Abstract
Inflammation coincides with diminished marrow function, vasodilation of blood vessels, and bone mass. Intermittent parathyroid hormone (PTH) administration independently improves marrow and vascular function, potentially impacting bone accrual. Currently, the influence of marrow and intermittent PTH administration on aged bone blood vessels has not been examined. Vasodilation of the femoral principal nutrient artery (PNA) was assessed in the presence and absence of marrow. Furthermore, we determined the influence of PTH 1–34 on 1) endothelium-dependent vasodilation and signaling pathways [i.e., nitric oxide (NO) and prostacyclin (PGI2)], 2) endothelium-independent vasodilation, 3) cytokine production by marrow cells, and 4) bone microarchitecture and bone static and dynamic properties. Young (4–6 mo) and old (22–24 mo) male Fischer-344 rats were treated with PTH 1–34 or a vehicle for 2 wk. In the absence and presence of marrow, femoral PNAs were given cumulative doses of acetylcholine, with and without the NO and PGI2 blockers, and diethylamine NONOate. Marrow-derived cytokines and bone parameters in the distal femur were assessed. Exposure to marrow diminished endothelium-dependent vasodilation in young rats. Reduced bone volume and NO-mediated vasodilation occurred with old age and were partially reversed with PTH. Additionally, PTH treatment in old rats restored endothelium-dependent vasodilation in the presence of marrow and augmented IL-10, an anti-inflammatory cytokine. Endothelium-independent vasodilation was unaltered, and PTH treatment reduced osteoid surfaces in old rats. In conclusion, the marrow microenvironment reduced vascular function in young rats, and PTH treatment improved the marrow microenvironment and vasodilation with age.
NEW & NOTEWORTHY This study investigated the influence of the marrow microenvironment on bone vascular function in young and old rats. An inflamed marrow microenvironment may reduce vasodilator capacity of bone blood vessels, diminishing delivery of blood flow to the skeleton. In young rats, the presence of the marrow reduced vasodilation in the femoral principal nutrient artery (PNA). However, intermittent parathyroid hormone administration (i.e., a treatment for osteoporosis) improved the marrow microenvironment and vasodilator capacity in old PNAs.
Keywords: aging, cytokines, marrow, PTH, vasodilation
INTRODUCTION
Parathyroid hormone (PTH) plays a central role in the regulation of bone cellular communications and bone remodeling (18) and is an anabolic agent used to treat osteoporosis (31). However, a number of studies highlighted that intermittent PTH administration also modifies the marrow microenvironment (28). For example, when administered intermittently for 3 wk to Bmi1-null mice (i.e., mice with defects in hematopoiesis), PTH 1–34 partially restored trabecular bone volume above that observed in vehicle-treated Bmi1-null mice (28). In addition to increasing osteoblast parameters (e.g., osteoblast number, type I collagen, osteopontin, etc.), the numbers of hematopoietic stem cells and adipocytes were partially increased and decreased, respectively, compared with the Bmi1-null, vehicle-treated animals (28). Furthermore, PTH 1–34 administration suppressed and prevented adipogenesis in the marrow microenvironment of ovariectomized rats (19). Thus, it is speculated that intermittent PTH administration modulates the accessory cells in the marrow (24), which may contribute to bone accrual.
Bone blood vessels reside within and rest just external to the marrow microenvironment, and they are profoundly influenced by PTH. Previous investigations have demonstrated alterations in the bone vascular network following direct application of PTH to blood vessels and following in vivo, intermittent administration of PTH for a given length of time. For example, PTH 1–34, PTH 1–84, and parathyroid hormone-related peptide (PTHrP) 1–34 delivered in vitro to isolated femoral principal nutrient arteries [i.e., PNA, the primary conduit for blood flow to long bones (4)] elicited modest-to-strong vasodilator responses (2, 34). In addition to enhancing trabecular bone volume (33, 34), 2–4 wk of intermittent PTH administration improved vasodilator capacity (34), sensitized vascular endothelial cells to agonist other than PTH (34), augmented bone angiogenesis (38), and relocated bone marrow blood vessels closer to bone-forming sites (33). All of these vascular alterations presumably serve to improve skeletal perfusion (30, 38) and aid in the precise delivery of oxygen, nutrients, and precursor cells to sites of bone formation (33). Many of the investigations were conducted in young animals (33, 34, 38); thus, the influence of intermittent PTH administration on the vascular system in advanced age (i.e., the target population for the treatment of osteoporosis) remains to be determined.
Alterations in bone vascular function and morphology such as reduced endothelium-dependent vasodilation and augmented bone marrow blood vessel ossification occur with old age (12, 32, 35–37), potentially contributing to declines in skeletal perfusion (12, 16, 37). Whereas recent investigations have focused attention on age- and disease-related aspects of the bone vasculature and the impact on bone (12, 32, 35–37, 43), to our knowledge no studies have considered the impact of the marrow microenvironment on bone vascular function and how this may alter delivery of blood and nutrients to the skeleton. Modifications to the bone vasculature with age, disease, and/or intermittent PTH administration were presumed to result from direct action of these stimuli on vascular endothelial and/or smooth muscle cells. However, the possibility remains that some of the modifications result from the bone marrow cells and the factors they may release. Many vascular pathologies (i.e., arterial stiffness, atherosclerosis, disordered vascular patterns, enhanced vasoconstriction, etc.) are related to enhanced production of inflammatory cytokines. For example, chronic exposure to interleukin (IL)-17A promoted a proinflammatory environment (14), whereas inhibition of IL-17A and knockout of IL-1α and IL-1β in mice led to declines in the progression of atherosclerotic lesions (14, 21). Cytokines are produced by many cell types, including endothelial, vascular smooth muscle, and bone marrow cells (10, 11, 21). mRNA expression of monocyte chemoattractant protein-1 (MCP-1) was upregulated in human umbilical vein endothelial cells following treatment of the culture medium with IL-13 and tumor necrosis factor (39). Cytokines also induced vascular dysfunction, such that exposure of rabbit carotid arteries to IL-1β diminished endothelium-dependent relaxation (22).
Since cytokines are produced by bone marrow-derived cells (21), their production may influence bone vascular function in old age and/or with intermittent PTH administration. For example, enhanced levels of circulating cytokines and proinflammatory markers were observed in advanced age (29), and T cell activation (and the subsequent production of tumor necrosis factor) is involved in bone loss associated with continuous PTH administration (44). In addition, interleukin IL-17A mRNA expression in peripheral blood cells was threefold higher in patients with primary hyperparathyroidism, and mice treated with a neutralizing antibody against IL-17A had attenuated bone loss with continuous administration of PTH (27). Thus, enhanced production of proinflammatory cytokines may contribute to the diminished bone vascular function observed in old animals. Furthermore, the positive impact of intermittent PTH administration on the marrow microenvironment (19, 28) suggests a similar beneficial outcome for the bone vasculature. The purpose of the present study was to determine whether the marrow microenvironment, via proinflammatory cytokine production, alters vasodilation in the femoral PNA in young and old rats. Since the focus of this investigation was to examine changes in the bone vascular system, a short time course of PTH treatment was chosen to assess any vascular alterations that may precede those occurring in bone. Thus, we assessed the effects of 2 wk of intermittent PTH 1–34 administration on 1) vasodilator capacity of the femoral PNA in the presence and absence of marrow, 2) cytokine production by bone marrow cells, and 3) bone microarchitecture and bone static and dynamic properties. We hypothesized that the marrow microenvironment would diminish vascular function in old PNAs that would be associated with enhanced proinflammatory cytokine production by bone marrow cells. Furthermore, we hypothesized that intermittent PTH 1–34 administration would enhance endothelium-dependent vasodilation and reduce the production of proinflammatory cytokines.
MATERIALS AND METHODS
The procedures employed in this study were approved by the University of Delaware and the University of Texas at Arlington’s Institutional Animal Care and Use Committees and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication no. 85-23, revised 1996). Young (4–6 mo old) and old (22–24 mo old) male Fischer-344 rats were obtained from the National Institutes of Aging and housed in standard cages in a temperature- (23 ± 2°C) and light- (12:12-h light-dark) controlled room. Tap water and regular rat chow were given ad libitum.
Experimental protocol.
Rats were matched-paired according to age and body mass and randomly assigned to the following groups: 1) young control (Young CON), 2) Young PTH, 3) Old CON, and 4) Old PTH. Thus, mean body masses at the start of the experiment were similar between Young CON and Young PTH and similar between Old CON and Old PTH. According to the treatment group, rats received injections of either 43 µg·kg−1·day−1 of PTH 1–34 subcutaneously or 100 µl/day of phosphate-buffered saline (vehicle) subcutaneously for 2 wk (5 days/wk). A dose of 43 µg·kg−1·day−1 of PTH 1–34 is molecularly equivalent to 100 µg·kg−1·day−1 of PTH 1–84 (45). PTH and vehicle were administrated at the same time daily. Rats were anesthetized with isoflurane (3% to O2 balance). Following a thoracotomy to expose the heart, a vial of blood was drawn from the left ventricle via vacutainer. Whole blood samples were centrifuged at 1,000 g (4°C) for 10 min. The plasma was then stored at −80°C until analysis for PTH and calcium (Ca2+). All rats were euthanized by cardiectomy.
Plasma PTH and calcium analysis.
Plasma samples were thawed and kept on ice. Samples were quantified with RayBio Human/Mouse/Rat Parathyroid Hormone EIA Kit (no. EIA-PTH-1; RayBiotech, Norcross, GA) and a calcium (Ca2+) assay kit (no. 701220; Cayman Chemical, Ann Arbor, MI) per the manufacturers’ instructions. The sensitivities for the PTH and Ca2+ assay kits were 1.27 pg/ml and 0.25 mg/dl, respectively. Luminescence was measured using a BioTek microplate reader. All standards and samples were run in triplicate.
Removal of the marrow from the femoral diaphysis.
The distal femur was removed with a Dremel rotary tool and a diamond grinding disk. The remaining bone was placed in a 1.5-ml microcentrifuge tube containing phosphate-buffered saline (70–140 μl). The tube was spun briefly in a minicentrifuge to dislodge the marrow from the shaft. Microsurgical forceps were used to gently remove the marrow from the tube, which was placed into a square of filter paper. The two ends of the filter paper were tied together with suture. During assessment of the vasodilator responses of the PNA, the marrow was secured to the inside of the vessel chamber, ensuring that the marrow was completely submerged in physiological saline solution (PSS) within the chamber but unable to escape the filter paper. During the assessment of vasodilator function in the absence of marrow, squares of filter paper without marrow were placed inside the vessel chamber, submerged in the PSS as described above, and served as a control condition. Immediately following the ACh dose-response experiments (described below), the PSS from both conditions (i.e., in the presence and absence of marrow) was collected from the vessel chamber, placed into a microcentrifuge tube, snap-frozen in liquid nitrogen, and stored at −80°C until assay analysis.
Endothelium-dependent vasodilation of the femoral PNA in the presence and absence of marrow.
In vitro experiments were performed to evaluate endothelium-dependent vasodilator responses and endothelial signaling pathways. Evaluation of endothelium-dependent vasodilation allows for examination of whether vasodilation of the femoral PNA occurred through modulation of vascular endothelial cells as opposed to vascular smooth muscle cells. Right femora and surrounding muscles were carefully dissected from the animals and placed in cold (4°C) PSS. The PNA was isolated from the femur and placed in a vessel chamber containing PSS at room temperature. Isolated PNAs were cannulated onto glass micropipette tips and secured with microfilament suture, placed in a vessel chamber, and viewed with an inverted microscope (Omano OM900; The Microscope Store, Wirtz, VA). The microscope was equipped with a video camera (Color CCD Video Camera; World Precision Instruments, Sarasota, FL) and video caliper (model 307; Colorado Video, Boulder, CO) to measure the intraluminal diameter of the PNA.
To maintain intraluminal diameter and mimic in vivo blood pressure, the PNAs were pressurized to 60 cmH2O (44 mmHg) by two different hydrostatic pressure reservoirs. The vessel chamber was warmed with a circulating water bath to maintain 37°C. The PNA was equilibrated for ≥1 h to allow for the development of percent spontaneous tone. PNAs were excluded if they leaked (i.e., failure to maintain intraluminal diameter when the hydrostatic pressure reservoirs were closed) or did not develop at least 20% spontaneous tone. Endothelium-dependent vasodilation of the PNA was assessed by the cumulative additions of acetylcholine (ACh; 10−9–10−4 M) following a 25-min incubation with one of the following: 1) PSS alone, 2) PSS containing the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10−5 M), or 3) PSS containing l-NAME (10−5 M) and the cyclooxygenase (COX) inhibitor indomethacin (INDO; 10−5 M). l-NAME prevents the production of endothelial NO, and INDO inhibits cyclooxygenase, which catalyzes the production of prostacyclin (PGI2) from endothelial cells to elicit vasodilation. Following the ACh dose-response experiment with PSS alone, the PSS was collected, snap-frozen, and stored at −80°C. After completion of all dose-response experiments, PNAs were incubated in Ca2+-free PSS for 30 min to obtain and record the maximal diameters. Sodium nitroprusside (SNP; 10−5 M) was added to the bath during the last 10 min to obtain full vascular smooth muscle relaxation.
In separate PNAs, the same experimental procedures were followed as above except the ACh dose-response experiments were conducted in the presence of marrow. Following the 1-h equilibration period and development of percent spontaneous tone, PNAs were exposed to a bolus dose of ACh (10−5 M) to assess functional viability. Subsequent to several washes, the marrow from the femoral shaft was incubated in the PSS with the PNA for ≥30 min. Marrow and PNAs from the same animal were incubated together. In other words, PNAs from the young and old rats were incubated with their own marrow. The PNA was allowed to develop at least 20% spontaneous tone. Endothelium-dependent vasodilation of the PNA was assessed by the cumulative additions of ACh (10−9–10−4 M) following a 25-min incubation with one of the following: 1) PSS alone, 2) PSS containing l-NAME (10−5 M), and 3) PSS containing l-NAME (10−5 M) and INDO (10−5 M). Following the ACh dose-response experiment in PSS buffer alone, the PSS was collected, snap-frozen, and stored at −80°C. After completion of the experimental protocols, the maximal diameters of the PNAs were determined subsequent to a 30-min incubation period in Ca2+-free PSS supplemented with SNP (10−5 M) during the last 10 min.
Endothelium-independent vasodilation of the femoral PNA in the presence and absence of marrow.
In vitro experiments were performed to evaluate endothelium-independent vasodilator responses. Evaluation of endothelium-independent vasodilation allows for the examination of whether vasodilation of the femoral PNA occurred as a result of modulation of vascular smooth muscle cells as opposed to vascular endothelial cells. Isolated PNAs were placed under an inverted microscope, pressurized to 60 cmH2O (44 mmHg), and equilibrated for ≥1 h. PNAs that were leaking or that did not develop at least 20% spontaneous tone were discarded. PNAs were subsequently exposed to cumulative doses of diethylamine (DEA) NONOate (10−10–10−4 M), which donates NO to vascular smooth muscle cells to elicit relaxation. Following several washes to remove residual DEA NONOate from the PNA and the vessel chamber, the marrow from the femoral shaft was incubated with the PNA for ≥30 min. Marrow and PNAs from the same animal were incubated together. In other words, PNAs from the young and old rats were incubated with their own marrow. Subsequent to the PNA developing at least 20% spontaneous tone, a second DEA NONOate dose-response experiment was conducted. After completion of the experimental protocols, the maximal diameters of the PNAs were determined subsequent to a 30-min incubation period in Ca2+-free PSS containing SNP (10−5 M) during the last 10 min.
Analysis of cytokines in PSS incubated with and without marrow.
The microcentrifuge tubes containing PSS (Young CON, n = 3; Young CON+Marrow, n = 3; Young PTH, n = 3; Young PTH+Marrow, n = 3, Old CON, n = 3; Old CON+Marrow, n = 3; Old PTH, n = 3; Old PTH+Marrow, n = 3) were thawed to room temperature. PSS samples were quantified with a Bio-Plex Pro Rat Cytokine 24-plex Assay (Bio-Rad, cat. no. 171-1001M) for simultaneous determination of the following cytokines: erythropoietin (EPO), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), growth-regulated protein-α (GRO/KC), interferon-γ (IFN-γ), interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 subunit p70 (IL-12p70), IL-13, IL-17A, IL-18, macrophage colony-stimulating factor (M-CSF), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-3α, regulated on activation normal T cell expressed and secreted (RANTES), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF). The cytokine kit was run per the manufacturer’s instructions and analyzed with a Luminex 100/200 instrument running xPONENT v3.1 software. PSS spiked with ACh at the same concentration of ACh administered during the dose-response protocols was used for the background, and all standards and samples were run in duplicate. The assay sensitivities for each cytokine were as follows: EPO (8.0 pg/ml), G-CSF (0.2 pg/ml), GM-CSF (0.6 pg/ml), GRO/KC (0.6 pg/ml), IFN-γ (1.0 pg/ml), IL-1α (1.0 pg/ml), IL-1β (2.0 pg/ml), IL-2 (3.0 pg/ml), IL-4 (1.0 pg/ml), IL-5 (6.0 pg/ml), IL-6 (10.0 pg/ml), IL-7 (0.4 pg/ml), IL-10 (5.0 pg/ml), IL-12p70 (0.7 pg/ml), IL-13 (0.9 pg/ml), IL-17A (0.1 pg/ml), IL-18 (4.0 pg/ml), M-CSF (0.4 pg/ml), MCP-1 (4.0 pg/ml), MIP-1α (12.0 pg/ml), MIP-3α (0.7 pg/ml), RANTES (3.0 pg/ml), TNF-α (3.0 pg/ml), and VEGF (0.3 pg/ml). A rat cytokine 24-plex assay was chosen because of the complexity of the marrow microenvironment and the various factors produced therein.
Bone microarchitecture and bone static and dynamic properties.
Bone histomorphometry analyses were conducted according to the guidelines set forth by the Nomenclature Committee of the American Society of Bone and Mineral Research (9). To assess bone microarchitecture and bone static and dynamic properties, right femora were cleaned of surrounding muscle and connective tissue, placed in 10% formalin for 3 days, and stored in 70% ethanol. Undecalcified bones were dehydrated and embedded in methylmethacrylate as previously described (33). Frontal sections (10 µm) of the distal femur were cut with a microtome (Leica RM2255; Leica Microsystems, Buffalo Grove, IL), and histological slides were created. Two sections were stained with Goldner’s trichrome (Young CON, n = 7; Young PTH, n = 10; Old CON, n = 7; Old PTH, n = 9) to measure bone microarchitecture [i.e., bone volume-to-total volume ratio (BV/TV, %), trabecular thickness (Tb.Th, µm), trabecular number (Tb.N, /mm2), and trabecular separation (Tb.Sp, µm)]. Additionally, the Goldner’s trichrome-stained sections were used to assess bone static properties related to osteoblast activity, i.e., osteoid surface-to-bone surface ratio (OS/BS, %) and osteoblast surface-to-bone surface ratio (Ob.S/BS, %).
To assess bone static properties related to osteoclast activity, two sections were used for the histoenzymatic reaction with tartrate-resistant acid phosphatase (Young CON, n = 8; Young PTH, n = 12; Old CON, n = 10; Old PTH, n = 12) to evaluate osteoclast surface-to-bone surface ratio (Oc.S/BS, %).
To assess bone dynamic properties, rats (Young CON, n = 8; Young PTH, n = 8; Old CON, n = 8; Old PTH, n = 8) received intraperitoneal injections of tetracycline (50 mg/kg body wt) 7 and 2 days before euthanasia. Bone dynamic properties were measured under UV light microscopy from tetracycline-labeled bone (2 unstained sections per rat) to assess mineral apposition rate (MAR, µm/day), single-labeled surface-to-bone surface ratio (sLS/BS, %), and double-labeled surface-to-bone surface ratio (dLS/BS, %). Single- and double-labeled surfaces were used to calculate the percentage of mineralizing surfaces-to-bone surface ratio [MS/BS (%) = dLS/BS + 1/2 sLS/BS]. Bone formation rate (BFR/BS, µm3·µm−2·day−1) was calculated as MS/BS × MAR. All measurements were conducted with OsteoMeasure bone histomorphometry analysis system (OsteoMetrics, Decatur, GA).
Solutions and drugs.
Stock solutions of PTH 1–34, ACh, SNP, and DEA NONOate were prepared and stored at −20°C. Serial dilutions of ACh and DEA NONOate were prepared daily. ACh, l-NAME, INDO, SNP, and DEA NONOate were purchased from Sigma Chemical (St. Louis, MO). PTH 1–34 was purchased from Phoenix Pharmaceuticals (Burlingame, CA).
Statistical analysis.
Vasodilator responses were expressed as a percentage of maximal relaxation according to the following formula:
where Dm is the maximal inner diameter recorded at 60 cmH2O in Ca2+-free PSS, Ds is the steady-state inner diameter recorded after each addition of the vasodilator substance, and Db is the initial baseline inner diameter recorded immediately before the first addition of ACh or DEA NONOate, with or without marrow and blockers.
Percent spontaneous tone for the ACh and DEA NONOate dose-response experiments was calculated according to the following formula:
where Dm is the maximal inner diameter recorded at 60 cmH2O in Ca2+-free PSS and Db is the initial baseline inner diameter recorded immediately before the first addition of ACh or DEA NONOate, with or without marrow.
EC50 was calculated for all conditions and represents the concentration of an agonist needed to elicit a 50% maximal vasodilator response.
One-way ANOVAs were used to determine significant differences in body mass, plasma PTH, plasma Ca2+, bone microarchitecture, and bone static and dynamic properties among groups (i.e., Young CON, Young PTH, Old CON, and Old PTH). One-way ANOVAs were used to determine significant differences in percent spontaneous tone, maximal diameter, percent maximal dilation, EC50, and cytokine concentration among conditions for young (i.e., Young CON, Young CON+Marrow, Young PTH, and Young PTH+Marrow) and old (i.e., Old CON, Old CON+Marrow, Old PTH, and Old PTH+Marrow) animals. Student-Newman-Keuls post hoc analyses were performed. One-way repeated-measures ANOVAs with pairwise comparisons were used to determine significant differences among conditions for vasodilator responses in the young (i.e., Young CON, Young CON+Marrow, Young PTH, and Young PTH+Marrow) and old (i.e., Old CON, Old CON+Marrow, Old PTH, and Old PTH+Marrow) groups. All data are presented as means ± SE. Significance was defined a priori as P ≤ 0.050. Tendencies for significant differences (P ≤ 0.100) are reported.
RESULTS
Animal characteristics.
Mean body and marrow masses are presented in Table 1. Mean body masses were higher in the old groups compared with the young groups for both the ACh and DEA NONOate experimental protocols. Marrow mass did not differ among groups for both the ACh and DEA NONOate experimental protocols.
Table 1.
Body characteristics of young and old male Fischer-344 rats
| Young |
Old |
|||
|---|---|---|---|---|
| CON | PTH | CON | PTH | |
| ACh | ||||
| Body mass, g | 361 ± 8 (n = 13) | 369 ± 4 (n = 15) | 449 ± 9a (n = 12) | 439 ± 12a (n = 14) |
| Marrow mass, mg | 52 ± 8 (n = 17) | 53 ± 6 (n = 14) | 93 ± 23 (n = 10) | 91 ± 20 (n = 13) |
| Diethylamine NONOate | ||||
| Body mass, g | 361 ± 11 (n = 8) | 364 ± 5 (n = 10) | 448 ± 12a (n = 8) | 427 ± 14a (n = 7) |
| Marrow mass, mg | 47 ± 12 (n = 7) | 57 ± 7 (n = 7) | 93 ± 40 (n = 6) | 108 ± 37 (n = 7) |
| Blood chemistry | ||||
| Plasma PTH, pg/ml | 6.12 ± 0.05 (n = 5) | 5.70 ± 0.05b (n = 5) | 5.95 ± 0.07 (n = 8) | 4.67 ± 0.08b (n = 8) |
| Plasma Ca2+, mg/dl | 12.6 ± 0.5 (n = 7) | 12.4 ± 0.3 (n = 9) | 14.3 ± 0.6c (n = 5) | 12.4 ± 0.6 (n = 7) |
Values are means ± SE; the sample size n represents the number of rats per group. CON, control; PTH, parathyroid hormone.
P < 0.050 vs. Young CON and Young PTH.
P < 0.050 vs. all other groups.
P = 0.070 vs. all other groups.
Plasma PTH and Ca2+ concentrations.
Plasma PTH and Ca2+ concentrations are shown in Table 1. Plasma PTH was reduced (P < 0.050) in Young PTH and Old PTH versus Young CON and Old CON. Furthermore, plasma PTH was lower (P < 0.050) in Old PTH versus Young PTH. Plasma Ca2+ was similar among Young CON, Young PTH, and Old PTH; however, plasma Ca2+ tended (P = 0.070) to be higher in Old CON versus the other groups.
Bone microarchitecture and bone static and dynamic properties.
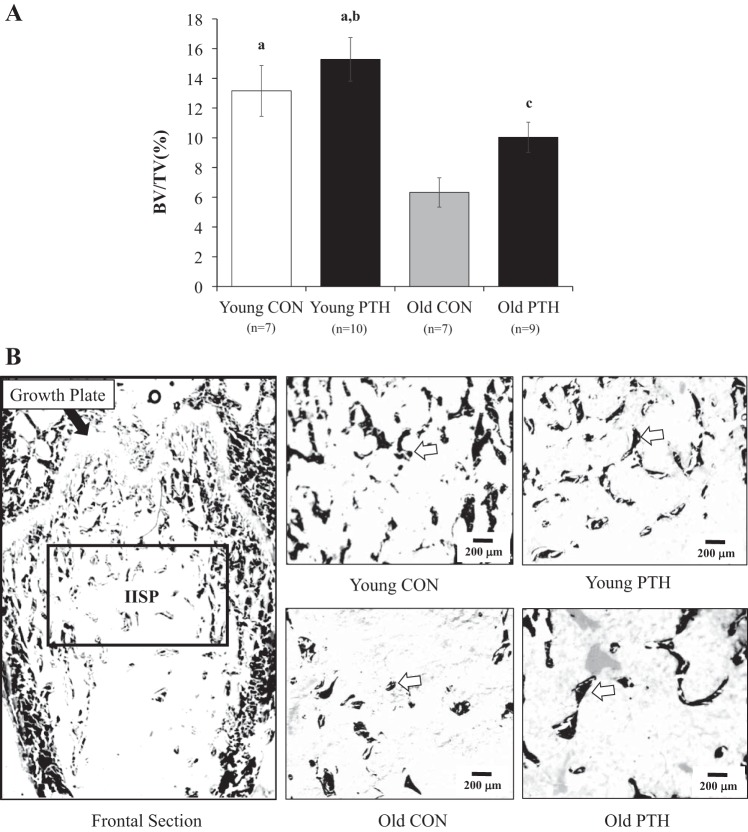
Figure 1 and Table 2 present data and representative two-dimensional images for bone microarchitecture and bone static and dynamic properties. Trabecular bone volume (i.e., BV/TV) declined 52% (P < 0.050) with advanced age (Fig. 1A), resulting in augmented (P < 0.050) Tb.Sp (Table 2). Figure 1B illustrates the two-dimensional bone microarchitecture of the area of analysis [i.e., the secondary spongiosa (IISP)] in the distal femur. The IISP denotes mature bone, and representative images are provided for each group. Following intermittent PTH administration in old rats, the 59% increase in BV/TV was not significant (P = 0.073); however, bone volume was restored to a degree such that age-related differences (i.e., Young CON vs. Old CON) were no longer observed. Bone volume in Young PTH was higher (P < 0.050) than in Old CON and Old PTH. Furthermore, PTH treatment tended (P = 0.068) to increase Tb.N in Young PTH versus Old CON. No significant differences in Ob.S/BS were observed among groups; however, OS/BS was higher (P < 0.050) in Old CON versus Old PTH. Osteoclast activity (i.e., Oc.S/BS) tended (P = 0.078) to be higher in Young PTH versus Old PTH. No differences in MAR, sLS/BS, and BFR/BS were observed among groups. However, dLS/BS and MS/BS were higher (P < 0.050) in Young PTH versus Old CON and dLS/BS tended (P = 0.055) to be higher in Young PTH versus Young CON and Old PTH.
Fig. 1.
A: bone volume-to-total volume ratio (BV/TV) in the distal femoral metaphysis following 2 wk of intermittent parathyroid hormone (PTH) 1–34 administration in young and old rats. Data were analyzed by one-way ANOVA. Values are means ± SE. The sample size n represents the number of rats per group. aP < 0.050 vs. Old Control (CON), bP < 0.050 vs. Old PTH, cP = 0.073 vs. Old CON. B: two-dimensional frontal section of the distal femoral metaphysis. The black box illustrates the area of analysis [i.e., the secondary spongiosa (IISP)]. Representative images for each group are shown. The white arrows denote examples of trabecular bone.
Table 2.
Bone microarchitecture and bone static and dynamic properties
| Young |
Old |
|||
|---|---|---|---|---|
| CON | PTH | CON | PTH | |
| Bone microarchitecture | ||||
| n | 7 | 10 | 7 | 9 |
| Tb.Th, µm | 21.2 ± 2.0 | 23.2 ± 2.4 | 19.6 ± 3.7 | 21.5 ± 3.0 |
| Tb.N, /mm2 | 6.6 ± 1.1 | 7.5 ± 1.2e | 3.6 ± 0.6 | 5.5 ± 1.1 |
| Tb.Sp, µm | 158.6 ± 29.4 | 149.8 ± 29.1 | 309.8 ± 52.5a | 220.6 ± 41.3 |
| Bone static properties: osteoblast activity | ||||
| n | 7 | 10 | 7 | 9 |
| OS/BS, % | 5.7 ± 1.1 | 5.1 ± 1.0 | 7.9 ± 1.6c | 3.2 ± 0.7 |
| Ob.S/BS, % | 0.7 ± 0.5 | 0.8 ± 0.3 | 0.3 ± 0.2 | 0.4 ± 0.1 |
| Bone static properties: osteoclast activity | ||||
| n | 8 | 11 | 9 | 9 |
| Oc.S/BS, % | 1.8 ± 0.3 | 2.5 ± 0.6d | 1.4 ± 0.3 | 1.0 ± 0.3 |
| Bone dynamic properties | ||||
| n | 8 | 8 | 8 | 8 |
| MAR, µm/day | 0.90 ± 0.30 | 1.18 ± 0.30 | 0.57 ± 0.05 | 0.55 ± 0.04 |
| sLS/BS, % | 1.44 ± 0.28 | 1.51 ± 0.13 | 0.78 ± 0.06 | 1.53 ± 0.38 |
| dLS/BS, % | 0.70 ± 0.19 | 1.41 ± 0.29b,d,f | 0.34 ± 0.07 | 0.88 ± 0.22 |
| MS/BS, % | 1.42 ± 0.28 | 2.16 ± 0.32b | 0.85 ± 0.18 | 1.65 ± 0.39 |
| BFR/BS, µm3·µm−2·day−1 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.01 |
Values are means ± SE; the sample size n represents the number of rats per group. CON, control; PTH, parathyroid hormone; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; OS/BS, osteoid surface-to-bone surface ratio; Ob.S/BS, osteoblast surface-to-bone surface ratio; Oc.S/BS, osteoclast surface-to-bone surface ratio; MAR, mineral apposition rate, sLS/BS, single-labeled surface-to-bone surface ratio; dLS/BS, double-labeled surface-to-bone surface ratio; MS/BS, mineralizing surfaces-to-bone surface ratio; BFR/BS, bone formation rate-to-bone surface ratio.
P < 0.050 vs. Young CON and Young PTH,
P < 0.050 vs. Old CON,
P < 0.050 vs. Old PTH,
P < 0.100 vs. Old PTH,
P < 0.100 vs. Old CON,
P < 0.050 vs. Young CON.
PNA characteristics.
Maximal diameter, percent spontaneous tone, and EC50 were not different among conditions for each age group for the ACh and DEA NONOate experimental protocols (Tables 3 and 4, respectively).
Table 3.
Maximal diameter, percent spontaneous tone, and EC50 of the femoral principal nutrient artery following the ACh dose-response experiment in young and old CON and PTH-treated rats in the absence and presence of marrow
| CON | CON+Marrow | PTH | PTH+Marrow | |
|---|---|---|---|---|
| Young | ||||
| Maximal diameter, µm | 188 ± 11 (n = 14) | 187 ± 9 (n = 18) | 198 ± 8 (n = 15) | 190 ± 7 (n = 14) |
| Spontaneous tone, % | 27 ± 4 (n = 14) | 34 ± 4 (n = 16) | 36 ± 4 (n = 14) | 29 ± 5 (n = 8) |
| EC50 | 7.48 × 10−7 ± 2.56 × 10−7(n = 14) | 1.09 × 10−6 ± 3.74 × 10−7(n = 13) | 4.60 × 10−7 ± 1.85 × 10−7(n = 12) | 8.75 × 10−7 ± 4.54 × 10−7(n = 7) |
| Old | ||||
| Maximal diameter, µm | 196 ± 12 (n = 12) | 196 ± 16 (n = 10) | 210 ± 8 (n = 13) | 224 ± 8 (n = 11) |
| Spontaneous tone, % | 37 ± 5 (n = 12) | 28 ± 2 (n = 10) | 33 ± 2 (n = 14) | 32 ± 3 (n = 13) |
| EC50 | 1.98 × 10−7 ± 1.13 × 10−7(n = 12) | 6.36 × 10−6 ± 4.66 × 10−6(n = 10) | 2.98 × 10−7 ± 1.30 × 10−7(n = 13) | 1.88 × 10−6 ± 1.04 × 10−6(n = 11) |
Values are means ± SE; the sample size n represents the number of rats per group. CON, control; PTH, parathyroid hormone.
Table 4.
Maximal diameter, percent spontaneous tone, and EC50 of the femoral principal nutrient artery following the diethylamine NONOate dose-response experiment in young and old CON and PTH-treated rats in the absence and presence of marrow
| CON | CON+Marrow | PTH | PTH+Marrow | |
|---|---|---|---|---|
| Young | ||||
| Maximal diameter, µm | 210 ± 11 (n = 8) | 206 ± 8 (n = 11) | 197 ± 13 (n = 8) | 197 ± 9 (n = 8) |
| Spontaneous tone, % | 36 ± 6 (n = 8) | 36 ± 5 (n = 11) | 29 ± 2 (n = 10) | 34 ± 10 (n = 8) |
| EC50 | 6.21 × 10−6 ± 4.89 × 10−6(n = 8) | 6.44 × 10−5 ± 5.75 × 10−5(n = 11) | 3.31 × 10−6 ± 2.70 × 10−6(n = 8) | 5.39 × 10−6 ± 3.96 × 10−6(n = 8) |
| Old | ||||
| Maximal diameter, µm | 215 ± 10 (n = 7) | 211 ± 26 (n = 7) | 201 ± 13 (n = 8) | 217 ± 10 (n = 8) |
| Spontaneous tone, % | 34 ± 4 (n = 7) | 28 ± 8 (n = 6) | 34 ± 6 (n = 8) | 37 ± 6 (n = 9) |
| EC50 | 4.97 × 10−6 ± 4.45 × 10−6(n = 7) | 3.16 × 10−6 ± 2.15 × 10−6(n = 7) | 1.59 × 10−6 ± 1.38 × 10−6(n = 8) | 2.21 × 10−7 ± 1.20 × 10−7(n = 8) |
Values are means ± SE; the sample size n represents the number of rats per group. CON, control; PTH, parathyroid hormone.
Endothelium-dependent vasodilator responses.
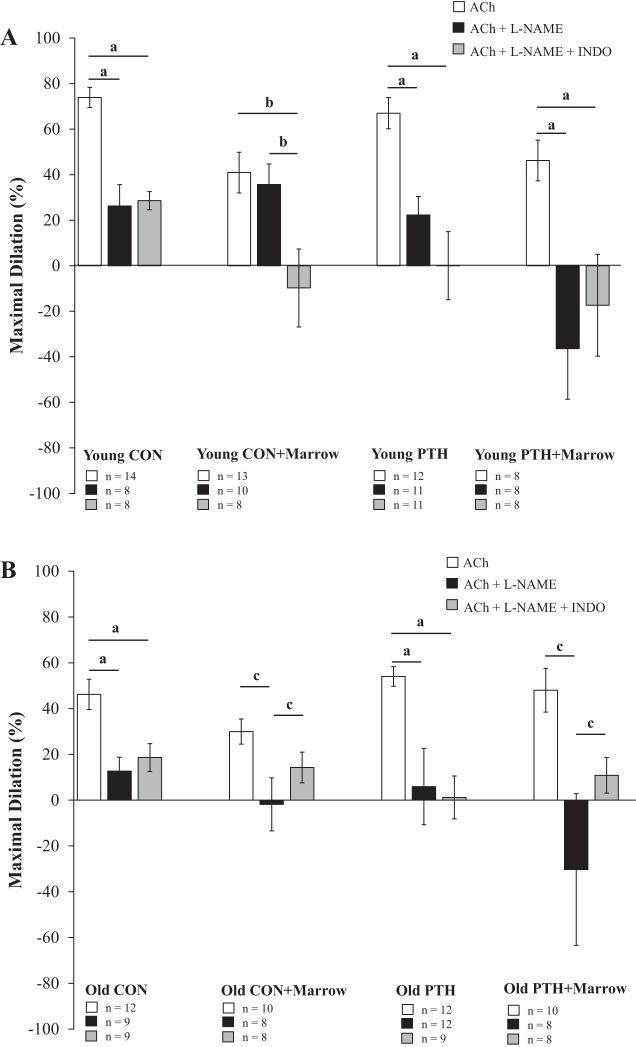
The presence of marrow reduced endothelium-dependent vasodilation by 44% (P < 0.050) in the young rats (i.e., Young CON vs. Young CON+Marrow; Fig. 2A). Intermittent PTH administration had no effect on endothelium-dependent vasodilation in young rats, such that vasodilator capacity did not differ from Young CON. Additionally, vasodilation in Young PTH did not differ from Young PTH+Marrow but was higher (P < 0.050) than Young CON+Marrow. Significant differences were not evident between Old CON and Old CON+Marrow, despite a 16% decline in vasodilator capacity in the presence of marrow (Fig. 2B). Intriguingly, 2 wk of intermittent PTH 1–34 administration in old rats tended (P = 0.060) to augment endothelium-dependent mediated vasodilation by 17% versus Old CON, which abolished the age-related declines in relation to Young CON. Interestingly, endothelium-dependent vasodilation in femoral PNAs from Old PTH+Marrow peaked at 48% of maximal dilation, despite the presence of marrow. In other words, PNAs from Old PTH+Marrow had a 60% higher (P < 0.050) vasodilator capacity than PNAs from Old CON+Marrow and similar vasodilator profiles to Old CON and Old PTH.
Fig. 2.
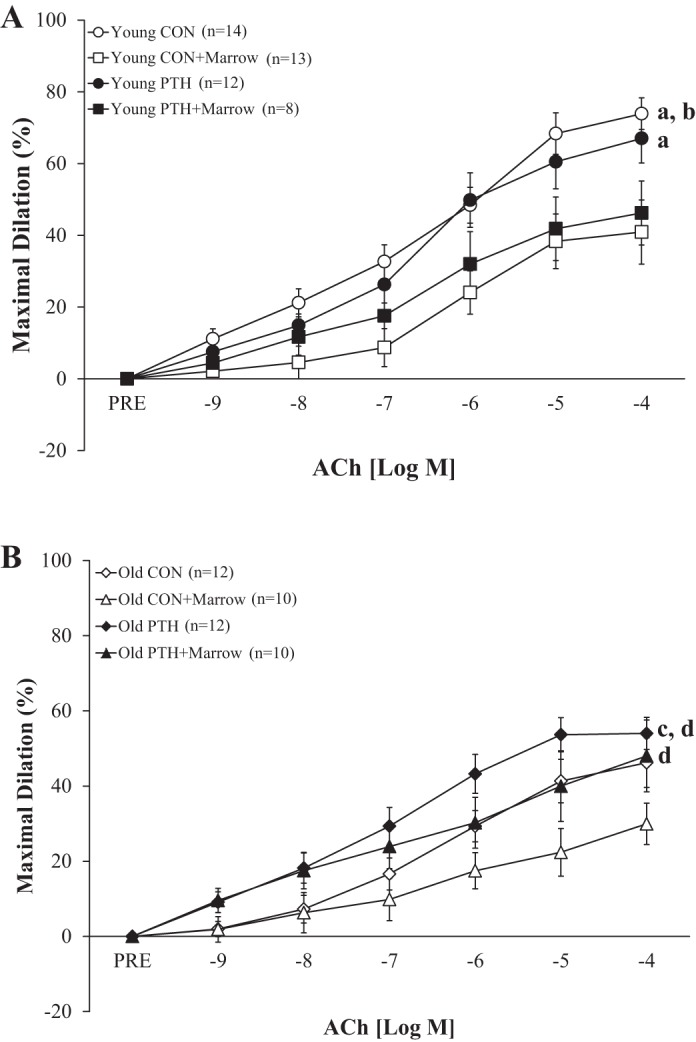
Effects of intermittent parathyroid hormone (PTH) administration and the marrow microenvironment on endothelium-dependent vasodilation of the femoral principal nutrient artery to ACh in young and old rats. A: no differences in vasodilation were observed between Young Control (CON) and Young PTH. Incubation with marrow reduced vasodilation in Young CON+Marrow and Young PTH+Marrow. B: vasodilation in Old PTH tended to be higher than in Old CON and was higher than in Old CON+Marrow. Vasodilator responses differed between Old PTH+Marrow and Old CON+Marrow. Data were analyzed by repeated-measures ANOVAs with pairwise comparisons. Values are means ± SE. PRE, before ACh administration. aP < 0.050 vs. Young CON+Marrow; bP < 0.050 vs. Young PTH+Marrow; cP = 0.060 vs. Old CON; dP < 0.050 vs. Old CON+Marrow.
Endothelium-dependent vasodilator signaling mechanisms.
Data indicate reliance on the NO signaling pathway for vasodilation to ACh in Young CON and Young PTH. For example, incubation of the femoral PNA with the NOS inhibitor l-NAME diminished (P < 0.050) maximal dilation to ACh by 65 and 66% in Young CON and Young PTH, respectively (Fig. 3A). Double incubation with l-NAME and the COX inhibitor INDO did not further reduce maximal dilation versus l-NAME alone. Interestingly, the presence of marrow elicited alterations in the vasodilator signaling pathways or exaggerated responses to ACh in young PNAs. For example, blockade of NO produced a substantial (P < 0.050) decline in vasodilation (178%) in Young PTH+Marrow, such that vasoconstriction occurred. In contrast, the presence of l-NAME did not alter maximal dilation in PNAs from Young CON+Marrow. Furthermore, double incubation with l-NAME and INDO did not further constrict PNAs from Young PTH+Marrow but reduced (P < 0.050) vasodilation by 124% in PNAs from Young CON+Marrow, resulting in vasoconstriction. These data highlight the importance of NO signaling under CON and PTH conditions.
Fig. 3.
Signaling pathways (i.e., NO and PGI2) involved in ACh-mediated, endothelium-dependent vasodilation in young (A) and old (B) rats following intermittent parathyroid hormone (PTH) administration and/or exposure to marrow. NG-nitro-l-arginine methyl ester (l-NAME) denotes that the ACh dose-response experiment was conducted in the presence of the NO synthase (NOS) inhibitor. l-NAME + indomethacin (INDO) denotes that the ACh dose-response experiment was conducted in the presence of the NOS and cyclooxygenase (COX) inhibitors. Data were analyzed by one-way ANOVAs. Values are means ± SE. The sample size n represents the number of rats per group. CON, control. aP < 0.050 between groups; bP < 0.050 between groups; cP < 0.050 between groups.
Similarly, the production of NO is of primary importance for endothelium-dependent vasodilation to ACh in Old CON and Old PTH (Fig. 3B). For example, incubation of femoral PNAs with the NOS inhibitor l-NAME diminished (P < 0.050) maximal dilation by 73 and 89% in Old CON and Old PTH, respectively (Fig. 3B). These data reveal that the improvements in endothelium-dependent vasodilation with intermittent PTH 1–34 administration in old rats occurred via an upregulation of the NOS signaling pathway. The addition of INDO did not further reduce endothelium-dependent vasodilation to ACh in either group. Incubation of femoral PNAs with l-NAME in the presence of marrow reduced (P < 0.050) vasodilation by 107 and 162% in Old CON+Marrow and Old PTH+Marrow, respectively, producing overall vasoconstrictor effects. Additionally, these data highlight the continued reliance on NO signaling in old animals, despite the presence of marrow. Interestingly, and in contrast to responses in young rats, the inhibition of PGI2 production with double blockade allowed for vasodilation in marrow-incubated old PNAs. In fact, maximal dilation under these conditions did not differ from maximal dilation observed with ACh alone in both Old CON+Marrow and Old PTH+Marrow.
Endothelium-independent vasodilator responses.
Endothelium-independent vasodilator responses to the NO donor, DEA NONOate, did not differ among conditions (Fig. 4).
Fig. 4.
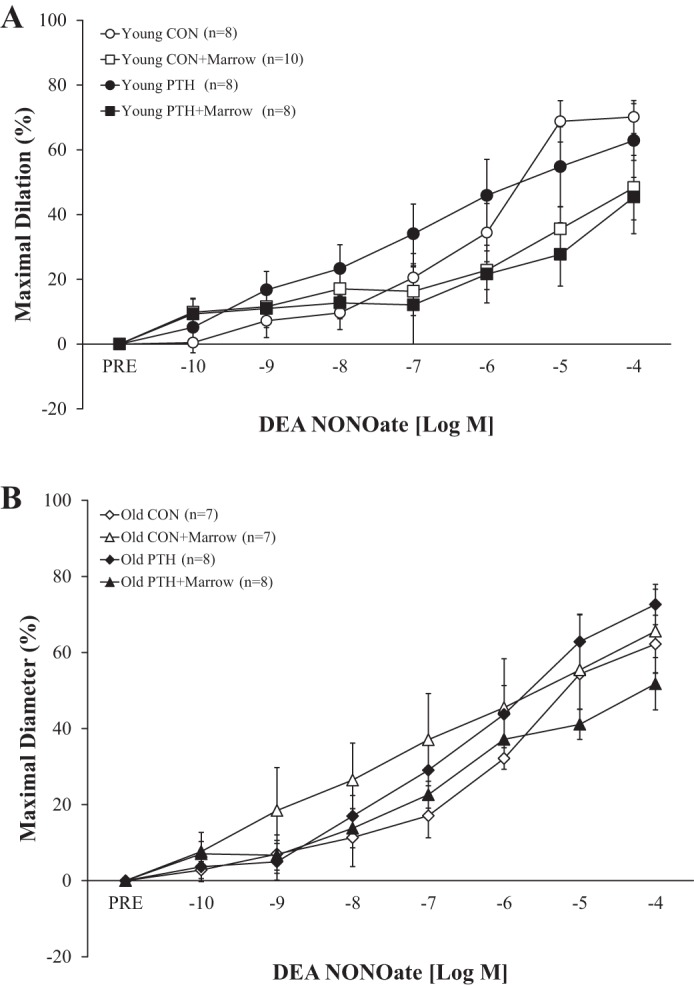
Effects of intermittent parathyroid hormone (PTH) administration and the marrow microenvironment on endothelium-independent vasodilation of the femoral principal nutrient artery to diethylamine (DEA) NONOate in young and old rats. A: vasodilator responses to DEA NONOate were unaltered in young rats following intermittent PTH administration and exposure to marrow. B: vasodilator responses to DEA NONOate were unaltered in old rats following intermittent PTH administration and exposure to marrow. Data were analyzed by repeated-measures ANOVAs with pairwise comparisons. Values are means ± SE. The sample size n represents the number of rats per group. CON, control; PRE, before DEA NONOate administration.
Cytokine production in the absence and presence of marrow following intermittent PTH administration.
Eleven (i.e., EPO, G-CSF, GRO/KC, IFN-γ, IL-2, IL-5, IL-7, IL-12p70, IL-17A, IL-18, and TNF-α) of the 24 cytokines were undetectable in the samples of PSS and were not analyzed. Nine of the 24 cytokines (i.e., IL-1α, IL-6, RANTES, M-CSF, MIP-1α, MIP-3α, VEGF, IL-4, and IL-13) did not differ among conditions for both the young and old groups (Table 5).
Table 5.
Cytokine concentration in the absence and presence of marrow following intermittent PTH 1–34 administration in young and old rats
| CON | CON+Marrow | PTH | PTH+Marrow | |
|---|---|---|---|---|
| Young | ||||
| n | 3 | 3 | 3 | 3 |
| Proinflammatory cytokines, pg/ml | ||||
| IL-1α | 1.00 ± 0.29 | 3.45 ± 0.68 | 0.68 ± 0.26 | 8.43 ± 5.81 |
| IL-6 | 4.18 ± 0.08 | 4.66 ± 0.39 | 3.60 ± 0.65 | 22.25 ± 11.23 |
| RANTES | 15.04 ± 0.74 | 20.00 ± 0.51 | 15.60 ± 0.65 | 27.20 ± 5.34 |
| M-CSF | 2.07 ± 0.09 | 2.11 ± 0.02 | 1.75 ± 0.15 | 5.23 ± 2.04 |
| MIP-1α | 2.81 ± 0.09 | 3.08 ± 0.03 | 2.81 ± 0.09 | 5.09 ± 1.93 |
| MIP-3α | 18.99 ± 0.43 | 19.83 ± 0.50 | 18.91 ± 1.15 | 29.40 ± 10.40 |
| VEGF | 0.63 ± 0.12 | 1.31 ± 0.36 | 0.96 ± 0.67 | 5.32 ± 4.14 |
| Anti-inflammatory cytokines, pg/ml | ||||
| IL-4 | 0.30 ± 0.03 | 0.58 ± 0.03 | 0.31 ± 0.03 | 2.61 ± 1.28 |
| IL-13 | 0.43 ± 0.12 | 0.48 ± 0.21 | 0.44 ± 0.33 | 12.56 ± 12.15 |
| Old | ||||
| n | 3 | 3 | 3 | 3 |
| Proinflammatory cytokines, pg/ml | ||||
| IL-1α | 0.67 ± 0.27 | 1.91 ± 0.56 | 0.49 ± 0.07 | 2.49 ± 0.95 |
| IL-6 | 2.11 ± 0.11 | 1.77 ± 0.31 | 2.07 ± 0.41 | 4.41 ± 1.49 |
| RANTES | 13.35 ± 0.59 | 89.59 ± 26.50 | 12.27 ± 0.59 | 82.19 ± 38.53 |
| M-CSF | 1.59 ± 0.14 | 1.80 ± 0.02 | 1.42 ± 0.11 | 1.99 ± 0.17 |
| MIP-1α | 2.85 ± 0.04 | 8.77 ± 5.51 | 2.65 ± 0.03 | 4.29 ± 0.27 |
| MIP-3α | 17.76 ± 0.58 | 19.92 ± 1.24 | 16.96 ± 0.21 | 20.30 ± 1.04 |
| VEGF | 0.32 ± 0.05 | 1.94 ± 0.75 | 0.24 ± 0.00* | 2.49 ± 0.35 |
| Anti-inflammatory cytokines, pg/ml | ||||
| IL-4 | 0.26 ± 0.01 | 0.32 ± 0.01 | 0.19 ± 0.04 | 0.54 ± 0.16 |
| IL-13 | 0.21 ± 0.03 | 0.24 ± 0.10 | 0.11 ± 0.00* | 1.90 ± 0.00* |
Values are means ± SE; the sample size n represents the number of rats per group. CON, control; M-CSF, macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; PTH, parathyroid hormone; RANTES, regulated on activation normal T cell expressed and secreted.
Sample size was reduced to n = 1 because of undetectable limits for the respective cytokines in the physiological saline solution.
In young animals, IL-1β was significantly (P < 0.05) higher in Young PTH+Marrow versus Young CON and Young PTH (Fig. 5A). Furthermore, IL-1β tended (P = 0.079) to be higher in Young PTH+Marrow versus Young CON+Marrow and tended (P = 0.085) to be higher in Young CON+Marrow versus Young CON and Young PTH.
Fig. 5.
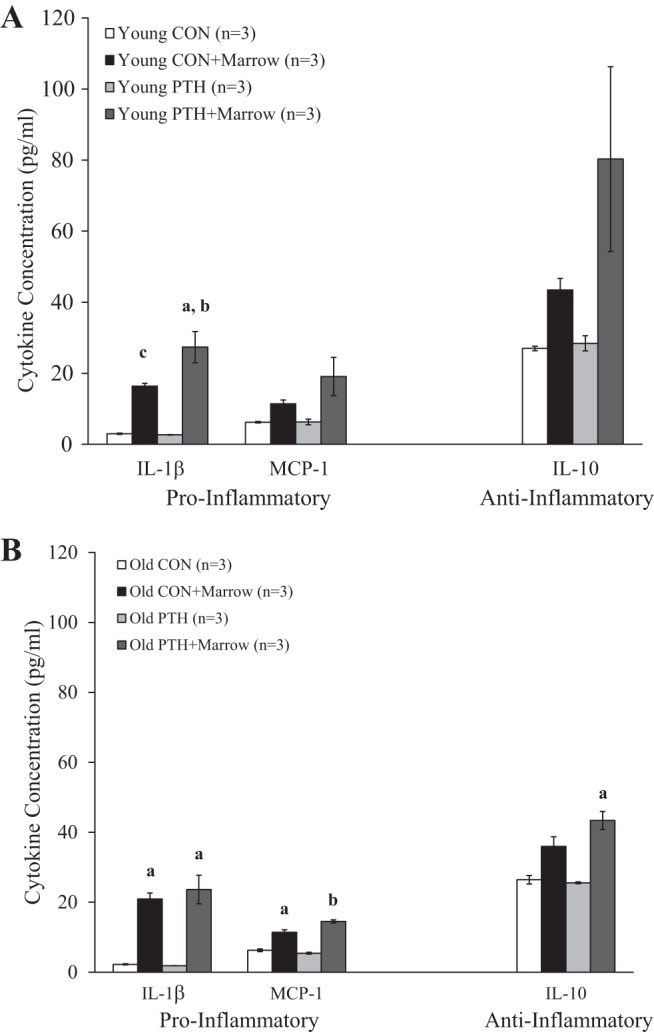
A: cytokine concentration in the physiological saline solution (PSS) in the absence and presence of marrow in Young Control (CON) and Young Parathyroid Hormone (PTH). aP < 0.050 vs. Young CON and Young PTH; bP = 0.079 vs. Young CON+Marrow; cP = 0.085 vs. Young CON and Young PTH. B: cytokine concentration in the PSS in the absence and the presence of marrow in Old CON and Old PTH. aP < 0.050 vs. Old CON and Old PTH; bP < 0.050 vs. all other groups. Data were analyzed by one-way ANOVAs. Values are means ± SE. The sample size n represents the number of rats per group. MCP-1, monocyte chemoattractant protein-1.
Correspondingly, IL-1β was significantly (P < 0.05) augmented in Old CON+Marrow and Old PTH+Marrow versus Old CON and Old PTH (Fig. 5B). Furthermore, the concentration of MCP-1 was enhanced (P < 0.050) in the presence of the marrow (Old CON+Marrow vs. Old CON and Old PTH) and further enhanced (P < 0.050) following PTH treatment (i.e., Old PTH+Marrow vs. all other groups). In contrast, intermittent PTH 1–34 administration in old rats increased (P < 0.050) the concentration of the anti-inflammatory cytokine, IL-10 (i.e., Old PTH+Marrow vs. Old CON and Old PTH; Fig. 5B).
DISCUSSION
This investigation demonstrates several key findings. 1) The reduced trabecular bone volume (Fig. 1) in old rats corresponded with diminished endothelium-dependent vasodilation in old femoral PNAs (data not shown) related to attenuated NO signaling (Fig. 3B). 2) Intermittent PTH administration in old rats tended to enhance endothelium-dependent vasodilation, such that vasodilator capacity no longer differed from Young CON. 3) PTH impacted endothelial cell function via improved NO signaling, as vascular smooth muscle cell responsiveness was unchanged. 4) The presence of marrow corresponded with diminished vasodilation in young PNAs. 5) The dysfunction caused by the marrow was restricted to the vascular endothelium, since vascular smooth muscle cell function did not differ among conditions (Fig. 4). 6) Intermittent PTH 1–34 administration altered the marrow microenvironment in old rats, such that vasodilator capacity differed between Old PTH+Marrow and Old CON+Marrow (Fig. 2B). 7) The proinflammatory cytokines measured in this investigation were not reduced with PTH treatment (Fig. 5, A and B); however, the anti-inflammatory cytokine, IL-10 (Fig. 5B), was enhanced in old marrow. To our knowledge, these are the first data demonstrating that intermittent PTH 1–34 administration improves vasodilator capacity, as related to NO signaling, in bone blood vessels from old rats. In addition, these are the first data to demonstrate an undesirable influence of the marrow microenvironment on vascular function, which can be ameliorated with intermittent PTH administration in old animals.
Previous investigations have made the link between the marrow microenvironment and bone remodeling (8, 19, 24, 28), suggesting paracrine signaling between bone marrow and bone cells. For example, restoration of hematopoietic stem cells in the tibia following intermittent PTH administration in mice served to augment bone volume (28). Similarly, previous reports have speculated that bone cellular activity is regulated by the vascular system (3, 7, 37). Extending upon these insights, this investigation demonstrated that the marrow microenvironment modulates the functional properties of bone blood vessels. Incubation of the marrow alongside the femoral PNA reduced (P < 0.050) endothelium-dependent vasodilation by 44% in young rats, an unanticipated finding. The lack of statistical significance with the 16% decline in vasodilator capacity in Old CON+Marrow may relate to the already diminished vasodilator capacity in old PNAs, which coincides with previous reports (12, 37). Vasodilation peaked at 46 versus 74% of maximum in Old CON and Young CON, respectively. Vasodilation in Old CON+Marrow peaked at 30% of maximum, illustrating the retention of some vasodilator responsiveness. It should be noted that segments of the PNA from young and old animals were exposed to the entire marrow content of the femoral shaft. In other words, exposure at these volumes of marrow may represent exposure at supraphysiological levels to segments of blood vessels. Since blood vessels normally rest within the marrow microenvironment and are exposed to the entire marrow content under in vivo conditions, it is unlikely to have influenced the vascular responses obtained. However, this possibility represents a potential limitation in the present study design.
The augmentation of IL-1β in the presence of marrow corresponded with reduced endothelium-dependent vasodilation in young animals. These findings coincide with reports of cytokine-induced vascular dysfunction (13, 22, 25). For example, 7 h of IL-1β incubation of carotid arteries from New Zealand White rabbits reduced endothelium-dependent relaxation to ACh and substance P (22). Similarly, endothelium-independent relaxation was unaltered with IL-1β incubation (22), suggesting no effect on vascular smooth muscle cell function.
The presence of proinflammatory cytokines was hypothesized to reduce vasodilator capacity for the old animals as opposed to the young. However, the proinflammatory milieu of the marrow microenvironment observed coincides with severe calcification of the bone marrow vasculature (32). Bone marrow blood vessels progressively ossify (i.e., calcify) with advancing age and theoretically convert into bone (32). Although the volume of ossification was markedly lower in young versus old male Fischer-344 rats, the presence of ossified bone marrow blood vessels in the young animals demonstrates that the disease process begins at an early age (32). Furthermore, bone marrow blood vessel ossification presumably shares similar pathological mechanisms to atherosclerosis and arteriosclerosis (32), both of which can be initiated by proinflammatory environments and commence via impaired vasodilator capacity. Thus, the marrow microenvironment may contribute to the initial stages of disease processes that lead to vascular calcification.
The proinflammatory cytokines augmented in the present investigation have been linked to vascular diseases related to calcification. For example, deficiency of IL-1α and/or IL-1β in ApoE knockout mice prevented atherosclerosis (21), and monocyte attraction to sites of vascular injury was presumably related to the IL-1β-induced increased mRNA expression of MCP-1 (39). Furthermore, increased gene expression of MCP-1, VCAM-1, E-selectin, and IL-6 (i.e., genes associated with inflammation) in human umbilical vein endothelial cells resulted from in vitro exposure to IL-4 (26). It should be noted that IL-4 is a mediator of anti-inflammatory responses (6) but has been demonstrated to have proinflammatory properties as well (1, 5, 26). These investigations illustrate that proinflammatory cytokines participate in vascular disease.
Other functional and structural modifications of the PNA were not altered by exposure to the marrow or intermittent PTH administration. For example, endothelium-independent vasodilation did not differ among conditions for both the young and old groups. Furthermore, intermittent PTH 1–34 administration and the marrow microenvironment had no effects on the maximal diameter of the PNA, percent spontaneous tone, or the sensitivity (EC50) of the PNA to the agonists ACh and DEA NONOate. Noteworthy, however, is the link between the age-related declines in trabecular bone volume and endothelium-dependent vasodilation (−52 and −38%, respectively). Similar to previous reports (2, 34), these modifications in vasodilator capacity were mediated through the vascular endothelium and NO signaling. Although 2 wk of PTH treatment did not augment endothelium-dependent vasodilation (+17%; P = 0.060) and trabecular bone volume (+59%; P = 0.073) in old rats, a longer time course of treatment may have yielded significant changes. Most interestingly, intermittent PTH 1–34 administration in old rats altered the marrow microenvironment such that endothelium-related vasodilator capacity was not impaired in the presence of marrow and coincided with elevated production of IL-10. Thus, intermittent PTH administration directly influenced vascular function and the marrow microenvironment.
It is difficult to ascertain how the marrow microenvironment was altered by PTH treatment in the present investigation. However, the vasodilator responsiveness of old femoral PNAs in the presence of the marrow following PTH treatment may provide some insight. The anti-inflammatory cytokine IL-10 has beneficial effects on vascular function. For example, mice (≥9 mo) deficient in IL-10 demonstrated increased blood pressure, arterial stiffness, and reduced endothelium-dependent vasorelaxation in aortic rings versus age-matched, wild-type controls (41). Recent evidence suggests that IL-10 plays a pivotal role in repressing oxidative stress and attenuating the impairment in endothelium-dependent vasorelaxation in carotid arteries of mice >22 mo of age (23). Additionally, knockout of IL-10 in these aged mice was associated with increased gene expression of IL-6, i.e., a proinflammatory cytokine associated with hypertension and vascular pathology (23). Furthermore, 14 days of TNF-α administration to IL-10 knockout mice enhanced vasoconstriction in mouse arteries as mediated through endothelin-1 and the ERK1/2 pathway (15). Since IL-10 protected endothelial cell function by reducing superoxide production during an acute inflammatory stimulus (17) and counterbalances vasoconstrictor pathways (15), enhanced production of IL-10 following intermittent PTH 1–34 administration in old rats may have provided some protection against the proinflammatory cytokines. Although the mechanisms by which IL-10 is elevated with PTH treatment are unknown, the interactions between PTH and IL-10 in senescence may provide a new avenue for investigation.
Senescence was also associated with bone loss in the present investigation, which coincides with other reports in the literature illustrating reduced bone mass in advanced age (12, 35, 37). The age-related declines in bone volume coincided with increased trabecular separation; however, the bone static and dynamic properties did not differ between young and old rats except for OS/BS, which was augmented in Old CON. The increased osteoid surface may represent an inability of old animals to properly mineralize bone, a condition not present following intermittent PTH administration. Furthermore, OS/BS, Oc.S/BS, and plasma Ca2+ were not enhanced in Young CON, and plasma Ca2+ was unchanged following intermittent PTH 1–34 administration in both age groups. These data are consistent with findings in elderly individuals (42) and in ovariectomized, osteoporotic rats (47) following 18 mo and 12 wk, respectively, of PTH treatment. In both studies, serum Ca2+ remained within normal ranges (42, 47).
In contrast to plasma Ca2+, intermittent PTH 1–34 administration reduced endogenous PTH 1–84, an effect more exaggerated in advanced age. Since PTH 1–84 is released by the parathyroid glands to mobilize Ca2+ from bone and given augmented bone resorption with constant PTH and PTHrP infusion (20), the attenuated endogenous production of PTH 1–84 coupled with intermittent administration of PTH 1–34 may eventually serve to enhance bone accrual in old rats. Bone volume was not augmented in Young PTH versus age-related controls. However, bone volume was higher than both old groups, a trend not evident in Young CON.
In conclusion, the marrow-induced impairment of vasodilation coincided with the production of proinflammatory cytokines. Interestingly, intermittent PTH administration altered the marrow microenvironment in old rats such that endothelium-dependent vasodilation was enhanced despite the presence of marrow. PTH treatment also augmented the production of the anti-inflammatory cytokine IL-10. Although given as a treatment for osteoporosis, the mechanisms by which intermittent PTH administration achieves bone accrual may include its effects on bone vasodilator properties and the marrow microenvironment. This is the first investigation to report improved endothelium-dependent vasodilation of bone arteries in old animals with intermittent PTH administration and to highlight the importance of the marrow microenvironment on bone vascular function.
GRANTS
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 7R15-AR-062882-02 (R. D. Prisby) and National Institute of General Medical Sciences Core Access Award P20-GM-103446 (R. D. Prisby).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.P. conceived and designed research; S.L., A.B., B.H., J.K., and R.D.P. performed experiments; S.L., A.B., B.H., J.R., J.K., and R.D.P. analyzed data; S.L. and R.D.P. interpreted results of experiments; S.L. and R.D.P. prepared figures; S.L. and R.D.P. drafted manuscript; S.L., A.B., B.H., J.R., J.K., and R.D.P. edited and revised manuscript; S.L., A.B., B.H., J.R., J.K., and R.D.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. John Guers for the measurement of plasma parathyroid hormone and calcium.
REFERENCES
- 1. Beekhuizen H, Verdegaal EM, Blokland I, van Furth R. Contribution of ICAM-1 and VCAM-1 to the morphological changes in monocytes bound to human venous endothelial cells stimulated with recombinant interleukin-4 (rIL-4) or rIL-1 alpha. Immunology 77: 469–472, 1992. [PMC free article] [PubMed] [Google Scholar]
- 2.Benson T, Menezes T, Campbell J, Bice A, Hood B, Prisby R. Mechanisms of vasodilation to PTH 1–84, PTH 1–34, and PTHrP 1–34 in rat bone resistance arteries. Osteoporos Int 27: 1817–1826, 2016. doi: 10.1007/s00198-015-3460-z. [DOI] [PubMed] [Google Scholar]
- 3.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res 21: 183–192, 2006. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 4.Brookes M, Revell WJ. Blood Supply of Bone: Scientific Aspects. London: Springer-Verlag, 1998. doi: 10.1007/978-1-4471-1543-4. [DOI] [Google Scholar]
- 5.Chalubinski M, Wojdan K, Luczak E, Gorzelak P, Borowiec M, Gajewski A, Rudnicka K, Chmiela M, Broncel M. IL-33 and IL-4 impair barrier functions of human vascular endothelium via different mechanisms. Vascul Pharmacol 73: 57–63, 2015. doi: 10.1016/j.vph.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, Mitchell BM. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens 28: 135–142, 2015. doi: 10.1093/ajh/hpu100. [DOI] [PubMed] [Google Scholar]
- 7.Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol (1985) 89: 1046–1054, 2000. doi: 10.1152/jappl.2000.89.3.1046. [DOI] [PubMed] [Google Scholar]
- 8.Compston JE. Bone marrow and bone: a functional unit. J Endocrinol 173: 387–394, 2002. doi: 10.1677/joe.0.1730387. [DOI] [PubMed] [Google Scholar]
- 9.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28: 2–17, 2013. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 87: 2095–2147, 1996. [PubMed] [Google Scholar]
- 11.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27: 519–550, 2009. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez JM II, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone 46: 813–819, 2010. doi: 10.1016/j.bone.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 94: 2157–2163, 2009. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A, Hakimi M, Dengler TJ, Giese T, Blessing E, Katus HA, Gleissner CA. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol 193: 4344–4355, 2014. doi: 10.4049/jimmunol.1400181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giachini FR, Zemse SM, Carneiro FS, Lima VV, Carneiro ZN, Callera GE, Ergul A, Webb RC, Tostes RC. Interleukin-10 attenuates vascular responses to endothelin-1 via effects on ERK1/2-dependent pathway. Am J Physiol Heart Circ Physiol 296: H489–H496, 2009. doi: 10.1152/ajpheart.00251.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith JF, Yeung DK, Tsang PH, Choi KC, Kwok TC, Ahuja AT, Leung KS, Leung PC. Compromised bone marrow perfusion in osteoporosis. J Bone Miner Res 23: 1068–1075, 2008. doi: 10.1359/jbmr.080233. [DOI] [PubMed] [Google Scholar]
- 17.Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol 279: H1555–H1562, 2000. doi: 10.1152/ajpheart.2000.279.4.H1555. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone 44: 1026–1033, 2009. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 19.Hillel I, Binderman I, Sarda Y, Nevo U. Monitoring of cellular changes in the bone marrow following PTH(1–34) treatment of OVX rats using a portable stray-field NMR scanner. J Osteoporos 2017: 7910432, 2017. doi: 10.1155/2017/7910432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz MJ, Tedesco MB, Sereika SM, Prebehala L, Gundberg CM, Hollis BW, Bisello A, Garcia-Ocaña A, Carneiro RM, Stewart AF. A 7-day continuous infusion of PTH or PTHrP suppresses bone formation and uncouples bone turnover. J Bone Miner Res 26: 2287–2297, 2011. doi: 10.1002/jbmr.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamari Y, Shaish A, Shemesh S, Vax E, Grosskopf I, Dotan S, White M, Voronov E, Dinarello CA, Apte RN, Harats D. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1α. Biochem Biophys Res Commun 405: 197–203, 2011. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Kessler P, Bauersachs J, Busse R, Schini-Kerth VB. Inhibition of inducible nitric oxide synthase restores endothelium-dependent relaxations in proinflammatory mediator-induced blood vessels. Arterioscler Thromb Vasc Biol 17: 1746–1755, 1997. doi: 10.1161/01.ATV.17.9.1746. [DOI] [PubMed] [Google Scholar]
- 23.Kinzenbaw DA, Chu Y, Peña Silva RA, Didion SP, Faraci FM. Interleukin-10 protects against aging-induced endothelial dysfunction. Physiol Rep 1: e00149, 2013. doi: 10.1002/phy2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh AJ, Novince CM, Li X, Wang T, Taichman RS, McCauley LK. An irradiation-altered bone marrow microenvironment impacts anabolic actions of PTH. Endocrinology 152: 4525–4536, 2011. doi: 10.1210/en.2011-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotowicz K, Callard RE, Friedrich K, Matthews DJ, Klein N. Biological activity of IL-4 and IL-13 on human endothelial cells: functional evidence that both cytokines act through the same receptor. Int Immunol 8: 1915–1925, 1996. doi: 10.1093/intimm/8.12.1915. [DOI] [PubMed] [Google Scholar]
- 26.Lee YW, Eum SY, Chen KC, Hennig B, Toborek M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol Med 10: 19–27, 2004. doi: 10.2119/2004-00024.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JY, D’Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T, Tyagi AM, Yu M, Reott M, Sassi F, Buondonno I, Adams J, Weitzmann MN, Isaia GC, Pacifici R. IL-17A is increased in humans with primary hyperparathyroidism and mediates PTH-induced bone loss in mice. Cell Metab 22: 799–810, 2015. doi: 10.1016/j.cmet.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu R, Wang Q, Han Y, Li J, Yang XJ, Miao D. Parathyroid hormone administration improves bone marrow microenvironment and partially rescues haematopoietic defects in Bmi1-null mice. PLoS One 9: e93864, 2014. doi: 10.1371/journal.pone.0093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14: 877–882, 2013. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Moore AE, Blake GM, Taylor KA, Rana AE, Wong M, Chen P, Fogelman I. Assessment of regional changes in skeletal metabolism following 3 and 18 months of teriparatide treatment. J Bone Miner Res 25: 960–967, 2010. doi: 10.1359/jbmr.091108. [DOI] [PubMed] [Google Scholar]
- 31.Neuprez A, Reginster JY. Bone-forming agents in the management of osteoporosis. Best Pract Res Clin Endocrinol Metab 22: 869–883, 2008. doi: 10.1016/j.beem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Prisby RD. Bone marrow blood vessel ossification and “microvascular dead space” in rat and human long bone. Bone 64: 195–203, 2014. doi: 10.1016/j.bone.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prisby R, Guignandon A, Vanden-Bossche A, Mac-Way F, Linossier MT, Thomas M, Laroche N, Malaval L, Langer M, Peter ZA, Peyrin F, Vico L, Lafage-Proust MH. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res 26: 2583–2596, 2011. doi: 10.1002/jbmr.459. [DOI] [PubMed] [Google Scholar]
- 34.Prisby R, Menezes T, Campbell J. Vasodilation to PTH (1–84) in bone arteries is dependent upon the vascular endothelium and is mediated partially via VEGF signaling. Bone 54: 68–75, 2013. doi: 10.1016/j.bone.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Prisby RD, Dominguez JM II, Muller-Delp J, Allen MR, Delp MD. Aging and estrogen status: a possible endothelium-dependent vascular coupling mechanism in bone remodeling. PLoS One 7: e48564, 2012. doi: 10.1371/journal.pone.0048564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prisby RD, Muller-Delp J, Delp MD, Nurkiewicz TR. Age, gender, and hormonal status modulate the vascular toxicity of the diesel exhaust extract phenanthraquinone. J Toxicol Environ Health A 71: 464–470, 2008. doi: 10.1080/15287390701839349. [DOI] [PubMed] [Google Scholar]
- 37.Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM II, Donato AJ, Allen MR, Delp MD. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res 22: 1280–1288, 2007. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- 38.Roche B, Vanden-Bossche A, Malaval L, Normand M, Jannot M, Chaux R, Vico L, Lafage-Proust MH. Parathyroid hormone 1–84 targets bone vascular structure and perfusion in mice: impacts of its administration regimen and of ovariectomy. J Bone Miner Res 29: 1608–1618, 2014. doi: 10.1002/jbmr.2191. [DOI] [PubMed] [Google Scholar]
- 39.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol 136: 1229–1233, 1990. [PMC free article] [PubMed] [Google Scholar]
- 41.Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, Fraser CD III, Ellis C, Ross D, Vandegaer K, Bedja D, Gabrielson K, Walston JD, Berkowitz DE, Barouch LA. Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Exp Gerontol 48: 128–135, 2013. doi: 10.1016/j.exger.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sridharan M, Cheung J, Moore AE, Frost ML, Fraser WD, Fogelman I, Hampson G. Circulating fibroblast growth factor-23 increases following intermittent parathyroid hormone (1–34) in postmenopausal osteoporosis: association with biomarker of bone formation. Calcif Tissue Int 87: 398–405, 2010. doi: 10.1007/s00223-010-9414-8. [DOI] [PubMed] [Google Scholar]
- 43.Stabley JN, Prisby RD, Behnke BJ, Delp MD. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J Endocrinol 225: 47–58, 2015. doi: 10.1530/JOE-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tawfeek H, Bedi B, Li JY, Adams J, Kobayashi T, Weitzmann MN, Kronenberg HM, Pacifici R. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. PLoS One 5: e12290, 2010. doi: 10.1371/journal.pone.0012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhaar HJ, Lems WF. PTH-analogs: comparable or different? Arch Gerontol Geriatr 49: e130–e132, 2009. doi: 10.1016/j.archger.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Rong H, Ji H, Wang D, Wang J, Zhang W, Zhang Y. Effects of different dosages of parathyroid hormone-related protein 1–34 on the bone metabolism of the ovariectomized rat model of osteoporosis. Calcif Tissue Int 93: 276–287, 2013. doi: 10.1007/s00223-013-9755-1. [DOI] [PubMed] [Google Scholar]