Abstract
It is suggested that the frequent strain the airways undergo in asthma because of repeated airway smooth muscle (ASM)-mediated constrictions contributes to airway wall remodeling. However, the effects of repeated constrictions on airway remodeling, as well as the ensuing impact of this presumptive remodeling on respiratory mechanics, have never been investigated in subjects without asthma. In this study, we set out to determine whether repeated constrictions lead to features that are reminiscent of asthma in mice without asthma. BALB/c mice were subjected to a 30-min constriction elicited by aerosolized methacholine every other day over 6 wk. Forty-eight hours after the last constriction, the mechanics of the respiratory system was evaluated at baseline and in response to incremental doses of nebulized methacholine with the flexiVent. The whole-lung lavages, the tracheas, and the lungs were also collected to evaluate inflammation, the contractile capacity of ASM, and the structural components of the airway wall, respectively. The resistance and the compliance of the respiratory system, as well as the Newtonian resistance and the resistive and elastic properties of the lung tissue, were not affected by repeated constrictions, both at baseline and in response to methacholine. All the other examined features also remained unaltered, except the number of goblet cells in the epithelium and the number of macrophages in the whole-lung lavages, which both increased with repeated constrictions. This study demonstrates that, despite causing goblet cell hyperplasia and a mild macrophagic inflammation, repeated constrictions with methacholine do not lead to structural changes that adversely impact the physiology.
NEW & NOTEWORTHY Repeated airway constrictions led to signs of remodeling that are typically observed in asthma, which neither altered respiratory mechanics nor the contractile capacity of airway smooth muscle. These findings shed light on a debate between those claiming that constrictions induce remodeling and those convinced that methacholine challenges are harmless. Insofar as our results with mice relate to humans, the findings indicate that repeated challenges with methacholine can be performed safely.
Keywords: airway hyperresponsiveness, airway remodeling, asthma, bronchoconstriction, respiratory mechanics, specific airway resistance
INTRODUCTION
Asthma is a common respiratory disorder for which the pathogenesis is highly variable between afflicted patients (13). The symptoms of asthma, although variable in terms of nature and severity, normally originate from excessive narrowing of the lumen in airways dispersed either throughout the lung or in focal regions of the lung (7, 30, 31). Excessive narrowing of the airways in asthma stems from multiple causes, but inflammation, airway constriction mediated by airway smooth muscle (ASM) contraction, and remodeling of the airway wall are often invoked. In turn, there are several and differing underlying mechanisms leading to these typical causes of asthma.
An emerging paradigm suggests that the strain the airway wall undergoes during constriction contributes to some features of asthma, particularly airway wall remodeling. This was first shown in volunteers with asthma in whom repeated constrictions elicited by three bouts of inhaled methacholine, spaced apart by 2 days, were shown to thicken the basement membrane and to increase the number of goblet cells in the epithelium (14). Similar findings were also reported in different animal models of allergic asthma (6, 17, 24, 27). In the latter, airway remodeling was prevented by interfering with either the muscarinic receptors, which presumably contribute to the natural constriction that occurs after allergen exposure in those animal models (6, 17, 24), or the integrin αvβ5, which is required for the constriction-induced activation of transforming growth factor β (27). Interestingly, some findings even imply that airway remodeling caused by ASM contraction occurs in vitro (19, 23, 27). Combined with the in vivo studies, this provides further evidence that airway constriction alone (i.e., in the absence of inflammation) may be one of the common causes of airway remodeling in asthma. However, the effect of repeated constrictions on airway remodeling and other reminiscent features of asthma has never been investigated systematically in vivo in mice without asthma. In this study, we set out to determine whether repeated constrictions elicited by methacholine lead to some features of asthma in mice without asthma. It is important to understand that this was not an attempt to mimic a real-life situation in humans, but rather a model used to measure the isolated effect of repeated constrictions on airway structure and function.
METHODS
Animals.
Fifty-two female BALB/c mice were purchased from Charles River (Saint-Constant, PQ, Canada) at 6 wk old. The mice were provided food and water ad libitum, and they were housed for at least one week before the investigation. All the procedures were approved by the Committee of Animal Care of Université Laval in accordance with the guidelines of the Canadian Council on Animal Care.
Kinetics of the induced airway constriction.
An initial set of experiments was conducted to quantify the magnitude and the duration of the constriction triggered by a 30-min exposure to methacholine. The mice were acclimated to the double-chamber plethysmograph (SCIREQ, Montreal, PQ, Canada) to measure specific airway resistance (sRaw) on 3 separate days the week before the beginning of the exposures. On the days of exposure, sRaw was measured for each mouse at baseline. The mice were then placed into a customized Plexiglas exposure chamber all at once. The internal volume of the chamber was 3 liters. During the exposure, phosphate-buffered saline (PBS) or methacholine at 7 mg/ml was aerosolized into the chamber using an up-mist nebulizer (Hospitak, Lindenhurst, NY) running at a constant rate of 120 µl/min. Fresh air was also provided at all times from a bias flow set at 7.5 l/min. In this initial set of experiments, the mice were removed one by one at 5-min intervals for the first 30 min of exposure to measure sRaw. The remaining mice were then all removed from the exposure chamber at 30 min but tested one by one at 5-min intervals to measure sRaw until the last mouse was evaluated. This whole experiment was repeated 6 times with PBS and 6 times with methacholine in alternating fashion on 12 separate days (not necessarily consecutive) on the same 12 mice.
Six weeks of treatment with repeated constrictions.
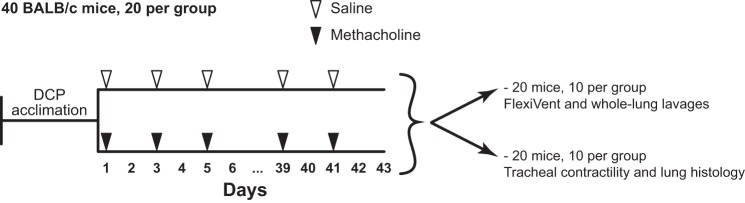
The next set of experiments was designed to assess the effect of repeated constrictions on respiratory mechanics, airway remodeling, and inflammation. Forty mice were used for this set of experiments. The protocol is depicted in Fig. 1. As described above, the mice were acclimated to the double-chamber plethysmograph the week before the beginning of the experiments. The mice were then divided into 2 groups of 20 to receive either aerosolized PBS (shammed procedure) or methacholine at 7 mg/ml for 30 min every other day over 6 weeks starting on day 1.
Fig. 1.
Experimental design that was used to investigate the effect of repeated airway constrictions on features of experimental asthma. DCP, double-chamber plethysmograph. See methods for a detailed description.
In this set of experiments, all the mice could not be exposed at once. Since we own two double-chamber plethysmographs, sRaw can only be measured for up to two mice at a time. The mice went through the procedures 2 by 2 at 5-min intervals. Hence, the first 2 mice were added in the exposure chamber at time zero and removed at 30 min. The next 2 mice were added at 5 min and removed at 35 min, and so on. To easily distinguish the mice within the chamber (as we had to keep track of which ones had been added at 0, 5, 10, or 15 min), they were 1) exposed in smaller groups of only 6 to 8 and 2) labeled with either 1 or 2 hole punches on either the left or the right ear within the week of acclimation. As we had 20 mice per group, there were 3 rounds of exposure for both the saline and the methacholine groups (6 + 6 + 8 = 20) at every tested day. We are aware that the chamber was opened from time to time to add or remove mice, which may have affected the concentration of saline/methacholine in the chamber. However, the same procedures took place in the first set of experiments described above, which was the protocol used to characterize the constriction.
sRaw was measured both before and after each exposure to confirm airway constriction on every tested day, as well as to determine whether the effect of methacholine changed over the 6 wk of treatment. On day 43, 48 h after the last exposure, the mice from each group were further divided into two subgroups. One subgroup of 10 mice was used to assess respiratory mechanics at baseline and in response to incremental doses of methacholine, as well as to collect the whole-lung lavages. The other subgroup of 10 mice was euthanized to collect the trachea and the left lobe of the lungs.
Respiratory mechanics.
Baseline respiratory mechanics and airway responsiveness to methacholine was assessed using the flexiVent (SCIREQ) (22). The mice were anesthetized, tracheotomized, mechanically ventilated, paralyzed, and kept in a supine position throughout the testing. More specifically, the mice were anesthetized with ketamine and xylazine at 100 and 10 mg/kg, respectively. They were then tracheotomized and connected to the flexiVent. The ventilation was set at a breathing frequency of 150 breaths/min, a tidal volume of 10 ml/kg, and a positive end-expiratory pressure of 3 cmH2O. Once the ventilation was established, the mice were paralyzed with 0.1 mg/kg of pancuronium bromide injected intramuscularly. Heart rate was monitored continuously by electrocardiography throughout the experiment to ensure proper anesthesia.
Baseline respiratory mechanics was measured 10 s after two deep inflations to 25 cmH2O, which are required for lung recruitment. Two distinct volume-perturbation maneuvers were then used to test the mechanics of the respiratory system, called the SnapShot-150 and the Quick Prime-3. They were both actuated twice in an alternating fashion, each being intercalated by 8 s of tidal breathing to prevent desaturation. The volume perturbation imparted by the SnapShot-150 is a single sine wave oscillation that allows one to infer values for the resistance (Rrs) and compliance (Crs) of the respiratory system based on the single-compartment model (5). The volume perturbation imparted by the Quick Prime-3 is a composite signal constituted of several sine waves, all at different frequencies, amplitudes, and phases, that allows one to infer values for Newtonian resistance (RN), tissue damping (G), and tissue elastance (H) based on the one-phase constant model (15).
The degree of airway responsiveness was assessed by monitoring the changes in respiratory mechanics during incremental doses of methacholine. The series of doses consisted of PBS and 1, 3, 10, and 30 mg/ml of methacholine. Again, respiratory mechanics were assessed by the SnapShot-150 and the Quick Prime-3. Each of these volume-perturbation maneuvers was actuated 10 times in an alternating fashion after each dose, starting 10 s after dose delivery. Eight seconds of tidal breathing was intercalated between each volume-perturbation maneuver. A deep inflation was also imposed after the last volume-perturbation maneuver ~2 min before the subsequent dose. The peak values for each inferred parameter (Rrs, Crs, RN, G, and H) after each dose were used to assess the response.
Whole-lung lavages.
The whole-lung lavages were collected in 20 mice, 10 from the PBS-exposed group and 10 from the methacholine-exposed group, immediately after the measurements of respiratory mechanics. The lavages were performed by 3 consecutive cycles of injection and aspiration of 1.0 ml of sterile PBS. The aspirated volumes were pooled and centrifuged at 500 g for 5 min. The recovered pellet was then resuspended in 100 µl of PBS, and a small fraction (10 µl) was used to estimate the total number of cells using a hemacytometer. The remaining cells were cytospun on a microscopic slide, which was then stained with modified May-Grünwald Giemsa stain (HemaStain Set, Fisher Scientific, Kalamazoo, MI) to assess the percentage by which the macrophages, lymphocytes, neutrophils, and eosinophils accounted for the total cell number.
Contractile capacity of airway smooth muscle.
The trachea was collected in 20 mice, 10 from the PBS-exposed group and 10 from the methacholine-exposed group. The mice were euthanized with ketamine and xylazine at 200 and 10 mg/kg, respectively. Thoracotomy was performed immediately after death. The entire trachea was excised and immersed into Krebs solution [pH 7.4, 111.9 mM NaCl, 5.0 mM KCl, 1.0 mm KH2PO4, 2.1 mM MgSO4, 29.8 mM NaHCO3, 11.5 mM glucose, and 2.9 mM CaCl2]. The trachea was then mounted in a 5-ml organ bath containing Krebs solution maintained at 37°C. The whole trachea was connected by a surgical thread to a force transducer (Harvard Apparatus, St-Laurent, PQ, Canada), which allowed measurements of isometric force deployed by the ASM in response to contractile activation. A distending force of 5 mN (the resting tension) was applied. Prior to any recorded measurement, the trachea was subjected to a period of equilibration, during which time the ASM was stimulated to contract repeatedly for 5 min at 10-min intervals with 10−5 M of methacholine until a reproducible force was recorded.
Cumulative concentration-response curves were generated with three distinct spasmogens, namely methacholine, KCl, and endothelin-1. The order was randomized. Methacholine was added in half-log increments at 5-min intervals from 10−8 to 10−4 M. Endothelin-1 was added in half-log increments at 10-min intervals from 10−10 to 10−7 M. The concentration of KCl was incrementally increased 1.41-fold at 5-min intervals. The peak force obtained at each concentration was used to generate the concentration-response curves. At least 30 min were left between the different spasmogens, over which time the trachea was repeatedly washed with fresh Krebs.
Histology on lung slices.
Lung histology was performed on the same mice as the ones used to measure the contractile capacity of the tracheas. Immediately after euthanasia, the left lobe was immersed in formalin for 24 h for fixation. They were then dehydrated in 50% ethanol until further processed. The lobe was embedded in paraffin, cut transversally in 5-µm-thick sections, and stained with either Masson trichrome, hematoxylin-eosin (H&E) or periodic acid shift (PAS). The sections were then imaged with a NanoZoomer Digital scanner (Hamamastu Photonics, Bridgewater, NJ). The sections stained with Masson trichrome were used to quantify both the amount of ASM within the airway wall and the content of collagen within the lung. For the ASM, six sections from each mouse were used. All the airways cut transversally were analyzed with the NDP View Software (Hamamastu Photonics). An average of 5.9 ± 2.3 airways per mouse were analyzed. For each analyzed airway, the total area occupied by the ASM was divided by the square of the basement membrane length. The values obtained for all individual airways were then compiled to obtain an average value per mouse. For the collagen, two sections in each mouse were used. The entire section of the lung was analyzed. The quantification was done using NDP View Software and ImageJ (version 1.50i). The two sections from each mouse were then compiled to obtain an average value per mouse. The sections stained with H&E were used to qualitatively assess tissue infiltration with inflammatory cells. Two sections from each mouse were examined. The sections stained with PAS were used to count the number of goblet cells in the epithelium, as well as to quantify the thickness of the epithelium as previously described (4). Two sections in each mouse were used. All the airways cut transversally were analyzed with the NDP View Software. An average of 4.2 ± 2.5 airways per mouse were analyzed. For each analyzed airway, the number of goblet cells was divided by the length of the basement membrane. The values obtained for all individual airways were then compiled to obtain an average value per mouse.
Statistical analyses.
Data are shown as means ± SD. Two-way ANOVAs with Sidak’s multiple comparison tests were used to assess the kinetics of the constriction triggered by aerosolized methacholine. The same statistical tests were used to assess the effect of treatment (PBS vs. methacholine) on the changes in Rrs, Crs, RN, G, and H induced by incremental doses of nebulized methacholine, as well as to assess the effect of treatment on the force generated by the tracheas in response to increasing concentrations of methacholine, KCl, and endothelin-1. A two-way ANOVA was used to assess the magnitude of the induced constriction over the 6 wk of treatment. Mann-Whitney tests were performed to assess the effect of treatment on the total cell count and the differential cell counts in the whole-lung lavages, as well as to assess the effect of treatment on the thickness of the epithelium and the content of ASM, collagen and goblet cells in histologic sections. All statistical analyses were performed using Prism 7 (version 7.0a, GraphPad, San Diego, CA). P < 0.05 was considered sufficient to reject the null hypothesis.
RESULTS
Constriction triggered by aerosolized methacholine.
The kinetics of the airway constriction triggered by aerosolized methacholine is depicted in Fig. 2. In contrast to the lack of effect of PBS exposure, sRaw progressively increased following the onset of methacholine exposure. The gain in sRaw was significant after 10 min. This gain in sRaw was then maintained throughout the rest of the exposure. At 30 min, sRaw amounted to 3.14 ± 1.12 cmH2O/s after methacholine exposure compared with 1.49 ± 0.26 cmH2O/s after PBS exposure (a difference of 1.65 cmH2O/s). The increase in sRaw then waned after methacholine exposure. However, sRaw in methacholine-exposed mice remained significantly higher than the PBS-exposed mice for another 10 min.
Fig. 2.
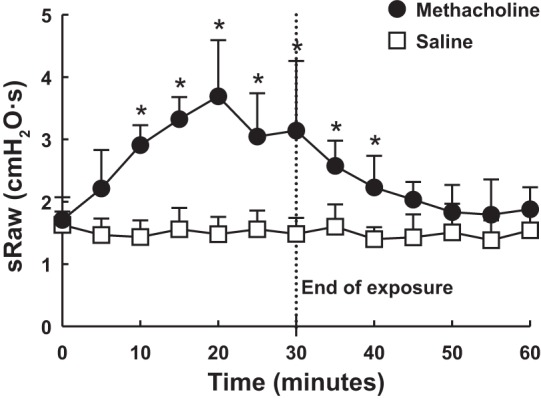
Kinetics of the induced airway constriction. Twelve mice were exposed to either aerosolized PBS (□) or methacholine at 7 mg/ml (●) in an exposure chamber. Mice were removed one by one at 5-min intervals for the first 30 min of exposure to measure specific airway resistance (sRaw) using the double-chamber plethysmograph. Remaining mice were then all removed from the exposure chamber at 30 min but tested one by one in the double-chamber plethysmograph at 5-min intervals to measure sRaw until the last mouse was evaluated. This experiment was repeated 6 times with PBS and 6 times with methacholine in alternating fashion on 12 separate days. Results show that the constriction was significant after 10 min and remained significant for at least 10 min after the end of methacholine exposure. *Statistically significant difference during exposure to aerosolized methacholine compared with the corresponding time point during the shammed procedure.
The magnitude of the repeated constrictions induced by aerosolized methacholine, as well as the effect of shammed procedures with aerosolized PBS, on every day of exposure during the entire 6 wk of treatment is depicted in Fig. 3. At the end of the 30-min exposure, sRaw was on average 1.71 ± 0.27 cmH2O/s higher in mice exposed to methacholine compared with PBS-exposed mice (P < 0.0001). There was no change in the response to methacholine over time (P = 0.49 for the interaction treatment vs. time).
Fig. 3.
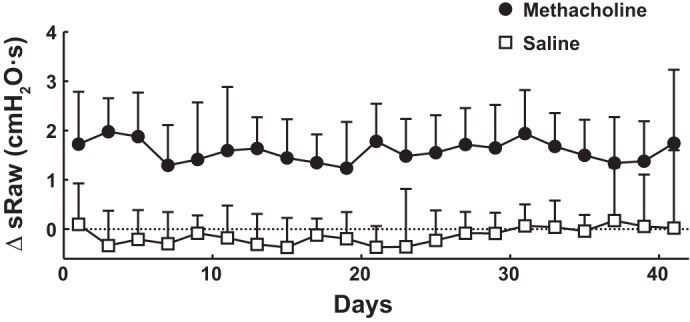
Airway response to either PBS (□) or methacholine (●) was monitored throughout the 6 wk of treatment. More specifically, 2 groups of mice were exposed to either PBS or methacholine at 7 mg/ml for 30 min every other day. Specific airway resistance (sRaw) for each mouse was measured using the double-chamber plethysmograph both before and after each exposure. Average difference in sRaw (ΔsRaw = sRaw at postexposure – sRaw at preexposure) at every tested day is displayed. Results demonstrate that although PBS did not affect sRaw, methacholine consistently increased sRaw by an average of 1.71 ± 0.27 cmH2O/s. n = 20 mice per group.
Respiratory mechanics.
The values of Rrs, Crs, RN, G, and H after the nebulization of PBS and in response to incremental doses of nebulized methacholine in mice subjected to either repeated constrictions or shammed procedures over 6 wk are depicted in Fig. 4. Although each of these parameters changed significantly in response to incremental doses of methacholine, no differences were observed in mice subjected to repeated constrictions compared with the mice subjected to shammed procedures.
Fig. 4.
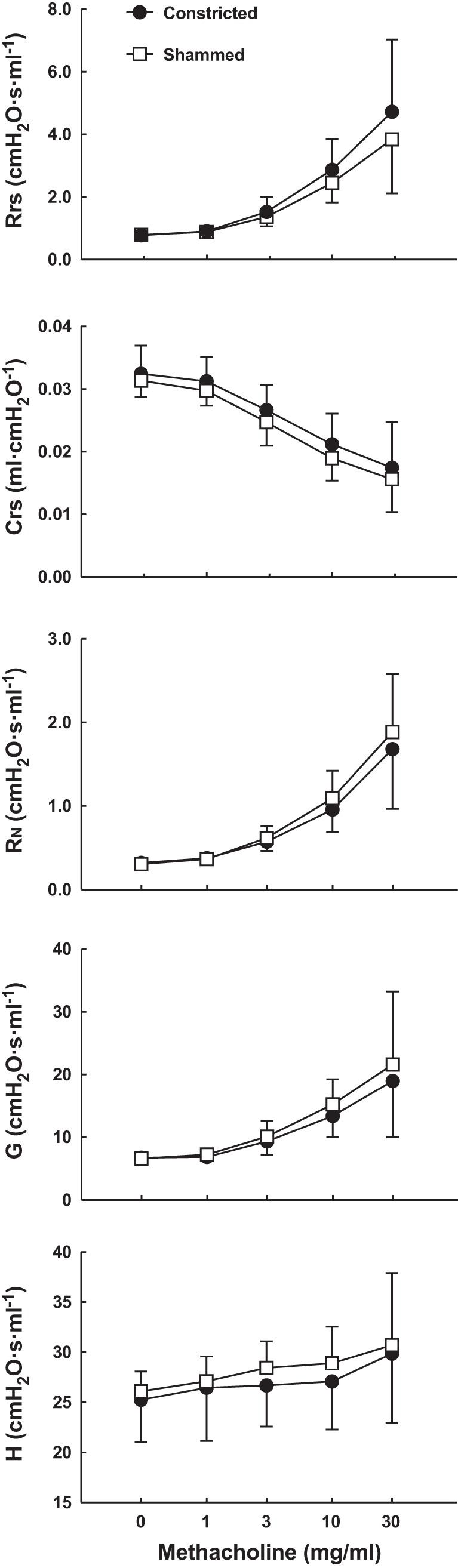
In vivo responsiveness to methacholine after 6 wk of treatment with either a 30-min constriction (●) or the shammed procedure (□) every other day. Respiratory system resistance (Rrs), respiratory system compliance (Crs), Newtonian resistance (RN), tissue damping (G), and tissue elastance (H) were measured after PBS nebulization and in response to incremental doses of nebulized methacholine. Note that the y-axis for H does not start at zero. n = 10 mice per group.
Inflammation.
The inflammatory cells collected in the whole-lung lavages after 6 wk of treatment are depicted in Fig. 5. The total number of cells was significantly higher (P = 0.02) in the mice subjected to repeated constrictions compared with the mice subjected to shammed procedures. This was entirely due to an increased number (P = 0.01) and percentage (P = 0.002) of macrophages. The numbers of lymphocytes, neutrophils, and eosinophils were not affected by repeated constrictions. Examination of the airway wall and the parenchyma by histology in lung sections stained with H&E revealed no difference in tissue inflammation in mice subjected to repeated constrictions compared with mice subjected to shammed procedures (data not shown).
Fig. 5.
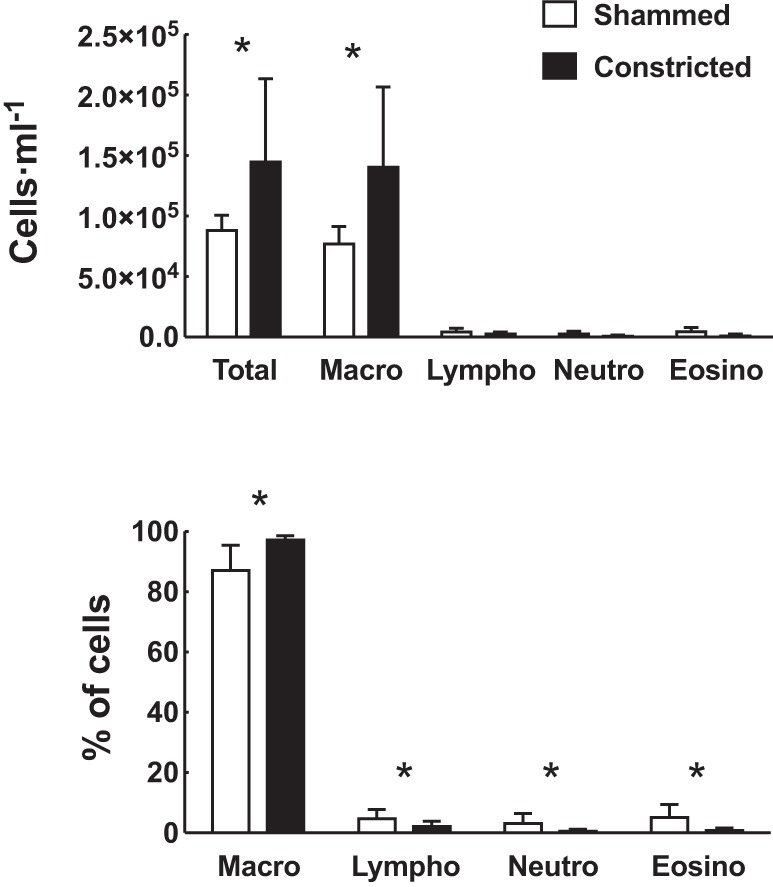
Inflammatory cells within the luminal compartments of the lungs after 6 wk of treatment with either a 30-min constriction (closed bars) or the shammed procedure (open bars) every other day. Total number of inflammatory cells per milliliter of whole-lung lavages is displayed in the upper panel. Percentage of the total cells that is constituted by macrophages, lymphocytes, neutrophils, and eosinophils is displayed at bottom. Number of macrophages, lymphocytes, neutrophils, and eosinophils at top was estimated based on the total number of cells and the computed proportions made up by each cell type. *Statistically significant difference in mice subjected to repeated constrictions vs. mice subjected to shammed procedures. n = 10 mice per group.
Contractile capacity of airway smooth muscle.
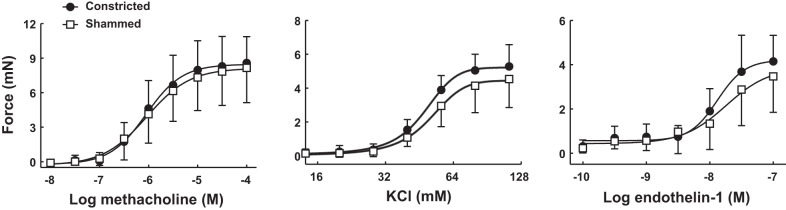
The ex vivo contractile capacity of the isolated tracheas after 6 wk of treatment is depicted in Fig. 6. Although the force generated by the tracheas increased significantly in response to increasing concentrations of methacholine, KCl, and endothelin-1, no differences were observed in the tracheas derived from mice subjected to repeated constrictions compared with the tracheas derived from mice subjected to shammed procedures.
Fig. 6.
Contractile capacity of airway smooth muscle after 6 wk of treatment with either a 30-min constriction (●) or the shammed procedure (□) every other day. Excised tracheas were mounted in an organ bath, and the isometric force generated in response to escalading concentrations of either methacholine (left), KCl (middle), or endothelin-1 (right) was measured. n = 10 mice per group.
Structural components of the airway wall and the lung.
The amount of ASM within the airway wall, the content of collagen throughout the lung, the number of goblet cells in the epithelium, and the thickness of the epithelium after 6 wk of treatment are depicted in Fig. 7. The amount of ASM, the content of collagens, and the thickness of the epithelium were not affected by repeated constrictions. However, the number of goblet cells significantly increased (P = 0.002) in mice subjected to repeated constrictions compared with mice subjected to shammed procedures.
Fig. 7.
Structural components of the airway wall and the lung after 6 wk of treatment with either the shammed procedure or a 30-min constriction every other day. A: representative lung sections derived from mice subjected to either shammed procedures (left) or repeated constrictions (right) stained with trichrome. B: average area of airway smooth muscle per square length of basement membrane (BM) is displayed for mice subjected to shammed procedures (open bar) and repeated constrictions (closed bar). C: average of collagen content throughout the lung in fraction of the total tissue area is displayed for mice subjected to shammed procedures (open bar) and repeated constrictions (closed bar). D: representative lung sections derived from mice subjected to either shammed procedures (left) or repeated constriction (right) stained with periodic acid shift. Arrowheads point toward goblet cells. E: average number of goblet cell per millimeter of basement membrane is displayed for mice subjected to shammed procedures (open bar) and repeated constrictions (closed bar). F: average thickness of the airway epithelium is displayed for mice subjected to shammed procedures (open bar) and repeated constrictions (closed bar). *Statistically significant difference in mice subjected to repeated constrictions vs. mice subjected to shammed procedures. n = 10 mice per group.
DISCUSSION
This study demonstrates that although repeated constrictions of the airways led to goblet cell hyperplasia and a mild macrophagic inflammation, these alterations were not sufficient to cause physiological alterations. This was shown by the lack of differences in Rrs, Crs, RN, G, and H at baseline and in response to incremental doses of nebulized methacholine in mice subjected to a constriction every other day over 6 wk compared with mice subjected to shammed procedures. This was further supported by the lack of differences in the ex vivo contractile capacity of the tracheas excised from these two groups of mice. The fact that the magnitude of the constriction elicited by aerosolized methacholine was stable throughout the 6 wk of treatment also supports the lack of progressive deterioration of lung function over time. Other typical features of asthma, such as increased amounts of ASM and collagen within the lung, were also not observed following repeated constrictions.
The kinetics of the induced constriction in our study was clearly characterized. Aerosolized methacholine at 7 mg/ml in our exposure chamber triggered a significant constriction that lasted for at least 30 min. The constriction was also monitored throughout the entire 6 wk of treatment. Together, these results confirm that a measurable airway constriction lasting at least 30 min took place every other day as intended.
The regimented pattern of constrictions that we have opted for may resemble some real-life situations in humans, such as elite endurance athletes with asthma who are subjected to repetitive airway constrictions because of the epithelial damage associated with their frequent bouts of intense exercise (3). One can still argue that the type of constriction inflicted by acute exposure to aerosolized methacholine does not realistically emulate the kinetics and the nature of the constrictions that occur naturally in asthma. However, the results of a study conducted on human volunteers with mild atopic asthma demonstrated that only three bouts of acute airway constriction elicited by inhaled methacholine were conducive to airway wall remodeling (14). We were thus surprised to notice very few signs of airway wall remodeling in mice, apart from goblet cell hyperplasia, after 21 bouts of methacholine-induced constriction.
Uncontestably, the strain imparted by constriction is an important mechanical signal that may drive airway remodeling (26, 28, 29). In fact, the ability to respond to mechanical strain is allegedly required for adaptation of the organ’s stress-bearing elements. In turn, this may be required to preserve the integrity and function of the organ in the dynamic environment in which it resides and operates. By extending this reasoning, some have proposed that the more frequent and pronounced constrictions that characterize asthma may promote the structural changes of the airway wall that are typical of this lung disorder. The remodeling induced by repeated constrictions reported by Grainge and coworkers (14) was thus not very surprising to some.
The putative role played by constrictions in airway wall remodeling is further substantiated by studies conducted with animals (6, 17, 24, 27). It is important to mention though that for both the human and the animal studies, the effect of constriction on airway remodeling has been mainly examined in the presence of allergic inflammation (in patients with atopic asthma or in mice and guinea pigs with experimental allergic asthma). This is in contrast to our study, in which the effect of repeated constrictions was tested in mice without asthma. One can thus postulate that a permissive interaction is required, in which the presence of allergic inflammation is needed for the constriction to elicit remodeling. However, the results of in vitro studies also imply that airway remodeling is induced by ASM contraction (19, 23, 27). This suggests that constriction alone (i.e., in the absence of airway inflammation) leads to airway remodeling. More recently, it was demonstrated that repeated in vivo constrictions elicited by nebulized methacholine increase the in vitro contractility of small airways in isolated lung slices of young, but not adult, mice without asthma (25). We were thus surprised to notice no sign of fibrosis, no enlargement of the ASM, no increase in the contractile capacity of ASM, and no airway hyperresponsiveness in mice subjected to repeated constrictions.
Notwithstanding the aforementioned discrepancies, our findings are consistent with the longstanding conviction that bronchoprovocation with methacholine is innocuous. A recent report actually demonstrated that patients who underwent repeated methacholine challenges over the course of a few years did not exhibit an accelerated decline in lung function over time (16). This report was reassuring, as methacholine testing is now common practice in clinical settings because of its usefulness in casting aside the diagnosis of asthma (11). The remodeling induced by three bouts of constrictions reported by Grainge and coworkers (14) was thus very surprising to some.
The conclusions drawn from Grainge and coworkers (14) and Janssen and coworkers (16) appear to conflict diametrically. We argue that the disagreement is merely semantic. The results of our study are perfectly in line with the findings (but not the interpretations) of each study, as repeated constrictions in our mice led to an increased number of goblet cells, as observed in Grainge and coworkers’ study (14), without impacting lung function, as observed in Janssen and coworkers’ study (16). Therefore, repeated constrictions may well increase the number of goblet cells and transiently affect the expression of genes that are reputed to be involved in airway wall remodeling, such as transforming growth factor (14). However, such acute constrictions do not seem sufficient to alter lung function (16).
The lack of airway hyperresponsiveness in the presence of goblet cell hyperplasia may also seem counterintuitive. Goblet cell hyperplasia is associated with mucus hypersecretion (2). In turn, excessive production of mucus is expected to increase the propensity of airway closure and thereby increase the degree of airway responsiveness (32). In fact, mucus obstruction accounted for ~50% of the methacholine-induced fall in specific airway conductance in mice (1). One is thus justified to anticipate a tight association between goblet cell hyperplasia and airway hyperresponsiveness. Our study was not design to address this question. Therefore, we do not have a definitive explanation for this lack of association. Speculatively, there may be different endotypes of goblet cell hyperplasia. That is to say that the etiologic factors leading to this final outcome may vary, and the molecular origin from which it stems may define its functional purpose. The caveat is that the readout used to quantify the number of goblet cells (plus staining with PAS) may not discriminate between the endotypes. Yet, different types of goblet cells may exist, some seemingly inoffensive and others supposedly detrimental for airway responsiveness. Alternatively, the increased number of goblet cells observed in constricted mice may be a sign of impaired secretion rather than hyperplasia of mucus-producing cells. Another possibility is that the increased number of macrophages that we observed may have been protective. These additional macrophages may have been recruited into the airways to clear the excess of mucus, which could have concomitantly preserved the normal functioning of the respiratory system. In support of this last conjecture, alterations of macrophages have been reported in experimental asthma (9, 21). Additionally, normal macrophages transferred into sensitized mice were shown to greatly attenuate airway hyperresponsiveness (8, 10). The dysfunction of macrophages induced by allergic inflammation may thus be a prerequisite that enables the excess of mucus because of goblet cell hyperplasia to cause airway hyperresponsiveness. Further studies that are specifically designed to address these questions are required.
Goblet cell hyperplasia and marcrophagic inflammation may have also arisen as a result of repetitive exposures to methacholine independently of airway constriction. Airway epithelial cells (12, 18) and macrophages (20) express muscarinic receptors and likely respond to inhaled methacholine. In fact, stimulation of human bronchial tissue and differentiated epithelial cells with the muscarinic agonist carbachol was shown to rapidly increase the number of goblet cells by acting through the M3 receptor (12). A direct pharmacologic effect of methacholine rather than an indirect effect mediated through airway constriction is thus very likely. In humans, however, the constriction seems to be required (14). More studies are warranted.
In conclusion, this study provides clear evidence that repeated constrictions of the airways in the absence of allergic inflammation do not lead to physiological alterations. Insofar as mice relate to humans, our results suggest that it is safe to expose volunteers without asthma to inhaled methacholine, even though some transient lung features that are reminiscent of asthma, such as goblet cell hyperplasia, are to be expected.
GRANTS
This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). S. Mailhot-Larouche was supported by a bursary from the Canadian Institutes of Health Research (CIHR). L. Deschênes was supported by a bursary from NSERC. M. Gazzola was supported by a bursary from the Respiratory Health Network of the Fonds de Recherche du Québec – Santé (FRQS). M. Morissette and Y. Bossé were supported by a research scholar from FRQS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.-L., B.S.B., M.C.M., and Y.B. conceived and designed research; S.M.-L., L.D., M.G., K.L., and C.H. performed experiments; S.M.-L., L.D., M.G., K.L., C.H., and Y.B. analyzed data; S.M.-L., L.D., M.G., K.L., C.H., B.S.B., M.C.M., and Y.B. interpreted results of experiments; S.M.-L., M.G., K.L., C.H., and Y.B. prepared figures; S.M.-L. and Y.B. drafted manuscript; S.M.-L., M.G., K.L., C.H., B.S.B., M.C.M., and Y.B. edited and revised manuscript; S.M.-L., L.D., M.G., K.L., C.H., B.S.B., M.C.M., and Y.B. approved final version of manuscript.
References
- 1.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol (1985) 102: 399–405, 2007. doi: 10.1152/japplphysiol.00630.2006. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101: 916–921, 1992. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol 122: 225–235, 2008. doi: 10.1016/j.jaci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bai A, Eidelman DH, Hogg JC, James AL, Lambert RK, Ludwig MS, Martin J, McDonald DM, Mitzner WA, Okazawa M, et. Proposed nomenclature for quantifying subdivisions of the bronchial wall. J Appl Physiol (1985) 77: 1011–1014, 1994. doi: 10.1152/jappl.1994.77.2.1011. [DOI] [PubMed] [Google Scholar]
- 5.Bates HT. The linear single-compartement model. In: Lung Mechanics: An Inverse Modeling Approach. New York: Cambridge University Press, 2009, p. 37–61. doi: 10.1017/CBO9780511627156.004. [DOI] [Google Scholar]
- 6.Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, Zaagsma J. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J 30: 653–661, 2007. doi: 10.1183/09031936.00004907. [DOI] [PubMed] [Google Scholar]
- 7.Campana L, Kenyon J, Zhalehdoust-Sani S, Tzeng YS, Sun Y, Albert M, Lutchen KR. Probing airway conditions governing ventilation defects in asthma via hyperpolarized MRI image functional modeling. J Appl Physiol (1985) 106: 1293–1300, 2009. doi: 10.1152/japplphysiol.91428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 31: 22–27, 2004. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 9.Careau E, Proulx LI, Pouliot P, Spahr A, Turmel V, Bissonnette EY. Antigen sensitization modulates alveolar macrophage functions in an asthma model. Am J Physiol Lung Cell Mol Physiol 290: L871–L879, 2006. doi: 10.1152/ajplung.00219.2005. [DOI] [PubMed] [Google Scholar]
- 10.Careau E, Turmel V, Lauzon-Joset JF, Bissonnette EY. Alveolar macrophages reduce airway hyperresponsiveness and modulate cytokine levels. Exp Lung Res 36: 255–261, 2010. doi: 10.3109/01902140903410757. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft DW. Direct challenge tests: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138, Suppl: 18S–24S, 2010. doi: 10.1378/chest.10-0088. [DOI] [PubMed] [Google Scholar]
- 12.Cortijo J, Mata M, Milara J, Donet E, Gavaldà A, Miralpeix M, Morcillo EJ. Aclidinium inhibits cholinergic and tobacco smoke-induced MUC5AC in human airways. Eur Respir J 37: 244–254, 2011. doi: 10.1183/09031936.00182009. [DOI] [PubMed] [Google Scholar]
- 13.Dean K, Niven R. Asthma phenotypes and endotypes: implications for personalised therapy. BioDrugs 31: 393–408, 2017. doi: 10.1007/s40259-017-0242-5. [DOI] [PubMed] [Google Scholar]
- 14.Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 364: 2006–2015, 2011. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 15.Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985) 72: 168–178, 1992. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 16.Janssen LJ, Gauvreau GM, Killian KJ, O’Byrne PM. The effects of repeated bronchoprovocation on FEV1 in subjects with asthma. Ann Am Thorac Soc 12: 1589–1591, 2015. doi: 10.1513/AnnalsATS.201506-325LE. [DOI] [PubMed] [Google Scholar]
- 17.Kistemaker LE, Bos ST, Mudde WM, Hylkema MN, Hiemstra PS, Wess J, Meurs H, Kerstjens HA, Gosens R. Muscarinic M3 receptors contribute to allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 50: 690–698, 2014. doi: 10.1165/rcmb.2013-0220OC. [DOI] [PubMed] [Google Scholar]
- 18.Kistemaker LE, Hiemstra PS, Bos IS, Bouwman S, van den Berge M, Hylkema MN, Meurs H, Kerstjens HA, Gosens R. Tiotropium attenuates IL-13-induced goblet cell metaplasia of human airway epithelial cells. Thorax 70: 668–676, 2015. doi: 10.1136/thoraxjnl-2014-205731. [DOI] [PubMed] [Google Scholar]
- 19.Kistemaker LEM, Oenema TA, Baarsma HA, Bos IST, Schmidt M, Facchinetti F, Civelli M, Villetti G, Gosens R. The PDE4 inhibitor CHF-6001 and LAMAs inhibit bronchoconstriction-induced remodeling in lung slices. Am J Physiol Lung Cell Mol Physiol 313: L507–L515, 2017. doi: 10.1152/ajplung.00069.2017. [DOI] [PubMed] [Google Scholar]
- 20.Koarai A, Traves SL, Fenwick PS, Brown SM, Chana KK, Russell RE, Nicholson AG, Barnes PJ, Donnelly LE. Expression of muscarinic receptors by human macrophages. Eur Respir J 39: 698–704, 2012. doi: 10.1183/09031936.00136710. [DOI] [PubMed] [Google Scholar]
- 21.Lauzon-Joset JF, Marsolais D, Langlois A, Bissonnette EY. Dysregulation of alveolar macrophages unleashes dendritic cell-mediated mechanisms of allergic airway inflammation. Mucosal Immunol 7: 155–164, 2014. doi: 10.1038/mi.2013.34. [DOI] [PubMed] [Google Scholar]
- 22.McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp: e50172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oenema TA, Maarsingh H, Smit M, Groothuis GM, Meurs H, Gosens R. Bronchoconstriction induces TGF-β release and airway remodelling in guinea pig lung slices. PLoS One 8: e65580, 2013. doi: 10.1371/journal.pone.0065580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohta S, Oda N, Yokoe T, Tanaka A, Yamamoto Y, Watanabe Y, Minoguchi K, Ohnishi T, Hirose T, Nagase H, Ohta K, Adachi M. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin Exp Allergy 40: 1266–1275, 2010. doi: 10.1111/j.1365-2222.2010.03478.x. [DOI] [PubMed] [Google Scholar]
- 25.Patel KR, Bai Y, Trieu KG, Barrios J, Ai X. Targeting acetylcholine receptor M3 prevents the progression of airway hyperreactivity in a mouse model of childhood asthma. FASEB J 31: 4335–4346, 2017. doi: 10.1096/fj.201700186R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci USA 98: 6180–6185, 2001. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, Knox AJ, Pang L, Sheppard D, Huang X, Jenkins G. Integrin αvβ5-mediated TGF-β activation by airway smooth muscle cells in asthma. J Immunol 187: 6094–6107, 2011. doi: 10.4049/jimmunol.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschumperlin DJ, Drazen JM. Mechanical stimuli to airway remodeling. Am J Respir Crit Care Med 164, Suppl 2: S90–S94, 2001. doi: 10.1164/ajrccm.164.supplement_2.2106060. [DOI] [PubMed] [Google Scholar]
- 29.Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol 282: L904–L911, 2002. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 30.Tzeng YS, Lutchen K, Albert M. The difference in ventilation heterogeneity between asthmatic and healthy subjects quantified using hyperpolarized 3He MRI. J Appl Physiol (1985) 106: 813–822, 2009. doi: 10.1152/japplphysiol.01133.2007. [DOI] [PubMed] [Google Scholar]
- 31.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 32.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol (1985) 96: 2019–2027, 2004. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]