Abstract
Patients with peripheral artery disease show an exaggerated pressor response to mild exercise, an effect attributable to the exercise pressor reflex, whose afferent arm comprises the thinly myelinated group III and unmyelinated group IV afferents. Previously, we found that DAMGO, a µ-opioid agonist injected into the femoral artery, attenuated the exaggerated exercise pressor reflex in rats with ligated femoral arteries, a preparation that simulates the blood flow patterns to muscle that is seen in patients with peripheral artery disease. Continuing this line of investigation, we recorded the responses of group III and IV afferents to static contraction before and after injecting DAMGO (1 µg) into the superficial epigastric artery in rats with patent femoral arteries and in rats with ligated femoral arteries. In rats with patent arteries, DAMGO did not change the responses to contraction of either group III (n = 9; P = 0.83) or group IV (n = 8; P = 0.34) afferents. In contrast, in rats with ligated femoral arteries, DAMGO injection (1 µg) significantly decreased the responses to contraction of both group III afferents (n = 9, P < 0.01) and group IV afferents (n = 9; P < 0.01). DAMGO did not significantly attenuate the responses of either group III or IV afferents to capsaicin in rats with either patent or ligated femoral arteries. These findings are in agreement with our previous studies that showed that peripheral DAMGO injection attenuated the exercise pressor reflex in rats with ligated femoral arteries but had only a modest effect on the exercise pressor reflex in rats with patent femoral arteries.
NEW & NOTEWORTHY In an animal model of peripheral artery disease, we show that the µ-opioid agonist, DAMGO reduces the afferent response rate resulting from stimulated static contraction. These results suggest that peripherally active opioid agonists that do not cross the blood-brain barrier may be therapeutic for treatment of peripheral artery disease without the negative and addictive side effects associated with opioids in the central nervous system.
Keywords: autonomic control, DAMGO, exercise pressor reflex, sympathetic nervous system, thin fiber muscle afferents
INTRODUCTION
The cardiovascular responses to exercise in patients with peripheral artery disease are exaggerated compared with the responses to exercise of their healthy age matched counterparts (Bakke et al. 2007; Muller et al. 2012; Reggiani et al. 1999). In part, if not in total, the exaggerated cardiovascular responses in these patients are attributed to the exercise pressor reflex, which is evoked by mechanical and metabolic stimuli arising in contracting leg muscles. The sensory arm of the exercise pressor reflex comprises thinly myelinated group III afferents and unmyelinated group IV afferents (Coote et al. 1971; McCloskey and Mitchell 1972). For the most part, the endings of group III afferents are located in connective tissue, whereas the endings of the group IV afferents are located either in the muscle interstitium or in the walls of small vessels (von During and Andres 1990). Group III afferents are also called Aδ fibers and group IV afferents are also called C fibers; together, group III and IV afferents are often called “thin fiber afferents.”
Terjung and colleagues (Prior et al. 2004; Taylor et al. 2008) developed a rat model of peripheral artery disease in which they ligated a femoral artery to simulate the blood flow patterns to skeletal muscle that is seen in patients with this disease. In this model, arterial blood flow to the hind limb whose femoral artery was ligated was adequate to meet metabolic demand at rest but was inadequate to meet demand during exercise. Our laboratory has adapted this rat model of arterial insufficiency to examine its effect on the exercise pressor reflex. We found that static contraction of the triceps surae muscles evoked a larger reflex in decerebrate unanesthetized rats whose femoral arteries were ligated than did contraction in decerebrate unanesthetized rats whose femoral arteries were patent. The exaggeration of the reflex in “ligated rats” appeared to be mediated in part by ASIC 3 and EP 4 receptors on the peripheral endings of thin fiber muscle afferents (Tsuchimochi et al. 2010a, 2011; Yamauchi et al. 2013).
In subsequent studies, our laboratory found that injection of a µ-opioid receptor agonist into the arterial supply of the triceps surae muscles attenuated the exercise pressor reflex in rats with ligated femoral arteries to a greater extent than it did in rats with patent femoral arteries (Tsuchimochi et al. 2010b). This finding raised the possibility that the peripheral endings and axons of the group III and IV muscle afferents had µ-opioid receptors, which when activated functioned to inhibit their discharge. To test this possibility, we recorded the responses of group III and IV afferents to static contraction before and after intra-arterial injection of [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO), a peptide agonist of µ-opioid receptors. We compared the effect of DAMGO on the afferents’ responses to contraction in rats whose femoral arteries were ligated with the effect of DAMGO on the afferents’ responses to contraction in rats whose femoral arteries were freely perfused.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine approved all procedures. Male Sprague-Dawley rats (434 ± 9 g) were provided ad libitum access to food and water and had constant supervision by animal care staff. In 15 rats, we ligated one femoral artery just distal the inguinal ligament 72 h before the experiment. Rats were anesthetized with a mixture of isoflurane (4–5%) in oxygen. The femoral artery was isolated and 6-0 suture was tied tightly around it. We refer to rats whose femoral artery was occluded as ligated, and rats whose femoral artery was patent as “freely perfused.”
On the day of the experiment, rats were anesthetized initially with isoflurane (5%) in oxygen and a tracheal catheter was inserted. The lungs were then ventilated with isoflurane (2–3%) in a balance of oxygen. Both carotid arteries and a jugular vein were cannulated (PE-50) to record arterial pressure and to administer drugs and fluids, respectively. A 2-0 suture snare was placed around the left iliac artery and vein, and the left superficial epigastric artery, which is a side branch of the femoral artery, was cannulated (PE-8).
Rats were secured in a customized stereotaxic frame (David Kopf Instruments; Terjung, CA). Arterial pressure was monitored by connecting one carotid catheter to a Statham P23XL (Gould-DATAQ; Akron, OH) strain gauge. A precollicular decerebration was performed by removing all neural tissue rostral to the colliculi. The skin of the left hindlimb was used to create a pool, which was filled with mineral oil. The left sciatic nerve was isolated, and a shielded stimulating electrode placed under it. The gastrocnemius muscle was cleared of surrounding tissues and connected to a Grass FT 03 force transducer (Natus Neurology–Grass; Warwick, RI) via the calcaneal bone, which was severed.
Afferent recording.
The concentration of isoflurane used to ventilate the lungs was reduced to 0.5% and continued throughout the duration of the experiment. The fibrous sheath enclosing the sciatic nerve was removed, and a small filament of nerve was pulled away starting near the hip and continuing distal to the placed stimulating electrode. From this section, a small filament was suspended across one foot of a bipolar electrode, with a saline soaked thread tied to the opposite foot as a ground. Afferent impulses from the nerve filament were passed through a high-impedance probe, and were subsequently amplified and filtered (30 Hz low, 3–10 kHz high; Grass P511: Natus Neurology–Grass). Individual fibers were isolated by splitting the filament and discriminated by their action potential waveform and amplitude. Conduction time was measured by stimulating the afferent’s receptive field in the triceps surae muscles with a handheld stimulating electrode (0.5–1.0 ms; 0.5 Hz). Conduction distance was then measured by placing a string between the receptive field and the proximal foot of the bipolar electrode. Conduction velocity was then calculated by dividing conduction distance by conduction time. Group III afferents conducted impulses between 10 and 1.5 m/s; likewise, group IV afferents conducted impulses at less than 1.5 m/s.
Static contraction.
Motor threshold was obtained by slowly increasing the current of a single pulse (0.01 ms) applied to the sciatic nerve (1 Hz) until the triceps surae muscles twitched. Following 30 s of stable baseline arterial pressure, we contracted the triceps surae muscles for 30 s by stimulating the sciatic nerve at 15–20 Hz with a current twice that of twitch threshold. The duration of each pulse applied to the sciatic nerve was always 0.01 ms.
Intra-arterial capsaicin injection.
The iliac snare was tightened and 30 s of stable baseline was achieved. Capsaicin (0.2 µg in 0.1 ml) was injected into the superficial epigastric artery, followed immediately by a saline flush (0.1 ml). After 30–45 s of afferent activity was recorded, the snare was released.
DAMGO injection.
DAMGO (Tocris Bioscience; Bristol, UK) was dissolved in saline (0.9%). Following control experiments, and before DAMGO injection, the iliac snare was tightened. DAMGO (1 µg in 0.1 ml) was injected into the superficial epigastric artery, followed by a 0.1-ml saline flush of the catheter. The iliac snare was released 10 min after injection of DAMGO, and an additional 10 min was allowed to pass before repeating the control experiments.
Data analysis and statistics.
Arterial pressure, contractile tension, and afferent activity data were collected using Spike2 software (Cambridge Electronic Design; Cambridge, UK). When applicable, spike waveforms were generated and counted using software wave sorting. Statistical analyses were conducted in Prism 6 (GraphPad Software; La Jolla, CA) using repeated-measures two-way ANOVA with Holm-Sidak post hoc analysis. Tension-time indices were compared using two-tailed paired t-tests.
RESULTS
A total of 49 thin-fiber afferents were recorded from 28 Sprague-Dawley rats. Because the recording life of the fibers was limited, we were not able to determine whether every afferent was responsive to both static contraction (for example see Fig. 1) and capsaicin injection. Also in a few instances, electrical stimulation of the tibial nerve failed to contract the triceps surae muscles, thereby limiting our investigation to capsaicin.
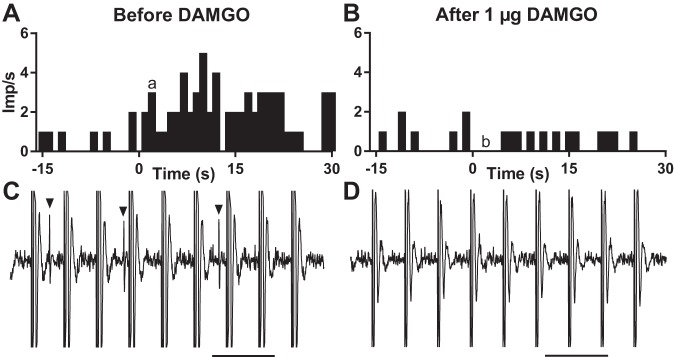
Fig. 1.
Individual example of the responses to static contraction of a group IV afferent before and after DAMGO. A and B: histograms showing the responses to contraction of a group IV afferent before (A) and after (B) injecting 1 µg DAMGO into the superficial epigastric artery. Contraction, which lasted for 30 s, was initiated at time zero. C and D: recordings of the impulse activity of the group IV afferent 3 s after the initiation of contraction. Arrowheads signify action potentials. Stimulation artifacts are the large equally spaced vertical lines. Scale bars = 100 ms. Lower case a and b correspond to time points shown in C and D, respectively. Imp, impulses.
Freely perfused rats.
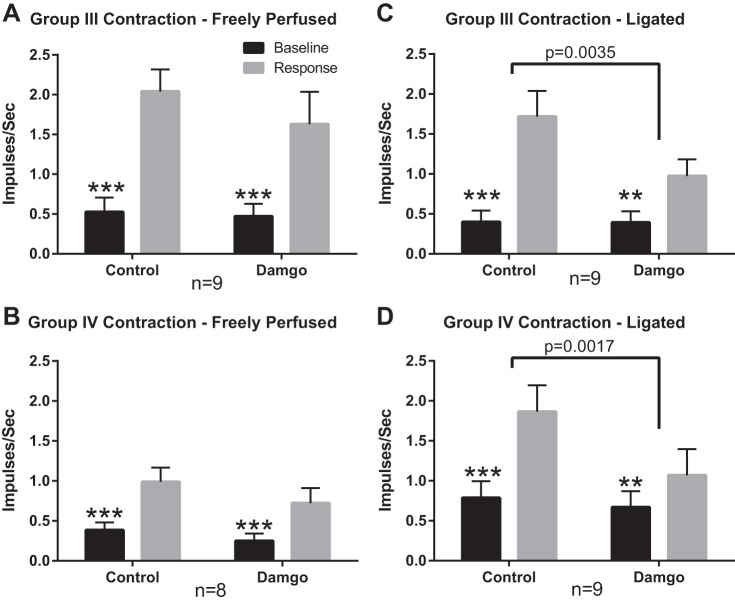
Before DAMGO injection into the hindlimb circulation of freely perfused rats, static contraction stimulated 9 of the 10 group III afferents tested (conduction velocity = 3.0 ± 0.4 m/s; n = 10), activity increasing for these nine afferents from 0.5 ± 0.2 to 2.0 ± 0.3 impulses/s (P < 0.001, Fig. 2A). After DAMGO injection (1 µg), static contraction still stimulated these same nine group III afferents, activity increasing from 0.5 ± 0.2 to 1.6 ± 0.4 impulses/s (P < 0.001). On average, DAMGO did not significantly decrease the responses of the nine group III afferents’ to contraction (P = 0.30; Fig. 3A). The tension-time indices remained consistent before and after DAMGO injection (11.4 ± 1.3 kg*s before DAMGO vs. 11.2 ± 1.2 kg*s after DAMGO; P = 0.83).
Fig. 2.
Summary data showing that the responses to contraction are reduced by DAMGO in group III and IV afferents whose impulse activity was recorded from “ligated” rats, but was not reduced in group III and IV afferents whose impulse activity was recorded from “freely perfused” rats. Black vertical bars represent mean values for the 30 s of control preceding contraction, whereas gray vertical bars represent mean values for the 30 s of contraction. Vertical brackets over each bar represent the standard error of the mean, whereas horizontal brackets represent significant differences between before and after DAMGO injection (1 µg) into the superficial epigastric artery. **P < 0.01, ***P < 0.001 vs. contraction for same condition.
Fig. 3.
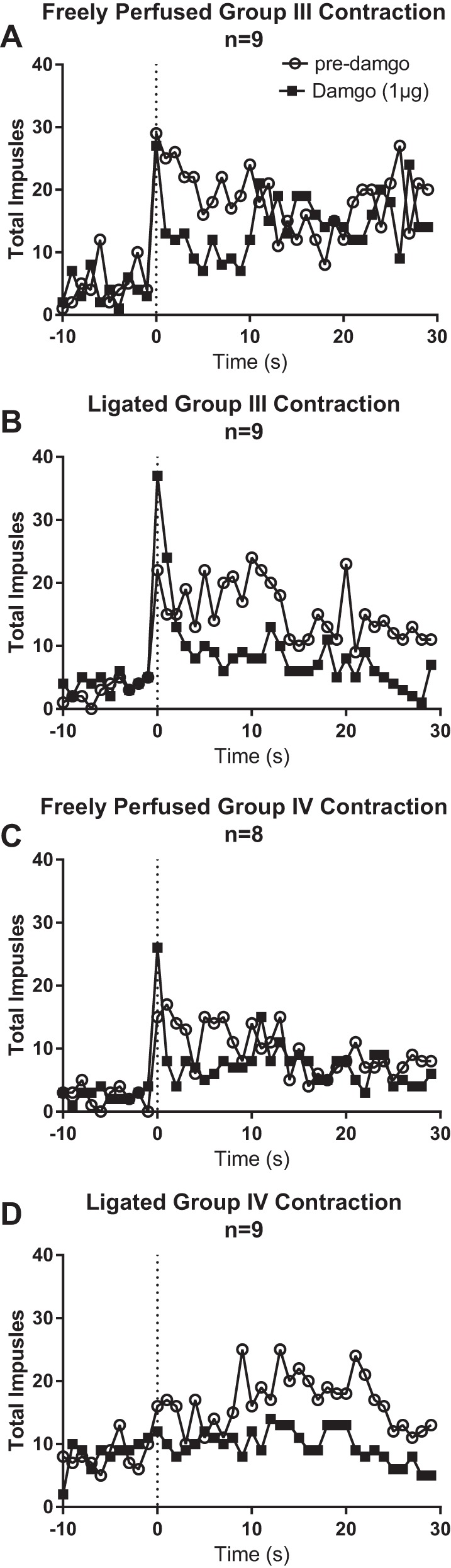
Second-by-second plots of the effect of DAMGO on the summed activity of the group III and IV afferents responsive to contraction. Open circles represent summed activity before injecting DAMGO, whereas closed squares represent summed activity after injecting 1 µg DAMGO into the superficial epigastric artery. “Freely perfused” rats show similar discharge rates for group III (A) and group IV (C) afferents before and after DAMGO. “Ligated” rats show reduced discharge rates for both group III (B) and group IV (D) afferents.
Before DAMGO injection, static contraction stimulated eight of the nine group IV afferents tested (conduction velocity = 0.9 ± 0.2 m/s; n = 9), activity increasing for these eight afferents from 0.4 ± 0.1 to 1.0 ± 0.2 impulses/s (P < 0.001, Fig. 2B). After DAMGO injection (1 µg), static contraction still stimulated these eight group IV afferents, activity increasing from 0.3 ± 0.1 to 0.7 ± 0.2 impulses/s (P < 0.001). On average, DAMGO did not significantly decrease the responses of these eight group IV afferents to contraction (P = 0.29; Fig. 3A). The tension-time indices did not differ before and after DAMGO (10.1 ± 1.6 kg*s before DAMGO vs. 9.3 ± 1.8 kg*s DAMGO; P = 0.34).
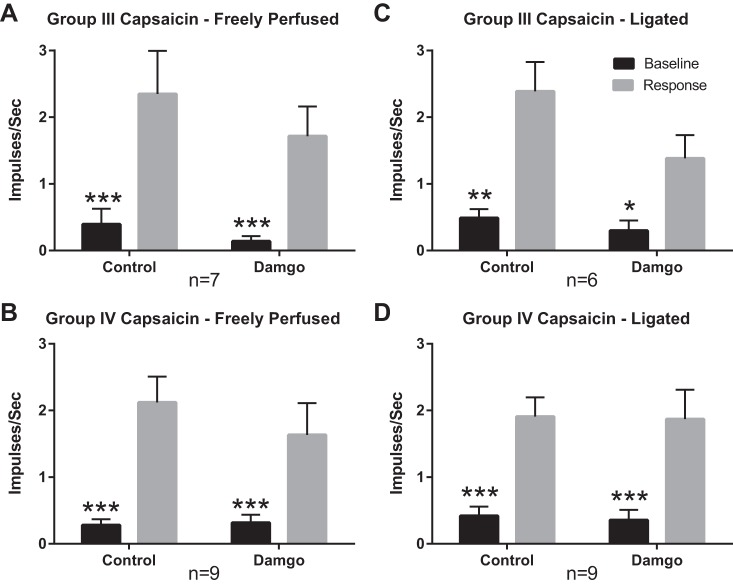
In addition to contraction, we examined in freely perfused rats, the responses of group III afferents to injecting capsaicin (0.2 µg in 0.1 ml) into the hindlimb circulation. Before DAMGO injection, capsaicin stimulated seven of the 13 group III afferents tested (conduction velocity = 3.5 ± 1.3 m/s; n = 13). Activity in the seven group III afferents responsive to capsaicin increased from 0.4 ± 0.2 to 2.4 ± 0.6 impulses/s (P < 0.001; Fig. 4A). After DAMGO injection (1 µg) into the hindlimb circulation, capsaicin still stimulated these seven group III afferents, activity increasing from 0.1 ± 0.08 to 1.7 ± 0.4 impulses/s (P < 0.01). On average, DAMGO did not significantly reduce the responses to capsaicin of these seven group III afferents (P = 0.34).
Fig. 4.
Summary data showing that the responses to capsaicin of group III and IV afferents were not reduced by DAMGO in either “freely perfused” or “ligated rats.” Black vertical bars represent mean values for the 30 s of control preceding capsaicin injection, whereas the gray vertical bars represent mean values for the 30 s following capsaicin (0.2 µg) injection into the superficial epigastric artery. Vertical brackets over each bar represent the standard error of the mean. Injecting DAMGO (1 µg) into the superficial epigastric artery did not significantly change the response in afferent fibers from freely perfused (A, B) or ligated (C, D) rats. *P < 0.05, **P < 0.01, ***P < 0.001 vs. capsaicin for same condition.
We also examined in freely perfused rats the responses of group IV afferents to injecting capsaicin (0.2 µg in 0.1 ml) into the hindlimb circulation. Before DAMGO injection, capsaicin stimulated nine of the 10 group IV afferents tested (conduction velocity = 0.9 ± 0.1 m/s; n = 10). Activity in the nine group IV afferents responsive to capsaicin increased from 0.3 ± 2.1 to 2.1 ± 0.4 impulses/s (P < 0.001; Fig. 4B). After DAMGO injection (1 µg) into the hindlimb circulation, capsaicin still stimulated these nine group IV afferents, activity increasing from 0.3 ± 0.1 to 1.6 ± 0.5 impulses/s (P < 0.001). On average, DAMGO did not significantly decrease the responses of the nine group IV afferents to capsaicin (p = 0.09).
Ligated rats.
Before DAMGO injection into the hindlimb circulation of ligated rats, static contraction stimulated nine of the nine group III afferents tested (conduction velocity = 3.9 ± 0.8 m/s; n = 9), activity increasing from a baseline of 0.4 ± 0.1 to 1.7 ± 0.3 impulses/s (P < 0.001, Fig. 2C). After DAMGO injection (1 µg), static contraction still stimulated each of the nine group III afferents, activity increasing from 0.4 ± 0.1 to 1.0. ± 0.2 impulses/s (P < 0.01). On average, DAMGO significantly decreased the responses of these nine group III afferents to static contraction (P < 0.01; Fig. 3C). The tension-time indices did not differ significantly (12.3 ± 1.4 kg*s before DAMGO vs. 12.8 ± 1.9 kg*s after DAMGO, P = 0.59).
Before DAMGO injection into the hindlimb circulation of ligated rats, static contraction stimulated nine of the 10 group IV afferents (conduction velocity = 1.1 ± 0.1 m/s; n = 9). The activity of these nine group IV afferents responsive to contraction increased from 0.8 ± 0.2 to 1.9 ± 0.3 impulses/s (P < 0.001, Fig. 2D). After DAMGO injection (1 µg), static contraction still stimulated these nine group IV afferents, activity increasing from 0.7 ± 0.2 to 1.1 ± 0.3 impulses/s (P < 0.01). On average, DAMGO significantly decreased the responses of these nine group IV afferents to static contraction (P < 0.01; Fig. 3D). The tension-time indices did not differ significantly (10.9 ± 1.2 kg*s before DAMGO vs. 10.7 ± 1.0 kg*s after DAMGO, P = 0.76).
In addition to contraction, we examined in ligated rats, the responses of group III afferents to injecting capsaicin (0.2 µg in 0.1 ml) into the hindlimb circulation. Before DAMGO injection, capsaicin stimulated six of the 12 group III afferents tested. The activity of the six group III afferents responsive to capsaicin increased from 0.5 ± 0.1 to 2.4 ± 0.4 impulses/s (P < 0.01; Fig. 4C). After DAMGO injection (1 µg) into the hindlimb circulation, capsaicin increased the activity in the six responsive afferents from 0.3 ± 0.2 to 1.4 ± 0.3 impulses/s (P < 0.05). On average, DAMGO injection did not significantly reduce the responses of the six group III afferents to capsaicin (P = 0.11).
We also examined in ligated rats the responses of group IV afferents to injecting capsaicin (0.2 µg in 0.1 ml) into the hindlimb circulation. Before DAMGO injection, capsaicin stimulated nine of the 11 group IV afferents tested (conduction velocity = 1.0 ± 0.1; n = 11), with activity increasing in the nine responsive afferents from 0.4 ± 0.1 to 1.9 ± 0.3 impulses/s (P < 0.001; Fig. 4D). After DAMGO injection (1 µg) into the hindlimb circulation, capsaicin increased activity in the nine responsive afferents from 0.4 ± 0.2 to 1.9 ± 0.4 impulses/s (P < 0.001). On average, DAMGO injection did not reduce the responses to capsaicin of these nine group IV afferents (P = 0.94).
Because it was possible that a 1-µg dose of DAMGO was inadequate to see an effect on group III and IV afferent responses to capsaicin, we performed additional testing using 10 µg DAMGO on a small set of afferents from ligated rats. In two group III afferents (conduction velocity = 2.0 ± 0.2 m/s), injecting capsaicin (0.2 µg in 0.1 ml) into the hindlimb circulation increased activity from 0.5 ± 0.4 to 2.3 ± 0.3 impulses/s. Repeating the capsaicin injection after injecting 10 µg DAMGO into the hindlimb circulation produced similar results, with activity increasing from 0.3 ± 0.1 to 2.1 ± 0.6 impulses/s. Similarly, in two group IV afferents (conduction velocity = 1.1 ± 0.01 m/s), injecting capsaicin (0.2 µg in 0.1 ml) into the hindlimb circulation increased activity from 0.3 ± 0.3 to 2.9 ± 0.6 impulses/s. Repeating the capsaicin injection after injecting 10 µg DAMGO into the hindlimb circulation produced similar results, with activity increasing from 0.4 ± 0.1 to 2.7 ± 0.4 impulses/s.
DISCUSSION
Conventional wisdom dictates that µ-opioid agonists exert their inhibitory effect by a central action rather than by a peripheral one. To be specific, the receptors for these agonists are located on spinal endings of primary afferents where they slow neurotransmitter release by blocking N-type calcium channels, or postsynaptically in the dorsal horn where they decrease impulse generation and neurotransmitter release by either blocking N-type calcium channels or opening inwardly rectifying potassium channels. The evidence for a central neural action of µ-opioid receptor agonists is strong. For example, stimulation of µ-opioid receptors by intrathecal injection of DAMGO, a µ-opioid receptor agonist, has been shown to attenuate the exercise pressor reflex in cats (Hill and Kaufman 1990), dogs (Pomeroy et al. 1986), and rats (Estrada and Kaufman In press). Likewise, intrathecal injection of fentanyl, a frequently prescribed µ-opioid receptor agonist, has been shown to attenuate the reflex in humans (Amann et al. 2010).
µ-Opioid receptors are also found on the peripheral endings and the axons of primary afferents (Coggeshall et al. 1997). This finding prompted us in a previous study to determine whether the stimulation of these receptors, which was accomplished by injecting DAMGO into the arterial supply of the triceps surae muscles, could attenuate the exercise pressor reflex in decerebrate unanesthetized rats. In rats with freely perfused femoral arteries, we found that DAMGO had no effect on the peak pressor response to static contraction but did exert a modest attenuation on the topography of the response. In contrast, in rats with ligated femoral arteries, DAMGO markedly attenuated the peak pressor response to contraction and substantially attenuated the topography of the response (Tsuchimochi et al. 2010b). In addition, we found that the DAMGO-induced attenuation of the exercise pressor reflex in ligated rats was prevented by prior injection of naloxone, an opioid receptor antagonist (Tsuchimochi et al. 2010b). Furthermore, intravenous injection of DAMGO did not attenuate the reflex, a finding that demonstrated the dose of DAMGO we injected intra-arterially did not circulate to the spinal cord and brain to exert its attenuating effect on the exercise pressor reflex. Together these findings provided strong indirect evidence that stimulation of µ-opioid receptors on either the peripheral endings or axons of group III and IV muscle afferents attenuated their responses to contraction.
The goal of our current experiments was to provide an electrophysiological basis for the “reflex findings” reported by Tsuchimochi et al. (2010b). In addition, we sought to determine whether the DAMGO-induced attenuation of the exercise pressor reflex in ligated rats was attributable to a decrease in responsiveness to contraction by group III afferents or by group IV afferents. The best way to make this determination was to count the total number of impulses generated by an afferent during the 30-s contraction period compared with the total number of impulses generated by that afferent during the 30-s control period immediately preceding the contraction. Using this method of analysis, we found that DAMGO significantly attenuated the responses of both group III and group IV afferents to contraction in rats whose femoral arteries were ligated. In contrast, DAMGO had no significant effect on the responses of either group III or group IV afferents in rats whose femoral arteries remained patent.
Hassan et al. (2014) compared the DAMGO-induced inhibition of calcium currents between isolated dorsal root ganglion (DRG) neurons innervating gastrocnemius muscles whose arterial supply was ligated and isolated DRG neurons innervating gastrocnemius muscles whose arterial supply was patent. Each of the neurons tested had NaV 1.8 channels and TRPV1 channels, findings which are consistent with the possibility that these neurons were the cell bodies of group III and IV muscle afferents. Hassan et al. (2014) found that a given concentration of DAMGO had a greater inhibitory effect on the inward calcium current evoked by depolarizing pulses applied to DRG neurons taken from rats with ligated femoral arteries than that in DRG neurons taken from rats with patent femoral arteries. Our in vivo findings in decerebrate unanesthetized rats, in which we activated group III and IV afferents by contracting the triceps surae muscles, confirm and extend the in vitro findings of Hassan et al. (2014), who activated group III and IV afferent cell bodies by depolarizing them with an electrical pulse.
We found that DAMGO injected into the arterial supply of the triceps surae muscles had no effect on the responses of group IV afferents to capsaicin, a TRPV1 agonist. This was the case regardless of whether we were recording afferent activity arising from triceps surae muscles whose arterial supply was patent or ligated. Moreover, in a few instances we increased the dose of DAMGO tenfold, but this still did not attenuate the responses of the afferents to capsaicin. As a result, we speculate that any decreases in the thin fiber afferents’ responses to capsaicin subsequent to injecting DAMGO into the femoral arterial circulation were probably attributable to tachyphylaxis rather than to stimulation of μ-opioid receptors. In rat spinal cord slices, Chen et al. (2018) reported that neither DAMGO nor ω-conotoxin GVIA, an N-type calcium channel antagonist, had any effect on the internalization of neurokinin 1 receptors, which is a measure of substance P release by thin fiber primary afferents. Chen et al. (2018) concluded that capsaicin opened TRPV1 receptors, which in turn allowed the passage of sufficient calcium to release substance P from primary afferents. Extending this conclusion to our experiments suggests that DAMGO, which blocks N-type calcium receptors, would have no effect on capsaicin-induced stimulation of group III and IV afferents because the TRPV1 receptor does not rely on the N-type calcium receptor to pass calcium ions across the afferents’ cell membranes.
In our experiments in rats, capsaicin stimulated ~50% of the group III afferents tested, whereas it stimulated almost 90% of the group IV afferents tested. This finding in rats is similar to that in cats (Kaufman et al. 1983) and dogs (Kaufman et al. 1982), in which capsaicin was found to stimulate significantly more group IV muscle afferents than group III muscle afferents. Nevertheless, capsaicin stimulated a higher percentage of group III afferents in rats (i.e., 50%) than it did in either cats (i.e., 26%) or dogs (i.e., 25%). This difference might be attributable, at least in part, to the fact that afferents conducting impulses at 1.5 m/s or more were classified by us as group III in rats, whereas afferents conducting impulses at 2.5 m/s or more were classified as group III in dogs and cats. Consequently, some slowly conducting group III afferents in rats might have unmyelinated axons and may therefore have response profiles more similar to group IV fibers than to group III fibers.
The cutoff distinguishing group III from group IV afferents is somewhat arbitrary and is usually based on compound action potential measurements. In rats, this cutoff has been placed at less than 1.5 m/s by some (Diehl et al. 1993; Reinöhl et al. 2003), and at less than 2.0 m/s by others (Lawson and Waddell 1991; Waddell et al. 1989). In our experiments, we used the lower of the two conduction velocities as the cutoff for group IV afferents; however, had we used the upper cutoff, the percentage of group III afferents responsive to capsaicin in rats would have been similar to that of either cats or dogs.
The exercise pressor reflex is exaggerated in patients with peripheral artery disease. Moreover, some of these patients report leg pain (i.e., claudication) when exercising. Our finding that intra-arterial injection of a μ-opioid agonist, namely DAMGO, decreased the responses of group III and IV afferents to contraction in rats with ligated femoral arteries raises the possibility that stimulation of these peripheral opioid receptors by an orally administered μ-opioid agonist that does not cross the blood-brain barrier might be a useful treatment for both the claudication and the exaggerated exercise pressor reflex occurring in patients with peripheral artery disease. Over time, the latter effect can lead to left ventricular hypertrophy and cardiac arrhythmias in these patients. In addition, a peripherally active μ-opioid agonist that does not cross the blood-brain barrier might prevent the dependence and ventilatory depression known to occur with opioids that have access to the central nervous system.
GRANTS
This work was supported by NIH grants R01 AR 059397 and P01 HL 134609.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.H., A.J.S., and M.P.K. conceived and designed research; J.E.H. and A.J.S. performed experiments; J.E.H. and A.J.S. analyzed data; J.E.H., A.J.S., and M.P.K. interpreted results of experiments; J.E.H. prepared figures; J.E.H. and M.P.K. drafted manuscript; J.E.H., A.J.S., and M.P.K. edited and revised manuscript; J.E.H., A.J.S., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joyce Kim and Andrea Noel for technical assistance.
REFERENCES
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke EF, Hisdal J, Jørgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007. doi: 10.1016/j.ejvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Chen W, Ennes HS, McRoberts JA, Marvizón JC. Mechanisms of μ-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord. Neuropharmacology 128: 255–268, 2018. doi: 10.1016/j.neuropharm.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res 764: 126–132, 1997. doi: 10.1016/S0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Exp Brain Res 92: 431–440, 1993. doi: 10.1007/BF00229031. [DOI] [PubMed] [Google Scholar]
- Estrada JA, Kaufman MP. Mu opioid receptors inhibit the exercise pressor reflex by closing N-type calcium channels but not by opening GIRK channels in rats. Am J Physiol Regul Integr Comp Physiol. In press. doi: 10.1152/ajpregu.00380.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan B, Kim JS, Farrag M, Kaufman MP, Ruiz-Velasco V. Alteration of the mu opioid receptor: Ca2+ channel signaling pathway in a subset of rat sensory neurons following chronic femoral artery occlusion. J Neurophysiol 112: 3104–3115, 2014. doi: 10.1152/jn.00630.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol (1985) 68: 2466–2472, 1990. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res 50: 133–139, 1982. doi: 10.1161/01.RES.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435: 41–63, 1991. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res 381: 385–389, 1986. doi: 10.1016/0006-8993(86)90095-8. [DOI] [PubMed] [Google Scholar]
- Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M, Baccelli G. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- Reinöhl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett 338: 25–28, 2003. doi: 10.1016/S0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008. doi: 10.1113/jphysiol.2007.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010a. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Kaufman MP. Peripheral μ-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010b. doi: 10.1152/ajpheart.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von During M, Andres KH. Topography and ultrastructure of group III and IV nerve terminals of cat’s gastrocnemius-soleus muscle. In: The Primary Afferent Neuron: A Survey of Recent Morpho-Functional Aspects, edited by Zenker W, Neuhuber WL. New York: Plenum, 1990, p. 35–41. doi: 10.1007/978-1-4613-0579-8_3. [DOI] [Google Scholar]
- Waddell PJ, Lawson SN, McCarthy PW. Conduction velocity changes along the processes of rat primary sensory neurons. Neuroscience 30: 577–584, 1989. doi: 10.1016/0306-4522(89)90152-8. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. doi: 10.1113/jphysiol.2012.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]