Abstract
Individuals who have experienced a stroke often demonstrate inappropriate muscle activity phasing in the paretic leg during locomotion. Past research has demonstrated that inappropriate paretic phasing varies between behavioral contexts and is reduced during unilateral pedaling with the nonparetic leg inactive. We investigated whether individuals could voluntarily alter activity in a target muscle of the paretic limb in a consistent behavioral context and whether this voluntary change differed between bilateral and unilateral pedaling. During a fixed-speed motorized pedaling task, participants were asked to use visual feedback to deactivate the vastus medialis (VM) before a 90° target region of the pedaling cycle, as measured by surface electromyography and by change in fraction of total cycle amplitude in the target region. We based the start of this target region on the earliest observed deactivation for this muscle (found in fast pedaling), which allowed us to challenge both the paretic and nonparetic VM. During visual feedback, participants significantly reduced the fraction of activity found in the target region, with no significant difference in degree of reduction between paretic and nonparetic legs or between bilateral and unilateral pedaling. Surprisingly, in bilateral pedaling, individuals with greater clinical impairment demonstrated greater paretic limb response to feedback. Our results demonstrated that during this tightly constrained task, the paretic VM showed a surprisingly similar flexibility of muscle activity to the nonparetic VM. Our findings show that participants were able to use provided visual feedback to modulate the degree of an observed poststroke muscle-phasing impairment.
NEW & NOTEWORTHY This study demonstrates that by using visual feedback during a constrained task with minimized kinematic control requirements, participants with poststroke hemiplegia can voluntarily change muscle activity phase in the vastus medialis. Surprisingly, we did not observe a significant difference in ability to alter phasing between paretic and nonparetic legs or between bilateral and unilateral pedaling. In this visual feedback task, participants appear to modify muscle activity well in both the paretic and nonparetic legs.
Keywords: EMG, locomotor control, stroke
INTRODUCTION
Stroke affects nearly 800,000 people each year in the United States (Mozaffarian et al. 2016), and over half of survivors experience lasting locomotor impairment (Jørgensen et al. 1995; Wade et al. 1987). Impaired muscle activity phasing has long been noted as an important contributor to locomotor impairment in walking following unilateral stroke (Brunnström 1970; Knutsson and Richards 1979; Olney and Richards 1996; Yelnik et al. 1999) and manifests as muscles being active or inactive during inappropriate phases of the locomotor cycle (as measured by surface electromyography, or EMG). Inappropriate phasing during locomotion results in muscle forces being generated at points in the locomotor cycle that either oppose progression or reduce output force magnitude when needed to progress (Kautz and Brown 1998). For example, inappropriately prolonged muscle activity in the vastus medialis (VM) contributes to a stiff-knee pattern during the swing phase of gait (Olney and Richards 1996) and causes resistive torque during the upstroke of pedaling (Kautz and Brown 1998). The magnitude of phasing impairment also has been shown to correlate with the synergy subscore of the Fugl-Meyer assessment, a functional measure of motor recovery (Fugl-Meyer et al. 1975; Kautz and Patten 2005).
As an experimental approach to investigate locomotor control, pedaling tasks that provide motorized crank rotation at fixed speeds are a consistent, mechanically constrained task for the study of inappropriate muscle phasing in individuals with impaired locomotor movement patterns (Alibiglou and Brown 2011; Jain et al. 2013; Kautz and Patten 2005; Kautz et al. 2006; Liang and Brown 2013; Rogers, Brown and Gruben 2004; Rogers et al. 2011; Schindler-Ivens et al. 2008; Van der Loos et al. 2010). When the hip is securely fixed to a seat, ankle angle secured by a boot, and pedals driven at a constant speed, each limb follows a consistent kinematic pattern. Forces exerted against the pedal in either leg do not impact the trajectory of the other limb due to the servomotor-driven crank. This isolation allows the study of neural interaction between the limbs without interlimb mechanical compensation, enabling mechanically comparable one-legged or two-legged pedaling (Kautz and Patten 2005; Rogers et al. 2011; “human motor” Ting et al. 1998). Motorized pedaling also allows participants with a wide range of impairment to be studied during a comparable mechanical task.
Several past studies in pedaling have demonstrated that following stroke, individuals exhibit changes in muscle activity phasing when compared between different behavioral contexts. For example, changing the relative angle between the paretic and nonparetic limbs during bilateral motorized pedaling (e.g., one leg leading the other by 90° rather than 180°) significantly changes the relative phase of muscles in the paretic limb, despite an unchanged mechanical task for that limb (Alibiglou and Brown 2011). In another example, during motorized unilateral pedaling, individuals alter the relative muscle activity phasing in the paretic limb, again with no change in its mechanical task (Kautz and Patten 2005; Rogers et al. 2011). These and other studies demonstrate that although muscle activity phasing is significantly impaired during many tasks, individuals can alter muscle activity phasing in response to varying behavioral contexts.
However, it is unclear from prior work whether individuals can generate voluntary flexibility in muscle activity within a single behavioral context or whether neural impairment poststroke may reduce the range of muscle activity available within a particular behavioral context. Any voluntary flexibility accessible to poststroke participants may be affected by recruitment of alternate control pathways to supplement the impaired corticospinal tract and resulting changes to the neural interaction of the paretic and nonparetic legs. The question of voluntary flexibility of muscle activity control is critical to understanding the capability of the poststroke nervous system to adapt new motor control strategies during the motor relearning process.
Stroke that affects the motor cortex or corticospinal tract can impair the control of locomotion. The impaired coordination of muscle activity poststroke has been suggested to stem from compensatory reliance on alternate (often ipsilateral) descending pathways such as the reticulospinal and bulbospinal tracts (Clark et al. 2010; Dewald et al. 2001) or from increased inappropriate interhemispheric inhibition of the lesioned cortex (Murase et al. 2004). These proposed mechanisms for impairment may all occur, but individuals may be able to modulate control of these mechanisms by recruiting other available resources. For this experiment, visuomotor pathways could provide an alternative control method, supplementing tactile and proprioceptive feedback, which are often impaired poststroke (Connell et al. 2008; Tyson et al. 2007). Some prior research suggests that, given appropriate feedback, individuals poststroke may be able to demonstrate voluntary flexibility in muscle activity within a task. Prior work on obstacle avoidance following stroke (Den Otter et al. 2005) and other gait visual feedback experiments (Cho et al. 2007; Ferrante et al. 2011; see for review Teasell et al. 2003) suggest that despite these impairments, the alternate pathways available poststroke can provide sufficient control to allow modulation of behavior with visual feedback, although with less precision than nonimpaired participants (Den Otter et al. 2005). Additionally, studies of split-belt treadmill adaptation following stroke suggest that adaptation mediated by proprioceptive feedback may primarily rely on subcortical pathways that link the cerebellum and brain stem/spinal cord (Reisman et al. 2007). These pathways are often relatively intact after stroke affecting the motor cortex or corticospinal tract, and these findings may suggest that we should expect some persistence of muscle activity phase changes after visual feedback, similar to those observed after split-belt adaptation.
To probe the degree of voluntary flexibility available in the paretic limb poststroke, the current study was designed to test hypotheses utilizing a visuomotor feedback task during motorized pedaling. We proposed two primary hypotheses. First, we hypothesized that the paretic limb would demonstrate significant reduction in targeted muscle activity following visual feedback during bilateral pedaling. Second, as a result of reduced inappropriate interference from control of the nonparetic leg, we hypothesized that in unilateral pedaling, the paretic limb would demonstrate a greater degree of reduction in the target deactivation region than that observed during bilateral pedaling. Finally, we expected to observe that as a result of relatively intact spinocerebellar interactions, the paretic limb would demonstrate a significant persistent change in targeted muscle activity following removal of feedback.
To test these hypotheses, participants were asked to alter activity of the vastus medialis (VM), a uniarticular knee extensor, as measured by surface EMG. We focused on the VM because it demonstrates significantly prolonged activation poststroke during typical bilateral pedaling and during walking but also has demonstrated a wide range of potential phasing during varying behavioral tasks (Alibiglou and Brown 2011; Rogers et al. 2011; Schindler-Ivens et al. 2008). Participants were asked to reduce muscle activity in the VM during a target region from 105° to 195° while maintaining a consistent peak EMG amplitude. This task required modulation of muscle activity in the target limb (whether paretic or nonparetic) and allowed participants to vary muscle activity duration during each cycle while maintaining effort. Elements of this work have been presented previously in posters at Society for Neuroscience annual meetings in 2014 and 2015.
MATERIALS AND METHODS
Participants
We recruited 15 participants who had experienced a unilateral stroke, all more than 5 mo poststroke at the time of participation (Table 1). Participants had lower limb hemiparesis following stroke but demonstrated the ability to walk at least 10 m (with or without a walking aid) and were able to sustain mild physical activity such as pedaling a stationary bicycle for up to 1 h, broken up by regular rests. Individuals were excluded from participation for safety reasons if they demonstrated uncontrolled hypertension (>180 mmHg resting systolic blood pressure) or cardiac arrhythmia or had other serious medical conditions (such as severe cardiac disease or poorly controlled diabetes) that would prevent them from safe participation. Exclusion criteria also included lower limb orthopedic conditions (prior joint replacement, limited range of motion) and difficulty generating detectable EMG muscle activity in the VM during pedaling, which would limit participant ability to perform the task. The lower limb component of the Fugl-Meyer (FM) assessment (Fugl-Meyer et al. 1975) was used as an estimate of poststroke motor recovery and was performed by a trained physical therapist blinded to experimental procedures before participation. As in past studies (Clark et al. 2010; Kautz and Brown 1998; Kautz and Patten 2005), we focused on the Fugl-Meyer synergy subscore (referred to as FMS), which estimates the ability to independently activate joints and muscles. All protocols were submitted to and approved by the University of Alabama at Birmingham Institutional Review Board, and participants provided written informed consent before both evaluation and participation in experimental protocols.
Table 1.
Participant information
| Participant | Starting Condition | Age, yr | Time Poststroke, mo | Sex | Paretic Side | 10-m Walk Test, m/s | LE FM | LE FMS |
|---|---|---|---|---|---|---|---|---|
| A | Bilateral | 62 | 286 | F | L | 0.24 | 16 | 14 |
| B | Bilateral | 61 | 55 | F | R | 0.47 | 20 | 15 |
| C | Bilateral | 69 | 11 | F | R | 0.94 | 18 | 14 |
| D | Unilateral | 68 | 147 | M | R | 0.48 | 8 | 4 |
| E | Unilateral | 64 | 20 | F | L | 0.63 | 32 | 21 |
| F | Unilateral | 24 | 34 | F | L | 0.90 | 19 | 12 |
| G | Unilateral | 36 | 34 | F | L | 0.15 | 17 | 10 |
| H | Unilateral | 57 | 48 | M | L | 0.32 | 22 | 14 |
| I | Bilateral | 44 | 13 | M | L | 0.69 | 11 | 5 |
| J | Bilateral | 49 | 5 | M | L | 0.41 | 12 | 7 |
| K | Unilateral | 53 | 14 | M | R | 0.89 | 26 | 21 |
| L | Bilateral | 65 | 63 | F | L | 0.86 | 29 | 21 |
| M | Bilateral | 48 | 13 | F | R | 0.92 | 18 | 10 |
| Mean | 54 ± 13 | 57 ± 75 | 0.61 ± 0.27 | 19 ± 7 | 13 ± 6 |
Data are individual participant information, with means ± SD for all participants whose data were analyzed [n = 13 total; starting condition: 7 bilateral, 6 unilateral; sex: 8 female (F), 5 male (M); paretic side: 8 left (L), 5 right (R)]. Data for participants D and J are partly excluded from analysis: as described in materials and methods, Data Processing, participant J is excluded from muscle activity phase estimates, and participant D is excluded from all feedback estimates. LE FM, lower extremity component of Fugl-Meyer assessment (maximum score = 34); LE FMS, synergy subscore of LE FM (maximum score = 22).
Apparatus and Equipment
Pedaling was performed on a custom-designed reclining motorized cycle ergometer, first described in Schindler-Ivens et al. (2004), with an added EMG visual feedback system (Fig. 1). Individuals pedaled while resting at an incline of 30° above horizontal, at a constant 40 rpm in all pedaling trials. The angular velocity of the crank and pedals was kept constant using a servomotor (12:1 gearing, 3.7 horsepower; model MT506B1-S1C1; Kollmorgen, Radford, VA), which was controlled via a custom LabVIEW software program (National Instruments, Austin TX). The control program used feedback from a 1,024-increment optical encoder geared to the central crank (model EX116-1024-2; BEI Sensors, Thousand Oaks, CA), which determined angular position to ±0.3° precision. The participant’s ankle position was fixed by using ankle constraint boots bolted to the pedals (Donjoy Walkabout Walker, model 11-0461; DJO Global, Vista, CA). Because the position of the crank was dictated by the motor position, the limbs were mechanically isolated, preventing the pedal forces exerted by one leg from influencing the crank motion and reaction forces experienced by the opposite leg. In addition, unilateral pedaling was performed with the non-target foot resting on a platform adjacent to the device, at the same position as that occurring at 270° during pedaling (Fig. 1). If participants were unable to consistently maintain this position for the non-target foot, the experimenter constrained its position manually.
Fig. 1.
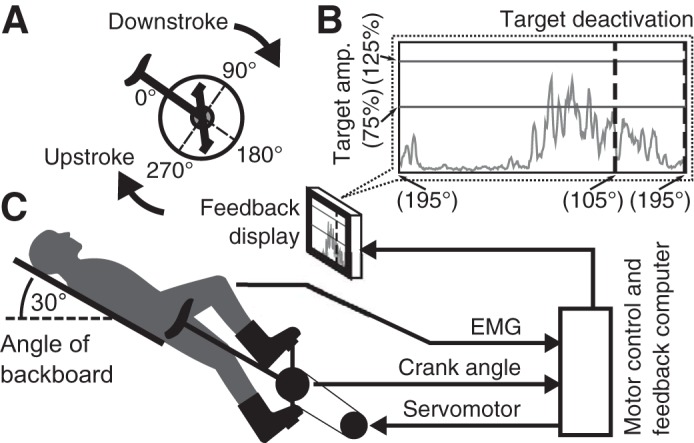
Experimental apparatus and conceptual diagram. A: motorized ergometer crank angle reference. All angles are relative to the seat tube, which is in line with the center of the crank. B: visual electromyography (EMG) feedback display of ongoing EMG relative to crank angle. The displayed EMG signal updates as participants progress through the pedaling cycle, starting over at 195°. Target deactivation region follows the first vertical dashed line, which corresponds to a crank angle of 105°. Target amplitude region is reported as a percentage of the 25% maximum voluntary contraction peak cycle amplitude target. C: experimental setup, including bicycle ergometer and servomotor. amp., Amplitude.
EMG activity was recorded in the target muscle, VM, of either the paretic or nonparetic leg, after standard skin preparation (remove hair, abrade, and clean with alcohol). EMG was also recorded from vastus lateralis (VL), rectus femoris (RF), and biceps femoris (BF). EMG recordings for RF and BF were poor (low amplitude or inconsistent activation were common), and no further analysis of EMG from RF or BF is presented in this report. Signals were collected via a Trigno Wireless EMG System (4 silver contacts, 5 × 1 mm; Delsys, Natick, MA) at 1,000-Hz, 900× amplification, which provided analog output at a fixed 48-ms delay. This delay was included as a correction factor in the data analysis. All EMG was whitened with a fourth-order linear predictive filter (comparable to Bonato et al. 1998) and filtered with a 10-Hz high-pass filter and 60-Hz bandstop filter (59–61 Hz) to minimize noise.
Protocol
Experimental conditions.
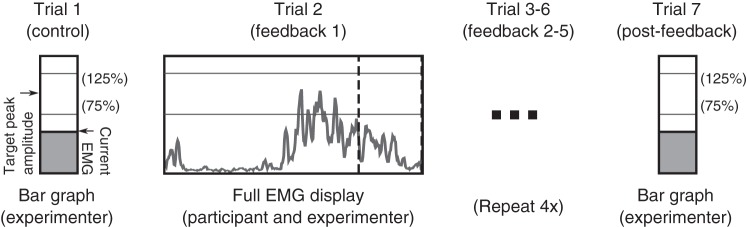
Each participant performed a stationary and moving maximum voluntary contraction (MVC) to calibrate EMG and effort, followed by several 3-min pedaling periods, referred to as epochs: an acclimation epoch to familiarize them with pedaling, one baseline epoch, five visual feedback epochs, and one postfeedback epoch (Fig. 2). There was a brief rest between epochs, typically less than 5 min, which ended when participants reported they were ready to continue. This protocol was performed during each of four distinct pedaling conditions: 1) bilateral pedaling with feedback about the nonparetic limb (BNP), 2) bilateral pedaling with feedback about the paretic limb (BP), 3) unilateral nonparetic pedaling with feedback on same (UNP), and 4) unilateral paretic pedaling with feedback on same (UP). These conditions were performed on 4 different days to avoid carryover of effects. Each participant was randomized into one of two groups for condition order: BNP/BP/UNP/UP or UNP/UP/BNP/BP. In this way participants consistently first received feedback about the nonparetic limb but did not all start with the bilateral condition.
Fig. 2.
Experiment schematic. Electromyogram (EMG) is smoothed across 20 ms and displayed to experimenter or experimenter and participant, depending on trial. The target peak amplitude is 25% comparably smoothed maximum voluntary contraction; the target deactivation for visual feedback is 105°–195° [value not displayed].
Maximum voluntary contraction.
Participants were asked to generate an MVC with the target leg, in both stationary and moving conditions, following 1–2 min of acclimation to moving in the device, at which time VM EMG was recorded. Shoulder restraints were provided during all MVC testing to allow maximum force during limb extension. For stationary pedaling, the target limb was oriented at a crank angle of 90° (halfway through downstroke). Participants were asked to start with a light push against the pedal and then ramp up to pushing as hard as they were able for 2–3 s, with the final push accompanied by verbal encouragement (“push Push PUSH,” typically with 5 repetitions of “push”). To obtain a more behaviorally relevant EMG MVC measure, we also asked participants to perform a moving MVC during bilateral pedaling at 40 rpm. Participants were asked to start by pedaling comfortably and then ramp up to pushing “as hard as you can, like you were going up a very steep hill” for 3–5 cycles, with verbal encouragement of a single extended “Push!” per downstroke. This moving MVC was used to calculate the target EMG amplitude level used to control effort across epochs for the rest of the experiment, with participants asked to generate a peak EMG equivalent to 25% of MVC during each pedaling cycle. EMG for the MVC and EMG for the visual feedback were both smoothed over a 20-ms window, to ensure compatible target amplitude.
Baseline epoch.
After a short rest following MVC, participants were then asked to pedal comfortably for a 3-min acclimation epoch, in which the experimenter provided verbal feedback to ensure that the participants were generating some EMG activity in the VM, but not performing exhausting effort. Following this acclimation period, a 3-min baseline pedaling epoch was performed. Using the target amplitude acquired from the MVC, participants were provided with regular verbal feedback to maintain peak amplitude near 25% of the peak EMG from the moving MVC condition. During baseline epochs, the EMG signal was converted to a simple bar graph of amplitude relative to target and presented only to the experimenter, with an indicated region of between 75% and 125% of the target amplitude. No feedback was provided on timing of muscle activity to experimenter or participant during the baseline epoch.
Visual EMG feedback epochs.
During each of five visual feedback epochs, the crank angle and EMG signals were used to generate visual feedback to the participant through a LabVIEW program (Fig. 1). For all feedback, EMG was rectified and smoothed over the prior 20 ms, to reduce visual noise while maintaining reasonable signal delay. During feedback epochs, participants were shown a live-updating plot of their EMG activity relative to the current crank position and asked to accomplish two concurrent tasks: first, they were presented with a large vertical line and asked to “turn the muscle off before reaching the vertical line” and to keep it inactive during the following 90° (the end of the display). Second, participants were also provided with two horizontal lines marking the target amplitude range (75% to 125% of target) and asked to attempt to achieve a peak EMG amplitude during each cycle that was between the two lines. Participants were asked to report what they were observing intermittently during the first visual feedback condition to assess visual comprehension. Unknown to the participant, the target deactivation line was set to correspond to 105° past top dead center. This crank angle approximates the earliest VM deactivation found in the literature during typical bilateral, self-driven pedaling for nonimpaired individuals, after correcting for differing angle references, from work by Neptune et al. (1997). It occurred during high-speed pedaling (120 rpm) and was 30° earlier than the deactivation angle estimated for 45 rpm pedaling. This advanced deactivation target allowed us to present a significant but still behaviorally anchored challenge to both the paretic and nonparetic limbs.
Postfeedback epoch.
Following the fifth feedback epoch, participants were asked to “pedal comfortably” for one additional 3-min epoch, without visual feedback but with verbal feedback on amplitude similar to that provided during the baseline epoch.
Data Processing
For each epoch of data collection, the initial data consisted of 110–118 pedaling cycles of EMG and crank angle. The minimum number of cycles in any epoch with clear delineation between activity and noise was determined by visual inspection to be 74 cycles. The peak amplitude of each cycle was determined from data smoothed over 20 ms to match visual feedback, and the 74 cycles with the least difference in peak amplitude from the target amplitude were then automatically selected from all epochs. The first 20 cycles of matched-amplitude pedaling for all epochs were then discarded, leaving 54 samples per epoch. This was done to reduce the impact of rapid adaptation effects, because participants began pedaling after a variable-length rest and because they calibrated effort in response to verbal or visual feedback. We originally recruited 15 participants but excluded data in full from 2 participants and in part from 2 additional participants. Two participants were excluded in full because they did not generate appreciable activity during the downstroke of pedaling. One participant (D) was excluded from feedback epoch analysis because of communication challenges, which prevented experimenters from adequately assessing task comprehension or visual impairment during visual feedback epochs. One participant (J) was excluded from muscle activity phase analysis because of their specific pattern of EMG activity, described below, which was not suitable for the method used to estimate muscle activity phase.
To calculate the experimental variables, we performed two primary types of analysis on the EMG data collected during appropriate-amplitude cycles. First, to compare EMG during baseline epochs between conditions and to compare EMG during postfeedback epochs with the baseline epochs, we estimated relative phase of VM muscle activity for each cycle by calculating the overall relative muscle activity phase delay or advancement compared with the bilateral nonparetic baseline for that participant. The phase measure was calculated by cross-correlating each individual cycle with an averaged reference cycle (the average of normalized cycles from the baseline of the reference condition, BNP) after rectifying and averaging across single degree bins. This is similar to a cross-correlation method used by Alibiglou and Brown (2011) on averaged data. The peak correlation between the two was taken as the relative delay or advancement of muscle activity phase for the cycle (Fig. 2). The phase estimation method allowed us to avoid discarding behavioral variation from cycle to cycle, as with average-based methods used in prior research (Alibiglou and Brown 2011; Rogers et al. 2011). This method does not, however, effectively process EMG with multiple well-separated activity bursts of similar amplitude at different phases within a single pedaling cycle, and for this reason data from one participant (J) were excluded in full from the phase analysis portion of the results.
Second, to measure the amplitude of muscle activity that occurred after the targeted deactivation point in the pedaling cycle, we calculated the fraction of total activation in each cycle that occurred during the 90° window following the target, in which participants were asked to keep VM inactive. The degree of success in this deactivation task was calculated from the mean change in amplitude between the baseline and the comparison epoch, normalized to the maximum possible reduction in amplitude from the baseline epoch. For example, if a participant reduced target region VM amplitude from 20% of total cycle activation to 5% of activation, they demonstrated a 75% reduction from the initial value. Participants were exposed to 5 separate visual feedback epochs of 3 min each, from which we determined the epoch with the greatest reduction in target region amplitude for each individual. This epoch was used as the reference for degree of change in amplitude with feedback. We chose the epoch with greatest amplitude change because we expected participants to alter their response to feedback across experimental epochs. Maintaining lower amplitude for a 3-min epoch containing 54 analyzed cycles is unlikely to solely reflect random cycle-to-cycle variation in EMG or behavior. This analysis was also performed for VL.
The two EMG activity measures were then checked to determine whether they were appropriate for standard statistical comparisons that assume normality. Phase data can potentially require circular statistics for accurate comparisons, but in practice the angle data were sufficiently clustered that circular statistics gave negligibly different results. Standard measures are presented for simplicity. Additionally, this phase data approximated a normal distribution. To verify that normality assumptions did not affect our conclusions for amplitude, the analysis of control vs. feedback amplitude change was repeated using the Wilcoxon rank sum test. However, amplitude data represented the fraction of total activity during the cycle present in a given region and were thus strictly limited to a 0–1 range. Data routinely approached zero, particularly as participants responded to feedback, but did not pass 0.5, so the upper limit did not practically constrain the data. Variance also increased with amplitude. These factors reasonably justified a log transformation of the data (Tabachnick et al. 2001), which as expected resulted in an approximately normal distribution after data transformation.
We tested the normality of the amplitude transformation for control and final feedback epochs across all participants and conditions. We found that skewness magnitude was less than 2 for all control cases and all but 5 of 48 feedback cases (which were between 2 and 3), and kurtosis was less than 7 for all control cases and all but 8 of 48 cases (which were between 7 and 13). The majority of values were below common rule-of-thumb estimates that suggest normal statistics are robust at skewness of 2 or less and kurtosis of 7 or less (Curran et al. 1996). Higher values in most cases increase the likelihood of false negatives, which suggests that any significant observations are likely still valid. Statistical analyses (including means) were calculated on the basis of the log-transformed data, but reported amplitude-based values have been transformed back to fractional changes to allow clearer interpretation of results.
Statistical Analysis
For all comparisons, we assumed P < 0.05 as the threshold for significance. Where appropriate, we report an adjusted P value after controlling for multiple comparisons. Any test with the number of comparisons specified is adjusted for multiple comparisons using the Benjamini-Hochberg method to control false discovery rate (Benjamini and Hochberg 1995; via MATLAB implementation by Groppe 2010). The false discovery rate was set to 0.05.
Four sets of comparisons were performed. First, we compared between baseline conditions: the mean muscle activity phases of the four different baseline conditions (BNP, UNP, BP, and UP) for each participant were calculated, and BP, UNP, and UP conditions were compared with the BNP condition by paired t-test. The BNP condition mean phase is approximately zero, because it is the source of the mean reference cycle. Additionally, these mean muscle activity phase data were tested for correlation with Fugl-Meyer synergy subscore (FMS). The mean relative phases for each non-BNP condition were tested for correlation with FMS (t-test, via MATLAB function “corrcoef”) to determine whether there was a relationship between observed muscle activity phasing and poststroke motor impairment. Comparison of mean differences between paretic and nonparetic baseline conditions was also performed for amplitude data (BNP vs. BP, UNP vs. UP).
Second, we compared the degree of change observed during visual feedback. To determine whether individuals were able to significantly alter muscle activity in response to feedback within each condition, the lowest amplitude of the five visual feedback epochs for each participant was compared against the baseline epoch for that participant, by two-sample t-test (48 total comparisons, 4 conditions each for 12 participants). We repeated the above comparison with the final epoch, rather than the lowest amplitude epoch, and noted the few cases where significance changed.
Third, to compare the effect of the individual pedaling conditions on the degree of response to visual feedback, we compared mean amplitude change from baseline to the reference feedback epoch and postfeedback epoch (scaled as described in data processing) between conditions with a one-way ANOVA (P value derived from F statistic). We repeated the comparison for mean phase change from baseline to reference feedback epoch and postfeedback periods. The mean participant amplitude and phase changes were tested for correlation with FMS score (4 comparisons: FMS vs. BNP, BP, UNP, UP), to determine whether there was a relationship between feedback response and poststroke motor impairment. Mean participant amplitude changes were also compared with one another, to determine whether performance in one condition correlated with performance in another condition, for BNP vs. BP and UNP vs. UP (2 comparisons). We then compared the phase delay measure for the initial baseline period with the degree of change in amplitude with feedback for the BP condition, to determine whether initial phase impairment correlated with response to feedback.
Finally, we compared the degree of change in muscle activity during the postfeedback epoch with the corresponding baseline epoch, comparing mean muscle activity phase difference and target region muscle amplitude (4 comparisons each, BNP, BP, UNP, UP, post vs. own baseline). Because we did not predict that unilateral and bilateral pedaling would differently affect postfeedback changes, we also compared grouped data from both paretic limb conditions vs. zero (t-test), repeated for both nonparetic limb conditions.
RESULTS
VM Muscle Activity Phasing and Amplitude During Baseline Pedaling
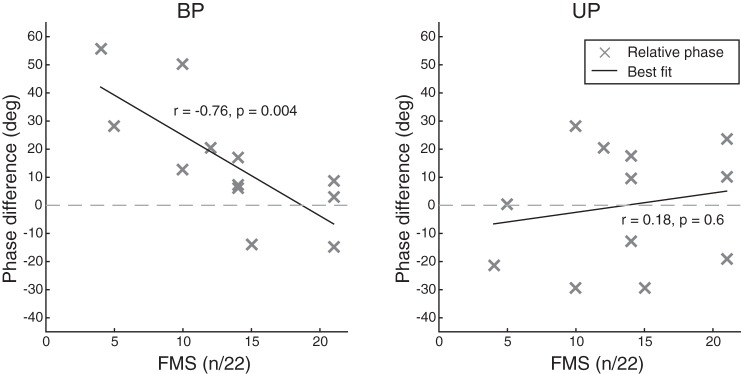
We first calculated VM muscle activity phase and amplitude measures in the baseline condition, during both bilateral and unilateral pedaling. We found that inappropriate paretic VM muscle activity occurred during the upstroke of the pedaling phase in bilateral pedaling, but not in unilateral pedaling, and these observations agreed with previously reported results (Kautz and Brown 1998; Kautz and Patten 2005; Rogers et al. 2011). For baseline VM muscle activity phase measurements (n = 12) relative to reference activity of the nonparetic VM during bilateral pedaling, we found that paretic VM muscle activity phase was significantly delayed during bilateral pedaling (15.1° ± 21.6°, mean ± SD, P = 0.03), but not during unilateral pedaling (−0.1° ± 21.3°, P = 0.98; Fig. 3). During bilateral pedaling in the paretic limb, the degree of VM phase delay correlated with impairment severity as measured by Fugl-Meyer assessment synergy subscore (FMS; r = −0.76, P = 0.004; Fig. 4) such that participants with less motor recovery demonstrated greater baseline period phase delay. In the nonimpaired limb, we did not observe a significant difference in VM muscle activity phase in UNP compared with BNP, as expected, or a relationship between FMS and muscle activity phase. For baseline VM amplitude measurements (n = 13), we observed a significantly greater mean percentage of total amplitude in the 105°–195° region in the paretic compared with the nonparetic VM during bilateral pedaling (P < 0.01; 24.2 ± 6.5% for paretic vs. 16.8 ± 6.1% for nonparetic), but in unilateral conditions we did not observe a significant amplitude difference between paretic and nonparetic VM (P = 0.17). These findings agree with prior work demonstrating prolonged VM activity (Kautz and Brown 1998; Rogers et al. 2011), reduced inappropriate activity in unilateral pedaling (Kautz and Patten 2005; Rogers et al. 2011), and a correlation between greater inappropriate activity and greater motor impairment (Kautz and Patten 2005).
Fig. 3.
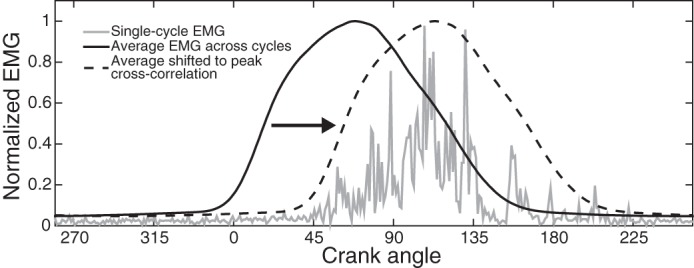
Estimation procedure to determine relative muscle activity phase shift. Example electromyographic (EMG) activation from participant A, showing single-cycle data from the paretic limb, average EMG across baseline epoch cycles of the nonparetic limb, and average EMG shifted to the maximum correlation with the single-cycle data. EMG data were averaged across single-degree bins. This cycle demonstrated a relative phase shift of +43°, a large delay.
Fig. 4.
Baseline epoch muscle activity phase relative to clinical impairment. Values are means for each participant (n = 12, excluding participant J). Relative vastus medialis (VM) muscle activity phase in the paretic limb was calculated in comparison with the average during bilateral pedaling of the nonparetic VM (BNP). A: VM muscle activity phase during bilateral pedaling in the paretic limb (BP), relative to the Fugl-Meyer synergy subscore (FMS). B: VM muscle activity phase during unilateral pedaling in the paretic limb (UP), relative to FMS.
VM Muscle Activity During Visual Feedback Epochs
For visual feedback epochs compared with the baseline epoch, data demonstrated that all participants were able to use the provided feedback to significantly reduce VM activation amplitude in the target region (105°–195°) during at least one of the four types of pedaling conditions, and typically in all four conditions (BNP, BP, UNP, UP). A representative unilateral paretic VM example comparing a standard baseline cycle with a feedback cycle from participant A is presented (Fig. 5), and demonstrates a 38% reduction in target region amplitude. Most participants demonstrated a progression across feedback epochs best described as either a progressive reduction in amplitude or a reduction followed by a plateau in most conditions. Participants B, E, and H demonstrated more complex patterns for multiple conditions. Although the paretic limb began at a higher fraction of amplitude in the target region, there was no systematic difference in the pattern of reduction between paretic and nonparetic limbs. Thus the specific feedback epoch with the lowest target region amplitude across 48 total participant-condition combinations (12 participants with 4 conditions each) was not always the fifth (last) (Table 2). In fact, 29 of 48 combinations demonstrated the lowest amplitude in 1 of the first 4 feedback epochs. Note that when comparing mean values, we performed statistical comparisons on log-transformed data, whereas the specific values reported in tables and figures have been converted back to relative fraction of activity in the target region. For each participant, we also calculated the greatest change for each limb in either the bilateral or unilateral condition. The greatest reduction for each participant averaged −46.1 ± 21.3% for the nonparetic limb and −42.9 ± 22.1% for the paretic limb. Mean change for all nonparetic conditions was −40.5 ± 21.1%, compared with −35.2 ± 21.9% for paretic conditions.
Fig. 5.
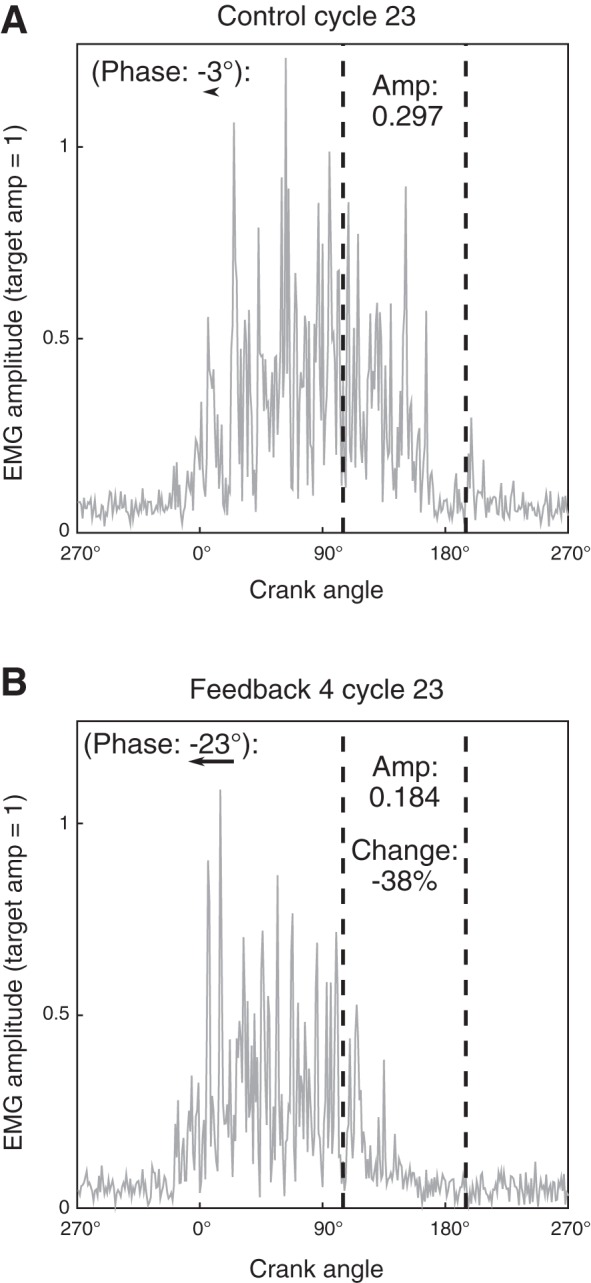
Example response to feedback. A: a representative cycle from the baseline epoch for participant A in the unilateral paretic (UP) condition. B: a representative cycle from the same condition during feedback epoch 4 (FB4). FB4 demonstrated the greatest average reduction in target deactivation region amplitude for this participant and condition. “Amp” is the fraction of total cycle activation in the target deactivation region, and this feedback cycle demonstrates a 38.0% reduction compared with this baseline cycle. Relative muscle activity phase for each cycle is included at top left (vs. UP baseline averaged reference cycle). An arrow beneath each phase shift shows the size of that shift. EMG, electromyography.
Table 2.
Feedback epoch with minimum target region activity
| Epoch 1 | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 5 | |
|---|---|---|---|---|---|
| BNP | 1 | 2 | 2 | 2 | 5 |
| BP | 1 | 0 | 1 | 3 | 7 |
| UNP | 2 | 1 | 1 | 2 | 6 |
| UP | 3 | 0 | 1 | 7 | 1 |
| Total occurrences | 7 | 3 | 5 | 14 | 19 |
Data are the number of participants for each condition who demonstrated their lowest target region activity amplitude epoch in the given epoch (n = 12, excluding participant D). Number of participant-condition combinations totals 48. BNP, bilateral pedaling with feedback about the nonparetic limb; BP, bilateral pedaling with feedback about the paretic limb; UNP, unilateral nonparetic pedaling with feedback on same limb; UP, unilateral paretic pedaling with feedback on same limb.
In general, participants were able to successfully reduce target region activity under all conditions. To determine the degree of change during visual feedback for each participant, we compared the set of all cycles for the lowest-amplitude epoch with the set of all cycles of the matched baseline epoch and found that in 10 of 12 participants, every condition showed significant amplitude reduction in the lowest-amplitude feedback epoch (adjusted P < 0.01, all but 2 comparisons adjusted P < 0.001). Each comparison significant to P < 0.001 was also significant according to the Wilcoxon rank sum test rather than a two-sample t-test. In the remaining two participants, the paretic and nonparetic limbs each demonstrated at least one condition in which amplitude was significantly reduced (participants B and E, Table 3). Although we are confident that selecting the 3-min epoch of best performance most accurately reflects participant ability to modulate muscle activity, we tested this comparison for the final feedback epoch, as well. Participants B, E, and H showed differences: participant B did not demonstrate significant reduction in amplitude in any condition (reduction for BNP, adjusted P = 0.053), participant E demonstrated significant reduction in only the BP condition, and participant H demonstrated significant reduction in only the BNP and UNP conditions. This indicates that for 9 of 12 participants, every condition showed significant reduction in amplitude even when the final epoch was selected. All further comparisons of feedback epochs are performed with the lowest-amplitude feedback epoch.
Table 3.
Change in target region activity with feedback
| Change in Target Region Activity, % |
||||
|---|---|---|---|---|
| Participant | BNP | BP | UNP | UP |
| A | −47.2 ± 4.1 | −18.6 ± 8.6 | −47.7 ± 4.33 | −41.1 ± 7.5 |
| B | −7.6 ± 9.5* | −6.0 ± 6.7* | −4.4 ± 10.3† | +2.6 ± 7.2† |
| C | −50.0 ± 14.1 | −48.0 ± 8.8 | −66.6 ± 10.1 | −53.2 ± 7.4 |
| E | +5.4 ± 7.1* | −12.6 ± 7.9 | −13.0 ± 9.3 | +0.8 ± 10.7† |
| F | −66.2 ± 7.2 | −55.4 ± 10.8 | −58.6 ± 5.6 | −66.6 ± 7.7 |
| G | −29.1 ± 4.3 | −20.7 ± 5.3 | −14.9 ± 5.6 | −9.0 ± 4.5 |
| H | −43.3 ± 12.8 | −14.0 ± 19.1* | −38.5 ± 13.6 | −33.3 ± 16.4 |
| I | −50.8 ± 7.0 | −65.6 ± 11.6 | −54.7 ± 4.7 | −20.7 ± 6.7 |
| J | −36.7 ± 10.4 | −58.9 ± 7.4 | −46.7 ± 7.3 | −51.6 ± 9.5 |
| K | −60.6 ± 8.2 | −13.6 ± 16.8* | −78.8 ± 8.1 | −50.6 ± 21 |
| L | −21.2 ± 15.9 | −32.7 ± 13.5 | −41.7 ± 17.9 | −32.8 ± 15.1 |
| M | −40.8 ± 6.3 | −70.2 ± 6.1 | −58.3 ± 4.6 | −73.1 ± 5.8 |
Values are means ± SD, where the mean is percent change from the control period amplitude and SD is the percentage of mean control period amplitude, for total cycle amplitude found in the target region for the feedback epoch with the lowest amplitude compared with baseline (n = 12, excluding participant D). Significance tests were performed on log-transformed cycle amplitude.
P < 0.05, significant difference (adjusted P value);
No significant difference.
Values that do not indicate a reduction in the target region with P < 0.001 significance are bold; differences for all other (unmarked) values are significant to adjusted P < 0.001. BNP, bilateral pedaling with feedback about the nonparetic limb; BP, bilateral pedaling with feedback about the paretic limb; UNP, unilateral nonparetic pedaling with feedback on same limb; UP, unilateral paretic pedaling with feedback on same limb.
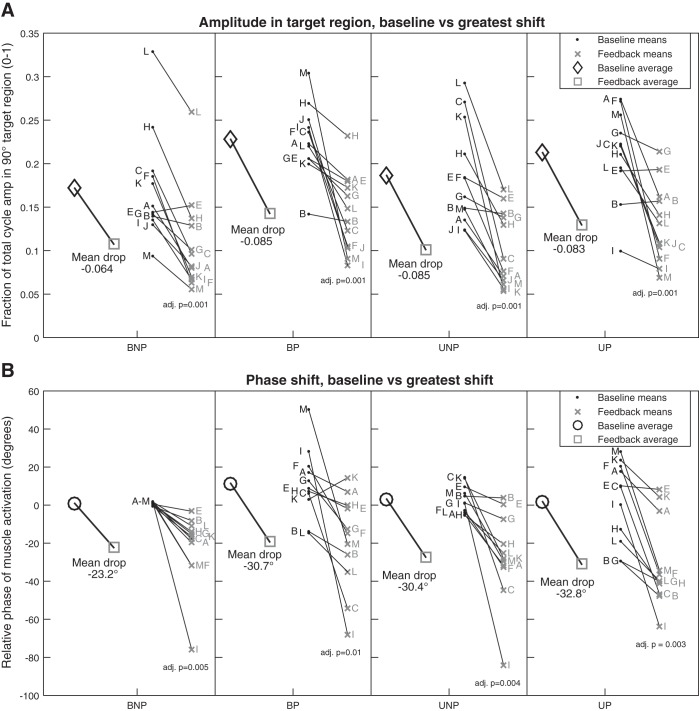
We then calculated the mean reduction in amplitude in the target region for each condition for each participant and compared group data for all four conditions (BNP, BP, UNP, UP). We found that the amplitude in the target region during the reference feedback epoch was significantly reduced (adjusted P < 0.001) for all conditions (Fig. 6). Muscle activity phase in the corresponding epoch was also significantly advanced for all conditions (adjusted P < 0.01). The amplitude was reduced by a comparable fraction of the total cycle amplitude in all conditions, and there were no significant differences between the amplitude reductions of each condition for amplitude (ANOVA, P = 0.76) or phase (P = 0.82).
Fig. 6.
Change in muscle activity: baseline epoch vs. reference feedback epoch. Data points for each participant, indicated by participant identifiers, are adjacent horizontally to the matched data point in the same vertical order for each condition (BNP, bilateral pedaling with feedback about the nonparetic limb; BP, bilateral pedaling with feedback about the paretic limb; UNP, unilateral nonparetic pedaling with feedback on same limb; UP, unilateral paretic pedaling with feedback on same limb). Some participants were omitted, as described in materials and methods, Data Processing. A: fraction of cycle amplitude (amp) in the target deactivation region (105°–195°; n = 12, excluding participant D). Significance of baseline vs. feedback epoch by paired t-test (with multiple comparison correction; adj. p, adjusted P value) is shown below feedback epoch data. B: relative shift in muscle activity phase compared with the averaged reference BNP baseline cycle (n = 11, excluding participants D and J). BNP baseline cycle average relative phase is therefore clustered around zero. Both change in amplitude and muscle activity phase show significant changes during the feedback epoch.
We then sought to determine whether participants who performed well with the nonparetic leg also performed well with the paretic leg. We compared bilateral conditions between the paretic and nonparetic leg (BNP vs. BP) and unilateral conditions between the paretic and nonparetic leg (UNP vs. UP). During bilateral pedaling, there was no significant correlation between degree of change in BNP and BP conditions (r = 0.38, adjusted P = 0.22), but during unilateral pedaling, the degree of change in the paretic and nonparetic legs was correlated (r = 0.67, adjusted P = 0.04). This suggests that during unilateral pedaling, participant properties relevant to both limbs may explain some of the variation in degree of change with feedback.
We then tested to see whether the degree of change was related to impairment as measured by FMS. The degree of change was not correlated with FMS (adjusted P = 0.67) for BNP, UNP, and UP conditions; however, the BP condition demonstrated a correlation of r = 0.69, P = 0.014 (adjusted P = 0.056), which may indicate that greater amplitude reduction in the paretic limb was correlated with greater impairment. To verify this possible finding for the BP condition, we then compared for that condition the baseline epoch estimated muscle activity phase delay of the VM vs. the degree of change in response to feedback and found a significant relationship (r = −0.73, P = 0.01, greater muscle activity phase delay correlated with greater reduction). Baseline epoch phase delay is thus correlated with both FMS and degree of change in the BP condition, supporting the result that in the BP condition, greater impairment as measured by FMS is positively correlated with greater degree of amplitude reduction.
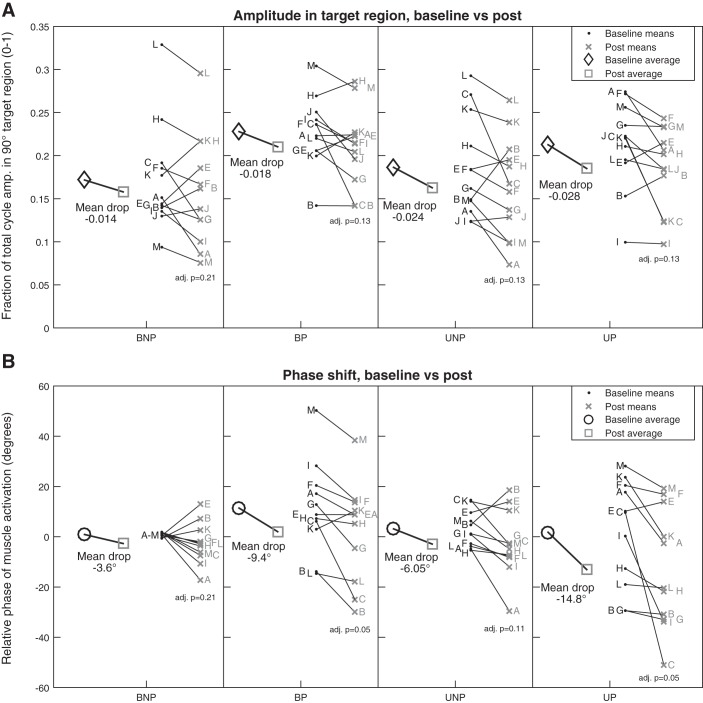
VM Muscle Activity During Postfeedback Epoch
Although visual feedback provided in this experiment was not designed to produce durable behavioral change, we did examine muscle activity phasing and amplitude during the postfeedback epoch, to compare persistent effects for each condition after exposure to feedback (Fig. 7). We first compared data from each limb for combined unilateral and bilateral conditions. Both paretic and nonparetic limb showed a significantly advanced muscle activity phase during the postfeedback epoch (nonparetic: −4.8° ± 9.6°, P = 0.03, paretic: −12.1° ± 15.2°, P = 0.001), as well as muscle amplitude (nonparetic: −7.9 ± 14.0%, P = 0.01, paretic: −8.11 ± 13.7%, P = 0.01). With focus on the four individual conditions, bilateral and unilateral conditions showed similar muscle activity phase significance for each limb. For the paretic limb, bilateral (P = 0.013, adjusted P = 0.052) and unilateral (P = 0.027, adjusted P = 0.054) conditions may have both demonstrated a difference in phase between the baseline and postfeedback epochs, whereas the smaller degree of change in the nonparetic limb was not significant in individual conditions (P > 0.1, adjusted P > 0.1). Individual condition muscle amplitude comparisons were nonsignificant (P > 0.1). When change in mean phase values for each participant between conditions, none of the four conditions were significantly different from one another in phase (ANOVA, P = 0.21) or amplitude (P = 0.86).
Fig. 7.
Change in muscle activity: baseline epoch vs. postfeedback epoch. Data points for each participant, indicated by participant identifiers, are adjacent horizontally to the matched data point in the same vertical order for each condition (BNP, bilateral pedaling with feedback about the nonparetic limb; BP, bilateral pedaling with feedback about the paretic limb; UNP, unilateral nonparetic pedaling with feedback on same limb; UP, unilateral paretic pedaling with feedback on same limb). Some participants were omitted, as described in materials and methods, Data Processing. A: amplitude in target deactivation region (105°–195°) for baseline vs. postfeedback epoch (n = 12, excluding participant D). Significance of paired t-test of baseline vs. post-feedback epoch for the condition is shown below each postfeedback epoch (adj. p, adjusted P value). B: relative shift in muscle activity phase against the averaged reference BNP baseline cycle for that condition for baseline vs. postfeedback epoch (n = 11, excluding participants D and J).
Feedback-Targeted VM Muscle Activity Phase and Nontargeted VL Muscle Activity Phase Were Strongly Correlated
We calculated the muscle activity phase estimate for VL, for which participants did not receive feedback, and analyzed the correlation between VL and VM muscle activity phase during control, feedback, and postfeedback periods for 10 participants. Participant G was excluded due to extremely small-amplitude recorded VL activity. Participant J was previously excluded from phase analysis for VM as described in materials and methods. For these 10 participants, we observed a strong correlation between VL and VM for all conditions. Correlation between VM and VL was r = 0.84 ± 0.09 in the nonparetic limb and r = 0.79 ± 0.18 in the paretic limb. Individual epoch correlation between VM and VL was significant for every epoch but 3 of 280 (10 participants, 4 conditions, 7 epochs per condition).
DISCUSSION
During visual feedback conditions, we initially proposed two hypotheses that posited the paretic limb would demonstrate impaired flexibility of muscle activity. First, we hypothesized that in bilateral pedaling, the paretic VM would demonstrate significant voluntary flexibility of muscle activity. This hypothesis was supported; we found the paretic limb demonstrated a significant reduction in VM muscle activity in the target region. In fact, the amount of reduction was not significantly different between the paretic limb and the nonparetic limb. Related to this hypothesis, we also found that in the paretic limb, greater clinical impairment (lower FMS score) was unexpectedly associated with greater muscle activity flexibility in response to feedback. Second, we hypothesized that the paretic VM would demonstrate greater voluntary flexibility of VM muscle activity during unilateral pedaling than in bilateral pedaling. This hypothesis was not supported; instead, we observed comparable voluntary changes in paretic VM activity during both conditions, with no significant difference between the two. Finally, both the paretic and nonparetic limbs showed a significant change in muscle activity phasing during the postfeedback epoch when unilateral and bilateral conditions were combined. Prior research has demonstrated reduced task success in individuals poststroke during walking obstacle avoidance, a more complex visually mediated locomotor task (Den Otter et al. 2005). This reduced success suggested that participants’ ability to modulate muscle activity in the paretic limb might be impaired, particularly during bilateral control, but this was not supported by our findings.
Delayed Paretic VM Activity During Pedaling in Baseline Conditions
During baseline conditions with no visual feedback, our results confirm prior findings during pedaling and establish that the participant group showed behavior similar to that in previous studies. We demonstrated 1) muscle activity phase delay in the VM of the paretic leg in bilateral pedaling, 2) correlation between degree of VM phase delay and clinical impairment, and 3) reduced VM phase delay in the paretic limb during unilateral pedaling. In prior research, prolonged VM activity has been quantified in broad regions and/or across multiple cycles (gait, Den Otter et al. 2007; pedaling, Kautz and Brown 1998; Kautz, Patten and Neptune 2006; Rogers et al. 2011). In the present study, however, we calculated an estimate of a single muscle’s average muscle activity phase for each cycle relative to a reference cycle, with a method that sought to limit the effect of signal to noise ratio on phase estimates.
Reduction in Target Deactivation Region VM Activity During Visual Feedback
This study focused on participants’ ability to use visual EMG feedback to reduce the inappropriate VM activity initially observed in the paretic limb during baseline pedaling. We first hypothesized that participants would demonstrate ability to reduce targeted muscle activity in the paretic limb, and this hypothesis was supported. We also hypothesized that participants would demonstrate a greater reduction during unilateral pedaling compared with bilateral pedaling, and this hypothesis was not supported. Previous research demonstrated inappropriate control of muscle activity phase in the paretic limb (Kautz and Brown 1998) and interlimb interference with control of the paretic limb during bilateral pedaling (Kautz and Patten 2005; Kautz et al. 2006; Rogers et al. 2011), but when participants were presented with visual feedback on the EMG activity of the VM during a kinematically constrained task, they were able to reduce target region activity with no significant difference between paretic and nonparetic limbs and no difference between unilateral and bilateral conditions.
This comparable reduction was surprising given our predictions based on prior theories related to impairment of muscle phasing poststroke. Two neural mechanisms have been suggested to play a role in inappropriate muscle activity phasing in prior research: recruitment of branching bulbospinal pathways (gait, Clark et al. 2010; reaching, Dewald et al. 2001; ankle task, Madhavan et al. 2010) and inappropriate interhemispheric inhibition (finger movement, Bütefisch et al. 2008; Duque et al. 2005; Murase et al. 2004). Functionally, these mechanisms could cause the previously observed contralateral interference between commands to the nonparetic limb and activity in the paretic limb in prior studies (Kautz and Patten 2005; Rogers et al. 2011), which we also observed in the baseline condition of this study. Increased drive of the nonparetic limb may result in recruitment of branching bulbospinal pathways that innervate both sides of the spinal cord, whereas increased activity of the contralesional cortex may increase inappropriate inhibition of the lesioned cortex (Bütefisch et al. 2008; Murase et al. 2004). In light of the findings of this study, however, it may be possible that with appropriate feedback, participants are able to modulate motor control strategies in a way that reduces the role of these mechanisms.
The visual EMG feedback task presented in this study may have allowed participants to reduce reliance on the mechanisms proposed to explain poststroke impairment of muscle phase. This task differed from prior research in several ways. First, the target region for deactivation began well before typical deactivation at the experimental crank speed, which allowed us to present a challenge to both the paretic and nonparetic limb. Prior studies have used activity of the nonparetic limb as a target, to attempt to generate symmetrical patterns between the two limbs (Ferrante et al. 2011). Second, the task was performed under tight kinematic control that allowed participants to freely vary muscle activity, and feedback was provided only on the target muscle, which simplified the mechanical task. Third, the task relied strongly on visual feedback, unlike most prior pedaling work in which proprioceptive feedback and mechanical task changed between varying behavioral contexts (Alibiglou and Brown 2011; Rogers et al. 2011; Schindler-Ivens et al. 2004). Individuals with poststroke motor impairment have been shown to rely to a greater degree on visual feedback over somatosensory feedback during postural control in balance-based tests (Bonan et al. 2013; Corriveau et al. 2004), and visual feedback on the target muscle may allow more effective voluntary control of muscle activity phase than alternate feedback methods.
The EMG data we obtained allows only limited assessment of the specific neural mechanisms recruited during this task. However, our results may suggest that attending to visual information encouraged participants to preferentially recruit visual premotor processing pathways. Visuomotor processing can be generalized as occurring in the dorsal stream of the cortex’s visual processing (Goodale and Milner 1992), with the posterior parietal cortex and premotor cortices playing a critical role (see for review, Wurtz and Kandel 2000). Visual feedback on paretic limb muscle activity may have allowed participants to reduce reliance on mechanisms that have been proposed to impair muscle activity phase during prior work, such as by reducing inappropriate inhibition of the lesioned cortex (by reducing lateralization of feedback processing). This is in part supported by the significant correlation between reduction of target region EMG during unilateral pedaling in the paretic and nonparetic limb, which suggests that central mechanisms that affect both limbs play a significant role. Participants also may have been able to more appropriately recruit existing descending corticospinal pathways and/or more intact indirect locomotor circuits, such as descending connections from the mesencephalic locomotor region that directly activate pattern/rhythm generating elements of the spinal cord (see for review, Grillner et al. 2008).
If participants altered muscle activity phasing in part by recruiting alternate pathways for the control of muscle activity phase, we would expect that participants with greater impairment of muscle activity phase (reflecting greater impairment of typically recruited pathways) could demonstrate a larger reduction in target region muscle activity in response to feedback as a result of recruiting these alternate pathways. We observed just that: participants with greater impairment demonstrated greater reduction in activity in the target region in response to feedback. This finding stands in contrast to our initial expectations (derived from prior work relating clinical impairment with poststroke muscle phase impairment: Kautz and Brown 1998; Kautz and Patten 2005) and supports our current interpretation that visual feedback on muscle activity allows for recruitment of alternate control strategies that result in flexible muscle activity phasing control in the paretic limb.
Other work on locomotion poststroke, focusing on adaptation, also has shown comparable changes in the paretic and nonparetic limbs. During split-belt treadmill walking, poststroke participants demonstrated a relatively similar degree of response to the task to that of nonimpaired participants (Reisman et al. 2007). Those authors suggested that the comparable changes they observed could be due to recruitment of spinocerebellar pathways that play a role in motor adaptation, and are spared in cortical stroke. We suggest that in both split-belt treadmill adaptation and the current experiment, whereas the specific pathways recruited are notably different, participants demonstrated recruitment of less-impaired alternate motor control pathways that allowed performance in the paretic limb that was comparable to that in the nonparetic limb.
Short-Term Persistence of Muscle Activity Phase Changes Immediately After Removal of Visual Feedback
Although our experiment was primarily designed to test changes in activity during feedback, rather than to encourage persistent effects, the presence of changes in muscle activity phase after feedback removal suggests the potential for further study. Participants demonstrated significantly earlier VM activity phase and reduced target region amplitude in both limbs after removal of feedback compared with the baseline epoch, despite the fact that the protocol was not designed to produce motor learning. Participants were exposed for a relatively short period (15 min total of feedback), consistently provided full knowledge of results, and were not tested for transfer or other common hallmarks of motor learning (see for review, Schmidt and Wrisberg 2004). The persistence of less-delayed activity in the paretic limb after feedback suggests that future studies with greater focus on training could result in durable reduction in inappropriate muscle activity during pedaling tasks and might potentially have rehabilitation applications in reduction of inappropriately prolonged muscle activation, such as that participants reduced in the VM, if longer term effects are observed. The extent to which this visual EMG feedback paradigm could be relevant to other more complex locomotor tasks such as walking remains to be determined.
Correlation of VM and VL Activity
During the course of our experiment, we did not observe evidence to suggest that VM and VL were performing separate control tasks. Changes in relative muscle activity phase of the two muscles correlated tightly before, during, and after feedback. Activation of the VM and VL has been well studied, given their relationship to patellofemoral pain, and past studies have used prolonged training periods to prompt changes in the relative activation amplitude of the two muscles (e.g., LeVeau and Rogers 1980). There also may be specific functional reasons that the motor system would avoid altering the relative timing of VM and VL: delayed activity of VM relative to VL appears to correlate with patellofemoral pain (Pal et al. 2011). It is perhaps unsurprising, then, that we observed such a close correlation between activity of the VM and VL throughout the experiment.
Limitations and Future Work
The current experiment studied poststroke participants and so did not include nonimpaired individuals. We cannot assume in general that control of the nonparetic VM was unimpaired, since previous researchers have observed motor control deficits in the nonparetic side (e.g., Winstein and Pohl 1995). However, we did not observe a significant relationship between clinical impairment of the paretic leg and reduction in muscle activity amplitude in the nonparetic leg. Combined with a study design that emphasizes comparisons within participants, the lack of evidence that impairment affected the nonparetic limb suggests that, in this study, the non-paretic limb provided reasonable comparison data. A second possible concern is that we limited our selection of participants to individuals who were able to walk independently, which could bias participant selection. If participants with greater impairment who recover the ability to walk independently tend to be more effective in general at altering locomotor strategies, our participant pool might be biased toward greater flexibility among more impaired participants. However, despite our finding of a correlation between muscle flexibility of an individual between the two unilateral conditions (which suggests a centralized element of control), the previously mentioned observation that we did not find a significant relationship between nonparetic limb degree of change and impairment suggests as well that this potential bias may not have greatly affected the results. Finally, this study focused closely on changes in activity in a single muscle. Prior work has often compared the relationships between muscles, which have been suggested to function synergistically (Clark et al. 2010; Dewald et al. 2001). We have chosen to target a uniarticular muscle with a clear biomechanical role during pedaling, to provide a model of individual muscle flexibility poststroke, but additional investigation will be necessary to determine how the changes in VM are reflected in other muscles of the target or contralateral leg and the wider implications of the single-muscle flexibility observed for motor control.
Conclusions
Participants showed significant flexibility in reducing their muscle activity in a targeted region using visual EMG feedback. Surprisingly, participants did not show a significant difference in the ability to change muscle activity amplitude between the paretic and nonparetic legs. We did not observe a significant difference when comparing between unilateral and bilateral pedaling in either leg. In this task, when provided visual EMG feedback focusing on a single muscle, and in which there was limited necessary mechanical control, participants were able to reduce VM muscle activity in a behaviorally relevant target region in both legs. This comparable flexibility provides a useful framework for further study of behavioral flexibility following stroke and suggests that at this basic level the paretic limb and nonparetic limbs may show similar motor control flexibility. Future studies will help to elaborate how this core behavioral flexibility functions (or is impaired) during more complex tasks as individuals face the challenges of locomotion poststroke.
GRANTS
This work was supported by American Heart Association Pre-doctoral Training Grant 14PRE18870084 (to C. H. Mullens), NIH Institutional Training Grant T32HD057845 (to D. A. Brown), and National Institute on Disability and Rehabilitation Research Grant H133G120297 (to D. A. Brown).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.H.M. and D.A.B. conceived and designed research; C.H.M. performed experiments; C.H.M. analyzed data; C.H.M. and D.A.B. interpreted results of experiments; C.H.M. prepared figures; C.H.M. drafted manuscript; C.H.M. and D.A.B. edited and revised manuscript; C.H.M. and D.A.B. approved final version of manuscript.
REFERENCES
- Alibiglou L, Brown DA. Impaired muscle phasing systematically adapts to varied relative angular relationships during locomotion in people poststroke. J Neurophysiol 105: 1660–1670, 2011. doi: 10.1152/jn.00290.2010. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol 57: 289–300, 1995. [Google Scholar]
- Bonan IV, Marquer A, Eskiizmirliler S, Yelnik AP, Vidal PP. Sensory reweighting in controls and stroke patients. Clin Neurophysiol 124: 713–722, 2013. doi: 10.1016/j.clinph.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Bonato P, D’Alessio T, Knaflitz M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Trans Biomed Eng 45: 287–299, 1998. doi: 10.1109/10.661154. [DOI] [PubMed] [Google Scholar]
- Brunnström S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. New York: Harper & Row, 1970. [Google Scholar]
- Bütefisch CM, Wessling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22: 4–21, 2008. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Cho SH, Shin HK, Kwon YH, Lee MY, Lee YH, Lee CH, Yang DS, Jang SH. Cortical activation changes induced by visual biofeedback tracking training in chronic stroke patients. NeuroRehabilitation 22: 77–84, 2007. [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil 22: 758–767, 2008. doi: 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- Corriveau H, Hébert R, Raîche M, Prince F. Evaluation of postural stability in the elderly with stroke. Arch Phys Med Rehabil 85: 1095–1101, 2004. doi: 10.1016/j.apmr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Curran PJ, West SG, Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Methods 1: 16–29, 1996. doi: 10.1037/1082-989X.1.1.16. [DOI] [Google Scholar]
- Den Otter AR, Geurts AC, de Haart M, Mulder T, Duysens J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res 161: 180–192, 2005. doi: 10.1007/s00221-004-2057-0. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture 25: 342–352, 2007. doi: 10.1016/j.gaitpost.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Sheshadri V, Dawson ML, Beer RF. Upper-limb discoordination in hemiparetic stroke: implications for neurorehabilitation. Top Stroke Rehabil 8: 1–12, 2001. doi: 10.1310/WA7K-NGDF-NHKK-JAGD. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 28: 940–946, 2005. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ferrante S, Ambrosini E, Ravelli P, Guanziroli E, Molteni F, Ferrigno G, Pedrocchi A. A biofeedback cycling training to improve locomotion: a case series study based on gait pattern classification of 153 chronic stroke patients. J Neuroeng Rehabil 8: 47, 2011. doi: 10.1186/1743-0003-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975. [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci 15: 20–25, 1992. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates–an overview. Brain Res Brain Res Rev 57: 2–12, 2008. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Groppe DM. fdr_bh (Online). https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr-bh. 2010. [21 June 2016].
- Jain S, Gourab K, Schindler-Ivens S, Schmit BD. EEG during pedaling: evidence for cortical control of locomotor tasks. Clin Neurophysiol 124: 379–390, 2013. doi: 10.1016/j.clinph.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 76: 27–32, 1995. doi: 10.1016/S0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Brown DA. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain 121: 515–526, 1998. doi: 10.1093/brain/121.3.515. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol 93: 2460–2473, 2005. doi: 10.1152/jn.00963.2004. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C, Neptune RR. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J Neurophysiol 95: 3154–3163, 2006. doi: 10.1152/jn.00951.2005. [DOI] [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain 102: 405–430, 1979. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- LeVeau BF, Rogers C. Selective training of the vastus medialis muscle using EMG biofeedback. Phys Ther 60: 1410–1415, 1980. doi: 10.1093/ptj/60.11.1410. [DOI] [PubMed] [Google Scholar]
- Liang JN, Brown DA. Impaired foot-force direction regulation during postural loaded locomotion in individuals poststroke. J Neurophysiol 110: 378–386, 2013. doi: 10.1152/jn.00005.2013. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Rogers LM, Stinear JW. A paradox: after stroke, the non-lesioned lower limb motor cortex may be maladaptive. Eur J Neurosci 32: 1032–1039, 2010. doi: 10.1111/j.1460-9568.2010.07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Hull ML. The effect of pedaling rate on coordination in cycling. J Biomech 30: 1051–1058, 1997. doi: 10.1016/S0021-9290(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: characteristics. Gait Posture 4: 136–148, 1996. doi: 10.1016/0966-6362(96)01063-6. [DOI] [Google Scholar]
- Pal S, Draper CE, Fredericson M, Gold GE, Delp SL, Beaupre GS, Besier TF. Patellar maltracking correlates with vastus medialis activation delay in patellofemoral pain patients. Am J Sports Med 39: 590–598, 2011. doi: 10.1177/0363546510384233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LM, Brown DA, Gruben KG. Foot force direction control during leg pushes against fixed and moving pedals in persons post-stroke. Gait Posture 19: 58–68, 2004. doi: 10.1016/S0966-6362(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Rogers LM, Stinear JW, Lewis GN, Brown DA. Descending control to the nonparetic limb degrades the cyclic activity of paretic leg muscles. Hum Mov Sci 30: 1225–1244, 2011. doi: 10.1016/j.humov.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Brown DA, Brooke JD. Direction-dependent phasing of locomotor muscle activity is altered post-stroke. J Neurophysiol 92: 2207–2216, 2004. doi: 10.1152/jn.01207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler-Ivens S, Brown DA, Lewis GN, Nielsen JB, Ondishko KL, Wieser J. Soleus H-reflex excitability during pedaling post-stroke. Exp Brain Res 188: 465–474, 2008. doi: 10.1007/s00221-008-1373-1. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Wrisberg CA. Motor Learning and Performance (3rd ed.). Champaign, IL: Human Kinetics, 2004. [Google Scholar]
- Tabachnick BG, Fidell LS, Osterlind SJ. Using Multivariate Statistics (5th ed.). Boston, MA: Pearson, 2001. [Google Scholar]
- Teasell RW, Bhogal SK, Foley NC, Speechley MR. Gait retraining post stroke. Top Stroke Rehabil 10: 34–65, 2003. doi: 10.1310/UDXE-MJFF-53V2-EAP0. [DOI] [PubMed] [Google Scholar]
- Ting LH, Raasch CC, Brown DA, Kautz SA, Zajac FE. Sensorimotor state of the contralateral leg affects ipsilateral muscle coordination of pedaling. J Neurophysiol 80: 1341–1351, 1998. doi: 10.1152/jn.1998.80.3.1341. [DOI] [PubMed] [Google Scholar]
- Tyson S, Hanley M, Chillala J, Selley AB, Tallis RC. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabil Neural Repair 22: 166–172, 2007. doi: 10.1177/1545968307305523. [DOI] [PubMed] [Google Scholar]
- Van der Loos HF, Worthen-Chaudhari L, Schwandt D, Bevly DM, Kautz SA. A split-crank bicycle ergometer uses servomotors to provide programmable pedal forces for studies in human biomechanics. IEEE Trans Neural Syst Rehabil Eng 18: 445–452, 2010. doi: 10.1109/TNSRE.2010.2047586. [DOI] [PubMed] [Google Scholar]
- Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med 19: 25–30, 1987. [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res 105: 163–174, 1995. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Kandel ER. Central visual pathways. In: Principles of Neural Science, edited by Kandel ER, Schwartz JH, Jessell T. New York: McGraw-Hill, 2000, p. 523–545. [Google Scholar]
- Yelnik A, Albert T, Bonan I, Laffont I. A clinical guide to assess the role of lower limb extensor overactivity in hemiplegic gait disorders. Stroke 30: 580–585, 1999. doi: 10.1161/01.STR.30.3.580. [DOI] [PubMed] [Google Scholar]