Abstract
To study how changes in baroreceptor afferent activity affect patterns of sympathetic neural activation, we manipulated arterial blood pressure with intravenous nitroprusside (NTP) and phenylephrine (PE) and measured action potential (AP) patterns with wavelet-based methodology. We hypothesized that 1) baroreflex unloading (NTP) would increase firing of low-threshold axons and recruitment of latent axons and 2) baroreflex loading (PE) would decrease firing of low-threshold axons. Heart rate (HR, ECG), arterial blood pressure (BP, brachial catheter), and muscle sympathetic nerve activity (MSNA, microneurography of peroneal nerve) were measured at baseline and during steady-state systemic, intravenous NTP (0.5–1.2 µg·kg−1·min−1, n = 13) or PE (0.2–1.0 µg·kg−1·min−1, n = 9) infusion. BP decreased and HR and integrated MSNA increased with NTP (P < 0.01). AP incidence (326 ± 66 to 579 ± 129 APs/100 heartbeats) and AP content per integrated burst (8 ± 1 to 11 ± 2 APs/burst) increased with NTP (P < 0.05). The firing probability of low-threshold axons increased with NTP, and recruitment of high-threshold axons was observed (22 ± 3 to 24 ± 3 max cluster number, 9 ± 1 to 11 ± 1 clusters/burst; P < 0.05). BP increased and HR and integrated MSNA decreased with PE (P < 0.05). PE decreased AP incidence (406 ± 128 to 166 ± 42 APs/100 heartbeats) and resulted in fewer unique clusters (15 ± 2 to 9 ± 1 max cluster number, P < 0.05); components of an integrated burst (APs or clusters per burst) were not altered (P > 0.05). These data support a hierarchical pattern of sympathetic neural activation during manipulation of baroreceptor afferent activity, with rate coding of active neurons playing the predominant role and recruitment/derecruitment of higher-threshold units occurring with steady-state hypotensive stress.
NEW & NOTEWORTHY To study how changes in baroreceptor afferent activity affect patterns of sympathetic neural activation, we manipulated arterial blood pressure with intravenous nitroprusside and phenylephrine and measured sympathetic outflow with wavelet-based methodology. Baroreflex unloading increased sympathetic activity by increasing firing probability of low-threshold axons (rate coding) and recruiting new populations of high-threshold axons. Baroreflex loading decreased sympathetic activity by decreasing the firing probability of larger axons (derecruitment); however, the components of an integrated burst were unaffected.
Keywords: baroreflex, microneurography, modified Oxford technique, sympathetic outflow
INTRODUCTION
Sympathetic neural recordings obtained from skeletal muscle in humans [via the technique of microneurography (Vallbo et al. 1979; Salmanpour et al. 2010)] are often rectified and integrated to give multiunit muscle sympathetic nerve activity (MSNA) “bursts” that represent activity of several axons recorded simultaneously. With this, a fundamental question in motor unit physiology (Adrian and Bronk 1929; Henneman 1957) has also emerged in sympathetic nervous system activity: Are increases in the incidence and/or size of multiunit integrated MSNA bursts the result of an increased firing rate of previously active neurons [rate coding (Macefield and Wallin 1999; Salmanpour et al. 2011a)] and/or recruitment of additional neurons (Badrov et al. 2015; Steinback et al. 2010b; Wallin et al. 1994)? The frequency of neural discharge determines neurotransmitter release patterns (Lambert et al. 2011), and the number of active neurons can vary with each integrated, multiunit MSNA burst (Macefield and Elam 2003). In this way, altered sympathetic neuronal firing patterns may have consequences in the cardiovascular system that are not detected with integrated MSNA recordings.
The activity of the sympathetic nervous system can vary significantly when blood pressure increases and decreases. Despite robust alterations in sympathetic activity with manipulation of baroreceptor afferent activity, changes in blood pressure are more closely related to integrated, multiunit MSNA burst occurrence than burst size (Kienbaum et al. 2001). These data led researchers to hypothesize that the baroreflex is more important in determining whether a sympathetic burst occurs, while another unknown factor determines the number of axons recruited (Keller et al. 2006; Kienbaum et al. 2001; Malpas 2010). Consistent with this concept, Salmanpour and colleagues used wavelet-based methodology to show that small to medium-sized, low-threshold axons were present in most integrated, multiunit MSNA bursts at baseline and increased their firing rates during orthostatic stress (Salmanpour et al. 2011a, 2011b; Salmanpour and Shoemaker 2012). In contrast, the authors found that larger, faster-conducting axons had a low probability of firing at baseline and were minimally affected by baroreceptor pathways engaged during moderate orthostatic stress (Salmanpour et al. 2011a; Salmanpour and Shoemaker 2012).
These recent results support a relatively small impact of moderate lower body suction on sympathetic action potential (AP) recruitment, compared with other reflexes (Steinback et al. 2010a, 2010b). In contrast, during more severe orthostasis (−80-mmHg lower body negative pressure), the majority of individuals studied recruited a new population of larger-amplitude, faster-conducting postganglionic axons (Badrov et al. 2015; Salmanpour et al. 2011a). However, until now, the study of baroreflex control of MSNA AP recruitment has occurred under stable baseline conditions and/or during nonhypotensive lower body negative pressure, where the baroreflex stimulus for elevations in MSNA involves changes in pulse pressure but little change in diastolic or mean pressures. To advance our understanding of the contribution of the baroreflex to sympathetic discharge patterns in humans, we systematically manipulated arterial blood pressure by using intravenous nitroprusside and phenylephrine to elicit a large range of primarily arterial baroreflex stress and measured sympathetic outflow with wavelet-based methodology (Salmanpour et al. 2010). We hypothesized that baroreflex unloading with nitroprusside would increase firing of low-threshold axons (rate coding) and would recruit latent axons. We further hypothesized that baroreflex loading with phenylephrine would decrease firing of low-threshold axons.
METHODS
Research subjects.
All subjects were 18- to 45-yr-old, healthy (no acute or chronic conditions) nonsmokers with a body mass index <30 kg/m2 and not taking any medications. All experiments were performed in the Clinical Research and Trials Unit (CRTU) at Mayo Clinic, were approved by the Institutional Review Board at the Mayo Clinic, and conformed to the Declaration of Helsinki. On a screening visit, each subject gave written informed consent, followed by a review of medical history and a brief physical exam performed by a laboratory physician that included baseline measurements of height, weight, blood pressure, and heart rate.
Instrumentation.
On the study day, subjects arrived at the laboratory after a 4-h fast and after abstaining from exercise, caffeine, and alcohol for at least 24 h. Subjects were semirecumbent and were instrumented with a three-lead electrocardiogram to measure heart rate (Cardiocap/5; Datex-Ohmeda) and a nose clip/mouthpiece connected to a turbine for measurements of respiratory rate and tidal volume (Universal Ventilation Meter; Vacumetrics). A 20-gauge, 5-cm catheter was placed in the brachial artery under aseptic conditions after local anesthesia (2% lidocaine) to measure arterial blood pressure and for periodic blood sampling (epinephrine, norepinephrine). Standard blood assays were performed by the Immunochemistry Core Laboratory of the CRTU of the Mayo Clinic Center for Clinical and Translational Science.
Resting MSNA was recorded with the technique of microneurography (Salmanpour et al. 2010; Vallbo et al. 1979). Multiunit MSNA was recorded with a tungsten microelectrode (200-µm shaft diameter tapering to 1–5 µm at the uninsulated tip, 2.0 ± 0.4 M impedance; FHC, Bowdoin, ME) placed percutaneously into the peroneal nerve, posterior to the fibular head under direct two-dimensional live ultrasound guidance with a 12–15 MHz linear probe (Curry and Charkoudian 2011). A reference electrode was positioned subcutaneously ~4 cm from the recording electrode. The recorded signal was amplified 80,000-fold, band-pass filtered (700–2,000 Hz), rectified, and integrated (resistance-capacitance integrator circuit, time constant 0.1 s) by a nerve traffic analyzer (662C-3; Dept. of Bioengineering, University of Iowa). Sympathetic bursts in the integrated neurogram were identified with a custom-manufactured automated analysis program (Salmanpour et al. 2010). Multiunit MSNA is reported as bursts per minute (burst frequency), bursts per 100 heartbeats (burst incidence), and burst area (arbitrary units per min). Only sections of data without ectopic beats were included in the analysis.
Action potential detection and analysis.
AP patterns were detected and extracted with wavelet-based methodology on the raw, band-pass-filtered neurogram (Salmanpour et al. 2010). Briefly, a continuous wavelet transform using a “mother wavelet” with the same morphology as a physiological postganglionic sympathetic AP was used for AP detection (Salmanpour et al. 2010). The continuous wavelet transform with the matched mother wavelet was applied to the filtered MSNA signal to provide a wavelet coefficient between the AP of interest and the mother wavelet such that the wavelet coefficient was largest in the presence of the APs and negligible when applied to noise. Wavelet coefficients related to APs and noise were separated on the basis of thresholding analysis (Salmanpour et al. 2010). Extracted APs were grouped as overlapped (summated signal of 2 co-occurring APs) or single APs, based on their morphology. Nonoverlapped APs were ordered on the basis of peak-to-peak amplitude (32-point k-means), and histogram analysis was performed to separate APs into amplitude-based clusters based on Scott’s rule (Scott 1979; proposes optimal histogram bin width based on the sample size and an estimate of the standard deviation of the data). The number of AP clusters in the neurogram varied between maneuvers. Thus bin characteristics (minimum histogram bin width, maximum bin center, and total number of bins) were normalized within each individual to ensure that corresponding clusters at baseline and pharmacological intervention contained APs with similar peak-to-peak amplitudes (Badrov et al. 2015). With this, an increase in the number of clusters is considered equal to increased recruitment of additional populations of neurons.
Outcome variables include APs per burst (reflecting firing rate), clusters per burst (reflecting recruitment), and conduction latency of each cluster (reflecting synaptic delays and verifying large amplitude clusters as new populations). Conduction latency was calculated as the time delay between the R wave of the electrocardiogram of the preceding cardiac cycle and the negative peak of the AP waveform. Because the number of total clusters varies by individual, each participants’ number of total clusters was normalized to 10 total clusters with methods published previously (Badrov et al. 2015). In this way, each cluster group contained a 10% range of the largest detected cluster (which was given a value of 100%). For example, cluster 0–10% contained an average of all cluster numbers that were 0–10% of the largest cluster (Badrov et al. 2015).
Intravenous infusions.
An intravenous catheter was placed under local anesthesia for systemic intravenous infusion of nitroprusside and phenylephrine, administered separately. Nitroprusside (nitric oxide donor) was infused intravenously after 3 min of quiet rest at an individualized dose (0.5–1.2 µg·kg−1·min−1) intended to achieve a blood pressure fall of 10 mmHg (Grassi et al. 1995, 2004, 2005; Grossman et al. 1991; Heindl et al. 2006). Phenylephrine (α1-adrenergic agonist) was infused intravenously after 3 min of quiet rest at a range of doses (0.2–1.0 µg·kg−1·min−1) selected to achieve varied levels of baroreceptor activity (Cui et al. 2002; Heindl et al. 2006; Wilkins et al. 2008). For example, 1.5 µg·kg−1·min−1 has been shown to increase mean blood pressure ~15 mmHg and silence MSNA via baroreceptor loading (Cui et al. 2002; Wehrwein et al. 2010). In contrast, low doses of phenylephrine (0.1 µg·kg−1·min−1) may increase blood pressure only slightly (~1 mmHg) (Wilkins et al. 2008). With such small changes in baroreceptor stretch changes in multiunit MSNA may be undetectable, but changes in the firing of individual axons may be observed with the AP analysis approach.
Study visit.
Subjects completed two protocols separated by a minimum of 30 min. Protocol order was randomized and counterbalanced. For each protocol, respiratory rate was held at baseline levels with a metronome. Protocol 1 consisted of intravenous infusion of sodium nitroprusside. All individuals started at a dose of 0.8 µg·kg−1·min−1, which was increased 0.1 µg·kg−1·min−1 every 2–3 min until a 10-mmHg fall in blood pressure and an increase in MSNA were observed. If blood pressure fell >10 mmHg, the nitroprusside dose was reduced in 0.1 µg·kg−1·min−1 increments until a stable 10-mmHg fall in blood pressure was reached (time to achieve desired effect = 6.2 ± 1.1 min; average final dose 0.90 ± 0.05 µg·kg−1·min−1). Data are reported as a 5-min average during the final dose (total infusion time = 11.3 ± 1.1 min).
Protocol 2 consisted of intravenous infusion of phenylephrine at 0.2 µg·kg−1·min−1 for 3 min. The dose of phenylephrine was increased by 0.2 µg·kg−1·min−1 every 3 min until arterial blood pressure was increased 5–10 mmHg and multiunit MSNA signal was silenced. After the final 3-min infusion, phenylephrine infusion was stopped and the MSNA signal was allowed to return, ensuring that the fall in MSNA was not the result of signal decay. Data are reported as a 3-min average during the final dose (phenylephrine dose 2, range 0.4–1.0, average 0.62 ± 0.08) and during approximately half of the maximum dose (phenylephrine dose 1, range 0.2–0.6, average 0.29 ± 0.05).
Three individuals from protocol 1 and six naive individuals were recruited to complete a third protocol. On a separate study day, subjects arrived to the laboratory after a 12-h fast and after abstaining from exercise, caffeine, and alcohol for at least 24 h. In the three returning individuals, visits were separated by 18 ± 2 mo (range 14–20 mo). Instrumentation for protocol 3 was identical to protocols 1 and 2, although subjects wore a mask rather than a nose clip/mouthpiece and respiratory rate was not controlled. After instrumentation, individuals completed a 5-min quiet resting period. An intravenous bolus of sodium nitroprusside (100 µg) was then administered, followed by an intravenous bolus of phenylephrine (150 µg) (modified Oxford technique) (Ebert 1990; Rudas et al. 1999; Smyth et al. 1969). This approach is currently considered the “gold standard” to examine sensitivity of the arterial baroreflex (Rudas et al. 1999). By incorporating the modified Oxford technique, we are able to examine the robustness of observations made under more transient decreases/increases in blood pressure. Data are reported as an average during the last 1 min of rest; after nitroprusside infusion, when blood pressure was falling; and during the subsequent phenylephrine infusion, when blood pressure was increasing but MSNA integrated bursts could still be detected.
Data analysis.
Data were recorded at 10,000 Hz with a computer data acquisition system (PowerLab; ADInstruments) and stored for off-line analysis (Salmanpour et al. 2010). Statistical analyses were conducted with SigmaPlot version 14.0 (Systat Software). Data presented in Tables 1–3 were analyzed by using a one-way repeated-measures analysis of variance (ANOVA) to determine the main effect of condition (baseline, drug) on main outcome variables. Normality was assessed with the Shapiro-Wilk test. If not normally distributed, a Friedman repeated-measures ANOVA on ranks was conducted. Pairwise comparisons were done by the Holm-Sidak method. Data presented in Figs. 2, 4, 6, and 7 were analyzed by using a two-way repeated-measures ANOVA to determine the main effect of relative cluster size (10–100% of total clusters) and condition (baseline, drug) and the interaction of cluster and condition. Pairwise comparisons were done with the Holm-Sidak method. All data are presented as means ± SE. P values ≤0.05 were considered statistically significant.
Table 1.
Cardiorespiratory and sympathetic responses to systemic nitroprusside
| Baseline | Nitroprusside | |
|---|---|---|
| Heart rate, beats/min | 61 ± 2 | 80 ± 3* |
| Systolic blood pressure, mmHg | 146 ± 3 | 136 ± 4* |
| Diastolic blood pressure, mmHg | 77 ± 2 | 73 ± 2* |
| Mean blood pressure, mmHg | 99 ± 2 | 91 ± 2* |
| Respiratory rate, breaths/min | 11 ± 1 | 11 ± 1 |
| Tidal volume, l/breath | 0.70 ± 0.14 | 0.81 ± 0.16 |
| Minute ventilation, ml/min | 6.7 ± 0.8 | 7.9 ± 1.0 |
| Epinephrine, pg/ml | 67 ± 11 | 82 ± 16 |
| Norepinephrine, pg/ml | 174 ± 14 | 215 ± 26 |
| Burst frequency, bursts/min | 25 ± 3 | 41 ± 3* |
| Burst incidence, bursts/100 heartbeats | 41 ± 5 | 53 ± 4* |
| Mean burst area, AU/min | 1.4 ± 0.2 | 2.8 ± 0.4* |
| Action potential frequency, APs/min | 191 ± 35 | 439 ± 90* |
| Action potential incidence, APs/burst | 8 ± 1 | 11 ± 2* |
| Action potential incidence, APs/100 heartbeats | 326 ± 66 | 579 ± 129* |
| Mean cluster incidence, clusters/burst | 4.5 ± 0.5 | 5.5 ± 0.7* |
| Max cluster incidence, clusters/burst | 9.5 ± 1.0 | 10.9 ± 1.0* |
| Max cluster number | 21.6 ± 2.7 | 24.4 ± 3.0* |
Data are means ± SE from n = 13. Average final dose was 0.90 ± 0.05 µg·kg−1·min−1. Data were analyzed with a 1-way repeated-measures ANOVA. Normality was assessed with the Shapiro-Wilk test. If not normally distributed, a Friedman repeated-measures ANOVA on ranks was conducted [respiratory rate, minute ventilation, action potential (AP) incidence (APs/burst), max cluster number]. AU, arbitrary units. Pairwise comparisons were done with the Holm-Sidak method.
P < 0.05 vs. baseline.
Table 3.
Cardiorespiratory and sympathetic responses during modified Oxford test
| Baseline | Nitroprusside | Phenylephrine | |
|---|---|---|---|
| Heart rate, beats/min | 58 ± 2 | 73 ± 4*† | 61 ± 3 |
| Systolic blood pressure, mmHg | 138 ± 4 | 130 ± 4*† | 139 ± 5 |
| Diastolic blood pressure, mmHg | 75 ± 3 | 65 ± 3*† | 75 ± 3 |
| Mean blood pressure, mmHg | 96 ± 3 | 86 ± 3*† | 95 ± 3 |
| Respiratory rate, breaths/min | 13 ± 1 | 12 ± 1 | 12 ± 1 |
| Tidal volume, l/breath | 0.58 ± 0.10 | 0.77 ± 0.07 | 0.98 ± 0.17* |
| Minute ventilation, ml/min | 7.1 ± 1.0 | 8.9 ± 1.1 | 11.5 ± 2.1* |
| Burst frequency, bursts/min | 21 ± 1 | 36 ± 2*† | 22 ± 4 |
| Burst incidence, bursts/100 heartbeats | 37 ± 4 | 53 ± 4*† | 37 ± 7 |
| Mean burst area, AU/min | 1.1 ± 0.2 | 2.1 ± 0.2*† | 1.2 ± 0.2 |
| Action potential frequency, APs/min | 222 ± 36 | 454 ± 71*† | 254 ± 74 |
| Action potential incidence, APs/burst | 10.3 ± 1.2 | 12.2 ± 1.8 | 10.7 ± 1.6 |
| Action potential incidence, APs/100 heartbeats | 398 ± 75 | 678 ± 120* | 431 ± 127 |
| Mean cluster incidence, clusters/burst | 5.3 ± 0.6 | 5.4 ± 0.6 | 5.0 ± 0.5 |
| Max cluster incidence, clusters/burst | 9.8 ± 0.8 | 10.0 ± 0.9 | 10.3 ± 1.0 |
| Max cluster number | 10.7 ± 1.2 | 11.3 ± 1.2 | 10.6 ± 1.2 |
Data are means ± SE from n = 9. Data were analyzed with a 1-way repeated-measures ANOVA. Normality was assessed with the Shapiro-Wilk test. If not normally distributed, a Friedman repeated-measures ANOVA on ranks was conducted (heart rate). Pairwise comparisons were done with the Holm-Sidak method.
P < 0.05 vs. baseline.
P < 0.05 vs. phenylephrine.
Fig. 2.
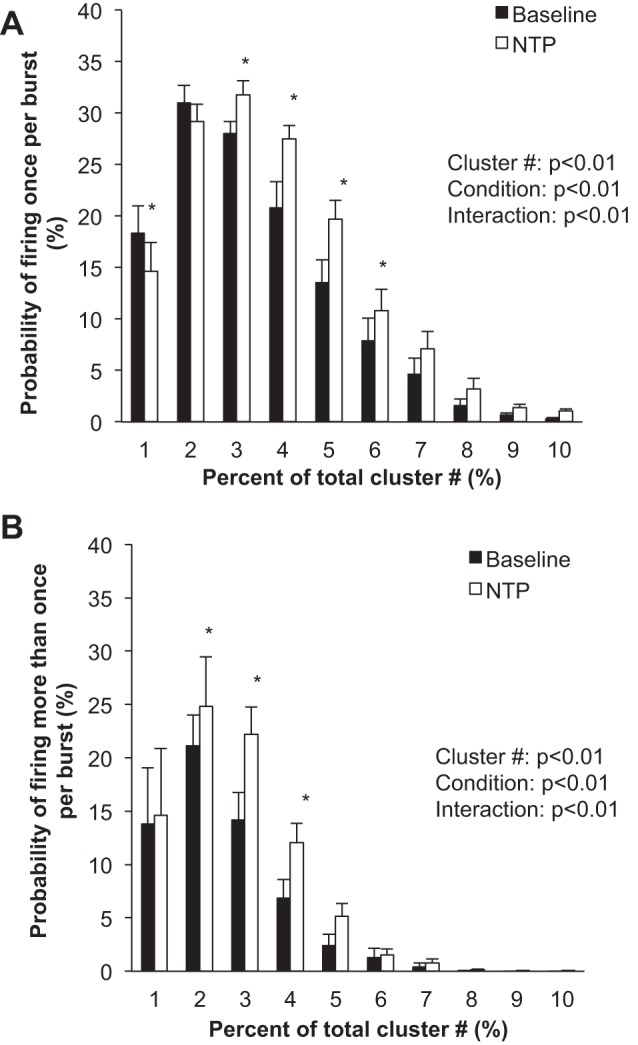
Sympathetic responses to steady-state systemic nitroprusside (NTP). Data are means ± SE from n = 13. Data were analyzed with a 2-way repeated-measures ANOVA to determine the main effect of relative cluster size (10–100% of total clusters) and condition (baseline, drug) and the interaction of cluster and condition. A: probability of a relative cluster (10–100% of total clusters) firing once per integrated MSNA burst. B: probability of a relative cluster (10–100% of total clusters) firing more than once per integrated MSNA burst. *P < 0.05 vs. baseline.
Fig. 4.
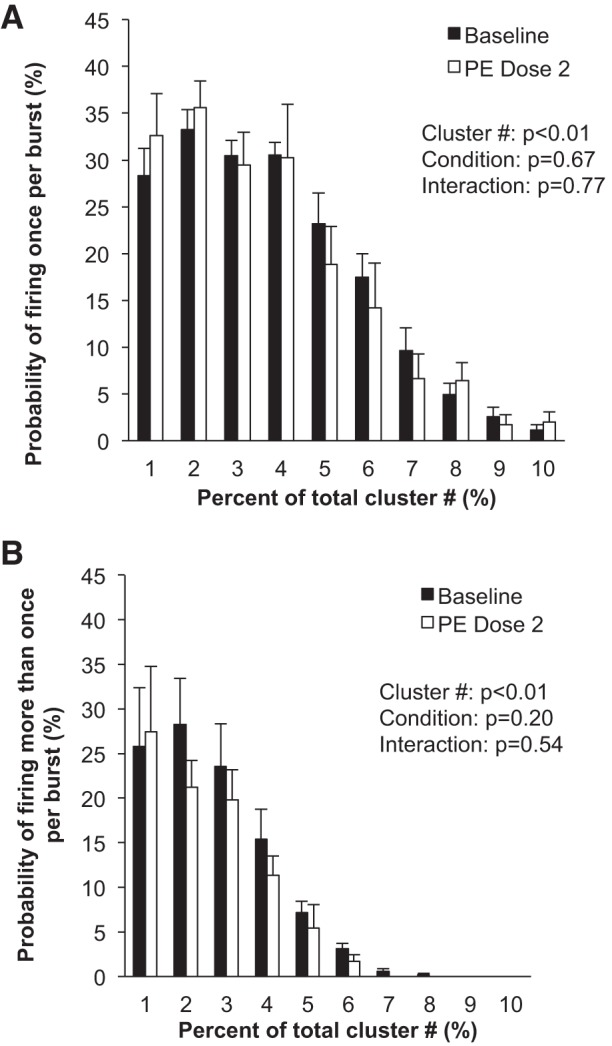
Sympathetic responses to steady-state systemic phenylephrine (PE). Data are means ± SE from n = 9. Data were analyzed with a 2-way repeated-measures ANOVA to determine the main effect of relative cluster size (10–100% of total clusters) and condition (baseline, drug) and the interaction of cluster and condition. A: probability of a relative cluster (10–100% of total clusters) firing once per integrated MSNA burst. B: probability of a relative cluster (10–100% of total clusters) firing more than once per integrated MSNA burst.
Fig. 6.
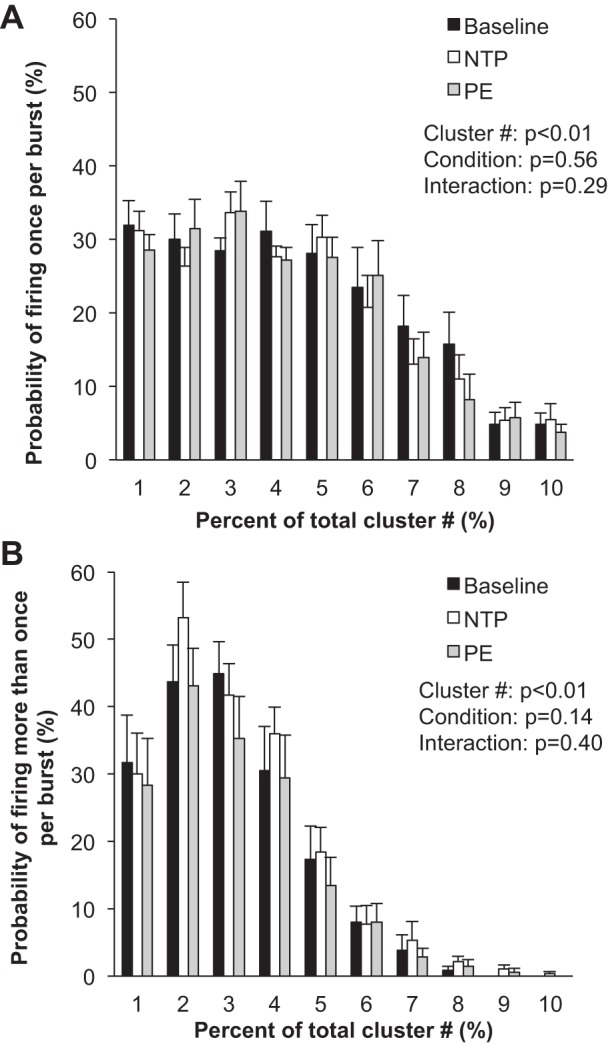
Sympathetic responses to the modified Oxford procedure. Data are means ± SE from n = 9. Data were analyzed with a 2-way repeated-measures ANOVA to determine the main effect of relative cluster size (10–100% of total clusters) and condition (baseline, drug) and the interaction of cluster and condition. A: probability of a relative cluster (10–100% of total clusters) firing once per integrated MSNA burst. B: probability of a relative cluster (10–100% of total clusters) firing more than once per integrated MSNA burst.
Fig. 7.
Synaptic delays during systemic infusions. A: steady-state nitroprusside (NTE; n = 13). B: steady-state phenylephrine (PE; n = 9). C: modified Oxford (n = 9). Sample sizes for clusters in which not all subjects are included (clusters were not present) are indicated. Data are means ± SE. Data were analyzed with a 2-way repeated-measures ANOVA to determine the main effect of relative cluster size (10–100% of total clusters) and condition (baseline, drug) and the interaction of cluster and condition.
RESULTS
Subject demographics.
The initial protocol recruited 19 individuals, of which high-quality nerve recordings were made in 14 individuals. Thirteen male subjects (30 ± 2 yr, 181 ± 2 cm, 82 ± 3 kg, 25 ± 1 kg/m2) successfully completed protocol 1 (1 subject was excluded because of failure to achieve desired fall in blood pressure). Of those 13 individuals, 9 individuals successfully completed protocol 2 (30 ± 2 yr, 181 ± 2 cm, 82 ± 3 kg, 25 ± 1 kg/m2) (data from 4 individuals were excluded because of shifts in the MSNA baseline during the infusion protocol). Three individuals returned for a second visit to complete protocol 3. An additional six male subjects were recruited to complete protocol 3 only (n = 9 total; 31 ± 2 yr, 183 ± 2 cm, 89 ± 3 kg, 26 ± 1 kg/m2).
Nitroprusside.
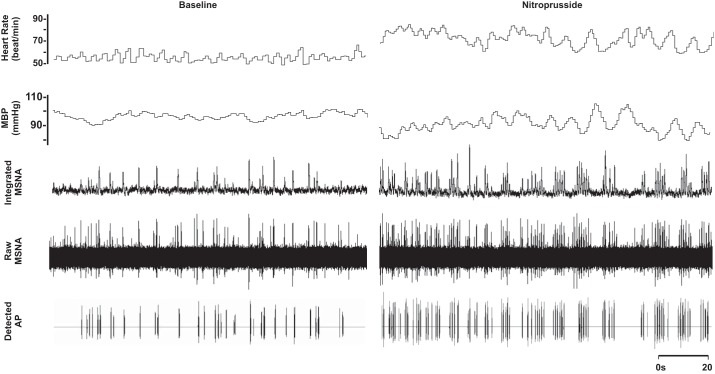
Compared with baseline, blood pressure decreased and heart rate increased with nitroprusside (P < 0.01; Table 1 and Fig. 1). Nitroprusside elicited a progressive increase in integrated MSNA bursts (P < 0.01; Table 1 and Fig. 1). Underlying nitroprusside-mediated increases in integrated MSNA were increases in neuronal discharge rate (APs/min, APs/100 heartbeats) and AP content per integrated burst (P < 0.05; Table 1). The probability of APs from the same cluster firing once per integrated MSNA burst (single firing; Fig. 2A) and more than once per burst (multiple firing; Fig. 2B) increased with nitroprusside, and recruitment of latent, high-threshold axons was observed (max cluster number; max clusters/burst) (P < 0.05 for all; Table 1).
Fig. 1.
Representative sample of data from 1 subject (26 yr, 21 kg/m2) collected at baseline and during steady-state nitroprusside infusion (1.1 µg·kg−1·min−1). MBP, mean blood pressure.
Phenylephrine.
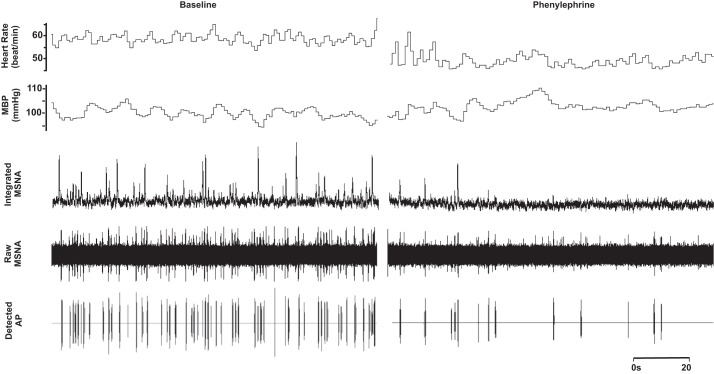
No changes in neuronal recruitment and/or firing patterns were observed at the lower dose (dose 1) of phenylephrine, despite an ~2-mmHg increase in diastolic blood pressure (Table 2). Compared with baseline, blood pressure increased and heart rate decreased with the highest dose (dose 2) of phenylephrine (P < 0.01; Table 2 and Fig. 3). The highest dose of phenylephrine elicited a reduction in integrated MSNA (P < 0.05; Table 2 and Fig. 3). Underlying the phenylephrine-mediated decreases in integrated MSNA at the highest dose were decreases in neuronal discharge rate (APs/min, APs/100 heartbeats) and fewer unique clusters (max cluster number, max clusters/burst), suggesting derecruitment of axons (P < 0.05 for all; Table 2). The firing probability (single and multiple) of low-threshold axons was not altered with phenylephrine (Fig. 4), and the components of an integrated burst were not altered (APs/burst, clusters/burst, P > 0.05; Table 2).
Table 2.
Cardiorespiratory and sympathetic responses to systemic phenylephrine
| Baseline | Phenylephrine Dose 1 | Phenylephrine Dose 2 | |
|---|---|---|---|
| Heart rate, beats/min | 61 ± 2 | 60 ± 2 | 56 ± 3*† |
| Systolic blood pressure, mmHg | 145 ± 4 | 144 ± 4 | 150 ± 4*† |
| Diastolic blood pressure, mmHg | 77 ± 2 | 79 ± 2* | 83 ± 2*† |
| Mean blood pressure, mmHg | 98 ± 2 | 100 ± 2 | 106 ± 3*† |
| Respiratory rate, breaths/min | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| Tidal volume, l/breath | 0.51 ± 0.08 | 0.51 ± 0.08 | 0.57 ± 0.07 |
| Minute ventilation, ml/min | 5.8 ± 0.8 | 6.0 ± 0.7 | 6.7 ± 0.8 |
| Epinephrine, pg/ml | 58 ± 10 | 60 ± 7 | 54 ± 9 |
| Norepinephrine, pg/ml | 160 ± 18 | 132 ± 19* | 132 ± 20 |
| Burst frequency, bursts/min | 24 ± 2 | 23 ± 2 | 11 ± 2*† |
| Burst incidence, bursts/100 heartbeats | 41 ± 3 | 39 ± 4 | 21 ± 3*† |
| Mean burst area, AU/min | 1.3 ± 0.4 | 1.2 ± 0.4 | 0.5 ± 0.1 |
| Action potential frequency, APs/min | 238 ± 73 | 222 ± 63 | 91 ± 25*† |
| Action potential incidence, APs/burst | 8.9 ± 2.3 | 8.9 ± 2.0 | 7.7 ± 1.8 |
| Action potential incidence, APs/100 heartbeats | 406 ± 128 | 377 ± 102 | 166 ± 42*† |
| Mean cluster incidence, clusters/burst | 4.6 ± 0.7 | 4.6 ± 0.7 | 3.9 ± 0.5 |
| Max cluster incidence, clusters/burst | 8.6 ± 1.0 | 8.6 ± 1.0 | 6.9 ± 0.7*† |
| Max cluster number | 14.6 ± 2.3 | 15.1 ± 2.2 | 8.9 ± 0.8*† |
Data are means ± SE from n = 9 unless otherwise noted (dose 2: epinephrine and norepinephrine, n = 6). Data are reported as a 3-min average during the final dose (phenylephrine dose 2, range 0.4–1.0, average 0.62 ± 0.08 µg·kg−1·min−1) and during approximately half of the maximum dose (phenylephrine dose 1, range 0.2–0.6, average 0.29 ± 0.05 µg·kg−1·min−1). Data were analyzed with a 1-way repeated-measures ANOVA. Normality was assessed with the Shapiro-Wilk test. If not normally distributed, a Friedman repeated-measures ANOVA on ranks was conducted [minute ventilation, mean burst area, action potential incidence (AP/burst), max cluster incidence]. Pairwise comparisons were done with the Holm-Sidak method.
P < 0.05 vs. baseline.
P < 0.05 vs. phenylephrine dose 1.
Fig. 3.
Representative sample of data from 1 subject (26 yr, 21 kg/m2) collected at baseline and during phenylephrine infusion at the highest dose (0.6 µg·kg−1·min−1).
Modified Oxford procedure.
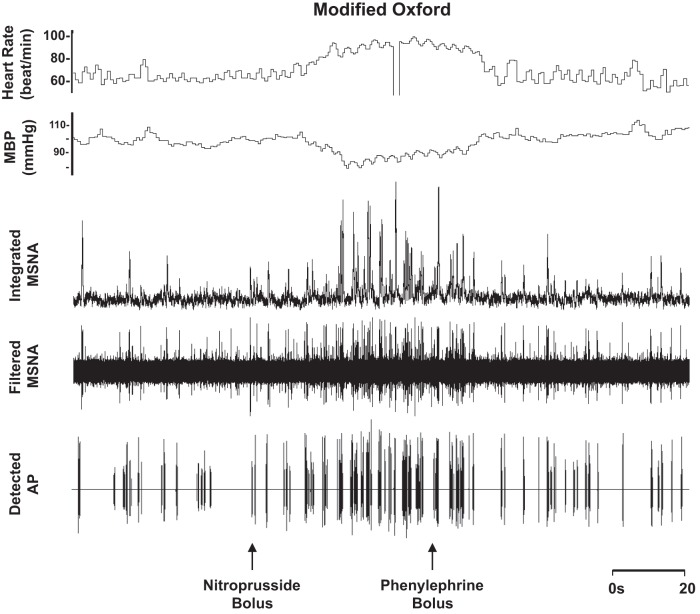
Blood pressure decreased and heart rate increased with nitroprusside, and values returned to baseline levels with subsequent phenylephrine (main effect, P < 0.01; Table 3 and Fig. 5). Nitroprusside elicited a robust increase in integrated MSNA bursts that returned to baseline levels with phenylephrine (main effect, P < 0.01; Table 3 and Fig. 5). Underlying changes in integrated MSNA were increases in neuronal discharge rate with nitroprusside (APs/min, APs/100 heartbeats) that returned to baseline with subsequent phenylephrine (main effect, P < 0.05; Table 3). No changes were observed in AP recruitment (max cluster number P = 0.69, max clusters/burst P = 0.25; Table 3), and components of a burst were not altered (APs/burst P = 0.22; mean clusters/burst P = 0.36) during the modified Oxford procedure. Furthermore, the firing probabilities (single and multiple) were not altered (P value range 0.14–0.56; Fig. 6).
Fig. 5.
Representative sample of data from 1 subject (24 yr, 24 kg/m2) collected at baseline and during the modified Oxford procedure.
Synaptic delays.
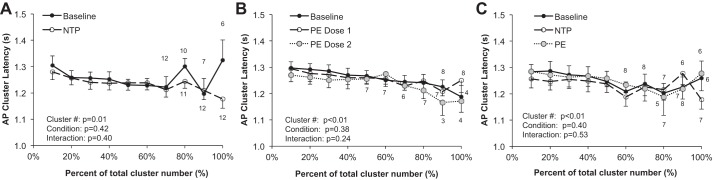
AP cluster latency decreased with cluster size in all protocols (P < 0.01) but was not altered with nitroprusside (P = 0.42) or phenylephrine (P = 0.39) or during the modified Oxford procedure (P = 0.40) (see Fig. 7).
DISCUSSION
The main new finding in this study is that baroreflex unloading via a reduction in arterial pressure can elicit recruitment of larger, faster-conducting sympathetic neurons. Conversely, baroreflex loading via increases in arterial pressure can derecruit neurons that are present at baseline. In both cases, we confirm prior observations demonstrating that ongoing rate coding of actively firing sympathetic neurons is under baroreflex control and is especially important during transient decreases/increases in blood pressure. We also show synaptic delays, which have been observed previously to be reduced during physical stress [handgrip exercise (Badrov et al. 2016a), voluntary apnea (Steinback et al. 2010a)] and somewhat prolonged during −80-mmHg lower body negative pressure (Badrov et al. 2015), are unaffected by baroreflex loading/unloading by pharmacological methods.
Nitroprusside.
In the present investigation, we found that pharmacological baroreflex unloading with systemic nitroprusside increases sympathetic neuronal discharge rate (rate coding) by increasing the firing probability of low-threshold axons (Fig. 2), in addition to recruitment of latent, higher-threshold axons (Table 1). This is similar to what has been observed previously during severe chemoreflex stress (Steinback et al. 2010a, 2010b) but is in contrast to previous work from Salmanpour and colleagues showing that larger, faster-conducting axons are minimally affected by baroreceptor pathways (Salmanpour et al. 2011a, 2011b; Salmanpour and Shoemaker 2012). However, the majority of these data were obtained under baseline conditions, thus relying on spontaneous fluctuations and/or low-level lower body negative pressure. In contrast, during more severe orthostasis (−80-mmHg lower body negative pressure), the majority of individuals studied recruited a new population of larger-amplitude, faster-conducting postganglionic axons (Badrov et al. 2015; Salmanpour et al. 2011a).
Comparisons of outcomes of the present pharmacological approach with observations using nonpharmacological models (i.e., orthostatic stress evoked by lower body negative pressure; Badrov et al. 2015; Salmanpour et al. 2011a) suggest that a consistent physiological response can be achieved under conditions of relatively stable baroreflex stress. With this, in the present study we show that an ordered, hierarchical pattern of neuronal recruitment is possible during intravenous infusion of nitroprusside in healthy male adults and further support the concept that subpopulations of larger-amplitude, faster-conducting sympathetic axons that are not present at baseline can be recruited during steady-state baroreflex stress. This pattern of recruitment is not unlike recruitment of motor units during muscular contraction (Adrian and Bronk 1929; Henneman 1957). It is important to acknowledge, however, that although recruitment occurs, it expresses interindividual variations. For example, previous work found that only 60–65% of individuals recruit latent subpopulations of axons during severe orthostasis (−80 mmHg lower body negative pressure; Badrov et al. 2015; Salmanpour et al. 2011a). Similarly, the present data show an increase in maximum clusters per burst (indicating recruitment) in 75% of individuals (n = 10 of 13) studied with pharmacological methods.
We also observed an increase in the probability of multiple firing (APs from the same cluster firing more than once per burst) during steady-state nitroprusside infusion (Fig. 2B). Macefield and colleagues have shown, using single-unit MSNA recordings, that neurons typically fire only once per integrated burst at rest (Macefield et al. 1994; Macefield and Wallin 1999) and the same neuron can fire multiple times within a single integrated burst during stress (Macefield and Wallin 1999). Although the present analysis approach cannot infer single-axon behavior, we show a relatively low probability of multiple firing at baseline, which is augmented during nitroprusside infusion.
Given that the frequency of neural discharge determines neurotransmitter release patterns (Lambert et al. 2011), these data may have important implications for neurovascular transduction. More specifically, one would expect an increase in release of norepinephrine and colocalized neurotransmitters (i.e., neuropeptide Y) with an increase in multiple firing—a concept that may be supported by the present data. With this, it would be especially interesting to examine similar responses in a group of individuals with known impairments in sympathetic baroreflex sensitivity and/or neurovascular transduction. For example, older individuals have a reduced capacity to recruit latent neural subpopulations compared with young adults (Badrov et al. 2016a). When combined with our new observations, it is reasonable to speculate that impairments in sympathetic baroreflex sensitivity observed with aging may be related to attenuated recruitment of latent, high-threshold axons in response to baroreflex unloading.
Phenylephrine.
To our knowledge, this is the first study of its kind to examine derecruitment strategies of sympathetic axons in humans. As noted above, a key strategy of the sympathetic nervous system to alter neuronal activity is to change the number of active neurons and/or the firing probability of neurons (Macefield and Wallin 1999). Previous work in motor units has shown that the firing rate of earlier-recruited motor units is greater than that of units recruited later (Henneman et al. 1965). In support of this idea, our data show that the firing probability of larger-diameter, higher-threshold axons is attenuated during pharmacological loading of the baroreflex. Specifically, there were fewer unique clusters present during the highest dose of phenylephrine infused, compared with baseline, and AP incidence was reduced (Table 2). In light of this, we can conclude that neurons present at baseline exhibiting the highest threshold activation (larger diameter, shorter latency) are the first lost under conditions of baroreflex loading. Based on the understanding that sympathetic neurons directed to skeletal muscle vasculature are vasoconstrictor signals, we surmise that, although changes in cluster number are relatively subtle in the present model, they should have an impact on regulation of vascular tone. Such findings may have important implications for clinical conditions such as hypertension, where one could speculate that patients may be unable to derecruit high-threshold axons with increases in arterial blood pressure. However, it is important to acknowledge that, contrary to our hypothesis, any changes in neuronal firing and recruitment were only observed at the highest dose of phenylephrine and relatively small (~2 mmHg) changes in blood pressure had minimal effect on AP recruitment.
Modified Oxford procedure.
Three returning subjects and six naive individuals completed a third protocol that consisted of a bolus infusion of nitroprusside followed immediately by a bolus infusion of phenylephrine (modified Oxford technique) (Ebert 1990; Rudas et al. 1999; Smyth et al. 1969). This approach is often used experimentally to examine sensitivity of the arterial baroreflex and is considered the “gold standard” (Rudas et al. 1999). With this, results from the modified Oxford approach vary from conclusions obtained during individual infusions in the following areas: 1) bolus nitroprusside did not increase recruitment of latent axons and 2) bolus phenylephrine did not decrease firing of larger, faster-conducting axons. The severity of the stimulus in terms of a change in blood pressure is relatively consistent between the protocols, and thus we speculate that differences in the response may be due to differing lengths of time over which the stress is occurring (seconds vs. minutes). From this, we can conclude that transient changes in blood pressure rely on rate coding rather than recruitment to achieve changes in sympathetic activation, which is in contrast to responses observed during steady-state infusions, where axonal recruitment/derecruitment was observed. It is also possible that recruitment/derecruitment occurs briefly in one or two bursts at the nadir/peak blood pressure during the modified Oxford procedure that are not detectable by current analysis methods. However, previous published work has shown that short (8–47 s) sections of data do not significantly impact detectable clusters (Badrov et al. 2016a), and thus this is a minor limitation.
Synaptic delays.
Wallin and colleagues have shown an inverse relationship between burst latency (measured as the delay of an integrated MSNA burst from a representative ECG R wave) and burst size, with larger bursts exhibiting shorter reflex latencies (Wallin et al. 1994). Consistent with this, we observed an inverse relationship between AP cluster amplitude and conduction velocity, with larger clusters exhibiting a shorter latency (Fig. 7). Macefield and colleagues have also shown that the latency of a single axon is highly variable at rest (Macefield et al. 1994), and in situations of severe physiological stress altered synaptic delays and/or central processing times have been suggested (Badrov et al. 2015; Coote and Macleod 1974; Fagius et al. 1987; Salmanpour et al. 2011b; Wallin et al. 1994). Along these lines, Badrov and colleagues found that AP clusters were recruited more slowly (longer latency) during severe orthostasis (Badrov et al. 2015). In the present investigation, synaptic delays were unaffected by pharmacological baroreflex loading/unloading. We hypothesize that clear shifts in cluster latency profiles may be primarily the result of changes in central processing and thus likely occur only in situations where an individual level of perceived stress and/or tolerance is present (Badrov et al. 2015, 2016a, 2016b, 2017); however, future work in this area is needed.
Experimental considerations.
Fagius and colleagues (Fagius et al. 1985) showed by blocking baroreceptor afferent activity that the normal cardiac rhythmicity of MSNA was disrupted and impulses instead occurred in a slow, irregular rhythm. Thus it may be expected that nitroprusside infusion, via arterial baroreflex unloading, would reduce the pulse-synchronous nature of MSNA and augment observed latencies. We did not observe augmented latencies in the present study; however, APs that are not pulse synchronous or not comprised within a burst are not included in the present analysis. Future work will be necessary to examine changes in activity of APs that are not pulse synchronous or incorporated into a multiunit MSNA burst.
The present approach classifies the contribution of multiple vasomotor neurons to multiunit integrated MSNA bursts morphologically based on peak-to-peak amplitude (Salmanpour et al. 2010). This approach compliments the single-unit approach (Macefield et al. 1994; Macefield and Wallin 1999), although it cannot determine whether APs with the same size/morphology are from the same or different neurons (Salmanpour et al. 2010). Furthermore, detection of APs by this method requires adequate signal-to-noise ratio. The average signal-to-noise ratio for the data was between 4.25 ± 0.21 (phenylephrine) and 4.51 ± 0.22 (nitroprusside). Based on previous work (Salmanpour et al. 2010), this level of signal-to-noise ratio would produce a correct detection rate of ~90% and a false positive detection rate of <3%. Finally, these studies were all conducted in young, healthy male participants with relatively normal baroreflex function. Whether these findings translate to women and/or individuals with impaired baroreflex function has yet to be assessed.
Conclusions.
In the present investigation we used a pharmacological approach (the “gold standard”) to elicit targeted increases/decreases in arterial blood pressure. Our data point to an important role for baroreflex mechanisms in modifying AP rate coding patterns. In addition, we show that the sympathetic nervous system has the ability to recruit latent subpopulations of large APs during steady-state unloading of the baroreceptors in healthy male participants and derecruit during baroreflex loading, with some interindividual variation. Conversely, recruitment was not apparent during large, but transient, fluctuations in arterial pressure. Further work will be needed to see whether these results translate to conditions with known baroreflex impairments.
GRANTS
This study was supported by American Heart Association Grant 15SDG25080095 (J. K. Limberg), National Heart, Lung, and Blood Institute (NHLBI) Grant HL-130339 (J. K. Limberg), NHLBI Grant HL-083947 (M. J. Joyner), the Mayo Clinic Department of Anesthesiology and Perioperative Medicine, and the Natural Sciences and Engineering Research Council of Canada Discovery Grant program (J. K. Shoemaker). J. K. Shoemaker is a Tier 1 Canada Research Chair.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., T.B.C., W.T.N., M.J.J., and J.K.S. conceived and designed research; J.K.L., W.W.H., S.E.B., T.B.C., W.T.N., M.J.J., and J.K.S. performed experiments; J.K.L. and E.P.O. analyzed data; J.K.L., E.P.O., W.W.H., S.E.B., M.J.J., and J.K.S. interpreted results of experiments; J.K.L. and E.P.O. prepared figures; J.K.L. drafted manuscript; J.K.L., W.W.H., S.E.B., M.J.J., and J.K.S. edited and revised manuscript; J.K.L., E.P.O., W.W.H., S.E.B., T.B.C., W.T.N., M.J.J., and J.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Many thanks to our research participants as well as individuals involved in recruitment, scheduling, and data collection: Nancy Meyer, Sarah Wolhart, Shelly Roberts, Christopher Johnson, Andrew Miller, Zachariah Scruggs, Gabrielle Dillion, Humphrey Petersen-Jones, and Lauren Newhouse. Thanks as well to Mark Badrov and Stephen Klassen for assistance with data analysis.
REFERENCES
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibres. Part II. The frequency of discharge in reflex and voluntary contractions. J Physiol 67: 3–151, 1929. doi: 10.1113/jphysiol.1929.sp002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrov MB, Barak OF, Mijacika T, Shoemaker LN, Borrell LJ, Lojpur M, Drvis I, Dujic Z, Shoemaker JK. Ventilation inhibits sympathetic action potential recruitment even during severe chemoreflex stress. J Neurophysiol 118: 2914–2924, 2017. doi: 10.1152/jn.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrov MB, Lalande S, Olver TD, Suskin N, Shoemaker JK. Effects of aging and coronary artery disease on sympathetic neural recruitment strategies during end-inspiratory and end-expiratory apnea. Am J Physiol Heart Circ Physiol 311: H1040–H1050, 2016a. doi: 10.1152/ajpheart.00334.2016. [DOI] [PubMed] [Google Scholar]
- Badrov MB, Olver TD, Shoemaker JK. Central vs. peripheral determinants of sympathetic neural recruitment: insights from static handgrip exercise and postexercise circulatory occlusion. Am J Physiol Regul Integr Comp Physiol 311: R1013–R1021, 2016b. doi: 10.1152/ajpregu.00360.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrov MB, Usselman CW, Shoemaker JK. Sympathetic neural recruitment strategies: responses to severe chemoreflex and baroreflex stress. Am J Physiol Regul Integr Comp Physiol 309: R160–R168, 2015. doi: 10.1152/ajpregu.00077.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Macleod VH. Evidence for the involvement in the baroreceptor reflex of a descending inhibitory pathway. J Physiol 241: 477–496, 1974. doi: 10.1113/jphysiol.1974.sp010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Phenylephrine-induced elevations in arterial blood pressure are attenuated in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 283: R1221–R1226, 2002. doi: 10.1152/ajpregu.00195.2002. [DOI] [PubMed] [Google Scholar]
- Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci 162: 89–93, 2011. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ. Differential effects of nitrous oxide on baroreflex control of heart rate and peripheral sympathetic nerve activity in humans. Anesthesiology 72: 16–22, 1990. doi: 10.1097/00000542-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Fagius J, Sundlöf G, Wallin BG. Variation of sympathetic reflex latency in man. J Auton Nerv Syst 21: 157–165, 1987. doi: 10.1016/0165-1838(87)90018-X. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG, Sundlöf G, Nerhed C, Englesson S. Sympathetic outflow in man after anaesthesia of the glossopharyngeal and vagus nerves. Brain 108: 423–438, 1985. doi: 10.1093/brain/108.2.423. [DOI] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 48: 1359–1365, 2005. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M, Mancia G. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995. doi: 10.1161/01.CIR.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell’Oro R, Facchini A, Ilardo V, Mancia G. Sympathetic and baroreflex function in hypertensive or heart failure patients with ventricular arrhythmias. J Hypertens 22: 1747–1753, 2004. doi: 10.1097/00004872-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Grossman E, Chang PC, Hoffman A, Tamrat M, Goldstein DS. Evidence for functional alpha 2-adrenoceptors on vascular sympathetic nerve endings in the human forearm. Circ Res 69: 887–897, 1991. doi: 10.1161/01.RES.69.4.887. [DOI] [PubMed] [Google Scholar]
- Heindl S, Holzschneider J, Hinz A, Sayk F, Fehm HL, Dodt C. Acute effects of aldosterone on the autonomic nervous system and the baroreflex function in healthy humans. J Neuroendocrinol 18: 115–121, 2006. doi: 10.1111/j.1365-2826.2005.01392.x. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28: 599–620, 1965. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert EA, Schlaich MP, Dawood T, Sari C, Chopra R, Barton DA, Kaye DM, Elam M, Esler MD, Lambert GW. Single-unit muscle sympathetic nervous activity and its relation to cardiac noradrenaline spillover. J Physiol 589: 2597–2605, 2011. doi: 10.1113/jphysiol.2011.205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Elam M. Why do human postganglionic neurones primarily only fire once during a sympathetic burst? Acta Physiol Scand 177: 247–253, 2003. doi: 10.1046/j.1365-201X.2003.01078.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol 516: 293–301, 1999. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol 481: 799–809, 1994. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Shoemaker JK. Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach. J Neurosci Methods 193: 343–355, 2010. doi: 10.1016/j.jneumeth.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R, Shoemaker JK. Relationship between size and latency of action potentials in human muscle sympathetic nerve activity. J Neurophysiol 105: 2830–2842, 2011a. doi: 10.1152/jn.00814.2010. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Frances MF, Goswami R, Shoemaker JK. Sympathetic neural recruitment patterns during the Valsalva maneuver. Conf Proc IEEE Eng Med Biol Soc 2011: 6951–6954, 2011b. doi: 10.1109/IEMBS.2011.6091757. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Shoemaker JK. Baroreflex mechanisms regulating the occurrence of neural spikes in human muscle sympathetic nerve activity. J Neurophysiol 107: 3409–3416, 2012. doi: 10.1152/jn.00925.2011. [DOI] [PubMed] [Google Scholar]
- Scott D. On optimal and data-based histograms. Biometrika 66: 605–610, 1979. doi: 10.1093/biomet/66.3.605. [DOI] [Google Scholar]
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ Res 24: 109–121, 1969. doi: 10.1161/01.RES.24.1.109. [DOI] [PubMed] [Google Scholar]
- Steinback CD, Breskovic T, Banic I, Dujic Z, Shoemaker JK. Autonomic and cardiovascular responses to chemoreflex stress in apnoea divers. Auton Neurosci Basic Clin 156: 138–143, 2010a. doi: 10.1016/j.autneu.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol 588: 4825–4836, 2010b. doi: 10.1113/jphysiol.2010.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Burke D, Gandevia S. Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol 474: 331–338, 1994. doi: 10.1113/jphysiol.1994.sp020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Joyner MJ, Hart EC, Wallin BG, Karlsson T, Charkoudian N. Blood pressure regulation in humans: calculation of an “error signal” in control of sympathetic nerve activity. Hypertension 55: 264–269, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Hesse C, Charkoudian N, Nicholson WT, Sviggum HP, Moyer TP, Joyner MJ, Eisenach JH. Autonomic cardiovascular control during a novel pharmacologic alternative to ganglionic blockade. Clin Pharmacol Ther 83: 692–701, 2008. doi: 10.1038/sj.clpt.6100326. [DOI] [PubMed] [Google Scholar]