO mice and their age-matched WT controls. G, glomerulus; JGA, juxtaglomerular apparatus; TAL, thick ascending limb; MD, macula densa. Note: 1) in young WT mice, NKCC2 and THP colocalized at the apical surface of TAL; MD was positive for NKCC2 but lacked THP; 2) in young THP KO mice, MD did not have apparent upregulation of NKCC2 and JGA was negative for renin; 3) in aged WT mice, NKCC2 and THP colocalized at the apical surface of TAL and MD was positive for NKCC2 but lacked THP; and 4) in aged THP KO mice, NKCC2 remained at the apical surface in MD, along with marked upregulation of renin in JGA, but it was primarily cytoplasmic in TAL cells. All the panels are of the same magnification, and bar = 30 μm.
Elevated serum renin, angiotensin II, aldosterone, and blood pressure in older THP KO mice.
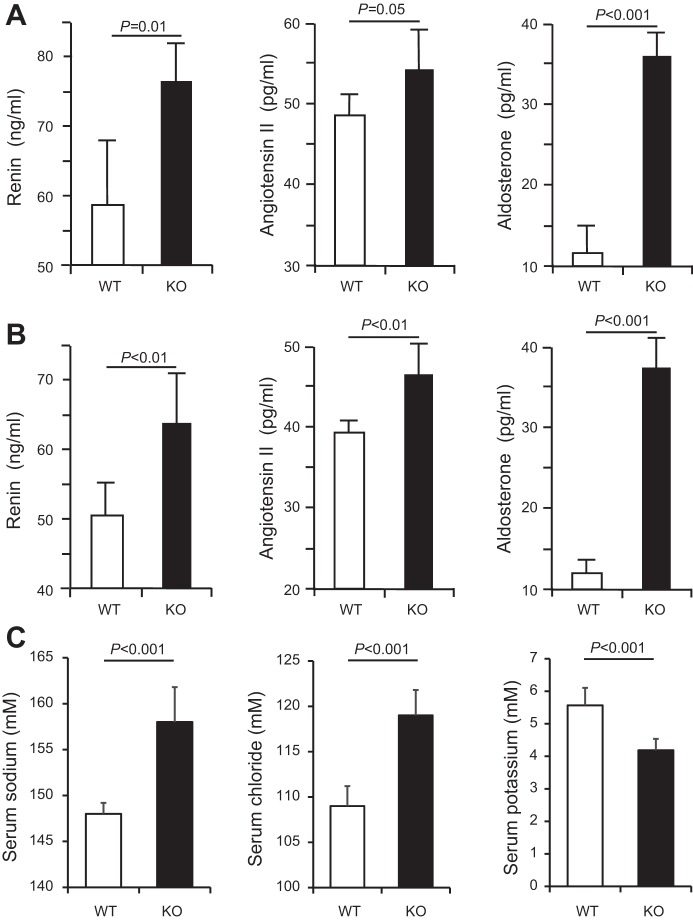
To examine the potential systemic effects of the renal compensatory responses in older THP mice, we measured the serum levels of renin, angiotensin II, and aldosterone. In 5-mo-old mice (Fig. 6A), renin and aldosterone were both significantly higher in KO mice than in WT mice. Angiotensin II was also elevated in KO mice although the increase did not reach statistical significance. In 12-mo-old mice (Fig. 6B), all the three factors were significantly elevated in THP KO mice. Interestingly, the concentrations of serum sodium and chloride were significantly higher and the concentration of potassium significantly lower in 12-mo-old THP KO mice than in age-matched WT mice (Fig. 6C). When we measured the blood pressure using a noninvasive tail-cuff procedure, we found that both systolic and diastolic pressure readings were higher in 12-mo-old KO mice than in age-matched WT mice (Fig. 7, A and B), although the latter did not reach statistical significance (Fig. 7B).
Fig. 6.
Measurement of serum renin, angiotensin II and aldosterone as well as sodium, chloride, and potassium in THP KO mice. Note that in A the significantly higher levels of renin and aldosterone in 5-mo-old THP KO mice (n = 4) than age-matched WT mice (n = 4). Angiotensin II was also higher although it was less statistically significant. Note that in B serum renin, angiotensin II, and aldosterone were all significantly higher in 12-mo old THP KO mice (n = 4) than in age-matched WT mice (n = 4). Also, note in C the significantly increased sodium and chloride and decreased potassium concentrations in 12-mo-old THP KO mice compared with age-matched WT mice.
Fig. 7.
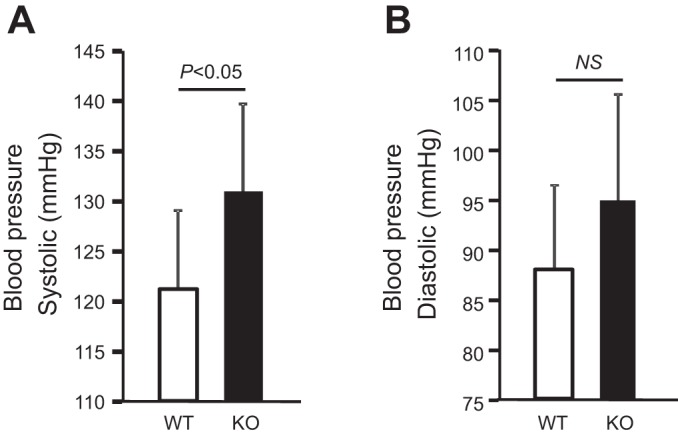
Blood pressure measurement in aged THP KO mice. THP KO mice and WT mice (both 12-mo old; n = 8) were subjected to measurement of systolic (A) and diastolic (B) blood pressure using a noninvasive tail cuff system (see materials and methods for details). Note that THP KO mice had higher blood pressure than WT controls.
Urate transporters and NHE3 were significantly upregulated in renal proximal tubules of aged THP KO mice.
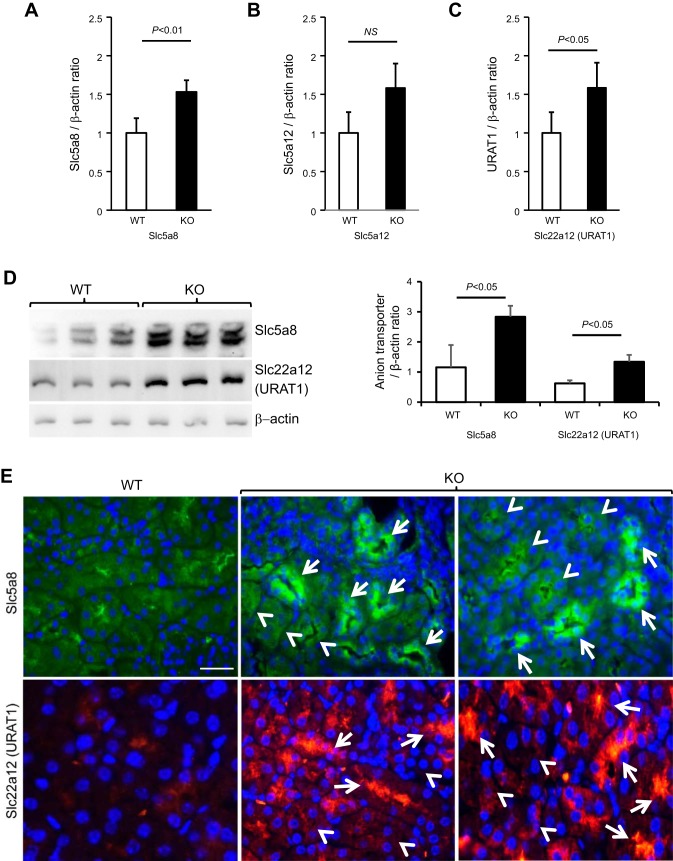
Because sodium reabsorption in the proximal tubule is functionally coupled with urate reabsorption (1, 32, 40), we assessed the expression of key urate transporters in aged THP KO mice by quantitative real-time PCR (Fig. 8, A–C), Western blotting (Fig. 8D), and immunofluorescent staining (Fig. 8E). The mRNA levels of Slc5a8 and Slc22a12 were both significantly higher in aged THP KO than in age-matched WT mice (Fig. 8, A and C). Slc5a12 was also upregulated, although the difference did not reach statistical significance (Fig. 8B). Western blotting and semiquantification by densitometry of antibody-reactive bands showed that Slc5a8 and Slc22a12 were significantly upregulated in the aged THP KO mice (Fig. 8D). Upon immunofluorescent staining, Slc5a8 and Slc22a12 were localized on the luminal surface of proximal tubules with much stronger intensity in aged THP KO mice than those in age-matched WT mice (Fig. 8E). Interestingly, only a portion of the proximal tubules overexpressed the two urate transporters (Fig. 8E), suggesting that not all nephrons were involved in or necessary for compensatory responses in the absence of THP.
Fig. 8.
Renal expression and localization of urate transporters in aged THP KO mice. Total RNAs extracted from 12-mo-old THP KO mice (n = 4) and age-matched WT mice (n = 4) were subjected to quantitative real-time PCR using oligonucleotide primers specific for Slc5a8 (A), Slc5a12 (B), and Slc22a12 (URAT1; C). Values were calculated using ΔΔCt formula and expressed as ratios against simultaneously amplified β-actin. Note the increased expression of all three urate transporters, with the differences of Slc5a8 and Slc22a12 between KO and WT mice reaching statistical significance. D: Western blotting detection (left) and densitometry (right) of Slc5a8 and Slc22a12 showing significantly their increased expression on the protein level in aged THP KO mice. Each lane was from an individual mouse. E: representative images of immunofluorescent staining of Slc5a8 and Slc22a12 (URAT1) in 12-mo-old WT and THP KO mouse kidneys. Note the markedly increased expression of both urate transporters at the luminal surface of some of the proximal tubules of the KO mice. Arrows denote the proximal tubules with urate transporter overexpression; whereas arrowheads denote those without significant overexpression. Bar = 30 μm.
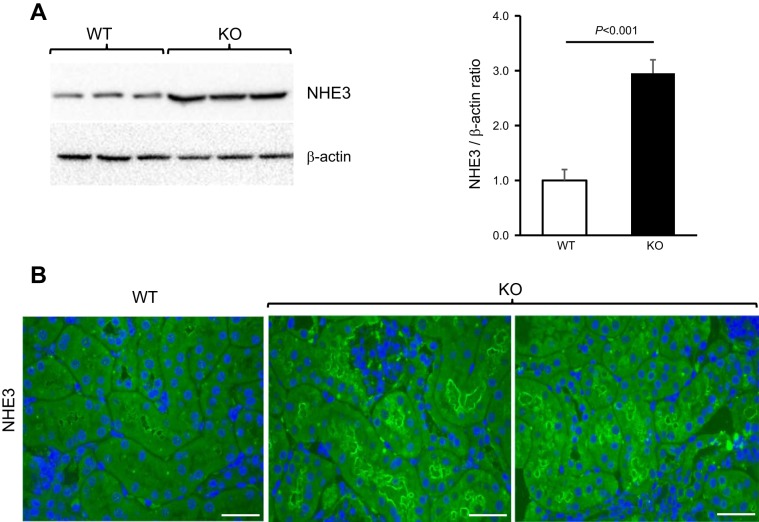
We also assessed the expression of NHE3 by Western blotting and immunofluorescence staining in mouse kidneys (Fig. 9) and found it to be significantly higher in aged THP KO mice than age-matched WT controls (Fig. 9A). While NHE3 was only weakly labeled in WT mice, it was prominently labeled at the apical surface of the proximal tubules in aged KO mice (Fig. 9B).
Fig. 9.
Expression of sodium-hydrogen antiporter 3 (NHE3) in aged THP KO mice. Western blotting (A) and immunofluorescence staining (B) using an anti-NHE3 antibody showing elevated expression of NHE3 in 12-mo old THP KO mice, compared with age-matched WT mice. A, left: each lane is from an individual mouse. A, right: densitometry result from left. B: representative images from 5 mice examined per genotype; bars = 30 μm.
Uric acid and allantoin were elevated in aged THP KO mice.
To assess whether the upregulation of the key urate transporters in the proximal tubules of older THP KO mice was associated with an increase in urate reabsorption, we measured the serum levels of uric acid and its catabolic product, allantoin. No significant difference was observed between 5-mo old KO mice and age-matched controls (Fig. 10, A and B). However, both uric acid and allantoin were significantly higher in 12-mo old THP KO mice than in age-matched WT controls (Fig. 10, C and D). The elevation of serum uric acid was particularly notable, given the fact that mice express the uric acid-degrading enzyme, uricase (59). In other words, had the mice not expressed the uricase, hyperuricemia due to THP deficiency would have been even more pronounced.
Fig. 10.
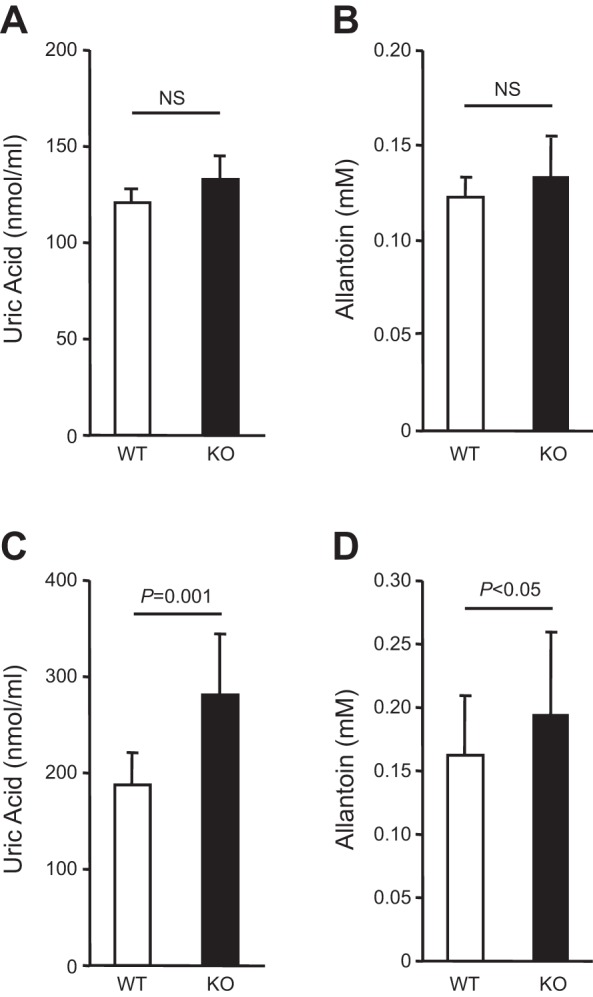
Measurement of serum levels of uric acid and allantoin in THP KO mice. Note the lack of significant difference in serum uric acid and allantoin levels between 5-mo old THP KO and WT mice (A and B; n = 10/genotype). Also, note the significantly higher levels of uric acid and its metabolic product allantoin in 12-mo-old THP KO mice (n = 8) than in age-matched WT mice (C and D; n = 8/genotype).
DISCUSSION
In the present study, we provide several key pieces of in vivo experimental evidence substantiating the physiological functions of THP and the effects of THP deficiency on renal pathophysiology and diseases. The first is the subcellular process by which THP affects the reabsorptive functions in the TAL of Henle and the progressive nature of the tubular dysfunctions in the absence of THP, a topic that had not been systematically explored in the past. In our young (1-mo-old) mice, loss of THP resulted in a defect of antinatriuresis with polyuria and salt wasting (Fig. 1; Table 1). This is typical of a reabsorptive dysfunction of sodium, chloride, and potassium in the TAL due to reduced NKCC2 activity. Such an effect would be expected from patients with inherited mutations in the THP gene (6, 7) or sporadic mutations in the NKCC2 gene that cause type I Bartter syndrome (23). The young THP KO mice were nevertheless somewhat responsive to the loop-acting furosemide leading to diuresis (Fig. 1A), suggesting that NKCC2 was only partially dysfunctional in the young animals, a suggestion verified by immunofluorescent staining showing only reduced apical localization of NKCC2 (Figs. 1C and 11). In striking contrast, the aged THP KO mice were oliguric and completely unresponsive to furosemide (Figs. 2A and 3, A–C). This was not due to the lack of NKCC2 in the TAL per se, but its failed translocation from the cytoplasm to the apical surface to partition in the lipid rafts, as evidenced by our lipid raft floatation assay (Fig. 4A), plasma membrane-associated/cytosol-associated fractionation (Fig. 4B), confocal immunofluorescence (Fig. 4C), and immunoelectron microscopy (Figs. 4D and 11). As has been established, the presence of NKCC2 on the apical surface is a prerequisite for the cotransporter to perform its reabsorptive function (22, 36). Conversely, the reduced or absent apical presence would render NKCC2 dysfunctional. Our data functionally linking THP with NKCC2 thus broaden the scenarios whereby other reabsorptive functions of TAL may also be affected during THP deficiency (see below).
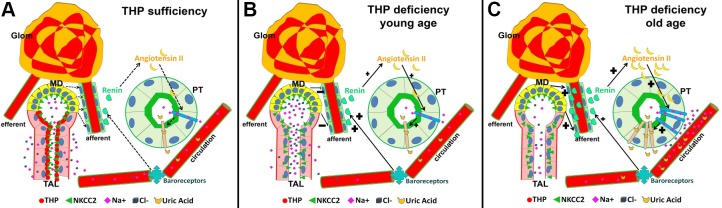
Fig. 11.
Schematic diagram depicting the age-dependent physiological responses during THP deficiency. A: under normal conditions when THP is sufficient, THP helps anchor NKCC2 to the apical surface of TAL where NKCC2 transports physiological amounts of Na, Cl, and K (K not drawn for brevity). Salt sensing at MD, renin synthesis at JGA, and angiotensin synthesis and uric acid reabsorption at PT are all kept at physiological levels. B: when THP is deficient in young animals, NKCC2 becomes partially cytoplasmic, hence partially dysfunctional. This results in salt wasting and increased urine output. Since NKCC2 is only partially dysfunctional, the physiological disturbances as well as the compensatory responses including the activation of renin-angiotensin axis are minimum. C: when THP is deficient in aged animals, NKCC2 is completely dysfunctional at TAL. Lower filtered load plus higher reabsorption of NaCl at PT led to robust response at MD and marked upregulation of renin and angiotensin. The salt wasting is compensated in part by increased reabsorption of NaCl at PT and functionally linked increased reabsorption of uric acid, leading to hyperuricemia.
Based on our present study and those published earlier, there appears to be a consensus on the critical importance of THP in NKCC2’s apical translocation. Mutig et al. (36) demonstrated that, in oocytes and cultured TAL cells, the expression of THP significantly increased the activity of NKCC2, thus functionally linking THP with NKCC2 activity. Renigunta et al. (47) showed, using a yeast two-hybrid system, that THP actually bound to and increased the surface translocation of ROMK2, a renal outer medullary potassium channel also expressed by TAL under normal conditions. Thus far, no evidence exists that THP binds to NKCC2 and directly affects its apical translocation. However, THP is a GPI-linked protein that has a major presence on the luminal surface of TAL. GPI-linked proteins are known to preferentially reside in and act as a protein organizer in the lipid microdomains or rafts of the cell surface (49, 55). It is conceivable that the loss of THP could alter the content of phospholipids and cholesterol and compromise the integrity or stability of the lipid rafts on the TAL surface, leading to reduced exocytosis and/or increased endocytosis of NKCC2. Further studies are needed to pinpoint the exact mechanisms.
Although NKCC2 was nonfunctional in the TAL of aged THP KO mice, oliguria but not polyuria was the principal manifestation (Figs. 2A and 3, A–C). We believe this was a result of combined effects of 1) markedly reduced GFR as we demonstrated earlier (29), 2) vasoconstriction caused by elevated renin/angiotensin II (Figs. 5 and 6), and 3) compensatory increase of salt and water reabsorption by the proximal and distal tubules (Fig. 3, B and C, and 11). The key to these physiological responses in aged THP KO mice may lie in the NKCC2 at the MD, which lacks THP expression and the apical translocation and reabsorptive function of which are unaffected in the absence of THP (Fig. 5). Thus the renin-angiotensin axis likely remains intact in aged THP KO mice (Figs. 5 and 6). This situation is distinctly different from sporadic NKCC2 mutations that cause type 1 Bartter syndrome. In these patients, the salt and water wasting is uncompensated (12), likely due to the fact that NKCC2 at both TAL and MD is dysfunctional.
The second key observation we made in this study was that THP deficiency can lead to hyperuricemia. We found both uric acid and its metabolic product allantoin in the serum to be significantly higher (Fig. 10) and uric acid in the urine significantly lower (Table 1) in aged THP KO mice than in age-matched WT mice. This is very much in line with the marked upregulation of two of the three urate transporters in the proximal tubules (Fig. 8). It is likely that increased serum renin and angiotensin II are involved in upregulating the expression of these urate transporters, as suggested by others (20, 43). The fact that upregulated renin-angiotensin-aldosterone axis preceded the increase in serum uric acid/allantoin in 5-mo-old KO mice further supports the sequence of events leading to hyperuricemia that we proposed here (Fig. 11). While mice lacking THP or expressing a mutated THP have been available for some time (4, 5, 35, 57), to date the status of uric acid had never been evaluated. This lack of attempt is probably due to the assumption that mice synthesize a functional uricase (59) and would be expected to have a low serum uric acid concentration. Our results showing elevated serum uric acid and allantoin even in the presence of uricase are therefore highly significant, because they provide direct experimental proof that THP deficiency leads to hyperuricemia and may recapitulate the human phenotype. It should be noted that hepatic uricase in mice can be modulated under different conditions and may affect serum uric acid level. This is an important caveat that we did not examine in our present study but will do so in the future.
Mechanistically, the increased urate reabsorption from the proximal tubule in the aged THP KO mice might have occurred in two functionally coupled events (1, 6, 32, 40, 51). The first is the increased Na+/H+ exchange due to the angiotensin II-mediated compensatory increase of sodium reabsorption. Our data on the robust diuretic response of the aged THP KO mice to acetazolamide (Fig. 3C), a carbonic anhydrase inhibitor, strongly support this possibility. Indeed, NHE3 is strongly upregulated at the apical surface of the proximal tubules in aged THP KO mice (Fig. 9). The second event is the increased H+/urate exchange, leading to inward transfer of urate. While these concepts are not entirely new, our present study is significant as it provides experimental evidence linking THP deficiency to hyperuricemia (Fig. 11).
Finally, data from our present study may have broader implications than explaining the pathogenesis for patients bearing THP mutations. Reduced levels of THP expression in the absence of mutations have been noted in a number of pathophysiological conditions, including acute kidney injury, chronic kidney disease, diabetic nephropathy, lupus nephritis, tubulointerstitial nephropathy, kidney transplantation, polycystic kidney disease, acute tubular necrosis, hypothyroidism, and hyperprostaglandin E syndrome (46, 50, 55). Certain single nucleotide polymorphisms of the THP gene that have been identified in the general population are associated with varied levels of urinary THP (21, 25, 26, 37, 45, 58). There is reason to believe that even altered levels of THP might affect the apical translocation, hence, the activities of the various ion transporters in the TAL, leading to compensatory responses, uric acid imbalance, and renal insufficiency. In this regard, it is of particular interest that Garimella et al. (19) recently demonstrated that higher urinary THP in patients is significantly associated with lower risk of estimated GFR decline and mortality. The authors suggested that lower urinary THP may help identify people at risk of progressive kidney disease and mortality better than estimated GFR and albumin/creatinine ratio. Similarly, Steubl et al. (56) recently showed that the plasma THP is a robust biomarker for renal secretion and may help identify early stage CKD. Based on these recent observations in patients, it is therefore not impossible that urinary and/or plasma THP may actually reflect more accurately the overall kidney function for early stage CKD than GFR, whose reduction may be associated with late-stage CKD, as Steubl et al. (56) suggested. Further studies are clearly warranted to sort out how the different types and degrees of THP abnormalities may affect the kidney responses to diuretics, tubular responses, uric acid metabolism, and GFR and at which pathogenic stages. Results from these studies could have important implications for how these conditions can be rationally managed.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-056903; Veterans Affairs Office of Research and Development, Biomedical Laboratory Research and Development Service Grant 1I01BX002049; and a grant-in-aid from the Goldstein Fund for Urological Research of the New York University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and X.-R.W. conceived and designed research; Y.L. and J.C.L. performed experiments; Y.L., D.G., T.M.E.-A., J.C.L., and X.-R.W. analyzed data; Y.L., D.G., T.M.E.-A., J.C.L., and X.-R.W. interpreted results of experiments; Y.L., T.M.E.-A., and X.-R.W. prepared figures; Y.L., D.G., T.M.E.-A., J.C.L., and X.-R.W. approved final version of manuscript; X.-R.W. drafted manuscript; X.-R.W. edited and revised manuscript.
ACKNOWLEDGMENTS
The immunoelectron microscopic work was carried out at the Microscopy Core of the Office of Collaborative Science, New York University School of Medicine.
REFERENCES
- 1.Anzai N, Jutabha P, Amonpatumrat-Takahashi S, Sakurai H. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol 16: 89–95, 2012. doi: 10.1007/s10157-011-0532-z. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985. doi: 10.1007/BF00492456. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005. doi: 10.1152/ajprenal.00143.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bates JM Jr, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65: 791–797, 2004. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 5.Bernascone I, Janas S, Ikehata M, Trudu M, Corbelli A, Schaeffer C, Rastaldi MP, Devuyst O, Rampoldi L. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet 19: 2998–3010, 2010. doi: 10.1093/hmg/ddq205. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer AJ, Kmoch S. Tamm Horsfall glycoprotein and uromodulin: it is all about the tubules! Clin J Am Soc Nephrol 11: 6–8, 2016. doi: 10.2215/CJN.12201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleyer AJ, Zivná M, Kmoch S. Uromodulin-associated kidney disease. Nephron Clin Pract 118: c31–c36, 2011. doi: 10.1159/000320889. [DOI] [PubMed] [Google Scholar]
- 8.Brown D, Waneck GL. Glycosyl-phosphatidylinositol-anchored membrane proteins. J Am Soc Nephrol 3: 895–906, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng J, Santambrogio S, Perrier R, Li S, Bokhove M, Bachi A, Hummler E, Devuyst O, Wu Q, Jovine L, Rampoldi L. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. eLife 4: e08887, 2015. doi: 10.7554/eLife.08887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron JS, Simmonds HA. Hereditary hyperuricemia and renal disease. Semin Nephrol 25: 9–18, 2005. doi: 10.1016/j.semnephrol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Capasso G, Jaeger P, Robertson WG, Unwin RJ. Uric acid and the kidney: urate transport, stone disease and progressive renal failure. Curr Pharm Des 11: 4153–4159, 2005. doi: 10.2174/138161205774913219. [DOI] [PubMed] [Google Scholar]
- 12.Castrop H, Schießl IM. Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 307: F991–F1002, 2014. doi: 10.1152/ajprenal.00432.2014. [DOI] [PubMed] [Google Scholar]
- 13.Cavallone D, Malagolini N, Monti A, Wu XR, Serafini-Cessi F. Variation of high mannose chains of Tamm-Horsfall glycoprotein confers differential binding to type 1-fimbriated Escherichia coli. J Biol Chem 279: 216–222, 2004. doi: 10.1074/jbc.M308821200. [DOI] [PubMed] [Google Scholar]
- 14.Devuyst O, Bochud M. Uromodulin, kidney function, cardiovascular disease, and mortality. Kidney Int 88: 944–946, 2015. doi: 10.1038/ki.2015.267. [DOI] [PubMed] [Google Scholar]
- 15.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013. doi: 10.1152/ajprenal.00543.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol 300: F999–F1007, 2011. doi: 10.1152/ajprenal.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012. doi: 10.1053/j.ajkd.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, Kestenbaum BR, Siscovick DS, Jensen MK, Shlipak MG, Chaves PH, Sarnak MJ. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 88: 1126–1134, 2015. doi: 10.1038/ki.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gersch M, Mutig K, Bachmann S, Kumar S, Ouyang X, Johnson R. Is salt-wasting the long awaited answer to the hyperuricaemia seen in uromodulin storage diseases? Nephrol Dial Transplant 21: 2028–2029, 2006. doi: 10.1093/ndt/gfk081. [DOI] [PubMed] [Google Scholar]
- 21.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, de Vegt F, d’Ancona FC, den Heijer M, Wetzels JF, Franzson L, Rafnar T, Kristjansson K, Bjornsdottir US, Eyjolfsson GI, Kiemeney LA, Kong A, Palsson R, Thorsteinsdottir U, Stefansson K. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet 6: e1001039, 2010. doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas M, Forbush B 3rd. The Na-K-Cl cotransporters. J Bioenerg Biomembr 30: 161–172, 1998. doi: 10.1023/A:1020521308985. [DOI] [PubMed] [Google Scholar]
- 23.Kemter E, Rathkolb B, Bankir L, Schrewe A, Hans W, Landbrecht C, Klaften M, Ivandic B, Fuchs H, Gailus-Durner V, Hrabé de Angelis M, Wolf E, Wanke R, Aigner B. Mutation of the Na+-K+-2Cl− cotransporter NKCC2 in mice is associated with severe polyuria and a urea-selective concentrating defect without hyperreninemia. Am J Physiol Renal Physiol 298: F1405–F1415, 2010. doi: 10.1152/ajprenal.00522.2009. [DOI] [PubMed] [Google Scholar]
- 24.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci 9: 1450–1482, 2004. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 25.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Köttgen A, Hwang SJ, Larson MG, Van Eyk JE, Fu Q, Benjamin EJ, Dehghan A, Glazer NL, Kao WH, Harris TB, Gudnason V, Shlipak MG, Yang Q, Coresh J, Levy D, Fox CS. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol 21: 337–344, 2010. doi: 10.1681/ASN.2009070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Muchmore A. Tamm-Horsfall protein–uromodulin (1950–1990). Kidney Int 37: 1395–1401, 1990. doi: 10.1038/ki.1990.128. [DOI] [PubMed] [Google Scholar]
- 28.Lhotta K. Uromodulin and chronic kidney disease. Kidney Blood Press Res 33: 393–398, 2010. doi: 10.1159/000320681. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem 287: 16365–16378, 2012. doi: 10.1074/jbc.M112.348243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR, Lieske JC, Wu XR. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol 299: F469–F478, 2010. doi: 10.1152/ajprenal.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malagolini N, Cavallone D, Serafini-Cessi F. Intracellular transport, cell-surface exposure and release of recombinant Tamm-Horsfall glycoprotein. Kidney Int 52: 1340–1350, 1997. doi: 10.1038/ki.1997.459. [DOI] [PubMed] [Google Scholar]
- 32.Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol 77: 323–345, 2015. doi: 10.1146/annurev-physiol-021113-170343. [DOI] [PubMed] [Google Scholar]
- 33.Micanovic R, Chitteti BR, Dagher PC, Srour EF, Khan S, Hato T, Lyle A, Tong Y, Wu XR, El-Achkar TM. Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J Am Soc Nephrol 26: 2172–2182, 2015. doi: 10.1681/ASN.2014070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66: 1159–1166, 2004. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 35.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802, 2004. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 36.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castañeda-Bueno M, Gamba G, Bachmann S. Activation of the bumetanide-sensitive Na+,K+,2Cl− cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 286: 30200–30210, 2011. doi: 10.1074/jbc.M111.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olden M, Corre T, Hayward C, Toniolo D, Ulivi S, Gasparini P, Pistis G, Hwang SJ, Bergmann S, Campbell H, Cocca M, Gandin I, Girotto G, Glaudemans B, Hastie ND, Loffing J, Polasek O, Rampoldi L, Rudan I, Sala C, Traglia M, Vollenweider P, Vuckovic D, Youhanna S, Weber J, Wright AF, Kutalik Z, Bochud M, Fox CS, Devuyst O. Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J Am Soc Nephrol 25: 1869–1882, 2014. doi: 10.1681/ASN.2013070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276: 9924–9930, 2001. doi: 10.1074/jbc.M008610200. [DOI] [PubMed] [Google Scholar]
- 39.Paladino S, Sarnataro D, Zurzolo C. Detergent-resistant membrane microdomains and apical sorting of GPI-anchored proteins in polarized epithelial cells. Int J Med Microbiol 291: 439–445, 2002. doi: 10.1078/1438-4221-00151. [DOI] [PubMed] [Google Scholar]
- 40.Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 31: 542–552, 2011. doi: 10.1016/j.semnephrol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Peach RJ, Day WA, Ellingsen PJ, McGiven AR. Ultrastructural localization of Tamm-Horsfall protein in human kidney using immunogold electron microscopy. Histochem J 20: 156–164, 1988. doi: 10.1007/BF01746679. [DOI] [PubMed] [Google Scholar]
- 42.Puschett JB. Pharmacological classification and renal actions of diuretics. Cardiology 84, Suppl 2: 4–13, 1994. doi: 10.1159/000176450. [DOI] [PubMed] [Google Scholar]
- 43.Quan A, Baum M. Regulation of proximal tubule transport by angiotensin II. Semin Nephrol 17: 423–430, 1997. [PubMed] [Google Scholar]
- 44.Raffi H, Bates J, Kumar S, Laszik Z, Buffington CA. Tamm-Horsfall protein knockout mice have increased stress induced micturition. Neurourol Urodyn 28: 469, 2009. doi: 10.1002/nau.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rampoldi L, Köttgen A, Devuyst O. The effect of common uromodulin variants on urinary protein level and gene transcription. Kidney Int 84: 410–411, 2013. doi: 10.1038/ki.2013.162. [DOI] [PubMed] [Google Scholar]
- 46.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011. doi: 10.1038/ki.2011.134. [DOI] [PubMed] [Google Scholar]
- 47.Renigunta A, Renigunta V, Saritas T, Decher N, Mutig K, Waldegger S. Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem 286: 2224–2235, 2011. doi: 10.1074/jbc.M110.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rindler MJ, Naik SS, Li N, Hoops TC, Peraldi MN. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem 265: 20784–20789, 1990. [PubMed] [Google Scholar]
- 49.Saha S, Anilkumar AA, Mayor S. GPI-anchored protein organization and dynamics at the cell surface. J Lipid Res 57: 159–175, 2016. doi: 10.1194/jlr.R062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitt R, Kahl T, Mutig K, Bachmann S. Selectively reduced expression of thick ascending limb Tamm-Horsfall protein in hypothyroid kidneys. Histochem Cell Biol 121: 319–327, 2004. doi: 10.1007/s00418-004-0638-4. [DOI] [PubMed] [Google Scholar]
- 51.Scolari F, Caridi G, Rampoldi L, Tardanico R, Izzi C, Pirulli D, Amoroso A, Casari G, Ghiggeri GM. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis 44: 987–999, 2004. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Sedor JR. Uromodulin and translational medicine: will the SNPs bring zip to clinical practice? J Am Soc Nephrol 21: 204–206, 2010. doi: 10.1681/ASN.2009121283. [DOI] [PubMed] [Google Scholar]
- 53.Seely JF, Dirks JH. Site of action of diuretic drugs. Kidney Int 11: 1–8, 1977. doi: 10.1038/ki.1977.1. [DOI] [PubMed] [Google Scholar]
- 54.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003. doi: 10.1016/S0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 55.Serafini-Cessi F, Monti A, Cavallone D. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J 22: 383–394, 2005. doi: 10.1007/s10719-005-2142-z. [DOI] [PubMed] [Google Scholar]
- 56.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, Angermann S, Hasenau AL, Stecher L, Heemann U, Renders L, Scherberich J. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 95: e3011, 2016. doi: 10.1097/MD.0000000000003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takiue Y, Hosoyamada M, Yokoo T, Kimura M, Shibasaki T. Progressive accumulation of intrinsic mouse uromodulin in the kidneys of transgenic mice harboring the mutant human uromodulin gene. Biol Pharm Bull 31: 405–411, 2008. doi: 10.1248/bpb.31.405. [DOI] [PubMed] [Google Scholar]
- 58.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, Citterio L, Demaretz S, Trevisani F, Ristagno G, Glaudemans B, Laghmani K, Dell’Antonio G, Loffing J, Rastaldi MP, Manunta P, Devuyst O, Rampoldi L; SKIPOGH team . Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 19: 1655–1660, 2013. doi: 10.1038/nm.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C Jr, Jones P, Bradley A, Caskey CT. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci USA 91: 742–746, 1994. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]