Abstract
Animals must perform spatial navigation for a range of different behaviors, including selection of trajectories toward goal locations and foraging for food sources. To serve this function, a number of different brain regions play a role in coding different dimensions of sensory input important for spatial behavior, including the entorhinal cortex, the retrosplenial cortex, the hippocampus, and the medial septum. This article will review data concerning the coding of the spatial aspects of animal behavior, including location of the animal within an environment, the speed of movement, the trajectory of movement, the direction of the head in the environment, and the position of barriers and objects both relative to the animal’s head direction (egocentric) and relative to the layout of the environment (allocentric). The mechanisms for coding these important spatial representations are not yet fully understood but could involve mechanisms including integration of self-motion information or coding of location based on the angle of sensory features in the environment. We will review available data and theories about the mechanisms for coding of spatial representations. The computation of different aspects of spatial representation from available sensory input requires complex cortical processing mechanisms for transformation from egocentric to allocentric coordinates that will only be understood through a combination of neurophysiological studies and computational modeling.
Keywords: boundary cells, entorhinal cortex, grid cells, head direction cells, speed cells
BRAIN REGIONS INVOLVED IN SPATIAL NAVIGATION
Navigation through space is important for a range of different behavioral functions. Navigation can be defined as selecting a behavioral trajectory to transition from a current location to a goal location. As reviewed previously, navigation can involve different levels of complexity (Eichenbaum 2017; O’Keefe and Nadel 1978; Trullier et al. 1997), ranging from navigation to a visible goal (taxis), to following a previously learned route (route-following), to flexible selection of novel trajectories to a goal in a two-dimensional environment (map-based navigation). Goal-directed behavior based on map-based navigation will be the focus of this review, with an emphasis on available data and outstanding questions concerning the mechanisms for coding spatial representations, which include coding based on self-motion information or coding based on sensory input (Fig. 1), and the associated problems of transformation between different egocentric vs. allocentric coordinate systems (Byrne et al. 2007).
Fig. 1.
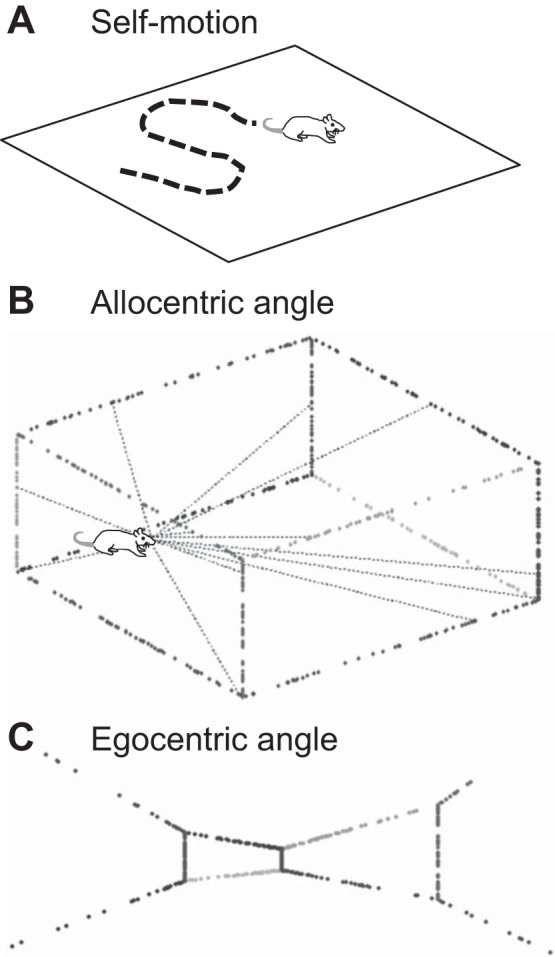
A: computation of the location of an animal in an environment can be determined by obtaining a velocity signal based on the self-motion of the animal. Integration of velocity will provide an update of the current location (McNaughton et al. 2006). B: alternately, location can be determined by cortical computations based on the allocentric angle and distance to visual features (Raudies and Hasselmo 2015). C: the computation of location based on feature angle requires transformation from the coordinates of feature angle in egocentric coordinates to feature angle in allocentric coordinates based on the current head direction of the animal.
As an example of map-based navigation, consider an animal that starts at one location and encounters a food source in a specific spatial location in an environment. On a subsequent day, if the animal starts at a different location, the animal could use map-based navigation to select a novel trajectory back to the previously visited food location. Rodents provide a particularly good species for the study of navigation, due to factors such as their natural behavior in foraging for food in multiple locations, and due to their small size that allows testing of navigation across environments multiple times larger than their body size. However, studies in humans have utilized virtual environments to analyze navigation behavior.
This review will focus on neurophysiological data, with an emphasis on the mechanisms of generation of spatial representations observed in different brain regions, which could depend on complex computations based on self-motion or the egocentric angle of sensory features (Fig. 1). Historically, the behavioral effects of damage to different brain structures provided first hints to the brain regions possibly involved in map-based navigation. Theories about the functional and computational roles of these structures and their connections to other brain regions prompted experimental and modeling studies aiming to identify the physiological mechanisms of navigation. In particular, a range of studies show that damage to the hippocampus causes impairments in the Morris water maze, which requires learning of a specific goal location and navigating to that location from a range of starting locations (Eichenbaum 2017; Morris et al. 1982). As described below, this relates to important physiological data on coding of location by place cells in the hippocampus. But beyond the hippocampus, additional structures are involved in coding spatial representations for goal-directed behavior. In particular, impairments of goal finding in the Morris water maze are also observed after lesions of the entorhinal cortex (Steffenach et al. 2005), after pharmacological inactivation of the medial septum (Brioni et al. 1990) or lesions of the medial septum (Marston et al. 1993), and after lesions of the dorsal presubiculum (Taube et al. 1992). Performance in this task is also impaired after lesions of the retrosplenial cortex (Czajkowski et al. 2014) and learning of the task is impaired after parietal lesions (Hoh et al. 2003).
Neurophysiological studies of spatial representation often require that animals cover a wide range of locations and directions during recording, so commonly the spatial responses of neurons are evaluated during foraging in an open field (Hafting et al. 2005; Muller et al. 1987). If this foraging were completely random, it would not require memory-based navigation, but in many cases the memory of previously visited locations that have been depleted of food must be maintained to allow navigation to avoid recently visited previous locations. This is particularly true in tasks such as the delayed spatial alternation tasks (Ainge et al. 2007) or the eight-arm radial maze (Otto et al. 1997), which both require that animals select trajectories to reward locations but also that they remember the response from preceding trials to avoid repetition of a response to a location depleted of reward. These tasks are also impaired by lesions of the hippocampus (Aggleton et al. 1986; Olton et al. 1979) and the medial septum (Chrobak et al. 1989; Givens and Olton 1990). Thus, the water maze, open field, spatial alternation, and radial maze require encoding and recognizing information about the overall spatial environment to remember the context of the specific behavioral task in that environment.
CODING OF SPATIAL REPRESENTATIONS FOR GOAL-DIRECTED BEHAVIOR
Successful performance of both goal directed behavior and foraging behavior require some internal representation of spatial aspects of an animal’s interaction with the environment. These spatial representations have been studied with recordings in behaving animals in structures including hippocampus, entorhinal cortex, medial septum, and retrosplenial cortex. Before considering the neurophysiological recording, we will consider insights about necessary spatial representations that can be obtained from robotics research, in which the robotic engineer must develop systems for coding specific spatial representations to guide behavior by an autonomous agent (Milford et al. 2010; Milford and Wyeth 2010). For example, robotic navigation commonly uses coding of the “pose” of the agent, which includes both the spatial location of the agent (usually in Cartesian coordinates) as well as the current heading direction of the agent. Robotics research also contains important clues about potential mechanisms for coding of spatial representations, indicating that these representations can be computed from self-motion information or from the angle of sensory features relative to the agent. These computations require complex transformations for example between the egocentric angle of a visual feature and the Cartesian coordinates of that same feature in an internal representation of allocentric space (Byrne et al. 2007; Milford et al. 2010; Milford and Wyeth 2010).
In particular, robotics focuses on coding of the location of an agent using frameworks such as simultaneous localization and mapping (SLAM) (Milford and Wyeth 2010). Robotics commonly computes location by convergence of information about self-motion and sensory input. The velocity of self-motion of an agent can be computed using “wheel odometry,” in which the speed and direction of self-motion are computed from the means of propulsion, or “visual odometry,” in which the speed and direction of movement are computed from egocentric sensory input, usually concerning visual features. Alternately, location can be coded by the angle of visual features independent of movement velocity. Vision-based navigation requires a range of complex computations, including the recognition of visual features, the coding of barriers and objects, and the transformation from the coordinates relative to the heading of the agent (egocentric coordinates) into coordinates of the absolute position of the agent relative to the overall environment (allocentric coordinates). These essential components of robotics navigation suggest that computations based on self-motion or computations based on the angle of sensory features provide potential mechanisms for the coding of spatial representations shown by neurons in different regions of the rodent brain. The following paragraphs will focus on the neurophysiological data showing spatially tuned neurons and their implementation in computational models for navigation.
Place cells.
Influential work by John O’Keefe and colleagues demonstrated that neurons in the hippocampus termed place cells respond selectively on the basis of the spatial location of a rat (O’Keefe and Dostrovsky 1971; O’Keefe 1976). This motivated an influential theoretical framework for behavior in which the hippocampus serves as a cognitive map (O’Keefe and Nadel 1978). In line with the role of the hippocampal formation in establishing a cognitive map of the environment, the hippocampus is essential for spatial navigation tasks if map-based navigation is used as the strategy. Despite many decades of research, the mechanism for generation of hippocampal place cells has not been fully described, though data indicates that place cells respond based on both self-motion cues and sensory input cues (Gothard et al. 1996; Muller and Kubie 1987; Terrazas et al. 2005). Studies have proposed that place cells could arise from path integration in other regions that drive the hippocampal place representation (Redish and Touretzky 1997, 1998; Touretzky and Redish 1996) or from attractor dynamics in recurrent loops of excitatory connectivity within region CA3 (Samsonovich and McNaughton 1997). These attractor dynamics were proposed to perform path integration based on self-motion (Conklin and Eliasmith 2005). As an alternative, earlier models proposed that place cells could be driven by the learned response to visual features in particular configurations (Byrne et al. 2007; Hetherington and Shapiro 1993). These responses would require a transformation of egocentric feature angle to allocentric position based on the current heading direction of the animal (Byrne et al. 2007). Recent studies have shown that, after extensive behavioral training, hippocampal neurons can map other sensory dimensions such as auditory frequency space (Aronov et al. 2017), but it should be noted that place cells automatically respond to space even in tasks which do not require map-based navigation or foraging (Kentros et al. 2004).
Head direction cells.
The theory of a cognitive map was initially based on the findings of hippocampal place cells but was further supported by the discovery of cells coding additional spatial aspects of behavior. For example, neurons coding the head direction of rodents were discovered in the dorsal presubiculum by Ranck and Taube (Taube et al. 1990) and have been described in a range of structures including the anterior thalamus (Taube 1995), the lateral mammillary nucleus (Stackman and Taube 1998), the entorhinal cortex (Brandon et al. 2013; Giocomo et al. 2014; Sargolini et al. 2006), and the retrosplenial cortex (Chen et al. 1994; Cho and Sharp 2001). Head direction cells respond selectively based on the current allocentric direction of the animal’s head (Fig. 2), regardless of the location of the animal in the environment and independent of the relative position of individual landmarks or features. The existence of head direction cells is particularly important to the mechanisms for transformation of egocentric coordinates into allocentric coordinates, as it is essential to know allocentric head direction if one is to transform egocentric coordinates of sensory feature angle into allocentric location (Byrne et al. 2007; Touretzky and Redish 1996). Consistent with this, lesions of the dorsal presubiculum causes destabilization of hippocampal place cells (Goodridge and Taube 1997). On the other hand, papers that propose that self-motion can be used for path integration commonly cite head direction cells as the directional signal for velocity. Unfortunately, the head direction signal is not equivalent to a movement direction signal, as behavioral head direction does not always directly match behavioral movement direction, and analysis of periods where head direction does not match movement direction shows that these cells primarily code head direction (Raudies et al. 2015).
Fig. 2.
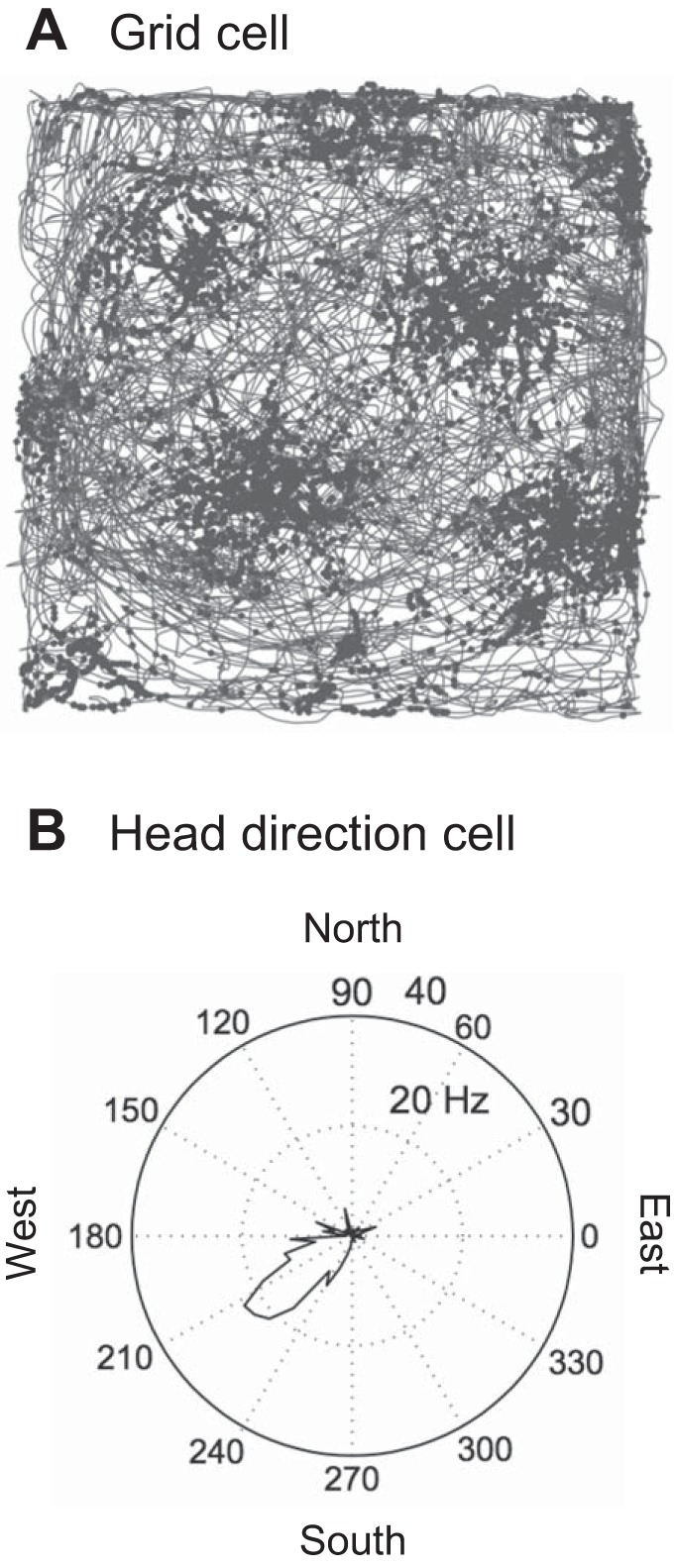
A: firing of a grid cell during foraging in a two-dimensional environment, shown in example data from Brandon et al. (2011). Grid cells were discovered in the Moser laboratory (Hafting et al. 2005). The gray line indicates the trajectory of the animal during foraging in an open field environment with barriers. The dots indicate the location of the animal each time an individual grid cell fires a spike. B: response of a head direction cell in example data from entorhinal recording in Brandon et al. (2013). Head direction cells were discovered by Ranck and Taube (Taube et al. 1990). This head direction cell changes firing rate based on head direction at any location in the environment. The firing rate is plotted in polar coordinates for 360° of head direction of the animal (gathering data across all locations in the environment). This neuron shows higher firing rates when the animal’s head is pointed toward the southwest, with a peak firing rate of ~20 Hz.
In addition to analysis of their role in generation of place cells, the mechanism of generation of head direction cells has been described extensively (Taube et al. 1996; Taube 1998). Signals coding the angular velocity of the head arise from both vestibular sensory input and motor efference copy and are computed in brain stem structures (Clark and Taube 2012). This angular velocity signal based on self-motion appears to be integrated to drive head direction neurons in structures such as the anterior thalamus, lateral mammillary nucleus, and dorsal tegmental nucleus (Clark and Taube 2012; Taube and Bassett 2003). The influences of the angle of sensory cues on head direction responses involve interactions with cortical sensory input in structures such as the dorsal presubiculum (Calton et al. 2003) that receive input from association cortices such as the retrosplenial cortex as described further below under spatial navigation in posterior parietal and retrosplenial association cortices.
Grid cells.
Neurons in the entorhinal cortex termed grid cells were demonstrated to respond when a foraging animal visits an array of spatial locations in the environment (Hafting et al. 2005). The firing fields fall in a regular array of locations that can be described as falling on the vertices of tessellated equilateral triangles, or in a hexagonal pattern (Fig. 2). These neurons provided an exciting new perspective on mechanisms of spatial coding because their repeating firing fields indicated a potential metric code for position across the full environment. Different grid cells show different spatial scales, allowing a population of grid cells to code a single location (Barry et al. 2007; Hafting et al. 2005; Sargolini et al. 2006; Stensola et al. 2012). Many grid cells conjunctively code both location and the current head direction of the animal (Sargolini et al. 2006). Similar to place cells, the mechanism of grid cell firing remains uncertain, but the regular firing pattern immediately suggested different mechanisms.
For example, grid cells could arise from attractor dynamics. These models build on extensive previous models of continuous attractors (Amari 1977) which were used in a ring configuration to model the response of head direction cells (Redish et al. 1996; Zhang 1996). Attractor models were extended to grid cells based on two-dimensional circularly symmetric inhibitory interactions between neurons, which result in a pattern of “bumps” of activity (Burak and Fiete 2009; Conklin and Eliasmith 2005; Fuhs and Touretzky 2006; Giocomo et al. 2011; McNaughton et al. 2006; Widloski and Fiete 2014; Yoon et al. 2013). These attractor bumps can be shifted across the population by neurons responding to speed and movement direction, providing a mechanism for integration of velocity based on self-motion (path integration). Data supporting a role for attractor dynamics in generation of grid cell firing includes the properties of distinct modules of grid cells that share properties such as spacing and orientation (Barry et al. 2007; Stensola et al. 2012) and the change in correlation of grid cell firing with different anatomical distances between grid cells (Heys et al. 2014). Path integration models of grid cells and place cells suffer from the integration of error over time, but this can be reduced by reset of path integration based on sensory cues (Bush and Burgess 2014; Hardcastle et al. 2015; Touretzky and Redish 1996).
Alternately, path integration based on self-motion can be obtained by coding velocity by a shift in the frequency of different oscillators and then computing the interference between oscillators to obtain a peak of activity at the current location (Burgess et al. 2007; Burgess 2008; Bush and Burgess 2014; Giocomo et al. 2011; Hasselmo 2008, 2014). These mechanisms link very effectively to data on the firing of grid cells relative to theta rhythm oscillations in the entorhinal cortex (Hafting et al. 2008) and the fact that reductions in theta rhythm oscillations associated with inactivation of the medial septum are associated with loss of the spatial coding by grid cells (Brandon et al. 2011; Koenig et al. 2011), whereas the coding of head direction in entorhinal cortex (Brandon et al. 2011) and place cell firing in hippocampus is spared (Brandon et al. 2014).
The role of oscillatory dynamics in coding of grid cells is supported by evidence that the intrinsic rhythmicity of entorhinal neurons differs with spatial scale (Jeewajee et al. 2008) and shifts with running speed (Hinman et al. 2016; Jeewajee et al. 2008). The integration of self-motion would be sensitive to any noise in the system, but the influence of internal noise can be reduced by redundant coding in a population of neurons (Zilli and Hasselmo 2010) or combining interference with attractor dynamics (Bush and Burgess 2014; Hasselmo and Shay 2014). The influence of external noise can be reduced by resetting location based on the angle of sensory cues (Bush and Burgess 2014). Grid cells show more accurate coding near environmental boundaries than at a distance from boundaries (Hardcastle et al. 2015), consistent with an influence of sensory cues near boundaries. The role of sensory input angle in grid cell firing is further supported by evidence that grid cells lose spatial coding during inactivation of regions providing head direction input to medial entorhinal cortex (Winter et al. 2015a). Head direction cells may be more important for coding sensory feature angle than self-motion given that they do not code path direction in locomotion (Raudies et al. 2015), though they can code angular head motion (Clark and Taube 2011).
Yet another different proposed mechanism for grid cells involves the self-organization of input from place cells (Kropff and Treves 2008; Si et al. 2012). In this model, the spike-frequency accommodation of entorhinal neurons results in a time-varying response to input from place cells that causes spatially varying modification of excitatory inputs from hippocampus to entorhinal cortex that self-organizes into the pattern of grid cell responses. This model would specifically depend on the mechanism of generation of place cell responses, which could depend on either self-motion or sensory feature angle. These and other models that generate grid cells based on place cell input (Dordek et al. 2016; Krupic et al. 2014) are supported by evidence from developmental studies showing that place cells appear earlier than grid cells (Wills et al. 2010, 2012), and evidence that place cell inactivation reduces grid cell firing (Bonnevie et al. 2013), whereas grid cell inactivation does not prevent place cell firing (Brandon et al. 2014), though changes in grid cell scale do influence place cell scale (Mallory et al. 2018).
Boundary cells.
One striking set of data concerning the mechanism of place cell firing shows that they depend on the position of the walls of the environment (O’Keefe and Burgess 1996), leading to the novel proposal that a specific class of neurons might code the position of an animal relative to boundaries (Burgess et al. 2000; Hartley et al. 2014; Savelli et al. 2008). This novel prediction was later supported by data showing the existence of boundary cells that respond at a specific distance and angle from boundaries (Lever et al. 2009; Savelli et al. 2008; Solstad et al. 2008). Alterations of visual boundaries also influence the location of grid cell firing fields. The spacing of grid cell firing fields is compressed or expanded by movements of the environment walls (Barry et al. 2007), and this change may be specific to grid cells with larger spacing while sparing smaller spacing (Stensola et al. 2012) though an earlier study showed compression of cells with small spacing (Barry et al. 2007). The response of grid cells to barrier movement has been modeled based on selective influences of the angle and optic flow of visual cues from different parts of the visual field (Raudies and Hasselmo 2015). This is related to models showing how egocentric visual input about boundaries could be transformed to code allocentric spatial location (Byrne et al. 2007).
Speed cells.
The mechanisms for coding of the spatial representations described above are not yet fully understood, but there are neuronal responses that seem to correspond to wheel odometry, to visual odometry, and to location coding based on visual feature angle. For example, we described the importance of coding of speed and direction for using velocity to code location. As described in detail in mechanisms for coding of running speed below, running speed has been shown to be coded by a number of different functional classes of neurons in the entorhinal cortex (Fig. 3) (Hinman et al. 2016; Kropff et al. 2015; Wills et al. 2012) and hippocampus (McNaughton et al. 1983). This could clearly provide a component of a velocity code. However, it is not clear how effectively the directional component of velocity is computed, as head direction cells code head direction rather than the movement direction required to integrate the velocity of an animal (Raudies et al. 2015).
Fig. 3.
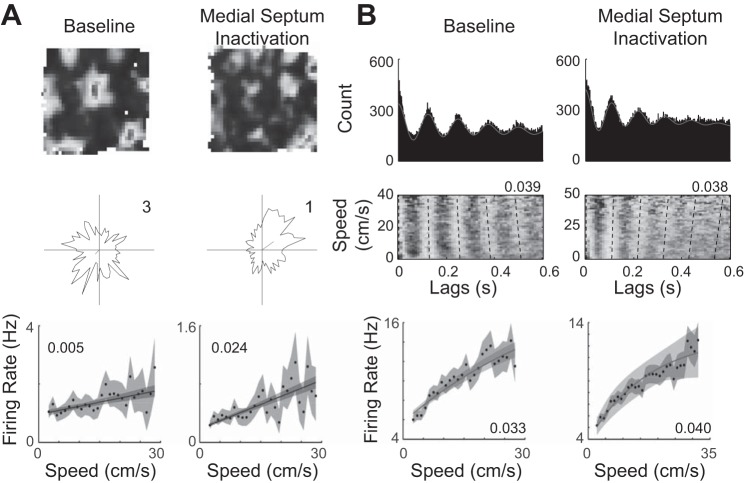
Coding of running speed by neurons in the medial entorhinal cortex. A: the three rows show a spatial rate map (top), head direction polar plot (middle), and running speed tuning curve for an example grid cell during a baseline recording (left) and from recording during inactivation of the medial septum (right). The grid cell loses its spatial periodicity during medial septum inactivation but has an increased linear firing rate speed signal. B: a spike time autocorrelation (top), density plot of spike time lags as a function of running speed (middle), and running speed tuning curve are shown during a baseline recording (left) and from recording during inactivation of the medial septum (right). During the baseline recording the oscillation frequency increases as a function of running speed, but in the absence of medial septum input the oscillatory frequency decreases as a function of running speed. Similar to the linear firing rate speed signal in A, the saturating firing rate speed signal of this cell increases in strength during medial septum inactivation. Example data adapted from Hinman et al. (2016) with permission from Elsevier.
Context-dependent cells.
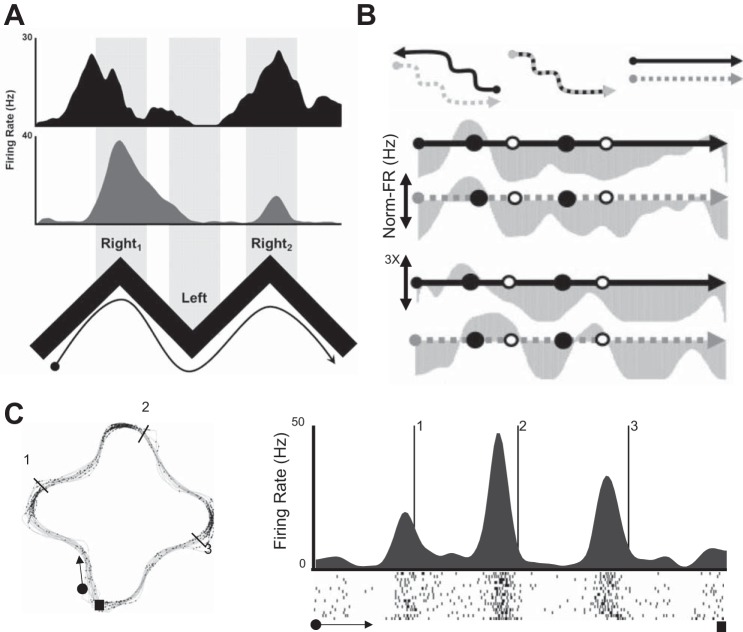
Extensive data shows that the coding of spatial representations is highly context dependent. Place cells in one environment will often turn off in a different environment and be replaced by other place cells that were inactive in the first environment (Lever et al. 2002; Muller and Kubie 1987). Even within an environment, neurons will show strongly context-dependent activity. For example, when a rat runs on the stem of a T maze during a spatial alternation task, individual neurons will fire selectively based on the past or future turning response in hippocampus (Ferbinteanu and Shapiro 2003; Wood et al. 2000) and entorhinal cortex (Frank et al. 2000). Neurons also code other features of the environment such as the identity of odors or the presence of reward (Eichenbaum et al. 1987; Wood et al. 1999). Additionally, recent work has revealed that spatially specific but nongrid cells of the medial entorhinal cortex (MEC) globally remapped in response to contextual changes to an environment (Diehl et al. 2017). Grid cells, on the other hand, have been found to exhibit either nodal rate alterations or translational shifts of the full map in response to contextual alterations to the environment (Diehl et al. 2017; Marozzi et al. 2015). There is some evidence that contextually sensitive or nonsensitive MEC neurons actually form molecularly and anatomically distinct subpopulations within the region (Kitamura et al. 2014, 2015; Ray et al. 2014). Together, these data indicate that spatial representations are not coded in isolation but are combined with many other features relevant to performance of a behavioral task.
Time cells.
The term “time cells” refers to neurons that respond at specific time intervals during behavioral tasks. The models of grid cell firing in entorhinal cortex can account for the fact that many grid cells and place cells will also respond at specific time intervals during running (Burgess et al. 2007; Hasselmo 2008). For example, in a spatial alternation task, neurons will fire at specific intervals during running on a running wheel (Pastalkova et al. 2008) or a treadmill (Kraus et al. 2013, 2015). These cells have therefore been described as time cells (MacDonald et al. 2011). Consistent with models of grid cells dependent on oscillatory input, the inactivation of medial septum also prevents the temporal specificity of firing by time cells (Wang et al. 2015).
Mechanisms for goal-directed behavior.
Once spatial representations of the environment have been formed based on self-motion or external sensory cues, they can be used to guide behavior. The available data on coding of spatial representations have inspired proposals for a number of different mechanisms for goal-directed behavior. Early models of goal-directed behavior were based on place cells, requiring the full environment to be tiled by the place fields of a large population of place cells (Burgess et al. 1997; Gerstner and Abbott 1997; Redish and Touretzky 1998), so that navigation could be guided by the spread of activity from a goal, or by asymmetric connections between neurons. The discovery of grid cells provides an advantage over these mechanisms, as the repeating firing patterns of grid cells suggest that they code a metric for location across the full environment. Models of grid cells were used to simulate how the forward readout of locations based on grid cell firing patterns allow selection of either the direct vector to a goal location (Bush et al. 2015; Erdem and Hasselmo 2012, 2014; Kubie and Fenton 2012) or a pathway dependent on obstacles (Erdem and Hasselmo 2012; Spiers and Gilbert 2015). Experimental data suggests that replay of place cell sequences may be associated with goal-directed navigation (Johnson and Redish 2007; Pfeiffer and Foster 2013; Ólafsdóttir et al. 2015). Sequential firing of place cells within a theta cycle have been found to reflect future trajectories to a goal location providing one possible neural mechanism for prospective planning (Wikenheiser and Redish 2015).
Human data.
These models of goal directed navigation are supported by functional MRI (fMRI), magnetoencephalography (MEG), and intracranial EEG data in humans. fMRI data demonstrated hippocampal and parahippocampal activity at the start of a trajectory when a subject retrieves a previously learned overlapping trajectory (Brown et al. 2010; Brown and Stern 2014). Subsequent work showed that retrieval of a planned trajectory involves reactivation of intermediate locations on the trajectory (Brown et al. 2016). These data are consistent with MEG data in humans demonstrating that hippocampal and parahippocampal theta activity correlates with goal-directed navigation performance in a virtual Morris water maze task with hippocampal theta activity at the start of navigation predicting performance and hippocampal theta activity being higher during the first trials during learning of the task (Cornwell et al. 2008). Likewise, theta oscillations recorded in the medial temporal lobe of epileptic patients have been shown to increase during navigation through real-world and virtual environments (Bohbot et al. 2017; Ekstrom et al. 2005; Kahana et al. 1999). Studies show coding of navigationally relevant information in fMRI activity, with hippocampus coding path length and entorhinal cortex coding the Euclidean distance to the goal (Chrastil et al. 2015; Howard et al. 2014). Modeling shows how interaction of brain regions can allow performance based on semantic memory for the allocentric position of landmarks translated to the egocentric position of landmarks (Byrne et al. 2007). These models and other work on the influence of visual cues (Raudies and Hasselmo 2015) are relevant to data showing the interaction of regions coding visual cues such as optic flow and feature angle with regions involved in navigation (Chrastil et al. 2015; Sherrill et al. 2015).
The subsequent sections will discuss potential neural mechanisms for coding of spatial representations. First, we will discuss neural coding in subcortical regions based on self-motion similar to odometry, which could provide coding of the speed and direction of movement. Second, we will discuss neural coding in cortical regions based on sensory cues that could transform visual input into either location or velocity codes, making transitions from egocentric coding of movements to their position along a trajectory or their allocentric position within an environment.
CODING BASED ON SELF-MOTION: ROLE OF THE MEDIAL SEPTUM
The medial septum plays an important role in the coding of spatial representations, as demonstrated by both behavioral and neurophysiological data. Behavioral studies demonstrate effects of lesions of the medial septum or the fornix, which is the primary fiber pathway between the medial septum and the hippocampus. These lesions cause impairments in spatial memory tasks similar to lesions of the hippocampus. Lesions of the medial septum and fornix cause memory impairments in tasks including delayed spatial alternation (Aggleton et al. 1995; Givens and Olton 1990), delayed nonmatch to position (Markowska et al. 1989), delayed alternation (Numan and Quaranta 1990), spatial reversal (M’Harzi et al. 1987), the Morris water maze (Martin et al. 2007), and the eight-arm radial maze (Mitchell et al. 1982). These same lesions of medial septum or fornix reduce theta power in the hippocampus (Rawlins et al. 1979) and entorhinal cortex (Mitchell et al. 1982). Reduction in hippocampal theta rhythm correlates with impairments in memory (Winson 1978) that are specific for recently experienced episodes but not for highly familiar memories (Givens and Olton 1994; M’Harzi et al. 1987). Temporary inactivation of the medial septum impairs spatial memory and reduces theta rhythm in both the hippocampus (Brioni et al. 1990; Chrobak et al. 1989; Mizumori et al. 1990) and entorhinal cortex (Jeffery et al. 1995). These data indicate an important behavioral role of the medial septum in spatial memory function.
Neurophysiological data also supports this important functional role. Pharmacological inactivation of medial septal activity results in a disruption of grid cell spatial response properties (Brandon et al. 2011; Koenig et al. 2011), though the response of head direction cells in medial entorhinal cortex is retained (Brandon et al. 2011) as well as the firing of place cells (Brandon et al. 2014). These data indicate that input from the medial septum is important for the coding of spatial location by grid cells, though the specific mechanisms requiring medial septal input are still being studied, as reviewed below. This loss of grid cell location specificity could underlie the spatial memory impairments mentioned above with lesions of the medial septum or the fornix (Brioni et al. 1990; Chrobak et al. 1989; Mizumori et al. 1990).
THETA RHYTHM AND SPATIAL CODING
In rabbits and rodents, spatial exploration and the formation of spatial memory is strongly correlated with a prominent theta oscillation of 6–10 Hz in hippocampal structures. Theta rhythmic oscillatory activity might contribute to navigation and spatial memory formation in several ways. First, it can act as a global synchronizing mechanism within the hippocampal formation itself, but also across different brain regions. For instance, theta rhythmic activity coordinates hippocampal-prefrontal cortex interactions in spatial working memory tasks (Benchenane et al. 2010; Hyman et al. 2005; Jones and Wilson 2005). Second, theta rhythmic activity is thought to provide a temporal framework for the relative timing of spikes inside or across regions (Skaggs et al. 1996; Lisman and Jensen 2013) in phenomena such as theta phase precession. Theta phase precession is different from sequential firing of place cells (theta sequences), since it has been shown to be an experience-independent phenomenon observable already on the first experience of a novel linear track environment, whereas theta sequences developed only in subsequent laps, further synchronizing phase precession (Feng et al. 2015). Third, theta oscillations provide temporal windows for local circuit computations (Mizuseki et al. 2009). Fourth, the different phases of the theta cycle provide control over the modification of synaptic weights. The same high-frequency stimulus can induce both long-term potentiation or long-term depression depending on the phase of the theta oscillation at which the stimulus is given (Huerta and Lisman 1995; Hyman et al. 2003). Fifth, the opposite phases of theta oscillations might reflect separate computational temporal windows for encoding and retrieval (Hasselmo et al. 2002), as supported by empirical data as described below in cholinergic neurons in the medial septum (Douchamps et al. 2013; Siegle and Wilson 2014).
Theta oscillations largely depend on the integrity of the medial septum/diagonal band of Broca (MSDB). Electrolytic lesions of the MSDB (Winson 1978) or pharmacological inactivation (Chrobak et al. 1989) of MSDB neuronal activity diminish hippocampal theta rhythmic activity to a large extent. As noted above, the reduction of hippocampal theta oscillatory power by medial septal inactivation is accompanied by spatial memory deficits (Givens and Olton 1990), and the successful encoding of memory traces is accompanied by increases in theta oscillatory power (Berry and Thompson 1978). Whereas targeted lesions of either the GABAergic or the cholinergic MSDB neurons only slightly affect performance in spatial memory tasks, the combined lesions of both GABAergic and cholinergic subpopulations in the MSDB severely impaired task acquisition and performance in the radial arm and water maze spatial memory tasks (Pang et al. 2001). These data point to an important synergistic role of cholinergic activity and theta rhythmic drive by GABAergic MSDB neurons.
MECHANISMS FOR CODING OF RUNNING SPEED
A critical component for self-localization is knowledge of the speed of movements through the environment. Path integration has been proposed as a mechanism for spatial localization for many years (Gallistel 1990; Mittelstaedt and Mittelstaedt 1980; Redish 1999) and comprehensive reviews of this research are available elsewhere (Redish 1999). As reviewed previously (Redish 1999), path integration requires integration over time of the speed and direction of movement to have a moment-to-moment accounting of location within an environment. In different species, path integration exhibits systematic errors (Müller and Wehner 1988; Séguinot et al. 1993). The overwhelming majority of computational models of grid cells employ a path integration mechanism to track motion and generate the regularly repeating spatial firing fields.
Computational models have generally employed one of two different types of speed signals when modeling the generation of grid cells: either a firing rate or an oscillatory frequency based speed signal are used. An additional difference across models can be noted in that some models instantiate the speed signal in principal cells (Zilli and Hasselmo 2010) such as conjunctive grid by head direction cells (Burak and Fiete 2009), while others implement the speed signal in interneurons (Guanella and Verschure 2007; Hasselmo and Shay 2014). Firing rate speed signals are most often found in attractor dynamics models where a set of modeled neurons have firing rates that increase linearly as a function of running speed activity (Burak and Fiete 2009; Giocomo et al. 2011; McNaughton et al. 2006; Widloski and Fiete 2014). Alternatively, oscillatory interference models of grid cell generation make use of neurons whose oscillation frequency varies as a function of running speed (Blair et al. 2008; Burgess et al. 2007; Burgess 2008). Originally these two classes of models were viewed as mutually exclusive, but more recently a number of hybrid models have been created that utilize aspects of each class (Bush and Burgess 2014; Hasselmo and Brandon 2012; Hasselmo and Shay 2014; Schmidt-Hieber and Häusser 2013). While the type of speed signal used, firing rate, or oscillatory frequency differs across models, the purpose is generally the same: to be a component in a path integration computation.
A speed signal, whether a firing rate or an oscillatory frequency speed signal, should have two characteristics to allow for the accurate estimation of location needed to dynamically update grid cell activity in path integration models. First, the signal must vary linearly as a function of speed. Linear variation as a function of speed ensures that a specific incremental increase in speed, say 2 cm/s, is coded by a specific incremental change in the speed signal, whether from 10 to 12 cm/s or 35 to 37 cm/s. Second, the signal should be context invariant, which ensures that speed is tracked the same across different environments. A speed signal that differs across contexts will result in running speed being tracked differently across the environments and this would ultimately result in different distance estimates across those environments. If such a context-dependent speed signal were implemented in a grid cell model the result would be individual grid cells having different grid field scales across environments or a general disruption of the spatial periodicity. While running speed signals have played a major role in computational models of spatial coding and navigation, only recently have experiments specifically addressed these two critical aspects of the speed signal.
Several studies have shown cells to be speed modulated in hippocampus (McNaughton et al. 1983; O’Keefe et al. 1998) and MEC (Buetfering et al. 2014; Sargolini et al. 2006; Wills et al. 2012), but recently a dedicated subset of cells that faithfully track the speed of a rat as it runs through different environments was demonstrated (Kropff et al. 2015). By having rats systematically run at different speeds across a 4-m-long track in a bottomless car (regulating its speed), it was shown that ~15% of MEC cells are speed cells that do not code other spatial properties, including both principal cells and interneurons. Thus, the Kropff study focused on neurons that coded speed without showing the additional coding properties of grid cells or head direction cells, even though many grid cells and head direction cells also show clear coding by running speed (Buetfering et al. 2014; Hinman et al. 2016; Jeewajee et al. 2008; Sargolini et al. 2006; Wills et al. 2012). Perhaps more importantly than demonstrating the presence of speed cells in MEC was the demonstration that speed cell tuning curves did not vary across different open fields or between the open field and the bottomless car on the linear track. Additionally, the rat’s running speed in one context could be decoded using speed cell tuning curves from a different context. Thus, Kropff et al. (2015) showed the context invariance of this speed signal.
In subsequent work we explicitly tested the linearity of the firing rate speed signal in MEC (Hinman et al. 2016). While many MEC cells have linear speed tuning curves as needed for path integration (Fig. 3A), the majority of speed-modulated cells have tuning curves that are nonlinear (Fig. 3B). These nonlinear firing rate responses to running speed saturate once the animal reaches a moderate running speed. The saturating nature of these responses does not fit current model instantiations of a speed signal used for path integration. However, despite only making up a minority of speed-modulated cells in MEC there is a subset of cells, including both principal cells and interneurons, which match the linear speed signal employed by computational models.
Cells throughout the hippocampal formation spike theta rhythmically and, in addition to the firing rate speed signal in MEC, the oscillatory frequency speed signal has been shown experimentally in single cells in MEC (Fig. 3B) (Hinman et al. 2016; Jeewajee et al. 2008) and the local field potential (Jeewajee et al. 2008). The oscillatory frequency of single cell firing increases as a rat runs faster, which has been used in oscillatory interference models. Given that both speed signals have been identified in MEC, we investigated whether the same cells express both speed signals similarly and found that the two signals are actually independently expressed in single cells (Hinman et al. 2016). The presence of two independent running speed signals in MEC raises questions as to where these signals originate from and whether they are actually critical for the generation of grid cells.
In an attempt to elucidate the neural source of the speed signals in MEC, our laboratory (Hinman et al. 2016) inactivated the medial septum while recording single units in MEC. The medial septum contains glutamatergic neurons that have been shown to carry a running speed signal to MEC (Justus et al. 2016) and stimulation of the medial septal glutamatergic neurons elicits running at speeds in proportion to the frequency of stimulation that initiates running (Fuhrmann et al. 2015). In our study, medial septal inactivation had opposing effects on the firing rate and oscillatory frequency speed signals in MEC. During inactivation, the firing rate signal of running speed became statistically stronger whereas the oscillatory frequency signal became weaker (Hinman et al. 2016). Concomitant with the changes in the speed signals was degradation in the spatial periodicity of the grid cells (Brandon et al. 2011) suggesting that the reduction of the oscillatory frequency speed signal played a role in the loss of grid cells.
There are numerous potential ways to generate a speed signal such as motor efference copy, proprioceptive feedback, optic flow, and somatosensation. The first two would likely reach MEC via subcortical routes, while the latter two via cortical pathways. Support for a role of self-motion, thus incorporating motor efference and/or proprioceptive feedback, comes from recent work showing that passively moving a rat around an environment is not sufficient to generate either the firing rate speed signal in grid cells or oscillatory frequency speed signal in the LFP in MEC (Winter et al. 2015b). In the absence of these speed signals there was also a loss of grid cell spatial tuning. This supports the idea that self-motion is a critical mechanism of the speed signals observed in MEC and for the expression of grid cell spatial tuning though the self-motion signal could also come via hippocampal place cells. The importance of self-motion has also been shown for hippocampal place cell responses (Terrazas et al. 2005) and head direction responses (Stackman et al. 2003; Zugaro et al. 2002).
In addition to self-motion being important for generating speed signals, recent evidence also supports a role for sensory information. Running speed-modulated activity is observed in primary visual cortex where the gain of principal cells is increased during locomotion relative to stationary behavior (Niell and Stryker 2010). Our laboratory has modeled how optic flow could be used to generate a speed signal that is amenable with various different grid cell models (Raudies et al. 2012). Having a mouse run in an open field in the dark illustrates the importance of visual information for both the firing rate speed signal and grid cell spatial tuning, as both become degraded in darkness in mice (Chen et al. 2016; Pérez-Escobar et al. 2016). In mice, both the strength and slope of the firing rate speed signal are reduced in the absence of visual input and simultaneously the grid cell spatial representation is disrupted. However, this data is potentially in conflict with that from rats where there are examples of grid cells maintaining their spatial firing patterns (Hafting et al. 2005) and speed cells maintaining their speed tuning curves in the absence of visual input (Kropff et al. 2015). These discrepancies could be due to species differences, specifically relating to several observations that suggest mice pay less attention to distal cues than rats (Cho et al. 1998; Kentros et al. 2004; Whishaw 1995). Given these results, it is possible that mouse grid cell patterning is disrupted in darkness because speed signals and other contributing factors rely on local visual information obtained from optic flow during movement that is lost or unreliable in darkness. In contrast, rats potentially have preserved grid firing under these conditions because the animal is able to orient itself relative to nonvisual distal cues (e.g., auditory cues). Differences in the effects of visual information on the grid fields between mice and rats could also result from methodological differences across studies, as discussed further under studies of spatial coding in darkness below.
The last several years have seen much progress toward understanding the coding of running speed in MEC, yet several critical questions remain. Are both the firing rate and oscillatory frequency speed signals necessary for grid cell generation or is only one of the speed signals needed? What neural circuit propagates each of the speed signals to MEC? Are the speed signals sensory or motor driven? Likely these questions have multiple or blended answers such that speed signals in MEC are influenced by a mix of sensory and motor information and as such reach MEC via several anatomical routes, but such answers will be critical to understanding how grid cells are generated and the coding mechanisms for self-localization in general.
COMPONENTS OF MEDIAL SEPTAL CIRCUITRY
As noted above, grid cell coding is lost with inactivation of the medial septum. Understanding the potential functional role of the medial septum benefits from understanding the different components of the medial septum. In rodents, the MSDB is located in the dorsal rostral and intermediate basal forebrain with projections to the allocortex and isocortex (Woolf 1991). The medial septum lies along the midline of the septum, whereas the diagonal band of Broca appears as a boomerang-shaped triangular structure along the ventrolateral border of the medial septum. Three main subpopulations of cell types can be distinguished within the MSDB, namely cholinergic, GABAergic, and glutamatergic neurons. Each one of these cell populations has specific properties and contributes in its own way to the regulation of theta rhythmic activity in the septohippocampal memory system, but all act together in concert. In the following section we will therefore give an overview of each population’s most characteristic properties and discuss how they work together on modulating hippocampal network activity.
Cholinergic neurons in the medial septum.
Approximately 30–50% and 50–75% of cells within the medial septum and vertical diagonal band are positive for the acetylcholine synthesis enzyme choline-acetyltransferase (ChAT) (Mesulam et al. 1983) and are therefore considered cholinergic cells. Studies using retrograde tracing from the hippocampus to identify cholinergic cells that project to the hippocampus have counted 30–35% and 45–55% of cells as ChAT+ within the medial septum and diagonal band, respectively (Wainer et al. 1985). The MSDB also sends prominent cholinergic projections to the entorhinal cortex (Alonso and Köhler 1984). Cholinergic MSDB neurons are characterized in vitro by a slow regular firing pattern, a broad action potential width, and a prolonged slow afterhyperpolarization (Griffith and Matthews 1986; Markram and Segal 1990).
Acetylcholine released in the hippocampal formation and entorhinal cortex acts via different subtypes of nicotinic and muscarinic acetylcholine receptors expressed differentially on different cellular compartments by different interneuron subtypes and principal cells—thus exhibiting complex effects on the cellular and network level. Acetylcholine regulates the spread of excitatory activity within hippocampal and cortical circuits via presynaptic inhibition of glutamatergic synaptic transmission (Hasselmo et al. 1995; Hasselmo 1999, 2006) mediated by M4 receptors (Dasari and Gulledge 2011). Another prominent effect on the cellular level is a depolarization of principal neurons mediated by M1 and M3 muscarinic receptors (Dasari and Gulledge 2011; Gulledge and Kawaguchi 2007). Interneurons display a great diversity of postsynaptic membrane potential changes after cholinergic stimulation. In acute slices, optogenetic stimulation of acetylcholine release caused mixed responses in interneurons via muscarinic acetylcholine receptor activation, namely hyperpolarizing, biphasic, and depolarizing effects (Bell et al. 2013). Shorter trains of stimuli evoked hyperpolarizing responses, but the depolarizing responses showed a steeper increase with increasing stimulation length. This muscarinic receptor-mediated depolarization of a subset of interneurons resulted in an increase of inhibitory postsynaptic currents (IPSCs) onto CA1 pyramidal neurons (Bell et al. 2015b; Nagode et al. 2011). Interestingly, optogenetic stimulation of acetylcholine release also resulted in the activation of interneuron-selective interneurons via nicotinic α4β2 receptors leading to disinhibition of principal cells (Bell et al. 2011, 2015a). Taken together, these data support a model proposed by McQuiston (2014), in which low levels of acetylcholine favor disinhibition via muscarinic hyperpolarization of interneurons and nicotinic activation of interneuron-selective interneurons, whereas higher levels of acetylcholine favor inhibition of hippocampal principal cells via muscarinic depolarization of a different subset of interneurons.
The nicotinic effects described above could be related to data showing increases in firing of VIP-positive interneuron-selective interneurons with running speed in the rodent visual cortex, which were dependent on nicotinic receptor activation and were proposed to cause disinhibition of excitatory neurons (Fu et al. 2014; Niell and Stryker 2010). The muscarinic depolarization of interneurons match with data from single unit recordings in the hippocampus and dentate gyrus showing increased inhibition of pyramidal neurons and dentate gyrus granule cells during optogenetic activation of cholinergic MSDB neurons (Dannenberg et al. 2015; Pabst et al. 2016). These cholinergic effects promote a hippocampal network state supporting theta rhythmic temporal organization of activity with important consequences for spatial coding. Importantly, cholinergic activity is accompanied by theta rhythmic brain activity (Monmaur et al. 1997), which can be paced by rhythmic activity of the GABAergic MSDB neurons (Stewart and Fox 1990; Tóth et al. 1997). Historically, two types of theta have been distinguished: lower frequency type II theta, which occurs during immobility and under urethane anesthesia and is blocked by the muscarinic antagonist atropine, and type I theta, which has a higher frequency, occurs during running, and is only partially affected by atropine (Buzsáki 2002; Kramis et al. 1975). Septal cholinergic neurons affect hippocampal theta via intraseptal activation of septal GABAergic neurons and via acetylcholine release in the hippocampus (Dannenberg et al. 2015). Consistent with an intraseptal relay, intraseptal carbachol infusions have been shown to induce hippocampal theta rhythmic activity during relaxed immobility (Monmaur and Breton 1991) and, conversely, atropine infusions into the MSDB decreased the amplitude of type II theta oscillations (Lawson and Bland 1993; Monmaur et al. 1997). However, infusions of cholinergic antagonists into either the MSDB or the hippocampus was sufficient to block the induction of type II theta oscillations (Dannenberg et al. 2015) showing the requirement of hippocampal acetylcholine release for type II theta generation. Furthermore, the amplitude of type I theta in freely moving mice is also reduced by atropine infusions into the hippocampus due to blocking M1 muscarinic receptors on pyramidal neurons (Gu et al. 2017). In contrast, NMDA receptors in the entorhinal cortex (EC) are critical for the atropine-resistant component of type I hippocampal theta generation, as previously suggested (Buzsáki 2002; Leung and Desborough 1988; Leung and Shen 2004), and recently supported by experimental data (Gu et al. 2017).
The higher cholinergic modulation and associated enhancement of theta rhythmicity present during active exploration may enhance dynamics for the encoding of new associations. The muscarinic cholinergic presynaptic inhibition of excitatory recurrent connections in the hippocampus (Hasselmo et al. 1995; Hasselmo 2006) could prevent retrieval of previous associations from interfering with the accurate formation of new associations between adjacent place cells or between representations of items and place codes (Hasselmo 2006, 2009). Similarly, rapid shifts between encoding and retrieval dynamics on different phases of theta rhythm oscillations could also be important for allowing encoding of new associations without interference from previously coded associations (Hasselmo et al. 2002). In this model, encoding preferentially occurs at the peak of CA1 pyramidal cell layer theta coincident EC input, and retrieval preferentially occurs at the theta trough, when input from CA3 is strongest. Key predictions of this model have been tested in rats (Douchamps et al. 2013) and mice (Siegle and Wilson 2014). Using an end-to-end T-maze with a separable encoding and retrieval segments, Siegle and Wilson (2014) showed that optogenetic activation of parvalbumin (PV+) interneurons improved task performance when applied during the theta phase of EC input in the T-maze retrieval segment, or when applied during the theta phase of CA3 input in the T-maze encoding segment. The improved performance could result from phase-specific inhibition blocking encoding input from EC during retrieval and blocking retrieval mediated by CA3 during encoding (Siegle and Wilson 2014). In data from novel environments, the preferred theta phase of CA1 place cells was closer to the peak of the theta cycle consistent with a higher demand for encoding novel information, and this shift was disrupted by injections of scopolamine, a cholinergic antagonist (Douchamps et al. 2013).
Microdialysis studies show that cholinergic modulation decreases during quiet waking or slow wave sleep. This would release the presynaptic inhibition of excitatory feedback, allowing increased generation of sharp wave ripple activity (Hasselmo 1999; Vandecasteele et al. 2014). Medial septal neurons decrease their activity specifically during sharp wave ripple events (Dragoi et al. 1999). The reduction of cholinergic presynaptic inhibition allows generation of sharp wave ripples and allows a strong influence of retrieval based on previously modified recurrent connections that can drive the retrieval of sequences of activity (Hasselmo 1999). These replay events could allow sequences of place cell activity encoded during waking to be consolidated during periods of quiet waking or slow-wave sleep (Craig et al. 2015; Dannenberg et al. 2015; Dewar et al. 2009, 2014; Jadhav et al. 2012; Lee and Wilson 2002; Skaggs and McNaughton 1996).
GABAergic neurons in the medial septum.
The role of medial septum in regulating theta rhythm oscillations depends strongly on GABAergic neurons. According to data obtained by retrograde fluorescent tracing combined with glutamic acid decarboxylase (GAD)-immunohistochemistry, the GABAergic MSDB cell population accounts for ~30% of hippocampus projecting cells in the MSDB (Amaral and Kurz 1985; Köhler et al. 1984). Inside the medial septum, the GABAergic cells mainly harbor the medial portion along the midline, which is relatively spared by the cholinergic cells. These data are consistent with retrograde tracer experiments (Kiss et al. 1990a, 1990b) that show that 33% of the neurons retrogradely labeled after hippocampal tracer injection were immunoreactive for PV, which has been established as a marker protein for medial septal GABAergic neurons (Freund 1989). Whereas the cholinergic projection terminates both on principal cells and interneurons (Frotscher and Léránth 1985), the septal GABAergic neurons selectively innervate hippocampal interneurons (Freund and Antal 1988). GABAergic MSDB neurons display fast- or burst-firing properties and a large hyperpolarization-activated inward current (Ih) with action potentials of significantly shorter duration than the ones from regular-spiking putative cholinergic neurons (Morris et al. 1999; Sotty et al. 2003). The Ih could underlie rhythmic rebound spiking of medial septal neurons (Varga et al. 2008) that drives theta rhythm in hippocampus and entorhinal cortex. This rhythmicity could interact with resonance and rebound spiking due to Ih in stellate cells of medial entorhinal cortex (Giocomo et al. 2007; Hasselmo and Shay 2014).
Septohippocampal projections of PV+ MSDB neurons terminate selectively on hippocampal interneurons, mainly on perisomatic inhibitory basket cells (Freund and Antal 1988; Serafin et al. 1996; Tóth et al. 1997). This connectivity allows optogenetic activation of PV+ neurons in the MSDB to precisely pace hippocampal rhythmic activity, so that field potential oscillations follow optogenetic stimulation frequencies ranging from theta to slow gamma (Dannenberg et al. 2015). PV+ neurons have also been shown to lead the hippocampal network in phase during endogenous theta oscillations (Hangya et al. 2009; Huh et al. 2010; Varga et al. 2008). GABAergic MSDB neurons can be activated by acetylcholine (Alreja et al. 2000) but also receive excitatory input from local glutamatergic MSDB neurons.
Glutamatergic neurons in the medial septum.
As noted above, the influence of medial septum in coding aspects of speed could depend on glutamatergic neurons (Fuhrmann et al. 2015; Justus et al. 2016). Glutamatergic MSDB neurons were initially described as a population of neurons expressing transcripts for the vesicular glutamate transporters VGLUT1 or VGLUT2, but no transcripts for GAD or ChAT (Sotty et al. 2003). Retrograde tracing via FluoroGold injections into the hippocampus or dentate gyrus (DG) in combination with immunohistochemistry of septal slices with antiglutamate antiserum showed that ~23% of the septohippocampal fibers are indeed glutamatergic projections (Colom et al. 2005). The glutamatergic MSDB neurons are a heterogeneous group displaying a range of electrophysiological properties with the majority having slow firing characteristics (Sotty et al. 2003) and nearly half of them exhibiting a unique cluster firing characteristic (Huh et al. 2010).
Glutamatergic MSDB neurons can provide excitatory synaptic input to hippocampal principal neurons (Huh et al. 2010), but multisynaptic inhibitory postsynaptic potentials (IPSPs) were also observed frequently after activation of glutamatergic neurons, indicating that the glutamatergic MSDB neurons may excite hippocampal interneurons. This is consistent with experimental data showing that optogenetic activation of projection fibers from glutamatergic MSDB neurons in acute hippocampal slices resulted in disinhibition of CA1 principal neurons (Fuhrmann et al. 2015). Glutamatergic fibers projecting to the entorhinal cortex can excite both interneurons and principal cells, but the most effective input-output coupling was detected in the layer 2/3 pyramidal cells (Fuhrmann et al. 2015). Optogenetic activation of glutamatergic MSDB neurons has been shown to initiate locomotion in head-restrained mice running on a treadmill with the onset time of locomotion negatively and the speed of locomotion positively correlated with stimulation strength (Fuhrmann et al. 2015), thereby conveying a speed signal to the hippocampus and medial entorhinal cortex (Justus et al. 2016).
Importantly, both the activation of cholinergic MSDB neurons (Dannenberg et al. 2015) and the activation of glutamatergic MSDB neurons (Robinson et al. 2016) result in rhythmic activity of the population of GABAergic MSDB neurons within the theta range. This activation is mediated by local intraseptal connections (Alreja et al. 2000). Thus, at the same time when hippocampal activity is tuned by direct septohippocampal projection fibers, the simultaneous activation of GABAergic MSDB neurons conveys theta rhythmic activity to the hippocampal formation. One consequence of this synergistic action is higher temporal organization of spiking activity in the hippocampus reflected as increased spike-theta phase coupling (Dannenberg et al. 2015) (Fig. 4). If this model holds true, inhibition of the cholinergic activity should disrupt the organization of spike timing in the hippocampus. Recent experimental data (Newman et al. 2017) indeed show that systemic application of the muscarinic cholinergic receptor antagonist scopolamine in rats reduced phase precession of CA1 place cells due to a decrease of spiking rhythmicity.
Fig. 4.
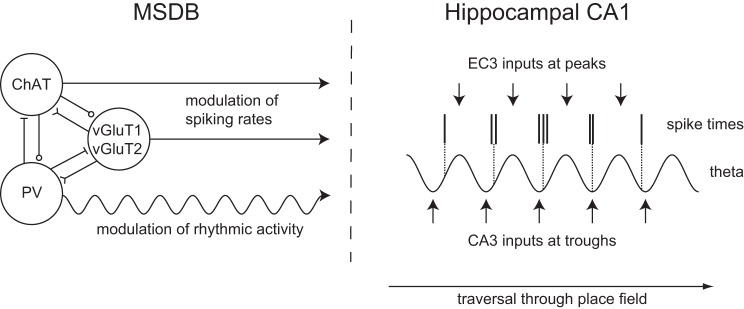
Regulation of hippocampal theta rhythm and spike-phase coupling by medial septal inputs. Left: parvalbumin-positive GABAergic, ChAT-positive cholinergic, and vGluT1- or vGluT2-positive glutamatergic MSDB neurons interact via local intraseptal connections and project to the hippocampus. PV-positive neurons can be activated by local cholinergic and glutamatergic inputs. Right: rhythmic inputs from GABAergic MSDB neurons pace hippocampal theta rhythmic activity via disinhibition. Inputs from EC3 and CA3 onto CA1 arrive at the peak and trough of the extracellular theta cycle measured at the pyramidal cell layer, respectively. The balance between EC3 and CA3 relative input strengths might determine the precise spike timing as suggested by different models for phase precession and models of encoding and retrieval. Changes in this balance during the traversal of the place field result in spikes occurring at progressively earlier phases of the theta cycle, i.e., phase precession. ChAT, choline-acetyltransferase; EC3, entorhinal cortex layer 3; MSDB, medial septum and diagonal band of Broca; PV, parvalbumin; vGluT, vesicular glutamate transporter.
GABAergic MSDB neurons transfer rhythmic activity to the hippocampus by impinging on PV+ hippocampal interneurons (Amilhon et al. 2015; Tóth et al. 1997). In addition to the hippocampal PV+ neurons which can pace the hippocampal theta rhythm, somatostatin positive hippocampal oriens-lacunosum-moleculare interneurons modulate the entrainment of intrinsic theta rhythm by EC inputs (Amilhon et al. 2015; Leão et al. 2012; Lovett-Barron et al. 2014). Thus, the two main theta rhythm generators in the hippocampus, the MSDB and the EC, depend on local inhibitory microcircuits to provide temporal organization of spiking activity.
The temporal organization of spiking activity relative to the phase of the underlying theta oscillation is a prominent feature observed in animals during navigation. The spiking of place cells display phase precession, i.e., their spikes occur at earlier and earlier phases in subsequent theta cycles (Eggink et al. 2014; O’Keefe and Recce 1993; Skaggs et al. 1996). There are a number of different models of generation of theta phase precession based on oscillatory interference of intracellular depolarization with network oscillations (Bose et al. 2000; Lengyel et al. 2003; O’Keefe and Recce 1993), or based on retrieval of previously encoded sequences of place cells (Hasselmo and Eichenbaum 2005; Jensen and Lisman 1996; Tsodyks et al. 1996; Wallenstein and Hasselmo 1997). Simultaneous intra- and extracellular recordings from the hippocampus of mice running in a virtual environment showed membrane potential oscillations in place cells whose amplitude and frequency increased while the mouse was running through the place field consistent with the oscillatory interference model of phase precession (Harvey et al. 2009). This model is challenged, however, by observations that transient disruption of hippocampal activity by single-pulse stimulation does not disrupt phase precession after that perturbation as would be expected by a reset of oscillatory activity (Zugaro et al. 2005). Likewise, the model of previously encoded sequences is challenged by phase precession in open field environments, where arbitrary routes can be taken. Another class of models (Chance 2012; Fernández-Ruiz et al. 2017; Magee 2001) suggest that the phase-precession phenomenon in the hippocampal CA1 region might be seen as part of a more general spike timing mechanism involving the temporal coordination of CA3 and EC3 inputs in conjunction with local inhibitory microcircuits. In these dual input models, timing of CA1 pyramidal cell spikes is tuned by the relative strengths of EC3 and CA3 inputs, whereas EC inputs are strongest during the peak of the theta cycle and CA3 inputs are strongest around the trough of the theta cycle. This is in line with the encoding of novel information coming from the entorhinal cortex during the peak of the theta cycle and retrieval of information previously stored in the autoassociative CA3 network during the trough of the theta cycle. An important difference between models is that in many models (Jensen and Lisman 1996; Tsodyks et al. 1996; Wallenstein and Hasselmo 1997) the CA3 input is strongest at the entry to the place field and the EC3 input is strongest at the exit of the place field, whereas in some models (Fernández-Ruiz et al. 2017; Hasselmo and Eichenbaum 2005) the EC3 input is strongest on entry to the field and CA3 input is strongest at exit.
Experimental data shows that the spike sequences of place cells during one theta cycle are temporally compressed representations of an animal’s trajectory (Dragoi and Buzsáki 2006; Skaggs et al. 1996), and theta cycle length is correlated with sequence length and path length as well as the number of nested gamma cycles (Gupta et al. 2012). However, as noted above, theta phase precession appears on a novel linear track, whereas theta sequences only develop over several laps (Feng et al. 2015).
STUDIES OF SPATIAL CODING IN DARKNESS
An important feature of theta oscillations recorded during movement is the linear increase of theta amplitude and frequency with running speed (Vanderwolf 1969; Hinman et al. 2011). The speed modulation of hippocampal theta frequency also correlates with spatial memory performance (Richard et al. 2013), indicating a role of theta rhythmic activity for the integration of sensorimotor signals into memory processes. The speed modulation of theta rhythm shows a clear dissociation from emotion-sensitive contributions to theta rhythm that affect overall frequency but not speed modulation (Monaghan et al. 2017; Wells et al. 2013). Recent data indicates that this speed-theta frequency correlation is abolished under conditions of complete darkness (Chen et al. 2016). A similar study by Pérez-Escobar et al. (2016) found that the speed code reflected by the slope of the regression line between speed and firing rate of entorhinal cortex speed cells was reduced in addition to an overall reduction in firing rate of all MEC neurons in darkness. Furthermore, passive transportation instead of active running was found to diminish speed tuning of theta rhythmicity in the hippocampus (Terrazas et al. 2005) and to abolish speed tuning in the parahippocampal cortex (Winter et al. 2015b). Intriguingly, the studies by Pérez-Escobar et al. (2016) and Chen et al. (2016) both show a disruption of grid cell firing characteristics in mice during exploration in darkness, while the head direction system was largely spared. Furthermore, pharmacological inactivation of the medial septum has been shown to result in a large reduction of theta rhythmic activity and the loss of grid cell spatial firing patterns while sparing the head direction system (Brandon et al. 2011; Koenig et al. 2011). These findings support the hypothesis by Winter et al. (2015b), which states hexagonal grid cell firing characteristics are disrupted when the hippocampal theta rhythm is no longer accurately representing the linear velocity due to a mismatch with the information in the head directional system. However, early experimental data from rats shows that grid cells can remain stable for 10 min under conditions of complete darkness and are even immediately stable in a dark novel room (Hafting et al. 2005). In addition, not all grid cells recorded by Chen et al. (2016) showed a complete loss of grid cell firing characteristics and also preliminary data from our laboratory show that grid cells in mice can be stable in total darkness. Notably, the studies by Pérez-Escobar et al. (2016) and Chen et al. (2016) used elevated mazes with no walls, whereas walls were present in the recordings by Hafting et al. (2005) and in the preliminary recordings from our laboratory. This difference in the experimental setup raises the question of whether the insertion of walls might provide additional cues helping grid cells to form stable hexagonal firing patterns. Stable grid fields in total darkness can be modeled by a probabilistic learning model demonstrating that the persistence of grid cells in darkness may be largely explained by the fusion of self-motion and learned boundary information (Cheung 2016). It would be interesting to see whether the stability of grid cells in darkness correlates with the stability of the speed codes reflected by the speed tuning of theta frequency and the speed tuning of speed cells in the entorhinal cortex. Stable grid cell activity in darkness may also be explained by the existence of a subset of grid cells that depend more on self-motion cues than visual inputs. This is consistent with evidence of different classes of place cells responding to self-motion cues or to visual input in a virtual world (Chen et al. 2013). Additional experiments could differentiate between these possibilities.
The experimental findings that the hexagonal firing pattern of grid cells is disrupted by either inactivating the medial septum in rats, by passive transportation in rats, or under the condition of total darkness on an elevated maze in mice are congruent with the hypothesized functions of the cholinergic and GABAergic medial septal neurons in modulating network dynamics and pacing theta rhythmic activity during movement. These findings also support the hypothesized role of the glutamatergic medial septal neurons in initiating movement and transmitting a speed signal to the medial entorhinal cortex. Taken together, these data indicate visual cues and self-motion cues may be working in conjunction to modulate the frequency of theta oscillations during movement, and speed tuning of hippocampal theta activity is important for the integration of sensorimotor signals into a spatial representation of the environment underlying map-based navigation.
SPATIAL NAVIGATION IN POSTERIOR PARIETAL AND RETROSPLENIAL ASSOCIATION CORTICES
As noted above, sensory processing in cortical structures could contribute to coding of many spatial representations, including the computation of location, head direction, running speed, and position relative to barrier. These processes could involve computation of velocity of self-motion or computation of position and head direction based on the egocentric angle of sensory features. Thus, it is important to consider the potential role of cortical regions in computation of spatial representations.
Multiple navigational strategies, including both path integration and landmark-based taxon, praxic, or route navigation methods, require the integration of sensory input and motor output with spatial maps of the external environment observed in subcortical structures (Andersen and Mountcastle 1983; O’Keefe and Nadel 1978; Redish 1999). Associative cortical regions, such as the retrosplenial cortex (RSC) and posterior parietal cortex (PPC), serve as multisensory processing hubs potentially capable of combining sensory and motor information encoded in egocentric coordinates with allocentric spatial information for effective navigation (Byrne et al. 2007; Oess et al. 2017; Sporns and Tononi 2007). Accordingly, lesion or inactivation of either PPC or RSC results in a variety of spatial navigation impairments (Cooper et al. 2001; DiMattia and Kesner 1988; Hindley et al. 2014; Hoh et al. 2003; Kolb and Walkey 1987; Pothuizen et al. 2009; Save and Poucet 2000; Takahashi et al. 1997; Vann et al. 2003; Vann and Aggleton 2005). The following section considers the anatomy of these two regions with respect to multiple spatial variables represented by their neural populations and discusses possible implications of these representations for navigation.
Anatomy of retrosplenial and parietal cortex.
As association cortices, both RSC and PPC are defined by a vast array of afferent and efferent connections to cortical regions known to be critical for executive function, sensory processing, and motor output. In rats, RSC is composed of two interconnected subregions: dysgranular (RSCd) and granular RSC (RSCg) (Shibata et al. 2004, 2009; van Groen and Wyss 1990, 1992, 2003). PPC forms the lateral border of RSC and is composed of three distinct subregions forming lateral, medial, and caudolateral portions that are defined by differing thalamic connections and cytoarchitecture (Olsen and Witter 2016; Olsen et al. 2017; Reep et al. 1994; Wilber et al. 2015).
Both RSC and PPC are interconnected with cortical regions important for sensory processing and form efferent projections to motor areas. RSC sensory inputs are dominated by the visual system, with RSCd in particular receiving strong projections from visual cortices (Shibata et al. 2004; Vogt and Miller 1983). Recent imaging work has identified RSC as a component of the visual network critical for associative learning, a property that may function to store relative positions of visual landmarks for spatial processing (Makino and Komiyama 2015; Robinson et al. 2011; Sulpizio et al. 2013; Zhuang et al. 2017). In rat, other forms of sensory information likely reach RSC via projections from the PPC which itself is innervated by more diverse sensory inputs from secondary visual, somatosensory, and auditory cortices (Reep et al. 1994). Accordingly, PPC has been implicated in multisensory integration (Avillac et al. 2005; Robinson and Bucci 2012) important for attention (Bucci et al. 1998; Fox et al. 2003), motor execution (Whitlock et al. 2012; Wilber et al. 2017), and navigation (Kolb and Walkey 1987). Many of these PPC functional properties have been observed or were originally elucidated in primate parietal cortex (Avillac et al. 2005; Behrmann et al. 2004; Gnadt and Andersen 1988; Mountcastle et al. 1981). PPC efferent projections reach both premotor and motor regions where neural activity has been shown to correspond with or anticipate a variety of actions including those driven by sensory stimuli or associated with higher-order decision making (Andersen 1997; Erlich et al. 2015; Harvey et al. 2012; Platt and Glimcher 1999; Raposo et al. 2014; Reep et al. 1994; Shadlen and Newsome 2001).
RSC and PPC projection patterns diverge with respect to the hippocampal formation (HPC), rhinal cortices, and thalamic nuclei, which are known to map spatial relationships with respect to the external world. RSC forms a significant cortical feedback loop with the HPC via reciprocal projections to anterior thalamic nuclei that directly innervate the HPC. Furthermore, RSC forms both afferent and efferent projections with the subiculum, the primary output structure of the HPC, and receives sparse direct projections from HPC subfield CA1 (van Groen and Wyss 1990, 1992, 2003; Wyss and Van Groen 1992). Accordingly, RSC is ideally positioned to integrate cortical processing with the HPC for navigation but also may function to consolidate both spatial and nonspatial mnemonic processing of the HPC (Cowansage et al. 2014; Czajkowski et al. 2014; Tse et al. 2011). Beyond direct HPC connectivity, RSC is reciprocally connected to the entorhinal, postrhinal, and perirhinal cortices, as well as all subicular subfields (Burwell and Amaral 1998; Czajkowski et al. 2013; Kononenko and Witter 2012; Sugar et al. 2011; van Groen and Wyss 1990, 1992, 2003; Wyss and Van Groen 1992). PPC also exhibits reciprocal connectivity to allocentric spatial systems but with lower density than that observed in RSC. PPC receives sparse afferent projections from the rhinal cortices and primarily projects to the presubiculum (Burwell and Amaral 1998; Olsen et al. 2017). However, the region is strongly reciprocally connected to dysgranular RSC, which may provide a mode by which PPC computations can reach the hippocampus and associated parahippocampal input and output structures (Olsen et al. 2017).
Spatial representations in retrosplenial and parietal cortex.
The convergence of sensory, motor, and spatial inputs into PPC and RSC implicate these regions as important for sensorimotor coordination and reference frame transformations critical for navigation relevant processes (Byrne et al. 2007). Accordingly, PPC and RSC populations represent a multitude of spatial variables relevant to fluid navigation not observed in HPC, including action state, route progression, distance, and heading orientation. Here, we place special emphasis on the prevalence of conjunctive spatial representations, afforded by associative connectivity, that are observed in both cortical regions. Conjunctive sensitivity is often manifested in neural populations as “gain fields,” wherein the firing rate magnitude of a neuron in response to one spatial variable (e.g., position of a visual stimulus on a screen) is decreased or increased (i.e., gain-modulated) as a function of a second spatial variable (e.g., head orientation of the animal (Andersen et al. 1985). Several lines of research have indicated that networks of neurons exhibiting conjunctive encoding can be linearly combined to approximate reference frame transformations that are nonlinear in nature (Pouget and Sejnowski 1997; Pouget and Snyder 2000). This property is likely critical for converting allocentric spatial representations to egocentric motor commands and vice versa (Andersen et al. 1993; Buneo et al. 2002). Furthermore, reference frame transformations computed in associative cortices may be especially important for the formation and maintenance of grid, border, object, or place cell representations, which are necessarily abstractions of egocentric sensory information.
Egocentric representations of actions in the association cortices.
Subsets of neurons in both RSC and PPC exhibit firing correlates with components of self-motion (e.g., angular velocity) which might reflect integration of optic flow, motor efference, vestibular input, or some combination of the aforementioned variables (Fig. 5A) (Alexander and Nitz 2015, 2017; Cho and Sharp 2001; McNaughton et al. 1994; Nitz 2006, 2009, 2012). Tracking of self-motion information has also been observed in human RSC (Chrastil et al. 2016; Sherrill et al. 2013). Self-motion correlates can be highly context dependent, varying greatly for a single neuron in track-running vs. free foraging in an open field. As such, self-motion encoding in these networks is flexibly recruited, indicating that a designated population of neurons that solely encode specific behavioral variables is unlikely to exist.
Fig. 5.
Retrosplenial cortex (RSC) and posterior parietal cortex (PPC) self-motion encoding and route representations. A: mean linear firing rates for two RSC neurons exhibiting right turn sensitivity on traversals across a W-shaped track. In black, a neuron that exhibits similar firing rate peaks for both instances of a right turn. In gray, a neuron that exhibits gain modulation of firing activity related to right turns. This neuron consistently exhibits greater activation for the first right turn vs. the second right turn, a form of gain modulation that concurrently represents action state and position within the route. Bottom: schematic of the track with turn sites labeled. B: spatial representation of route trajectory in PPC. Top left: schematic of track running procedure. Animal runs along two paths, shown in dashed gray and black, that are identical in shape but require the traversal of different allocentric positions and heading orientations. Top middle: schematic of the two paths overlaid following 180° rotation of the black path. Top right: example track linearization for corresponding spatial firing rate profile below. Bottom plots: example firing rate profiles of 2 PPC neurons during traversals of both tracks. Gray shading depicts normalized firing activity as a function of position within the route. Black circles indicate right turn sites; open circles indicate left turn sites. Spatial firing patterns are complex, yet highly similar along the two routes despite their differing allocentric positions and heading directions. This indicates that the spatial activity is referenced within route space. Furthermore, firing rate fluctuations are unrelated to any particular behavioral variable (i.e., turn-type or straight running). C: example RSC neuron exhibiting subroute encoding with gain modulation to distinguish route locations sharing highly similar local structure. Left: tracking data in gray of smooth traversals during route running along a plus-shaped track. In black, positions where the RSC neuron fired an action potential. Starting and ending locations are indicated with a black circle and square, respectively. Lines and numbers indicate the second right turn in repeating double right turn sequences. Right: linearized firing rate profile of the same neuron shows spatially periodic activation corresponding to positions within the route sharing analogous local structure (i.e., occurring between two right turns). Gain modulation along the route generates reliably different activity peaks among the firing fields, enabling a representation of position within local topology and the full route simultaneously. Data adapted from Alexander and Nitz (2015); Nitz (2009); Alexander and Nitz (2017).
Action-specific firing can exhibit reliably distinct activity peaks across multiple positions associated with a specific action (i.e., individual retrosplenial or parietal neurons may respond differently for all instances of a right turn on a labyrinthian maze), suggesting that the self-motion encoding observed in these associative structures is gain modulated by other spatial variables (Fig. 5A) (Alexander and Nitz 2015; Nitz 2006). In some cases, these egocentric action responses can be modulated by allocentric position within an environment (Alexander and Nitz 2015). This is one form by which responses of RSC and PPC neurons conjunctively encode across spatial coordinate systems, in this case simultaneously representing the execution of an action (egocentric) and the position of that action within a route (route-centric) or relative to the boundaries of the observable environment (allocentric). This form of conjunctive encoding provides a mechanism by which self-motion is registered with respect to external location or heading orientation of the animal, which could be critical for path integration. Specifically, gain modulation on turn-related activation within traversal of a well-known trajectory is one mechanism by which RSC and PPC represent the animal’s position within a route.
Importantly, a systematic analysis of the source of self-motion correlates in these regions has not been conducted at this time, and, thus, it is unclear whether these regions are involved in the engagement of motor systems for action execution or represent the current action state for downstream spatial updating, or both. PPC turn-related activation has been shown to anticipate action execution, which indicates PPC might be involved in signaling motor circuits about navigational plans (Whitlock et al. 2012). A similar analysis has not yet been conducted in RSC. However, given dense connectivity from RSC to regions critical for spatial representation, it is possible that RSC self-motion encoding is more involved in updating self-location estimates to be utilized by these structures.
Representation of progression through routes.
For most animals, day-to-day spatial navigation occurs along constrained routes (e.g., tunnels, paths, or streets) leading to and from behaviorally relevant goal locations. Neural representations of well-known routes are observed in both PPC and RSC (Alexander and Nitz 2015, 2017; Nitz 2006, 2009, 2012; Whitlock et al. 2012). Neurons that encode position within a route often exhibit complex, yet spatially reliable, activation patterns that generate distinct rates for each position within the route (Fig. 5B). Ensembles of route-encoding neurons can be utilized to accurately reconstruct the animal’s location within a known trajectory on the order of centimeters. These firing rate profiles cannot be accounted for purely by self-motion or spatial position within the broader environment, indicating that this form of RSC and PPC activity is anchored to locations within the route itself.
Route encoding differs between RSC and PPC in at least one critical way. PPC route encoding is invariant to the spatial position or orientation of the route within the allocentric space, generating highly similar patterns of activation for similarly shaped routes positioned in distinct environmental locations (Nitz 2006, 2009). Conversely, RSC population activity will robustly encode route progression at a single allocentric position but will exhibit alterations in activity when the same route is translated or rotated to a different position in the environment (Alexander and Nitz 2015). Thus, route-referenced activity in RSC is conjunctively sensitive to the animal’s position within a route as well as the external position of the route within the environment. Furthermore, RSC route encoding has solely been observed on constrained environments, but PPC neurons have been shown to exhibit similar route activation patterns on trajectories with or without route-defining boundaries (e.g., such as in open field environments; Whitlock et al. 2012). Further analysis of route representation in animal’s traversing known routes in open environments is needed to more completely describe this form of spatial representation.
Interestingly, when a route contains multiple segments that are similarly shaped, PPC and RSC networks may employ “gain field” modulation to distinguish route locations sharing highly similar local structure (Fig. 5C) (Alexander and Nitz 2015, 2017; Nitz 2012). Thus, RSC and PPC ensembles can simultaneously encode the position of an animal within a subroute of the trajectory as well as the position of that subroute within the entire path. RSC and PPC subroute representations are similar to observations in humans that blood-oxygen-level dependent (BOLD) activity patterns reflect occupation of analogous positions and heading orientations relative to the locally defined topology (Marchette et al. 2014).
Recent work has demonstrated that RSC route representations can manifest as spatial firing rate profiles with symmetric characteristics, which in turn can be utilized to decode the animal’s distance from any point within the trajectory (Alexander and Nitz 2017). This property is consistent with recent fMRI work demonstrating that RSC BOLD activity is related to distance traveled along a virtual track (Chrastil et al. 2015; Wiener et al. 2016). RSC distance encoding of this form could underlie path integration deficits following lesions or inactivation of the RSC (Cooper et al. 2001; Elduayen and Save 2014).
Conjunctive allocentric spatial representations: beyond place cells and head direction.
Currently there have been limited reports of place cells and no reports of grid cells in either PPC or RSC during free foraging in two-dimensional environments (Cho and Sharp 2001; Jacob et al. 2017; Mao et al. 2017). The lack of these responses is likely the result of the diversity of inputs to both regions and strong egocentric influences that vary independently of the animal’s position within the environment. Implementation of statistical methods that characterize activity beyond spatial tuning curves would likely further reveal spatial sensitivity that transcends multiple spatial frames of reference (Hardcastle et al. 2017; Raposo et al. 2014; Rigotti et al. 2013).
In contrast to the absence of place cells or grid cells, allocentric head direction cells similar to those observed in anterior thalamic nuclei (Taube 1995) or subicular subfields (Taube et al. 1990) have been observed in both PPC and RSC, albeit in greater numbers in the RSC (Alexander and Nitz 2015, 2017; Chen et al. 1994; Cho and Sharp 2001; Jacob et al. 2017; Shine et al. 2016; Wilber et al. 2014). Recent work has revealed subsets of neurons in RSC and PPC that extend our understanding beyond the standard head direction neuron. Wilber et al. (2014) identified a population of PPC neurons that encode the angle to a behaviorally relevant landmark relative to the animal, irrespective of the navigational state or position of the rat within the recording environment. These “egocentric cue direction” neurons are likely imperative for goal-directed navigation and navigation strategies that require computations of vectors relative to distal landmarks. Additionally, a subset of parietal neurons encoding egocentric cue direction exhibited conjunctive representations of the animal’s allocentric heading orientation in the environment. That is, the egocentric cue direction and allocentric heading direction of the animal are solely encoded by the neuron when both tuning preferences are satisfied simultaneously. These conjunctive responses suggest that PPC neurons can potentially map the position of the landmark within the broader environment as computed from the position of the landmark relative to the animal. Importantly, neurons with this form of spatial sensitivity are likely critical for orientation behavior and trajectory updating during goal-directed navigation. It is unclear whether similar responses are observed in RSC, although recent reports suggest that the region may encode navigational cues (Vedder et al. 2016).
A recent study identified a subpopulation of dysgranular RSC neurons that encoded heading orientation relative to local visual landmarks in a two-compartment environment with rotational symmetry (Jacob et al. 2017). Following exposure to the environment in the light, this population continued to exhibit local head direction tuning in darkness. Additional experiments are needed to assess what influence visual information has on the initial construction of local-cue referenced head direction representations given the preponderance of visual inputs to dysgranular RSC. Nevertheless, the existence of local head direction tuning could prove vastly important for multiple landmark-based navigation strategies that may require offset detection between proximal and distal cues. The same study replicated the existence of RSC place-specific responses that are head direction sensitive, a conjunctive representation thought to be potentially critical for the calibration and stable representation of the broader head direction network (Bicanski and Burgess 2016; Cho and Sharp 2001; Jacob et al. 2017).
CONCLUSIONS
This review demonstrates the wide range of data concerning the potential mechanisms for coding of different aspects of the spatial pose of an animal in both cortical and subcortical structures. The data indicate that distributed circuits of neurons can gate complex transformations of neuronal activity to generate internal spatial representations based on external sensory input, resulting in neurons that exhibit response properties relevant to many features of spatial behavior. Data shows intriguing relationships and differences in the neural coding between different regions. Data shows place cells largely confined to hippocampus, as well as more broadly distributed head direction cells in dorsal presubiculum, entorhinal cortex, and retrosplenial cortex as well as anterior thalamus. Research has demonstrated grid cells and boundary cells in entorhinal cortex and subiculum. Neuronal responses consistent with the transformation between egocentric and allocentric representations have been shown in retrosplenial cortex and parietal cortex, including neurons coding direction of motor actions and the position within a route, as well as allocentric position and interactions between these spatial features.
A combination of new neurophysiological data and sophisticated computational modeling will be necessary to understand how complex transformations generate internal spatial representations based on external sensory input. As noted above, spatial coding of location and head direction could arise from self-motion information coded by proprioceptive feedback about self-motion or the computation of self-motion based on changes in the relative angle of sensory features. This process may involve ascending pathways through the medial septum and the regulation of theta rhythmic activity by running speed and direction but might also involve cortical computations based on the optic flow of sensory features. Alternately, spatial coding of allocentric location and head direction can arise from complex transformations of the egocentric angle of sensory features to determine location and head direction. This could involve the interaction of a number of different cortical regions, including the interaction of sensory cortices, retrosplenial cortex, and parietal cortex with the coding of location and head direction in the medial entorhinal cortex. Understanding these processes will require detailed computational theories of the generation, selection, and propagation of multiple forms of transformations mediated by neuronal circuits, as well as neurophysiological data testing hypotheses about the mechanism of these neuronal transformations.
GRANTS
This work supported by the National Institutes of Health, grant numbers R01 MH60013, R01 MH61492 and by the Office of Naval Research MURI N00014-16-1-2832.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.H., H.D., A.S.A., and M.E.H. prepared figures; J.R.H., H.D., A.S.A., and M.E.H. drafted manuscript; J.R.H., H.D., A.S.A., and M.E.H. edited and revised manuscript; J.R.H., H.D., A.S.A., and M.E.H. approved final version of manuscript.
REFERENCES
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res 19: 133–146, 1986. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Hunt PR. A comparison of the effects of anterior thalamic, mammillary body and fornix lesions on reinforced spatial alternation. Behav Brain Res 68: 91–101, 1995. doi: 10.1016/0166-4328(94)00163-A. [DOI] [PubMed] [Google Scholar]
- Ainge JA, van der Meer MA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus 17: 988–1002, 2007. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci 18: 1143–1151, 2015. doi: 10.1038/nn.4058. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA. Spatially periodic activation patterns of retrosplenial cortex encode route sub-spaces and distance traveled. Curr Biol 27: 1551–1560, 2017. doi: 10.1016/j.cub.2017.04.036. [DOI] [PubMed] [Google Scholar]
- Alonso A, Köhler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J Comp Neurol 225: 327–343, 1984. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: implications for learning and memory. J Neurosci 20: 8103–8110, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol 240: 37–59, 1985. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Amari S. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern 27: 77–87, 1977. doi: 10.1007/BF00337259. [DOI] [PubMed] [Google Scholar]
- Amilhon B, Huh CYL, Manseau F, Ducharme G, Nichol H, Adamantidis A, Williams S. Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron 86: 1277–1289, 2015. doi: 10.1016/j.neuron.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Andersen RA. Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc Lond B Biol Sci 352: 1421–1428, 1997. doi: 10.1098/rstb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science 230: 456–458, 1985. doi: 10.1126/science.4048942. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Mountcastle VB. The influence of the angle of gaze upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci 3: 532–548, 1983. doi: 10.1523/JNEUROSCI.03-03-00532.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Li CS, Stricanne B. Coordinate transformations in the representation of spatial information. Curr Opin Neurobiol 3: 171–176, 1993. doi: 10.1016/0959-4388(93)90206-E. [DOI] [PubMed] [Google Scholar]
- Aronov D, Nevers R, Tank DW. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543: 719–722, 2017. doi: 10.1038/nature21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avillac M, Denève S, Olivier E, Pouget A, Duhamel J-R. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci 8: 941–949, 2005. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci 10: 682–684, 2007. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol 14: 212–217, 2004. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits. Neuropharmacology 61: 1379–1388, 2011. doi: 10.1016/j.neuropharm.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LA, Bell KA, McQuiston AR. Synaptic muscarinic response types in hippocampal CA1 interneurons depend on different levels of presynaptic activity and different muscarinic receptor subtypes. Neuropharmacology 73: 160–173, 2013. doi: 10.1016/j.neuropharm.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LA, Bell KA, McQuiston AR. Acetylcholine release in mouse hippocampal CA1 preferentially activates inhibitory-selective interneurons via α4β2* nicotinic receptor activation. Front Cell Neurosci 9: 115, 2015a. doi: 10.3389/fncel.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LA, Bell KA, McQuiston AR. Activation of muscarinic receptors by ACh release in hippocampal CA1 depolarizes VIP but has varying effects on parvalbumin-expressing basket cells. J Physiol 593: 197–215, 2015b. doi: 10.1113/jphysiol.2014.277814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron 66: 921–936, 2010. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science 200: 1298–1300, 1978. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- Bicanski A, Burgess N. environmental anchoring of head direction in a computational model of retrosplenial cortex. J Neurosci 36: 11601–11618, 2016. doi: 10.1523/JNEUROSCI.0516-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Gupta K, Zhang K. Conversion of a phase- to a rate-coded position signal by a three-stage model of theta cells, grid cells, and place cells. Hippocampus 18: 1239–1255, 2008. doi: 10.1002/hipo.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, Copara MS, Gotman J, Ekstrom AD. Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat Commun 8: 14415, 2017. doi: 10.1038/ncomms14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. Grid cells require excitatory drive from the hippocampus. Nat Neurosci 16: 309–317, 2013. doi: 10.1038/nn.3311. [DOI] [PubMed] [Google Scholar]
- Bose A, Booth V, Recce M. A temporal mechanism for generating the phase precession of hippocampal place cells. J Comput Neurosci 9: 5–30, 2000. doi: 10.1023/A:1008976210366. [DOI] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332: 595–599, 2011. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Schultheiss NW, Hasselmo ME. Segregation of cortical head direction cell assemblies on alternating θ cycles. Nat Neurosci 16: 739–748, 2013. doi: 10.1038/nn.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron 82: 789–796, 2014. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioni JD, Decker MW, Gamboa LP, Izquierdo I, McGaugh JL. Muscimol injections in the medial septum impair spatial learning. Brain Res 522: 227–234, 1990. doi: 10.1016/0006-8993(90)91465-S. [DOI] [PubMed] [Google Scholar]
- Brown TI, Carr VA, LaRocque KF, Favila SE, Gordon AM, Bowles B, Bailenson JN, Wagner AD. Prospective representation of navigational goals in the human hippocampus. Science 352: 1323–1326, 2016. doi: 10.1126/science.aaf0784. [DOI] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci 30: 7414–7422, 2010. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Stern CE. Contributions of medial temporal lobe and striatal memory systems to learning and retrieving overlapping spatial memories. Cereb Cortex 24: 1906–1922, 2014. doi: 10.1093/cercor/bht041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci 18: 8038–8046, 1998. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetfering C, Allen K, Monyer H. Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat Neurosci 17: 710–718, 2014. doi: 10.1038/nn.3696. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature 416: 632–636, 2002. doi: 10.1038/416632a. [DOI] [PubMed] [Google Scholar]
- Burak Y, Fiete IR. Accurate path integration in continuous attractor network models of grid cells. PLOS Comput Biol 5: e1000291, 2009. doi: 10.1371/journal.pcbi.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus 18: 1157–1174, 2008. doi: 10.1002/hipo.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Barry C, O’Keefe J. An oscillatory interference model of grid cell firing. Hippocampus 17: 801–812, 2007. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Donnett JG, Jeffery KJ, O’Keefe J. Robotic and neuronal simulation of the hippocampus and rat navigation. Philos Trans R Soc Lond B Biol Sci 352: 1535–1543, 1997. doi: 10.1098/rstb.1997.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Jackson A, Hartley T, O’Keefe J. Predictions derived from modelling the hippocampal role in navigation. Biol Cybern 83: 301–312, 2000. doi: 10.1007/s004220000172. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol 398: 179–205, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Bush D, Barry C, Manson D, Burgess N. Using grid cells for navigation. Neuron 87: 507–520, 2015. doi: 10.1016/j.neuron.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, Burgess N. A hybrid oscillatory interference/continuous attractor network model of grid cell firing. J Neurosci 34: 5065–5079, 2014. doi: 10.1523/JNEUROSCI.4017-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron 33: 325–340, 2002. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev 114: 340–375, 2007. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. J Neurosci 23: 9719–9731, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS. Hippocampal phase precession from dual input components. J Neurosci 32: 16693–16703, 2012. doi: 10.1523/JNEUROSCI.2786-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, King JA, Burgess N, O’Keefe J. How vision and movement combine in the hippocampal place code. Proc Natl Acad Sci USA 110: 378–383, 2013. doi: 10.1073/pnas.1215834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Manson D, Cacucci F, Wills TJ. Absence of visual input results in the disruption of grid cell firing in the mouse. Curr Biol 26: 2335–2342, 2016. doi: 10.1016/j.cub.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res 101: 8–23, 1994. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Cheung A. Probabilistic learning by rodent grid cells. PLOS Comput Biol 12: e1005165, 2016. doi: 10.1371/journal.pcbi.1005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci 115: 3–25, 2001. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in alphaCaMKIIT286A and CREBalphaDelta- mice. Science 279: 867–869, 1998. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- Chrastil ER, Sherrill KR, Hasselmo ME, Stern CE. There and back again: hippocampus and retrosplenial cortex track homing distance during human path integration. J Neurosci 35: 15442–15452, 2015. doi: 10.1523/JNEUROSCI.1209-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrastil ER, Sherrill KR, Hasselmo ME, Stern CE. Which way and how far? Tracking of translation and rotation information for human path integration. Hum Brain Mapp 37: 3636–3655, 2016. doi: 10.1002/hbm.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Stackman RW, Walsh TJ. Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behav Neural Biol 52: 357–369, 1989. doi: 10.1016/S0163-1047(89)90472-X. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Intact landmark control and angular path integration by head direction cells in the anterodorsal thalamus after lesions of the medial entorhinal cortex. Hippocampus 21: 767–782, 2011. doi: 10.1002/hipo.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Front Neural Circuits 6: 7, 2012. doi: 10.3389/fncir.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 58: 151–164, 2005. doi: 10.1002/syn.20184. [DOI] [PubMed] [Google Scholar]
- Conklin J, Eliasmith C. A controlled attractor network model of path integration in the rat. J Comput Neurosci 18: 183–203, 2005. doi: 10.1007/s10827-005-6558-z. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Manka TF, Mizumori SJ. Finding your way in the dark: the retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behav Neurosci 115: 1012–1028, 2001. doi: 10.1037/0735-7044.115.5.1012. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci 28: 5983–5990, 2008. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron 84: 432–441, 2014. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M, Dewar M, Della Sala S, Wolbers T. Rest boosts the long-term retention of spatial associative and temporal order information. Hippocampus 25: 1017–1027, 2015. doi: 10.1002/hipo.22424. [DOI] [PubMed] [Google Scholar]
- Czajkowski R, Jayaprakash B, Wiltgen B, Rogerson T, Guzman-Karlsson MC, Barth AL, Trachtenberg JT, Silva AJ. Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci USA 111: 8661–8666, 2014. doi: 10.1073/pnas.1313222111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski R, Sugar J, Zhang SJ, Couey JJ, Ye J, Witter MP. Superficially projecting principal neurons in layer V of medial entorhinal cortex in the rat receive excitatory retrosplenial input. J Neurosci 33: 15779–15792, 2013. doi: 10.1523/JNEUROSCI.2646-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H. Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J Neurosci 35: 8394–8410, 2015. doi: 10.1523/JNEUROSCI.4460-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Gulledge AT. M1 and M4 receptors modulate hippocampal pyramidal neurons. J Neurophysiol 105: 779–792, 2011. doi: 10.1152/jn.00686.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M, Alber J, Cowan N, Della Sala S. Boosting long-term memory via wakeful rest: intentional rehearsal is not necessary, consolidation is sufficient. PLoS One 9: e109542, 2014. doi: 10.1371/journal.pone.0109542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M, Garcia YF, Cowan N, Della Sala S. Delaying interference enhances memory consolidation in amnesic patients. Neuropsychology 23: 627–634, 2009. doi: 10.1037/a0015568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GW, Hon OJ, Leutgeb S, Leutgeb JK. Grid and nongrid cells in medial entorhinal cortex represent spatial location and environmental features with complementary coding schemes. Neuron 94: 83–92, 2017. doi: 10.1016/j.neuron.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMattia BV, Kesner RP. Role of the posterior parietal association cortex in the processing of spatial event information. Behav Neurosci 102: 397–403, 1988. doi: 10.1037/0735-7044.102.3.397. [DOI] [PubMed] [Google Scholar]
- Dordek Y, Soudry D, Meir R, Derdikman D. Extracting grid cell characteristics from place cell inputs using non-negative principal component analysis. eLife 5: e10094, 2016. doi: 10.7554/eLife.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douchamps V, Jeewajee A, Blundell P, Burgess N, Lever C. Evidence for encoding versus retrieval scheduling in the hippocampus by theta phase and acetylcholine. J Neurosci 33: 8689–8704, 2013. doi: 10.1523/JNEUROSCI.4483-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50: 145–157, 2006. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsáki G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J Neurosci 19: 6191–6199, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggink H, Mertens P, Storm E, Giocomo LM. Hyperpolarization-activated cyclic nucleotide-gated 1 independent grid cell-phase precession in mice. Hippocampus 24: 249–256, 2014. doi: 10.1002/hipo.22231. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The role of the hippocampus in navigation is memory. J Neurophysiol 117: 1785–1796, 2017. doi: 10.1152/jn.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci 7: 716–732, 1987. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus 15: 881–889, 2005. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Elduayen C, Save E. The retrosplenial cortex is necessary for path integration in the dark. Behav Brain Res 272: 303–307, 2014. doi: 10.1016/j.bbr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Erdem UM, Hasselmo M. A goal-directed spatial navigation model using forward trajectory planning based on grid cells. Eur J Neurosci 35: 916–931, 2012. doi: 10.1111/j.1460-9568.2012.08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem UM, Hasselmo ME. A biologically inspired hierarchical goal directed navigation model. J Physiol Paris 108: 28–37, 2014. doi: 10.1016/j.jphysparis.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Brunton BW, Duan CA, Hanks TD, Brody CD. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. eLife 4: e05457, 2015. doi: 10.7554/eLife.05457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Silva D, Foster DJ. Dissociation between the experience-dependent development of hippocampal theta sequences and single-trial phase precession. J Neurosci 35: 4890–4902, 2015. doi: 10.1523/JNEUROSCI.2614-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron 40: 1227–1239, 2003. doi: 10.1016/S0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, Buzsáki G. Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling. Neuron 93: 1213–1226, 2017. doi: 10.1016/j.neuron.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci 23: 676–681, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27: 169–178, 2000. doi: 10.1016/S0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res 478: 375–381, 1989. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336: 170–173, 1988. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Léránth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol 239: 237–246, 1985. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell 156: 1139–1152, 2014. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron 86: 1253–1264, 2015. doi: 10.1016/j.neuron.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci 26: 4266–4276, 2006. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press, 1990. [Google Scholar]
- Gerstner W, Abbott LF. Learning navigational maps through potentiation and modulation of hippocampal place cells. J Comput Neurosci 4: 79–94, 1997. doi: 10.1023/A:1008820728122. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Moser MB, Moser EI. Computational models of grid cells. Neuron 71: 589–603, 2011. doi: 10.1016/j.neuron.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Stensola T, Bonnevie T, Van Cauter T, Moser MB, Moser EI. Topography of head direction cells in medial entorhinal cortex. Curr Biol 24: 252–262, 2014. doi: 10.1016/j.cub.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Zilli EA, Fransén E, Hasselmo ME. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315: 1719–1722, 2007. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B, Olton DS. Local modulation of basal forebrain: effects on working and reference memory. J Neurosci 14: 3578–3587, 1994. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behav Neurosci 104: 849–855, 1990. doi: 10.1037/0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Gnadt J, Andersen RA. Memory related motor planninng activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220, 1988. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J Neurosci 17: 9315–9330, 1997. doi: 10.1523/JNEUROSCI.17-23-09315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci 16: 823–835, 1996. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith WH, Matthews RT. Electrophysiology of AChE-positive neurons in basal forebrain slices. Neurosci Lett 71: 169–174, 1986. doi: 10.1016/0304-3940(86)90553-7. [DOI] [PubMed] [Google Scholar]
- Gu Z, Alexander GM, Dudek SM, Yakel JL. Hippocampus and entorhinal cortex recruit cholinergic and NMDA receptors separately to generate hippocampal theta oscillations. Cell Reports 21: 3585–3595, 2017. doi: 10.1016/j.celrep.2017.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guanella A, Verschure PF. Prediction of the position of an animal based on populations of grid and place cells: a comparative simulation study. J Integr Neurosci 6: 433–446, 2007. doi: 10.1142/S0219635207001556. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Kawaguchi Y. Phasic cholinergic signaling in the hippocampus: functional homology with the neocortex? Hippocampus 17: 327–332, 2007. doi: 10.1002/hipo.20279. [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal θ sequences. Nat Neurosci 15: 1032–1039, 2012. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453: 1248–1252, 2008. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature 436: 801–806, 2005. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilágyi N, Freund TF, Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci 29: 8094–8102, 2009. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle K, Ganguli S, Giocomo LM. Environmental boundaries as an error correction mechanism for grid cells. Neuron 86: 827–839, 2015. doi: 10.1016/j.neuron.2015.03.039. [DOI] [PubMed] [Google Scholar]
- Hardcastle K, Maheswaranathan N, Ganguli S, Giocomo LM. A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron 94: 375–387, 2017. doi: 10.1016/j.neuron.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci 369: 20120510, 2014. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484: 62–68, 2012. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461: 941–946, 2009. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci 3: 351–359, 1999. doi: 10.1016/S1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16: 710–715, 2006. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus 18: 1213–1229, 2008. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. A model of episodic memory: mental time travel along encoded trajectories using grid cells. Neurobiol Learn Mem 92: 559–573, 2009. doi: 10.1016/j.nlm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex. Philos Trans R Soc Lond B Biol Sci 369: 20120523, 2014. doi: 10.1098/rstb.2012.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14: 793–817, 2002. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Brandon MP. A model combining oscillations and attractor dynamics for generation of grid cell firing. Front Neural Circuits 6: 30, 2012. doi: 10.3389/fncir.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw 18: 1172–1190, 2005. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci 15: 5249–5262, 1995. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Shay CF. Grid cell firing patterns may arise from feedback interaction between intrinsic rebound spiking and transverse traveling waves with multiple heading angles. Front Syst Neurosci 8: 201, 2014. doi: 10.3389/fnsys.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington PA, Shapiro ML. A simple network model simulates hippocampal place fields: II. Computing goal-directed trajectories and memory fields. Behav Neurosci 107: 434–443, 1993. doi: 10.1037/0735-7044.107.3.434. [DOI] [PubMed] [Google Scholar]
- Heys JG, Rangarajan KV, Dombeck DA. The functional micro-organization of grid cells revealed by cellular-resolution imaging. Neuron 84: 1079–1090, 2014. doi: 10.1016/j.neuron.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley EL, Nelson AJD, Aggleton JP, Vann SD. The rat retrosplenial cortex is required when visual cues are used flexibly to determine location. Behav Brain Res 263: 98–107, 2014. doi: 10.1016/j.bbr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JR, Brandon MP, Climer JR, Chapman GW, Hasselmo ME. Multiple running speed signals in medial entorhinal cortex. Neuron 91: 666–679, 2016. doi: 10.1016/j.neuron.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Long LL, Escabí MA, Chrobak JJ. Septotemporal variation in dynamics of theta: speed and habituation. J Neurophysiol 105: 2675–2686, 2011. doi: 10.1152/jn.00837.2010. [DOI] [PubMed] [Google Scholar]
- Hoh TE, Kolb B, Eppel A, Vanderwolf CH, Cain DP. Role of the neocortex in the water maze task in the rat: a detailed behavioral and Golgi-Cox analysis. Behav Brain Res 138: 81–94, 2003. doi: 10.1016/S0166-4328(02)00237-1. [DOI] [PubMed] [Google Scholar]
- Howard LR, Javadi AH, Yu Y, Mill RD, Morrison LC, Knight R, Loftus MM, Staskute L, Spiers HJ. The hippocampus and entorhinal cortex encode the path and Euclidean distances to goals during navigation. Curr Biol 24: 1331–1340, 2014. doi: 10.1016/j.cub.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15: 1053–1063, 1995. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Huh CYL, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J Neurosci 30: 15951–15961, 2010. doi: 10.1523/JNEUROSCI.3663-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci 23: 11725–11731, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus 15: 739–749, 2005. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Jacob P-Y, Casali G, Spieser L, Page H, Overington D, Jeffery K. An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nat Neurosci 20: 173–175, 2017. doi: 10.1038/nn.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science 336: 1454–1458, 2012. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewajee A, Barry C, O’Keefe J, Burgess N. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus 18: 1175–1185, 2008. doi: 10.1002/hipo.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Donnett JG, O’Keefe J. Medial septal control of theta-correlated unit firing in the entorhinal cortex of awake rats. Neuroreport 6: 2166–2170, 1995. doi: 10.1097/00001756-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn Mem 3: 279–287, 1996. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci 27: 12176–12189, 2007. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol 3: e402, 2005. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus D, Dalügge D, Bothe S, Fuhrmann F, Hannes C, Kaneko H, Friedrichs D, Sosulina L, Schwarz I, Elliott DA, Schoch S, Bradke F, Schwarz MK, Remy S. Glutamatergic synaptic integration of locomotion speed via septoentorhinal projections. Nat Neurosci 20: 16–19, 2016. doi: 10.1038/nn.4447. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399: 781–784, 1999. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron 42: 283–295, 2004. doi: 10.1016/S0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Baimbridge KG, Freund TF. Topographical localization of neurons containing parvalbumin and choline acetyltransferase in the medial septum-diagonal band region of the rat. Neuroscience 36: 61–72, 1990a. doi: 10.1016/0306-4522(90)90351-4. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Freund TF. Distribution of septohippocampal neurons containing parvalbumin or choline acetyltransferase in the rat brain. J Comp Neurol 298: 362–372, 1990b. doi: 10.1002/cne.902980308. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. Island cells control temporal association memory. Science 343: 896–901, 2014. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Sun C, Martin J, Kitch LJ, Schnitzer MJ, Tonegawa S. Entorhinal cortical ocean cells encode specific contexts and drive context-specific fear memory. Neuron 87: 1317–1331, 2015. doi: 10.1016/j.neuron.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332: 592–595, 2011. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- Köhler C, Chan-Palay V, Wu JY. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat Embryol (Berl) 169: 41–44, 1984. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behav Brain Res 23: 127–145, 1987. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Witter MP. Presubiculum layer III conveys retrosplenial input to the medial entorhinal cortex. Hippocampus 22: 881–895, 2012. doi: 10.1002/hipo.20949. [DOI] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol 49: 58–85, 1975. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Kraus BJ, Brandon MP, Robinson RJ II, Connerney MA, Hasselmo ME, Eichenbaum H. During running in place, grid cells integrate elapsed time and distance run. Neuron 88: 578–589, 2015. doi: 10.1016/j.neuron.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ II, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron 78: 1090–1101, 2013. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Carmichael JE, Moser MB, Moser EI. Speed cells in the medial entorhinal cortex. Nature 523: 419–424, 2015. doi: 10.1038/nature14622. [DOI] [PubMed] [Google Scholar]
- Kropff E, Treves A. The emergence of grid cells: intelligent design or just adaptation? Hippocampus 18: 1256–1269, 2008. doi: 10.1002/hipo.20520. [DOI] [PubMed] [Google Scholar]
- Krupic J, Bauza M, Burton S, Lever C, O’Keefe J. How environment geometry affects grid cell symmetry and what we can learn from it. Philos Trans R Soc Lond B Biol Sci 369: 20130188, 2014. doi: 10.1098/rstb.2013.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubie JL, Fenton AA. Linear look-ahead in conjunctive cells: an entorhinal mechanism for vector-based navigation. Front Neural Circuits 6: 20, 2012. doi: 10.3389/fncir.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson VH, Bland BH. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: analysis by the intraseptal microinfusion of carbachol, atropine, and procaine. Exp Neurol 120: 132–144, 1993. doi: 10.1006/exnr.1993.1047. [DOI] [PubMed] [Google Scholar]
- Leão RN, Mikulovic S, Leão KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort ABL, Kullander K. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci 15: 1524–1530, 2012. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36: 1183–1194, 2002. doi: 10.1016/S0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Lengyel M, Szatmáry Z, Erdi P. Dynamically detuned oscillations account for the coupled rate and temporal code of place cell firing. Hippocampus 13: 700–714, 2003. doi: 10.1002/hipo.10116. [DOI] [PubMed] [Google Scholar]
- Leung LS, Shen B. Glutamatergic synaptic transmission participates in generating the hippocampal EEG. Hippocampus 14: 510–525, 2004. doi: 10.1002/hipo.10199. [DOI] [PubMed] [Google Scholar]
- Leung LW, Desborough KA. APV, an N-methyl-D-aspartate receptor antagonist, blocks the hippocampal theta rhythm in behaving rats. Brain Res 463: 148–152, 1988. doi: 10.1016/0006-8993(88)90538-0. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci 29: 9771–9777, 2009. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416: 90–94, 2002. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Jensen O. The θ-γ neural code. Neuron 77: 1002–1016, 2013. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science 343: 857–863, 2014. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M’Harzi M, Palacios A, Monmaur P, Willig F, Houcine O, Delacour J. Effects of selective lesions of fimbria-fornix on learning set in the rat. Physiol Behav 40: 181–188, 1987. doi: 10.1016/0031-9384(87)90205-8. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71: 737–749, 2011. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic mechanisms of phase precession in hippocampal CA1 pyramidal neurons. J Neurophysiol 86: 528–532, 2001. doi: 10.1152/jn.2001.86.1.528. [DOI] [PubMed] [Google Scholar]
- Makino H, Komiyama T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci 18: 1116–1122, 2015. doi: 10.1038/nn.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory CS, Hardcastle K, Bant JS, Giocomo LM. Grid scale drives the scale and long-term stability of place maps. Nat Neurosci 21: 270–282, 2018. doi: 10.1038/s41593-017-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Kandler S, McNaughton BL, Bonin V. Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat Commun 8: 243, 2017. doi: 10.1038/s41467-017-00180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette SA, Vass LK, Ryan J, Epstein RA. Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nat Neurosci 17: 1598–1606, 2014. [Erratum. Nat Neurosci 18: 926, 2015.] 10.1038/nn.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Murray EA, Gaffan D. A comparative analysis of the role of fornix and cingulate cortex in memory: rats. Exp Brain Res 74: 187–201, 1989. doi: 10.1007/BF00248292. [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Electrophysiological characteristics of cholinergic and non-cholinergic neurons in the rat medial septum-diagonal band complex. Brain Res 513: 171–174, 1990. doi: 10.1016/0006-8993(90)91106-Q. [DOI] [PubMed] [Google Scholar]
- Marozzi E, Ginzberg LL, Alenda A, Jeffery KJ. Purely translational realignment in grid cell firing patterns following nonmetric context change. Cereb Cortex 25: 4619–4627, 2015. doi: 10.1093/cercor/bhv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston HM, Everitt BJ, Robbins TW. Comparative effects of excitotoxic lesions of the hippocampus and septum/diagonal band on conditional visual discrimination and spatial learning. Neuropsychologia 31: 1099–1118, 1993. doi: 10.1016/0028-3932(93)90035-X. [DOI] [PubMed] [Google Scholar]
- Martin MM, Horn KL, Kusman KJ, Wallace DG. Medial septum lesions disrupt exploratory trip organization: evidence for septohippocampal involvement in dead reckoning. Physiol Behav 90: 412–424, 2007. doi: 10.1016/j.physbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O’Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52: 41–49, 1983. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci 7: 663–678, 2006. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Mizumori SJ, Barnes CA, Leonard BJ, Marquis M, Green EJ. Cortical representation of motion during unrestrained spatial navigation in the rat. Cereb Cortex 4: 27–39, 1994. [DOI] [PubMed] [Google Scholar]
- McQuiston AR. Acetylcholine release and inhibitory interneuron activity in hippocampal CA1. Front Synaptic Neurosci 6: 20, 2014. doi: 10.3389/fnsyn.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10: 1185–1201, 1983. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Milford MJ, Wiles J, Wyeth GF. Solving navigational uncertainty using grid cells on robots. PLOS Comput Biol 6: e1000995, 2010. doi: 10.1371/journal.pcbi.1000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milford MJ, Wyeth G. Persistent navigation and mapping using a biologically inspired SLAM system. Int J Robot Res 29: 1131–1153, 2010. doi: 10.1177/0278364909340592. [DOI] [Google Scholar]
- Mitchell SJ, Rawlins JN, Steward O, Olton DS. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J Neurosci 2: 292–302, 1982. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstaedt M, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften 67: 566–567, 1980. doi: 10.1007/BF00450672. [DOI] [Google Scholar]
- Mizumori SJY, Perez GM, Alvarado MC, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res 528: 12–20, 1990. doi: 10.1016/0006-8993(90)90188-H. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 64: 267–280, 2009. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan CK, Chapman GW IV, Hasselmo ME. Systemic administration of two different anxiolytic drugs decreases local field potential theta frequency in the medial entorhinal cortex without affecting grid cell firing fields. Neuroscience 364: 60–70, 2017. doi: 10.1016/j.neuroscience.2017.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monmaur P, Breton P. Elicitation of hippocampal theta by intraseptal carbachol injection in freely moving rats. Brain Res 544: 150–155, 1991. doi: 10.1016/0006-8993(91)90898-6. [DOI] [PubMed] [Google Scholar]
- Monmaur P, Collet A, Puma C, Frankel-Kohn L, Sharif A. Relations between acetylcholine release and electrophysiological characteristics of theta rhythm: a microdialysis study in the urethane-anesthetized rat hippocampus. Brain Res Bull 42: 141–146, 1997. doi: 10.1016/S0361-9230(96)00200-6. [DOI] [PubMed] [Google Scholar]
- Morris NP, Harris SJ, Henderson Z. Parvalbumin-immunoreactive, fast-spiking neurons in the medial septum/diagonal band complex of the rat: intracellular recordings in vitro. Neuroscience 92: 589–600, 1999. doi: 10.1016/S0306-4522(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683, 1982. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Andersen RA, Motter BC. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci 1: 1218–1225, 1981. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Wehner R. Path integration in desert ants, Cataglyphis fortis. Proc Natl Acad Sci USA 85: 5287–5290, 1988. doi: 10.1073/pnas.85.14.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7: 1951–1968, 1987. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB Jr. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci 7: 1935–1950, 1987. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagode DA, Tang A-H, Karson MA, Klugmann M, Alger BE. Optogenetic release of ACh induces rhythmic bursts of perisomatic IPSCs in hippocampus. PLoS One 6: e27691, 2011. doi: 10.1371/journal.pone.0027691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Venditto SJC, Climer JR, Petter EA, Gillet SN, Levy S. Precise spike timing dynamics of hippocampal place cell activity sensitive to cholinergic disruption. Hippocampus 27: 1069–1082, 2017. doi: 10.1002/hipo.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65: 472–479, 2010. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D. Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiol Learn Mem 91: 179–185, 2009. doi: 10.1016/j.nlm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Nitz DA. Tracking route progression in the posterior parietal cortex. Neuron 49: 747–756, 2006. doi: 10.1016/j.neuron.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Nitz DA. Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nat Neurosci 15: 1365–1367, 2012. doi: 10.1038/nn.3213. [DOI] [PubMed] [Google Scholar]
- Numan R, Quaranta JR Jr. Effects of medial septal lesions on operant delayed alternation in rats. Brain Res 531: 232–241, 1990. doi: 10.1016/0006-8993(90)90779-B. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol 51: 78–109, 1976. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature 381: 425–428, 1996. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci 353: 1333–1340, 1998. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175, 1971. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon, 1978. [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3: 317–330, 1993. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Oess T, Krichmar JL, Röhrbein F. A computational model for spatial navigation based on reference frames in the hippocampus, retrosplenial cortex, and posterior parietal cortex. Front Neurorobot 11: 4, 2017. doi: 10.3389/fnbot.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ólafsdóttir HF, Barry C, Saleem AB, Hassabis D, Spiers HJ. Hippocampal place cells construct reward related sequences through unexplored space. eLife 4: e06063, 2015. doi: 10.7554/eLife.06063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GM, Ohara S, Iijima T, Witter MP. Parahippocampal and retrosplenial connections of rat posterior parietal cortex. Hippocampus 27: 335–358, 2017. doi: 10.1002/hipo.22701. [DOI] [PubMed] [Google Scholar]
- Olsen GM, Witter MP. Posterior parietal cortex of the rat: architectural delineation and thalamic differentiation. J Comp Neurol 524: 3774–3809, 2016. doi: 10.1002/cne.24032. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space and memory. Behav Brain Sci 2: 313–322, 1979. doi: 10.1017/S0140525X00062713. [DOI] [Google Scholar]
- Otto T, Wolf D, Walsh TJ. Combined lesions of perirhinal and entorhinal cortex impair rats’ performance in two versions of the spatially guided radial-arm maze. Neurobiol Learn Mem 68: 21–31, 1997. doi: 10.1006/nlme.1997.3778. [DOI] [PubMed] [Google Scholar]
- Pabst M, Braganza O, Dannenberg H, Hu W, Pothmann L, Rosen J, Mody I, van Loo K, Deisseroth K, Becker AJ, Schoch S, Beck H. Astrocyte intermediaries of septal cholinergic modulation in the hippocampus. Neuron 90: 853–865, 2016. doi: 10.1016/j.neuron.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Pang KC, Nocera R, Secor AJ, Yoder RM. GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus 11: 814–827, 2001. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science 321: 1322–1327, 2008. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escobar JA, Kornienko O, Latuske P, Kohler L, Allen K. Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex. eLife 5: e16937, 2016. doi: 10.7554/eLife.16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497: 74–79, 2013. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature 400: 233–238, 1999. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Davies M, Albasser MM, Aggleton JP, Vann SD. Granular and dysgranular retrosplenial cortices provide qualitatively different contributions to spatial working memory: evidence from immediate-early gene imaging in rats. Eur J Neurosci 30: 877–888, 2009. doi: 10.1111/j.1460-9568.2009.06881.x. [DOI] [PubMed] [Google Scholar]
- Pouget A, Sejnowski TJ. Spatial transformations in the parietal cortex using basis functions. J Cogn Neurosci 9: 222–237, 1997. doi: 10.1162/jocn.1997.9.2.222. [DOI] [PubMed] [Google Scholar]
- Pouget A, Snyder LH. Computational approaches to sensorimotor transformations. Nat Neurosci 3, Suppl: 1192–1198, 2000. doi: 10.1038/81469. [DOI] [PubMed] [Google Scholar]
- Raposo D, Kaufman MT, Churchland AK. A category-free neural population supports evolving demands during decision-making. Nat Neurosci 17: 1784–1792, 2014. doi: 10.1038/nn.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudies F, Brandon MP, Chapman GW, Hasselmo ME. Head direction is coded more strongly than movement direction in a population of entorhinal neurons. Brain Res 1621: 355–367, 2015. doi: 10.1016/j.brainres.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudies F, Hasselmo ME. Differences in visual-spatial input may underlie different compression properties of firing fields for grid cell modules in medial entorhinal cortex. PLOS Comput Biol 11: e1004596, 2015. doi: 10.1371/journal.pcbi.1004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudies F, Mingolla E, Hasselmo ME. Modeling the influence of optic flow on grid cell firing in the absence of other cues. J Comput Neurosci 33: 475–493, 2012. doi: 10.1007/s10827-012-0396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins JN, Feldon J, Gray JA. Septo-hippocampal connections and the hippocampal theta rhythm. Exp Brain Res 37: 49–63, 1979. doi: 10.1007/BF01474253. [DOI] [PubMed] [Google Scholar]
- Ray S, Naumann R, Burgalossi A, Tang Q, Schmidt H, Brecht M. Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science 343: 891–896, 2014. doi: 10.1126/science.1243028. [DOI] [PubMed] [Google Scholar]
- Redish AD, Elga AN, Touretzky DS. A coupled attractor model of the rodent head direction system. Network 7: 671–685, 1996. doi: 10.1088/0954-898X_7_4_004. [DOI] [Google Scholar]
- Redish AD, Touretzky DS. Cognitive maps beyond the hippocampus. Hippocampus 7: 15–35, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput 10: 73–111, 1998. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Redish D. Beyond the Cognitive Map: From Place Cells to Episodic Memory. Cambridge, MA: MIT Press, 1999. [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res 100: 67–84, 1994. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Richard GR, Titiz A, Tyler A, Holmes GL, Scott RC, Lenck-Santini P-P. Speed modulation of hippocampal theta frequency correlates with spatial memory performance. Hippocampus 23: 1269–1279, 2013. doi: 10.1002/hipo.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature 497: 585–590, 2013. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, Williams S. Optogenetic activation of septal glutamatergic neurons drive hippocampal theta rhythms. J Neurosci 36: 3016–3023, 2016. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Bucci DJ. Damage to posterior parietal cortex impairs two forms of relational learning. Front Integr Nuerosci 6: 45, 2012. doi: 10.3389/fnint.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behav Neurosci 125: 578–587, 2011. doi: 10.1037/a0024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci 17: 5900–5920, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312: 758–762, 2006. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B. Involvement of the hippocampus and associative parietal cortex in the use of proximal and distal landmarks for navigation. Behav Brain Res 109: 195–206, 2000. doi: 10.1016/S0166-4328(99)00173-4. [DOI] [PubMed] [Google Scholar]
- Savelli F, Yoganarasimha D, Knierim JJ. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus 18: 1270–1282, 2008. doi: 10.1002/hipo.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Häusser M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat Neurosci 16: 325–331, 2013. doi: 10.1038/nn.3340. [DOI] [PubMed] [Google Scholar]
- Séguinot V, Maurer R, Etienne AS. Dead reckoning in a small mammal: the evaluation of distance. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 173: 103–113, 1993. doi: 10.1007/BF00209622. [DOI] [PubMed] [Google Scholar]
- Serafin M, Williams S, Khateb A, Fort P, Mühlethaler M. Rhythmic firing of medial septum non-cholinergic neurons. Neuroscience 75: 671–675, 1996. doi: 10.1016/0306-4522(96)00349-1. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sherrill KR, Chrastil ER, Ross RS, Erdem UM, Hasselmo ME, Stern CE. Functional connections between optic flow areas and navigationally responsive brain regions during goal-directed navigation. Neuroimage 118: 386–396, 2015. doi: 10.1016/j.neuroimage.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J Neurosci 33: 19304–19313, 2013. doi: 10.1523/JNEUROSCI.1825-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Honda Y, Sasaki H, Naito J. Organization of intrinsic connections of the retrosplenial cortex in the rat. Anat Sci Int 84: 280–292, 2009. doi: 10.1007/s12565-009-0035-0. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kondo S, Naito J. Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci Res 49: 1–11, 2004. doi: 10.1016/j.neures.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Shine JP, Valdés-Herrera JP, Hegarty M, Wolbers T. The human retrosplenial cortex and thalamus code head direction in a global reference frame. J Neurosci 36: 6371–6381, 2016. doi: 10.1523/JNEUROSCI.1268-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si B, Kropff E, Treves A. Grid alignment in entorhinal cortex. Biol Cybern 106: 483–506, 2012. doi: 10.1007/s00422-012-0513-7. [DOI] [PubMed] [Google Scholar]
- Siegle JH, Wilson MA. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. eLife 3: e03061, 2014. doi: 10.7554/eLife.03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271: 1870–1873, 1996. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6: 149–172, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science 322: 1865–1868, 2008. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol 551: 927–943, 2003. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Gilbert SJ. Solving the detour problem in navigation: a model of prefrontal and hippocampal interactions. Front Hum Neurosci 9: 125, 2015. doi: 10.3389/fnhum.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G. Structural determinants of functional brain dynamics. In: Handbook of Brain Connectivity, edited by Jirsa VK, McIntosh AR. Berlin: Springer, 2007, p. 117–147. [Google Scholar]
- Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90: 2862–2874, 2003. doi: 10.1152/jn.00346.2003. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci 18: 9020–9037, 1998. doi: 10.1523/JNEUROSCI.18-21-09020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron 45: 301–313, 2005. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, Frøland K, Moser MB, Moser EI. The entorhinal grid map is discretized. Nature 492: 72–78, 2012. doi: 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci 13: 163–168, 1990. doi: 10.1016/0166-2236(90)90040-H. [DOI] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, Cappaert NL. The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform 5: 7, 2011. doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpizio V, Committeri G, Lambrey S, Berthoz A, Galati G. Selective role of lingual/parahippocampal gyrus and retrosplenial complex in spatial memory across viewpoint changes relative to the environmental reference frame. Behav Brain Res 242: 62–75, 2013. doi: 10.1016/j.bbr.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology 49: 464–469, 1997. doi: 10.1212/WNL.49.2.464. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15: 70–86, 1995. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55: 225–256, 1998. doi: 10.1016/S0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Taube JS, Bassett JP. Persistent neural activity in head direction cells. Cereb Cortex 13: 1162–1172, 2003. doi: 10.1093/cercor/bhg102. [DOI] [PubMed] [Google Scholar]
- Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW. Processing the head direction cell signal: a review and commentary. Brain Res Bull 40: 477–484; discussion 484–486, 1996. [DOI] [PubMed] [Google Scholar]
- Taube JS, Kesslak JP, Cotman CW. Lesions of the rat postsubiculum impair performance on spatial tasks. Behav Neural Biol 57: 131–143, 1992. doi: 10.1016/0163-1047(92)90629-I. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10: 420–435, 1990. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas A, Krause M, Lipa P, Gothard KM, Barnes CA, McNaughton BL. Self-motion and the hippocampal spatial metric. J Neurosci 25: 8085–8096, 2005. doi: 10.1523/JNEUROSCI.0693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol 500: 463–474, 1997. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touretzky DS, Redish AD. Theory of rodent navigation based on interacting representations of space. Hippocampus 6: 247–270, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Trullier O, Wiener SI, Berthoz A, Meyer JA. Biologically based artificial navigation systems: review and prospects. Prog Neurobiol 51: 483–544, 1997. doi: 10.1016/S0301-0082(96)00060-3. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RGM. Schema-dependent gene activation and memory encoding in neocortex. Science 333: 891–895, 2011. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus 6: 271–280, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol 300: 593–606, 1990. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 315: 200–216, 1992. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463: 249–263, 2003. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Varga V, Berényi A, Papp E, Barthó P, Venance L, Freund TF, Buzsáki G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci USA 111: 13535–13540, 2014. doi: 10.1073/pnas.1411233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol 26: 407–418, 1969. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci 119: 1682–1686, 2005. doi: 10.1037/0735-7044.119.6.1682. [DOI] [PubMed] [Google Scholar]
- Vann SD, Kristina Wilton LA, Muir JL, Aggleton JP. Testing the importance of the caudal retrosplenial cortex for spatial memory in rats. Behav Brain Res 140: 107–118, 2003. doi: 10.1016/S0166-4328(02)00274-7. [DOI] [PubMed] [Google Scholar]
- Varga V, Hangya B, Kránitz K, Ludányi A, Zemankovics R, Katona I, Shigemoto R, Freund TF, Borhegyi Z. The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum. J Physiol 586: 3893–3915, 2008. doi: 10.1113/jphysiol.2008.155242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder LC, Miller AMP, Harrison MB, Smith DM. Retrosplenial cortical neurons encode navigational cues, trajectories and reward locations during goal directed navigation. Cereb Cortex 27: 3713–3723, 2016. doi: 10.1093/cercor/bhw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216: 192–210, 1983. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Levey AI, Rye DB, Mesulam MM, Mufson EJ. Cholinergic and non-cholinergic septohippocampal pathways. Neurosci Lett 54: 45–52, 1985. doi: 10.1016/S0304-3940(85)80116-6. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME. GABAergic modulation of hippocampal population activity: sequence learning, place field development, and the phase precession effect. J Neurophysiol 78: 393–408, 1997. doi: 10.1152/jn.1997.78.1.393. [DOI] [PubMed] [Google Scholar]
- Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci 18: 282–288, 2015. doi: 10.1038/nn.3904. [DOI] [PubMed] [Google Scholar]
- Wells CE, Amos DP, Jeewajee A, Douchamps V, Rodgers J, O’Keefe J, Burgess N, Lever C. Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J Neurosci 33: 8650–8667, 2013. doi: 10.1523/JNEUROSCI.5040-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav 58: 687–693, 1995. doi: 10.1016/0031-9384(95)00110-5. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Pfuhl G, Dagslott N, Moser M-B, Moser EI. Functional split between parietal and entorhinal cortices in the rat. Neuron 73: 789–802, 2012. doi: 10.1016/j.neuron.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Widloski J, Fiete IR. A model of grid cell development through spatial exploration and spike time-dependent plasticity. Neuron 83: 481–495, 2014. doi: 10.1016/j.neuron.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Wiener M, Michaelis K, Thompson JC. Functional correlates of likelihood and prior representations in a virtual distance task. Hum Brain Mapp 37: 3172–3187, 2016. doi: 10.1002/hbm.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD. Hippocampal theta sequences reflect current goals. Nat Neurosci 18: 289–294, 2015. doi: 10.1038/nn.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Demecha AJ, Mesina L, Vos JM, McNaughton BL. Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Front Neural Circuits 8: 146, 2015. doi: 10.3389/fncir.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Forster TC, Tatsuno M, McNaughton BL. Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex. J Neurosci 34: 5431–5446, 2014. doi: 10.1523/JNEUROSCI.0511-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Skelin I, Wu W, McNaughton BL. Laminar organization of encoding and memory reactivation in the parietal cortex. Neuron 95: 1406–1419, 2017. doi: 10.1016/j.neuron.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Barry C, Cacucci F. The abrupt development of adult-like grid cell firing in the medial entorhinal cortex. Front Neural Circuits 6: 21, 2012. doi: 10.3389/fncir.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science 328: 1573–1576, 2010. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201: 160–163, 1978. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Winter SS, Clark BJ, Taube JS. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science 347: 870–874, 2015a. doi: 10.1126/science.1259591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SS, Mehlman ML, Clark BJ, Taube JS. Passive transport disrupts grid signals in the parahippocampal cortex. Curr Biol 25: 2493–2502, 2015b. doi: 10.1016/j.cub.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature 397: 613–616, 1999. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27: 623–633, 2000. doi: 10.1016/S0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475–524, 1991. doi: 10.1016/0301-0082(91)90006-M. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus 2: 1–11, 1992. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Yoon K, Buice MA, Barry C, Hayman R, Burgess N, Fiete IR. Specific evidence of low-dimensional continuous attractor dynamics in grid cells. Nat Neurosci 16: 1077–1084, 2013. doi: 10.1038/nn.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J Neurosci 16: 2112–2126, 1996. doi: 10.1523/JNEUROSCI.16-06-02112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Ng L, Williams D, Valley M, Li Y, Garrett M, Waters J. An extended retinotopic map of mouse cortex. eLife 6: e18372, 2017. doi: 10.7554/eLife.18372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilli EA, Hasselmo ME. Coupled noisy spiking neurons as velocity-controlled oscillators in a model of grid cell spatial firing. J Neurosci 30: 13850–13860, 2010. doi: 10.1523/JNEUROSCI.0547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro MB, Berthoz A, Wiener SI. Peak firing rates of rat anterodorsal thalamic head direction cells are higher during faster passive rotations. Hippocampus 12: 481–486, 2002. doi: 10.1002/hipo.10022. [DOI] [PubMed] [Google Scholar]
- Zugaro MB, Monconduit L, Buzsáki G. Spike phase precession persists after transient intrahippocampal perturbation. Nat Neurosci 8: 67–71, 2005. doi: 10.1038/nn1369. [DOI] [PMC free article] [PubMed] [Google Scholar]