Abstract
Poststroke pain syndrome (PSPS) is an often intractable disorder characterized by hemiparesis associated with unrelenting chronic pain. Although traditional analgesics have largely failed, integrative approaches targeting affective-cognitive spheres have started to show promise. Recently, we demonstrated that deep brain stimulation (DBS) of the ventral striatal area significantly improved the affective sphere of pain in patients with PSPS. In the present study, we examined whether electrophysiological correlates of pain anticipation were modulated by DBS that could serve as signatures of treatment effects. We recorded event-related fields (ERFs) of pain anticipation using magnetoencephalography (MEG) in 10 patients with PSPS preoperatively and postoperatively in DBS OFF and ON states. Simple visual cues evoked anticipation as patients awaited a painful (PS) or nonpainful stimulus (NPS) to the nonaffected or affected extremity. Preoperatively, ERFs showed no difference between PS and NPS anticipation to the affected extremity, possibly due to loss of salience in a network saturated by pain experience. DBS significantly modulated the early N1, consistent with improvements in affective networks involving restoration of salience and discrimination capacity. Additionally, DBS suppressed the posterior P2 (aberrant anticipatory anxiety) while enhancing the anterior N1 (cognitive and emotional regulation) in responders. DBS-induced changes in ERFs could potentially serve as signatures for clinical outcomes.
NEW & NOTEWORTHY We examined the electrophysiological correlates of pain affect in poststroke pain patients who underwent deep brain stimulation (DBS) targeting the ventral striatal area under a randomized, controlled trial. DBS significantly modulated early event-related components, particularly N1 and P2, measured with magnetoencephalography during a pain anticipatory task, compared with baseline and the DBS-OFF condition, pointing to possible mechanisms of action. DBS-induced changes in event-related fields could potentially serve as biomarkers for clinical outcomes.
Keywords: chronic pain, deep brain stimulation, event-related fields, magnetoencephalography, pain anticipation
INTRODUCTION
Neurostimulation therapies for chronic pain management, including deep brain stimulation (DBS), have largely focused on targeting the sensory sphere of pain, by either modulating pain signal transmission across ascending sensory pathways or augmenting the activity of intrinsic, inhibitory descending pathways. As such, current targets of DBS for patients with severe, refractory chronic pain typically include the sensory thalamic relay nuclei (Abreu et al. 2017) and the endorphin-releasing periventricular and periaqueductal areas (Adams 1976; Gerhart et al. 1981; Levy et al. 1987, 2010). Analgesia is the standard primary outcome for clinical trials using these approaches, with a successful trial outcome often defined as a reduction of ≥50% in pain magnitude as measured by the visual analog scale (VAS) in >50% of patients. However, despite significant advances, these interventions only partially alleviate pain or restore function, with patients often failing to experience long-term benefit (Kumar et al. 2008; Sears et al. 2011). Our scientific understanding of pain, based on the neuromatrix theory, suggests that the overall pain experience is determined by a complex integration of the sensory, affective and cognitive spheres of pain (Melzack 1999). In other words, pain is not a passive process determined solely by nociception, but rather an experience determined by a complex integration of sensory information along with an individual’s prior experience, cultural background, and emotional state. This renewed definition of pain has led to exploration of alternative DBS targets that play a role in regulating the affective component of pain (Boccard et al. 2017).
We recently investigated DBS of the ventral capsule/ventral striatum (VC/VS) region as a potential treatment option for patients with poststroke pain syndrome (PSPS) in a prospective, randomized, placebo-controlled trial (Lempka et al. 2017). PSPS is characterized by severe unrelenting anesthesia dolorosa on one hemibody and is known to be particularly refractory to medical and surgical treatment (Machado et al. 2007). The VC/VS region has a well-established role in the control of emotion and behavior (Modell et al. 1989), and DBS targeting this region is currently approved by the United States Food and Drug Administration (FDA) for the management of obsessive-compulsive disorder (Greenberg et al. 2010; Malone et al. 2009). Our study demonstrated significant improvements in outcome measures sensitive to pain affect, such as the Montgomery Åsberg Depression Rating Scale (MADRS), Beck Depression Index (BDI), and McGill Affective Pain Rating Index (Lempka et al. 2017). The findings corroborate the effects of VC/VS DBS in reducing suffering by directly modulating the affective components of pain.
In the present study we used magnetoencephalography (MEG) to assess the neural substrates underlying the effects of VC/VS DBS during pain anticipation in patients with PSPS. We used a heat-based stimulator to deliver a quantifiable, controllable, and repeatable pain stimulus. Event-related potentials or fields (ERF) offer an excellent tool to capture affective responses in chronic pain given their relevance to sensory, emotional, and cognitive processes (Campanella and Maurage 2016; Kappenman and Luck 2016). We have previously studied both normal controls (Gopalakrishnan et al. 2015; Machado et al. 2014) and patients with PSPS before surgical intervention (Gopalakrishnan et al. 2016a) with the same paradigm used in the present study. In normal controls, we found enhanced activation of the frontal associative and limbic areas during pain anticipation. Interestingly, we also found that the visual cortex was able to decode the contextual meaning of pain signaling cues independently, without the assistance of prefrontal areas. This phenomenon may represent a significant evolutionary leap that allows for more rapid processing of information associated with risk of injury and guides subsequent fight or flight behavior. In patients with PSPS, however, we found no difference when comparing pain anticipation to nonpainful stimulus anticipation on the affected extremity, suggesting a loss of cue salience (Gopalakrishnan et al. 2016a). In this study, we extended these previous results by using ERFs from MEG to investigate potential therapeutic mechanisms of action of VC/VS DBS targeting the affective component of pain.
MATERIALS AND METHODS
Subjects
The study was conducted on 10 patients (6 men, 4 women; mean age at implant: 52 ± 5 yr, disease duration 5 ± 3 yr) with unrelenting PSPS. One male patient dropped out of the study upon enrollment, and two female patients were excluded from the analysis because of cognitive/attentional limitations that precluded compliance with the MEG experimental procedures. The clinical characteristics and demographics of the patients with PSPS are summarized in Table 1. Additional details regarding the study design, recruitment, inclusion/exclusion criteria, and outcomes have been published previously (Lempka et al. 2017; Plow et al. 2013). All research activities were approved by the Cleveland Clinic Institutional Review Board with signed informed consent and were monitored under an Investigational Device Exemption (IDE) from the FDA.
Table 1.
Patients’ symptomology and clinical characteristics
| Patient ID | Age at Implant, yr/Sex | Location (Type) of Stroke | Time from Stroke to Baseline, yr | Pain Lateralization | Mean Pain Rating at Enrollment (1–10) | Sensory Deficit in Painful Zone |
|---|---|---|---|---|---|---|
| 01 | 50 (M) | Left thalamus (I) | 3 | Right | 7 | Severe |
| 02 | 49 (M) | Right Medulla (I) | 9 | Left | 6 | Severe |
| 03 | 48 (F) | Left basal ganglia (H) | 9 | Right | 9 | Severe |
| 05 | 53 (M) | Left thalamus (H) | 3 | Right | 7 | Severe |
| 06 | 56 (M) | Right thalamus (H) | 1 | Left | 10 | Moderate |
| 07 | 49 (M) | Left brain stem (H) | 1 | Right | 9 | Severe |
| 08 | 51 (M) | Left temporal stem (H) | 2 | Right | 10 | Severe |
| 09 | 43 (F) | Left thalamus (H) | 4 | Right | 9 | Severe |
| 10 | 59 (F) | Right middle cerebral artery (I) | 9 | Left | 10 | Severe |
| 11 | 60 (F) | Right basal ganglia (H) | 6 | Left | 8 | Severe |
Subject 01 dropped out of the study after enrollment, and subjects 03 and 10 were eliminated from data analysis due to lack of attention to experimental procedures. Subjects 02, 06, 08, 10, and 11 (bold type) were classified as responders on the basis of clinical outcome. M, male, F, Female, I, ischemic stroke; H, hemorrhagic stroke. Pain duration was the same as “Time from stroke to baseline” in all patients. Sensory deficits were defined as follows: mild, subjective mild hypoesthesia but able to distinguish sharp from dull; moderate, inability to consistently distinguish between sharp and dull; and severe, anesthesia dolorosa.
Clinical Trial Design
The clinical trial followed a prospective, double-blinded, randomized, sham-controlled, crossover design (Plow et al. 2013). DBS parameters were adjusted so that stimulation did not induce paresthesia or side effects. We also allowed 1 mo between the programming sessions and randomization to ON or OFF states to avoid potential unmasking of the treatment allocation. The unblinded investigators who performed DBS programming procedures were not involved in outcome assessment, which was performed by separate investigators blinded to DBS programming. Briefly, the study was conducted in five main phases, as follows:
Screening and enrollment: Enrollment was limited to 10 patients who had experienced chronic disabling PSPS for longer than 6 mo that was refractory to treatment with at least one antidepressant, one anti-seizure medication, and one opioid.
Baseline evaluation: The patients underwent several clinical evaluations, including extensive behavioral, psychological, psychiatric, and neuroimaging/electrophysiological assessments.
DBS surgery: Bilateral VC/VS DBS and nonrechargeable pulse generator implantation followed by 1 mo of recovery for resolution of any insertional (i.e., microlesional) effects.
Programming, randomization, sham-controlled double-blinded assessment and crossover: Detailed DBS stimulation parameters used during chronic stimulation for each patient can be found in our previous report (Lempka et al. 2017). Once the optimal settings were determined, DBS was switched OFF and remained off for 1 mo to reduce memory of stimulation effects. Patients were then randomized to either an active (optimal settings) or sham treatment group (amplitude set to 0 V with stimulator turned ON) for 3 months, followed by crossover for an additional 3 mo. Randomization was achieved using a computer-generated sequence created using block randomization with a size of 2. Patients and investigators collecting outcome measures were blinded to DBS status.
Unblinded follow-up: At the end of the blinded stimulation phase, all participants were reprogrammed in an attempt to continuously optimize outcomes in an unblinded fashion. At this point, there was no sham stimulation. Participants performed follow-up procedures at ~9, 12, 18, and 24 mo.
MEG Experimental Design
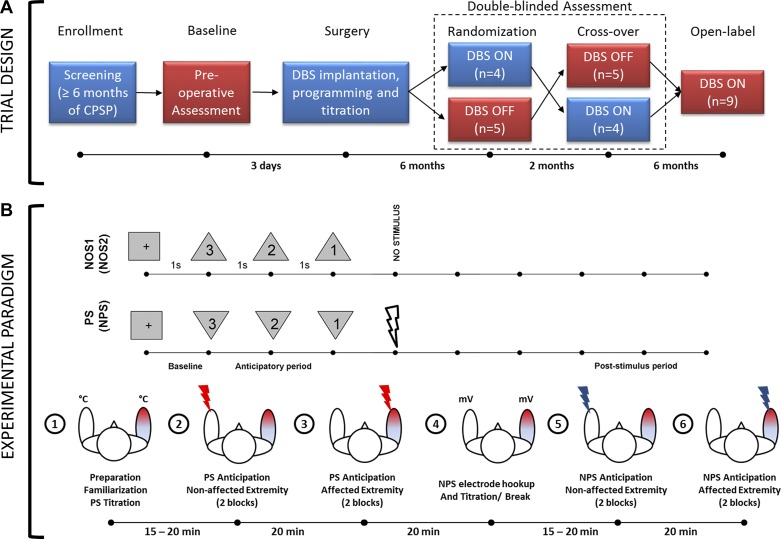
We collected MEG data during the preoperative baseline phase, during each arm of the blinded treatment phase (active or sham), and during the unblinded 12-mo follow-up phase. We were unable to analyze the data collected during blinded active treatment phase because of large stimulation artifacts present in the MEG data, which were caused by DBS having been delivered using a pseudo-monopolar configuration (i.e., pulse generator casing serving as the anode). At the 12-mo follow-up, we circumvented this issue by delivering DBS using a bipolar configuration that involved at least two separate contacts of the DBS lead. In this article, we present data from the preoperative baseline, blinded sham (OFF), and unblinded follow-up (ON) phases (Fig. 1A).
Fig. 1.
A: clinical trial design. Magnetoencephalography (MEG) data were collected during baseline, randomization, crossover, and open-label phase. MEG data reported in this paper are from stages highlighted in red. CPSP, central poststroke pain; DBS, deep brain stimulation; n = no. of patients. Time periods shown between phases are approximate. B: experimental paradigm. During the painful stimulus (PS) paradigm, visual countdown cues preceding a PS were presented as downward-pointing triangles, whereas those preceding a no-stimulus (NOS1) condition were presented as upward-pointing triangles. NOS2 was the same as NOS1, but corresponds to nonpainful stimulus (NPS) paradigm. Bottom row shows the flow of events along with approximate elapsed time during the data collection procedures.
We used a 306-channel Neuromag MEG system (Elekta, Stockholm, Sweden) to record neural activity while the subjects performed the experimental task inside a magnetically shielded room (Imedco, Hägendorf, Switzerland). We seated subjects in the upright position with the height of the chair adjusted such that the vertex of the scalp was in contact with the internal surface of the MEG apparatus. A screen was placed in the center of each subject’s field of view ~2 m away to display the visual conditioning cues relayed by the STIM2 stimulus presentation hardware (Compumedics Neuroscan, Charlotte, NC) in conjunction with a high-definition projector (Panasonic, Osaka, Japan). A technician affixed five head position indicator (HPI) coils to the subject’s scalp to continuously monitor patient movement throughout the study and recorded fiduciary points (nasion, right and left auricular), head surface points, and HPI locations using a Fastrack digitizer (Polhemus, Colchester, VT) to allow for postregistration to anatomical data using custom-built analysis tools.
Experimental Task
While seated in the MEG array, the subject was presented with visual cues in the form of a countdown, with the shape of the cue signifying whether the approaching stimulus (applied to the volar surface of the forearm) would be painful (PS), nonpainful (NPS), or absent [i.e., no stimulus (NOS)] (Fig. 1B). A downward-pointing triangle warned of a PS or an NPS (depending on the stimulation block), whereas an upward-pointing triangle signified that no stimulus would be delivered. Visual cues always accurately represented whether a PS, NPS, or NOS would follow. Different orientations of the same shape were used instead of different shapes to maintain the spatial properties of the visual cue (and their confounding effects on the visual evoked responses) across conditions. The countdown was 3 s long and marked by the numbers “3,” “2,” and “1,” presented in descending order at 1-s intervals within each geometric cue. Subjects were instructed to stay alert and focus on the cues and numbers presented during countdown to evoke anticipation. They were also asked to avoid blinking during the countdown and remain as still as possible inside the MEG array. The paradigm was explained verbally to the subjects before data collection.
PS paradigm.
The first paradigm consisted of 4 blocks of 60 pseudo-randomized trials, 60% of which signaled impending PS whereas the remaining 40% signaled NOS. PS was applied to the nonaffected extremity in the first two blocks and then switched to the affected extremity for the last two blocks (Fig. 1B). Data collection from consecutive blocks for each extremity helped minimize changes in head position inside the array. Each trial within a block was 8–9 s long, including a 1-s baseline, 3 s of prestimulus countdown or anticipatory period, and 4–5 s of poststimulus (recovery) period. Between blocks, subjects were asked (over an intercom) if they were ready to continue with the next block. At the conclusion of data collection for each extremity, the subjects were asked to rate the average pain experienced during PS on a numerical scale of 0–10 for each extremity. Subjects were monitored continuously with a video camera to ensure alertness and continued attention to visual cues. MEG recordings were acquired continuously during the four blocks.
A Contact Heat-Evoked Potential Stimulator (Medoc, Ramat-Yoshai, Israel) was used to elicit PS. Before data acquisition, the magnitude of the PS was established individually for each participant by applying ramp and hold stimuli (rise rate: 70°C/s, hold: 2 s, fall rate: 40°C/s) over a range of target temperatures from 40 to 50°C in 1°C increments, with a 1-s interstimulus gap. Patients were instructed to stop titration when they felt the temperature was painful enough to evoke anticipation. As a general rule, they were asked to report when their subjective perception of pain was ~8 on a numerical rating scale of 0–10 (0 being no pain and 10 being the worst pain imaginable). Subjects were specifically asked to not allow pain to reach severe or excruciating levels, and stimulation was never delivered above 50°C, with all procedures performed within the FDA-approved limits of the device.
NPS paradigm.
The paradigm above was repeated in a second set of four blocks, but the PS was replaced with an NPS consisting of electrical stimulation (Grass Instruments, Quincy, MA) delivered to the median nerve at the wrist. Given that sensory thresholds are known to be below motor thresholds, the intensity (voltage) of stimulation was gradually increased to the point where a thumb twitch was evident. On the basis of feedback from subjects, the voltage level was either maintained or lowered until subjects rated the pain associated with stimulation as 1–2 on a numerical scale of 0–10 while maintaining their attention. The visual cues for NPS were identical to those for PS in the prior paradigm (Fig. 1).
MEG Data Analysis
All MEG data were collected at either 1,000 (DC to 330 Hz) or 2,400 samples/s (DC to 800 Hz). Preprocessing was performed to ensure subsequent processing of artifact-free trials in sufficient numbers for each condition, with the following steps applied sequentially. Data were processed using the tSSS algorithm (Taulu and Simola 2006) to remove external and internal DBS artifacts. Data from 204 planar gradiometers were chosen for subsequent analysis because of their sensitivity to activity from local sources directly underneath rather than from distant environmental sources. The data were resampled to 400 samples/s or 200 Hz (using the FieldTrip function ft_resampledata with a built-in anti-aliasing low-pass finite impulse response filter), parsed and time-locked to the onset of the conditioned stimulus to distinguish the baseline and anticipatory periods (refer to Fig. 1) for each paradigm. Data trials were inspected visually using a semiautomated method (Oostenveld et al. 2011) to reject trials with SQUID jump artifacts by means of thresholding the z-transformed value of the raw data. The trials were subtracted for DC offset and bandpass filtered (1–70 Hz) using a fourth-order Butterworth zero-phase lag infinite impulse response filter in FieldTrip (Oostenveld et al. 2011) after mirror padding of the trials to avoid edge artifacts. Variance due to head movements away from mean head position were modeled and subtracted using a generalized linear model-based compensation algorithm (Stolk et al. 2013).
On average, 7%, 15%, and 14% of the trials were rejected from the baseline, OFF, and ON states, respectively, due to artifacts. MEG sensor data (data trials) were averaged to compute the ERFs for the PS and NPS conditions. Analyses were performed as described below.
Within-state analysis.
ERFs derived from anticipation of PS and NPS were compared to examine the spatiotemporal neural correlates of pain anticipation in each state (baseline, OFF, and ON state). Data from 204 orthogonal gradiometer pairs were combined (using function ft_combineplanar of the FieldTrip toolbox) in each subject before statistical analysis was performed.
Between-state analysis.
We compared the baseline with the DBS OFF state to isolate any effects related to DBS implantation and possible placebo effects given that patients were blinded to whether they were receiving stimulation or not. Thereafter, we compared DBS OFF vs. ON state to determine the effects of stimulation. Given that the baseline noise in each of the three states studied was different (due to presence/absence of DBS) and that combining planar gradients would amplify noise differentially between states, we performed our analysis on 204 orthogonal gradiometer pairs without combining the pairs.
Responder/nonresponder analysis.
In our previous report, we found that VC/VS DBS produced statistically significant effects on the affective sphere of chronic pain as indexed by the Montgomery Åsberg Depression Rating Scale (MADRS) (Lempka et al. 2017). Five of the patients studied showed a >50% reduction in their MADRS score during the ON stimulation phase. In this sample (Table 1), after exclusion of patients that were unable to stay attentive during the MEG task, 4 of the 7 patients studied met the “responder” criteria. To investigate the MEG correlates of those who presented with an improvement in the affective sphere of chronic pain, we repeated the within-state and between-state ERF analyses separately for responders (n = 4) and nonresponders (n = 3).
ERF Statistics
To identify sensor clusters that showed significant effects, the ERFs for each analysis listed above were subjected to a nonparametric permutation statistical analysis (Maris and Oostenveld 2007). Event-related neural activity between two conditions (e.g., PS vs. NPS, DBS-OFF vs DBS-ON, etc.) was clustered on the basis of adjacency in space and time (using a dependent samples two-sided t-test) to retain the cluster with largest sum of t values on either side. Data were then permuted (2,000×) to compute a Monte Carlo estimate of t statistics (Maris and Oostenveld 2007). For each randomization, the cluster with maximum sum was retained to compute the distribution histogram. Significance was established where test statistics of the observed data were larger than the permutation distribution (P ≥ 0.01). The minimum cluster size was set to three sensors, with no maximum limit. Evaluating the cluster-level statistics under the permutation distribution of the maximum cluster-level statistic controlled for multiple comparisons. Cluster topographies were plotted in intervals of 20 ms highlighting sensors that showed significant effect for at least 10 ms.
Each visual cue appeared for 250 ms and then disappeared for 750 ms (displaying blank screen with cross-hair) before the next countdown cue was presented (Fig. 1). This produced two sets of visual ERFs, one pertaining to appearance of the cue and another pertaining to disappearance of the cue. Depending on the latency, the effects were identified and labeled as P1, N1, P2, or N2. ERFs occurring at a latency <360 ms were attributed to cue appearance (early responses), whereas ERFs occurring between 360 and 500 ms were attributed to cue disappearance (late responses) (Gopalakrishnan et al. 2016b).
RESULTS
Pain Tolerance
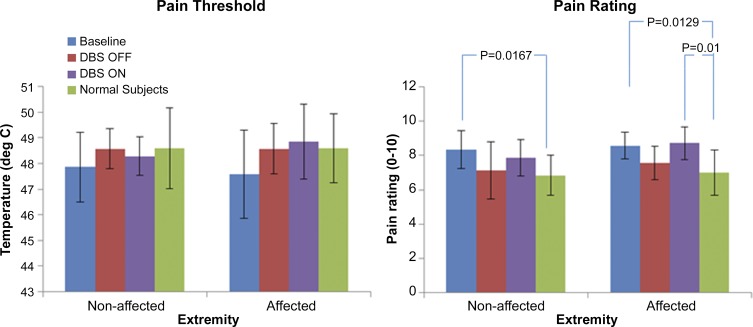
Measured average pain thresholds (°C) and pain rating (0–10) as measured at baseline, ON, and OFF states are presented in Fig. 2. Although patients with PSPS displayed lower pain thresholds and higher pain ratings than healthy controls irrespective of extremity, no significant differences were observed in either measure across the three states.
Fig. 2.
Pain tolerance results. Graph on the left shows the pain threshold (°C; means ± SD) for both the nonaffected and affected extremities as a function of treatment phase, whereas the plot on the right shows the overall pain rating (means ± SD) at the end of data collection for each extremity on a numerical scale of 0–10 for patients with poststroke pain syndrome (PSPS) at baseline, OFF, and ON states. Data for normal controls (n = 10, 7 men and 3 women; age 45 ± 15 yr) adapted from our previous publication (Machado et al. 2014) reflect the average overall pain experienced in the right and left extremities at the end of data collection. Statistical significance was based on a 2-sample t-test assuming equal variance. P values of significant differences are listed above bars.
Group Analysis
Within-state analysis (PS vs. NPS).
baseline state.
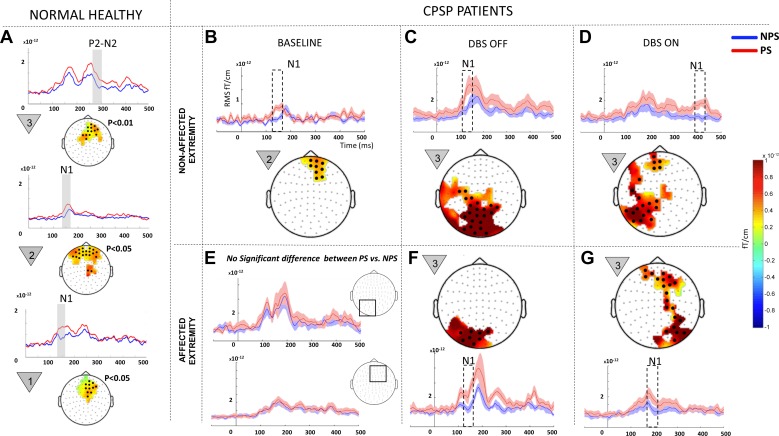
At baseline, pain anticipatory phenomenon (i.e., PS eliciting greater responses than NPS) was evident only on the nonaffected extremity (Fig. 3B, adapted from Gopalakrishnan et al. 2016a and shown for comparison). Significant clusters corresponding to the early N1 component ranging from 140 to 160 ms localized to medial prefrontal cortex were recorded. The affected extremity showed no significant difference in anticipating PS vs. NPS (Fig. 3E).
Fig. 3.
Within-state comparisons in all patients: painful (PS) vs. nonpainful stimulus (NPS) comparison within each state (baseline, OFF, and ON) for all patients (n = 7). A: data from normal healthy controls (n = 10, 7 men and 3 women; age 45 ± 15 yr) previously studied by our group using the same paradigm (Gopalakrishnan et al. 2016b). Baseline data (B and E) were modified from our earlier publication on the same patient population (Gopalakrishnan et al. 2016a). Because no significant difference was recorded between PS and NPS at baseline in the affected extremity (E), the mean event-related field (ERF) signal from 2 representative regions that displayed predominant effect in the OFF and ON states (shown in squares) are shown. The signal shows elevated ERF components during NPS (blue line) on the same scale compared with DBS OFF (F) and ON (G). Each panel shows a difference topography (PS − NPS) masked to show the sensors with significant effect (highlighted with black dots) and a line plot with the mean evoked responses from sensor cluster on the topography for each condition. Shading around the line plots show SE, whereas the dotted box highlights the temporal location of the significant effect. The countdown cue that evoked the significant effect is shown to the left of each topographic map. To better visualize slow changes during significant effect, the line plots were smoothed using a 10-ms moving average filter.
off state.
During the OFF state (Fig. 3, C and F), both the nonaffected (P = 0.0099) and affected extremities (P = 0.0019) displayed significant N1 clusters (100–160 ms) localized posteriorly, encompassing left parietal, visual, and visual association cortex, indicating that PS elicited greater responses than NPS in posterior cortical areas. Significant effects were confined to the first countdown cue.
on state.
During the ON state, ERFs recorded during stimulation of the nonaffected extremity (Fig. 3D) yielded significant clusters (P = 0.0019) corresponding to the late N1 component (360–420ms) localized both to left parietal and prefrontal cortices. The affected extremity (Fig. 3G) elicited significant (P = 0.0019) anticipatory phenomena during the early N1 component (160–200 ms) localized to both right parietal and prefrontal cortices, a finding that was only observed in the nonaffected extremity at baseline (Fig. 3B) and healthy controls (Fig. 3A). Significant effects were confined to the first countdown cue.
Between-state analysis (placebo, microlesional, and stimulation effects).
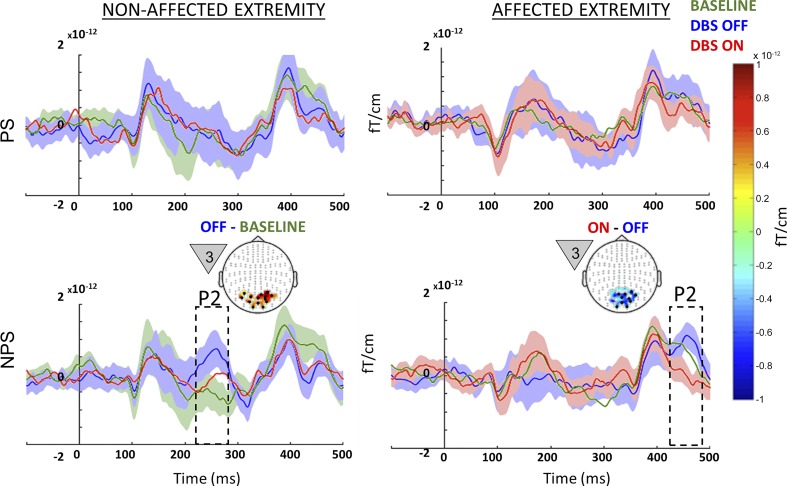
off vs. baseline (microlesional/placebo effects).
A significant microlesional/placebo effect (P = 0.01) was evident only for the nonaffected extremity when a NPS was anticipated (Fig. 4, bottom left). In the DBS OFF state, patients showed (in response to the first countdown cue) clusters of significantly increased activity corresponding to early P2 component (240–270 ms) localized to visual association cortex. Microlesional/placebo effects were not evident for the affected extremity.
Fig. 4.
Between-state comparison in all patients: event-related fields (ERF) recorded from the nonaffected (left) and affected extremities (right) for all patients studied (n = 7) during painful (PS; top) and nonpainful stimuli (NPS; bottom) from each of the 3 different states (baseline, green; OFF, blue; ON, red). Microlesional/placebo effect (OFF vs. baseline) recorded on the nonaffected extremity around 250 ms during NPS is highlighted in the bottom left panel, along with the topography highlighting posterior activation. The effect of deep brain stimulation (DBS) (ON vs. OFF) recorded during stimulation of the affected extremity around 450 ms during NPS is highlighted in the bottom right panel, along with the topography highlighting posterior suppression of activation. The countdown cue that evoked the significant effect is shown to the left of each topographic map. The topography shows the spatial location of the significant effect (difference between the 2 signals), whereas the line plots shows the mean evoked response from the channels that showed significant effect (black dots on topographic plot). Shading around line plots (of states that show significant difference) show the SE, whereas the dotted box highlights the temporal location of the significant effect. For comparison, the top plots show signal from the same sensors as the corresponding bottom plots. To better visualize slow changes during significant effect, the line plots were smoothed using a 10-ms moving average filter.
on vs. off (stimulation effects).
When analyzing the whole sample, we noted that a significant stimulation effect was only observed for the affected extremity when NPS was anticipated (Fig. 4, bottom right), with clusters of significantly decreased activity (P = 0.0019) localized to the visual association cortex pertaining to the late P2 (440–480 ms) component. An effect of DBS ON vs. OFF was not evident for the nonaffected extremity.
Responder/Nonresponder Analysis
Within-state analysis (PS vs. NPS).
baseline state.
No significant within-state differences were observed in responders and nonresponders at baseline.
off state.
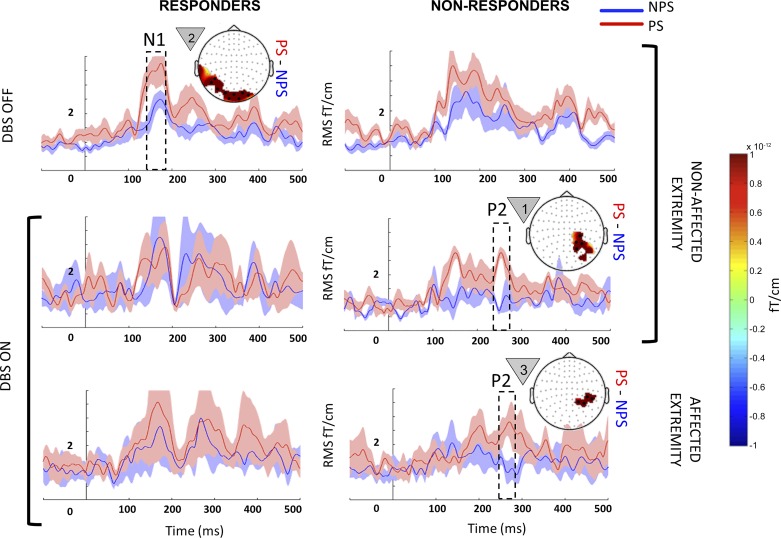
Responders showed significantly increased activity pertaining to early N1 (140–160 ms; P = 0.001) localized to posterior cortical areas when anticipating PS on their nonaffected extremity (Fig. 5, top left). Nonresponders did not show any significant difference between PS and NPS in the OFF state.
Fig. 5.
Within-state comparison in responders and nonresponders: event-related fields (ERF) from responders (left) and nonresponders (right) when they were anticipating painful (PS; red) and nonpainful stimuli (NPS; blue) on their nonaffected (top 2 rows) and affected extremities (bottom row) during the deep brain stimulation (DBS) OFF (top row) and ON states (bottom 2 rows). Each panel with a topography map shows the mean evoked responses from sensor clusters that showed significant responses (black dots), with shading around the line plot showing the SE. Each topographical map displays the average difference (PS − NPS) for the temporal window highlighted within the dotted box superimposed on the ERF. The countdown cue that evoked the significant effect is shown to the left of each topographic map. For comparison, in each row, the plot without a topography shows signal from sensors that showed significance in the opposite column. In the OFF state, PS vs. NPS did not show significant differences for the affected extremity in either responders or nonresponders, and hence the data are not shown. To better visualize slow changes during significant effect, the line plots were smoothed using a 10-ms moving average filter.
on state.
When anticipating PS, nonresponders showed clusters of increased activity corresponding to early P2 (260–280ms) that localized to portions of right superior parietal cortex in both affected and nonaffected (Fig. 5, bottom right and middle right) extremities (P = 0.0019). Responders did not show a significant difference between PS vs. NPS in the ON state.
Between-state analysis (microlesional/placebo and stimulation effects).
off vs. baseline (microlesional/placebo effects).
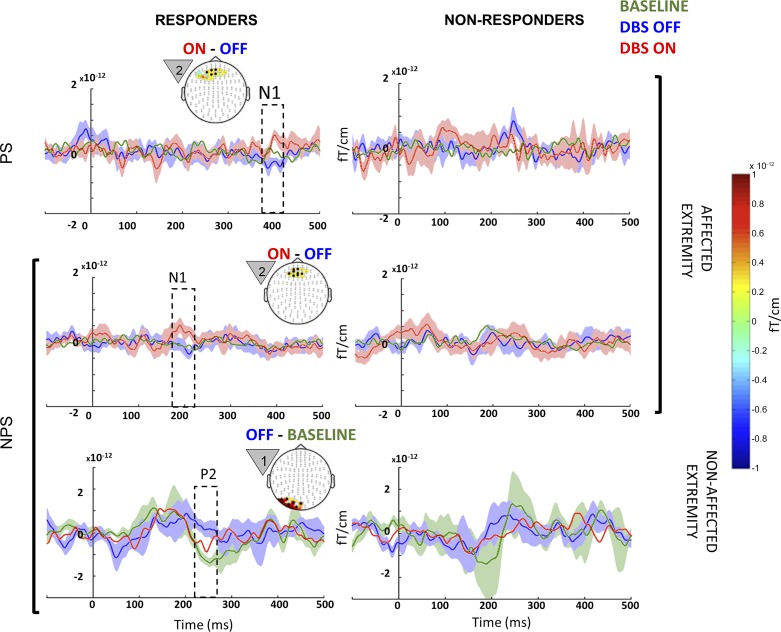
Similar to group analysis (Fig. 4), effects were confined to the nonaffected extremity (Fig. 6). A significant microlesional/placebo effect was evident only in responders with clusters of significantly increased activity (P = 0.002) when NPS was anticipated, which corresponded to early P2 (240–270 ms) localized to left lateral parieto-occipital cortex (Fig. 6, bottom left). Nonresponders did not show a significant effect.
Fig. 6.
Between-state comparison in responders and nonresponders: event-related fields (ERF) from therapeutic responders (left) and nonresponders (right) when they were anticipating painful (PS; top row) and NPS (bottom 2 rows) on their nonaffected (bottom row) and affected extremities (top 2 rows) from each of the 3 different states (baseline, green; OFF, blue; ON, red). Microlesional/placebo effect (OFF vs. baseline) recorded on the nonaffected extremity around 250 ms during NPS is highlighted in the bottom left panel, along with the topography highlighting posterior activation. Stimulation effects (DBS ON vs. OFF) recorded on the affected extremity around 400 ms during PS and at 180 ms during NPS are highlighted in the top left and middle left panels, respectively, along with the topography highlighting anterior activation. The countdown cue that evoked the significant effect is shown to the left of each topography map. The topography shows the spatial location of the significant effect (difference between the 2 signals), whereas the line plots shows the mean evoked response from the channels that showed significant effect (black circles). Shading around line plots (of states that show significant difference) shows the SE, whereas the dotted box highlights the temporal location of the significant effect. For comparison, plots on the right side show signal from same sensors as those of left, but in nonresponders. When anticipating PS on the nonaffected extremity, neither responders nor nonresponders showed significant differences between the 3 states, and hence the data are not shown. To better visualize slow changes during significant effect, the line plots were smoothed using a 10-ms moving average filter.
on vs. off (stimulation effects).
In agreement with the group analysis (Fig. 4), stimulation effects were confined to the affected extremity (P = 0.0019) and were evident only in responders when both PS and NPS were anticipated (Fig. 6, top left and middle left, respectively). When anticipating PS, responders showed an increased late N1 (380–400 ms), whereas when anticipating NPS, responders showed an increased early N1 component (180–200 ms). Both these stimulation effects were localized near midline, consistent with the topography of the medial portion of the anterior prefrontal cortex or anterior cingulate cortex. Nonresponders did not show significant stimulation effects.
DISCUSSION
In this study, we used event-related MEG data to characterize the effects of VC/VS DBS on pain anticipatory phenomena in PSPS patients with unilateral stroke (Paggiaro et al. 2016). To our knowledge, this is the first MEG study to report the effects of VC/VS DBS on neural processing of visual cues related to pain anticipation in this patient population. Our clinical hypothesis was that VC/VS DBS would modulate the affective sphere of pain, which was corroborated by our clinical data (Lempka et al. 2017). MEG was utilized to examine the neurophysiological correlates of our clinical findings during the ON and OFF phases of the randomized placebo-controlled trial. The key finding of the present study is that DBS restored pain anticipatory phenomena on the affected extremity, allowing the network to again distinguish cues associated with incoming PS from cues associated with incoming NPS. We speculate that patients with PSPS may have regained the salience to nociceptive stimulus that was possibly lost in the pretreatment phase (Gopalakrishnan et al. 2016a). Specifically, VC/VS DBS modulated the N1 and P2 component of the visually evoked anticipatory ERF.
Patients with PSPS demonstrate a reconfiguration of critical pain areas of the neuromatrix and altered pain anticipatory mechanisms compared with healthy controls (Gopalakrishnan et al. 2016a). These changes are greatest for brain areas related to their affected extremity, as evidenced by the lack of significant differences in the anticipatory ERFs derived from PS vs. NPS delivery at baseline. Furthermore, when anticipating NPS on their affected extremity, patients with PSPS showed enhanced activity in orbitofrontal cortex, a region implicated in fear response and anxiety (Gopalakrishnan et al. 2016a; Kringelbach and Rolls 2004). These observations led us to postulate that patients with PSPS experience a loss of salience to nociceptive stimuli due to repeated exposure to painful stimuli and subsequent “saturation” of pain neuromatrix areas.
DBS Restored Salience by Modulating N1 Component
ERF components have been widely studied in both the healthy controls and patient populations given their significant role in characterizing sensory as well as cognitive processing (Luck and Kappenman 2012). Though ERF components such as P1, N1, P2, and N2 have been implicated with attentional processing (Carretié 2014; Carretié et al. 2004), we have previously demonstrated that they also play a vital role in pain anticipatory phenomena (Gopalakrishnan et al. 2016b). Specifically, anteriorly localized N1 are indicative of heightened anticipatory attention including alertness, cognitive control, and emotion, whereas P2 is indicative of hypervigilance and anxiety.
At baseline, we found no significant pain anticipatory phenomena (PS vs. NPS) for stimuli delivered to the affected extremity (Fig. 3E). In the OFF state, the PS condition displayed significant N1 localized posteriorly (Fig. 3F and Fig. 5, top left). In the ON state, the PS condition exhibited significantly greater N1 that was localized both posteriorly (inferior parietal cortex) and anteriorly (dorsolateral prefrontal and prefrontal regions) (Fig. 3G). The spatial topography of the anterior spread was comparable to that observed in normal healthy subjects during the second and third countdown cues (Fig. 3A). Furthermore, it is evident that DBS widened the gap between PS and NPS at N1 by subduing NPS-related N1 rather than enhancing PS-related N1 (Fig. 3, E–G). Collectively, these results suggest that DBS restored the salience between the PS and NPS conditions that is present in normal individuals but lost in PSPS.
DBS Modulated Pain Affect by Suppressing Posterior P2
Our group analysis revealed that the effects of DBS were predominantly evident when NPS was anticipated on the affected extremity (Fig. 4). As mentioned earlier, patients with PSPS anticipate NPS on their affected extremity in a remarkably similar fashion to anticipating PS, and we hypothesized that DBS would modulate this maladaptive response and restore patients’ ability to differentially anticipate salient vs. nonsalient stimuli. In support of this hypothesis, we found that DBS suppressed the late P2 that was observed when NPS was anticipated at baseline and OFF states in the affected extremity (Fig. 4, bottom right). P2 was predominantly localized posteriorly in the visual association cortex, emphasizing its role in sensory motor and defense mechanisms to counter an emotionally salient event (Carretié et al. 2001). The P2 component of the ERF has also been attributed to a high degree of hypervigilance (Gopalakrishnan et al. 2016b) and affect processing (Carretié 2014). Presence of this posterior P2 in the OFF and baseline states once again clarifies the salient and emotional nature of NPS countdown cues in PSPS patients and the effects of DBS in suppressing these aberrant anticipatory phenomena. We note that P2 was preserved when PS was anticipated on the affected and nonaffected extremities in the DBS ON state (Fig. 4, top right, and Fig. 5, middle and bottom right), indicating that the effects of VC/VS stimulation were selective and did not obliterate physiological function.
Anterior N1 Represents Affective Benefit in Responders
When comparing ERFs between therapeutic responders and nonresponders to VC/VS DBS, we found that DBS effects were evident only among responders when they were anticipating PS or NPS on their affected extremity (Fig. 6). In the DBS ON state, significant early and late N1 components were evident during NPS and PS, respectively. Both effects were subtle, consistently localized to sensors above rostral anterior cingulate/medial prefrontal cortex (a critical structure of the affective component), and observed in response to the second countdown cue. This observation is in contrast to the first countdown cue responses seen in the group analysis (Fig. 3, C, D, F, and G, and Fig. 4), which plays a key role in cue classification, salience, and attention (Gopalakrishnan et al. 2016b). The subtle anterior N1 noted in this study could be indicative of affective benefit that responders derived from the VC/VS DBS treatment. The anterior N1 component is associated primarily with cognitive control and emotional regulation (Kalisch et al. 2005; MacDonald et al. 2000; Posner and Petersen 1990). Our earlier work in healthy individuals (Fig. 3A) as well as data from the nonaffected extremity of the patients with PSPS at preoperative baseline (Fig.3B) revealed an anterior N1 component during pain anticipation in response to the second countdown cue (Gopalakrishnan et al. 2016b). Taken together, these findings suggest that patients who responded to DBS may have regained the normal N1 component of neurophysiological processing of pain anticipation compared with nonresponders, and this is reflected in the second countdown cue. The enhancement of N1, together with suppression of P2, could represent the neurophysiological correlate of treatment effect in responders.
Limitations
Sample size.
For this study, our initial sample size was 10 participants, but our sample was reduced to 7 participants following patient exclusions. Although we recognize this small sample size as a limitation, this investigation included a very unique and homogenous population of patients who had participated in the only randomized controlled trial of DBS for chronic pain to date, targeting the VC/VS area. All patients had the same diagnosis and similar principal neurological presentation.
Trial design.
The MEG data collected during the blinded sham treatment phase (i.e., OFF state) were either before (3 participants; 4.7 ± 0.6 mo after implant) or after (4 participants; 9.3 ± 0.5 mo after implant) the blinded active treatment phase, and the unblinded follow-up phase (i.e., ON state) was 15.6 ± 1.3 mo after implant (Fig. 1A). This variance in the elapsed time between OFF and ON states was inherent in the nature of the trial design, but a potential limitation to consider.
DBS stimulation.
The neural effects of bipolar DBS used during the MEG data collection in the unblinded follow-up phase may have been slightly different from those of monopolar DBS that may have been used as part of patients’ treatment.
Nociceptive stimuli.
PS was evoked with a temperature stimulus, whereas NPS was evoked with an electrical stimulus. Although PS and NPS have different nociceptive mechanisms, we argue that the impact would be greater on neural correlates of pain processing (i.e., processing after the stimulus) rather than pain anticipation reported here.
Conditioning effect.
Another possible limitation was a potential conditioning effect that may have occurred because we tested the nonaffected extremity before the affected extremity in all subjects. However, we preferred this nonrandomized approach in an attempt to reduce pain-related carryover effects from stimulating the affected extremity first that could bias data interpretation for the nonaffected extremity.
Conclusions
To our knowledge, this is the first study to examine MEG neurophysiological correlates of pain anticipation in a cohort of patients with PSPS undergoing DBS targeting the affective sphere of chronic pain. Patients with PSPS displayed maladaptive anticipatory phenomena at baseline in response to cues that signaled NPS to their affected extremity. Our data indicate that DBS of the VC/VS restored the lost salience on the affected extremity through modulation of N1 component, allowing patients to differentially anticipate PS and NPS. Second, DBS suppressed the posterior P2 component in all patients during NPS anticipation while enhancing the anterior N1 component in responders. We postulate that early ERF such as N1 and P2 may serve as signatures (biomarkers) of aberrant pain expectations in the chronic pain state. Normalization of these signatures may serve as a guide to identify effectiveness in emerging neuromodulatory therapies.
GRANTS
This work was supported by National Institutes of Health New Innovator Award DO006469A.
DISCLOSURES
A.G.M has the following conflicts of interest: intellectual property distribution rights (ATI, Cardionomics, Enspire) and consulting agreement (St. Jude). K.B.B has the following conflicts of interest: ownership interest (Cardionomics) and consulting agreement/research grant (St. Jude). None of the above disclosures are pertinent to this research project or to this paper. Other authors have no disclosures to make.
AUTHOR CONTRIBUTIONS
R.G., R.C.B., D.A.M., J.T.G., and A.G.M. conceived and designed research; R.G. performed experiments; R.G. analyzed data; R.G., R.C.B., D.A.M., S.F.L., J.T.G., D.P.F., K.B.B., and A.G.M. interpreted results of experiments; R.G. prepared figures; R.G., S.F.L., and K.B.B. drafted manuscript; R.G., R.C.B., D.A.M., S.F.L., J.T.G., D.P.F., K.B.B., and A.G.M. edited and revised manuscript; R.G., R.C.B., D.A.M., S.F.L., J.T.G., D.P.F., K.B.B., and A.G.M. approved final version of manuscript.
REFERENCES
- Abreu V, Vaz R, Rebelo V, Rosas MJ, Chamadoira C, Gillies MJ, Aziz TZ, Pereira EAC. Thalamic deep brain stimulation for neuropathic pain: efficacy at three years’ follow-up. Neuromodulation 20: 504–513, 2017. doi: 10.1111/ner.12620. [DOI] [PubMed] [Google Scholar]
- Adams JE. Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 2: 161–166, 1976. doi: 10.1016/0304-3959(76)90111-1. [DOI] [PubMed] [Google Scholar]
- Boccard SG, Prangnell SJ, Pycroft L, Cheeran B, Moir L, Pereira EA, Fitzgerald JJ, Green AL, Aziz TZ. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurg 106: 625–637, 2017. doi: 10.1016/j.wneu.2017.06.173. [DOI] [PubMed] [Google Scholar]
- Campanella S, Maurage P. Cognitive event-related potentials in psychopathology: new experimental and clinical perspectives. Front Psychol 7: 1738, 2016. doi: 10.3389/fpsyg.2016.01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L. Exogenous (automatic) attention to emotional stimuli: a review. Cogn Affect Behav Neurosci 14: 1228–1258, 2014. doi: 10.3758/s13415-014-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Hum Brain Mapp 22: 290–299, 2004. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. Int J Psychophysiol 41: 75–85, 2001. [DOI] [PubMed] [Google Scholar]
- Gerhart KD, Yezierski RP, Wilcox TK, Grossman AE, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in ipsilateral or contralateral ventral posterior lateral (VPLc) thalamic nucleus. Brain Res 229: 514–519, 1981. doi: 10.1016/0006-8993(81)91014-3. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan R, Burgess RC, Lempka SF, Gale JT, Floden DP, Machado AG. Pain anticipatory phenomena in patients with central poststroke pain: a magnetoencephalography study. J Neurophysiol 116: 1387–1395, 2016a. doi: 10.1152/jn.00215.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan R, Burgess RC, Plow EB, Floden DP, Machado AG. A magnetoencephalography study of multi-modal processing of pain anticipation in primary sensory cortices. Neuroscience 304: 176–189, 2015. doi: 10.1016/j.neuroscience.2015.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan R, Burgess RC, Plow EB, Floden DP, Machado AG. Early event related fields during visually evoked pain anticipation. Clin Neurophysiol 127: 1855–1863, 2016b. doi: 10.1016/j.clinph.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA Jr, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 15: 64–79, 2010. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci 17: 874–883, 2005. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. Best practices for event-related potential research in clinical populations. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 110–115, 2016. doi: 10.1016/j.bpsc.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 72: 341–372, 2004. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 63: 762–770, 2008. doi: 10.1227/01.NEU.0000325731.46702.D9. [DOI] [PubMed] [Google Scholar]
- Lempka SF, Malone DA Jr, Hu B, Baker KB, Wyant A, Ozinga JG 4th, Plow EB, Pandya M, Kubu CS, Ford PJ, Machado AG. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol 81: 653–663, 2017. doi: 10.1002/ana.24927. [DOI] [PubMed] [Google Scholar]
- Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician 13: 157–165, 2010. [PubMed] [Google Scholar]
- Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery 21: 885–893, 1987. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES. The Oxford Handbook of Event-Related Potential Components, edited by Kappenman ES and Luck SJ. New York: Oxford University Press, 2012, p. xxii. [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838, 2000. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Machado A, Azmi H, Rezai AR. Motor cortex stimulation for refractory benign pain. Clin Neurosurg 54: 70–77, 2007. [PubMed] [Google Scholar]
- Machado AG, Gopalakrishnan R, Plow EB, Burgess RC, Mosher JC. A magnetoencephalography study of visual processing of pain anticipation. J Neurophysiol 112: 276–286, 2014. doi: 10.1152/jn.00193.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DA Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65: 267–275, 2009. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Melzack R. From the gate to the neuromatrix. Pain 82, Suppl 6: S121–S126, 1999. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Modell JG, Mountz JM, Curtis GC, Greden JF. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 1: 27–36, 1989. doi: 10.1176/jnp.1.1.27. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paggiaro A, Birbaumer N, Cavinato M, Turco C, Formaggio E, Del Felice A, Masiero S, Piccione F. Magnetoencephalography in stroke recovery and rehabilitation. Front Neurol 7: 35, 2016. doi: 10.3389/fneur.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Malone DA Jr, Machado A. Deep brain stimulation of the ventral striatum/anterior limb of the internal capsule in thalamic pain syndrome: study protocol for a pilot randomized controlled trial. Trials 14: 241, 2013. doi: 10.1186/1745-6215-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci 13: 25–42, 1990. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Sears NC, Machado AG, Nagel SJ, Deogaonkar M, Stanton-Hicks M, Rezai AR, Henderson JM. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation 14: 312–318, 2011. doi: 10.1111/j.1525-1403.2011.00372.x. [DOI] [PubMed] [Google Scholar]
- Stolk A, Todorovic A, Schoffelen JM, Oostenveld R. Online and offline tools for head movement compensation in MEG. Neuroimage 68: 39–48, 2013. doi: 10.1016/j.neuroimage.2012.11.047. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768, 2006. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]