Abstract
When a Gabor patch moves along a path in one direction while its internal texture drifts orthogonally to this path, it can appear to deviate from its physical path by 45° or more. This double-drift illusion is different from other motion-induced position shift effects in several ways: it has an integration period of over a second; the illusory displacement that accumulates over a second or more is orthogonal to rather than along the motion path; the perceptual deviations are much larger; and they have little or no effect on eye movements to the target. In this study we investigated the underlying neural mechanisms of the motion integration and position processing for this double-drift stimulus by testing possible anatomical constraints on its magnitude. We found that the illusion was reduced at the vertical and horizontal meridians when the perceptual path would cross or be driven toward the meridian, but not at other locations or other motion directions. The disruption of the accumulation of the position error at both the horizontal and vertical meridians suggests a central role of quadrantic areas in the generation of this type of motion-induced position shift.
NEW & NOTEWORTHY The remarkably strong double-drift illusion is disrupted at both the vertical and horizontal meridians. We propose that this finding is the behavioral consequence of the anatomical gaps at both meridians, suggesting that neural areas with quadrantic representations (e.g., V2, V3) are the initial locus of this motion-induced position shift. This result rules out V1 as the source of the illusion because it has an anatomical break only at the vertical meridian.
Keywords: anatomical constraints on behavior, extrastriate cortex, motion-induced position shift
INTRODUCTION
Perceived positions of visual inputs are not simply based on their current retinal locations but can be constructed by integrating other sources of information, such as motion signals from the target (Cavanagh and Anstis 2013; Eagleman and Sejnowski 2000). Although several neuroimaging studies in humans have studied the cortical locus of motion-induced position shifts, they mainly focused on early vs. late visual areas (Fischer et al. 2011; Kohler et al. 2017; Maus et al. 2013). The present study focuses on distinguishing the roles of visual areas by examining possible constraints on the magnitude of a perceptual motion-induced position shift by the type of visual field representation of involved cortical regions. In particular, we contrast 1) the meridian signatures of retinotopic hemifield representations, found in V1 and areas beyond V3, with 2) the quadrant-based representations found in V2 and V3 (see Winawer and Witthoft 2015 for a review on the type of representation in hV4), and possibly beyond (Leavitt et al. 2017), by comparing the magnitude of the illusion at the horizontal and vertical meridians with its magnitude in the quadrants between them. We tested a specific type of motion-induced position shift, the double-drift stimulus (Kwon et al. 2015; Lisi and Cavanagh 2015; Shapiro et al. 2010; Tse and Hsieh 2006), that is created when a Gabor patch moves along a path, typically on an equiluminant background, at the same time that its internal texture drifts orthogonally to this path (Fig. 1). In this case, a large perceived position displacement accumulates in the direction of the internal drift over at least a second, creating a deviation in the perceived path angle of 40°–50°. In comparison, other motion-induced position illusions studied so far only integrate motion signals over ~80–100 ms (Cavanagh and Anstis 2013; Eagleman and Sejnowski 2000; Mackay 1958; Nijhawan 1994).
Fig. 1.
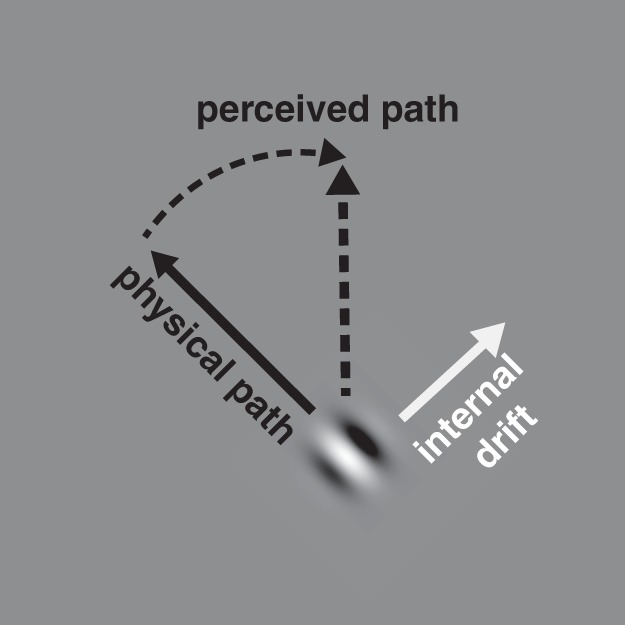
Double-drift stimulus. A Gabor patch that is moving obliquely (physical path) can be perceived to be moving vertically (perceived path) if its internal texture drifts orthogonally to the physical path (up and to the right).
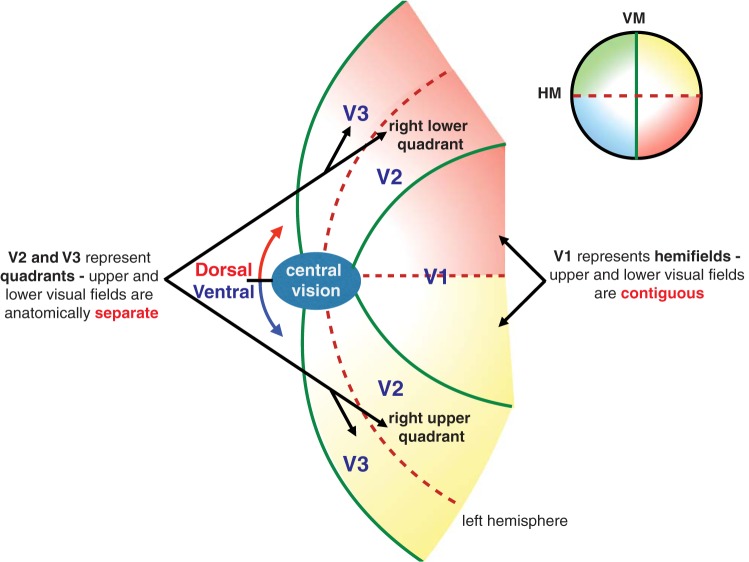
We first outline our hypothesis on the basis of the anatomical properties of early retinotopic cortices, but we later extend this to include the possibility of high-level quadrantic representations (e.g., dorsolateral prefrontal cortex; Leavitt et al. 2017). In particular, if lateral interactions between past and current cortical activity are responsible for the position shifts, then these interactions may be disrupted when the motion path crosses the anatomical gaps of the two visual meridians, placing past and present target positions at widely separated locations on the cortex. Because of the distinct anatomies of early visual retinotopic cortical areas (V1 and areas beyond V3 exhibit a hemifield representation, whereas V2, V3 and possibly hV4 exhibit a quadrant representation), variations in motion-induced position shift effects around the meridians may reveal the involvement of specific visual cortical areas. In particular, if the illusory position shift is reduced at the vertical meridian but not at the horizontal meridian, it would suggest that this effect emerges from V1 or areas beyond V3. However, if the illusory position shift is reduced at both the horizontal and vertical meridians, it would suggest that part of the process must operate in cortical areas that have an anatomical gap at the horizontal meridian (i.e., V2 and V3; Fig. 2) or in regions that take input from such areas.
Fig. 2.
Schematic representation of early visual areas V1–V3. V1 processes the contralateral visual hemifield (left hemisphere shown) with the upper and lower visual fields contiguous in each hemifield. V2 and V3 represent visual quadrants, with the upper and lower visual fields anatomically flanked by V1.
The representation of visual space in most retinotopic visual areas is divided by the vertical meridian so that each hemifield is represented by the contralateral hemisphere (see Wandell et al. 2007 for a review) with the callosal connections between the two lobes contributing to the integration of visual inputs from the opposing visual fields (Hubel and Wiesel 1967; Lavidor and Walsh 2004; Leicester 1968; Myers 1962; Payne 1990; Zeki 1969). Neurophysiological evidence has revealed that visual information on a narrow strip (~1°) along the vertical midline on the retina, a nasotemporal overlap (Blakemore 1969), is represented bilaterally in the cortex near the V1/V2 boundary (~1 cm around calcarine sulcus as shown in humans using functional MRI; Dougherty et al. 2003). Beyond this transition zone, each visual area has a separate retinotopic map of the hemifield so that visual features that are close together on the retina can be widely separated anatomically. Thus a stimulus that crosses the vertical meridian is partly represented in each hemisphere at locations that can be separated by several centimeters.
Behavioral effects due to the anatomical separation at the vertical meridian have been reported in a number of visual tasks. For instance, it was shown that behavioral performance in the multiple object tracking task (Pylyshyn and Storm 1988) is better when the targets are split evenly into separate hemifields (Alvarez and Cavanagh 2005). The strength of apparent motion (Anstis 1978; Newsome et al. 1986) also shows a cost of traversing the vertical meridian so that two stimuli straddling the vertical meridian act as if they are farther apart than two that are within one hemifield (Chaudhuri and Glaser 1991). Genç et al. (2011) found that this cost was correlated with the white matter integrity of the callosum segments connecting the two hemispheres in hMT/V5+, but not those in V1. In addition, Liu et al. (2009) have shown that visual crowding effects were greatly reduced when distractor stimuli were presented across the vertical meridian as opposed to within the same hemifield. Similarly, Pillow and Rubin (2002) showed that perceptual completion of illusory contours was disrupted across the vertical meridian. Clearly, results from these studies indicate that perceptual effects that rely on lateral interactions may be disrupted at the vertical meridian. Given that a contralateral hemifield representation is present in nearly all regions in early visual cortex and some higher order visual areas, the effects on stimuli that cross the vertical meridian could arise in practically any region with a contralateral retinotopic representation.
As illustrated in Fig. 2, the retinotopic map is further disrupted along the horizontal meridian in areas V2 and V3 such that the upper and lower quadrants are anatomically isolated and flanked by V1 (DeYoe et al. 1996; Horton and Hoyt 1991; Sereno et al. 1994; Shipp et al. 1995; Zeki 1977). Because the anatomical break along the horizontal meridian is only present in V2 and V3 (and possibly V4), any behavioral effects found for stimuli that cross the horizontal meridian therefore act as signatures of neural processing that is disrupted by the representation near the horizontal meridian or the poor lateral connections slightly farther from this meridian.
There have been only a few reports of behavioral effects related to the anatomical discontinuity at the horizontal meridian. It was demonstrated that performance in the multiple object tracking task was much better when targets were split evenly into separate quadrants than when the same number of targets was restricted to one quadrant (Carlson et al. 2007). This suggested that the capacity limits for attentional resources is independent, not just within left and right hemifields as demonstrated in Alvarez and Cavanagh (2005), but also within each quadrant as constrained by the anatomical gap across the horizontal meridian in V2 and V3 (and possibly V4). Rubin et al. (1996) showed that the processing of illusory contours differed across the horizontal meridian, being better in the lower field than the upper field, implying a role for areas V2 and V3, and areas that receive their input from V2 and V3. However, the study did not examine any losses for stimuli that straddled the horizontal meridian, as Pillow and Rubin (2002) did for the vertical meridian.
Beyond area V3, a few retinotopic visual areas in the dorsal (e.g., V3A/B, lateral occipital, LO-1 and LO-2) and ventral surface of the occipital lobe (e.g., ventral occipital, VO-1 and VO-2) have been identified with complete hemifield maps (see Wandell et al. 2007 for review), although there is some disagreement concerning the type of representation (quadrantic vs. hemifield) in hV4 (see Winawer and Witthoft 2015 for review). In addition, several retinotopically organized maps of the contralateral hemifield have been identified in human frontoparietal cortex using functional imaging, such as those along the intraparietal sulcus (e.g., IPS0, IPS1, IPS2, IPS3, IPS4, and area V7; Swisher et al. 2007) and in the superior (e.g., sPCS) and inferior portions of the precentral sulcus (e.g., iPCS) that include the frontal eye fields (FEF) (Mackey et al. 2017). Higher order visual areas such as the face- and object-selective areas of ventral visual cortex (e.g., fusiform face area, or FFA; occipital and lateral occipital cortex, or LOC) are traditionally considered to be position invariant given the relatively large receptive field sizes of neurons in these areas (Grill-Spector et al. 1998; Mishkin et al. 1983). However, a number of recent studies have reported that these high-level visual areas show some sensitivity to object positions, albeit limited to distinguishing the hemifields (Hemond et al. 2007) or quadrants of their preferred stimulus (Kravitz et al. 2010; Nichols et al. 2016), to a degree that varies across regions (Hasson et al. 2002; Levy et al. 2001).
Although the evidence concerning the organization of the meridian representations is controversial or mixed for many of these higher order areas, a recent neurophysiological study from Leavitt et al. (2017) showed specifically that dorsolateral prefrontal cortex (DLPFC) maintains a quadrantic representation of the visual-mnemonic space. Their tests involved information in visual working memory, and although there was clear evidence of response biases along both vertical and horizontal meridians, there was no evidence that the receptive fields were organized in a retinotopic manner. Nevertheless, given the unusual properties of the double-drift illusion, in particular its long integration period of up to a second or more, this high-level area that links visual memory and quadrantic effects suggests that we should also consider areas like the DLPFC as candidate areas for quadrantic effects, if any, on the illusion.
The present study investigated the neural locus of the double-drift effect by examining changes in its magnitude around the two meridians that may be driven by hemifield vs quadrantic representations. Specifically, we asked subjects to report the perceived direction of the double-drift stimulus at eight locations in the visual field and four motion directions at each location. We compared the magnitude of the double-drift illusion when its perceived position would cross either meridian compared with conditions within quadrants where it did not. We found that the magnitude of the effect was significantly lower when the direction of the internal drift was moving either orthogonally or obliquely toward either of the two meridians, suggesting that the accumulation of position errors is disrupted at these locations. This suggests the involvement of the quadrant-based visual areas (i.e., V2, V3, and possibly V4) and/or downstream areas that may take outputs from these areas (DLPFC; Leavitt et al. 2017) in generating this double-drift, motion-induced position shift.
MATERIALS AND METHODS
Participants.
Ten graduate students from Dartmouth College participated (4 women; age range: 24–32 yr, mean age: 27.5 ± 2.9 yr) who had normal or corrected-to-normal vision. All volunteered to participate and were naive to the purpose of the study. Participants signed an informed consent approved by the Institutional Review Board at Dartmouth College and received a compensation of $10/h. The protocol was approved by the Committee for the Protection of Human Subjects.
Apparatus.
Stimuli were generated using MATLAB R2015a (The MathWorks, Natick, MA) and PsychToolbox-3 (Kleiner et al. 2007) on an Apple iMac Intel Core i5 computer (Cupertino, CA) and were displayed in an otherwise dark room on a 16-in. ViewSonic G73f CRT monitor (1,024 × 768 pixels at 90 Hz) placed 57 cm from the observer. The participant’s head was stabilized with the use of a chinrest during the psychophysical experiment.
Stimuli.
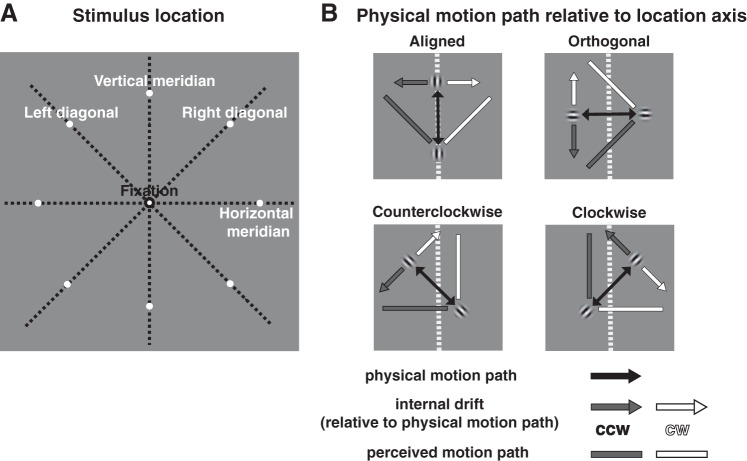
Stimuli were presented on a gray background (53 cd/m2). A fixation point [0.2° visual angle (dva) diameter] was presented throughout the experiment at the center of the screen. The stimulus was a Gabor patch (a sinusoidal grating within a Gaussian envelope) with a spatial frequency of 2 cycles/dva and 100% contrast. The standard deviation of the Gaussian envelope was 0.1 dva. In each trial, the Gabor patch oscillated back and forth along a 3-dva linear path with a speed of 3 dva/s (external motion). The patch moved parallel to the orientation of the internal grating. In the experimental (“double drift”) condition, the internal texture also drifted orthogonally to the direction of the external motion with a temporal frequency of 5 Hz (internal motion), reversing its direction at the same time as the external motion reversed. A control condition using the same stimulus where the internal texture remained static was also included to test participants’ accuracy in determining the physical path angle. Figure 3 illustrates the stimulus conditions. For both the double-drift and the control stimulus, the physical motion path of the stimulus was centered at one of the eight isoeccentric peripheral locations (8 dva from fixation) with two locations on each location axis (vertical meridian, horizontal meridian, right diagonal or left diagonal, as shown in Fig. 3A). The physical motion path of the stimulus was either aligned, orthogonal, or 45° clockwise (CW) or counterclockwise (CCW) with the diagonal axis through the center of a quadrant or a vertical or horizontal meridian axis (example shown at the vertical meridian in Fig. 3B). The double-drift stimulus had two conditions with opposite internal drift directions, creating an illusory motion path that deviated either CW or CCW relative to the physical motion path. Perceived orientation of the motion path was reported using a black line centered at fixation with 0.05 dva in width and 5 dva in length.
Fig. 3.
Stimulus conditions. A: the midpoint of the physical motion path was placed at 1 of the 8 peripheral locations with equal eccentricity (8° visual angle; 2 locations at each location axis: vertical meridian, horizontal meridian, right diagonal, left diagonal). B: the physical motion path of the Gabor patch had 4 orientations [aligned, orthogonal, 45° clockwise (cw), counterclockwise (ccw)] relative to each location axis (example shown at the vertical meridian). The internal drift had 2 conditions with opposite directions that could make the perceived motion path appear shifted either cw or ccw relative to the physical motion path.
Procedure.
Figure 4 shows a sample trial sequence. Participants were instructed to fixate at the center of the screen throughout the experiment. In each trial, a moving Gabor patch was shown in the periphery for 2 s and disappeared. The fixation point then turned green, and the response bar was presented. Participants rotated the response bar by pressing the up arrow key for CCW and down arrow key for CW until the bar’s direction matched the perceived path angle of the Gabor patch that was shown. Each participant completed 5 adjustment trials for each peripheral location, external motion path orientation, and internal motion direction (for the double-drift stimulus) in each experimental session for a total of 2 sessions on separate days, each composed of 320 trials that lasted ~40 min. Each experimental session contained 4 breaks, one every 64 trials with all conditions randomized and counterbalanced across the trials.
Fig. 4.
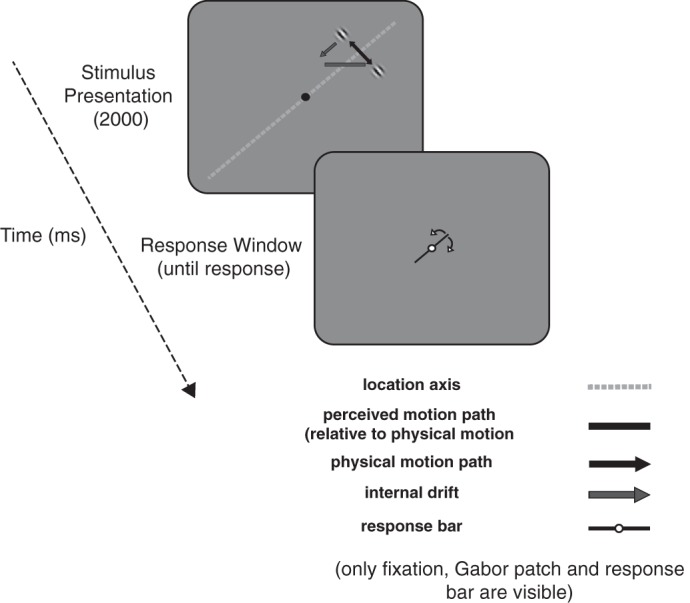
Trial sequence. Each trial began when a moving Gabor patch was presented in the periphery (example stimulus has a physical motion path that is orthogonal relative to the upper right diagonal with a possible counterclockwise perceived motion path due to the internal drift). The Gabor was presented for 2 s and then disappeared. Participants were asked to adjust the response bar at fixation until it was parallel to the perceived motion path of the Gabor path.
Analysis.
For each participant, the shift of the perceived direction under each condition was computed by taking the difference between the degrees of the adjusted path angle of the double-drift stimulus and that of the control stimulus in the corresponding condition. For the double-drift condition, a positive value indicates that the perceived motion direction was biased toward that of the internal drift. Group-averaged perceived direction shifts were then used in repeated-measures ANOVAs and post hoc Tukey’s honestly significant different and pairwise t-tests with multiple test correction using the false discovery rate (FDR; Benjamini and Hochberg 1995) to examine whether and how internal drift direction, stimulus location, and external motion direction relative to each location axis affected the perceived direction shift of the double-drift stimulus.
RESULTS
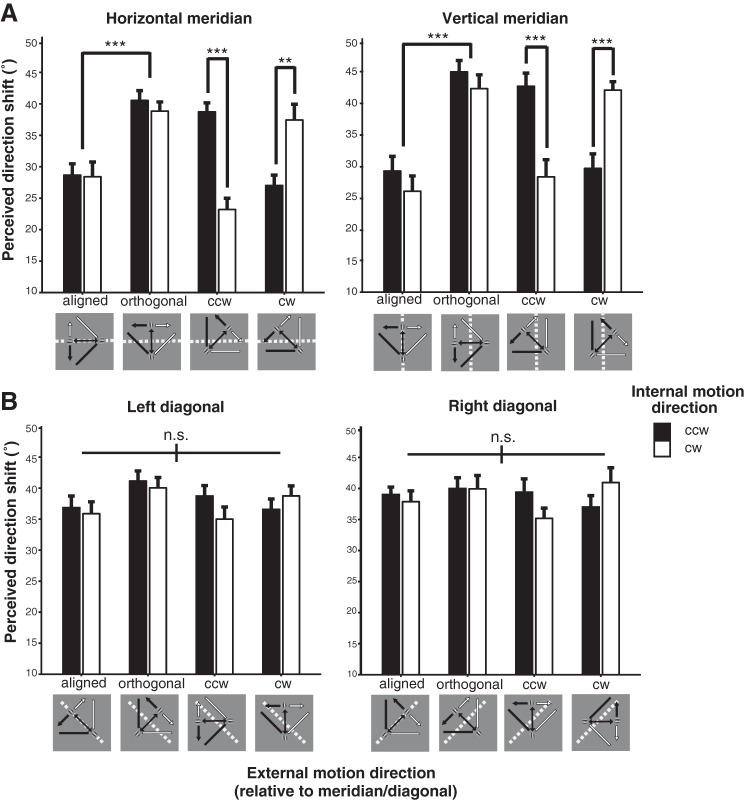
As shown in Fig. 5, there was a strong motion-induced position shift effect for the double-drift stimulus across all conditions (36.21 ± 2.66, mean ± SE). We found a significant three-way interaction between internal drift direction, stimulus location, and external motion direction [F(21, 189) = 3.26, P < 0.001, = 0.27).
Fig. 5.
Group-averaged perceived direction shift (°) of the double-drift stimulus for the 2 internal drift directions and 4 external motion directions at the meridian (A) and diagonal (B) locations. Error bars are SE. **P < 0.01; ***P < 0.001. ccw, Counterclockwise; cw, clockwise; n.s., no significant difference.
Two-way ANOVAs on the simple main effects of external motion direction and internal drift direction at each stimulus location revealed that the main effect of external motion direction and its interaction with internal drift direction was significant only at the four meridian locations (Fig. 5A; all FDR-adjusted P values <0.01), but not at the diagonal locations within quadrants (Fig. 5B; all FDR-adjusted P values >0.1). Next, one-way ANOVAs were performed to compare the effect of external motion direction for each internal drift direction at each meridian location to further examine the interaction between external and internal drift direction. Results showed that there was a significant main effect of external motion direction for both internal drift directions at all meridian locations (all FDR-adjusted P values <0.01). Critically, perceived direction shift was significantly lower when the external motion path was aligned with, rather than orthogonal to, either meridian (Fig. 5A; all P values <0.001). This indicates that participants were less affected (~45% less) by the motion-induced position displacement when the position shifts (that were orthogonal to the physical motion path) would cross the meridians to form the perceived motion path. The amount of reduction in perceived direction shift between the aligned and orthogonal external motion direction was not significantly different between the two meridians for either internal drift direction [CCW: t(9) = 1.08, P = 0.31; CW: t(9) = 1.73, P = 0.12].
The effect of the two oblique external motion directions on perceived direction shift at the two meridians differed depending on the direction of the internal drift: it was significantly lower when the internal drift direction was CCW than when it was CW for a stimulus with an external motion direction that was CCW to either meridian; conversely, it was higher when the internal drift direction was CCW than when it was CW for a stimulus with an external motion direction that was CW to either meridian (Fig. 5A; all FDR-adjusted P values <0.01). The interaction between internal drift and the two oblique external motion directions at the two meridians suggests that the accumulation of position shift errors was disrupted when they were crossing or driven toward, but not away from, the meridians. There is no significant difference in perceived direction shift between the two meridians for each external motion direction and internal drift direction (all FDR-adjusted P values >0.3).
DISCUSSION
Our results reveal that the perceived direction shift of the double-drift stimulus is ~50% weaker in the vicinity of the two meridians compared with similar configurations presented in the quadrants between the meridians. When the physical motion path of the double-drift stimulus was aligned with the vertical or horizontal meridian and the illusory shift would have to cross either meridian to form the perceptual path, the shift in the path angle was reduced significantly. In contrast, the direction shift was large and unaffected by the physical direction when present in the middle of a quadrant, suggesting that it did not arise from a general bias for vertical or horizontal over diagonal motion directions or for tangential over radial motion directions across the visual field (Supplemental Video S1, available in the data supplement online at the Journal of Neurophysiology Web site).
Several functional MRI studies in humans have examined the cortical locus of motion-induced position effects. For instance, Kohler et al. (2017) found that the perceived position of a flash-grab stimulus (Cavanagh and Anstis 2013) is represented in early visual areas V1, V2, and V3, but not in later areas such as hMT+ and IPS. Others reported that activations at higher levels such as area LO, posterior fusiform sulcus (pFS), and area MT+, but not early visual areas, code the perceived location of a Gabor patch that is shifted in the same direction of the motion of its internal texture (Fischer et al. 2011) or when the position of a static flash is shifted in the direction of surrounding moving texture (Maus et al. 2013). One possible explanation for the inconsistent results is that the observed blood oxygen level-dependent activation at the perceptual location in various visual areas might be driven by downward projection of attention signals from frontal and parietal regions to prioritize the expected locations rather than representing where the encoding of motion-induced position shifts starts. Indeed, it was shown that saccades and perception were both affected by the position effects tested in previous studies when the perceived position of a stimulus was shifted in the direction along the motion path of itself or that of surrounding patterns (de’Sperati and Baud-Bovy 2008; Schafer and Moore 2007; Zimmermann et al. 2012). Given the close link between attention and the saccade system (Awh et al. 2006; Moore and Zirnsak 2017), these results suggest that top-down attention signals might influence the coding of motion-induced position effects and hence could be a possible confound for disentangling the cortical regions where the bottom-up sensory encoding of the stimulus in the retinal coordinates differs from that of the top-down attentional effect.
In the present study, we used a specific type of motion-induced position shift, the double-drift stimulus, with a misperceived path angle as large as 40°–50° driven by motion signal from the target (Lisi and Cavanagh 2015). The perceived position offsets of the double-drift stimulus occur orthogonal to, rather than along the direction of, the physical motion path as in the other types of motion-induced position shifts tested previously. In addition, these double-drift offsets accumulate over 1 s or more (Kwon et al. 2015; Lisi and Cavanagh 2015; Shapiro et al. 2010; Tse and Hsieh 2006), whereas the others, such as the flash-grab stimulus, only integrate motion signals for ~90 ms (Cavanagh and Anstis 2013). Critically, it was shown that motion signals in the double-drift stimulus influence only the perceived, but not the saccade, landing position for target location (Lisi and Cavanagh 2015). Thus it may be advantageous to use this stimulus to study the emergence of perceived location coding in the early visual cortices because it may exclude top-down attentional influences if attention signals, such as saccades, are unaffected by the illusory shift.
Early visual areas exhibit distinct types of anatomical breaks along the two meridians. Starting from V1, an anatomical break along the vertical meridian separates visual space in the two hemispheres, whereas a second anatomical break along the horizontal meridian further divides the representation of the corresponding hemifield in each hemisphere into upper and lower quadrants in extrastriate cortical areas V2 and V3 (Figs. 2 and 6). Beyond V3, several areas have also been shown to have complete hemifield representations (e.g., V3A/B, VO-1, and VO-2), with some debate concerning the retinotopic map found in V4 (see Wandell et al. 2007 for review). Farther along the visual pathway, several higher order visual areas that are object sensitive (e.g., LOC) or face selective (e.g., FFA and occipital face area, or OFA) exhibit some contralateral (Hemond et al. 2007) and quadrant sensitivity to their preferred stimuli (Kravitz et al. 2010; Nichols et al. 2016) although without showing the anatomical breaks between hemifields and quadrants seen in early visual areas. In addition, several frontoparietal regions involved in executive cognitive functions such as working memory maintenance (e.g., DLPFC), attention (e.g., IPS), and eye movement planning (e.g., FEF) were also shown to maintain quadrantic (Leavitt et al. 2017) or hemilateral representations of the visual field (Mackey et al. 2017; Swisher et la. 2007).
Fig. 6.
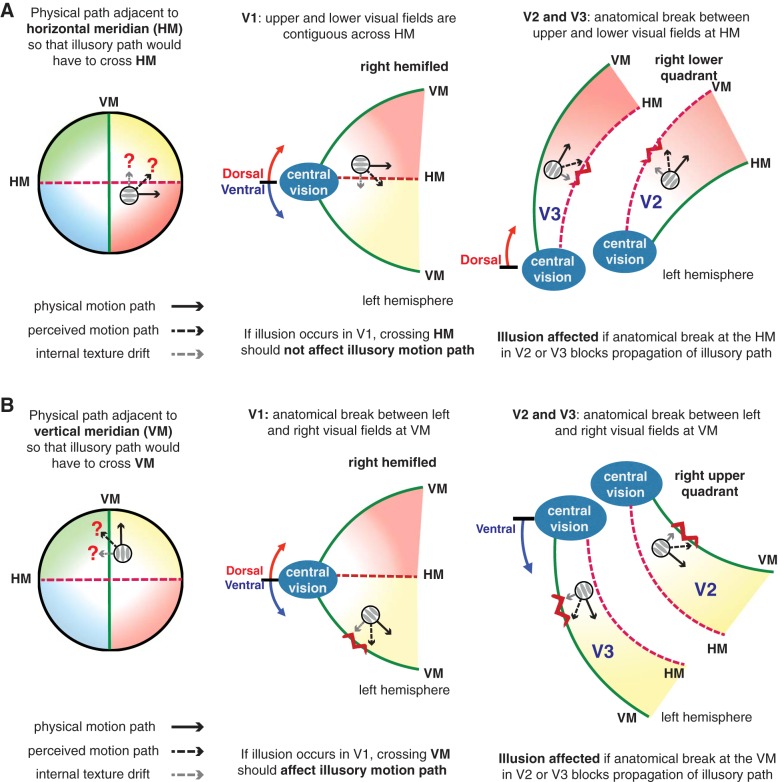
Schematic representation of the illusory motion path of the double-drift stimulus crossing the 2 meridians [A: horizontal meridian (HM); B: vertical meridian (VM); left) and their corresponding representation in V1 (middle) and in V2 and V3 (right). If the illusory position shifts of the double-drift stimulus happen at the hemifield-representing V1, they should not be affected when the perceived motion path would have to cross the HM but should be affected when it would have to cross the VM (middle). If quadrant-representing V2 and V3 are the sites of illusory position shifts, the illusion should be affected at both meridians (right).
Anatomical effects on behavior at the two meridians have been shown in a number of visual tasks, such as hemifield- and quadrant-level interference in the multiple object tracking task (Carlson et al. 2007; Cavanagh and Alvarez 2005), reduced perception of apparent motion (Chaudhuri and Glaser 1991), and reduced illusory contour completion across the vertical meridian (Pillow and Rubin 2002). Given the distinct anatomical features seen in early visual areas, modulation of behavioral effects at the two meridians, compared with other visual field locations, could place constraints on the possible cortical regions mediating the effect. If the effect is reduced for a stimulus straddling a meridian, it suggests that the processes depend on the lateral connections between regions representing adjacent locations and that these connections are compromised when they must traverse the anatomical break at a meridian. For the double-drift stimulus, if the position shift emerges across all visual areas and is reduced by the anatomical gap at both horizontal and vertical meridians, the behavioral loss should be greater when it is at the vertical than at the horizontal meridian. This is because all early visual areas starting from V1 are hemilateral, so the effects of the vertical meridian should accumulate over all of them. In contrast, only V2 and V3 (and possibly V4 and higher level areas with quadrantic representations, such as DLPFC) have quadrant representation (Fig. 6), so only these areas should contribute to effects at the horizontal meridian. Because our results show that there was no significant difference in the amount of reduction in perceived direction shift between the two meridians, we conclude that it is not anatomical gaps in all cortical areas that contribute to the loss near the meridians. The more plausible explanation of the equal loss near both horizontal and vertical meridians is that the underlying processes are affected by meridians only in quadrantic areas, where both are present and so would give an equal contribution to the loss. If correct, the observed reduction of the double-drift illusion with equal magnitude at the two meridians suggests that the starting point for the computation of perceived position of this stimulus, at least that part that can be disrupted by the anatomical gap at a meridian, relies on the specific interactions of local groups of neurons within quadrantic areas in the visual pathway and that this process does not start in V1 or in higher hemifield areas. This conclusion is consistent with human functional MRI studies (Moutoussis et al. 2005; Tse et al. 2005) and single-unit recording studies in primates (Sheinberg and Logothetis 1997; Leopold and Logothetis 1996) that suggest that activity in area V1 does not drive visual awareness even if it is necessary for its emergence in later areas. A quadrantic bias does not, however, establish that perceived position is resolved in V2/V3. The neural basis of perceived position may be resolved in downstream quadrantic areas that “inherit” the meridian biases of these areas, without necessarily being retinotopic or quadrantic in cortical organization themselves.
Because the double-drift position errors can accumulate for a second or more, it is unlikely that the neural basis of the double-drift illusion occurs solely in early quadrantic visual areas (V2, V3, and possibly V4), given the short decay constants of neurons found in these areas. Rather, it is more likely that the results of computations of the perceived motion path taking place in these early areas are then fed into some other brain region that acts as a buffer within which position errors can accumulate for a second or more. For instance, given that DLPFC has been shown to represent visual-mnemonic space in a quadrantic manner (Leavitt et al. 2017), it is possible that this area is involved in maintaining and accumulating these position errors in working memory that results in a large displacement in the perceived motion path. Future work will have to determine where in the visual processing hierarchy such an accumulator of position errors, with such a long time constant of informational integration, might occur.
Recent authors (Griffin and Nobre 2003; Landman et al. 2003; 2004; Makovski and Jiang 2007; Makovski et al. 2008; Matsukura et al. 2007; Sligte et al. 2008, 2009, 2011) have argued that there may be three stages of visual processing: 1) a high-capacity, retinotopic iconic buffer-realized processing in V1–V3 that encodes features that have not yet been bound into object representations, 2) a high-capacity “fragile visual short-term memory” buffer, perhaps realized in V4 or posterior inferotemporal areas, that encodes bound object representations that last a few seconds, and 3) a low-capacity, potentially viewpoint-invariant visual working memory buffer that can sustain information for as long as attention remains engaged with a tracked object, which may be realized in part in the frontoparietal attentional network (Sligte et al. 2011). Given the long duration over which position errors accumulate in the case of the double-drift stimulus, it would seem that the neural basis of this type of motion-induced position shift involves ongoing (mis)computations of position in early quadrantic buffers that are then integrated in frontal visual working memory in regions such as the DLPFC. Although speculative, this model of position error accumulation would make concrete testable predictions. For example, if position errors are accumulated in a frontal working memory area, then disrupting the function of such an area, using, for example, transcranial magnetic stimulation, might alter the perceived position of the double-drift stimulus or weaken the effect. In addition, patients with damage in lateral frontal areas might exhibit different degrees of the position mislocalization illusion. Such ideas must await future experimental testing.
To conclude, the present study revealed that the double-drift illusion is disrupted at the two meridians when the position offsets cross or are driven toward either meridian. This result suggests that some component of the double-drift effect, accounting for ~50% of the illusion strength, emerges in quadrant-representing areas such as V2 and V3 (and possibly V4 and/or DLPFC) and is disrupted at both meridians. The remaining 50% of the effect may also be based in these regions with anatomical gaps at both meridians while remaining immune to the effects of the meridians, or it may be based in any or all visual cortical areas, including V1, but again be immune to meridian effects. This finding furthers our understanding of where and how motion signals modulate position coding in the visual system by tagging them with the consequences of anatomical gaps arising at the two meridians.
GRANTS
The research leading to these results was funded by National Science Foundation Grant 1632738 (to P. U. Tse), Templeton Foundation Grant 14316 (to P. U. Tse), the European Research Council under the European Union’s Seventh Framework Program (FP7/2007–2013)/ERC Grant Agreement No. AG324070 (to P. Cavanagh), the Department of Psychological and Brain Sciences of Dartmouth College (to P. Cavanagh), and a VISTA Research Grant (York University) (to P. Cavanagh and J. Elder).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L. and P.C. conceived and designed research; S.L. performed experiments; S.L. analyzed data; S.L. interpreted results of experiments; S.L. prepared figures; S.L. drafted manuscript; S.L., P.U.T., and P.C. edited and revised manuscript; S.L., P.U.T., and P.C. approved final version of manuscript.
Supplemental Data
REFERENCES
- Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci 16: 637–643, 2005. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- Anstis SM. Apparent movement. In: Perception, edited by Held R, Leibowitz HW, Teuber HL. Berlin: Springer, 1978, p. 655–673. doi: 10.1007/978-3-642-46354-9_21. [DOI] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention Trends Cogn Sci 10: 124–130, 2006. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. [Google Scholar]
- Blakemore C. Binocular depth discrimination and the nasotemporal division. J Physiol 205: 471–497, 1969. doi: 10.1113/jphysiol.1969.sp008978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TA, Alvarez GA, Cavanagh P. Quadrantic deficit reveals anatomical constraints on selection. Proc Natl Acad Sci USA 104: 13496–13500, 2007. doi: 10.1073/pnas.0702685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention Trends Cogn Sci 9: 349–354, 2005. doi: 10.1016/j.tics.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Anstis S. The flash grab effect. Vision Res 91: 8–20, 2013. doi: 10.1016/j.visres.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Glaser DA. Metastable motion anisotropy. Vis Neurosci 7: 397–407, 1991. doi: 10.1017/S0952523800009706. [DOI] [PubMed] [Google Scholar]
- de’Sperati C, Baud-Bovy G. Blind saccades: an asynchrony between seeing and looking. J Neurosci 28: 4317–4321, 2008. doi: 10.1523/JNEUROSCI.0352-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA 93: 2382–2386, 1996. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis 3: 586–598, 2003. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- Eagleman DM, Sejnowski TJ. Motion integration and postdiction in visual awareness. Science 287: 2036–2038, 2000. doi: 10.1126/science.287.5460.2036. [DOI] [PubMed] [Google Scholar]
- Fischer J, Spotswood N, Whitney D. The emergence of perceived position in the visual system. J Cogn Neurosci 23: 119–136, 2011. doi: 10.1162/jocn.2010.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç E, Bergmann J, Singer W, Kohler A. Interhemispheric connections shape subjective experience of bistable motion. Curr Biol 21: 1494–1499, 2011. doi: 10.1016/j.cub.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Griffin IC, Nobre AC. Orienting attention to locations in internal representations. J Cogn Neurosci 15: 1176–1194, 2003. doi: 10.1162/089892903322598139. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum Brain Mapp 6: 316–328, 1998. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron 34: 479–490, 2002. doi: 10.1016/S0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Hemond CC, Kanwisher NG, Op de Beeck HP. A preference for contralateral stimuli in human object- and face-selective cortex. PLoS One 2: e574, 2007. doi: 10.1371/journal.pone.0000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol 109: 816–824, 1991. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J Neurophysiol 30: 1561–1573, 1967. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in psychtoolbox-3. Perception 36: 1–16, 2007. [Google Scholar]
- Kohler PJ, Cavanagh P, Tse PU. Motion-induced position shifts activate early visual cortex. Front Neurosci 11: 168, 2017. doi: 10.3389/fnins.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Kriegeskorte N, Baker CI. High-level visual object representations are constrained by position. Cereb Cortex 20: 2916–2925, 2010. doi: 10.1093/cercor/bhq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OS, Tadin D, Knill DC. Unifying account of visual motion and position perception. Proc Natl Acad Sci USA 112: 8142–8147, 2015. doi: 10.1073/pnas.1500361112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman R, Spekreijse H, Lamme VA. Large capacity storage of integrated objects before change blindness. Vision Res 43: 149–164, 2003. doi: 10.1016/S0042-6989(02)00402-9. [DOI] [PubMed] [Google Scholar]
- Landman R, Spekreijse H, Lamme VA. The role of figure-ground segregation in change blindness. Psychon Bull Rev 11: 254–261, 2004. doi: 10.3758/BF03196567. [DOI] [PubMed] [Google Scholar]
- Lavidor M, Walsh V. The nature of foveal representation. Nat Rev Neurosci 5: 729–735, 2004. doi: 10.1038/nrn1498. [DOI] [PubMed] [Google Scholar]
- Leavitt ML, Pieper F, Sachs AJ, Martinez-Trujillo JC. A quadrantic bias in prefrontal representation of visual-mnemonic space. Cereb Cortex 52: 1–17, 2017. doi: 10.1093/cercor/bhx142. [DOI] [PubMed] [Google Scholar]
- Leicester J. Projection of the visual vertical meridian to cerebral cortex of the cat. J Neurophysiol 31: 371–382, 1968. doi: 10.1152/jn.1968.31.3.371. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature 379: 549–553, 1996. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci 4: 533–539, 2001. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Lisi M, Cavanagh P. Dissociation between the perceptual and saccadic localization of moving objects. Curr Biol 25: 2535–2540, 2015. doi: 10.1016/j.cub.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Liu T, Jiang Y, Sun X, He S. Reduction of the crowding effect in spatially adjacent but cortically remote visual stimuli. Curr Biol 19: 127–132, 2009. doi: 10.1016/j.cub.2008.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DM. Perceptual stability of a stroboscopically lit visual field containing self-luminous objects. Nature 181: 507–508, 1958. doi: 10.1038/181507a0. [DOI] [PubMed] [Google Scholar]
- Mackey WE, Winawer J, Curtis CE. Visual field map clusters in human frontoparietal cortex. eLife 6: 2704, 2017. doi: 10.7554/eLife.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovski T, Jiang YV. Distributing versus focusing attention in visual short-term memory. Psychon Bull Rev 14: 1072–1078, 2007. doi: 10.3758/BF03193093. [DOI] [PubMed] [Google Scholar]
- Makovski T, Sussman R, Jiang YV. Orienting attention in visual working memory reduces interference from memory probes. J Exp Psychol Learn Mem Cogn 34: 369–380, 2008. doi: 10.1037/0278-7393.34.2.369. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP. Attention effects during visual short-term memory maintenance: protection or prioritization? Percept Psychophys 69: 1422–1434, 2007. doi: 10.3758/BF03192957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus GW, Fischer J, Whitney D. Motion-dependent representation of space in area MT+. Neuron 78: 554–562, 2013. doi: 10.1016/j.neuron.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci 6: 414–417, 1983. doi: 10.1016/0166-2236(83)90190-X. [DOI] [Google Scholar]
- Moore T, Zirnsak M. Neural mechanisms of selective visual attention. Annu Rev Psychol 68: 47–72, 2017. doi: 10.1146/annurev-psych-122414-033400. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Keliris G, Kourtzi Z, Logothetis N. A binocular rivalry study of motion perception in the human brain. Vision Res 45: 2231–2243, 2005. doi: 10.1016/j.visres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Myers RE. Commissural connections between occipital lobes of the monkey. J Comp Neurol 118: 1–16, 1962. doi: 10.1002/cne.901180102. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Maunsell JH, Van Essen DC. Ventral posterior visual area of the macaque: visual topography and areal boundaries. J Comp Neurol 252: 139–153, 1986. doi: 10.1002/cne.902520202. [DOI] [PubMed] [Google Scholar]
- Nichols DF, Betts LR, Wilson HR. Position selectivity in face-sensitive visual cortex to facial and nonfacial stimuli: an fMRI study. Brain Behav 6: e00542, 2016. doi: 10.1002/brb3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhawan R. Motion extrapolation in catching. Nature 370: 256–257, 1994. doi: 10.1038/370256b0. [DOI] [PubMed] [Google Scholar]
- Payne BR. Representation of the ipsilateral visual field in the transition zone between areas 17 and 18 of the cat’s cerebral cortex. Vis Neurosci 4: 445–474, 1990. doi: 10.1017/S0952523800005204. [DOI] [PubMed] [Google Scholar]
- Pillow J, Rubin N. Perceptual completion across the vertical meridian and the role of early visual cortex. Neuron 33: 805–813, 2002. doi: 10.1016/S0896-6273(02)00605-0. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat Vis 3: 179–197, 1988. doi: 10.1163/156856888X00122. [DOI] [PubMed] [Google Scholar]
- Rubin N, Nakayama K, Shapley R. Enhanced perception of illusory contours in the lower versus upper visual hemifields. Science 271: 651–653, 1996. doi: 10.1126/science.271.5249.651. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Moore T. Attention governs action in the primate frontal eye field. Neuron 56: 541–551, 2007. doi: 10.1016/j.neuron.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, McDonald CT, Allman JM. Analysis of retinotopic maps in extrastriate cortex. Cereb Cortex 4: 601–620, 1994. doi: 10.1093/cercor/4.6.601. [DOI] [PubMed] [Google Scholar]
- Shapiro A, Lu Z-L, Huang C-B, Knight E, Ennis R. Transitions between central and peripheral vision create spatial/temporal distortions: a hypothesis concerning the perceived break of the curveball. PLoS One 5: e13296, 2010. doi: 10.1371/journal.pone.0013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci USA 94: 3408–3413, 1997. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Watson JD, Frackowiak RS, Zeki S. Retinotopic maps in human prestriate visual cortex: the demarcation of areas V2 and V3. Neuroimage 2: 125–132, 1995. doi: 10.1006/nimg.1995.1015. [DOI] [PubMed] [Google Scholar]
- Sligte IG, Scholte HS, Lamme VA. Are there multiple visual short-term memory stores? PLoS One 3: e1699, 2008. doi: 10.1371/journal.pone.0001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligte IG, Scholte HS, Lamme VA. V4 activity predicts the strength of visual short-term memory representations. J Neurosci 29: 7432–7438, 2009. doi: 10.1523/JNEUROSCI.0784-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligte IG, Wokke ME, Tesselaar JP, Scholte HS, Lamme VA. Magnetic stimulation of the dorsolateral prefrontal cortex dissociates fragile visual short-term memory from visual working memory. Neuropsychologia 49: 1578–1588, 2011. doi: 10.1016/j.neuropsychologia.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci 27: 5326–5337, 2007. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse PU, Hsieh PJ. The infinite regress illusion reveals faulty integration of local and global motion signals. Vision Res 46: 3881–3885, 2006. doi: 10.1016/j.visres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Tse PU, Martinez-Conde S, Schlegel AA, Macknik SL. Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proc Natl Acad Sci USA 102: 17178–17183, 2005. doi: 10.1073/pnas.0508010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron 56: 366–383, 2007. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Winawer J, Witthoft N. Human V4 and ventral occipital retinotopic maps. Vis Neurosci 32: E020, 2015. doi: 10.1017/S0952523815000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Representation of central visual fields in prestriate cortex of monkey. Brain Res 14: 271–291, 1969. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Simultaneous anatomical demonstration of the representation of the vertical and horizontal meridians in areas V2 and V3 of rhesus monkey visual cortex. Proc R Soc Lond B Biol Sci 195: 517–523, 1977. doi: 10.1098/rspb.1977.0024. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Morrone MC, Burr D. Visual motion distorts visual and motor space. J Vis 12: 10, 2012. doi: 10.1167/12.2.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.