Abstract
Calcific aortic vasculopathy correlates with bone loss in osteoporosis in an age-independent manner. Prior work suggests that teriparatide, the bone anabolic treatment for postmenopausal osteoporosis, may inhibit the onset of aortic calcification. Whether teriparatide affects the progression of preexisting aortic calcification, widespread among this patient population, is unknown. Female apolipoprotein E-deficient mice were aged for over 1 yr to induce aortic calcification, treated for 4.5 wk with daily injections of control vehicle (PBS), 40 µg/kg teriparatide (PTH40), or 400 µg/kg teriparatide (PTH400), and assayed for aortic calcification by microcomputed tomography (microCT) before and after treatment. In a followup cohort, aged female apolipoprotein E-deficient mice were treated with PBS or PTH400 and assayed for aortic calcification by serial microCT and micropositron emission tomography. In both cohorts, aortic calcification detected by microCT progressed similarly in all groups. Mean aortic 18F-NaF incorporation, detected by serial micropositron emission tomography, increased in the PBS-treated group (+14 ± 5%). In contrast, 18F-NaF incorporation decreased in the PTH400-treated group (−33 ± 20%, P = 0.03). Quantitative histochemical analysis by Alizarin red staining revealed a lower mineral surface area index in the PTH400-treated group compared with the PBS-treated group (P = 0.04). Furthermore, Masson trichrome staining showed a significant increase in collagen deposition in the left ventricular myocardium of mice that received PTH400 [2.1 ± 0.6% vs. control mice (0.5 ± 0.1%), P = 0.02]. In summary, although teriparatide may not affect the calcium mineral content of aortic calcification, it reduces 18F-NaF uptake in calcified lesions, suggesting the possibility that it may reduce mineral surface area with potential impact on plaque stability.
NEW & NOTEWORTHY Parathyroid hormone regulates bone mineralization and may also affect vascular calcification, which is an important issue, given that its active fragment, teriparatide, is widely used for the treatment of osteoporosis. To determine whether teriparatide alters vascular calcification, we imaged aortic calcification in mice treated with teriparatide and control mice. Although teriparatide did not affect the calcium content of cardiovascular deposits, it reduced their fluoride tracer uptake.
Keywords: aortic calcification, fibrosis, parathyroid hormone, teriparatide, vascular calcification
INTRODUCTION
Calcific aortic vasculopathy is widespread, with increasing prevalence with age, and it increases the risk of cardiovascular and all-cause mortality (7, 23, 41, 45). Calcification reduces vascular compliance, leading to clinical consequences, such as hypertension, coronary ischemia, infarction, congestive heart failure, and ventricular hypertrophy (55). In coronary arteries, calcification independently predicts a 1.7-fold increase in mortality (45). The mechanism of increased plaque vulnerability may be compliance mismatch at the interface between compliant vascular tissue and rigid mineral (27, 37), which is increased by mineral surface area (MSA). Additionally, calcific valve disease is associated with ventricular arrhythmias, syncope, congestive heart failure, and ventricular hypertrophy, and about half of patients with severe aortic valve calcification die within 2 yr of symptom onset (11).
Epidemiological studies have shown that cardiovascular disease correlates with osteoporosis in an age-independent manner (22, 24, 26, 32, 43, 52). Secreted from the parathyroid gland, parathyroid hormone (PTH; 84-amino acid peptide) acts on bone and the kidney to maintain systemic calcium homeostasis (46, 47). In rodents and humans, treatment with teriparatide [PTH(1–34), the biologically active fragment of PTH] increases bone mass and strength, improves microarchitectural connectivity, and reduces fractures, leading to its successful clinical use as an anabolic agent for osteoporosis (14, 38, 40, 45).
Prior studies in mice and rats have raised the possibility that this bone anabolic treatment may also affect mineralization in cardiovascular tissues. Using a mouse model of diet-induced diabetes and hyperlipidemia, Towler and colleagues (54) found that treatment of young mice with PTH (400 µg/kg) attenuated the development of aortic valve leaflet calcification based on histochemical staining. In a rat model of chronic kidney disease, Friedman and colleagues (53) found that PTH (40 µg/kg) reduced the development of microcalcifications in the aorta and that, after controlling for hyperparathyroidism with parathyroidectomy, teriparatide had no significant effect on aortic calcification. In clinical use, most patients requiring teriparatide treatment are expected to have preexisting calcific cardiovascular disease (32, 43, 52), and its effects on preexisting calcific disease are unknown. Given the prevalence of calcific aortic vasculopathy and hyperlipidemia among osteoporotic individuals, our objective was to determine whether teriparatide halts and/or reverses the progression of preexisting atherosclerotic calcification. In the present study, we tested the effects of teriparatide on aged hyperlipidemic mice with preexisting cardiovascular calcification.
MATERIALS AND METHODS
Animals and Treatments
Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California-Los Angeles. Two independent cohorts of mice were studied. In the initial cohort, female apolipoprotein E (ApoE)-null mice (12 wk old, n = 23, C57BL/6 background, Jackson Laboratory, Bar Harbor, ME) were aged on a chow diet to 70–78 wk old to induce aortic calcification. Unfortunately, during the aging process, five mice had to be euthanized because of severe ulcerative dermatitis. The remaining mice were randomized into the following three groups (n = 6 mice/group): control (PBS), 40 µg/kg teriparatide (PTH40), and 400 µg/kg teriparatide (PTH400), and in vivo microcomputed tomography (microCT) imaging was performed before and after treatment for 4.5 wk. Despite the excess loss of mice during aging, the group sizes were comparable with those of prior studies of vascular disease in aged mice (10, 48, 54). Mice were euthanized at 75–82 wk of age. In the followup cohort, 52- to 70-wk-old female ApoE-null mice (PTH1rf/fApoE−/−) were divided into the following two groups: control (PBS; n = 5) and PTH400 (n = 6), and in vivo microCT/micropositron emission tomography (microPET) imaging as well as echocardiography were performed before and after treatment for 4.5 wk. In this cohort, mice were euthanized at 57–75 wk of age. In both initial and followup experiments, mice were fed a standard chow diet (6.2% fat and 18% protein, Teklad 7013, Envigo, Madison, WI) and weighed weekly, and teriparatide [human PTH(1–34), Bachem, Torrance, CA] was administered by subcutaneous injection (5 days/wk). In both cohorts, these aged mice developed aortic calcification spontaneously on the standard chow diet.
Histochemistry
Hearts were embedded in optimal cutting temperature compound, and 10-µm frozen sections were obtained. For the quantitation of myocardial fibrosis, Masson trichrome stain was performed per the manufacturer’s protocol (IMEB, San Marcos, CA). Note that the PTH400-treated group had one less set of data because of processing of the heart for light-sheet fluorescence microscopy. The extent of fibrosis was quantified using Metamorph for Olympus software (Waltham, MA), and the fibrotic regions (stained in blue) were segmented by a threshold value; the total left ventricular (LV) myocardial area was segmented manually before quantification of their areas. For the quantitation of aortic calcification, aortic root frozen sections underwent Alizarin red staining (1,25-dihydroxyanthraquinone, Sigma, St. Louis, MO). The calcified regions were segmented using ImageJ software, and the total perimeter and area of each calcified deposit were quantified using custom Matlab code (Mathworks, Natick, MA). A macrocalcium deposit was defined as any single deposit with an area of ≥1,950 µm2 (corresponding to an average diameter of ≥50 µm) and a microcalcium deposit had an area <1,950 µm2, similar to previously described thresholds (29).
Alkaline phosphatase activity and Alizarin red staining were performed as previously described (33, 34). To test for myofibroblast activation, we performed immunohistochemistry using antibody specific to α-smooth muscle actin (Santa Cruz Biotechnology, Dallas, TX), as previously described (33).
Echocardiography
Mice were anesthetized (3.0% isoflurane for initiation and 1.5–2.0% isoflurane for maintenance delivered via nose cone), and pulsed-wave Doppler echocardiography was performed using a VisualSonics Vevo 2100 equipped with a 30-MHz linear transducer. LV ejection fraction, LV wall thickness, and LV cavity dimensions were measured.
Ex Vivo MicroCT Imaging of Femurs
Right femoral bones were harvested, cleaned of tissues, and analyzed for cortical and trabecular bone surface normalized for total volume (BS/TV) and percent bone volume (BV/TV) by microCT (Skyscan 1172), as previously described (44).
Serial In Vivo MicroCT Imaging of Vascular Calcification
Mice were anesthetized by isoflurane (3% for initial sedation and 1.5–2.0% for maintenance via nose cone) and scanned by microCT. For the initial cohort, the in-house microCT (CrumpCAT, University of California-Los Angeles) (57) was used for pretreatment scans, and G8 microCT (Perkin-Elmer), the commercialized version of the CrumpCAT, was used for posttreatment scans. One mouse in the PBS-treated group was excluded, as it died before the end of the study. Images were acquired using a 50-kVp, 200-µA X-ray source with a 45° cone angle, positioned at 13–15 cm from the isocenter, reconstructed using the Feldkamp algorithm with a voxel size of 125 µm (CrumpCAT) and 200 µm (G8), and calibrated for Hounsfield units (HU). Calcium content was quantified from the images of the aortic root, aortic arch, and great vessels using AMIDE software (version 1.0.5) (36). Individual three-dimensional isocontour regions of interest (ROIs) were drawn around each calcified region, and a conservative minimum threshold was set at 350 HU (CrumpCAT) and 300 HU (G8) based on the density of soft tissue versus bone in mice (21). The comparable thresholds were determined, in a separate additional experiment, by scanning a water and air calibration phantom as well as by the same mice on both the CrumpCAT and G8 machines. The aortic volumetric calcium content (vHU) was defined as the product of mean ROI value (HU) and volume of the ROI (mm3), summed over all ROIs for each mouse. To establish soft tissue anatomic landmarks, 1 wk after the initial microCT scan, each mouse underwent contrast-enhanced microCT imaging preceded by a tail vein injection of Omnipaque 350 (5 ml/kg, GE Healthcare, Princeton, NJ).
Because the G8 microCT is a commercialized version of the low-resolution setting of the CrumpCAT scanner, the only differences that would potentially affect the two data sets are in the resolution settings (0.125 mm, 0.2 mm for CrumpCAT and 0.2 mm for G8) and the HU calibration. For validation, in a separate study, we analyzed the data from the same mice scanned on the CrumpCAT at high and low resolutions and found that differences in HU attributable to resolution were minimal. Because the HU scale is a linear transformation of the data based on water and air radiodensity, we identified the difference in the linear transformation between the two scanners to arrive at the different thresholding values for the isocontour ROI analyses. We further confirmed this correction in a third study by imaging the same three mice on the G8 scanner and on the CrumpCAT, at both high and low resolution, immediately one after the other.
Serial Fused In Vivo MicroPET/MicroCT Imaging
Fused microPET/microCT imaging was performed only in the followup cohort before and after teriparatide treatment. Mice were injected via tail vein with ~200 µCi 18F-NaF (prepared as previously described) (12), anesthetized (3% isoflurane for induction) 1 h postinjection, and imaged on the microPET (Focus 200 microPET, Concorde Microsystems, Knoxville, TN) and microCT (CrumpCAT) scanners. MicroPET images were acquired for 10 min with an energy window of 250–700 keV followed by three-dimensional histographic analysis and reconstruction with a zoom factor of 4.7452 using 3D-OSEM with two iterations followed by maximum a posteriori with 18 iterations and resolution set at 1 mm. Images were analyzed, and maximum-intensity projections (MIPs) were generated using AMIDE software. For each microPET/microCT image set, an initial volumetric ROI was drawn to isolate the cardiac and aortic regions from the remainder of the body, three-dimensional isocontour ROIs were then drawn of the calcified areas within this region, and, finally, quantification of the isocontour ROIs was performed on the original images. The minimum 18F-NaF isocontour threshold for aortic calcification was defined as 2% injected dose per gram (%ID/g), a value above 18F-NaF uptake in normal heart tissue. The minimum microCT isocontour threshold was 350 HU. The aortic calcium content (%ID for microPET, vHU for microCT) was defined as the product of the mean ROI value (%ID/g or HU) and volume of the ROI (mm3). Notably, one mouse in the PTH400-treated group had to be removed from the analysis because of a processing acquisition error with the microPET.
Light-Sheet Fluorescence Microscopy
Anesthetized mice (under 3% isoflurane) were injected retroorbitally with fluorescent bisphosphonate (5-FAM-ZOL, BioVinc, Pasadena, CA) 5 days before euthanasia. Hearts were carefully dissected, washed, and fixed overnight at 4°C in 4% paraformaldehyde. Hearts were rendered nearly transparent by chemical tissue clearing using the CLARITY technique, as previously described (56), for a duration of 2 mo. This optical clearing technique removes most of the lipid and equalizes the refractive indexes throughout the tissue with that of the mounting solution. Specimens were transferred to a borosilicate glass tube (Pyrex 7740, Corning, Corning, NY) and imaged using our in-house, light-sheet fluorescence microscope (LSFM) (17, 18). This LSFM uses orthogonal lenses for dual illumination and detection, allowing rapid scanning to achieve high axial resolution with low levels of photobleaching. This LSFM applies a 473-nm laser line (Laserglow Technologies, Toronto, ON, Canada) as the illumination source of 5-FAM-ZOL (maximum absorption: 493 nm, maximum emission: 521 nm), a stereomicroscope (MVX10, Olympus, Tokyo, Japan) with a ×1 magnification objective (numerical aperture = 0.25), and a scientific complementary metal-oxide semiconductor (ORCA-Flash4.0 LT, Hamamatsu, Bridgewater, NJ) image sensor as the detection unit. The final thickness of the light sheet was ~18 μm with an effective width of 40 mm. Green pseudocolor was set for values exceeding a threshold of 76% over background tissue autofluorescence.
Statistical Analysis
Values are expressed as means ± SD. Statistical analysis was performed on Prism software (GraphPad) using a Student’s t-test (2-tailed), paired t-test (2-tailed) for before and after comparisons within the same treatment group, and one-way ANOVA with Tukey’s multiple-comparison analysis for comparisons among the three groups. Values of P ≤ 0.05 were considered statistically significant.
RESULTS
Effects of Teriparatide on Cardiovascular Calcification
Initial cohort.
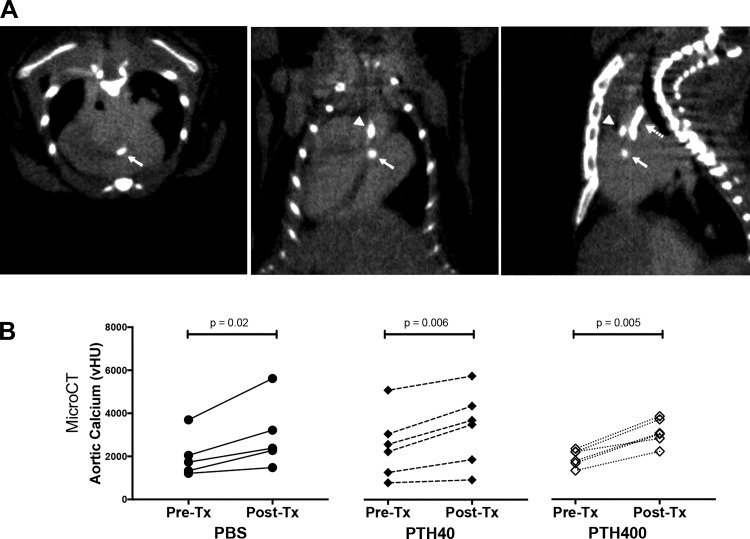
Calcium mineral deposits were demonstrated in the aortic root, ascending aorta, and great vessels by microCT scan (Fig. 1A). In hyperlipidemic (ApoE−/−) mice aged to 70–78 wk of age with preexisting aortic calcification, the amount of aortic calcium mineral before treatment was not significantly different among the three groups (PBS: 2,005 ± 1,003 vHU, PTH40: 2,496 ± 1,521 vHU, and PTH400: 1,923 ± 389 vHU, P = 0.63). Aortic calcium mineral increased significantly over the study period in all three groups (Fig. 1B), whereas the mean percent increase of aortic calcium mineral over the treatment period was not significantly different among the groups (PBS: 47.8 ± 18.4%, PTH40: 36.8 ± 17.4%, and PTH400: 63.5 ± 18.2%, P = 0.07).
Fig. 1.
Effects of teriparatide on aortic calcification in the initial cohort. A: representative microcomputed tomography images with an intravenous contrast agent, Omnipaque 350, of transverse (left), coronal (middle), and sagittal (right) cardiac sections showing calcium mineral deposits in the regions of the aortic valve (closed arrow), aortic root (arrowhead), and aortic arch (dashed arrow). B: quantitative comparisons of volumetric calcium deposition (vHU) before and after treatment (Tx) with PBS (n = 5), 40 µg/kg teriparatide (PTH40; n = 6), and 400 µg/kg teriparatide (PTH400; n = 6) for 4.5 wk. The aortic calcium deposition (vHU), determined as the product of mean region of interest (ROI) density [Hounsfield units (HU)] and volume of the ROI, includes mineral in the aortic valves, aortic root, and aortic arch. A paired two-tailed t-test was used for before and after comparisons within the same treatment group, and one-way ANOVA was used for comparisons among the three groups.
We have previously found that the skeletal anabolic effects of teriparatide are blunted in hyperlipidemic mice (28, 50). Consistent with these findings, microCT analysis of excised femoral bone from these mice revealed that treatment at the conventional dose (PTH40) for mice failed to increase BS/TV or BV/TV in cortical bone. Compared with the control (PBS) group, mice treated with PTH400 developed significantly greater cortical BS/TV but not cortical BV/TV, trabecular BS/TV, or trabecular BV/TV (data not shown).
Followup cohort.
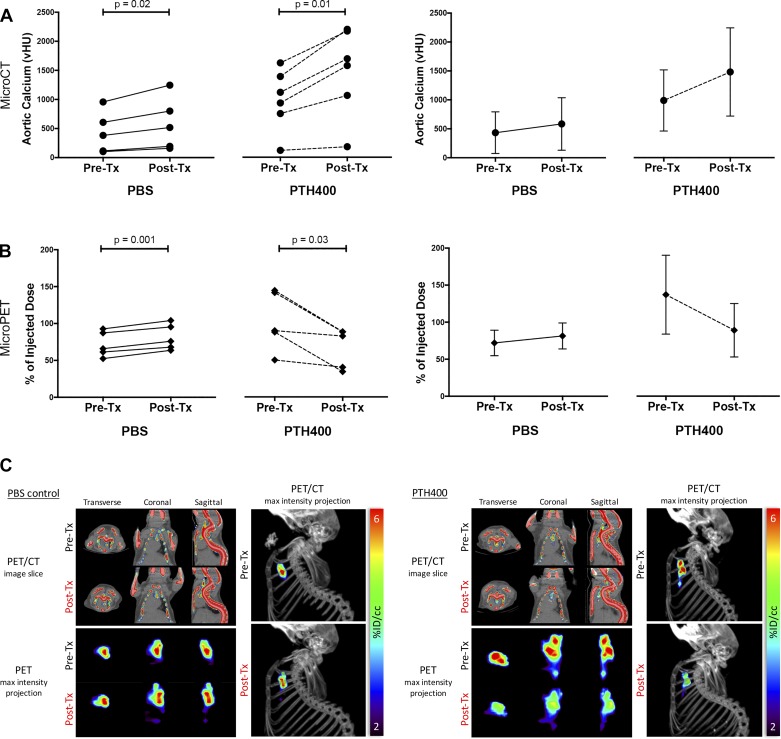
Although our initial cohort did not demonstrate any effects of teriparatide on the progression of aortic calcium mineral content as detected on in vivo microCT, we considered the possibility that microscopic calcium mineral deposits and their surface area, which is associated with plaque instability (27), may change independently of total calcium content based on recent work by others using 18F-NaF PET imaging (29, 30). Thus, we performed a followup cohort, incorporating fused microPET/microCT to determine the effects of teriparatide on the surface area of aortic calcification. In this study, we used a different cohort of aged hyperlipidemic mice to compare both aortic calcium deposition before and after treatment with either PBS or PTH400. Consistent with the findings in the initial cohort, microCT imaging showed that aortic calcification progressed significantly in both groups, and the mean percent change in microCT-detected aortic calcification was not significantly different between the two groups (PBS: 44 ± 17% and PTH400: 51 ± 12%, P = 0.46; Fig. 2A). In the PBS-treated group, microPET imaging also showed that the mean percent change in 18F-NaF incorporation increased in the aortic tissue of all mice (+14 ± 5% over pretreatment baseline, P = 0.001; Fig. 2, B and C). In contrast, in the PTH400-treated group, the mean percent change in 18F-NaF incorporation decreased significantly (−33 ± 20% compared with pretreatment baseline, P = 0.03; Fig. 2, B and C).
Fig. 2.
Effects of teriparatide on aortic calcification by microcomputed tomography (microCT) and micropositron emission tomography (microPET) in the followup cohort. A: quantitative microCT analysis of aortic calcium content before and after treatment (Tx). vHU, volumetric calcium deposition; PTH40, 40 µg/kg teriparatide; PTH400, 400 µg/kg teriparatide. B: quantitative microPET analysis of fluoride uptake in the aortic region pre- and post-Tx. C: representative fused microPET/microCT images showing fluoride uptake (representing calcium mineral surface area) in 12-mo-old PBS-treated control mice and 16-mo-old PTH400-treated mice. Top left: transverse, coronal, and sagittal slices of the chest. Bottom left: corresponding views of maximum-intensity projections of the mediastinal regions of interest. Right: lateral view of the microPET maximum-intensity projection superimposed on the microCT image of the skeleton. A paired two-tailed t-test was used for the statistical analysis.
Effects of Teriparatide on the Morphology of Aortic Calcium Deposits
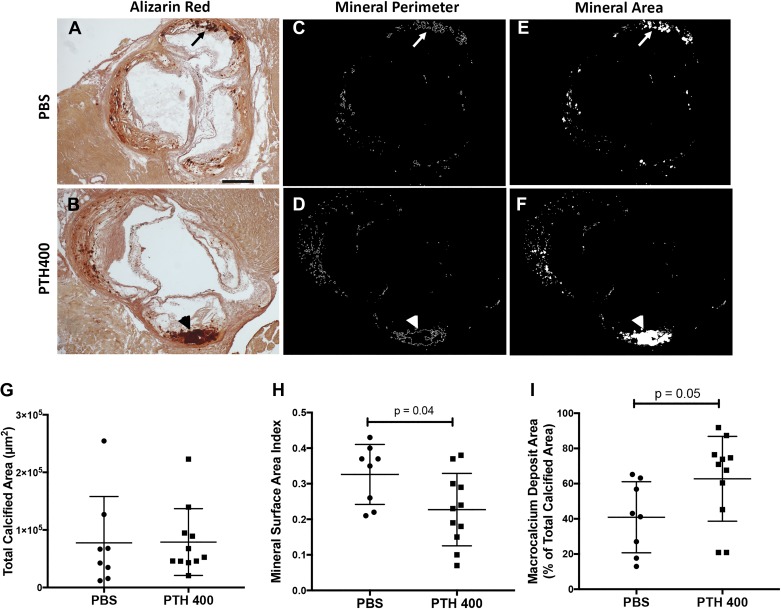
Because microPET analysis showed a reduction of 18F incorporation in the PTH400-treated group in the followup cohort, we further characterized the morphology of calcium deposits by Alizarin red staining of representative aortic root sections from control and PTH400-treated mice (Fig. 3, A and B). Consistent with our microCT findings, the mean calcified area in these sections was similar between the control and teriparatide-treated groups (Fig. 3, C–G). Although the total perimeter of the calcified deposits was similar between the two groups (PBS: 14.5 ± 9.8 mm and PTH400: 14.8 ± 7.9 mm, P = 0.94), because the mice have different total calcified area in each histological section, we normalized the perimeter for total calcified area. This quantitative analysis showed that the MSA index (total perimeter/total calcified area in each section) was significantly lower in the teriparatide-treated group (0.23 ± 0.10) than in the control group (0.33 ± 0.08, P = 0.04; Fig. 3H). Consistent with these results, a significantly higher percentage of total calcium area was present in larger deposits in the teriparatide-treated group (40.9 ± 20.2%) compared with the control group (62.7 ± 24.1%, P = 0.05; Fig. 3I), indicating that the reduction of 18F incorporation and reduction of the MSA index by teriparatide treatment may be a result of mineral deposit fusion and coalescence.
Fig. 3.
Effects of teriparatide on morphology of aortic calcium deposits. A and B: representative Alizarin red-stained aortic root sections from PBS-treated mice (A) and 400 µg/kg teriparatide (PTH400)-treated mice (B). The arrow in A denotes a region containing microcalcium deposits, whereas the arrowhead in B denotes a macrocalcium deposit. Scale bar = 500 µm. C–F: calcified deposits were computationally segmented, and their total perimeters (C and D) and areas (E and F) were drawn and measured in their respective groups. G–I: comparison of the total calcified area (G), mineral surface area index (total perimeter/total area; H), and percentage of calcified area present in macrocalcium deposits between groups (I). Statistical analysis was performed using a two-tailed Student’s t-test.
Location of Calcium Deposits by Light-Sheet Fluorescence Microscopy
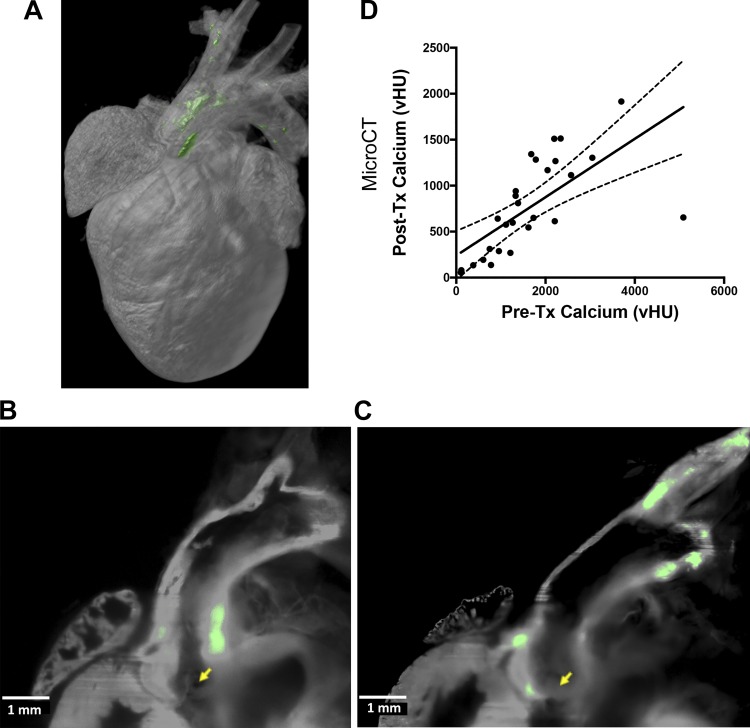
The patterns of distribution of calcium mineral deposits in the intact heart and aorta were demonstrated by LSFM, which revealed preferential location of high-intensity bisphosphonate fluorescence in the aortic valve, aortic root, lesser curvature of the aortic arch, and innominate artery (Fig. 4, A–C). To exclude signals from tissue autofluorescence, only areas exceeding a threshold of 76% over background were shown in green pseudocolor.
Fig. 4.
Location of calcium deposits and effect of baseline calcium mineral on progression. A: representative three-dimensional reconstruction of light-sheet fluorescence microscopic images showing calcium deposits in the aortic root and arch. The vertical dimension was ~1 cm. B and C: two-dimensional raw data of the distribution of calcium mineral in the aortic root (B) as well as the aortic valve, lesser curvature of the arch, and innominate artery (C). Aortic valve cusps are indicated by yellow arrows. Green pseudocolor indicates values >76% of background tissue autofluorescence. D: correlation between the baseline degree of calcification and progression over the 4.5-wk treatment (Tx) period in the aortic root, arch, and great vessels (Pre-Tx calcium) in mice from both initial and followup cohorts. MicroCT, microcomputed tomography; vHU, volumetric calcium deposition.
Effect of Baseline Calcium Mineral on Progression
To evaluate the influence of baseline calcium mineral on progression of calcification, we analyzed microCT data from all the groups combined (PBS-, PTH40-, and PTH400-treated groups in both the initial and followup cohorts). The results showed a significant correlation (r = 0.68, P < 0.0001, 95% confidence interval: 0.41−0.84) between pretreatment baseline aortic calcification and calcium mineral deposited over the duration of the treatment, suggesting that calcium deposition increases with the amount of preexisting aortic calcium (Fig. 4D). Each group alone also had a significant correlation between baseline aortic calcium and subsequent progression during treatment (data not shown).
Effect of Teriparatide on Myocardial Fibrosis
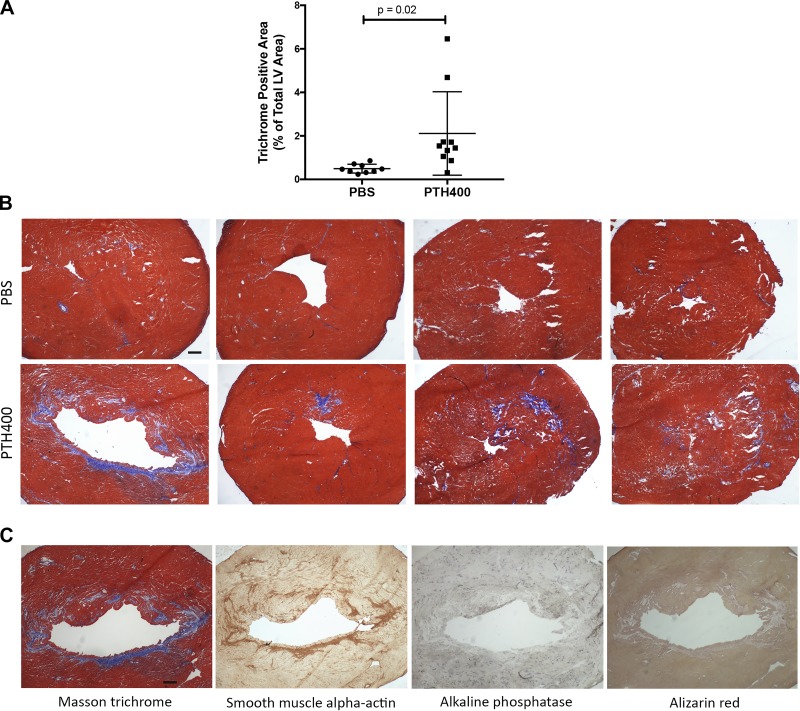
As an incidental finding, we observed fibrotic changes in the myocardium of some of the treated mice on routine histology of the aortic root. To objectively determine whether teriparatide affected myocardial fibrosis, we performed Masson trichrome staining of the ventricles. Quantitative analysis showed a significant increase in collagenous tissue in response to PTH400 treatment in the LV myocardium, suggesting that teriparatide may promote myocardial fibrosis (Fig. 5, A and B). To test for myofibroblast activation, we performed immunohistochemical staining for α-smooth muscle actin. The results showed that immunopositivity colocalized with the areas of collagen deposition. These areas were negative for osteogenic markers (alkaline phosphatase activity and Alizarin red stain) by histochemical staining, suggesting that the collagen deposition was due to fibroblastic activation but not due to osteogenic activation (Fig. 5C).
Fig. 5.
Effects of teriparatide on collagen deposition in the left ventricular (LV) myocardium. A: quantitative analysis of collagen deposition by trichrome-positive area (normalized to total tissue area) in the LV myocardium of mice (from both cohorts) treated with PBS (n = 9) or 400 µg/kg teriparatide (PTH400; n = 11). B: representative images of transverse sections stained for collagen (blue) by Masson trichrome stain showing interstitial fibrosis in the PTH400-treated group. Scale bar = 200 µm. C: representative images of transverse sections of a mouse in the PTH400-treated group. Scale bar = 200 µm. Statistical analysis was performed using a two-tailed Student’s t-test.
Given this unexpected finding, which raised the possibility that teriparatide may alter cardiac function, we also performed echocardiographic imaging in the followup cohort before and after treatment to assess LV systolic function. No significant differences in LV ejection fraction, internal diameter, and wall thickness were observed over the treatment period or between groups (data not shown), although this secondary analysis did not have the statistical power to detect small differences in these parameters.
DISCUSSION
Teriparatide is the most widely used bone anabolic therapy for osteoporosis treatment. It is used primarily in postmenopausal women, who, on the basis of epidemiological data, are likely to have preexisting calcific aortic vasculopathy. To model this population, we produced reproductively senescent hyperlipidemic female mice with aortic calcification by aging them on a chow diet to almost a year and half. These mice, in which we confirmed preexisting vascular calcification, were treated with teriparatide, the active subunit of PTH [PTH(1–34)]. The results from in vivo microCT imaging showed that the aortic calcium content increased in both control and treatment groups and that teriparatide treatment neither reduced nor enhanced the progression of aortic calcification. A particularly strong correlation was found between the progression of aortic calcium during the treatment period and the extent of aortic calcium at baseline. This finding is consistent with the clinical finding that the baseline calcium mineral predicts the rate of coronary artery calcium progression in humans (61).
Initial studies by Derlin and colleagues (15, 16) demonstrated that PET scanning with sodium fluoride (18F-NaF PET) labels atherosclerotic plaque, and 18F-NaF activity was subsequently correlated with early stages of plaque formation (20). More recently, 18F-NaF PET has been shown to identify vulnerable plaque in humans (30), where histological evidence suggests that this is by its correspondence with MSA rather than calcium content (29). Our PET scans of female, reproductively senescent, hyperlipidemic mice on teriparatide treatment showed a reduction in 18F-NaF uptake, suggesting a possible reduction in surface area of aortic calcium deposits. Although this finding may appear inconsistent with the in vivo microCT results, which showed increased calcium mineral content in this group, this could be explained by fusion and the coalescence of smaller calcium deposits, which reduces surface area. The 18F-NaF incorporation quantified by microPET likely reflects surface area (and perhaps degree of metabolic activity), whereas microCT reflects calcium mineral content regardless of shape/surface area or metabolic activity. Thus, the mismatch between microPET and microCT may indicate a reduction in available MSA (29). The kinetics of 18F-NaF, which depend on blood flow (rapid) and renal clearance (rapid), as well as exposure to MSA and ion exchange (slow) (4), support this concept. Consistent with this interpretation, although early calcification develops as a result of matrix vesicle formation and, possibly, its deposition on collagen fibers (2), teriparatide has been shown to increase the fusion and coalescence of mineral microparticles in rats, probably via production of collagen-based osteoid, resulting in increased “micro-interlocks” (31). Indeed, further analysis of the morphology of calcium deposits in aortic root sections by quantitative histochemistry analyses confirmed that teriparatide treatment was associated with a reduction in MSA despite similar levels of total calcification. Decreased MSA of calcified deposits in atherosclerotic lesions has important biomechanical significance, as the extent of surface area corresponds with the degree of mechanical compliance mismatch, which increases risk of plaque rupture (1, 25).
Our findings demonstrate that teriparatide reduced 18F uptake without affecting the progression of calcification seen on microCT. This suggests that teriparatide promoted growth preferentially at sites of existing calcium deposits, which, if juxtaposed, would have coalesced into larger deposits, thereby reducing their surface area. In contrast, the spontaneous calcification in controls may have arisen at random sites, leading to increased surface area and 18F uptake. It is also possible that teriparatide has a direct effect on plaque inflammation, which has been linked to ectopic calcification. Although chronically elevated serum PTH levels have been associated with increased inflammatory markers (9), the effects of teriparatide on inflammation are largely unknown. In addition, on the basis of our findings, we would speculate that, in younger mice, teriparatide may not have the same effect because calcium deposits may be too sparsely situated for coalescence.
In bone, teriparatide has been shown to promote collagen synthesis, as transient exposure to PTH increases synthesis of collagen type I in cultured osteoblasts (8), and teriparatide treatment increases collagen deposition in bone in rats (49). As an incidental finding, teriparatide (PTH400) treatment was associated with greater collagen deposition in the LV wall, suggesting that treatment may induce myocardial fibrosis. Indeed, our histochemical analyses revealed increased α-smooth muscle actin staining in the areas of collagen deposition, supporting increased myofibroblastic activation, with no evidence of adjacent osteogenic activity. The mechanisms by which teriparatide exerts this effect may be mediated by the cAMP pathway, which promotes cardiomyocyte necrosis from increased myocardial uptake of calcium that depresses mitochondrial respiration and oxidative phosphorylation (5, 6, 59). Such cardiomyocyte necrosis induced by activation of the cAMP pathway has been shown to stimulate both fibroblast proliferation and collagen production (3). In addition, although calcification progressed similarly in both control and teriparatide-treated groups, we cannot rule out the possibility that the increased myocardial fibrosis is secondary to valve dysfunction.
Despite the increased collagen deposition in the LV wall, we did not detect a significant difference in LV systolic function between PBS- and PTH400-treated groups by echocardiography although the analysis only had enough statistical power to detect a large change. Prior studies have demonstrated that PTH increases cardiomyocyte contractility in rodents (51, 58). However, in a small study of postmenopausal women (42), teriparatide treatment was associated with a mild decrease in LV fractional shortening. Nonetheless, given the established relationship between ventricular fibrosis and ventricular arrhythmias (39), further studies of the effects of teriparatide on myocardial function and fibrosis are warranted.
Some limitations of this study warrant discussion. First, because the echocardiographic analysis was added after our incidental finding of myocardial fibrosis, the sample size was small relative to the variance of the parameters. Further studies are needed to address the question of the effects of teriparatide on cardiac function. Second, because of the lack of diastolic function assessment in our study, whether LV diastolic function was affected by the increased collagen deposition seen in the treatment group is not clear. Additionally, despite some loss of animals during the aging protocol limiting group size, the numbers were comparable with those used in prior studies of vascular disease in aged mice (10, 48, 54). More importantly, because of our ability to perform serial imaging in each animal, we were able to use paired statistical analyses, which allowed us to demonstrate statistically significant differences in our primary end points over the treatment period. Furthermore, in the initial cohort, because of technical reasons, mice were scanned in different microCT scanners for the pretreatment and posttreatment images. Although this could introduce errors, corrective steps, as described in materials and methods, were taken to ensure comparability of the data. Indeed, the results obtained from the followup cohort, where the same microCT scanner was used for both pretreatment and posttreatment images, showed the same trends as the initial cohort.
Our findings have clinical importance given the complex metabolic interactions between the cardiovascular and skeletal systems. Although most postmenopausal women with osteoporosis have ectopic calcification, especially in the aorta (13), many of the osteoporosis treatment regimens, such as calcium and vitamin D supplements and hormone-replacement therapy, may conceivably aggravate extraskeletal calcification (60). Until recently, teriparatide was the only bone anabolic therapy available to these patients. However, with the recent Federal Drug Administration approval of abaloparatide, a PTH-related protein analog, to treat postmenopausal osteoporosis, it is increasingly important to understand the potential effects of these anabolic agents on preexisting ectopic calcification. In the major clinical trials (19, 35, 40) evaluating the efficacy of teriparatide on osteoporosis, there were no comments on cardiac fibrosis, and it was not systematically assessed in these trials. In the present study, our results raise the possibility that, although teriparatide does not reverse or attenuate the progression of preexisting vascular calcification, it may modify the morphology of calcified deposits by promoting fusion and coalescence of small deposits into fewer large ones. This effect may be beneficial in calcific atherosclerosis, specifically possible plaque stabilization through reduction of the size of the areas subject to rupture attributable to compliance mismatch.
In conclusion, the increase in overall aortic calcium mineral content in all groups suggests that teriparatide does not stop the progression or cause regression of preexisting aortic calcification. Meanwhile, on serial in vivo microPET imaging, although 18F uptake increased significantly in the control group, uptake decreased significantly in the teriparatide-treated group. The decreased MSA of calcified deposits in this latter group further suggests a possible effect of teriparatide on stability of calcific atherosclerotic plaque.
GRANTS
This work was supported by National Institutes of Health Grants HL-114709, HL-121019, R21-DE-023410, HL-129727, HL-007895, P30-AG-028748, P30-CA-016042, and KL2-TR-001882). J. Hsu is supported by an award from the University of California-Los Angeles Specialty Training and Advanced Research Program and a grant from the Claude Pepper Older American Independence Center at the University of California-Los Angeles.
DISCLOSURES
J.J.H., J.L., S.U., J.T.L., R.P.K., Y.D., C.C., T.H., A.H., I.G., L.L.D. and Y.T. have nothing to disclose. I.N. was a consultant to BioVinc, and S.T. serves as a consultant and receives research funding from Amgen.
AUTHOR CONTRIBUTIONS
J.J.H., J.L., S.U., J.T.L., R.K., Y.D., C.-C.C., T.K.H., A.H., I.G., S.T., I.N., and Y.T. performed experiments; J.J.H., S.U., J.T.L., L.L.D., and Y.T. analyzed data; J.J.H., J.T.L., and Y.T. interpreted results of experiments; J.J.H., J.T.L., Y.D., C.-C.C., and Y.T. prepared figures; J.J.H., S.U., J.T.L., L.L.D., and Y.T. edited and revised manuscript; J.J.H., J.L., S.U., J.T.L., R.K., Y.D., C.-C.C., T.K.H., A.H., I.G., S.T., I.N., L.L.D., and Y.T. approved final version of manuscript; Y.T. conceived and designed research; Y.T. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank K. Sung and Y. Shin for tissue clearance of the hearts and C. Zamilpa and R. Taschereau of the University of California-Los Angeles Crump Institute’s Preclinical Imaging Technology Center for assistance with microPET/microCT imaging.
REFERENCES
- 1.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24: 1161–1170, 2004. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 2.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep 5: 222–226, 2003. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin IJ, Jalil JE, Tan LB, Cho K, Weber KT, Clark WA. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ Res 65: 657–670, 1989. doi: 10.1161/01.RES.65.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med 2: 31–37, 1972. doi: 10.1016/S0001-2998(72)80005-9. [DOI] [PubMed] [Google Scholar]
- 5.Bogin E, Massry SG, Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest 67: 1215–1227, 1981. doi: 10.1172/JCI110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borkowski BJ, Cheema Y, Shahbaz AU, Bhattacharya SK, Weber KT. Cation dyshomeostasis and cardiomyocyte necrosis: the Fleckenstein hypothesis revisited. Eur Heart J 32: 1846–1853, 2011. doi: 10.1093/eurheartj/ehr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 49: 1860–1870, 2007. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 8.Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 83: 60–65, 1989. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng SP, Liu CL, Liu TP, Hsu YC, Lee JJ. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm 2014: 709024, 2014. doi: 10.1155/2014/709024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu Y, Lund DD, Doshi H, Keen HL, Knudtson KL, Funk ND, Shao JQ, Cheng J, Hajj GP, Zimmerman KA, Davis MK, Brooks RM, Chapleau MW, Sigmund CD, Weiss RM, Heistad DD. Fibrotic aortic valve stenosis in hypercholesterolemic/hypertensive mice. Arterioscler Thromb Vasc Biol 36: 466–474, 2016. doi: 10.1161/ATVBAHA.115.306912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowell SJ, Newby DE, Boon NA, Elder AT. Calcific aortic stenosis: same old story? Age Ageing 33: 538–544, 2004. doi: 10.1093/ageing/afh175. [DOI] [PubMed] [Google Scholar]
- 12.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med 51: 1826–1829, 2010. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demer LL, Tintut Y. Mechanisms linking osteoporosis with cardiovascular calcification. Curr Osteoporos Rep 7: 42–46, 2009. doi: 10.1007/s11914-009-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 16: 1846–1853, 2001. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 15.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, Klutmann S. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 51: 862–865, 2010. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 16.Derlin T, Tóth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, Mester J, Klutmann S. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. J Nucl Med 52: 1020–1027, 2011. doi: 10.2967/jnumed.111.087452. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y, Lee J, Ma J, Sung K, Yokota T, Singh N, Dooraghi M, Abiri P, Wang Y, Kulkarni RP, Nakano A, Nguyen TP, Fei P, Hsiai TK. Light-sheet fluorescence imaging to localize cardiac lineage and protein distribution. Sci Rep 7: 42209, 2017. doi: 10.1038/srep42209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei P, Lee J, Packard RR, Sereti KI, Xu H, Ma J, Ding Y, Kang H, Chen H, Sung K, Kulkarni R, Ardehali R, Kuo CC, Xu X, Ho CM, Hsiai TK. Cardiac light-sheet fluorescent microscopy for multi-scale and rapid imaging of architecture and function. Sci Rep 6: 22489, 2016. doi: 10.1038/srep22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): a randomized controlled trial. JAMA 280: 1067–1073, 1998. doi: 10.1001/jama.280.12.1067. [DOI] [PubMed] [Google Scholar]
- 20.Fiz F, Morbelli S, Piccardo A, Bauckneht M, Ferrarazzo G, Pestarino E, Cabria M, Democrito A, Riondato M, Villavecchia G, Marini C, Sambuceti G. 18F-NaF uptake by atherosclerotic plaque on PET/CT imaging: inverse correlation between calcification density and mineral metabolic activity. J Nucl Med 56: 1019–1023, 2015. doi: 10.2967/jnumed.115.154229. [DOI] [PubMed] [Google Scholar]
- 21.Freeman TA, Patel P, Parvizi J, Antoci V Jr, Shapiro IM. Micro-CT analysis with multiple thresholds allows detection of bone formation and resorption during ultrasound-treated fracture healing. J Orthop Res 27: 673–679, 2009. doi: 10.1002/jor.20771. [DOI] [PubMed] [Google Scholar]
- 22.Frye MA, Melton LJ III, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, Riggs BL. Osteoporosis and calcification of the aorta. Bone Miner 19: 185–194, 1992. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 23.Gondrie MJ, van der Graaf Y, Jacobs PC, Oen AL, Mali WP; PROVIDI Study Group . The association of incidentally detected heart valve calcification with future cardiovascular events. Eur Radiol 21: 963–973, 2011. doi: 10.1007/s00330-010-1995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol 20: 1926–1931, 2000. doi: 10.1161/01.ATV.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 25.He F, Hua L, Gao LJ. A computational model for biomechanical effects of arterial compliance mismatch. Appl Bionics Biomech 2015: 213236, 2015. doi: 10.1155/2015/213236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J 31: 1975–1984, 2010. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol 297: H802–H810, 2009. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang MS, Lu J, Ivanov Y, Sage AP, Tseng W, Demer LL, Tintut Y. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res 23: 1672–1679, 2008. doi: 10.1359/jbmr.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 6: 7495, 2015. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 383: 705–713, 2014. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 31.Kamo K, Miyakoshi N, Kasukawa Y, Nozaka K, Sasaki H, Shimada Y. Intermittent weekly administration of human parathyroid hormone (1-34) improves bone-hydroxyapatite block bonding in ovariectomized rats. J Bone Miner Metab 28: 634–640, 2010. doi: 10.1007/s00774-010-0178-z. [DOI] [PubMed] [Google Scholar]
- 32.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int 68: 271–276, 2001. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Lim J, Lu J, Pedego TM, Demer L, Tintut Y. Protective role of Smad6 in inflammation-induced valvular cell calcification. J Cell Biochem 116: 2354–2364, 2015. doi: 10.1002/jcb.25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim J, Ehsanipour A, Hsu JJ, Lu J, Pedego T, Wu A, Walthers CM, Demer LL, Seidlits SK, Tintut Y. Inflammation drives retraction, stiffening, and nodule formation via cytoskeletal machinery in a three-dimensional culture model of aortic stenosis. Am J Pathol 186: 2378–2389, 2016. doi: 10.1016/j.ajpath.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350: 550–555, 1997. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 36.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2: 131–137, 2003. doi: 10.1162/153535003322556877. [DOI] [PubMed] [Google Scholar]
- 37.Lutgens E, van Suylen RJ, Faber BC, Gijbels MJ, Eurlings PM, Bijnens AP, Cleutjens KB, Heeneman S, Daemen MJ. Atherosclerotic plaque rupture: local or systemic process? Arterioscler Thromb Vasc Biol 23: 2123–2130, 2003. doi: 10.1161/01.ATV.0000097783.01596.E2. [DOI] [PubMed] [Google Scholar]
- 38.Makras P, Delaroudis S, Anastasilakis AD. Novel therapies for osteoporosis. Metabolism 64: 1199–1214, 2015. doi: 10.1016/j.metabol.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Morita N, Mandel WJ, Kobayashi Y, Karagueuzian HS. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm 30: 389–394, 2014. doi: 10.1016/j.joa.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441, 2001. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 41.O’Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, Pohost GM, Shaw LJ, Weintraub WS, Winters WL Jr, Forrester JS, Douglas PS, Faxon DP, Fisher JD, Gregoratos G, Hochman JS, Hutter AM Jr, Kaul S, Wolk MJ. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 102: 126–140, 2000. doi: 10.1161/01.CIR.102.1.126. [DOI] [PubMed] [Google Scholar]
- 42.Passeri E, Dozio E, Mendola M, Costa E, Bandera F, Corsi Romanelli MM, Corbetta S. Treatment with teriparatide might be associated with cardiometabolic changes in postmenopausal severe osteoporotic women. J Biol Regul Homeost Agents 29: 931–940, 2015. [PubMed] [Google Scholar]
- 43.Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 15: 405–416, 2009. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Pirih F, Lu J, Ye F, Bezouglaia O, Atti E, Ascenzi MG, Tetradis S, Demer L, Aghaloo T, Tintut Y. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res 27: 309–318, 2012. doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 5: 185–197, 2009. doi: 10.2147/VHRM.S4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen CJ. The cellular and clinical parameters of anabolic therapy for osteoporosis. Crit Rev Eukaryot Gene Expr 13: 25–38, 2003. doi: 10.1615/CritRevEukaryotGeneExpr.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- 47.Rosen CJ. What’s new with PTH in osteoporosis: where are we and where are we headed? Trends Endocrinol Metab 15: 229–233, 2004. doi: 10.1016/j.tem.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol 20: 2587–2592, 2000. doi: 10.1161/01.ATV.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 49.Rowshan HH, Parham MA, Baur DA, McEntee RD, Cauley E, Carriere DT, Wood JC, Demsar WJ, Pizarro JM. Effect of intermittent systemic administration of recombinant parathyroid hormone (1-34) on mandibular fracture healing in rats. J Oral Maxillofac Surg 68: 260–267, 2010. doi: 10.1016/j.joms.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 50.Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, Demer LL, Tintut Y. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res 26: 1197–1206, 2011. doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlüter KD, Weber M, Piper HM. Parathyroid hormone induces protein kinase C but not adenylate cyclase in adult cardiomyocytes and regulates cyclic AMP levels via protein kinase C-dependent phosphodiesterase activity. Biochem J 310: 439–444, 1995. doi: 10.1042/bj3100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89: 4246–4253, 2004. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 53.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone 43: 1022–1030, 2008. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem 278: 50195–50202, 2003. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 55.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension 55: 579–592, 2010. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung K, Ding Y, Ma J, Chen H, Huang V, Cheng M, Yang CF, Kim JT, Eguchi D, Di Carlo D, Hsiai TK, Nakano A, Kulkarni RP. Simplified three-dimensional tissue clearing and incorporation of colorimetric phenotyping. Sci Rep 6: 30736, 2016. doi: 10.1038/srep30736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taschereau RVN, Chatziioannou A. Calibration and data standardization of a prototype bench-top preclinical CT. In: Nuclear Science Symposium and Medical Imaging Conference (IEEE) 20. Seattle, WA: 2014. [Google Scholar]
- 58.Tastan I, Schreckenberg R, Mufti S, Abdallah Y, Piper HM, Schlüter KD. Parathyroid hormone improves contractile performance of adult rat ventricular cardiomyocytes at low concentrations in a non-acute way. Cardiovasc Res 82: 77–83, 2009. doi: 10.1093/cvr/cvp027. [DOI] [PubMed] [Google Scholar]
- 59.Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, Gaksch M, Grübler M, Fahrleitner-Pammer A, Mrak P, Toplak H, Kraigher-Krainer E, März W, Pilz S. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism 63: 20–31, 2014. doi: 10.1016/j.metabol.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Yavropoulou MP, Pikilidou M, Yovos JG. Anti-osteoporotic drugs and vascular calcification: the bidirectional calcium traffic. J Vasc Res 51: 37–49, 2014. doi: 10.1159/000355204. [DOI] [PubMed] [Google Scholar]
- 61.Yoon HC, Emerick AM, Hill JA, Gjertson DW, Goldin JG. Calcium begets calcium: progression of coronary artery calcification in asymptomatic subjects. Radiology 224: 236–241, 2002. doi: 10.1148/radiol.2241011191. [DOI] [PubMed] [Google Scholar]