Abstract
New devices that use targeted electrical stimulation to treat refractory localization-related epilepsy have shown great promise, although it is not well known which targets most effectively prevent the initiation and spread of seizures. To better understand how the brain transitions from healthy to seizing on a local scale, we induced focal epileptiform activity in the visual cortex of five anesthetized cats with local application of the GABAA blocker picrotoxin while simultaneously recording local field potentials on a high-resolution electrocorticography array and laminar depth probes. Epileptiform activity appeared in the form of isolated events, revealing a consistent temporal pattern of ictogenesis across animals with interictal events consistently preceding the appearance of seizures. Based on the number of spikes per event, there was a natural separation between seizures and shorter interictal events. Two distinct spatial regions were seen: an epileptic focus that grew in size as activity progressed, and an inhibitory surround that exhibited a distinct relationship with the focus both on the surface and in the depth of the cortex. Epileptiform activity in the cortical laminae was seen concomitant with activity on the surface. Focus spikes appeared earlier on electrodes deeper in the cortex, suggesting that deep cortical layers may be integral to recruiting healthy tissue into the epileptic network and could be a promising target for interventional devices. Our study may inform more effective therapies to prevent seizure generation and spread in localization-related epilepsies.
NEW & NOTEWORTHY We induced local epileptiform activity and recorded continuous, high-resolution local field potentials from the surface and depth of the visual cortex in anesthetized cats. Our results reveal a consistent pattern of ictogenesis, characterize the spatial spread of the epileptic focus and its relationship with the inhibitory surround, and show that focus activity within events appears earliest in deeper cortical layers. These findings have potential implications for the monitoring and treatment of refractory epilepsy.
Keywords: epileptic focus, high-resolution ECoG, inhibitory surround, refractory epilepsy
INTRODUCTION
Approximately one-third of all epilepsy is medically refractory, meaning seizures cannot be adequately controlled with antiepileptic drugs (Kwan and Sander 2004). The majority of these patients have localization-related (focal) epilepsies, in which seizures arise from one or more distinct areas of the brain and often secondarily spread to other brain regions or generalize to the whole brain (Berg et al. 2010; French 2007; Kazemi et al. 2008). Resective surgery, the traditional treatment method for these patients, has modest long-term outcomes (de Tisi et al. 2011; Téllez-Zenteno et al. 2005) and a significant risk of cognitive deficits (Sherman et al. 2011). Newer, minimally invasive technologies such as neuroablation and neuromodulation are promising alternatives, but initial findings have not shown improved outcomes vs. surgery (Bergey et al. 2015; Fridley et al. 2012).
The clinical motivations for this work are straightforward: to improve the effectiveness of new treatments for refractory epilepsy, we must address the scientific questions underlying their mechanisms of action. With advancements in implantable electrode technologies and stereotaxic placement procedures, new devices can strategically deliver local therapy to a specific target in the brain. This ability, however, necessitates that we know what to record from and what to target (e.g., specific cell types, cortical layers, subcortical structures), as well as how to deliver therapy that can reach those targets and effectively disrupt seizure propagation. Advancing our understanding of how islands of abnormal cortical tissue generate seizures that affect large neuronal networks and generalize throughout the entire brain will help answer these questions and inform new treatment modalities.
One current limitation in our understanding of localization-related epilepsies is the relatively low spatial resolution of clinical recording electrodes. Clinical electrocorticography (ECoG) arrays are composed of electrodes that are typically 3 mm in diameter with 10-mm interelectrode spacing (Nuwer 2010). These arrays record primarily from the cortical surface, poorly resolving deeper brain structures that can generate seizures, such as the cingulate gyrus and insula. Stereotaxic electroencephalography (SEEG) utilizes arrays of stereotaxically placed, penetrating depth electrodes to map seizures throughout the cortex, offering a less invasive alternative to ECoG that can record from deeper structures (Lang et al. 2016; Podkorytova et al. 2016). Although the resolution of SEEG electrodes is higher than for ECoG, with typical interelectrode spacing of 3.5 mm (Narizzano et al. 2017), both recording methods can miss important epileptic activity occurring in the cortex on a submillimeter scale (Schevon et al. 2010; Stead et al. 2010; Viventi et al. 2011). An acute animal preparation allows us to create a controllable model of epilepsy and utilize high-resolution recording technologies. Although any such model has inherent differences from chronic human epilepsy, it is still extremely useful in investigating how seizure-generating networks arise focally in the cortex.
A common method of modeling epilepsy in animals is to introduce chemoconvulsants to the brain that create an imbalance between inhibitory and excitatory forces in networks of neurons, which is thought to be the basis of most epileptic activity (McCormick and Contreras 2001). Previous studies have shown that chemoconvulsants that locally block inhibition produce consistent epileptiform discharges on the cortex (Chagnac-Amitai and Connors 1989a; Miles et al. 1988), although more complicated responses are seen in tissue farther from the site of application due to competing depolarizing and hyperpolarizing influences in the network (Dichter and Spencer 1969a; Prince and Wilder 1967). These studies are limited, however, by their inability to continuously record from a large area of the brain and capture the spatiotemporal progression from healthy to seizing tissue.
In this study, we locally applied picrotoxin to produce focal epileptiform activity in the intact cat brain while simultaneously recording with a high-resolution ECoG array and penetrating laminar depth electrodes introduced through holes in the array. With these recordings, we were able to 1) quantify the temporal evolution of cortical activity from a healthy to a seizing state as a model of ictogenesis, 2) spatially map the spread of seizure activity on the cortical surface with high spatial resolution, and 3) characterize the cortical depth activity seen in response to this spatiotemporal progression. By elucidating how focal abnormalities expand into healthy tissue and progress to seizures, our findings may inform more effective therapies to prevent seizure initiation and spread in localization-related epilepsies.
MATERIALS AND METHODS
Animal Protocol
Experiments were conducted according to the guidelines of the National Institutes of Health and with the approval of the Institutional Animal Care and Use Committee of the University of Pennsylvania. Surgical protocols are similar to those previously published (Sedigh-Sarvestani et al. 2017). Adult male cats (2.5–3.5 kg, n = 5) were anesthetized with an intraperitoneal injection of Nembutal (25 mg/kg). Once animals reached a surgical plane of anesthesia, they were paralyzed with gallamine triethiodide (Flaxedil) and artificially ventilated (end-tidal CO2 held at 3.8–4.0%). Anesthesia was maintained by continuous infusion of propofol (3–4 mg·kg−1·h−1), fentanyl, and dexmedetomidine throughout the experiment. Local anesthetics (lidocaine, bupivacaine) were applied to all pressure points and incisions, and reapplied every 4 h. Heart rate and blood pressure were continuously monitored and anesthesia adjusted throughout the experiment to maintain a surgical plane. Rectal temperature was maintained at 37−38°C. A craniotomy centered at Horsley-Clarke posterior 4.0, lateral 2.0, followed by removal of the dura, was performed to expose an area of cortex of ~2 × 1.5 cm corresponding with the caudal portions of the lateral and suprasylvian gyri.
Electrophysiological Recordings
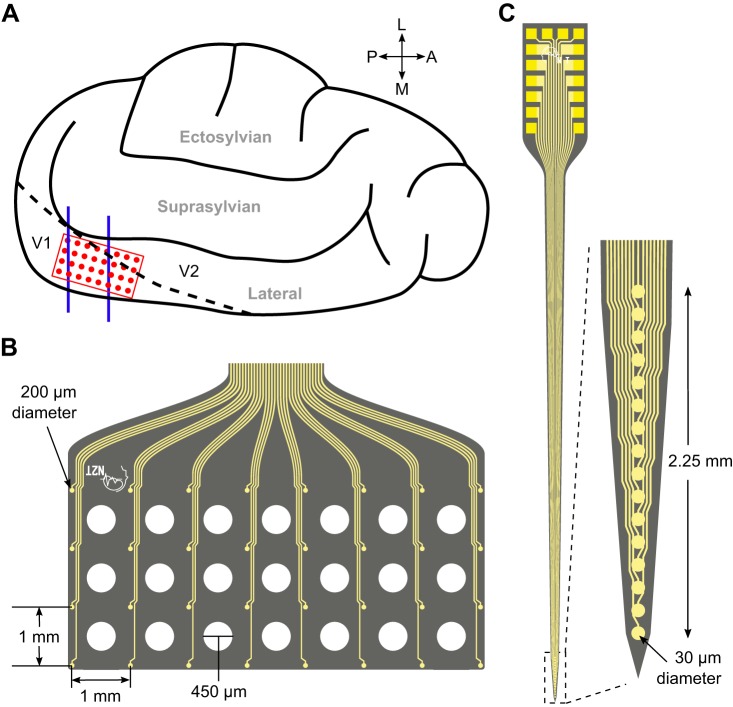
A flat, high-density ECoG array (model no. E32-1000-30-200, NeuroNexus Technologies; Fig. 1B) was placed on the surface of the exposed visual cortex over the caudal portion of the lateral and suprasylvian gyri (Fig. 1A). It consisted of 32 platinum-coated recording sites arranged in 4 rows and 6 columns on a flexible polyimide substrate. Individual electrodes were 200 µm in diameter with a spacing of 1 mm covering an area of 21 mm2. The ECoG array also contained 21 holes in the substrate between the electrodes through which silicon depth probes could be inserted into the cortex.
Fig. 1.
Recording electrode dimensions and placement on the cat visual cortex. A: illustrated left hemisphere of the cat brain with electrocorticography (EcoG) array (red) and depth probe (blue) placement over the primary visual cortex. B: dimensions of the ECoG array, showing holes in the substrate through which depth probes were inserted. C: dimensions of the depth probe. Electrode illustrations courtesy of NeuroNexus Technologies (NeuroNexus Technologies 2013).
The rigid, penetrating depth probes (model no. A1x16-5mm-150-703, NeuroNexus Technologies; Fig. 1C) consisted of 16 vertically arranged, iridium-coated contacts with a diameter of 30 µm on a silicon substrate. The electrodes spanned a vertical distance of 2.25 mm, approximately the thickness of cat primary visual cortex (Payne and Peters 2002). We inserted the probes perpendicular to the cortical surface using a stereotaxic micromanipulator until the top electrode was just below the pia as seen through an optical microscope. We recorded from one depth probe inserted through the posterior portion of the array in the first two experiments and from two probes, one posterior and one anterior, in the final three.
A Neuralynx digital data acquisition system was used to record local field potentials (LFPs) from the ECoG and depth electrodes with a sampling rate of 32 kHz and online bandpass filtering of 0.1–300 Hz. Reference electrodes consisted of cranial bone screws placed far from the visual cortex and ground electrodes were placed on exposed neck muscle. Data was downsampled to 2 kHz and analyzed using custom MATLAB scripts.
Chemoconvulsant Delivery
Picrotoxin (Sigma Aldrich), a GABAA receptor antagonist that produces epileptiform activity by blocking synaptic inhibition (Korshoej et al. 2010), was applied to the cortex of five cats. In the first 3 animals, 5–15 mg (~8–25 mM) of picrotoxin were deposited directly over the surface of the cortex in a confined area immediately posterior to the surface grid. In the final two animals, picrotoxin was dissolved in saline (10 mM) and injected (100 and 10 μl, respectively) with a Hamilton syringe at a depth of 1 mm under the posterior portion of the ECoG array. Recording time after application ranged from 45 to 120 min.
Data Analysis
Automated event detector.
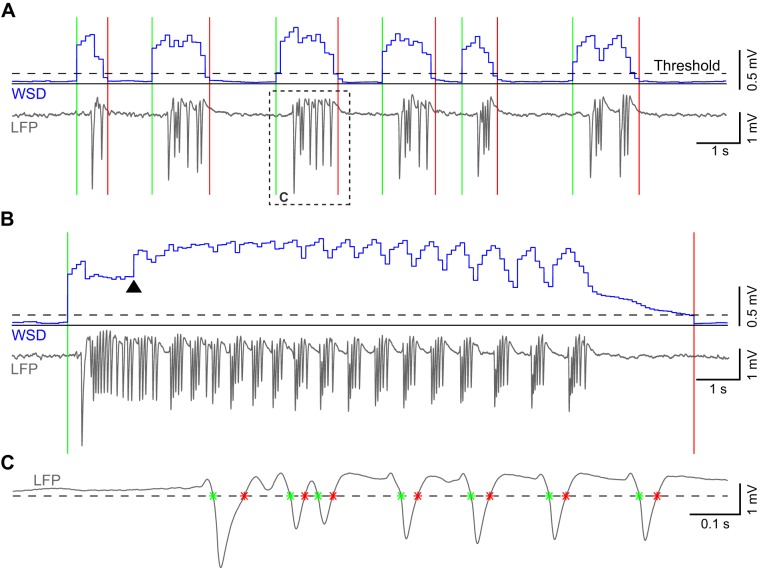
After the addition of picrotoxin, spontaneous high-amplitude epileptiform activity appeared on otherwise normal background LFP recording. The epileptiform activity was not continuous, but appeared as distinct events, separated by lower amplitude activity similar to that seen before picrotoxin application. We took advantage of the large amplitude difference between epileptiform and nonepileptiform activity to develop an automated event detector (detector code and example input data available at https://github.com/binkhank/eventDetector). After electronic artifacts unrelated to physiological activity were manually removed, the detector algorithm first calculated the standard deviation of the LFP signal within a sliding window 400 ms in length, shifting forward in steps of 100 ms for the duration of the recording [windowed standard deviation (WSD); Fig. 2, A and B]. Next, the algorithm separated epileptiform and nonepileptiform activity on the basis of a standard deviation threshold (WSDthresh) that was manually determined in each experiment (130, 275, 300, 285, and 200 μV for the 5 experiments). On average across all experiments, the standard deviation of the LFP signal in windows that were above threshold was 14 times greater than the standard deviation of windows devoid of epileptiform activity and considered baseline. An upward crossing of the WSD threshold indicated the beginning of an event, whereas the subsequent downward crossing marked the end of that event.
Fig. 2.
Epileptiform event and spike detection. A: trace of consecutive groups of spikes recorded on a single electrocorticography (EcoG) channel and the calculated standard deviation in overlapping windows (WSD; blue). Threshold (dashed line) crossings mark the beginning (green line) and end (red line) of individual events. The dotted box outlines an event trace enlarged in C. B: recorded local field potential (LFP) and the corresponding WSD of a longer event. Arrowhead marks the point at which the long-event correction factor in the detector takes effect. C: trace of a single event outlined in A and the voltage threshold (dashed line) used to detect the beginning and end of individual spikes (*).
In long events, the WSD would often briefly drop below WSDthresh before the true termination of the event, causing the detector to erroneously mark the end of that event. Thus we introduced a correction factor (Eq. 1) that increased the WSD based on prior history of the LFP in that event (Fig. 2B)
| (1) |
The WSD was calculated in window k by taking the standard deviation of the data within that window (Xk) and then, in the case of longer events, adding a factor equal to one-half of the average WSD in the preceding p windows. A given window was determined to be part of a long event if the WSD values in the p windows immediately preceding it were all above WSDthresh. The value of p was chosen to be 15 in all experiments because erroneous markings were only seen in events of at least that length.
Detector channel.
The electrode in the ECoG array close to the site of picrotoxin application with the largest amplitude difference between epileptiform and baseline activity was chosen to be the detector channel in each experiment. The event detector was run on the detector channel in each experiment, which was sufficient for determining the presence of epileptiform activity as it appeared concurrently across the array. Detections found on this channel were used to define common reference event times across all electrodes on the ECoG array and on the depth probes.
Detecting spikes within events.
Epileptiform events on the detector channel consisted of one or more high-amplitude, negative spikes. To detect the individual spikes within every event, we applied a voltage threshold to the raw LFP trace (Fig. 2C). The threshold was set to six times the pooled standard deviation (σp; Zar 1996) of baseline activity in each experiment, a value that allowed detection of lower amplitude spikes without false positives from baseline signal. σp was calculated according to Eq. 2, where WSDst represents the WSD values of all n baseline detector windows (WSD < WSDthresh) within an experiment.
| (2) |
Postprocessing of the threshold crossings was performed to remove erroneous spike detections due to artifact or noisy, near-threshold activity. Any event in which there were no detected spikes was removed.
First-spike extraction.
For each event on the detector channel, we used the threshold crossing of the first spike as a reference time to study concomitant LFP behavior across the ECoG array. We used the first spike on the detector channel as reference time because of its consistent shape across events as detailed in results. On the basis of the reference time (t = 0 ms), we extracted two 100-ms segments from the LFP of every ECoG electrode (Fig. 3A): 1) a first-spike segment (−20 to +80 ms) that included the first spike of each event as seen on the detector channel, which we will call simply “first spike,” and 2) a baseline segment (−200 to −100 ms), which we will call “baseline.” We subtracted the mean of the baseline from the first spike for every event to remove direct current (DC) offset.
Fig. 3.
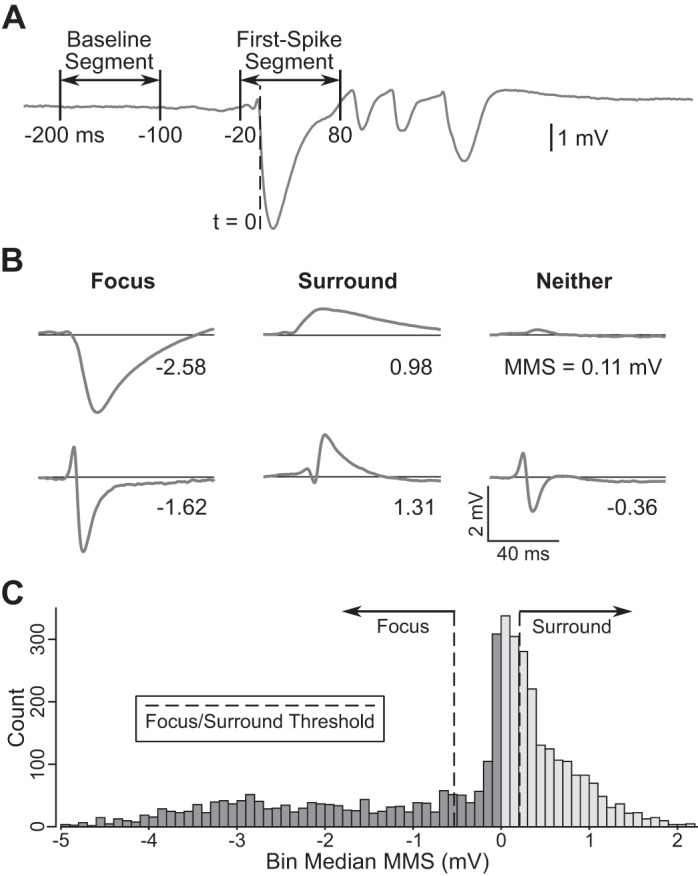
First-spike properties. A: local field potential (LFP) trace from a single event and timing of the baseline and first-spike segments that were extracted and used to quantify event shape. B: example first spikes recorded on various electrocorticography (EcoG) electrodes in the first and fifth experiments, with value for the sum of the maximum and minimum amplitude in the first spike (min-max sum, MMS) noted and arranged in columns based on classification into focus (left), surround (middle), or neither (right). C: distribution of negative (dark gray) and positive (light gray) median MMS values from significant bins across all ECoG electrodes and experiments (n = 5). Dashed lines indicated the focus and surround classification thresholds at the 33rd percentile of each distribution (−0.53 and 0.21 mV, respectively).
ECoG first-spike analysis.
First spikes on the detector channel typically consisted of a single high-amplitude spike with negative polarity, similar to the first spikes of events plotted in Fig. 2. On more distant electrodes of the ECoG array, activity concomitant with the first spike had predominantly positive polarity. We refer to these spatially distinct regions as the epileptic focus and the surround, respectively. We measured the dominant polarity of first spikes using the sum of the minimum and maximum amplitude (min-max sum, MMS). Figure 3B shows example first spikes from the first and fifth experiments and the MMS value of each. Electrodes in the focus had events with a large negative first-spike MMS (Fig. 3B, left), electrodes in the surround exhibited events with a large positive first-spike MMS (Fig. 3B, middle), and electrodes that recorded events with first-spike MMS values near zero were in neither region (Fig. 3B, right), due to either low-amplitude (Fig. 3B, top right) or mixed positive and negative first-spike activity (Fig. 3B, bottom right).
Because of the large number of detected events in each experiment (ranging from 283 to 1,596), we summarized the activity by calculating a moving average of first-spike MMS to more easily observe spatiotemporal trends as seizure activity evolved. The moving average was calculated over 24 consecutive bins per experiment, with 50% overlap in successive bins and each bin containing an equal numbers of events per experiment. In each bin, the median MMS was found to capture the trend across the scatter of consecutive events, revealing how the focus or surround evolved on a given electrode (see example in Fig. 6C).
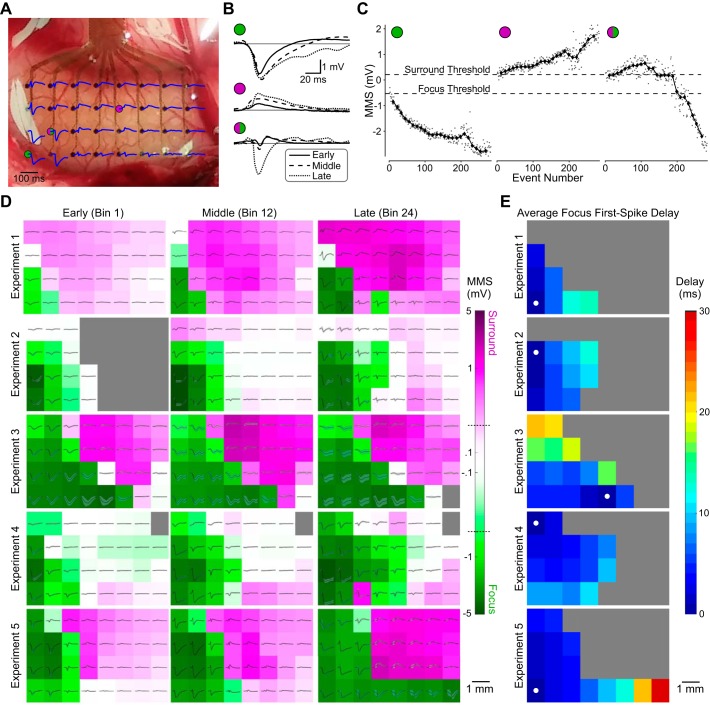
Fig. 6.
Spatiotemporal characteristics of epileptiform activity. A: activity during the first spike of one seizure in the first experiment as recorded across the electrocorticography (EcoG) array. Picrotoxin was applied near the bottom left of the array, where large negative spikes were seen. B: example first spikes from 3 electrodes at different points in the recording (early, middle, and late). Two electrodes were in either the focus (green circle) or surround (purple circle) throughout the recording; the third began in the surround but was eventually recruited into the focus (green/purple circle). C: the first-spike min-max sum (MMS) on the same electrodes for every event (gray dots) and the median MMS in each bin (black diamonds), with classification thresholds (dotted lines). Left and middle plots show data from electrodes that remained in the focus (green circle) and surround (purple circle) in this experiment, respectively, whereas the electrode in right plot began in the surround but was eventually recruited into the focus. D: ECoG array in 3 bins during early, middle, and late portions of each experiment (n = 5), with mean first-spike traces (black) and SD (gray) in the corresponding bin for each electrode. Background color, on a log scale, denotes the median bin MMS for each electrode, with green representing negative MMS (focus) and purple positive MMS (surround). Dotted lines on the color bar mark the MMS thresholds for focus and surround classification. E: delay maps showing the spread of the first spike of events in the focus on the ECoG array averaged across each experiment. Filled white circles mark the earliest-onset electrode.
Because first-spike activity became much smaller on electrodes outside the focus and surround, a criterion was required to determine if there was significant departure from baseline. We first calculated the variance of all baselines and first spikes from each experiment. Next, we ran a Wilcoxon rank sum test between the two variance distributions for each electrode and in each bin. A significant result (P < 0.002, Bonferroni corrected for the number of bins) indicated that activity above baseline was recorded on that electrode for the tested bin.
Spatial classification.
Once an ECoG electrode was determined to have recorded activity above baseline for a given bin, it was classified as focus, surround, or neither based on the median MMS of the events in that bin. Electrodes with a median MMS value more negative than the focus threshold were classified as focus, whereas those more positive than the surround threshold were classified as surround. Electrodes with a median MMS value between the two thresholds were classified as neither. Classification thresholds were manually determined on the basis of two separate distributions (Fig. 3C): one containing the negative median MMS values from significant bins across all electrodes and experiments, and another containing all positive values. The surround threshold was set at the 33rd percentile of the positive distribution (0.21 mV), whereas the focus threshold was set at the 33rd percentile of the negative distribution (−0.53 mV), ranking lower absolute values as lower percentiles.
Event propagation.
To illustrate the propagation of epileptiform events, we created delay maps based on voltage threshold crossings of first spikes in the focus recorded on the ECoG array. The voltage threshold was calculated as negative six times the standard deviation of baseline segments across the array in a given experiment. In every event, the first-spike segment on each focus electrode (classified as focus in at least one time bin) was DC shifted so the first point had a voltage of zero and the time of the first crossing below threshold was found. Crossing times were converted to delays in each event by subtracting the time of the earliest detection, and then the delays on each electrode were averaged across the experiment.
On the basis of ECoG delay values, we calculated the average propagation velocity of first spikes in the focus for each experiment. Within an event, we found the absolute difference in delay time and physical distance between each pair of focus electrodes on which a crossing was detected and calculated speed. The mean speed across all pairs represented the propagation velocity for a given event, and those values were averaged across all events in a given experiment to give an overall estimate of propagation velocity.
Cortical depth analysis.
Using the same methods described above for the ECoG array, we extracted first spikes and baselines from the recordings on each depth electrode using detector channel reference times and removed baseline DC offset. For every resulting baseline and first spike, we computed the average signal power of the LFP on every electrode and the average power of a single bipolar signal calculated on each depth probe. Bipolar signals were the difference between the LFP recorded on the most superficial electrode beneath the cortical surface and the deepest electrode. LFP power measured the magnitude of activity at each specific depth, whereas bipolar power estimated the activity within the cortical column by discounting most volume-conducted activity.
On every depth channel, we then calculated the 95th percentile of the baseline power distribution and divided all baseline and first spikes by that value. The resulting power ratio was converted to decibels so that power above 0 dB was considered above baseline and thus epileptiform. We estimated the average signal power on the cortical surface near a given depth probe by summing the average power on the four ECoG electrodes closest to it. The surface power was similarly converted to decibels using the 95th percentile of the summed baseline power.
The first spikes of events recorded on the most superficial electrodes of a depth probe were similar in shape to those recorded in the same events on nearby ECoG electrodes, as detailed in results. Thus, when a depth probe was in the focus, negative spikes were seen on its superficial electrodes, and often on deeper ones, as well. We ran a detector that found the first local minimum greater than six times the standard deviation of all baselines recorded on a given electrode. If a spike was detected in an event on the most superficial electrode, detections in that event on deeper electrodes were only found before that peak time. The detector was only run on probes with at least one of the four closest ECoG electrodes classified as focus (one probe in each animal).
RESULTS
Classification of Epileptiform Events
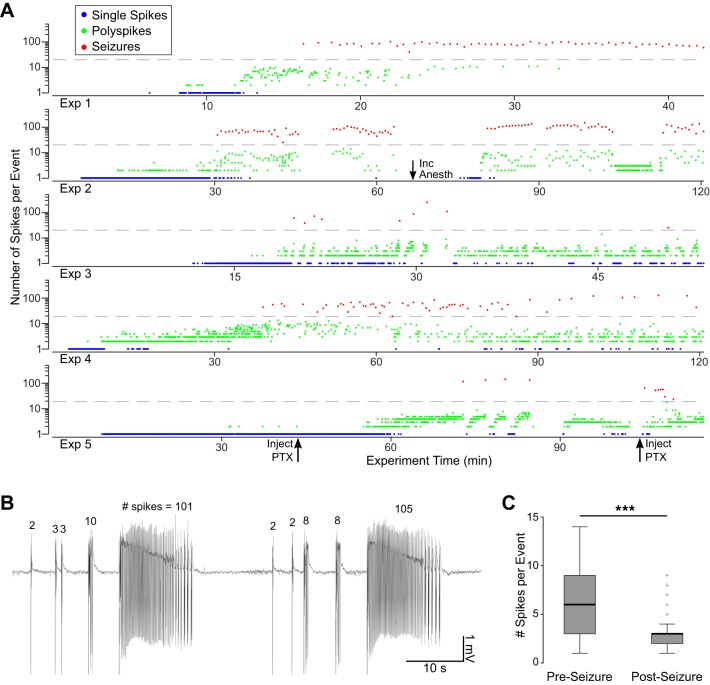
We quantified the spatiotemporal properties of picrotoxin-induced epileptiform activity in visual cortex of anesthetized cats (n = 5) from LFP recordings using a high-density ECoG array placed on the cortical surface and laminar depth probes inserted perpendicular to the cortical surface. In each animal, addition of picrotoxin to the visual cortex led to spontaneous epileptiform activity on the surface and depth LFPs of otherwise normal background activity. The epileptiform activity was not continuous but appeared in distinguishable, self-contained, discrete events. We classified these epileptiform events in three types (Fig. 4, A–C): 1) single spikes, which contained only one isolated, high-amplitude spike, 2) polyspikes, which consisted of an initial high-amplitude spike followed by a small number (<20) of lower amplitude, shorter duration spikes, and 3) seizures, which were longer events that began with a high-amplitude spike followed by a period of repetitive spiking of variable length, and continued with groupings of distinct, polyspike-like events that slowed until termination. Seizures were followed by a longer period of low or absent activity in the LFP.
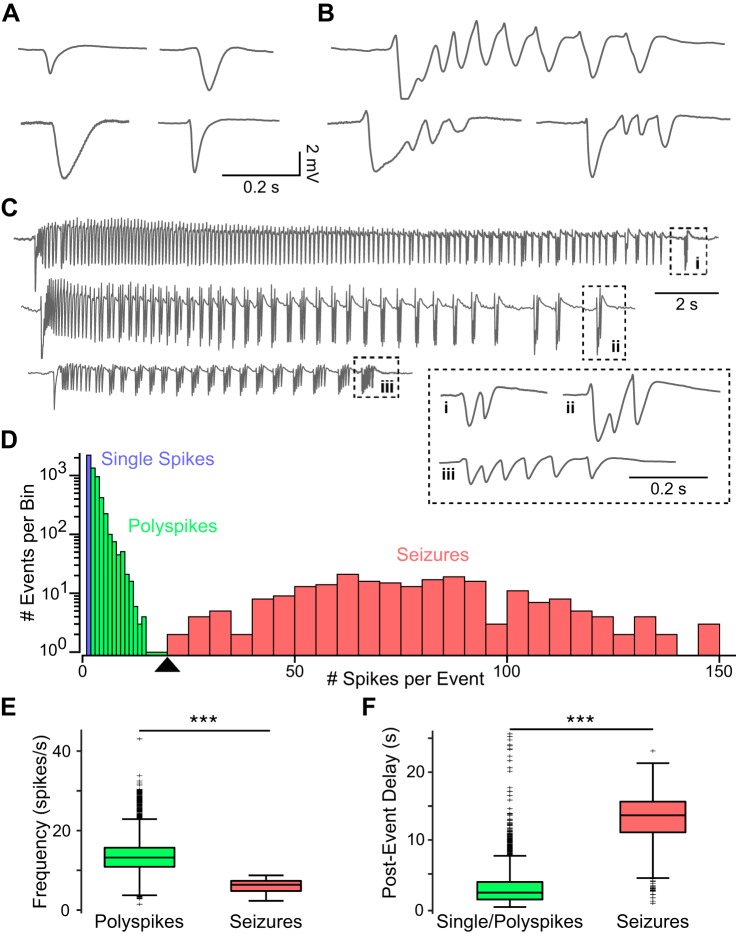
Fig. 4.
Distribution and characteristics of detected epileptiform events. A–C: example traces taken from all experiments (n = 5) showing the 3 types of detected events: single spikes (A), polyspikes (B), and seizures (C). The final grouping of spikes in each seizure (i–iii) is expanded in the inset to highlight their similarity to polyspikes. D: distribution of events across all animals as a function of number of spikes per event, with blue, green and red indicating single spikes, polyspikes, and seizures, respectively. The first 14 bins are 1 spike/event, and the remaining bins are 5 spikes/event. A threshold of 20 spikes/event was chosen to distinguish polyspikes from seizures based on the local minimum in the distribution (arrowhead). E: frequency of spikes within polyspike and seizure events. F: postevent delay, or time after the end of one event before the beginning of the next in single/polyspikes and seizures. ***P < 0.001 indicates significance with the Wilcoxon rank sum test.
We developed an automated detector that successfully identified the start and end points of epileptiform events in all three categories described above, as well as individual spikes within the events (described in detail in methods). We applied the detector to LFPs from a single ECoG electrode close to the picrotoxin application site with the largest amplitude activity in each experiment, which we call the detector channel. All detections on this channel were manually reviewed and any that appeared non-physiological (e.g., artifact caused by environmental noise or movement) were removed from the data set. A representative sample of events from all three categories was visually validated by a trained epileptologist (B. Litt). There were no false positive or false negative event detections due to the large amplitude of induced epileptiform activity on the detector channel compared with baseline. Some low-amplitude spikes within events were not detected, but these were rare enough to not affect the overall population statistics.
The distribution of the number of spikes per event (Fig. 4D) showed that 90% of all detected events contained between 1 and 5 spikes and that 96% had fewer than 20 spikes. A local minimum at 20 in the distribution of spikes per event preceded a second mode with a peak between 50 and 100 spikes per event. The second mode consisted of events characterized by an early phase of faster (10–15 spikes/s) spiking activity and a later phase of small, repetitive groups of spikes whose repetition rate slowed toward the end of the event. This electrographic pattern is common in seizures seen in humans and animal models (Kandratavicius et al. 2014; Sperling and Clancy 2008) and was not seen in events with fewer spikes, which typically exhibited only the faster spiking phase. Thus we used the local minimum in the distribution as a threshold to categorically define polyspikes as events containing between 2 and 20 spikes and seizures as events containing greater than 20 spikes. As indicated above, events with one spike were classified separately as single-spike events.
A total of 5,689 events were detected across all experiments, of which 2,210 were single spikes (38.8%), 3,254 were polyspikes (57.2%), and 225 were seizures (4.0%). The median number of spikes per event for polyspikes and seizures were 3 and 76, respectively. There was some difference in distributions between animals, but single spikes and polyspikes far outnumbered seizures in all five cases.
Polyspikes had a median spike frequency of 13.2 spikes/s, significantly faster than seizures (median = 6.4 spikes/s; P < 0.001 with Wilcoxon rank sum test; Fig. 4E). This difference was mainly due to the characteristic reduction in frequency toward the end of seizures that did not occur in polyspikes. The postevent delay, defined as the time after a given event terminated and before the next began, was much shorter for polyspikes than for seizures, with median values of 2.2 and 13.6 s, respectively (P < 0.001 with Wilcoxon rank sum test; Fig. 4F). This silent period after seizures aligns closely with postictal depression seen in human EEG recordings (So and Blume 2010). These results show that there were distinct differences between polyspikes and seizures and validate the chosen threshold distinguishing the two types of events.
Temporal Evolution of Ictogenesis
The temporal evolution of ictogenesis was consistent across experiments (Fig. 5A). Within minutes of application of picrotoxin (7.0 ± 3.3 min, mean ± SD), single spikes appeared in isolation, followed by polyspikes (15.0 ± 9.9 min after application), and, finally, seizures (35.7 ± 22.6 min after application). The number of spikes per event in polyspikes increased leading up to the first seizure: the first 10 polyspikes had a mean of 2.1 spikes per event across experiments, whereas the 10 polyspikes immediately preceding the first seizure had a mean of 4.5 spikes per event. Polyspikes and seizures continued consistently through the end of the experiment, although the appearance of single spikes was greatly reduced, with a mean of 84% of all single spikes occurring before the first seizure across experiments.
Fig. 5.
Temporal pattern of ictogenesis. A: progression of epileptiform events for each experiment. Single spikes (blue) appeared after initial picrotoxin application (t = 0), followed by polyspikes (green) and eventually seizures (red). Dashed gray lines mark the 20 spikes/event cutoff delineating polyspikes and seizures. Additional picrotoxin application (Inject PTX) and anesthesia increases (Inc Anesth) are noted with arrows. B: example trace from the second experiment shows polyspikes with an increasing number of spikes leading to seizures. C: the number of spikes in polyspikes immediately preceding (Pre) and following (Post) seizures. ***P < 0.001 indicates significance with the Wilcoxon rank sum test.
In addition to the polyspike buildup before the first seizure in an experiment, individual seizures were consistently preceded by polyspikes with an increasing number of spikes per event. Following the seizure and postevent delay, polyspikes restarted with one or a small number of spikes and the sequence repeated, leading to a subsequent seizure. An example of this pattern is shown in Fig. 5B. Over all experiments, polyspikes immediately preceding seizures had a median of 6 spikes per event, whereas those that immediately followed had 3 spikes per event (P < 0.001 with Wilcoxon rank sum test; Fig. 5C). This result, along with the increased postevent delay, indicates that seizures caused at least a temporary change in the local network that was not seen in shorter events.
Consecutive events exhibited similar first-spike shape that changed slowly over the course of the experiment and did not differ based on the number of spikes in individual events. Events were grouped into 24 overlapping, consecutive bins in each experiment (see methods), and the Pearson correlation was calculated between the shapes of every pair of first spikes within each bin. Across experiments, the median correlation in every bin was >0.95, demonstrating the strong consistency of the first-spike shape in events that occurred near each other in time and justifying our binning method.
Spatial Spread on the Cortical Surface
The shape of epileptiform events varied across the ECoG array relative to the site of picrotoxin application. As detailed in methods, electrodes were classified as focus, surround, or neither on the basis of their predominant first-spike polarity as measured by the sum of the maximum and minimum amplitude in the first spike (min-max sum, MMS). Events recorded on electrodes close to the application site contained large negative first spikes, which defined spatially the epileptic focus. Activity on further electrodes concomitant with first spikes in the focus was often positive, comprising the epileptic surround. An example of this spatial distribution can be seen in Fig. 6A, showing the first spike of a seizure from the first experiment across the entire ECoG array. It is clear that the focus is near the bottom left of the grid (close to the site of picrotoxin application) and the surround is concentrated in the top center.
In contrast to the relative shape constancy of first spikes on the detector electrode, first-spike shape on other electrodes often changed over the course of an experiment, thus changing classification between surround and focus. Examples spikes on three electrodes (Fig. 6B) show that two of them were firmly entrenched in either the focus (Fig. 6B, top) or surround (Fig. 6B, middle) throughout the recording (illustrated by first spikes from early, middle, and late in the first experiment), whereas a third started with positive first spikes in the surround but grew negative as it was recruited into the focus (Fig. 6B, bottom).
We plotted the MMS value of each event in the first experiment (Fig. 6C, data points). Because of the large number of events and their variable number across experiments, we grouped them into 24 overlapping bins containing an equal number of events in each experiment to calculate a moving average of MMS. This strategy allowed us to more easily observe spatiotemporal trends as seizure activity evolved throughout each experiment as seen in the moving average of the value of MMS (Fig. 6C, symbols and line) for the three example electrodes indicated in Fig. 6A. The third electrode was classified as surround in early bins while MMS values were above the surround threshold, and then a precipitous drop resulted in a focus classification from the 19th bin through the end of the recording (examples of this change in shape are illustrated in Fig. 6B, bottom).
Figure 6D illustrates the distribution of focus and surround over time for each experiment. Negative spikes defining the focus increased in amplitude and duration over time as the focus increased in size during each experiment except the third, in which it stayed constant. The percent increase in the number of electrodes classified as focus from the beginning of the experiment (median of first 3 bins) to the end (median of last 3 bins) was 100%, 67%, 0%, 100%, and 60% for experiments 1–5, respectively. The presence and progression of the surround, defined by positive spikes, was much less consistent. The odd-numbered experiments exhibited strong surround responses. Positive spikes in the surround increased in amplitude and duration throughout the first and fifth experiments, mirroring the progression of negative spikes in the focus, whereas both regions stayed constant in the third experiment. In the second and fourth experiments, however, very few electrodes were classified as surround, and those that were had small MMS values due to low-amplitude or mixed first spikes. The lack of a strong surround on the ECoG array did not appear to affect the progression of the focus relative to the other experiments.
The spatiotemporal dynamics within the focus were consistent across animals. We created delay maps for each experiment based on first-spike voltage threshold crossing and averaged across events (Fig. 6E). First-spike activity propagated systematically through the focus as a planar wave radiating out from the site of picrotoxin application, with the electrode close to the convulsant presenting the shortest delay indicated. In all cases, the fast propagation wave of the first spike stopped abruptly at the edge of the focus. Outside the focus, electrodes exhibited surround activity or no activity at all. The average first-spike propagation velocities in the focus of experiments 1–5 were 0.41, 0.51, 0.32, 0.60, and 0.68 m/s, respectively. The third experiment had a markedly slower speed and slightly different delay pattern possibly due to the different location and more diffuse application of picrotoxin. The faster speed in the fourth and fifth experiments may also have been the result of their unique picrotoxin application methods, via a syringe in the depth closer to the ECoG array.
Activity in the Cortical Depth
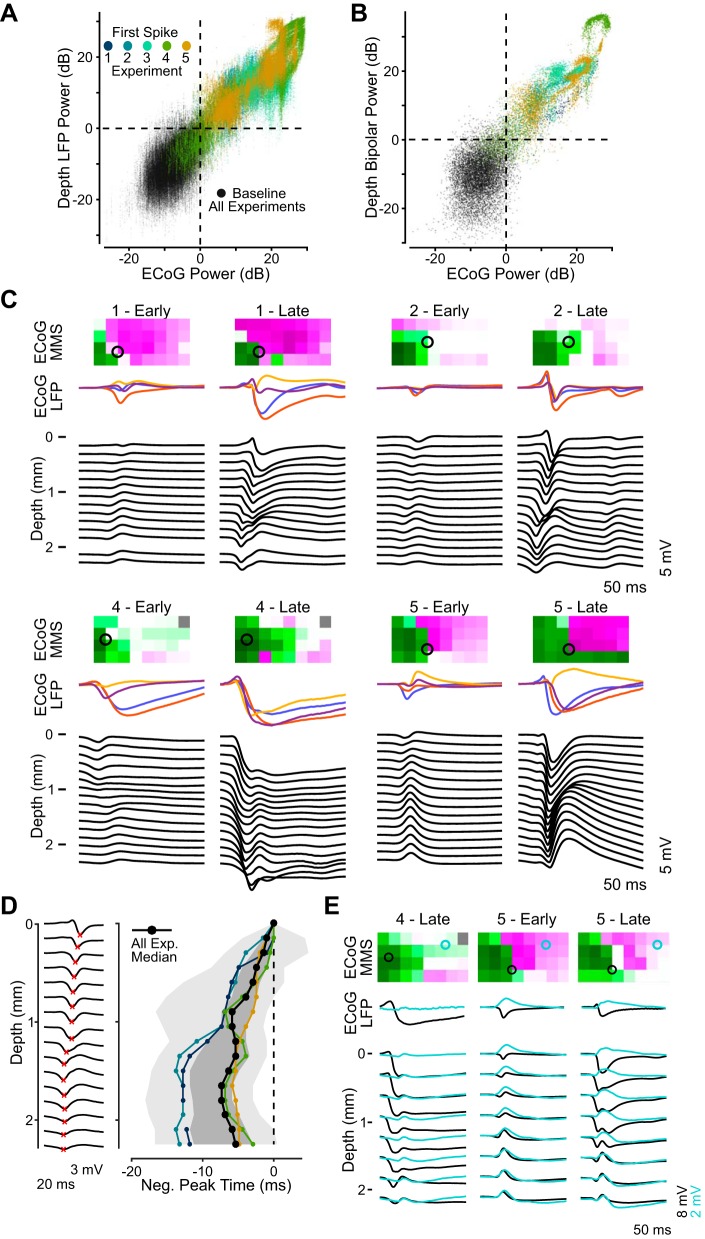
In each experiment, laminar depth probes were inserted perpendicular to the cortical surface to record activity from the cortical laminae. When epileptiform activity was seen on the surface, it was also seen in the corresponding depth, and likewise for baseline activity. To correlate activity between surface and depth, we measured average signal power of the baseline and first spike in all detected events across experiments. We plotted the sum of the signal power on the four surface electrodes surrounding the depth probe against the signal power from all depth electrodes (Fig. 7A). In 95.2% of values plotted, there was agreement between surface and depth: both showed either epileptiform activity (>0 dB; top right quadrant) or baseline activity (<0 dB; bottom left quadrant). Only in 1.6% of all first spikes was epileptiform activity seen on the surface but not on any of the electrodes of the corresponding depth probe. Even rarer was the case when epileptiform activity was seen on at least one of the depth electrodes of a probe but not on the surface, occurring in only 1.2% of first spikes.
Fig. 7.
Properties of activity recorded in the cortical depth. A: average power calculated for all baseline (black points) and first-spike (colored points by experiment) local field potentials (LFPs) recorded on every depth electrode plotted against surface electrocorticography (EcoG) power near a given depth probe, estimated as the sum of the average power on the 4 closest ECoG electrodes. Dotted lines are at 0 dB for each distribution, separating baseline from epileptiform activity. B: average power of all bipolar baselines and first spikes spanning each depth probe against summed ECoG power, with the same convention as in A. C: depth LFP profiles recorded on probes in the focus (black traces) and the corresponding LFP traces from the 4 nearby ECoG electrodes (colored traces) during 2 time points in 4 experiments. Each set of traces is the average first spike from 10 consecutive events. The corresponding min-max sum (MMS) maps (top) show the location of the depth probe (black open circle) relative to the focus and surround seen on the ECoG array during the bin in which the events were recorded. D: example of depth spike detections (red crosses) during one event in the second experiment (left). Plot on right shows the timing of negative peaks as a function of depth for focus probes (1–4 mm from picrotoxin application site) in 4 experiments with consistent depth activity. The peak time on the most superficial electrode below the cortical surface is set to t = 0 in each experiment. Plotted lines are the median peak timing for each experiment (colors match experiments in A) and the median across all experiments (black), and boundaries show the 50% and 95% edges of the distribution (dark and light gray shading, respectively). E: LFP traces from experiments with 2 depth probes, showing depth activity from both (bottom traces) averaged across 10 consecutive events (different events than in C). MMS maps (top) show each probe’s location (circles) relative to the focus (cyan) and surround (black) on the ECoG array during the events. The depth profiles omit every other electrode and have different voltage scales between probes to enable comparison. Surface LFP traces (middle) are from the ECoG electrode whose activity was most correlated with activity recorded on the most superficial depth electrode near it.
The first-spike power on the surface was highly correlated with first-spike LFP power on the top depth electrode, with a Pearson correlation coefficient of 0.96, calculated on all events aggregated across experiments (P < 0.001). The correlation of depth to surface power progressively decreased on each subsequently deeper electrode, but remained strong even at the deepest electrode (r = 0.80; P < 0.001). A strong correlation was also seen between first-spike power on the surface and the average power of bipolar signals calculated between the surface and depth of each vertical probe (r = 0.87; P < 0.001; Fig. 7B). This result indicates that epileptiform activity in the depth arose locally in the vicinity of the laminar probe with limited contribution from volume conduction. In both plots, variation in signal power between experiments reflects differences in spike amplitude recorded on the depth probes.
To better understand how epileptiform events arose and progressed in the cortical laminae, and how that activity was reflected on the surface, we explored the first-spike LFP signals recorded on the depth probes. Example first-spike traces from the surface and depth averaged over 10 consecutive events are shown in Fig. 7C at early and late periods in 4 experiments. The examples are from probes that were in the focus for the majority of the experiment, inserted 1–4 mm from the site of picrotoxin application. The shape of events recorded on electrodes near the top of the depth probe (Fig. 7C, “depth”) closely resembled those recorded on one or more of the nearby ECoG electrodes (Fig. 7C, “ECoG LFP”) in all examples. To test this, we calculated the Pearson correlation between first spikes recorded on the top three electrodes of a depth probe and those recorded on the closest four ECoG electrodes and chose the maximum value for every event. Across all five experiments, the median correlation coefficient was 0.96, with 5th and 95th percentiles of 0.67 and 1.00, respectively. This result validates the similarity between activity recorded on our two different types of electrodes when they were positioned close to one another.
The shape of first spikes in the laminae was dependent on the depth of the electrode on which they were recorded. The example depth profiles in Fig. 7C all exhibit first spikes on superficial probes that were dominated by negative spikes with little positivity, matching activity on nearby ECoG electrodes in the focus. Deeper electrodes in these example events recorded first spikes with large positive spiking, however, marking a phase reversal that is a common characteristic of laminar activity caused by synaptic current input at a specific layer (Buzsáki et al. 2012).
Activity in the depth of the cortex evolved in conjunction with the spatiotemporal evolution observed on the surface. MMS maps (Fig. 7C, top) show the location of the focus and surround recorded on the ECoG array in the bin that contained the corresponding events, along with the position of the depth probe. In each of the early examples, the depth probe was on the edge of the focus and the depth LFP profile was straightforward: a single small negative spike on the superficial electrodes that reversed to a single small positive spike on deeper electrodes. As the recordings progressed, the focus grew in size to further encompass the tissue in which the depth probe was placed, and focus spikes on the surface grew in amplitude and duration. Correspondingly, the amplitude and duration of first-spike activity in the depth increased in the late examples. The depth profiles also changed, with negative spikes appearing across the span of the depth probe that was followed by a positivity only on deeper electrodes.
When negative first spikes appeared across all electrodes on a depth probe, the timing of the spike was dependent on the depth of the electrode on which it was recorded. To quantify this relationship, we developed an algorithm to detect initial negative peaks throughout the depth during each event (example detection in Fig. 7D, left). The depth activity in the third experiment was inconsistent and did not exhibit clear peaks, so data from focus probes in the other four animals were used in this analysis. In those four animals, the peak time of spikes on deeper electrodes consistently preceded the peak time of spikes recorded on more superficial electrodes (Fig. 7D, right). Across experiments, the median peak time of first spikes on electrodes 1 mm or deeper below the cortical surface was 6.3 ms before the peak time on the most superficial electrode below the surface. This time difference between depths varied across experiments, as can be seen by the median peak time values from each experiment plotted in Fig. 7D. The fourth and fifth experiments had a smaller time difference between superficial and deep spikes than the first and second experiments. This could be due to the difference in picrotoxin application methods or the closer proximity of the depth probe to the application site in experiments four and five. Overall, the finding that focus epileptiform activity appeared earliest deeper in the gray matter suggests that deep layers are crucial in expanding the focus and recruiting healthy tissue into the epileptic network.
The depth analysis up to this point only consisted of depth electrodes in or near the focus that exhibited negative spiking activity. In the fourth and fifth experiments, a second depth probe recorded activity from tissue well outside of the focus. Figure 7E gives example first spikes averaged over 10 consecutive events in those experiments (different events than examples in Fig. 7D) showing depth LFP profiles from the probe in the focus and the probe outside the focus (bottom traces), as well as LFP from the ECoG electrode most correlated with each probe (middle traces). In the two examples from the fifth experiment, there was a positive peak throughout the depth that matched with the positive peak recorded on the nearby ECoG electrode. This probe was in the surround, as seen in the corresponding MMS maps (Fig. 7E, top). In the example from the fourth experiment, the nearby ECoG electrodes were not classified as surround and there was no evident positive peak on the surface, but there was a distinct positive peak through the laminae as seen on the depth LFP. The depth positivity was much lower amplitude than that observed in the surround in the fifth experiment, but its presence suggests an active surround that was not reflected on ECoG recordings.
The examples in Fig. 7E reveal a relationship between depth activity within the focus and outside of it. The positive peaks on the probes in the surround appear to have occurred concomitant with a corresponding positive peak deep on the probes in the focus. This deep focus positivity decreases in amplitude on more superficial electrodes and was not observed closer to the surface. In the surround, however, the shape of the positivity remains constant throughout the laminae. This result suggests that deeper layers are not just crucial for expanding the focus but also serve as a connection between focus and surround.
DISCUSSION
Using high-resolution surface ECoG arrays and laminar depth probes, we measured the transition from normal cortical activity to progressively more severe levels of electrographic epileptiform activity in a cat model of focal cortical seizures. The recordings revealed a consistent temporal pattern of ictogenesis, distinct focus and surround regions that evolved as seizures progressed, and earlier onset of activity in deep cortical layers relative to more superficial layers within events. These findings have implications for how epilepsy is treated clinically, highlighting the role of interictal events in the transition from healthy to seizing cortex and stressing the need for higher resolution recordings. Our data also suggest that deep layers may be involved in the recruitment of healthy tissue into the epileptic focus, and we therefore propose deep cortical layers as a potential target for future closed-loop stimulation devices.
Interictal events are commonly seen on human ECoG and EEG recordings and are often used in the diagnosis of epilepsy (Staley and Dudek 2006). Prior studies have reported spatial and temporal correlations between interictal events and the underlying seizure-generating network in humans (Baykan et al. 2000; Gambardella et al. 1996; Hufnagel et al. 2000; Karoly et al. 2016; Krendl et al. 2008; Litt et al. 1998; Noachtar et al. 2008; Rosati et al. 2003). The exact relationship between interictal activity and seizures, however, is not well understood and is a matter of debate in the field (Avoli et al. 2006; Staley et al. 2011). Similar to other models of epilepsy (Dyhrfjeld-Johnsen et al. 2010; White et al. 2010), we found that single spikes and polyspikes, two types of interictal activity, were precursors to initial seizure activity. This suggests that interictal events create an environment in the brain where seizures can thrive, potentially playing a significant role in epileptogenesis and helping sustain the epileptic focus, as has been suggested previously (Dichter and Spencer 1969b; Staley and Dudek 2006). Furthermore, the similarity in shape among the first spikes of interictal and ictal events recorded in the same time frame points toward a common generation mechanism for both.
There is a wide variety of interictal activity seen in human patients, and the distinction between interictal and ictal events is not always clear. For instance, similar electrographic recordings can be accompanied by different clinical manifestations, and interictal events can have a significant effect on behavior and cognitive abilities (Fisher et al. 2014; Gelinas et al. 2016; Ibrahim et al. 2014; Ung et al. 2017). In our study, polyspikes and seizures in the focus naturally separated into two groups based on the number of spikes per event, a finding that was consistent across experiments. Additionally, there was a distinct morphological difference between the event types. Both began with fast spiking activity, but only seizures contained a later phase of short bursts of spikes that slowed until termination, similar to patterns observed in human tonic-clonic seizures (Dreifuss 1997; Jirsa et al. 2014). Although it was not directly explored in this study, the clear quantitative and qualitative separation we observed between interictal and ictal activity suggests that there may be a true mechanistic distinction between seizures and interictal events and that they are not on a continuum based solely on duration.
This study was based on an acute model of ictogenesis in an anesthetized animal that received neuromuscular blocking agents, preventing behavioral manifestation of seizures. Because we do not know whether the seizures would eventually become chronic, our data provide no direct information on the process of epileptogenesis. However, because of the many parallels between our results and clinical observations (e.g., interictal spike and seizure waveforms, postictal depression, buildup of polyspikes leading to seizures), we believe this model of seizure generation is mechanistically analogous to human epilepsy and that interictal activity plays an important physiological role in the development of seizures. Additionally, the appearance of interictal events and seizures within a very small area of cortex corroborates the growing evidence supporting the need for higher resolution recording arrays when localizing epileptiform activity (Schevon et al. 2010; Stead et al. 2010; Viventi et al. 2011).
Early studies using GABA antagonists to reduce inhibition showed that large, predominantly negative LFP spikes near the chemoconvulsant (focus) were associated with a mass depolarization of neurons (Dichter and Spencer 1969a; Matsumoto and Marsan 1964; Miles et al. 1984; Prince and Wilder 1967). In the cat primary visual cortex, horizontal connections between regions are predominantly excitatory and mainly synapse on large pyramidal cells in layer V and layers II/III (Schmidt and Lowel 2002). Epileptic spikes are believed to be generated by the depolarization of pyramidal cells (McCormick and Contreras 2001), which suggests that activity in the focus is predominantly caused by intracortical excitatory connections to layers V and II/III. Our results found that negative spikes, markers of focus activity, were seen earliest on deeper electrodes. Thus we believe that synaptic activity in layer V plays a large role in generating epileptiform events and that connections in deep layers are vital in recruiting healthy tissue into the epileptic network and spreading the focus, which grew over time in all but one of our experiments (in which it remained constant). Results from other seizure models in disinhibited cortex showed that cells in layers IV/V were responsible for the initiation and propagation of synchronized epileptiform activity in a local population of neurons (Chagnac-Amitai and Connors 1989b; Pinto et al. 2005). Layer V also has been shown to be necessary for self-sustained horizontal propagation in healthy cortex and serves to amplify activity in layers II/III (Wester and Contreras 2012). These findings raise the idea that blocking synaptic transmission in deep layers may be a promising method to deter the spread of epileptiform activity in the network, potentially improving the ability of future closed-loop stimulation devices to prevent seizure propagation.
Our findings in the depth were based on LFP recordings, rather than current source density (CSD) analysis. We chose this method because we did not match the exact location of the depth probes relative to the cortical laminae, which is vital in interpreting the results of CSD calculations. Future work includes performing detailed histology and CSD analysis to verify what layers are responsible for the initiation and spread of epileptiform activity in this model.
In our study, negative spikes propagated as a planar wave radiating from the site of picrotoxin application to the edge of the focus, where inhibition was active. Previous experiments in a similar preparation recorded more complicated waveform patterns on the cortical surface (Vanleer et al. 2016; Viventi et al. 2011), which we believe was due to a more diffuse application of chemoconvulsant resulting in multiple foci generating activity. Such a model may be more similar to what is seen in humans with more advanced epileptic networks but is not useful in detailing the evolution of a single, well-defined focus. The propagation velocity we found of the planar waves in the focus (0.32–0.68 m/s) was on the order of that seen in human seizures (Eissa et al. 2017; González-Ramírez et al. 2015; Huberfeld et al. 2011; Martinet et al. 2017). It was also close to the measured axonal propagation velocity of traveling waves in the cat visual cortex (Hirsch and Gilbert 1991; Sato et al. 2012), further suggesting that the epileptiform events spread by lateral synaptic connections within V1.
Positive spiking on the cortical surface outside of the focus has been seen in many similar models, a phenomenon known as “surround inhibition” in which a barrage of synaptic currents from the focus results in strong, recurrent inhibition in nearby tissue where GABA is still active (Dichter and Spencer 1969a; Prince and Wilder 1967; Trevelyan et al. 2007). Studies using high-resolution recordings of seizures in humans posit that local failure of inhibition allows neurons to be recruited into the focus, whereas inhibition in tissue farther from the focus remains intact and plays a role in determining seizure dynamics (Eissa et al. 2017; Schevon et al. 2012). The nature of epileptiform activity observed on the ECoG array and depth probes in our model supports this idea of a direct relationship between the focus and surround. As negative spikes in the focus increased in amplitude and duration over the course of a recording, we saw a corresponding increase in the amplitude and duration of positive spikes in the surround, when they were evident on the ECoG array. When the focus grew in size, electrodes between the focus and surround that previously exhibited a mix of positive and negative spiking were the ones recruited into the focus, signaling a breakdown of inhibition locally while inhibition in more distant tissue remained strong. It is unclear why positive spiking was not recorded on the ECoG array in two experiments, but focus growth was similar to that in the other animals and positive spikes were recorded in the surround depth in one, which suggests that some form of surround inhibition was present but not reflected on the surface. Finally, simultaneous recordings from two depth probes revealed positive spiking that appeared at the same time throughout the laminae in the surround and only on the deepest electrodes in the focus. This concomitant activity indicates a synaptic connection between the deep layers in the focus and the surround, potentially feedback from the surround to the focus thought to be critical in sustaining seizure activity (Eissa et al. 2017). This was only seen in two experiments, however, and warrants further investigation.
The findings presented highlight the importance of interictal events in the development of seizures, stress the need for higher resolution cortical recording technologies, and propose deep layers as a potential target for stimulation devices. We hope that with continued research, we can help improve the clinical tools used to diagnose and treat patients with refractory epilepsy.
GRANTS
This work was supported by NIH Grant U01-NS094340 (to B. Litt), the Mirowski Family Foundation (to B. Litt), Neil and Barbara Smit (to B. Litt), a Citizens United for Research in Epilepsy Taking Flight Award (to F. Vitale), and NIH Gant R01-EY020765 (to D. Contreras).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.B., B.L., and D.C. conceived and designed research; H.B., M.S.-S., and I.F.-L. performed experiments; H.B. analyzed data; H.B., M.S.-S., L.K., H.U., D.K., F.V., and D.C. interpreted results of experiments; H.B. prepared figures; H.B. drafted manuscript; H.B., M.S.-S., L.K., H.U., F.V., B.L., and D.C. edited and revised manuscript; H.B., M.S.-S., H.U., F.V., and D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
Present addresses: M. Sedigh-Sarvestani, Max Planck Florida Institute for Neuroscience, 1 Max Planck Way, Jupiter, FL 33458; I. Fernandez-Lamo, Cajal Institute, Spanish National Research Council (CSIC), 28002 Madrid, Spain.
REFERENCES
- Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr 6: 203–207, 2006. doi: 10.1111/j.1535-7511.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykan B, Kinay D, Gökyigit A, Gürses C. Periodic lateralized epileptiform discharges: association with seizures. Seizure 9: 402–406, 2000. doi: 10.1053/seiz.2000.0435. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 51: 676–685, 2010. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O’Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84: 810–817, 2015. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: 407–420, 2012. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol 61: 747–758, 1989a. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol 62: 1149–1162, 1989b. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, Duncan JS. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378: 1388–1395, 2011. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. I. Characteristics and topographical features. J Neurophysiol 32: 649–662, 1969a. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. II. Mechanisms underlying origin and restriction. J Neurophysiol 32: 663–687, 1969b. doi: 10.1152/jn.1969.32.5.663. [DOI] [PubMed] [Google Scholar]
- Dreifuss FE. Classification of epileptic seizures. In: Epilepsy: A Comprehensive Textbook, edited by Engel J, Pedley T. Philadelphia, PA: Lippincott-Raven, 1997, p. 517–524. [Google Scholar]
- Dyhrfjeld-Johnsen J, Berdichevsky Y, Swiercz W, Sabolek H, Staley KJ. Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis. J Clin Neurophysiol 27: 418–424, 2010. doi: 10.1097/WNP.0b013e3181fe0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa TL, Dijkstra K, Brune C, Emerson RG, van Putten MJ, Goodman RR, McKhann GM Jr, Schevon CA, van Drongelen W, van Gils SA. Cross-scale effects of neural interactions during human neocortical seizure activity. Proc Natl Acad Sci USA 114: 10761–10766, 2017. [Erratum in Proc Natl Acad Sci USA 114: E9182, 2017.] 10.1073/pnas.1702490114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Scharfman HE, deCurtis M. How can we identify ictal and interictal abnormal activity? Adv Exp Med Biol 813: 3–23, 2014. doi: 10.1007/978-94-017-8914-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA. Refractory epilepsy: clinical overview. Epilepsia 48, Suppl 1: 3–7, 2007. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Fridley J, Thomas JG, Navarro JC, Yoshor D. Brain stimulation for the treatment of epilepsy. Neurosurg Focus 32: E13, 2012. doi: 10.3171/2012.1.FOCUS11334. [DOI] [PubMed] [Google Scholar]
- Gambardella A, Palmini A, Andermann F, Dubeau F, Da Costa JC, Quesney LF, Andermann E, Olivier A. Usefulness of focal rhythmic discharges on scalp EEG of patients with focal cortical dysplasia and intractable epilepsy. Electroencephalogr Clin Neurophysiol 98: 243–249, 1996. doi: 10.1016/0013-4694(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Khodagholy D, Thesen T, Devinsky O, Buzsáki G. Interictal epileptiform discharges induce hippocampal-cortical coupling in temporal lobe epilepsy. Nat Med 22: 641–648, 2016. doi: 10.1038/nm.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ramírez LR, Ahmed OJ, Cash SS, Wayne CE, Kramer MA. A biologically constrained, mathematical model of cortical wave propagation preceding seizure termination. PLoS Comput Biol 11: e1004065, 2015. doi: 10.1371/journal.pcbi.1004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat’s visual cortex. J Neurosci 11: 1800–1809, 1991. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci 14: 627–634, 2011. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Dümpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia 41: 467–478, 2000. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim GM, Cassel D, Morgan BR, Smith ML, Otsubo H, Ochi A, Taylor M, Rutka JT, Snead OC 3rd, Doesburg S. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain 137: 2690–2702, 2014. doi: 10.1093/brain/awu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsa VK, Stacey WC, Quilichini PP, Ivanov AI, Bernard C. On the nature of seizure dynamics. Brain 137: 2210–2230, 2014. doi: 10.1093/brain/awu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandratavicius L, Balista PA, Lopes-Aguiar C, Ruggiero RN, Umeoka EH, Garcia-Cairasco N, Bueno-Junior LS, Leite JP. Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat 10: 1693–1705, 2014. doi: 10.2147/NDT.S50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, Seneviratne U, Berkovic S, O’Brien T, Cook MJ. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain 139: 1066–1078, 2016. doi: 10.1093/brain/aww019. [DOI] [PubMed] [Google Scholar]
- Kazemi NJ, O’Brien TJ, Cascino GD. Localization-related epilepsies due to specific lesions. In: Epilepsy: A Comprehensive Textbook, edited by Engel J, Pedley T. Philadelphia, PA: Lippincott Williams & Wilkins, 2008, p. 2511–2523. [Google Scholar]
- Korshoej AR, Holm MM, Jensen K, Lambert JD. Kinetic analysis of evoked IPSCs discloses mechanism of antagonism of synaptic GABAA receptors by picrotoxin. Br J Pharmacol 159: 636–649, 2010. doi: 10.1111/j.1476-5381.2009.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology 71: 413–418, 2008. doi: 10.1212/01.wnl.0000310775.87331.90. [DOI] [PubMed] [Google Scholar]
- Kwan P, Sander JW. The natural history of epilepsy: an epidemiological view. J Neurol Neurosurg Psychiatry 75: 1376–1381, 2004. doi: 10.1136/jnnp.2004.045690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MJ, Chitale A, Sharan AD, Wu C. Advancements in stereotactic epilepsy surgery: Stereo-EEG, laser interstitial thermotherapy, and responsive neurostimulation. JHN J 11: 32–36, 2016. doi: 10.29046/JHNJ.011.2.005. [DOI] [Google Scholar]
- Litt B, Wityk RJ, Hertz SH, Mullen PD, Weiss H, Ryan DD, Henry TR. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia 39: 1194–1202, 1998. doi: 10.1111/j.1528-1157.1998.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Martinet LE, Fiddyment G, Madsen JR, Eskandar EN, Truccolo W, Eden UT, Cash SS, Kramer MA. Human seizures couple across spatial scales through travelling wave dynamics. Nat Commun 8: 14896, 2017. doi: 10.1038/ncomms14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Marsan CA. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol 9: 286–304, 1964. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol 63: 815–846, 2001. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Miles R, Traub RD, Wong RKS. Spread of synchronous firing in longitudinal slices from the CA3 region of the hippocampus. J Neurophysiol 60: 1481–1496, 1988. doi: 10.1152/jn.1988.60.4.1481. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RKS, Traub RD. Synchronized afterdischarges in the hippocampus: contribution of local synaptic interactions. Neuroscience 12: 1179–1189, 1984. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- Narizzano M, Arnulfo G, Ricci S, Toselli B, Tisdall M, Canessa A, Fato MM, Cardinale F. SEEG assistant: a 3DSlicer extension to support epilepsy surgery. BMC Bioinformatics 18: 124, 2017. doi: 10.1186/s12859-017-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NeuroNexus Technologies NeuroNexus 2013 Research Products Catalog. Ann Arbor, MI: NeuroNexus Technologies, 2012. [Google Scholar]
- Noachtar S, Bilgin O, Rémi J, Chang N, Midi I, Vollmar C, Feddersen B. Interictal regional polyspikes in noninvasive EEG suggest cortical dysplasia as etiology of focal epilepsies. Epilepsia 49: 1011–1017, 2008. doi: 10.1111/j.1528-1167.2008.01583.x. [DOI] [PubMed] [Google Scholar]
- Nuwer MR. Electrocorticography. In: Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, edited by Schomer DL, Lopes da Silva FH. Philadelphia, PA: Lippincott Williams & Wilkins, 2010, p. 715–724. [Google Scholar]
- Payne BR, Peters A. The concept of cat primary visual cortex. In: The Cat Primary Visual Cortex, edited by Payne BR, Peters A. San Diego, CA: Academic, 2002, p. 1–129. doi: 10.1016/B978-012552104-8/50002-X. [DOI] [Google Scholar]
- Pinto DJ, Patrick SL, Huang WC, Connors BW. Initiation, propagation, and termination of epileptiform activity in rodent neocortex in vitro involve distinct mechanisms. J Neurosci 25: 8131–8140, 2005. doi: 10.1523/JNEUROSCI.2278-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkorytova I, Hoes K, Lega B. Stereo-encephalograpy versus subdural electrodes for seizure localization. Neurosurg Clin N Am 27: 97–109, 2016. doi: 10.1016/j.nec.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol 16: 194–202, 1967. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- Rosati A, Aghakhani Y, Bernasconi A, Olivier A, Andermann F, Gotman J, Dubeau F. Intractable temporal lobe epilepsy with rare spikes is less severe than with frequent spikes. Neurology 60: 1290–1295, 2003. doi: 10.1212/01.WNL.0000058761.12715.0E. [DOI] [PubMed] [Google Scholar]
- Sato TK, Nauhaus I, Carandini M. Traveling waves in visual cortex. Neuron 75: 218–229, 2012. doi: 10.1016/j.neuron.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Goodman RR, McKhann G Jr, Emerson RG. Propagation of epileptiform activity on a submillimeter scale. J Clin Neurophysiol 27: 406–411, 2010. doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G Jr, Goodman RR, Yuste R, Emerson RG, Trevelyan AJ. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun 3: 1060, 2012. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KE, Lowel S. Long-range intrinsic connections in cat primary visual cortex. In: The Cat Primary Visual Cortex, edited by Payne BR, Peters A. San Diego, CA: Academic, 2002, p. 387–426. doi: 10.1016/B978-012552104-8/50011-0 [DOI] [Google Scholar]
- Sedigh-Sarvestani M, Vigeland L, Fernandez-Lamo I, Taylor MM, Palmer LA, Contreras D. Intracellular, in vivo, dynamics of thalamocortical synapses in visual cortex. J Neurosci 37: 5250–5262, 2017. doi: 10.1523/JNEUROSCI.3370-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Hader WJ, Jetté N. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia 52: 857–869, 2011. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- So NK, Blume WT. The postictal EEG. Epilepsy Behav 19: 121–126, 2010. doi: 10.1016/j.yebeh.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Sperling MR, Clancy RR. Ictal electroencephalogram. In: Epilepsy: A Comprehensive Textbook, edited by Engel J, Pedley T. Philadelphia, PA: Lippincott Williams & Wilkins, 2008, p. 825–854. [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr 6: 199–202, 2006. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, White A, Dudek FE. Interictal spikes: harbingers or causes of epilepsy? Neurosci Lett 497: 247–250, 2011. doi: 10.1016/j.neulet.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, Worrell GA. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain 133: 2789–2797, 2010. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 128: 1188–1198, 2005. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Baldeweg T, van Drongelen W, Yuste R, Whittington M. The source of afterdischarge activity in neocortical tonic-clonic epilepsy. J Neurosci 27: 13513–13519, 2007. doi: 10.1523/JNEUROSCI.3005-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H, Cazares C, Nanivadekar A, Kini L, Wagenaar J, Becker D, Krieger A, Lucas T, Litt B, Davis KA. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain 140: 2157–2168, 2017. doi: 10.1093/brain/awx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanleer AC, Blanco JA, Wagenaar JB, Viventi J, Contreras D, Litt B. Millimeter-scale epileptiform spike propagation patterns and their relationship to seizures. J Neural Eng 13: 026015, 2016. doi: 10.1088/1741-2560/13/2/026015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 14: 1599–1605, 2011. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester JC, Contreras D. Columnar interactions determine horizontal propagation of recurrent network activity in neocortex. J Neurosci 32: 5454–5471, 2012. doi: 10.1523/JNEUROSCI.5006-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Dudek FE, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia 51: 371–383, 2010. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis (3rd ed.). Upper Saddle River, NJ: Simon and Schuster, 1996. [Google Scholar]