Abstract
Head-down tilt bed rest (HDBR) has been used as a spaceflight analog to study some of the effects of microgravity on human physiology, cognition, and sensorimotor functions. Previous studies have reported declines in balance control and functional mobility after spaceflight and HDBR. In this study we investigated how the brain activation for foot movement changed with HDBR. Eighteen healthy men participated in the current HDBR study. They were in a 6° head-down tilt position continuously for 70 days. Functional MRI scans were acquired to estimate brain activation for foot movement before, during, and after HDBR. Another 11 healthy men who did not undergo HDBR participated as control subjects and were scanned at four time points. In the HDBR subjects, the cerebellum, fusiform gyrus, hippocampus, and middle occipital gyrus exhibited HDBR-related increases in activation for foot tapping, whereas no HDBR-associated activation decreases were found. For the control subjects, activation for foot tapping decreased across sessions in a couple of cerebellar regions, whereas no activation increase with session was found. Furthermore, we observed that less HDBR-related decline in functional mobility and balance control was associated with greater pre-to-post HDBR increases in brain activation for foot movement in several cerebral and cerebellar regions. Our results suggest that more neural control is needed for foot movement as a result of HDBR.
NEW & NOTEWORTHY Long-duration head-down bed rest serves as a spaceflight analog research environment. We show that brain activity in the cerebellum and visual areas during foot movement increases from pre- to post-bed rest and then shows subsequent recovery. Greater increases were seen for individuals who exhibited less decline in functional mobility and balance control, suggestive of adaptive changes in neural control with long-duration bed rest.
Keywords: fMRI, foot motor, head-down bed rest, microgravity analog
INTRODUCTION
Head-down bed rest (HDBR) has been extensively used as a microgravity analog research environment. For example, long-term HDBR is associated with intravascular and extravascular fluid shifts toward the upper body and head (Caprihan et al. 1999; Pavy-Le Traon et al. 2007), as occurs in microgravity (Jennings 1990; Nelson et al. 2014). HDBR also results in axial body unloading, which simulates the reduced somatosensory inputs to the axial body axis that occur in microgravity (Pavy-Le Traon et al. 2007).
Balance control and functional mobility dramatically decline after spaceflight (Cohen et al. 2012; Mulavara et al. 2010; Wood et al. 2015) and 30 days of HDBR (Dupui et al. 1992; Macaulay et al. 2016). The neural bases of such declines are unclear but could be related to alterations in sensory weighting and processing and motor control of the lower limbs. Although the motor cortices are not the only brain regions that are involved in balance control and functional mobility, the cortical areas that are related to foot movement clearly play a crucial role in adaptation to gravity change. In this study, we test whether there are changes in the neural control of foot movement over long-duration HDBR and, if so, whether they associate with concomitant declines in functional mobility and balance.
Previous studies have reported structural cortical change after spaceflight or HDBR. For example, we have recently reported spaceflight-induced gray matter decreases in temporal and frontal cortices as well as gray matter increases in bilateral medial primary somatosensory and motor cortices (Koppelmans et al. 2016), indicating neuroplasticity of central nervous system with alteration in gravity. Roberts et al. (2015) reported increased brain tissue density at the vertex of the brain as well as decreased brain tissue density at the base after HDBR of 42–90 days. The structural change in somatosensory and motor cortices may reflect sensorimotor neuroplasticity associated with adaptation to microgravity and HDBR.
Functional neural change with spaceflight and analog environments also has been documented with various techniques. For example, visual evoked potentials are altered in microgravity, suggesting gravity effects on visual processing (Cheron et al. 2014). A resting-state functional magnetic resonance imaging (MRI) study reported HDBR-induced decrease of intrinsic spontaneous fluctuations in the left thalamus (Liao et al. 2012). Decreased resting-state functional connectivity was also found in the left anterior insula and anterior middle cingulate cortex after 45 days of HDBR (Zhou et al. 2014). Another study in which motor evoked potentials (MEP) were induced in target muscles by application of transcranial magnetic stimulation (TMS) to the motor cortex reported facilitation of MEP response during 0 g parabolic flight, evidencing altered corticospinal excitability with microgravity (Davey et al. 2004). These studies demonstrate that microgravity and its analogs could affect sensorimotor neural functioning in the human brain. Thus it is plausible to hypothesize that HDBR affects the neural control of foot movement.
Studies on the neural control of foot movement during HDBR are scant. A 90-day HDBR study that included just 4 subjects reported increased cortical activation for leg movement post-HDBR compared with pre-HDBR (Roberts et al. 2010), although the brain region was not specified. The sample size of Roberts’s study was relatively small, and the HDBR-associated blood oxygen level-dependent (BOLD) signal change from pre- to post-HDBR varied substantially across subjects. Moreover, with this small sample size it is difficult to determine the functional behavioral consequences of such neural changes. Thus, to better understand the effects of HDBR on the neural control of foot movement, studies with larger sample sizes and neuroimaging protocols that allow more detailed cortical localization are necessary.
In the current study, we used fMRI to investigate HDBR-induced changes in the neural control of foot movement. We evaluated how the brain activation for foot movement changed as a function of 70 days of HDBR. To account for practice effects, we also evaluated a control group of 12 subjects who did not undergo HDBR, but rather went about their daily lives. Because long-term HDBR is associated with reduced somatosensory inputs, loss of bone mass, and reductions in skeletal muscle mass and strength (Bloomfield 1997; Ferrando et al. 1996; Leblanc et al. 1990; Rittweger et al. 2005), all of which are disadvantageous for foot movement, we hypothesized that the brain activation for foot movement would increase with HDBR to compensate for the adverse effects of HDBR, particularly in sensorimotor cortical and subcortical regions such as the cerebellum. In addition, we hypothesized compensatory adaptive changes would be reflected as greater HDBR-related increases in brain activation being associated with less HDBR-related deterioration of functional mobility and balance performance.
METHODS
Participants.
Eighteen healthy male subjects participated in a 70-day, 6° HDBR campaign. These subjects were right-handed and aged 31.1 ± 4.7 yr at the time of admission (range: 25.7–39.8 yr). They were admitted to the University of Texas, Medical Branch (UTMB) Galveston flight analog facility 13–23 days before the start of HDBR and released 14 days after HDBR. During the 70 days of bed rest, the participants remained in the head-down tilt position all the time except for each meal, when they were allowed to prop themselves up on their elbow. Weight-bearing activity beyond the testing and exercise sessions was not permitted. All subjects received financial compensation for their participation. Thirteen of these HDBR subjects participated in an exercise protocol (Koppelmans et al. 2015; Ploutz-Snyder et al. 2014), whereas the other five HDBR subjects did not exercise aside from stretching and physiotherapy. In the current study, all HDBR subjects’ data were analyzed as one group and exercise was not included as a factor because the majority of HDBR subjects exercised.
Another 11 healthy male subjects who did not undergo HDBR participated as controls. These control subjects were recruited by the Human Test Subject facility at NASA Johnson Space Center. Control participants were aged 41.4 ± 10.4 yr at the time of admission (range: 26.2–59.7 yr). All the HDBR and control participants passed an Air Force Class III equivalent physical examination. Both the HDBR and control studies were approved by the Institutional Review Boards of the University of Michigan, the UTMB, and NASA Johnson Space Center. Written informed consent was obtained from all participants.
Foot tapping and sensorimotor tests.
All the HDBR subjects and control subjects performed a series of tasks in the scanner after the acquisition of anatomical images and resting-state fMRI. The tasks remained the same for all subjects across all sessions, in the order of vestibular stimulation, dual tasking, sensorimotor adaptation, spatial working memory, and foot tapping. In the foot tapping task, subjects were instructed to tap their right foot, paced by a visual stimulus at 1 Hz. Subjects alternated between 20 s of foot movement and 20 s of rest within a run of 3 min. Functional MRIs were acquired during right-foot tapping and repeated at 7 sessions for HDBR participants: 14.1 ± 3.8 and 7.9 ± 2.0 days before the start of HDBR; 8.4 ± 1.0, 50.6 ± 0.9, and 66.8 ± 1.8 days after the onset of HDBR; and 6.7 ± 0.8 and 11.4 ± 1.6 days after HDBR. For control participants, the task was repeated 4 times at day 0 and 12.6 ± 9.7, 50.2 ± 12.8, and 84.8 ± 14.0 days afterward.
Subjects in both the HDBR and control groups performed a series of cognitive and sensorimotor tests. The influence of HDBR on performance of these tasks has been previously published from this sample (Koppelmans et al. 2015). In the current study, in addition to foot movement, we were particularly interested in the Functional Mobility Test (FMT) and Sensory Organization Test 5 (SOT-5 and SOT-5M) as potential correlates of brain activity for foot movement. The FMT and SOT-5/SOT-5M were repeated four times for control subjects and twice pre-HDBR and three times post-HDBR for the HDBR subjects.
In the FMT, subjects were directed to walk through an obstacle course as quickly and safely as possible. The course consisted of a series of foam obstacles such as bars, hurdles, and pylons (Koppelmans et al. 2013). The first part (FMT-1) was set up on a hard floor, and the second part (FMT-2) was on a base of medium-density foam for increased postural challenge. The completion times for each part were used as performance measures. FMT was repeated 10 times at each session. The completion times for the first trial of each session were used in this study because they were considered the most sensitive to change.
SOT-5/SOT-5M were used to assess changes in balance control. These tests were administered with the EquiTest System platform (NeuroCom, Clackamas, OR). During SOTs, subjects stood on a sway-referenced force platform and were instructed to maintain a stable upright posture for 20-s trials with their eyes closed, arms folded across the chest, and feet placed shoulder width apart. The platform swayed during the trials proportional to the ankle joint motion exhibited by the subjects, and their eyes were closed to disrupt somatosensory and visual feedback, emphasizing the role of vestibular input for balance control. The center of pressure was obtained from the force platform and filtered to estimate the center of mass (COM). The subject’s sway angle was then calculated from the COM, which was assumed to be above the support surface at ~55% of total height. The anterior-posterior peak-to-peak sway angle was used to calculate a continuous equilibrium score that was scaled relative to 12.5° and normalized on the basis of the percentage of the trial completed (Wood et al. 2012). This computer-generated score was used to quantify equilibrium, with higher scores indicating better balance performance. Each participant completed three trials with the head erect (referred to as SOT-5) and three trials with approximately ±20° head pitch movements at 0.33 Hz paced by an auditory tone (referred to as SOT-5M). For both SOT-5 and SOT-5M, the median score of three trials was used to prevent the influence of outliers.
Image acquisition and processing.
The fMRI scans for HDBR subjects were collected on a 3T Siemens Magnetom Verio MRI scanner, and the fMRI scans for control subjects were collected on a 3T Siemens Magnetom Skyra MRI scanner. A gradient echo T2*-weighted echo-planar imaging sequence was used to collect the fMRI scans with an identical protocol for all the subjects: TR = 3,660 ms, TE = 39 ms, flip angle = 90°, field of view (FOV) = 240 × 240 mm, slice thickness = 4 mm, slice gap = 1 mm, matrix = 94 × 94, voxel size = 2.55 × 2.55 × 5.0 mm, 36 axial slices. A T1-weighted gradient-echo pulse sequence was also collected. For HDBR subjects, we used the following parameters: TR = 1,900 ms, TE = 2.49 ms, flip angle = 9°, FOV = 270 × 270 mm, slice thickness = 0.9 mm, matrix = 288 × 288, voxel size = 0.94 × 0.94 mm, 192 slices, duration = ~4 min. For the control subjects, we used the following parameters: TR = 1,900 ms, TE = 2.32 ms, flip angle = 9°, FOV = 250 × 250 mm, slice thickness = 0.9 mm, 192 slices, matrix = 512 × 512, voxel size = 0.49 × 0.49 mm, 3-dimensional T1 axial overlay, duration = ~4 min.
The functional images were corrected for slice timing. The Artifact Detection Tool (ART; https://ww.nitrc.org/projects/artifact_detect/) was then used to quantify head motion and detect outliers of head motion and global brain activation. The head motion and outliers would be used later as nuisance variables in the first level statistical analyses. Next, the images were normalized to MNI152 common space. In addition to the whole brain normalization, a spatially unbiased atlas template of the cerebellum and brain stem (SUIT; Diedrichsen 2006) was used for cerebellar normalization. Spatial smoothing was then applied with an 8-mm full-width half-maximum 3-dimensional Gaussian kernel. The fMRI runs with large head motion (>3 mm) were omitted from further analyses. Five fMRI runs involving four HDBR subjects were omitted because of excessive head motion. Another four fMRI sessions involving three HDBR subjects were not analyzed because of missing data. For the control subjects, there was one missing session, but no fMRI run contained excessive head motion. Thus a total of nine runs involving six subjects were omitted in estimating HDBR-related brain activation changes. Those omitted or missing sessions constituted 7% of the fMRI data for HDBR subjects and 2% of the fMRI data for the control subjects.
The functional images were analyzed using SPM8. In the first-level analyses, we calculated brain activation for each subject on a voxel-by-voxel basis for foot tapping. In the second-level analyses, flexible factorial (SPM’s mixed model equivalent) analysis was used to determine brain activation changes during HDBR and subsequent recovery post-HDBR across sessions for the HDBR group. For the HDBR subjects, we sought to identify brain regions with two types of HDBR-related activation changes: immediate change and cumulative change (Yuan et al. 2016). The immediate change was hypothesized to be sensitive to HDBR status. It was assumed to arise shortly after the start of HDBR, to stay constant during HDBR, and to restore soon after the finish of HDBR. The cumulative change was hypothesized to grow gradually with the days in HDBR, peaking at the end of HDBR, and to recover progressively after HDBR. We set corresponding contrast vectors to test the hypothesized HDBR-related changes in brain activation across sessions. For the control subjects, we estimated linear increases and decreases in brain activation across sessions to inspect practice or other time-varying effects. For both groups, the first measurement session was regarded as a practice session, and thus the fMRI data in the first session were dropped. The second session then served as baseline. In the second-level analyses for HDBR subjects, we also computed the brain activation change from baseline to the end of HDBR (66.8 ± 1.8 days in HDBR), which we then entered into analyses examining brain and behavior change associations. For these correlation analyses between pre- and post-HDBR changes in performance and activation, we used fMRI data from both sessions 2 and 5. In these analyses we had to drop the 5 HDBR subjects who had missing data or excessive head motion in either session 2 or session 5. As a result, 13 HDBR subjects were included in the correlation analyses. For all the fMRI analyses, the alpha level was set at 0.001 (uncorrected for multiple comparisons), and the extent threshold was 10 voxels.
RESULTS
HDBR-associated declines in FMT and SOT-5/SOT-5M have been reported in our previous publication in the same sample (Koppelmans et al. 2015). Functional mobility and standing balance performance exhibited statistically significant decline with HDBR, compared with the pre-HDBR baseline level, consistent with findings from previous studies (Mulder et al. 2014; Reschke et al. 2009).
Baseline activation from the foot tapping task is presented in Fig. 1. A few brain regions exhibited significant HDBR-related immediate increases or cumulative increases in brain activation during foot tapping (Fig. 2A and Table 1). These activation increases subsequently recovered post-HDBR (Fig. 2B). However, no regions showed significant immediate or cumulative activation decreases.
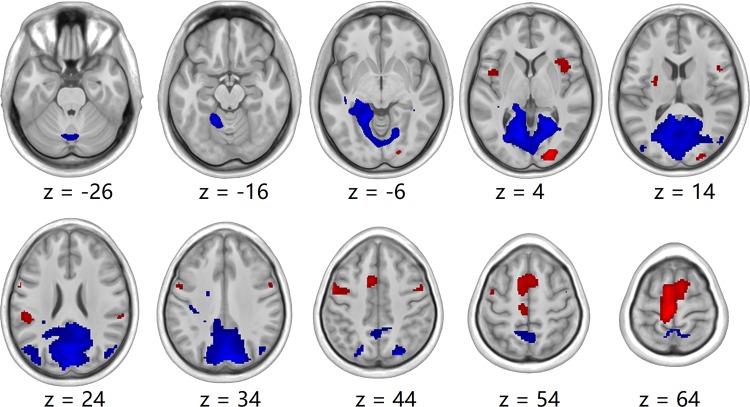
Fig. 1.
Group-level activation (red) and deactivation (blue) foot tapping maps at baseline. P < 0.001, uncorrected.
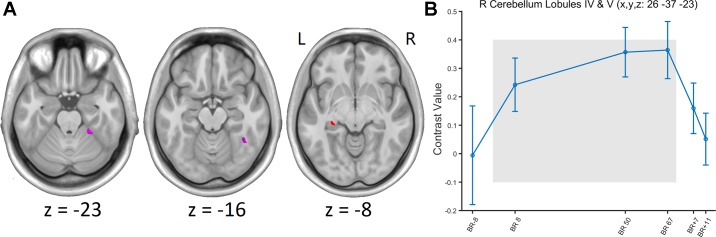
Fig. 2.
A: group t-statistic maps for the regions showing head-down bed rest (HDBR)-related changes in activation for foot tapping, thresholded at absolute value of the test statistic: abs(t) > 3.18 (P < 0.001, uncorrected). Red, HDBR-related cumulative increases; violet, HDBR-related immediate increases; L, left; R, right. B: example of HDBR-related activation increase and post-HDBR activation recovery at a voxel. The gray area indicates the period of HDBR.
Table 1.
Regions showing head-down bed rest-related increases in activation for foot movement
| Region | k | Peak t | Peak P | x, y, z, mm | ||
|---|---|---|---|---|---|---|
| Immediate increase | ||||||
| R cerebellum lobules IV and V | 127 | 4.428 | 1E-05 | 26 | −37 | −23 |
| R fusiform gyrus | 126 | 3.577 | 3E-04 | 40 | −50 | −16 |
| Cumulative increase | ||||||
| L hippocampus | 77 | 3.337 | 6E-04 | −27 | −29 | −8 |
| R middle occipital gyrus | 18 | 3.247 | 8E-04 | 37 | −62 | 6 |
Values are parameters for brain regions with peak t value (t value at the peak voxel). k, Cluster size; peak P, P value at the peak voxel; x, y, z, Montreal Neurological Institute coordinates of the peak voxel; L, left; R, right.
For the control subjects, several cerebellar regions showed decreased activation for foot tapping across sessions, suggestive of practice effects (Fig. 3 and Table 2). No activation increase with session was found for the control subjects.
Fig. 3.
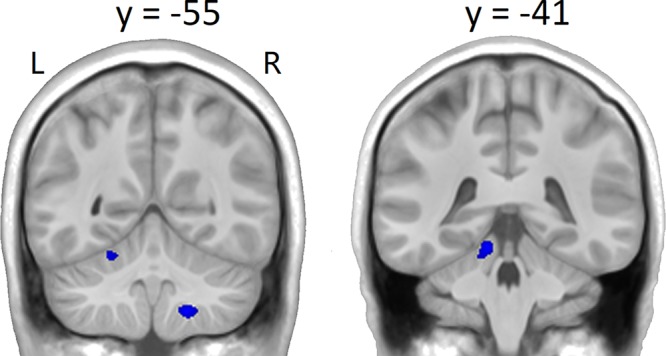
Group t-statistic maps for the regions showing activation decreases (blue) across sessions for foot movement in the control subjects, thresholded at absolute value of the test statistic: abs(t) > 3.41 (P < 0.001, uncorrected).
Table 2.
Regions showing activation decreases across sessions for foot movement in the control subjects
| Region | k | Peak t | Peak P | x, y, z, mm | ||
|---|---|---|---|---|---|---|
| L cerebellum lobules IV and V | 273 | 4.847 | 2E-05 | −10 | −41 | −16 |
| R cerebellum lobule VIII | 269 | 4.658 | 4E-05 | 18 | −55 | −49 |
| L cerebellum lobule VI | 149 | 4.104 | 2E-04 | −21 | −55 | −20 |
| R cerebellum lobule VIII | 20 | 3.650 | 5E-04 | 27 | −46 | −53 |
Values are parameters for brain regions with peak t value (t value at the peak voxel). k, Cluster size; peak P, P value at the peak voxel; x, y, z, Montreal Neurological Institute coordinates of the peak voxel; L, left; R, right.
The pre-to-post-HDBR difference in FMT performance, as defined by the change in FMT-1 completion times from baseline to the first post-HDBR measure, was significantly correlated with brain activation change from baseline to the end of HDBR in a distributed network, including the frontal, parietal, temporal, occipital, cingulate, insula, and cerebellum areas (Fig. 4 and Table 3). The association was in a pattern where less slowing of course completion time was associated with greater activation increases or lower activation decreases in these regions; i.e., less performance decline was related to greater increase or less decrement in brain activation. No opposite correlation was found between changes in FMT-1 completion time and brain activation.
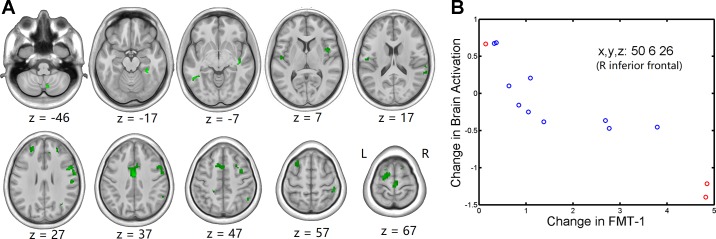
Fig. 4.
A: group t-statistic maps for regions showing negative correlations between pre- and post-head-down bed rest (HDBR) changes in functional mobility test (FMT)-1 completion time and brain activation (green) for foot movement, thresholded at P < 0.001, uncorrected. B: example of relationship between behavioral change and brain activation change. Red circles represent HDBR subjects who did not exercise during HDBR, whereas blue circles represent HDBR subjects who exercised during HDBR.
Table 3.
Regions showing correlations between pre- and post-head-down bed rest changes in FMT-1 completion time and brain activation for foot movement
| Region | k | Peak t | Peak P | x, y, z, mm | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| R precentral/inferior frontal | 375 | 7.331 | 7E-06 | 50 | 6 | 26 |
| R middle frontal | 67 | 6.462 | 2E-05 | 28 | 8 | 42 |
| L middle frontal | 77 | 6.289 | 3E-05 | −22 | 46 | 26 |
| L superior frontal | 27 | 5.852 | 6E-05 | −16 | 10 | 66 |
| R paracentral lobule | 200 | 5.658 | 7E-05 | 2 | −26 | 70 |
| R middle frontal | 55 | 5.605 | 8E-05 | 36 | 0 | 50 |
| L middle frontal | 63 | 5.398 | 1E-04 | −28 | 14 | 52 |
| R supplementary motor area | 54 | 5.390 | 1E-04 | 14 | 8 | 66 |
| L inferior frontal | 102 | 5.051 | 2E-04 | −48 | −10 | 6 |
| R middle frontal | 13 | 4.590 | 4E-04 | 26 | 44 | 28 |
| R inferior frontal | 13 | 4.588 | 4E-04 | 44 | 28 | 22 |
| R middle frontal | 14 | 4.559 | 4E-04 | 38 | 24 | 32 |
| L precentral | 12 | 4.268 | 7E-04 | −38 | −24 | 62 |
| Parietal | ||||||
| R inferior parietal | 193 | 6.018 | 4E-05 | 50 | −54 | 46 |
| R supramarginal | 26 | 5.752 | 6E-05 | 54 | −18 | 28 |
| R inferior parietal | 67 | 5.654 | 7E-05 | 46 | −34 | 54 |
| L postcentral | 16 | 4.545 | 4E-04 | −36 | −36 | 62 |
| Temporal | ||||||
| R superior temporal | 71 | 8.009 | 3E-06 | 40 | −20 | −8 |
| R superior temporal | 24 | 5.606 | 8E-05 | 60 | 2 | 4 |
| L inferior temporal | 174 | 4.972 | 2E-04 | −48 | −51 | −7 |
| R superior temporal | 165 | 4.793 | 3E-04 | 66 | −40 | 18 |
| L superior temporal | 18 | 4.710 | 3E-04 | −48 | −34 | 22 |
| R hippocampus | 10 | 4.227 | 7E-04 | 27 | −23 | −8 |
| Occipital | ||||||
| R fusiform | 234 | 5.641 | 8E-05 | 33 | −38 | −17 |
| R middle occipital | 12 | 4.505 | 4E-04 | 36 | −62 | 30 |
| Cingulate | ||||||
| Middle cingulate | 545 | 7.104 | 1E-05 | 0 | −6 | 36 |
| L anterior cingulate | 28 | 4.715 | 3E-04 | −6 | 18 | 20 |
| R anterior cingulate | 40 | 4.503 | 4E-04 | 6 | 32 | 20 |
| L middle cingulate | 15 | 4.480 | 5E-04 | −20 | −36 | 46 |
| Insula | ||||||
| R insula | 63 | 6.438 | 2E-05 | 38 | 6 | 10 |
| L insula | 16 | 4.971 | 2E-04 | −30 | −20 | 20 |
| Cerebellum | ||||||
| R cerebellum lobule VIII | 178 | 5.327 | 1E-04 | 7 | −69 | −46 |
| L cerebellum lobule IX | 58 | 4.757 | 3E-04 | −15 | −44 | −55 |
Values are parameters for brain regions with peak t value (t value at the peak voxel). k, Cluster size; peak P, P value at the peak voxel; x, y, z, Montreal Neurological Institute coordinates of the peak voxel; L, left; R, right, FMT-1, Functional Mobility Test 1.
The pre-to-post-HDBR change in SOT-5 scores was similarly associated with pre-to-post-HDBR change in brain activation for foot tapping in occipital, temporal, cerebellar, and insular regions (Fig. 5 and Table 4). Less HDBR-related impairment in SOT-5 was related to greater activation increases or lower activation decreases in these areas. Thus, for both FMT-1 and SOT-5, less HDBR-related performance deterioration was associated with greater brain activity increase or less brain activity decrease.
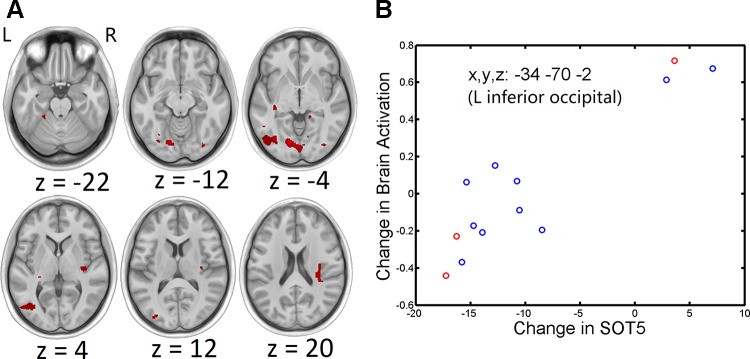
Fig. 5.
A: group t-statistic maps for regions showing positive correlations between pre- and post-head-down bed rest (HDBR) changes in Sensory Organization Test (SOT)-5 score and brain activation (red) for foot movement, thresholded at P < 0.001, uncorrected. B: example of relationship between behavioral change and brain activation change. Red circles represent HDBR subjects who did not exercise during HDBR, whereas blue circles represent HDBR subjects who exercised during HDBR.
Table 4.
Regions showing correlations between pre- and post-head-down bed rest changes in SOT-5 score and brain activation for foot movement
| Region | k | Peak t | Peak P | x, y, z, mm | ||
|---|---|---|---|---|---|---|
| Occipital | ||||||
| L inferior occipital | 1,962 | 7.610 | 5E-06 | −34 | −70 | −2 |
| L lingual | 1,848 | 6.437 | 2E-05 | −14 | −79 | −6 |
| R inferior occipital | 39 | 5.035 | 2E-04 | 33 | −81 | −12 |
| R inferior occipital | 63 | 5.023 | 2E-04 | 35 | −79 | −3 |
| R fusiform | 53 | 4.691 | 3E-04 | 36 | −52 | −16 |
| L middle occipital | 25 | 4.572 | 4E-04 | −30 | −84 | 12 |
| R fusiform | 78 | 4.364 | 6E-04 | 28 | −56 | −9 |
| R lingual | 68 | 4.319 | 6E-04 | 12 | −68 | −1 |
| L fusiform | 43 | 4.266 | 7E-04 | −30 | −75 | −11 |
| L fusiform | 12 | 4.245 | 7E-04 | −30 | −65 | −12 |
| Temporal | ||||||
| L hippocampus | 398 | 6.339 | 3E-05 | −35 | −29 | −7 |
| R parahippocampal | 73 | 4.884 | 2E-04 | 16 | −40 | −5 |
| L inferior temporal | 19 | 4.195 | 7E-04 | −50 | −53 | −4 |
| Insula | ||||||
| R insula | 203 | 6.083 | 4E-05 | 32 | −30 | 20 |
| Cerebellum | ||||||
| L cerebellum lobules IV and V | 94 | 5.159 | 2E-04 | −24 | −38 | −22 |
Values are parameters for brain regions with peak t value (t value at the peak voxel). k, Cluster size; peak P, P value at the peak voxel; x, y, z, Montreal Neurological Institute coordinates of the peak voxel; L, left; R, right, SOT-5, Sensory Organization Test 5.
DISCUSSION
The current study investigated the effects of 70-day HDBR on brain activation for foot movement. Right cerebellar lobules IV and V, the right fusiform gyrus, the left hippocampus, and the right middle occipital gyrus exhibited increased activation for foot tapping during HDBR, which then recovered after HDBR. The observed HDBR-related activation increase indicates that more neural resources are needed for foot movement during HDBR, as we hypothesized. These findings suggest that HDBR might result in reduced neural efficiency for control of foot movement or that there might be more neural responsivity for motor control and somatosensory processing with extended disuse. This concept of neural efficiency has been described, for example, during motor planning in expert vs. novice golfers, with novices exhibiting more activation than experts (Milton et al. 2007). It also has been reported in high vs. low performers under conditions of stress and high task difficulty, where lower performance was associated with more brain activity (Jaeggi et al. 2007).
The HDBR-associated increase in brain activation for foot movement parallels our recent findings of brain activations for dual tasking and for vestibular stimulation during HDBR (Yuan et al. 2016, 2018). In these studies, we found HDBR-related cumulative and immediate increases in brain activations when subjects simultaneously performed a hand motor task and a secondary cognitive counting task; furthermore, we also found HDBR-related cumulative increases in activation in response to vestibular stimulation. These findings all imply that long-term HDBR might reduce neural efficiency, which is recoverable post-HDBR. Such a reduction could be due to adaptation to the HDBR environment. First, when a subject is in a long-term head-down tilt supine position, the extravascular and intravascular fluids are shifted toward the upper body and the head, and the brain concurrently shifts into a new position toward the back of the skull, appearing as gray matter volumetric changes (Koppelmans et al. 2017). This shift with HDBR could lead to reductions in neural efficiency. Second, the HDBR-related changes might result from sensory reweighting during HDBR. The reduced pressure inputs from the soles of the feet, reduced loading at the ankle joints, and enhanced pressure inputs from the back during HDBR all could cause changes in somatosensory representations and then changes in neural efficiency for control of foot movement. Animal studies have documented that visual occlusion leads to synaptic functional changes in both visual and auditory cortices, providing a neural basis for cross-modal sensory reweighting (Carriot et al. 2015; Meng et al. 2015; Petrus et al. 2015). Lowrey et al. (2014) investigated skin sensitivity in astronauts and reported decreased skin sensitivity, or increased perceptual threshold, for vibration on the foot sole following short-duration spaceflight missions. Lowrey et al. (2014) also found increased skin sensitivity in a subset of astronauts who displayed a greater reduction in balance than the nonhypersensitive participants. Strzalkowski et al. (2015) also examined the perception of foot sole stimulation postflight in astronauts and observed increased vibration perception threshold, i.e., decreased sensitivity after flight. The results from both studies imply reweighting of sensory inputs with microgravity. In addition, it has been documented that long-term HDBR is linked to loss of bone mass, reduction of skeletal muscle mass, and decreases in muscle strength (Bloomfield 1997; Ferrando et al. 1996; Leblanc et al. 1990; Rittweger et al. 2005), all of which can also contribute to making foot movement more difficult. Thus greater neural control would be demanded for the control of foot movement during HDBR to compensate for all of these disadvantageous factors related to HDBR. In contrast, for the non-HDBR control subjects, activation decreases across sessions were found in several cerebellar regions, whereas no activation increases with session were found, precluding the possibility that HDBR-associated activation changes were due to practice of the foot tapping task. If there was a practice effect on the brain activation, it should be activation decrease with sessions, as observed in the control subjects. Consequently, the actual magnitude of HDBR-associated brain activation increase would be even greater if practice-induced activation decreases could be accounted for.
The cerebellar anterior lobe (lobules I–V), parts of medial lobule VI, and lobule VIII of the posterior lobe are recognized as serving predominantly sensorimotor functions (Stoodley and Schmahmann 2010). For the control subjects in the current study, practice-related activation decreases were evident within the sensorimotor cerebellar regions, suggesting higher neural efficiency in these brain regions with practice. For HDBR participants, immediate HDBR-related increases were also observed in cerebellar sensorimotor regions (ipsilateral cerebellum lobules IV and V), revealing the sensitivity of sensorimotor cerebellar activation to HDBR. In addition, cumulative HDBR-associated increase was found in the hippocampus, an area that is involved in motor learning (Albouy et al. 2008). Notably, HDBR-related activation increases in the left hippocampus were found not only for foot movement but also for finger tapping in our recent study (Yuan et al. 2016). However, HDBR-associated activation increase in the hippocampus was not evidenced for vestibular stimulation, which was also tested in the same project (Yuan et al. 2018). Thus, although the hippocampus also has been documented to be involved in cardiovascular control (Shoemaker and Goswami 2015), it is not likely the case in the current study. That is, if the activation increase in the hippocampus reflected altered cardiovascular control during HDBR, it should present for the vestibular stimulation, too.
In the current study, greater pre-to-post-HDBR activation increases were associated with less HDBR-related deterioration of functional mobility, as well as less HDBR-related deterioration or greater improvement of standing balance. HDBR causes changes in both brain activation and functional performance, indicative of the important influence HDBR has on neural plasticity that covaries with decrements in functional performance. Subjects who further exploited the brain areas for foot movement by increasing activation were likely to obtain less performance deteriorations with HDBR in functional mobility or balance control. The activation-behavior association was only found for SOT-5 but not for SOT-5M, perhaps because SOT-5M provides a greater challenge to vestibular control of postural equilibrium than SOT-5. These correlations between brain activation increases and less performance decline differed from the opposite associations of brain-behavior changes in our recent studies. We have previously found that subjects with more pre-to-post-HDBR increases in brain activation for dual tasking tended to exhibit greater deteriorations in task performance (Yuan et al. 2016) and that subjects with more pre-to-post-HDBR increases in brain activation for vestibular stimulation were more likely to show greater declines in functional mobility and balance control (Yuan et al. 2018). This discrepancy indicates that the relationship between pre-to-post-HDBR changes in brain activation and behavior depends on the characteristics of the tasks and the neural systems recruited to meet the task demands.
The current study used BOLD signals to estimate brain activation changes across sessions. HDBR affects basic characteristics of the blood flow in the brain, which might affect the BOLD response. HDBR could induce decreases in intravascular volume (Norsk 2005). Although these vascular changes likely differ by area (Pavy-Le Traon et al. 2007), any changes in blood flow in the brain could directly affect the BOLD signal. However, these effects should be present both during foot movement and during rest. Our estimation of brain activation was based on the relative BOLD signal change during task performance compared with that during rest. It is currently not clear how the relative BOLD signal change would be affected by HDBR-induced alterations in blood flow. However, the influence of HDBR-induced change in blood flow on brain activation changes, if any, is not likely to be pronounced. Otherwise, HDBR-related activation changes would be observed in a more widely distributed pattern.
Structural brain changes occurring with HDBR may also impact the BOLD signal. However, the pattern of changes that we observed in this study does not overlap with the structural brain changes that we (Koppelmans et al. 2016) and others (Roberts et al. 2015) have reported to occur with HDBR.
In the current study, the HDBR subjects were all men, because they served as controls for a separate investigator’s study of testosterone supplementation to reduce HDBR effects. This should be noted as a limitation, and generalization of the current study to the broader astronaut population should be done with caution.
In the current study, we observed both immediate and cumulative brain activation increases with HDBR, indicating that HDBR-related change in brain activation for foot movement could occur as soon as 7 days after the onset of HDBR. However, understanding the exact dynamic process will require further studies with more frequent measurement sessions during HDBR. Future HDBR studies with even larger sample sizes and both male and female subjects would make it possible to investigate potential moderators of HDBR-related changes in behavior and brain activation.
Conclusion.
The current study investigated the effects of 70 days of HDBR on brain activation for foot movement. We observed HDBR-associated brain activation increases in cerebellar lobules IV and V, fusiform gyrus, hippocampus, and middle occipital gyrus, suggesting greater neural effort and neural plasticity for the control of foot movement during HDBR. We also observed an association between greater pre-to-post-HDBR activation increases and less HDBR-related deterioration of functional mobility and standing balance, reflecting that neural plasticity and compensatory change in brain activation during HDBR may be helpful in executing functional tasks. We conjecture similar brain activation changes may occur during spaceflight.
GRANTS
This work was supported by National Aeronautics and Space Administration (NASA) Grant NNX11AR02G, the NASA Flight Analogs Project, National Space Biomedical Research Institute Grant NCC 9-58, and National Institutes of Health Grant 1UL1RR029876-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.B., A.M., and R.D.S. conceived and designed research; Y.D.D., N.G., R.R., and I.K. performed experiments; P.Y. and Y.D.D. analyzed data; P.Y., P.R.-L., J.B., A.M., and R.D.S. interpreted results of experiments; P.Y. prepared figures; P.Y. drafted manuscript; P.Y., V.K., P.R.-L., Y.D.D., N.G., R.R., I.K., J.B., A.M., and R.D.S. edited and revised manuscript; P.Y., V.K., P.R.-L., Y.D.D., N.G., R.R., I.K., J.B., A.M., and R.D.S. approved final version of manuscript.
REFERENCES
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, Luxen A, Maquet P. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58: 261–272, 2008. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29: 197–206, 1997. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- Caprihan A, Sanders JA, Cheng HA, Loeppky JA. Effect of head-down tilt on brain water distribution. Eur J Appl Physiol Occup Physiol 79: 367–373, 1999. doi: 10.1007/s004210050522. [DOI] [PubMed] [Google Scholar]
- Carriot J, Jamali M, Cullen KE. Rapid adaptation of multisensory integration in vestibular pathways. Front Syst Neurosci 9: 59, 2015. doi: 10.3389/fnsys.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Leroy A, Palmero-Soler E, De Saedeleer C, Bengoetxea A, Cebolla AM, Vidal M, Dan B, Berthoz A, McIntyre J. Gravity influences top-down signals in visual processing. PLoS One 9: e82371, 2014. doi: 10.1371/journal.pone.0082371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Kimball KT, Mulavara AP, Bloomberg JJ, Paloski WH. Posturography and locomotor tests of dynamic balance after long-duration spaceflight. J Vestib Res 22: 191–196, 2012. doi: 10.3233/VES-2012-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey NJ, Rawlinson SR, Nowicky AV, McGregor AH, Dubois K, Strutton PH, Schroter RC. Human corticospinal excitability in microgravity and hypergravity during parabolic flight. Aviat Space Environ Med 75: 359–363, 2004. [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33: 127–138, 2006. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Dupui P, Montoya R, Costes-Salon MC, Séverac A, Güell A. Balance and gait analysis after 30 days -6 degrees bed rest: influence of lower-body negative-pressure sessions. Aviat Space Environ Med 63: 1004–1010, 1992. [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Etienne A, Ozdoba C, Perrig WJ, Nirkko AC. On how high performers keep cool brains in situations of cognitive overload. Cogn Affect Behav Neurosci 7: 75–89, 2007. doi: 10.3758/CABN.7.2.75. [DOI] [PubMed] [Google Scholar]
- Jennings T. Space adaptation syndrome is caused by elevated intracranial pressure. Med Hypotheses 32: 289–291, 1990. doi: 10.1016/0306-9877(90)90108-Q. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, Bloomberg JJ, De Dios YE, Wood SJ, Reuter-Lorenz PA, Kofman IS, Riascos R, Mulavara AP, Seidler RD. Brain plasticity and sensorimotor deterioration as a function of 70 days head down tilt bed rest. PLoS One 12: e0182236, 2017. doi: 10.1371/journal.pone.0182236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD.. Brain structural plasticity with spaceflight. NPJ Microgravity 2: 2, 2016. doi: 10.1038/s41526-016-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Erdeniz B, De Dios YE, Wood SJ, Reuter-Lorenz PA, Kofman I, Bloomberg JJ, Mulavara AP, Seidler RD. Study protocol to examine the effects of spaceflight and a spaceflight analog on neurocognitive performance: extent, longevity, and neural bases. BMC Neurol 13: 205, 2013. doi: 10.1186/1471-2377-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Mulavara AP, Yuan P, Cassady KE, Cooke KA, Wood SJ, Reuter-Lorenz PA, De Dios YE, Stepanyan V, Szecsy DL, Gadd NE, Kofman I, Scott JM, Downs ME, Bloomberg JJ, Ploutz-Snyder L, Seidler RD. Exercise as potential countermeasure for the effects of 70 days of bed rest on cognitive and sensorimotor performance. Front Syst Neurosci 9: 121, 2015. doi: 10.3389/fnsys.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5: 843–850, 1990. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- Liao Y, Zhang J, Huang Z, Xi Y, Zhang Q, Zhu T, Liu X. Altered baseline brain activity with 72 h of simulated microgravity–initial evidence from resting-state fMRI. PLoS One 7: e52558, 2012. doi: 10.1371/journal.pone.0052558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey CR, Perry SD, Strzalkowski ND, Williams DR, Wood SJ, Bent LR. Selective skin sensitivity changes and sensory reweighting following short-duration space flight. J Appl Physiol (1985) 116: 683–692, 2014. doi: 10.1152/japplphysiol.01200.2013. [DOI] [PubMed] [Google Scholar]
- Macaulay TR, Macias BR, Lee SM, Boda WL, Watenpaugh DE, Hargens AR.. Treadmill exercise within lower-body negative pressure attenuates simulated spaceflight-induced reductions of balance abilities in men but not women. NPJ Microgravity 2: 16022, 2016. doi: 10.1038/npjmgrav.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Kao JP, Lee HK, Kanold PO. Visual deprivation causes refinement of intracortical circuits in the auditory cortex. Cell Reports 12: 955–964, 2015. doi: 10.1016/j.celrep.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton J, Solodkin A, Hlustík P, Small SL. The mind of expert motor performance is cool and focused. Neuroimage 35: 804–813, 2007. doi: 10.1016/j.neuroimage.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Mulavara AP, Feiveson AH, Fiedler J, Cohen H, Peters BT, Miller C, Brady R, Bloomberg JJ. Locomotor function after long-duration space flight: effects and motor learning during recovery. Exp Brain Res 202: 649–659, 2010. doi: 10.1007/s00221-010-2171-0. [DOI] [PubMed] [Google Scholar]
- Mulder E, Linnarsson D, Paloski WH, Rittweger J, Wuyts FL, Zange J, Clément G. Effects of five days of bed rest with and without exercise countermeasure on postural stability and gait. J Musculoskelet Neuronal Interact 14: 359–366, 2014. [PubMed] [Google Scholar]
- Nelson ES, Mulugeta L, Myers JG. Microgravity-induced fluid shift and ophthalmic changes. Life (Basel) 4: 621–665, 2014. doi: 10.3390/life4040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsk P. Cardiovascular and fluid volume control in humans in space. Curr Pharm Biotechnol 6: 325–330, 2005. doi: 10.2174/1389201054553734. [DOI] [PubMed] [Google Scholar]
- Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986-2006). Eur J Appl Physiol 101: 143–194, 2007. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- Petrus E, Rodriguez G, Patterson R, Connor B, Kanold PO, Lee HK. Vision loss shifts the balance of feedforward and intracortical circuits in opposite directions in mouse primary auditory and visual cortices. J Neurosci 35: 8790–8801, 2015. doi: 10.1523/JNEUROSCI.4975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Downs M, Ryder J, Hackney K, Scott J, Buxton R, Goetchius E, Crowell B. Integrated resistance and aerobic exercise protects fitness during bed rest. Med Sci Sports Exerc 46: 358–368, 2014. doi: 10.1249/MSS.0b013e3182a62f85. [DOI] [PubMed] [Google Scholar]
- Reschke MF, Bloomberg JJ, Paloski WH, Mulavara AP, Feiveson AH, Harm DL. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviat Space Environ Med 80, Suppl: A45–A54, 2009. doi: 10.3357/ASEM.BR06.2009. [DOI] [PubMed] [Google Scholar]
- Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36: 1019–1029, 2005. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Ramsey D, Johnson K, Kola J, Ricci R, Hicks C, Borckardt JJ, Bloomberg JJ, Epstein C, George MS. Cerebral cortex plasticity after 90 days of bed rest: data from TMS and fMRI. Aviat Space Environ Med 81: 30–40, 2010. doi: 10.3357/ASEM.2532.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Zhu X, Tabesh A, Duffy EW, Ramsey DA, Brown TR. Structural brain changes following long-term 6° head-down tilt bed rest as an analog for spaceflight. AJNR Am J Neuroradiol 36: 2048–2054, 2015. doi: 10.3174/ajnr.A4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Goswami R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front Physiol 6: 240, 2015. doi: 10.3389/fphys.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46: 831–844, 2010. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalkowski ND, Lowrey CR, Perry SD, Williams DR, Wood SJ, Bent LR. Selective weighting of cutaneous receptor feedback and associated balance impairments following short duration space flight. Neurosci Lett 592: 94–98, 2015. doi: 10.1016/j.neulet.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Paloski WH, Clark JB. Assessing sensorimotor function following ISS with computerized dynamic posturography. Aerosp Med Hum Perform 86, Suppl: 45–53, 2015. doi: 10.3357/AMHP.EC07.2015. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Reschke MF, Owen Black F. Continuous equilibrium scores: factoring in the time before a fall. Gait Posture 36: 487–489, 2012. doi: 10.1016/j.gaitpost.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Yuan P, Koppelmans V, Reuter-Lorenz PA, De Dios YE, Gadd NE, Wood SJ, Riascos R, Kofman IS, Bloomberg JJ, Mulavara AP, Seidler RD. Increased Brain Activation for Dual Tasking with 70-Days Head-Down Bed Rest. Front Syst Neurosci 10: 71, 2016. doi: 10.3389/fnsys.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Koppelmans V, Reuter-Lorenz PA, De Dios YE, Gadd NE, Wood SJ, Riascos R, Kofman IS, Bloomberg JJ, Mulavara AP, Seidler RD.. Vestibular brain changes with 70 days head down bed rest. Hum Brain Mapp. In press. doi: 10.1002/hbm.24037. [DOI] [PMC free article] [PubMed]
- Zhou Y, Wang Y, Rao LL, Liang ZY, Chen XP, Zheng D, Tan C, Tian ZQ, Wang CH, Bai YQ. Disrupted resting-state functional architecture of the brain after 45-day simulated microgravity. Front Behav Neurosci 8: 200, 2014. doi: 10.3389/fnbeh.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]