Abstract
Aging is a modern concept: human life expectancy has more than doubled in less than 150 yr in Western countries. Longer life span, however, reveals age-related diseases, including cerebrovascular diseases. The vascular system is a prime target of aging: the “wear and tear” of large elastic arteries exposed to a lifelong pulsatile pressure causes arterial stiffening by fragmentation of elastin fibers and replacement by stiffer collagen. This arterial stiffening increases in return the amplitude of the pulse pressure (PP), its wave penetrating deeper into the microcirculation of low-resistance, high-flow organs such as the brain. Several studies have associated peripheral arterial stiffness responsible for the sustained increase in PP, with brain microvascular diseases such as cerebral small vessel disease, cortical gray matter thinning, white matter atrophy, and cognitive dysfunction in older individuals and prematurely in hypertensive and diabetic patients. The rarefaction of white matter is also associated with middle cerebral artery pulsatility that is strongly dependent on PP and artery stiffness. PP and brain damage are likely associated, but the sequence of mechanistic events has not been established. Elevated PP promotes endothelial dysfunction that may slowly develop in parallel with the accumulation of proinflammatory senescent cells and oxidative stress, generating cerebrovascular damage and remodeling, as well as brain structural changes. Here, we review data suggesting that age-related increased peripheral artery stiffness may promote the penetration of a high PP to cerebral microvessels, likely causing functional, structural, metabolic, and hemodynamic alterations that could ultimately promote neuronal dysfunction and cognitive decline.
Keywords: cerebral arteries, endothelium, large elastic arteries, pulsatile pressure and flow
ORIGIN OF CEREBROVASCULAR AGING
The genus Australopithecus emerged from the hominid crowd ∼4,000,000 yr ago and slowly gave rise to different Homo genuses evolving through their ability to fight infections (98), to resist long periods of fasting, and to perform high levels of physical activity (11, 16, 18). Within 150 yr, healthiness and the discovery of vaccines and antibiotics boosted the average life expectancy of Homo sapiens from 35 to 70 yr by the mid-20th century in Western countries (26). Put simply, humans have not been selected to age naturally beyond ~50 yr (54). As an indirect evidence, longevity is linearly and inversely proportional to resting heart rate, and this applies to all mammals in the wild: the mouse has the highest heart rate (∼550 beats/min) and the smaller life expectancy (<1 yr), whereas the big whale has the longest life expectancy (>100 yr) and the lowest heart rate (∼15 beats/min) (58). Humans of the mid-18th century fitted the slope of this negative regression with an average maximal life span of 35 yr (58). This negative correlation between life expectancy and heart rate could suggest that the chronic lifelong mechanical stress induced by each heart beat induces the “wear and tear” of the cardiovascular system. The primary target would be the endothelium, damaging its dilatory and barrier functions: endothelial dilatory dysfunction is evidenced from the age of 40 yr in men and 50 yr in women (17), and blood-brain barrier breakdown is already detectable in the brain (hippocampus) of healthy volunteers older than 55 yr of age (69). Lifelong repeated cycles of vascular distension and recoil lead to vascular damage, fatigue, and fragmentation of elastin fibers, leaving stiffer collagen in the vascular wall (see Refs. 110 and 117). Cardiovascular and cerebrovascular diseases somehow reveal the limits of the physiological resistance of the cardiovascular system and brain. Here, we review evidence suggesting that the age-related increase in peripheral artery stiffness may promote the penetration of the pulse pressure wave deeper into cerebral microvessels (penetrating arterioles, capillaries, and venules), likely causing functional, structural, metabolic, and hemodynamic alterations in the brain that could ultimately induce neuronal death and cognitive decline.
STIFFNESS OF LARGE ARTERIES AND INCREASED CEREBRAL PULSE PRESSURE: THE “BIOMECHANICAL HYPOTHESIS” OF BRAIN DAMAGE IN AGING
Arterial blood pressure and blood flow oscillate at each heart beat, generating pulsatility. The pressure gradient (from systolic to diastolic), or in other words, the pulse pressure, translates into a pressure wave that propagates along the circulation, from the arterial tree to the microcirculation (110, 117). As the heart beats, pulsatile blood flow is converted into a relative continuous capillary flow and the efficiency of this conversion is mostly ensured by the compliance of large peripheral elastic arteries (the aorta and carotids) that absorb the pulsatility from the arterial tree to the brain microcirculation (called the Windkessel effect) (77), followed at the level of resistance arteries by the pressure-dependent myogenic response (130) that further dampens pulsatility. With aging, vascular stiffening of the large peripheral elastic arteries occurs (72), translating into decreased compliance (pressure-volume relationship) and a selective rise in systolic blood pressure (>160 mmHg) that are highly prevalent above the age of 70 yr (136). Pulse pressure dampening is consequently reduced, increasing its amplitude and creating more mechanical stress. Aging also affects myogenic contraction, an essential mechanism to protect the downstream cerebral microcirculation from the penetration of potentially damaging pressure waves. In cerebral arteries from aged mice, myogenic adaptation of large cerebral arteries in the presence of increased pulsatile pressure ex vivo is significantly impaired (109). Endothelial dysfunction is a hallmark of vascular aging (17), and we have reported that the endothelium senses pulse pressure and regulates myogenic tone, at least ex vivo (93). Finally, another factor that makes the cerebral circulation likely vulnerable to the pulse pressure is that intracranial cerebral arteries lack an external elastic lamina and have attenuated media (Ref. 56; for a review, see Ref. 104). This anatomic fragile structure may render cerebral arteries highly sensitive to abnormal chronic rises in blood pressure, as suggested by Heistad (43). Thus, with aging, pulse pressure increases and creates more mechanical stress that would not be attenuated by smaller resistance cerebral arteries with endothelial dysfunction (114) and impaired myogenic contraction (109). Consequently, the pulse pressure wave may propagate further along the fragile brain microcirculation (68, 100, 110, 135). Evidence for a pulse pressure penetration is given by the presence of pulsatility of cerebral blood flow (CBF) and white matter structure abnormalities observed in an elderly population in association with increased pulse pressure (91, 115). However, there is only indirect evidence that pulse pressure extends up to the penetrating arterioles, capillaries, and venules in the context of arterial stiffening. The penetration of pulse pressure in the cerebrovasculature, at the basis of the “biomechanical hypothesis” of brain damage (62), could have two hypothetical consequences: 1) microvascular damages and 2) neuronal injuries.
Increased Cerebral Pulsatility Induces Microvascular Damage
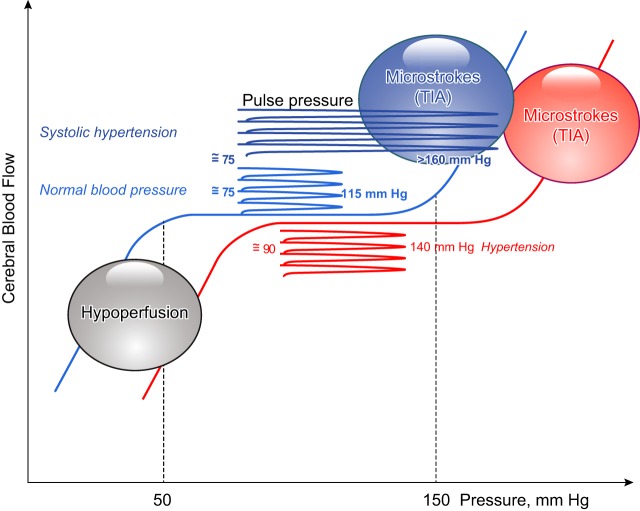
The biomechanical hypothesis of age-dependent brain damage can be illustrated (Fig. 1): under normal conditions, CBF is maintained constant from a pressure range of ~50 to ~150 mmHg (84). Below 50 mmHg, hypoxia dilates cerebral blood vessels (52) but the brain is hypoperfused; above 150 mmHg, blood vessels are passively dilated and CBF dangerously rises, favoring microbleeds or transient ischemic attacks development (108). A normal pulse pressure, i.e., within the lower (~50 mmHg) and upper (~150 mmHg) limits, does not affect CBF autoregulation. In contrast, an increase in pulse pressure needs to be accommodated by brain arteries to maintain CBF within the range of autoregulation. In hypertensive subjects, the curve of autoregulation is shifted to the right, toward higher pressures (84, 111) where transient ischemic attacks may occur (83). In the context of isolated systolic hypertension, the amplitude of the pulse pressure is dramatically increased toward pressures that do not permit CBF autoregulation and may further augment the risk of stroke and increase mortality (Fig. 1) (101, 107a). Thus, an increase in pulse pressure may induce brain microvascular damage by shifting blood pressure to levels where CBF autoregulation is not controlled and where ischemic attacks may occur. Accordingly, in nondemented individuals, the presence of microhemorrhages (i.e., cerebral capillary microbleeds) is associated with older age, hypertension, and cognitive impairment (39). In mice, aging combined with hypertension is associated with a deregulation of pressure-induced myogenic tone (118), cerebral microvascular injury, oxidative stress, neuroinflammation, and cerebral microhemorrhages (121–123). These studies therefore suggest that aging associated with high blood and pulse pressures impairs autoregulatory protection of the brain, permitting the pulse to penetrate deeper into the cerebral circulation, likely contributing to brain microvascular injuries.
Fig. 1.
Positioning of pulse pressure in the cerebral blood flow (CBF) autoregulation. In normal conditions, CBF is maintained constant from a pressure range of 50 to 150 mmHg; normal pulse pressure is within the “safe” zone of CBF autoregulation. Below 50 mmHg, the brain is hypoperfused; above 150 mmHg, blood vessels are passively dilated and CBF dangerously rise, favoring microbleeds or transient ischemic attacks (TIA). In hypertensive subjects, the curve of autoregulation is shifted to the right, toward higher pressures; pulse pressure is higher, toward pressure values where TIA may occur. In the context of systolic hypertension, the amplitude of the pulse pressure is dramatically increased toward pressures that do not permit CBF autoregulation and that may further induce TIA. Schematic representation was adapted from Pires et al. (84).
We know that in humans, a chronic increase in pulse pressure associated with aortic stiffening 1) is an independent risk factor for stroke (8) and 2) leads to so-called pulse-wave encephalopathy (44). In reviews entitled “Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain” (100) and “The mechanical cause of age-related dementia (Alzheimer’s disease): the brain is destroyed by the pulse” (110), it has been proposed that a chronic change in hemodynamics (i.e., an increase in pulse pressure or blood pressure variability) could be sufficient to damage the cerebral microcirculation and cause cerebral small vessel diseases associated white matter hyperintensity (WMH), microbleeds, and silent lacunar infarcts (100) and encephalopathies comprising cerebral hemorrhages, general cerebral hypoxia, and Alzheimer’s disease (110). Epidemiological data have indeed demonstrated a positive association between increased pulse wave velocity (PWV), a marker of aortic stiffening (for a review, see Ref. 76), and white and gray matter (62, 63) and/or microvascular damage (87, 107, 124, 125, 135). Moreover, risk factors for cardiovascular diseases potentiate this deleterious cerebrovascular impact of pulse pressure: several clinical studies have associated peripheral arterial stiffness and brain microvascular diseases such as cerebral small vessel disease, cortical gray matter thinning, white matter atrophy, and cognitive dysfunction in older individuals (44, 53) as well as hypertensive (46, 50) and diabetic (type 1) patients (127, 128). Thus, higher arterial stiffness is associated with microvascular damage.
Increased Pulsatility Induces Neuronal Damage
In humans, a chronic increase in pulse pressure is associated with cognitive decline and dementia (73, 79, 80, 82, 92, 107, 124, 131, 133, 134). More specifically, increased PWV is associated with memory loss (42), mild cognitive impairment (81), cognitive decline (29, 41), and dementia (for reviews, see Refs. 23 and 92). Indeed, since the cerebrovascular system is critically involved in the regulation of the neural network by feeding it, it has been proposed that age-related vascular dysfunction and/or remodeling in the brain may, as a consequence, promote a neurodegenerative process leading to cognitive failure (for a review, see Ref. 47). A decrease in neurovascular coupling with aging has indeed been reported (31). Neurovascular coupling (37) is defined by anatomic proximity between arterioles, neurons, astrocytes, glia and complex signaling mechanisms, permitting the local adjustment of blood flow to local neuronal activity in which endothelium-derived nitric oxide (NO) plays a central role (120). In a large community-based sample of older women and men, it has been reported that pulse pressure (assessed by carotid-femoral PWV) was positively associated with microvascular brain lesions (subcortical infarcts and WMH) and with reduced scores in multiple cognitive domains (68). Poels et al. (85) showed in patients that the number of microbleeds was specifically associated with worse performance on all domains of cognitive tests except memory. Eighty-five percent of 12 studies (n > 6,000 subjects) found a significant association between an increase in arterial stiffness and cognitive impairment after adjusting for age, education, or other factors influencing cognition (92). The conclusion of another meta-analysis, including four longitudinal studies, was similar (80). For example, in the Baltimore longitudinal study using nondemented stroke-free individuals, people with higher baseline PWV exhibited a prospective decline in cognitive tests during the followup (8–11 yr) (131), confirming previous cross-sectional studies (42, 86, 106). In a recent longitudinal study, the age-dependent rise in pulse pressure was associated with a decrease on a measure of executive functions and higher baseline pulse pressure was associated with smaller cerebral volume and was predictive of hippocampal atrophy (over a 5- to 7-yr period) (73). Of note, in contrast to the Baltimore study (131), McDade et al. (67) found that pulse pressure was either associated with no change, an increase, or a decrease in cognition depending on age, baseline systolic blood pressure, and the trajectory of pulse pressure. In addition, a recent meta-analysis showed that greater arterial stiffening was significantly associated with markers of cerebral vascular diseases but only weakly associated with cognition (129). Such a disparity is, however, not surprising considering large individual differences in brain characteristics and/or in the manner people process memory tasks, allowing some individuals to cope better than others despite brain pathology and, hence, to show preserved memory performance (75). Collectively, these studies suggest that both cerebral microvascular damage and parenchymal damage induced by the penetrating pulse pressure could contribute to the association between arterial stiffening and memory loss (23). Thus, there is a general consensus that higher arterial stiffness, which is associated with microvascular damage, predicts poor cognitive performance and a faster cognitive decline.
CAUSES OR CONSEQUENCES: MECHANICAL LINKS BETWEEN AGE- AND PULSE PRESSURE-INDUCED MICROCEREBROVASCULAR DAMAGE, NEURONAL DAMAGE, AND COGNITIVE IMPAIRMENT
Endothelial Dysfunction in the Aging Brain
In large to small cerebral resistance arteries, the endothelium and vascular smooth muscle cells sense flow rate and pressure, respectively, and they need precise coordination to achieve adequate blood supply to the cerebral arterioles and capillaries upon neuronal metabolic demand. Recently, we have reported that a physiological pulse pressure regulates endothelial NO synthase activity in isolated mouse cerebral arteries, affecting both myogenic tone and endothelial shear stress sensitivity (93). Importantly, the mechanism by which endothelial cells sense pulse pressure and transduce its signals is impaired by a rise in pulse pressure ex vivo (93). In humans, endothelial dysfunction, arterial remodeling, and reduced CBF are proposed to be associated with central nervous system inflammation and precocious vascular cognitive impairment (119). However, it is mostly from animal studies that the biomechanical hypothesis can be dissected out. For example, mice with severe dyslipidemia (LDLr−/−; hApoB100+/+) and characterized by carotid stiffening and mild hypertension (10) develop cerebrovascular endothelial dysfunction (28), cerebral artery wall remodeling associated with matrix metalloproteinase (MMP) activation (10), reduced CBF (9, 27), and premature cognitive impairment (27). In vivo, a 1-wk experimental increase in pulse pressure in the mouse brain disturbed the blood-brain barrier and induced inflammation and production of reactive oxygen species (ROS) (89). Another study reported that a 6-wk rise in pulse pressure leads to blood-brain barrier disruption and cognitive defects in mice (15). In aged and hypertensive mice, in which blood-brain barrier disruption is commonly observed, microbleeds were reported (121), confirming that high pressure renders small cerebral arteries and endothelial cells very vulnerable. In hypertensive insulin-like growth factor-1-deficient mice, impaired cerebrovascular autoregulation and lower myogenic responses were observed and associated with disruption of the blood-brain barrier, neuroinflammation (microglia-induced expression of cytokines and chemokines), and impaired learning capacity using an elevated plus maze (123).
Cerebral endothelial dysfunction is paralleled with altered neurovascular coupling, i.e., the association between local transient neural activity and local changes in CBF (114). In vivo, altered neurovascular coupling has been proposed to emerge from CBF maladaptation (for a review, see Ref. 119). The decline in cerebrovascular endothelial function observed in aging and in atherosclerotic mice (28, 55) parallels reduced resting CBF and cognitive dysfunctions (9, 27); on the other hand, prevention of endothelial dysfunction limits CBF maladaptation and slows the cognitive decline (27). In addition, we observed that endothelial dysfunction and alteration in the biomechanical properties of the vascular wall synergize in mouse cerebral arteries to limit the vascular dilatory response to the metabolic demand (10), which could potentially create chronic neuronal stress. In support of this hypothesis, it has been shown in patients that white matter lesions increase in size in regions where the dilatory response to a dilatory CO2 challenge is impaired (reduced cerebrovascular reactivity) (102). This cerebrovascular endothelial dysfunction may affect CBF regulation, limit the vascular dilatory response, and reduce neuronal perfusion, leading to neuronal stress and cognitive decline. This sequence of events is still partly speculative and remains to be fully demonstrated, as recently reviewed in Ref. 49.
Structural, Functional, Neurogenic, and Metabolic Changes in the Aging Brain
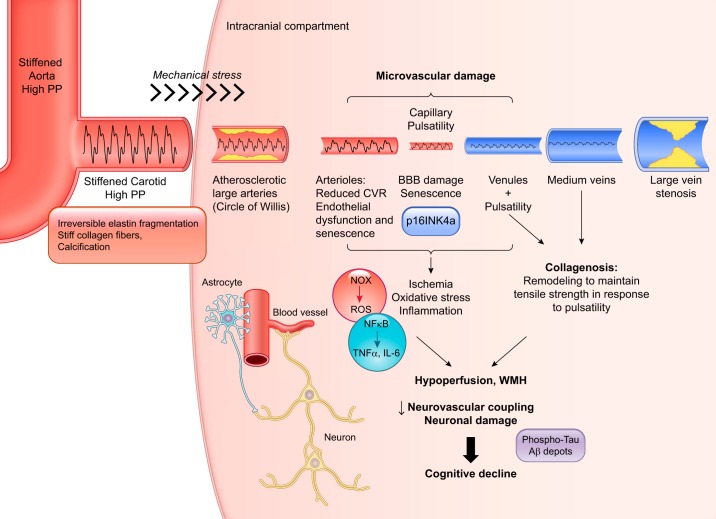
Because the endothelium senses pulse pressure (93), it is not surprising that the cerebral endothelium contributes to cerebral artery wall remodeling (10). The cerebral microvessel ultrastructure is altered in old subjects, including structural abnormalities of the basement membrane, perivascular collagen deposits called microvascular fibrosis (or collagenosis), and basement membrane thickening (Fig. 2) (for a review, see Ref. 32). Although less studied and less reported, collagenosis also occurs in cerebral veins, mostly in venules; it consists of an age-related increase in venous wall thickness by multiple concentric layers of collagen (12, 70). This remodeling is thought to maintain venous tensile strength in response to arteriolar and capillary pulsatility generated by the stiffening of large peripheral elastic arteries (90). The abnormal penetration of the arterial pulse wave through capillaries into the venous system may result in a pulsatile shear stress on the vessel walls that stimulates the production of collagen (45). Recent studies have suggested that venous collagenosis could contribute to white matter lesions in normal aging and in Alzheimer’s disease (51), another indirect evidence of the penetration of the pulse pressure into the cerebral microcirculation.
Fig. 2.
Schematization of the putative cellular and molecular events linking pulse pressure penetration in the cerebral microcirculation and the development of cognitive decline. Age-associated stiffening of large elastic arteries such as the aorta and carotids is due to irreversible elastin fragmentation induced by the lifelong exposure of the vascular wall to the mechanical stress inherent to the heart beat. Stiff collagen, which replaces elastin, and calcification of the vascular wall significantly reduce arterial elasticity and augment the amplitude of the pulse pressure (PP) that penetrates into the fragile low-resistance cerebral microcirculation. Arteriolar, venular, and capillary pulsatility is associated with endothelial nitric oxide synthase dysfunction and possibly endothelial senescence (p16INK4a expression), reduced cerebrovascular reactivity (CVR), and blood-brain barrier (BBB) disruption. The latter permits the infiltration of inflammatory cells and toxic molecules, leading to inflammation (through NF-κB), oxidative stress [via NADPH oxidase (NOX) activation], and ischemia. In the venules and medium-size veins, pulsatility promotes collagenosis that contributes to cerebral hypoperfusion. Altogether, this deleterious ischemic and inflammatory environment favors parenchymal damage [including white matter hyperintensity (WMH)], neurovascular uncoupling, and neuronal damage [phospho-tau and amyloid-β (Aβ) depots], ultimately leading to cognitive decline and dementia. ROS, reactive oxygen species.
Such changes in cerebrovascular structure could contribute to the decrease in regional resting CBF (57, 66; for a review, see Ref. 32). Resting CBF gradually declines in most areas of the brain between 21 and 81 yr of age in healthy volunteers (1). In addition, the capillary density decreases in the fifth decade in the human brain (3). Cerebrovascular reactivity (CVR), the change in CBF in response to a vasoactive stimulus (neurovascular coupling), also decreases with age, and a recent study has demonstrated that reduced CVR preceded age-related WMH, suggesting that CVR contributes to the pathogenesis of these brain lesions (102). Although not established, it is indeed possible that the lower resting CBF observed in old age is the long-term consequence of an early impairment in CVR associated with midlife apparition of risk factors for cardiovascular diseases that would lead to transient episodes of localized hypoxia and, with accumulation of these hypoxic episodes, favor neuronal loss. On average, aging is associated with changes in brain integrity, often nonlinearly, such that volume shrinkage and white matter pathologies accelerate as a function of age (94, 95). Hence, an age-related decline in CBF may reflect both an impaired CVR and a reduced metabolic demand due to neuronal loss.
The reduced resting CBF with age is associated with a decrease in cerebral O2 and glucose metabolism (64). The development of chronic brain hypoperfusion with aging (for a review, see Ref. 13) is indeed associated with progressive hypometabolism (71). This decline in metabolism has generally been interpreted as a consequence of neuronal failure and death because, not surprisingly, brain regions with dysfunctional or dead neurons need less energy. However, after partial correction for volume effect, it has been shown that cerebral metabolic rate of glucose is significantly decreased (by ∼7%) in cognitively normal older adults, specifically in the frontal cortex (74), suggesting that hypometabolism is not only associated with neuronal loss. Age-related hypometabolism, however, may be exacerbated in medical conditions associated with neurodegeneration and prodromal to Alzheimer disease (24). In a study performed in cognitively normal old subjects, a direct inverse relationship was detected between amyloid load (seen on Pittsburgh compound B-PET) and glucose metabolism (seen on [18F]fluorodeoxyglucose-PET) in “Alzheimer disease-signature” regions (61) that include the parietal, posterior cingulate, and temporal cortex and much less present in the occipital cortex and cerebellum (for a review, see Ref. 25). In young adults with normal cognitive functions but at risk of developing Alzheimer’s disease because they are carriers of the presenilin-1 mutation (105) or carriers of apolipoprotein E4 alleles (96), glucose metabolism is also reduced, which certainly argues for a defect in neuronal metabolism prodromal to Alzheimer’s disease. Of note, in mice with knockin for the human apolipoprotein E4 allele (4), brain perfusion was reduced prematurely at 4 mo of age compared with apolipoprotein E2 and E3 knockin mice, whereas glucose uptake was reduced by 29%. An early decline in brain perfusion could therefore precipitate glucose hypometabolism among other metabolic alterations favoring cognitive dysfunction and Alzheimer’s disease.
Consequently to perfusion and metabolic changes associated with age, numerous molecular imaging studies have linked the decline in neurotransmitter production (for a review, see Ref. 59) and particularly dopamine losses (for a review, see Ref. 5) to age-related deficits in multiple cognitive domains, including episodic and working memory. Specifically, in humans, the associations of nigrostriatal to mesolimbic and mesocortical dopamine pathways are reduced in aging along with slower responding in an interference resolution task (97). This pattern suggests that aging is associated with reduced connectivity among dopaminergic pathways. In old rats, whole brain dopamine levels are the most reduced, but norepinephrine and serotonin are also significantly decreased (40). These age-related changes in neurotransmitter production, however, have never been studied integrating arterial stiffness as a covariable.
Aging of the brain is therefore multifaceted, with structural (brain atrophy), functional (decreased endothelial function, CVR, myogenic tone, and neurovascular coupling), hemodynamic (lower CBF), neurogenic (reduction in neurotransmitters), and metabolic (decreased glucose and O2 metabolism) changes. Of note, while it seems established that pulse pressure affects endothelial function, myogenic tone, and CBF, whether an increase in pulse pressure is directly related to the age-related changes in neurotransmitters and metabolism is not known. Studying progeria syndromes, genetic disorders leading to accelerated aging such as Werner syndrome, Down syndrome, and Hutchinson-Gilford progeria syndrome, could help defining the mechanisms leading to cognitive decline with age. In these syndromes of accelerating aging, does pulse pressure increase and is this associated with cognitive dysfunction? As recently reviewed (48), the brain ages faster in patients with Down syndrome than in other organs due to the accumulation of β-amyloid deposits, but PWV has been reported to be lower in these patients (78). In contrast, despite their very young age, PWV increases dramatically in patients with Hutchinson-Gilford syndrome (36) to values comparable to PWV seen in very old subjects. Patients with Hutchinson-Gilford syndrome, who die around 13 yr of age, however, have no cognitive defects, as recently reviewed (126). In patients with Werner syndrome, the brain is apparently protected (88), and, to the best of our knowledge, no data on aortic stiffness or PWV are available. Fundamental differences, therefore, exist between genetically driven premature aging and normal aging.
Chronic “Cold” Inflammation and Senescence in the Aging Brain
Since the conceptualization of “inflamm-aging” by Franceschi et al. (34), it is accepted that the low-grade chronic inflammation associated with aging contributes to the development of neurodegenerative diseases, cardiovascular diseases, diabetes, and some cancers. Age-related endothelial dysfunction induced by pulse pressure leads to blood-brain barrier leakage (proposed in Refs. 62 and 123) that could trigger central nervous system low-grade inflammation associated with immune cell infiltration, a scheme that is supported by the inverse association between systemic IL-6 levels and cognitive performance in a healthy community-based population (65). Recently, an association between basal midlife C-reactive protein levels (a nonspecific marker of systemic inflammation) and high WMH volumes measured 21 yr later by MRI was observed in a cohort from the Atherosclerosis Risk in Communities Study (132). The authors concluded that inflammation might promote the development of chronic structural white matter abnormalities associated with aging (132). Not to forget that a heightened chronic inflammatory load and immune surveillance during aging constitutes potentially an important energy sink in the body (112), which can compete with the brain for a fixed (or declining) energy intake in older people with decreasing brain metabolism (25). Finally, one key element of age-related diseases may be the inability of our immune system to eliminate proinflammatory senescent cells that accumulate with time as recently demonstrated in healthy aging mice (6) and in atherosclerotic mice (20).
Telomere shortening, a marker of cellular replicative senescence and thus aging, is accelerated in patients with elevated pulse pressure and PWV (7). Conditions characterized by age-augmented peripheral artery stiffness could therefore be associated with accelerated senescence, structural changes, brain damage, and cognitive impairment. Yet, while the link between arterial stiffness and cognitive impairment has been clearly established, the precise mechanisms of brain damage induced by this vascular remodeling remain poorly understood. We believe that the cerebrovascular endothelium, a key element in regulating vascular tone, pressure, perfusion in the brain, and exchanges, is an important intermediate between pulse pressure and brain damage because of its strategic localization and chronic exposition to mechanical stress. We propose that in the brain, senescent endothelial cells could impair blood-brain barrier function (137) and promote infiltration of circulating monocytes, microglia activation, and neuronal damage (21). We believe that mechanical stress impacts directly the endothelium (116) that enters cycles of regeneration in response to damage, leading ultimately to endothelial cell replicative senescence. It is unknown whether this is true in the brain. However, it has been observed that brains from old subjects and from patients with Alzheimer’s disease stain for β-galoctasidase (a marker of senescent cells) and express higher levels of senescence-associated p21 and of senescence-associated secretory phenotype factors, suggesting a possible association of senescence with age-related cognitive impairment including Alzheimer diseases (hypothesis proposed in Refs. 38, 113, 114, and 139). Astrocytes, glial cells, and neurons have been the focus of these studies, and senescence of endothelial cells is only presupposed (21, 114, 137). In a recent study performed in a model of blood-brain barrier in cultured cells, the authors demonstrated that the accumulation of senescent endothelial cells and pericytes was associated with impaired permeability, which could contribute to the infiltration of immune, inflammatory cells (137). A meta-analysis showed that patients with Alzheimer’s disease have shorter telomeres (33), but only one study has suggested causality between senescence and cognitive impairment: using genetically modified aging mice in which cells expressing the senescence marker p16(Ink4a) could be removed by triggering apoptosis (p16INK-ATTAC mice), the authors demonstrated that elimination of p16 senescent cells from all origins led to a prolonged health span (increased median life span) associated with a reduction of markers of aging, including a better exploratory behavior in the open field test (6). This is the only study showing that senescence contributes to cognitive impairment. In a second study (60), the authors showed that resveratrol slowed the accumulation of senescent cells in the brain and that this was associated with a slower progression of cognitive decline in a mouse model of accelerated senescence. Nonetheless, it remains to be demonstrated whether senescent cerebrovascular endothelial cells specifically contribute by initiating age-related cognitive decline. In addition, there is no evidence in the literature that senescence in elderly neurons or glial cells are directly dependent on the chronic mechanical stress of the pulse pressure and changes in cerebral hemodynamics. Altogether, the role of cellular senescence in the process leading to cognitive decline is still hypothetical; however, knowing that pulse pressure promotes blood-brain barrier damage (62) and that cell damage contributes to aging by increasing cell death, dysfunction, or senescence (for a review, see Ref. 30), it is conceivable that endothelial, glial, and neuronal senescence contributes, at least partially, to the pathway leading to neuronal damage and cognitive decline.
Oxidative Stress and MMPs
Human pathological studies in infarcted and matched noninfarcted cerebral tissue have shown that ischemia associates with infiltrating macrophages and activated microglia, inflammatory cells that both release MMPs and ROS (22, 99). Similarly, MMP activity and ROS release are increased in brain microvessels of atherosclerotic mice with stiffer carotids (10, 27); in addition, MMP activity was specifically reduced and endothelial NO synthase function maintained if heart rate (mechanical stress) was lowered chronically (3 mo) using the If (funny current) inhibitor ivabradine (10). MMPs released by the macrophage/microglia directly attack the extracellular matrix of blood vessels, loosening tight junctions and breaking down the basal lamina; they also attack myelinated fibers, breaking down myelin and leading to loss of normal white matter function (138). This could explain both the disruption of the blood-brain barrier and demyelination induced by vascular disease, as proposed 40 yr ago (14), to a stage where brain cell dysfunction is irreversible. In vitro, in cultured endothelial cells, cyclic mechanical stretch to mimic pulse pressure promotes ROS generation (19) but also IκB degradation and nuclear translocation of NF-κB and, thus, the expression of inflammatory response genes. Moreover, in cultured human brain microvascular endothelial cells, exposure to a high shear stress and/or pulsatility mimicked blood-brain barrier impairment (35). Oxidative stress and inflammation, endothelial dysfunction and senescence, and vascular remodeling are therefore tightly linked and could characterize the molecular response to penetrating pulse pressure in aging cerebral microvessels (Fig. 2).
CONCLUSIONS
Increased peripheral artery stiffness that leads to a rise in pulse pressure could lead to microcerebrovascular endothelial dysfunction and senescence, blood-brain barrier leakiness with infiltration of inflammatory cells, and CBF dysregulation, alterations that, taken together, could ultimately promote downstream ischemia and brain structural changes, neurovascular uncoupling, neuronal death, and cognitive decline (Fig. 2). All these characteristics of the aging brain are confirmed, but the cause-effect links are tenuous. One proposition is that lifetime exposure to pulse pressure magnified by the age-related stiffening of elastic arteries damages cerebral microvessels (arterioles, capillaries, and venules), leading to cerebrovascular diseases, parenchymal damage, and cognitive impairment. Pharmacological treatments that correct or lower arterial stiffening do not exist yet but should offer some protection for the brain (for a review, see Ref. 49). This thought led to the following question by O’Rourke et al. (110): “If we could, in the aged, restore the elasticity of the great vessels, how long might we then live? And of what would [we] then die”? Natural aging, starting at conception (103), is a complex interplay between our genetic background and the environment leading to adaptive responses that determine, by their unique cause-effect interferences, our lifespan and brain health. It remains to be fully established whether this natural limit is ultimately dependent on the progressive wear and tear of the vasculature.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.T. conceived and designed research; A.R. performed experiments; O.d.M., A.P., and E.T. analyzed data; O.d.M., A.P., and E.T. interpreted results of experiments; N.T.-T. and E.T. drafted manuscript; N.T.-T. draw the figures N.T.-T., O.d.M., A.P., L.C., P.L., and E.T. edited and revised manuscript; N.T.-T., O.d.M., A.P., A.R., L.C., P.L., and E.T. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of A. Raignault: Universal Medica, Saint-Cloud 92210, France.
REFERENCES
- 1.Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jónsdottir KY, Møller A, Ashkanian M, Vafaee MS, Iversen P, Johannsen P, Gjedde A. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab 32: 1177–1187, 2012. doi: 10.1038/jcbfm.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akima M, Nonaka H, Kagesawa M, Tanaka K. A study on the microvasculature of the cerebral cortex. Fundamental architecture and its senile change in the frontal cortex. Lab Invest 55: 482–489, 1986. [PubMed] [Google Scholar]
- 4.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 35: 86–94, 2015. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev 34: 670–677, 2010. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530: 184–189, 2016. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37: 381–385, 2001. doi: 10.1161/01.HYP.37.2.381. [DOI] [PubMed] [Google Scholar]
- 8.Benjo A, Thompson RE, Fine D, Hogue CW, Alejo D, Kaw A, Gerstenblith G, Shah A, Berkowitz DE, Nyhan D. Pulse pressure is an age-independent predictor of stroke development after cardiac surgery. Hypertension 50: 630–635, 2007. doi: 10.1161/HYPERTENSIONAHA.107.095513. [DOI] [PubMed] [Google Scholar]
- 9.Bolduc V, Baraghis E, Duquette N, Thorin-Trescases N, Lambert J, Lesage F, Thorin E. Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr−/−:hApoB+/+ mice and improves cerebral blood flow. Am J Physiol Heart Circ Physiol 302: H1330–H1339, 2012. doi: 10.1152/ajpheart.01044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolduc V, Drouin A, Gillis MA, Duquette N, Thorin-Trescases N, Frayne-Robillard I, Des Rosiers C, Tardif JC, Thorin E. Heart rate-associated mechanical stress impairs carotid but not cerebral artery compliance in dyslipidemic atherosclerotic mice. Am J Physiol Heart Circ Physiol 301: H2081–H2092, 2011. doi: 10.1152/ajpheart.00706.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics 28: 146–157, 2007. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 12.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci 203−204: 159–163, 2002. doi: 10.1016/S0022-510X(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 13.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37: 56–74, 2011. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cammer W, Bloom BR, Norton WT, Gordon S. Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages: a possible mechanism of inflammatory demyelination. Proc Natl Acad Sci USA 75: 1554–1558, 1978. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60: 188–197, 2012. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrera-Bastos P, Fontes-Villaba M, O’Keefe J, Lindeberg S, Cordain L. The Western diet and lifestyle and diseases of civilization. Res Rep Clin Cardiol 2: 15, 2011. doi: 10.2147/RRCC.S16919. [DOI] [Google Scholar]
- 17.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol 96: 3–10, 2004. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 19.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension 31: 125–130, 1998. doi: 10.1161/01.HYP.31.1.125. [DOI] [PubMed] [Google Scholar]
- 20.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354: 472–477, 2016. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol 68: 3–7, 2015. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett 238: 53–56, 1997. doi: 10.1016/S0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 23.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension 67: 176–182, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 27: 3–20, 2011. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano CA. Can ketones help rescue brain fuel supply in later life? implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci 9: 53, 2016. doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutler D, Deaton A, Lleras-Muney A. The determinants of mortality. J Econ Perspect 20: 97–100, 2006. doi: 10.1257/jep.20.3.97. [DOI] [Google Scholar]
- 27.Drouin A, Bolduc V, Thorin-Trescases N, Bélanger É, Fernandes P, Baraghis E, Lesage F, Gillis MA, Villeneuve L, Hamel E, Ferland G, Thorin E. Catechin treatment improves cerebrovascular flow-mediated dilation and learning abilities in atherosclerotic mice. Am J Physiol Heart Circ Physiol 300: H1032–H1043, 2011. doi: 10.1152/ajpheart.00410.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drouin A, Farhat N, Bolduc V, Thorin-Trescases N, Gillis MA, Villeneuve L, Nguyen A, Thorin E. Up-regulation of thromboxane A2 impairs cerebrovascular eNOS function in aging atherosclerotic mice. Pflugers Arch 462: 371–383, 2011. doi: 10.1007/s00424-011-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension 53: 668–673, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdő F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab 37: 4–24, 2017. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage 85: 592–607, 2014. doi: 10.1016/j.neuroimage.2013.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64: 575–611, 2001. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- 33.Forero DA, González-Giraldo Y, López-Quintero C, Castro-Vega LJ, Barreto GE, Perry G. Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 71: 1069–1073, 2016. doi: 10.1093/gerona/glw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908: 244–254, 2000. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Polite F, Martorell J, Del Rey-Puech P, Melgar-Lesmes P, O’Brien CC, Roquer J, Ois A, Principe A, Edelman ER, Balcells M. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J Cereb Blood Flow Metab 37: 2614–2625, 2017. doi: 10.1177/0271678X16672482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhard-Herman M, Smoot LB, Wake N, Kieran MW, Kleinman ME, Miller DT, Schwartzman A, Giobbie-Hurder A, Neuberg D, Gordon LB. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension 59: 92–97, 2012. doi: 10.1161/HYPERTENSIONAHA.111.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27: 303–309, 2007. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 38.Golde TE, Miller VM. Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer’s and other neurodegenerative diseases. Alzheimers Res Ther 1: 5, 2009. doi: 10.1186/alzrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graff-Radford J, Simino J, Kantarci K, Mosley TH Jr, Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR Jr, Vemuri P, Knopman DS. Neuroimaging correlates of cerebral microbleeds: the ARIC Study (Atherosclerosis Risk in Communities). Stroke 48: 2964–2972, 2017. doi: 10.1161/STROKEAHA.117.018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, Sadir S, Liaquat L, Madiha S. Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Dordr) 36: 9653, 2014. doi: 10.1007/s11357-014-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension 67: 171–175, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke 36: 2193–2197, 2005. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- 43.Heistad DD. What’s new in the cerebral microcirculation? Landis Award lecture. Microcirculation 8: 365–375, 2001. doi: 10.1111/j.1549-8719.2001.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 44.Henry Feugeas MC, De Marco G, Peretti II, Godon-Hardy S, Fredy D, Claeys ES. Age-related cerebral white matter changes and pulse-wave encephalopathy: observations with three-dimensional MRI. Magn Reson Imaging 23: 929–937, 2005. doi: 10.1016/j.mri.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Henry-Feugeas MC, Koskas P. Cerebral vascular aging: extending the concept of pulse wave encephalopathy through capillaries to the cerebral veins. Curr Aging Sci 5: 157–167, 2012. doi: 10.2174/1874609811205020157. [DOI] [PubMed] [Google Scholar]
- 46.Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 52: 1120–1126, 2008. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 47.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120: 287–296, 2010. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaev NK, Genrikhs EE, Oborina MV, Stelmashook EV. Accelerated aging and aging process in the brain. Rev Neurosci 29: 233−240, 2018. doi: 10.1515/revneuro-2017-0051. [DOI] [PubMed] [Google Scholar]
- 49.Iulita MF, Noriega de la Colina A, Girouard H. Arterial stiffness, cognitive impairment and dementia: real risk or confounding factor? J Neurochem 144: 527−548, 2018. doi: 10.1111/jnc.14235. [DOI] [PubMed] [Google Scholar]
- 50.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke 40: 1229–1236, 2009. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- 51.Keith J, Gao FQ, Noor R, Kiss A, Balasubramaniam G, Au K, Rogaeva E, Masellis M, Black SE. Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol 76: 299–312, 2017. doi: 10.1093/jnen/nlx009. [DOI] [PubMed] [Google Scholar]
- 52.Kontos HA, Wei EP, Raper AJ, Rosenblum WI, Navari RM, Patterson JL Jr. Role of tissue hypoxia in local regulation of cerebral microcirculation. Am J Physiol Heart Circ Physiol 234: H582–H591, 1978. [DOI] [PubMed] [Google Scholar]
- 53.Kuo HK, Chen CY, Liu HM, Yen CJ, Chang KJ, Chang CC, Yu YH, Lin LY, Hwang JJ. Metabolic risks, white matter hyperintensities, and arterial stiffness in high-functioning healthy adults. Int J Cardiol 143: 184–191, 2010. doi: 10.1016/j.ijcard.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Lakatta EG. So! What’s aging? Is cardiovascular aging a disease? J Mol Cell Cardiol 83: 1–13, 2015. doi: 10.1016/j.yjmcc.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leblond F, Nguyen A, Bolduc V, Lambert J, Yu C, Duquette N, Thorin E. Postnatal exposure to voluntary exercise but not the antioxidant catechin protects the vasculature after a switch to an atherogenic environment in middle-age mice. Pflugers Arch 465: 197–208, 2013. doi: 10.1007/s00424-012-1206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee RM. Morphology of cerebral arteries. Pharmacol Ther 66: 149–173, 1995. doi: 10.1016/0163-7258(94)00071-A. [DOI] [PubMed] [Google Scholar]
- 57.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RS. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 58.Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol 30: 1104–1106, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Li SC, Rieckmann A. Neuromodulation and aging: implications of aging neuronal gain control on cognition. Curr Opin Neurobiol 29: 148–158, 2014. doi: 10.1016/j.conb.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Liu GS, Zhang ZS, Yang B, He W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sci 91: 872–877, 2012. doi: 10.1016/j.lfs.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 61.Lowe VJ, Weigand SD, Senjem ML, Vemuri P, Jordan L, Kantarci K, Boeve B, Jack CR Jr, Knopman D, Petersen RC. Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology 82: 1959–1967, 2014. doi: 10.1212/WNL.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Aortic stiffness, increased white matter free water, and altered microstructural integrity: a continuum of injury. Stroke 48: 1567–1573, 2017. doi: 10.1161/STROKEAHA.116.016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Effects of arterial stiffness on brain integrity in young adults from the Framingham Heart Study. Stroke 47: 1030–1036, 2016. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchal G, Rioux P, Petit-Taboué MC, Sette G, Travère JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 49: 1013–1020, 1992. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- 65.Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med 68: 895–903, 2006. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- 66.Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11: 684–689, 1991. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- 67.McDade E, Sun Z, Lee CW, Snitz B, Hughes T, Chang CC, Ganguli M. The association between pulse pressure change and cognition in late life: age and where you start matters. Alzheimers Dement (Amst) 4: 56–66, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296–302, 2015. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 194: 469–476, 1995. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 71.Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging 36: 811–822, 2009. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 73.Nation DA, Preis SR, Beiser A, Bangen KJ, Delano-Wood L, Lamar M, Libon DJ, Seshadri S, Wolf PA, Au R. Pulse pressure is associated with early brain atrophy and cognitive decline: modifying effects of APOE-ε4. Alzheimer Dis Assoc Disord 30: 210–215, 2016. doi: 10.1097/WAD.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nugent S, Castellano CA, Bocti C, Dionne I, Fulop T, Cunnane SC. Relationship of metabolic and endocrine parameters to brain glucose metabolism in older adults: do cognitively-normal older adults have a particular metabolic phenotype? Biogerontology 17: 241–255, 2016. doi: 10.1007/s10522-015-9595-7. [DOI] [PubMed] [Google Scholar]
- 75.Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci 16: 292–305, 2012. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 76.O’Rourke MF. Arterial aging: pathophysiological principles. Vasc Med 12: 329–341, 2007. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 77.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 78.Parra P, Costa R, de Asúa DR, Moldenhauer F, Suárez C. Atherosclerotic surrogate markers in adults with Down syndrome: a case-control study. J Clin Hypertens (Greenwich) 19: 205–211, 2017. doi: 10.1111/jch.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pase MP, Herbert A, Grima NA, Pipingas A, O’Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern Med J 42: 808–815, 2012. doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 81.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The Framingham Third Generation Cohort Study. Hypertension 67: 513–519, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pase MP, Pipingas A, Kras M, Nolidin K, Gibbs AL, Wesnes KA, Scholey AB, Stough C. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens 28: 1724–1729, 2010. doi: 10.1097/HJH.0b013e32833b1ee7. [DOI] [PubMed] [Google Scholar]
- 83.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990. [PubMed] [Google Scholar]
- 84.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304: H1598–H1614, 2013. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 78: 326–333, 2012. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 86.Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke 38: 888–892, 2007. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- 87.Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, Witteman JC, Breteler MM, Mattace-Raso FU, Ikram MA. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke 43: 2637–2642, 2012. doi: 10.1161/STROKEAHA.111.642264. [DOI] [PubMed] [Google Scholar]
- 88.Postiglione A, Soricelli A, Covelli EM, Iazzetta N, Ruocco A, Milan G, Santoro L, Alfano B, Brunetti A. Premature aging in Werner’s syndrome spares the central nervous system. Neurobiol Aging 17: 325–330, 1996. doi: 10.1016/0197-4580(96)00033-4. [DOI] [PubMed] [Google Scholar]
- 89.Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, Russo G, Echart C, Maffei A, De Simoni MG, Lembo G. Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab 26: 253–262, 2006. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- 90.Pries AR, Secomb TW. Control of blood vessel structure: insights from theoretical models. Am J Physiol Heart Circ Physiol 288: H1010–H1015, 2005. doi: 10.1152/ajpheart.00752.2004. [DOI] [PubMed] [Google Scholar]
- 91.Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS, Guttmann CR, Fisher ND, Lipsitz LA, Sorond FA. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab 34: 228–234, 2014. doi: 10.1038/jcbfm.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis 32: 541–549, 2012. [DOI] [PubMed] [Google Scholar]
- 93.Raignault A, Bolduc V, Lesage F, Thorin E. Pulse pressure-dependent cerebrovascular eNOS regulation in mice. J Cereb Blood Flow Metab 37: 413–424, 2017. doi: 10.1177/0271678X16629155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage 51: 501–511, 2010. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging 33: 429.e1– 429.e5, 2012. doi: 10.1016/j.neurobiolaging.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA 101: 284–289, 2004. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rieckmann A, Karlsson S, Karlsson P, Brehmer Y, Fischer H, Farde L, Nyberg L, Bäckman L. Dopamine D1 receptor associations within and between dopaminergic pathways in younger and elderly adults: links to cognitive performance. Cereb Cortex 21: 2023–2032, 2011. doi: 10.1093/cercor/bhq266. [DOI] [PubMed] [Google Scholar]
- 98.Rook GA. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol 160: 70–79, 2010. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosenberg GA, Sullivan N, Esiri MM, Sobel RA. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke 32: 1162–1168, 2001. doi: 10.1161/01.STR.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 100.Saji N, Toba K, Sakurai T. Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain? Pulse (Basel) 3: 182–189, 2016. doi: 10.1159/000443614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saklayen MG, Deshpande NV. Timeline of history of hypertension treatment. Front Cardiovasc Med 3: 3, 2016. doi: 10.3389/fcvm.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sam K, Crawley AP, Conklin J, Poublanc J, Sobczyk O, Mandell DM, Venkatraghavan L, Duffin J, Fisher JA, Black SE, Mikulis DJ. Development of white matter hyperintensity is preceded by reduced cerebrovascular reactivity. Ann Neurol 80: 277–285, 2016. doi: 10.1002/ana.24712. [DOI] [PubMed] [Google Scholar]
- 103.Sartori C, Rimoldi SF, Duplain H, Stuber T, Garcin S, Rexhaj E, Allemann Y, Scherrer U. Developmental origins of hypoxic pulmonary hypertension and systemic vascular dysfunction: evidence from humans. Adv Exp Med Biol 903: 17–28, 2016. doi: 10.1007/978-1-4899-7678-9_2. [DOI] [PubMed] [Google Scholar]
- 104.Schievink WI. Intracranial aneurysms. N Engl J Med 336: 28–40, 1997. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 105.Schöll M, Almkvist O, Bogdanovic N, Wall A, Långström B, Viitanen M, Nordberg A. Time course of glucose metabolism in relation to cognitive performance and postmortem neuropathology in Met146Val PSEN1 mutation carriers. J Alzheimers Dis 24: 495–506, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens 23: 1211–1216, 2005. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 107.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens 25: 1035–1040, 2007. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 107a.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 265: 3255–3264, 1991. doi: 10.1001/jama.1991.03460240051027. [DOI] [PubMed] [Google Scholar]
- 108.Smeda JS. Cerebral vascular changes associated with hemorrhagic stroke in hypertension. Can J Physiol Pharmacol 70: 552–564, 1992. doi: 10.1139/y92-070. [DOI] [PubMed] [Google Scholar]
- 109.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab 35: 527–530, 2015. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stone J, Johnstone DM, Mitrofanis J, O’Rourke M. The mechanical cause of age-related dementia (Alzheimer’s disease): the brain is destroyed by the pulse. J Alzheimers Dis 44: 355–373, 2015. doi: 10.3233/JAD-141884. [DOI] [PubMed] [Google Scholar]
- 111.Strandgaard S, Jones JV, MacKenzie ET, Harper AM. Upper limit of cerebral blood flow autoregulation in experimental renovascular hypertension in the baboon. Circ Res 37: 164–167, 1975. doi: 10.1161/01.RES.37.2.164. [DOI] [PubMed] [Google Scholar]
- 112.Straub RH, Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health 2016: 37–51, 2016. doi: 10.1093/emph/eow001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan FC, Hutchison ER, Eitan E, Mattson MP. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 15: 643–660, 2014. doi: 10.1007/s10522-014-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tarantini S, Tran CH, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 94: 52–58, 2017. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thorin E, Thorin-Trescases N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovasc Res 84: 24–32, 2009. doi: 10.1093/cvr/cvp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thorin-Trescases N, Thorin E. Lifelong cyclic mechanical strain promotes large elastic artery stiffening: increased pulse pressure and old age-related organ failure. Can J Cardiol 32: 624–633, 2016. doi: 10.1016/j.cjca.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 118.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–H1708, 2013. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: H1–H20, 2017. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol 309: H1837–H1845, 2015. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34: 1887–1897, 2014. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, Trivilou P, Zerva L, Stamboulis E, Kremastinos DT. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens 22: 525–530, 2009. doi: 10.1038/ajh.2009.35. [DOI] [PubMed] [Google Scholar]
- 125.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81: 984–991, 2013. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol 132: 249–264, 2015. doi: 10.1016/B978-0-444-62702-5.00018-4. [DOI] [PubMed] [Google Scholar]
- 127.van Elderen SG, Brandts A, van der Grond J, Westenberg JJ, Kroft LJ, van Buchem MA, Smit JW, de Roos A. Cerebral perfusion and aortic stiffness are independent predictors of white matter brain atrophy in type 1 diabetic patients assessed with magnetic resonance imaging. Diabetes Care 34: 459–463, 2011. doi: 10.2337/dc10-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Elderen SG, Brandts A, Westenberg JJ, van der Grond J, Tamsma JT, van Buchem MA, Romijn JA, Kroft LJ, Smit JW, de Roos A. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol 20: 1132–1138, 2010. doi: 10.1007/s00330-009-1655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 53: 121–130, 2015. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vrselja Z, Brkic H, Mrdenovic S, Radic R, Curic G. Function of circle of Willis. J Cereb Blood Flow Metab 34: 578–584, 2014. doi: 10.1038/jcbfm.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51: 99–104, 2008. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 132.Walker KA, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Selvin E, Jack CR Jr, Gottesman RF. Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: the Atherosclerosis Risk in Communities Study. Stroke 48: 3196–3202, 2017. doi: 10.1161/STROKEAHA.117.018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wardlaw JM, Allerhand M, Eadie E, Thomas A, Corley J, Pattie A, Taylor A, Shenkin SD, Cox S, Gow A, Starr JM, Deary IJ. Carotid disease at age 73 and cognitive change from age 70 to 76 years: a longitudinal cohort study. J Cereb Blood Flow Metab 37: 3042–3052, 2017. doi: 10.1177/0271678X16683693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watson NL, Sutton-Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM, Najjar SS, Launer LJ, Yaffe K, Atkinson HH, Satterfield S, Newman AB. Arterial stiffness and cognitive decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci 66: 1336–1342, 2011. doi: 10.1093/gerona/glr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke 43: 2631–2636, 2012. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 136.Wilking SV, Belanger A, Kannel WB, D’Agostino RB, Steel K. Determinants of isolated systolic hypertension. JAMA 260: 3451–3455, 1988. doi: 10.1001/jama.1988.03410230069030. [DOI] [PubMed] [Google Scholar]
- 137.Yamazaki Y, Baker DJ, Tachibana M, Liu CC, van Deursen JM, Brott TG, Bu G, Kanekiyo T. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke 47: 1068–1077, 2016. doi: 10.1161/STROKEAHA.115.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27: 697–709, 2007. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 139.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17: 324–328, 2014. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]