Abstract
Although systemic inflammation induced by even a low dose of lipopolysaccharide (LPS, 100 μg/kg) impairs respiratory motor plasticity, little is known concerning cellular mechanisms giving rise to this inhibition. Phrenic motor facilitation (pMF) is a form of respiratory motor plasticity elicited by pharmacological agents applied to the cervical spinal cord, or by acute intermittent hypoxia (AIH; 3, 5-min hypoxic episodes); when elicited by AIH, pMF is known as phrenic long-term facilitation (pLTF). AIH consisting of moderate hypoxic episodes (mAIH, arterial Po2 = 35–55 mmHg) elicits pLTF via the Q pathway to pMF, a mechanism that requires spinal serotonin (5HT2) receptor activation and new brain-derived neurotrophic factor (BDNF) protein synthesis. Although mild systemic inflammation attenuates mAIH-induced pLTF via spinal p38 MAP kinase activation, little is known concerning how p38 MAP kinase activity inhibits the Q pathway. Here, we confirmed that 24 h after a low LPS dose (100 μg/kg ip), mAIH-induced pLTF is greatly attenuated. Similarly, pMF elicited by intrathecal cervical injections of 5HT2A (DOI; 100 μM; 3 × 6 μl) or 5HT2B receptor agonists (BW723C86; 100 μM; 3 × 6 μl) is blocked 24 h post-LPS. When pMF was elicited by intrathecal BDNF (100 ng, 12 μl), pMF was actually enhanced 24 h post-LPS. Thus 5HT2A/2B receptor-induced pMF is impaired downstream from 5HT2 receptor activation, but upstream from BDNF/TrkB signaling. Mechanisms whereby LPS augments BDNF-induced pMF are not yet known.
NEW & NOTEWORTHY These experiments give novel insights concerning mechanisms whereby systemic inflammation undermines serotonin-dependent, spinal respiratory motor plasticity, yet enhances brain-derived neurotrophic factor (BDNF)/TrkB signaling in phrenic motor neurons. These insights may guide development of new strategies to elicit functional recovery of breathing capacity in patients with respiratory impairment by reducing (or bypassing) the impact of systemic inflammation characteristic of clinical disorders that compromise breathing.
Keywords: 5HT2A, 5HT2B, hypoxia, intermittent, long-term facilitation, motor neuron, phrenic, respiratory plasticity, serotonin
INTRODUCTION
A hallmark feature of neural systems is the ability to express plasticity, including the neural system controlling breathing (Abel et al. 2013; Allen and Barres 2005; Andero et al. 2014; Dale-Nagle et al. 2010; Feldman et al. 2003; Mitchell and Johnson 2003; Xing et al. 2013). Plasticity allows neural systems to adapt to diverse challenges throughout life, including a range of clinical disorders often associated with systemic inflammation.
Even mild inflammation impairs hippocampal synaptic plasticity and spinal respiratory motor plasticity induced by moderate acute intermittent hypoxia (Czerniawski et al. 2015; Hocker et al. 2017; Huxtable et al. 2011; Huxtable et al. 2013). Impaired respiratory motor plasticity may reduce the capacity to compensate for challenges such as injury or the onset of disease (Huxtable et al. 2011; Huxtable et al. 2013; Mitchell and Johnson 2003). Despite its potential importance, we know little concerning cellular mechanisms that undermine respiratory motor plasticity. Here, we investigate the impact of inflammation on key steps in the cellular cascade leading to a form of long-lasting phrenic motor facilitation (pMF).
Distinct mechanisms of pMF can be elicited by intrathecal drug administration or by acute intermittent hypoxia (AIH); when pMF is elicited by AIH, it is referred to as phrenic long-term facilitation (pLTF) (Dale-Nagle et al. 2010). When pLTF is elicited by moderate AIH [mAIH, arterial Po2 () = 35–55 mmHg], it is attenuated or even blocked by mild systemic inflammation (Huxtable et al. 2011; Huxtable et al. 2013). Although inflammation impairs mAIH-induced pLTF by a mechanism that requires spinal p38 MAP kinase activation (Huxtable et al. 2015), we know little else concerning mechanisms of this impairment.
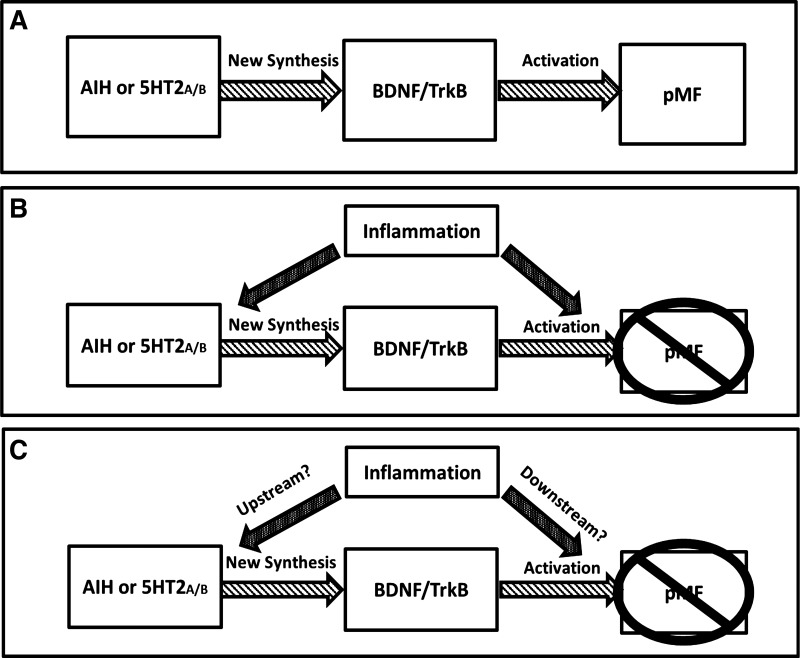
In our current working model of mAIH-induced pLTF, AIH activates raphe serotonergic neurons, triggering episodic serotonin release within the phrenic motor nucleus, thus activating Gq-protein coupled metabotropic serotonin receptors, particularly the 5HT2A/2B receptors (Baker-Herman and Mitchell 2002; MacFarlane et al. 2011). New synthesis of brain-derived neurotrophic factor (BDNF) (Baker-Herman et al. 2004) and TrkB signaling within phrenic motor neurons (Dale et al. 2017) play a central role in the cellular cascade downstream from serotonin receptor activation. This pathway is termed the Q pathway to pMF since it is initiated by Gq protein-coupled metabotropic receptors (Dale-Nagle et al. 2010). Our primary goal was to understand molecular targets of inflammation in the Q pathway to pMF. We addressed two specific questions: 1) does mild inflammation impair Q pathway-induced pMF downstream from 5HT2A/2B activation? and 2) does inflammation impair pMF upstream or downstream from BDNF/TrkB signaling? (Fig. 1).
Fig. 1.
Possible mechanisms whereby inflammation abolishes the Q pathway to phrenic motor facilitation (pMF). A: simple depiction of the serotonin-dependent Q pathway to pMF. Serotonin2A/B receptors activated by acute intermittent hypoxia (AIH) or intrathecal agonist injections cause new brain-derived neurotrophic factor (BDNF) synthesis and BDNF/TrkB signaling leading to pMF. B: pMF elicited by serotonin-dependent receptor activation is abolished or attenuated by systemic inflammation through unknown mechanisms; the goal of this study is to reveal steps in this cellular cascade that are undermined by mild systemic inflammation. C: possible mechanisms whereby inflammation impairs the Q pathway to pMF. Inflammation may affect pMF by inhibiting mechanisms upstream and/or downstream: either 1) 5HT2A/B receptor activation or function (upstream mechanisms); and/or 2) BDNF/TrkB signaling (downstream mechanisms).
To confirm the impact of mild systemic inflammation on mAIH-induced pLTF, we elicited inflammation with a low dose of lipopolysaccharide (LPS; 100 μg/kg ip) 24 h before an assessment of pLTF (Huxtable et al. 2013). LPS is a Toll-like receptor 4 agonist (Lu et al. 2008; Triantafilou and Triantafilou 2002) frequently used to elicit systemic inflammation and secondary neuroinflammation (Huxtable et al. 2011; Lu et al. 2008; Min et al. 2009; Triantafilou and Triantafilou 2002; Vinit et al. 2011).
After confirming that mild inflammation from low-dose LPS attenuates mAIH-induced pLTF, we addressed the question of whether this impairment is caused by failure to activate serotonin receptors by investigating the impact of LPS on pMF elicited by intrathecal injections of 5HT2A (DOI) or 5HT2B (BW723C86) receptor agonists (MacFarlane et al. 2011). To address the question of whether LPS impairment of the Q pathway to pMF is upstream vs. downstream from BDNF/TrkB signaling, we investigated the impact of LPS on pMF elicited by intrathecal BDNF injections (Baker-Herman et al. 2004). Collectively, our results demonstrate that LPS undermines the Q pathway to pMF downstream from 5HT2A/B receptor activation, but upstream of BDNF/TrkB signaling. Surprisingly, LPS increased the amplitude of BDNF-induced pMF through an unknown mechanism. We suggest that mild inflammation from low-dose LPS impairs the Q pathway to pMF by interfering with new BDNF synthesis.
MATERIALS AND METHODS
Animals.
All experiments were done using adult male Sprague-Dawley rats (3–4 mo of age; Harlan, Colony 211). Rats were housed in a controlled environment (12:12-h light/dark cycle, humidity and temperature monitoring), with food and water ad libitum. The University of Wisconsin Animal Care and Use Committee approved all experimental protocols.
Lipopolysaccharide (LPS).
Systemic inflammation was elicited by intraperitoneal (ip) injections of LPS (E. coli 0111: B4; 100 μg/kg); control rats were injected with vehicle (sterile saline). Injections were done 24 h before neurophysiology experiments (Huxtable et al. 2013). Rats were anesthetized with isoflurane before LPS injections. Injections were done in the lower left quadrant of the abdomen to avoid organ damage.
Experimental series.
The first experimental series confirmed previous findings that LPS attenuates moderate AIH-induced pLTF. This series consisted of four treatments: 1) saline (vehicle) + mAIH (n = 9); 2) LPS + mAIH (n = 8); 3) saline without hypoxia (time controls, n = 4); and 4) LPS without hypoxia (time controls, n = 4).
In a second series, we tested the hypothesis that intrathecal 5HT2A receptor agonist (DOI)-induced pMF is abolished 24 h post-LPS. This series consisted of four treatments: 1) saline + DOI (n = 7); 2) LPS + DOI (n = 7); 3) saline + 20% DMSO/80% aCSF (DOI vehicle) (n = 3); and 4) LPS + 20% DMSO/80% aCSF (n = 4).
In a third experimental series, we tested the hypothesis that intrathecal 5HT2B receptor agonist (BW723C86)-induced pMF is abolished 24 h post-LPS. This series consisted of four treatments: 1) saline + BW723C86 (n = 6); 2) LPS + BW723C86 (n = 6); 3) saline + 20% DMSO/80% aCSF (BW723C86 vehicle) (n = 3); and 4) LPS + 20% DMSO/80% aCSF (n = 4).
In a fourth series, we tested the hypothesis that intrathecal BDNF-induced pMF is abolished by LPS. This series consisted of four treatments: 1) saline + BDNF (n = 11); 2) LPS + BDNF (n = 6); 3) saline + aCSF + 0.1% BSA (BDNF vehicle) (n = 3); and 4) LPS + aCSF + 0.1% BSA (n = 3).
Experimental preparation.
Rats were initially anesthetized with isoflurane in a chamber, and then transferred to a temperature-regulated table where isoflurane was continued via nose cone throughout surgical preparations (3.5% isoflurane in 50% O2). Body temperature was monitored with a rectal probe and maintained between 36 and 38°C. A catheter was placed in a tail vein (24 gauge × 3/4 in., Surflo) or femoral vein (polyethylene PE-50, Intramedic), and intravenous infusion was administered continuously (0.5–3 ml·kg−1·h−1). The infusion solution consisted of a 1:2:0.13 solution of HesPan (6% Hetastarch in 0.9% sodium chloride), lactated Ringer, and sodium bicarbonate (8.4% sodium bicarbonate solution) to maintain fluid and acid-base balance. A tracheotomy was performed to enable artificial ventilation (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA; tidal volume 2.5 ml). Lungs were hyperinflated by 2 breaths every ~2 h, before baseline conditions were established to prevent alveolar collapse.
End-tidal Pco2 was monitored with a flow-through CO2 analyzer (Capnogard, Novametrix, Wallingford, CT), and end-tidal Pco2 was maintained constant between 40 and 45 mmHg. Bilateral vagotomy prevented entrainment of respiratory neural activity with the ventilator. A catheter in the right femoral artery (polyethylene catheter PE-50, Intramedic) was placed to monitor blood pressure and collection of arterial blood samples (0.2–0.4 ml) for blood gas and acid base analysis (ABL-800 Flex, Radiometer; Westlake, OH). A pressure transducer (Gould, P23ID) was used to monitor blood pressure.
Using a dorsal approach, the left phrenic nerve was isolated, cut distally, desheathed, and covered with a cotton ball soaked with saline until protocols began. At cervical level 2 (C2) a laminectomy was performed to all rats except those exposed to mAIH. To deliver drugs near the phrenic motor nucleus, a small incision was made in the dura, and a soft silicone catheter (2 Fr; Access Technologies, Skokie, IL) was inserted caudally 4–5 mm until the tip rested over the C4 segment. The catheter was attached to a 50-μl Hamilton syringe filled with drug or vehicle solutions and temperature and light isolated.
Once surgery was complete, the rats were converted to urethane anesthesia (1.85 g/kg) over a 15–20 min period. Anesthetic depth was assessed by lack of blood pressure and phrenic nerve response to toe pinch with a hemostat. After conversion to urethane anesthesia, a minimum of 1 h was allowed before commencing experimental protocols. Rats were euthanized via urethane overdose after the neurophysiological protocol was complete.
Neurophysiology protocol.
Pancuronium bromide (2.5 mg/kg iv) was used to paralyze the rats. Mineral oil was added to the phrenic nerve cavity and the desheathed left phrenic nerve was placed on bipolar silver electrodes to allow nerve recordings. Phrenic nerve signals were amplified (10,000×), band-pass filtered (100–10,000 Hz, model 1800, A-M Systems, Carlsborg, WA), rectified, and integrated (Paynter filter, time constant 50 ms, MA-821, CWE, Ardmore, PA). Integrated phrenic nerve bursts were digitized (8 kHz) and analyzed using WINDAQ data acquisition systems (DATAQ Instruments, Akron, OH).
For all protocols apneic threshold was determined by lowering end-tidal Pco2 until phrenic nerve activity ceased for ~1 min. The recruitment threshold was determined by slowly increasing end-tidal CO2 until nerve activity resumed. End-tidal Pco2 was raised 2 mmHg above the recruitment threshold and ~15–20 min were allowed to establish a stable baseline neural activity. During protocols, arterial blood samples were drawn and blood gases measured during baseline, the first hypoxic episode, and at 15, 30, 60, and 90 min posttreatment. Arterial Pco2 () was maintained ± 2 mmHg of baseline levels by adjusting inspired CO2 or ventilator rate.
Acute intermittent hypoxia.
Moderate acute intermittent hypoxia (mAIH) was delivered as three 5-min episodes of isocapnic (±2 mmHg) hypoxia. Episodes were separated by 5-min intervals of baseline oxygen levels [~55% inspired O2; >150 mmHg]. During hypoxic episodes, was between 35 and 45 mmHg as verified by blood gas analysis. After the third hypoxic episode, rats were returned to baseline inspired O2 levels and maintained for the duration of the experiment.
pMF elicited by intrathecal DOI or BW723C86.
Serotonin-2 receptor activation has significant effects on the respiratory network, and 5HT2A/B agonists are frequently used in studies of this nature (Günther et al. 2006; Niebert et al. 2011) For both drugs, doses had been previously established (concentration and injection volume) that do not affect XII nerve activity or phrenic burst frequency in this experimental preparation, suggesting the drugs remain localized in the cervical spinal cord (MacFarlane et al. 2011).
2,5-Dimethoxy-4-iodoamphetamine (DOI, 5-HT2A agonist) and BW723C86 (5-HT2B receptor agonist) were acquired from Sigma-Aldrich (St. Louis, MO). Both drugs were diluted in dimethyl sulfoxide (DMSO) to make stock solutions (50 mM in 100% DMSO) which were stored at −20°C until use; on the day of experiments, stock solution aliquots (2 μl each aliquot) were diluted with artificial cerebral spinal fluid (aCSF) and DMSO to the desired concentration. The compounds were diluted to 100 μM DOI (198 μl DMSO and 800 μl aCSF; 6 μl × 3 each 5 min) and 100 μM BW723C86 (198 μl DMSO and 800 μl aCSF; 6 μl × 3 each 5 min) (MacFarlane et al. 2011). This drug delivery paradigm mimics the mAIH protocol previously described to elicit pLTF (Bach and Mitchell 1996, Baker-Herman et al. 2004; Devinney MJ et al. 2013; MacFarlane et al. 2011).
Control groups received aCSF (in mM: 120 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 23 NaHCO3, 10 glucose bubbled with 95% O2-5% CO2) and DMSO. aCSF (800 μl) and DMSO (200 μl) was prepared, and a small volume of this solution was delivered intrathecally (6 μl × 3 each 5 min) to mimic experimental drug delivery.
Brain-derived neurotrophic factor.
Brain derived neurotrophic factor (BDNF) was acquired from Promega (Madison, WI). On arrival the protein was diluted with double distilled water (50 µl to create a stock solution), divided into 4-µl aliquots, and stored at −20°C. Stock solutions were not used if stored more than 1 mo. The day of use, stock solutions containing 100 ng of BDNF were diluted in 44 µl of aCSF with 0.1% BSA; rats received a single 12-µl intrathecal injection of this BDNF solution or vehicle (aCSF + 0.1% BSA). This drug delivery paradigm mimics BDNF release after mAIH protocol (Baker-Herman et al. 2004).
Time controls.
For assessment of time-dependent variations in phrenic nerve activity, time control experiments were performed for all experimental series. For group 1, rats received either saline or LPS intraperitoneal injections, without AIH. For groups 2–4, the rats received either saline or LPS intraperitoneal injections and intrathecal drug vehicle.
Statistical analysis.
Normalization of integrated phrenic nerve burst amplitude was done as a percent change from baseline. Respiratory frequency was normalized as an absolute change from baseline (bursts/min). Statistical analysis for the short-term hypoxic phrenic response was done using a two-way repeated-measures ANOVA (Prism 6; GraphPad Software). Statistical significance for baseline vs. 90 min values for the mean arterial pressure (MAP), temperature, pH, and blood gases were done using t-tests (Prism 6). Statistical analysis of time controls was done via two-way repeated-measures ANOVA to detect significant differences between saline and LPS time controls (Prism 6). Since there were no significant differences in phrenic burst amplitude or respiratory frequency at any time between time controls receiving saline or LPS, they were combined into a single time control group.
Statistical comparisons within and between treatment groups for phrenic burst amplitude and frequency (BL, 15, 30, 60, and 90 min) were made with a two-way ANOVA with a repeated-measures design (Prism 6). Phrenic burst amplitude and frequency were analyzed in periods of 60 s near the specified time points (BL, 15, 30, 60, and 90 min). Individual comparisons were made with Tukey’s post hoc test. For all analyses, significance was set at P < 0.05; results are shown as means ± 1 SE.
RESULTS
Moderate acute intermittent hypoxia.
during AIH episodes ranged from 35 to 45 mmHg, replicating our earlier study (Huxtable et al. 2013). was successfully regulated within ~2 mmHg of its baseline value during hypoxic episodes. Mean arterial pressure (MAP) decreased significantly during hypoxic episodes, as is typically observed in this experimental preparation (Huxtable et al. 2013) (Table 1). There were no significant differences between any group or any hypoxic episode for , temperature, or pH.
Table 1.
Arterial Pco2 and Po2, MAP, temperature, and pH during hypoxic episodes
| Experimental Groups | , mmHg | , mmHg | MAP, mmHg | Temp, °C | pH |
|---|---|---|---|---|---|
| Saline + mAIH | |||||
| HX1 | 46.7 ± 1.2 | 37.2 ± 1.6* | 64.0 ± 7.8* | 36.9 ± 0.0 | 7.4 ± 0.0 |
| HX2 | 45.1 ± 1.0 | 36.5 ± 1.5* | 51.0 ± 5.8* | 36.9 ± 0.1 | 7.4 ± 0.0 |
| HX3 | 44.8 ± 1.2 | 37.2 ± 1.0* | 51.8 ± 6.8* | 36.9 ± 0.1 | 7.4 ± 0.0 |
| LPS + mAIH | |||||
| HX1 | 47.1 ± 0.9 | 39.3 ± 3.0* | 106.8 ± 10.4 | 37.3 ± 0.1 | 7.4 ± 0.0 |
| HX2 | 47.1 ± 1.0 | 37.4 ± 1.4* | 92.8 ± 8.1* | 37.3 ± 0.1 | 7.4 ± 0.0 |
| HX3 | 47.2 ± 1.0 | 38.4 ± 1.5* | 84.6 ± 6.3* | 37.2 ± 0.1 | 7.4 ± 0.0 |
| Time control | |||||
| 1 | 47.5 ± 0.7 | 285.0 ± 16.5 | 110.8 ± 6.8 | 37.0 ± 0.1 | 7.4 ± 0.0 |
| 2 | 47.8 ± 0.7 | 281.8 ± 17.7 | 112.1 ± 5.3 | 37.0 ± 0.1 | 7.4 ± 0.0 |
| 3 | 47.4 ± 0.7 | 277.3 ± 16.5 | 112.7 ± 5.8 | 37.2 ± 0.1 | 7.4 ± 0.0 |
All values are expressed as means ± 1 SE. HX1, HX2, and HX3 are the moderate acute intermittent hypoxia (mAIH) episodes (hypoxia 1, hypoxia 2, and hypoxia 3), and the data are collected at each episode for arterial Pco2 (), arterial Po2, (), mean arterial pressure (MAP), temperature, and pH. All experimental groups are pretreated with either saline or LPS, and after 24 h the groups either received mAIH or normoxia (time control).
Significant difference from time control. Differences were considered significant if P ≤ 0.05.
Baseline and 90 min comparisons.
was successfully regulated within ±2 mmHg of its baseline value; was kept >150 mmHg (Table 2). and pH show no significant difference between baseline and 90-min value (Table 2). MAP and temperature show significant changes among groups at baseline and 90 min (Table 2).
Table 2.
Arterial Pco2 and Po2, MAP, temperature, and pH during baseline and 90 min of experimental groups
| Experimental Groups | , mmHg | , mmHg | MAP, mmHg | Temp, °C | pH |
|---|---|---|---|---|---|
| Saline + mAIH | |||||
| Baseline | 45.7 ± 0.8 | 295 ± 5.8 | 104.2 ± 6.9 | 36.9 ± 0.2 | 7.4 ± 0 |
| 90 min | 47.2 ± 1.5 | 270.5 ± 8.9* | 86.0 ± 4.2* | 37.3 ± 0.2* | 7.4 ± 0 |
| LPS + mAIH | |||||
| Baseline | 48.0 ± 0.3 | 318.1 ± 7.3 | 131.6 ± 9.2 | 37.1 ± 0.6 | 7.4 ± 0 |
| 90 min | 45.2 ± 1.3 | 287.3 ± 11.6* | 109.7 ± 5.5* | 37.5 ± 0.5* | 7.4 ± 0 |
| Time Control | |||||
| Baseline | 48.4 ± 0.7 | 283.1 ± 15.4 | 114.0 ± 5.1 | 36.9 ± 0.1 | 7.4 ± 0 |
| 90 min | 46.7 ± 1.2 | 271.0 ± 17.0 | 107.2 ± 5.3 | 37.1 ± 0.1 | 7.4 ± 0 |
| Saline + DOI | |||||
| Baseline | 45.3 ± 0.5 | 296.2 ± 14.7 | 92.5 ± 3.0 | 37.3 ± 0.1 | 7.4 ± 0 |
| 90 min | 45.6 ± 0.6 | 296.2 ± 9.4 | 78.0 ± 5.4* | 37.6 ± 0.1* | 7.4 ± 0 |
| LPS + DOI | |||||
| Baseline | 43.1 ± 1.0 | 299.2 ± 27.1 | 113.3 ± 5.8 | 37.3 ± 0.2 | 7.4 ± 0 |
| 90 min | 41.8 ± 0.7 | 309.4 ± 25.3 | 92.3 ± 6.1* | 37.1 ± 0.1 | 7.4 ± 0 |
| Saline + BW723C86 | |||||
| Baseline | 44.5 ± 0.8 | 298 ± 11.5 | 97.6 ± 5.7 | 37.8 ± 0.1 | 7.4 ± 0 |
| 90 min | 46.5 ± 1.9 | 313.3 ± 16.8 | 79.19 ± 7.7* | 37.8 ± 0.1 | 7.4 ± 0 |
| LPS + BW723C86 | |||||
| Baseline | 45.7 ± 0.4 | 296.6 ± 8.7 | 116.1 ± 5.1 | 37.3 ± 0.2 | 7.4 ± 0 |
| 90 min | 46.2 ± 2.1 | 298.5 ± 8.4 | 95.7 ± 5.9* | 37.3 ± 0.1 | 7.4 ± 0 |
| Time control (aCSF) | |||||
| Baseline | 45.6 ± 0.7 | 326.2 ± 6.3 | 108.9 ± 5.8 | 38.3 ± 1.1 | 7.4 ± 0 |
| 90 min | 44.9 ± 1.3 | 322.5 ± 8.7 | 92.3 ± 5.4* | 38.2 ± 0.9 | 7.4 ± 0 |
All values are expressed as means ± 1 SE. Animals were pretreated with either LPS (100 µg/kg) or saline and were either treated with moderate acute intermittent hypoxia (mAIH), normoxia (time control), 5HT2A agonist DOI, 5HT2b agonist BW723C86, or aCSF (DOI, BW723C86 vehicles)
Significant difference from baseline value; t-test was run to determine significance between baseline and 90 min. Differences were considered significant if P ≤ 0.05.
Progressive augmentation of the short-term hypoxic phrenic response.
Progressive augmentation is an increase in the magnitude of the hypoxic phrenic response with successive hypoxic episodes (Powell et al. 1998). There was no evidence for progressive augmentation of the short-term hypoxic phrenic response during successive hypoxic episodes of the mAIH + saline protocol in this study, similar to previous reports (Fig. 2F) (Huxtable et al. 2013).
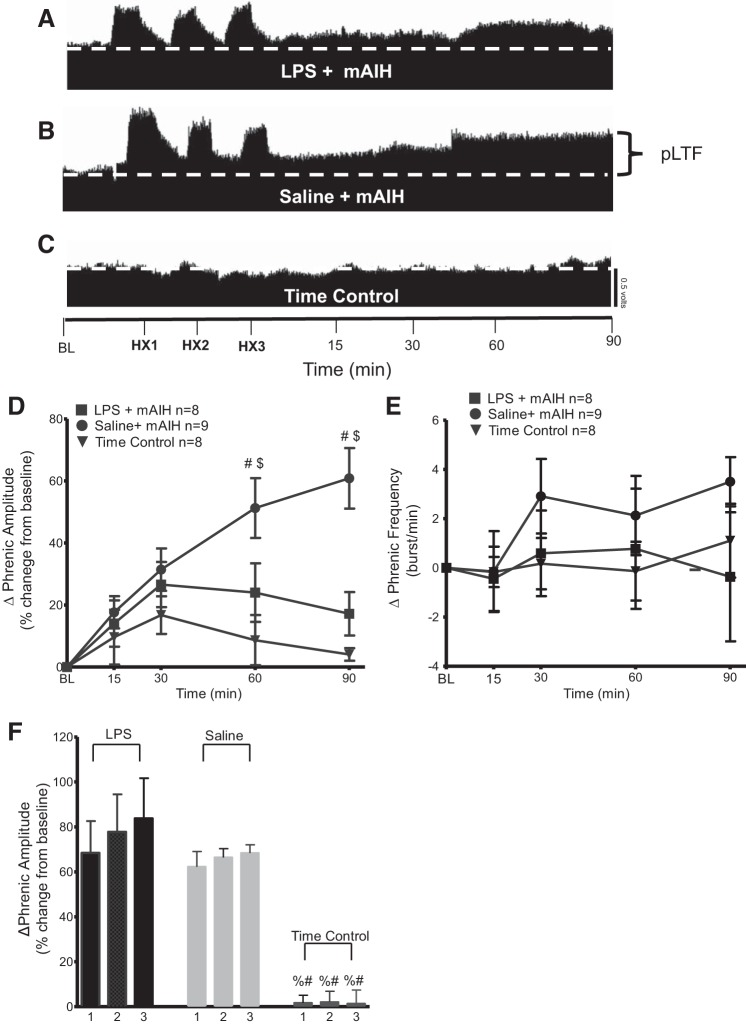
Fig. 2.
Systemic inflammation induced by LPS (100 μg/kg; 24 h postinjection) attenuates phrenic long-term facilitation (pLTF) elicited by moderate acute intermittent hypoxia (mAIH). A–C: representative traces of compressed integrated phrenic neurograms during and following mAIH. A: LPS-injected group was exposed to mAIH, and no longer exhibits pLTF. B: in contrast, the saline-injected group exposed to mAIH exhibits robust pLTF. C: time controls (LPS or saline groups without mAIH exposure) do not show any consistent, time-dependent changes in phrenic burst amplitude. HX1, HX2, and HX3 are hypoxia 1, hypoxia 2, and hypoxia 3, respectively; BL, baseline. D: group data for phrenic burst amplitude (percent change from baseline). LPS + mAIH (n = 8) and saline + mAIH (n = 9) were compared with time controls (n = 8). LPS + mAIH was significantly reduced vs. saline + mAIH at 60 and 90 min. LPS + mAIH rats were not significantly different from time controls at any time, but were significantly different from saline + mAIH at 60 and 90 min. Significance is P ≤ 0.05: #significance vs. time control; $significance vs. LPS. E: group data for phrenic burst frequency (bursts/min). LPS + mAIH (n = 8) and saline + mAIH (n = 9) were compared with time controls (n = 8). There were no significant differences between any group or time point (P > 0.05). F: there is no progressive augmentation of the short-term hypoxic phrenic response during mAIH + saline or mAIH + LPS. The numbers represent hypoxic episodes (1–3). Significance is P ≤ 0.05: %significance vs. saline; #significance vs. LPS.
mAIH-induced pLTF is attenuated by LPS.
In agreement with earlier studies, mAIH-induced pLTF was greatly attenuated by LPS. pLTF was significantly greater in rats receiving saline vs. LPS at 60 min (saline 51 ± 9%; LPS 24 ± 9%, P = 0.0148) and 90 min (saline 60 ± 9%; LPS 17 ± 6%, P < 0.0001) post-mAIH (Fig. 2, A–D; saline, n = 9; LPS, n = 8). Time control experiments did not receive mAIH, and do not exhibit significant pLTF at any time (4 ± 2%; P ≤ 0.05, n = 8; Fig. 2, C and D). Systemic LPS had no significant effect on frequency in any group (Fig. 2E).
pMF elicited by intrathecal DOI is attenuated by LPS.
Intrathecal 5HT2A receptor activation with DOI elicits pMF when delivered in three bolus injections (3 × 6 μl) (MacFarlane et al. 2011). The increase in nerve burst amplitude slowly develops and persists for at least 90 min postinjection (Fig. 3).
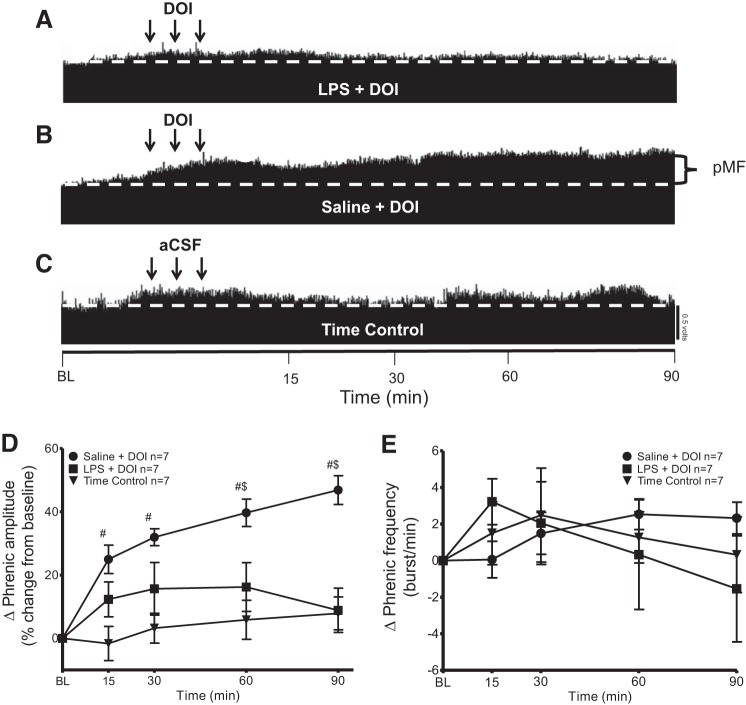
Fig. 3.
Systemic inflammation (24 h) induced by LPS (100 μg/kg) inhibits phrenic motor facilitation (pMF) elicited by 5HT2A agonist DOI. A–C: representative traces of compressed integrated phrenic neurograms after intrathecal administration of the 5HT2A receptor agonist DOI. A: LPS-injected group given intrathecal DOI did not show significant pMF. B: in contrast, the saline group given intrathecal DOI exhibits robust pMF. C: time controls given intrathecal vehicle (20% DMSO/80% aCSF) do not show a time-dependent change in amplitude vs. baseline (BL) values. D: group data for phrenic burst amplitude (percent change from baseline). LPS + DOI (n = 7) and saline + DOI (n = 7) were compared with time control (vehicle 20% DMSO/80% aCSF; triangle, n = 7). pMF following LPS + DOI was significant from saline + DOI at 60 and 90 min. There was no significant difference at any time between LPS + DOI and time control (P > 0.05). Significance is P ≤ 0.05: #significant difference vs. time control; $significance difference vs. LPS. Arrows represent intrathecal injections (3, 6-µl injections, 5-min intervals). E: group data for phrenic burst frequency (bursts/min). LPS + DOI (n = 7) and saline + DOI (n = 7), were compared with time control (vehicle 20% DMSO/80% aCSF) (n = 7) protocols. There was no significant difference between any group at any time (P > 0.05).
We confirm that intrathecal DOI elicits pMF and demonstrate that LPS attenuates this effect (Fig. 3). LPS + DOI was not significantly different from time controls at any point (P > 0.05; Fig. 3, A, C, and D). Saline + DOI was significantly greater than LPS + DOI at 60 min (saline 39 ± 4%; LPS 16 ± 7%; P = 0.0044) and 90 min (Saline 46 ± 4%; LPS 8 ± 6%, P < 0.0001) post-drug injection. (saline, n = 7; LPS, n = 7; Fig. 3, A and D). Time controls did not show pMF at any time (time control, n = 7; P > 0.05; Fig. 3, C and D). There was no significant difference in frequency between groups at any time (P > 0.05; Fig. 3E).
pMF elicited by spinal BW723C86 is attenuated by LPS.
The 5HT2B antagonist BW723C86 also elicits pMF following episodic intrathecal injections (MacFarlane et al. 2011). Similar to the 5HT2A agonist, increased nerve burst amplitude develops slowly and persists for at least 90 min postinjection.
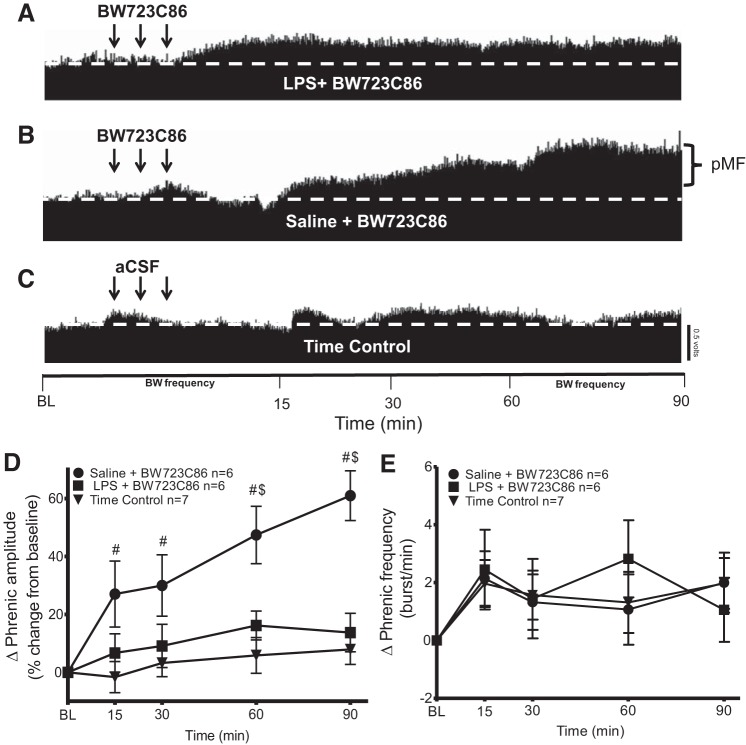
LPS abolished pMF elicited by BW723C86 (Fig. 4) since LPS + BW723C86 was not significantly different from time controls at any time (P > 0.05; Fig. 4, A, C, and D). Saline + BW723C86 was significantly greater than time controls at multiple points (P ≤ 0.05; Fig. 4, A and D). LPS + BW723C86 vs. saline + BW723C86 were significantly different at 60 (saline 47 ± 9%; LPS 16 ± 5%, P = 0.0050) and 90 min (saline 60 ± 8%; LPS 13 ± 6%, P < 0.0001) (Saline, n = 6; LPS, n = 6; time control, n = 7; Fig. 4, A and D). There were no significant differences in frequency between groups (P > 0.05; Fig. 4E).
Fig. 4.
LPS (100 μg/kg)-induced inflammation (24 h) inhibits phrenic motor facilitation (pMF) elicited by the 5HT2B agonist BW723C86. A–C: representative traces of compressed integrated phrenic neurograms after intrathecal BW723C86 injections. A: LPS + BW723C86 did not elicit pMF. B: saline + BW723C86 exhibited robust pMF. C: time controls given intrathecal vehicle (20% DMSO/80% aCSF) do not show time-dependent changes in phrenic burst amplitude. D: group data for phrenic burst amplitude (percent change from baseline). LPS + BW723C86 (n = 6) and saline + BW723C86 (n = 6) were compared with 20% DMSO/80% aCSF (n = 7). LPS + BW723C86 was significant to saline + BW723C86 at 90 min post-intrathecal injection. There were no significant differences at any time between LPS + BW723C86 and time controls (P > 0.05). Significance is P ≤ 0.05: #significant difference vs. time control; $significant difference vs. LPS. Arrows represent intrathecal drug injections (3, 6-µl injections, 5-min intervals). E: group data for phrenic burst frequency (bursts/min). LPS + BW723C86 (n = 6) and saline + BW723C86 (n = 6) were compared with 20% DMSO/80% aCSF (n = 7). There were no significant differences between groups at any time (P > 0.05).
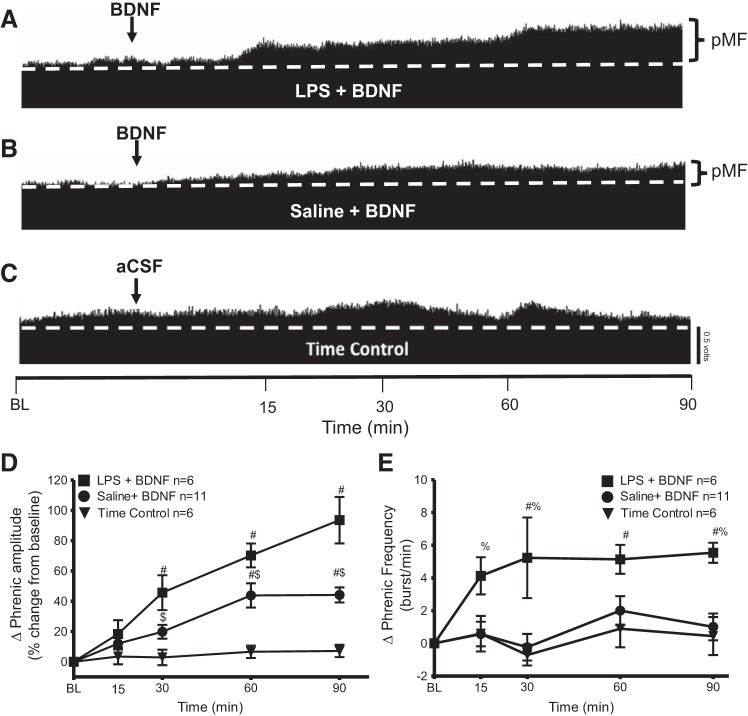
LPS enhances intrathecal BDNF induced pMF.
To elicit pMF we followed the previously published Baker-Herman protocol and injected a single bolus injection of BDNF (12 μl, 100 ng, intrathecal) (Baker-Herman et al. 2004).
To our surprise, the saline + BDNF group was significantly less than LPS + BDNF at 30 min (saline 19 ± 5%; LPS 45 ± 11%; P = 0.0279), 60 min (saline 43 ± 8%; LPS 70 ± 7%; P = 0.0241) and 90 min (saline 44 ± 5%; LPS 93 ± 15%; P < 0.0001) postinjection (LPS, n = 6; saline, n = 11; time control, n = 6; Fig. 5, A, B, and D). Both groups were significantly greater than time controls at 30, 60, and 90 min postinjection (P ≤ 0.05). There were no significant changes in time controls at any time (P > 0.05; Fig. 5, C and D).
Fig. 5.
Systemic inflammation (24 h) by LPS (100 μg/kg) enhances brain-derived neurotrophic factor (BDNF)-induced phrenic motor facilitation (pMF). A–C: representative traces of compressed integrated phrenic neurograms after intrathecal BDNF. A: LPS + BDNF shows increased phrenic burst amplitude vs. baseline (BL) values, demonstrating robust pMF. B: saline + BDNF also exhibits pMF. C: time control group given intrathecal vehicle (aCSF + 0.1% BSA) does not show time-dependent changes in phrenic burst amplitude. Arrow indicates intrathecal BDNF injection (12 µl, 100 ng). D: group data for phrenic burst amplitude (percent change from baseline). LPS + BDNF (n = 6) and saline + BDNF (n = 11) were compared with time control (n = 6) protocols. After intrathecal BNDF, there is a significant increase from baseline values for at least 90 min. LPS + BDNF was significantly greater than saline + BDNF at 30, 60, and 90 min, demonstrating that LPS enhanced BDNF-induced pMF. Significance is P ≤ 0.05: #significant difference vs. time control; $significant difference vs. LPS. E: group data for phrenic burst frequency (bursts/min). LPS + BDNF (n = 6) and saline + BDNF (n = 11) were compared with time control (n = 6) protocols. LPS + BDNF group increased more than saline + BDNF and time controls at 15, 30, 60, and 90 min postinjection. Significance is P ≤ 0.05: #significant difference vs. time control; %significant difference vs. saline treatment.
There were significant differences in phrenic burst frequency at multiple times post-BDNF injection. Saline + BDNF had a lower frequency response vs. LPS + BDNF at 15 min (saline 0.5 ± 0.7%; LPS 4 ± 1%; P = 0.0224), 30 min (saline −0.2 ± 0.8%; LPS 5 ± 2%; P = 0.0002), and 90 min (saline 1.0 ± 0.8%; LPS 5.5 ± 0.6%; P = 0.0025); LPS + BDNF had a significantly higher frequency than time controls at 30 (time control 0.7 ± 0.6%; LPS 5 ± 2%, P = 0.0004), 60 min (time controls 0.9 ± 1.1%; LPS 5.1 ± 0.8%; P = 0.0160), and 90 min (time control 0.4 ± 1.1%; LPS 5.5 ± 0.6%; P = 0.0029). There were no significant differences between saline + BDNF vs. time controls (P > 0.05; Fig. 5E).
DISCUSSION
We demonstrate that even mild systemic inflammation by LPS (100 μg/kg, 24 h) undermines 5HT2 receptor-dependent pMF (i.e., the Q pathway) downstream from serotonin receptor activation, but upstream from BDNF/TrkB signaling. Surprisingly LPS actually enhanced pMF elicited by TrkB receptor activation with BDNF. These findings enable us to triangulate on molecular targets undermined by systemic inflammation (Huxtable et al. 2013; Huxtable et al. 2015; Vinit et al. 2011).
With systemic inflammation, pMF induced by intrathecal 5HT2A or 5HT2B agonist injections is greatly attenuated, similar to mAIH-induced pLTF. Thus LPS pretreatment does not undermine serotonin release or receptor binding. Although 5HT2A receptors are necessary for mAIH-induced pLTF (Fuller et al. 2001), the requirement for 5HT2B receptor activation in mAIH-induced pLTF is less clear (MacFarlane et al. 2011). Both receptors are Gq protein-coupled metabotropic receptors, and both are sufficient to elicit pMF via the Q pathway to pMF (Dale-Nagle et al. 2010; MacFarlane et al. 2011). However, these receptors are not entirely similar in their effects since 5HT2B, but not the 5HT2A, receptors elicit pMF by a mechanism that requires NADPH oxidase activity (MacFarlane et al. 2011). Since mAIH-induced pLTF also requires NADPH oxidase activity (MacFarlane and Mitchell 2009), we suggest that both receptors may be involved in its underlying mechanism. If true, LPS impairs mAIH-induced pLTF downstream from both receptors, presumably converging at a common molecule in the Q pathway to pMF such as ERK MAP kinase (Dale-Nagle et al. 2010; Hoffman et al. 2012).
A critical step in the Q pathway to pMF is new BDNF protein synthesis and BDNF/TrkB signaling (Baker-Herman et al. 2004; Dale et al. 2017). Based on our results, systemic inflammation must undermine the Q pathway to pMF upstream from BDNF/TrkB signaling. One likely site of impairment with LPS-induced inflammation is new BDNF synthesis. Since BDNF is also implicated in hippocampal synaptic plasticity, and inflammation decreases hippocampal BDNF mRNA and protein levels, reduced BDNF likely undermines plasticity (Calabrese et al. 2014; Di Filippo et al. 2008; Leal et al. 2015; Tong et al. 2012; Yirmiya and Goshen, 2011). BDNF dysregulation may also be critical in the mechanism whereby inflammation impairs the Q pathway to pMF.
We were surprised that LPS actually enhanced BDNF-induced pMF, a finding with two major implications. First, LPS-induced inflammation impairs serotonin 2 receptor-induced pMF upstream from BDNF/TrkB signaling. Second, LPS-induced inflammation must upregulate BDNF/TrkB signaling through an, as yet, unknown mechanism.
LPS undermines mAIH-induced pLTF.
The same low dose of LPS used here elicits only transient inflammation in ventral spinal homogenates and microglia isolated near the phrenic motor nucleus (Huxtable et al. 2013). We confirm our previous report that low-dose LPS (100 μg/kg ip) undermines mAIH-induced pLTF 24 h later (Huxtable et al. 2013), similar to acute administration of a higher LPS dose (3 h, 3 mg/kg) (Vinit et al. 2011).
LPS attenuates pMF elicited by intrathecal serotonin receptor agonists.
Cervical spinal 5HT2A receptor activation elicits pMF (MacFarlane et al. 2011) and is necessary for mAIH-induced pLTF (Baker-Herman and Mitchell 2002; Fuller et al. 2001). We now provide evidence that systemic LPS undermines mAIH-induced pLTF downstream from serotonin release and 5HT2A receptor activation since spinal 5HT2A receptor-induced pMF is attenuated.
Cervical spinal 5HT2B receptor activation also elicits pMF, although this form of pMF requires NADPH oxidase activity (MacFarlane et al. 2011). Although 5HT2B receptors have not been demonstrated to play a necessary role in mAIH-induced pLTF, they likely do since mAIH-induced pLTF also requires NADPH oxidase activity (MacFarlane and Mitchell, 2009). The fact that LPS impairs 5HT2A and 5HT2B receptor-induced pMF suggests that 1) LPS impairs 5HT2A and 5HT2B receptor signaling through a common mechanism, or 2) LPS impairs each through an independent mechanism. We favor the former possibility and suggest that p38 MAP kinase (Huxtable et al. 2015) inhibits a kinase triggering new BDNF protein synthesis, a critical step in the Q pathway to pMF (Baker-Herman et al. 2004). This working hypothesis remains to be tested.
BDNF-induced pMF is enhanced by LPS.
A single bolus injection of BDNF elicits pMF (12 μl, 100 ng, intrathecal) for at least 90 min postinjection (Baker-Herman et al. 2004). At this dose and volume, BDNF does not affect XII nerve activity, suggesting that effective BDNF concentrations are localized in the cervical spinal cord, and that the effects on the phrenic nerve are not due to protein distribution to brain stem structures. Studies that specifically tested the effect of BDNF in the brain stem show that medullary BDNF administration increases breathing frequency, which would be reflected as changes in the hypoglossal nerve output (Fregosi 2011; Thoby-Brisson et al. 2003). We acknowledge that there are other brain stem areas related to breathing that are rich in TrkB receptors (Tang et al. 2010). The activation of these areas due to spinal BDNF administration might lead to effects that we are not aware of which directly affect spinal respiratory plasticity contributing to the results reported here.
Since inflammation lowers BDNF mRNA and protein levels in the hippocampus (Guan and Fang 2006; Lapchak et al. 1993), it may do the same in the phrenic motor nucleus. We speculate that a persistent BDNF deficit could initiate compensatory responses, such as TrkB upregulation within phrenic motor neurons. Subsequent BDNF administration would activate more TrkB receptors, thereby eliciting greater pMF.
Enhanced downstream BDNF/TrkB signaling is another possible explanation for these effects. For example, LPS may change coupling between TrkB and MEK/ERK, PI3K/AKT, and PLCγ pathways (Gottschalk et al. 1999; Minichiello 2009; Yoshii and Constantine-Paton 2010). LPS can alter cross-talk inhibition between MEK/ERK and PI3K/AKT pathways, diminishing their inhibitory balance and enabling 1) one signaling pathway to overpower the other, or 2) multiple pathways to contribute to the pMF (Dent 2014; Mendoza et al. 2011). These possible mechanisms remain to be explored.
Small but significant differences in phrenic burst frequency were observed after BDNF injections in LPS vs. saline and time control rats. Frequency LTF following mAIH is small and inconsistent (Baker-Herman and Mitchell 2008). However, if neuroinflammation increases BDNF/TrkB signaling in spinal dorsal horn neurons (Khan and Smith 2015; Lin et al. 2011) that transmit afferent information to CNS regions regulating breathing frequency, the impact of intrathecal BDNF on phrenic burst frequency may be enhanced (Arsenault et al. 2013; Ji et al. 2014).
Conclusions.
Even mild systemic inflammation undermines the Q pathway to phrenic motor facilitation, impairing an unknown process(es) downstream from serotonin release and receptor activation, but upstream of BDNF/TrkB signaling. Although the mechanism of impairment is unknown, we suggest that it relates to diminished capacity to synthesize BDNF protein following AIH or serotonin receptor activation, undermining the Q pathway to pMF. To our surprise, BDNF/TrkB-induced pMF was enhanced by inflammation through unknown mechanisms. These novel findings give useful insights in our efforts to discover mechanisms whereby inflammation impairs spinal respiratory motor plasticity.
GRANTS
Support was provided by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-111598 and HL-69064. I. M. Agosto-Marlin was supported by a National Institutes of Health (NIH) Supplement to HL-111598. N. L. Nichols was supported by Francis Families Foundation and NIH K99/R00-HL-119606.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.A.-M., N.L.N., and G.S.M. conceived and designed research; I.M.A.-M. and N.L.N. performed experiments; I.M.A.-M. and N.L.N. analyzed data; I.M.A.-M., N.L.N., and G.S.M. interpreted results of experiments; I.M.A.-M. prepared figures; I.M.A.-M. drafted manuscript; I.M.A.-M., N.L.N., and G.S.M. edited and revised manuscript; I.M.A.-M., N.L.N., and G.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bradley Wathen for expert technical assistance.
Present address of I. M. Agosto-Marlin: Center for Integrative Brain Research Seattle Children’s Research Institute, Seattle, WA 98101.
Present address of N. L. Nichols: Department of Biomedical Sciences University of Missouri, Columbia, MO 65211.
REFERENCES
- Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 23: R774–R788, 2013. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15: 542–548, 2005. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci 122: 169–192, 2014. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- Arsenault M, Ladouceur A, Lehmann A, Rainville P, Piché M. Pain modulation induced by respiration: phase and frequency effects. Neuroscience 252: 501–511, 2013. doi: 10.1016/j.neuroscience.2013.07.048. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci 8: 430, 2014. doi: 10.3389/fncel.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: Evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun 44: 159–166, 2015. doi: 10.1016/j.bbi.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol 287: 130–136, 2017. doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol Ther 15: 245–246, 2014. doi: 10.4161/cbt.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Sarchielli P, Picconi B, Calabresi P. Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol Sci 29: 402–412, 2008. doi: 10.1016/j.tips.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF. Respiratory related control of hypoglossal motoneurons—knowing what we do not know. Respir Physiol Neurobiol 179: 43–47, 2011. doi: 10.1016/j.resp.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90: 2001–2006, 2001. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Gottschalk WA, Jiang H, Tartaglia N, Feng L, Figurov A, Lu B. Signaling mechanisms mediating BDNF modulation of synaptic plasticity in the hippocampus. Learn Mem 6: 243–256, 1999. [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun 20: 64–71, 2006. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Günther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol 66: 949–961, 2006. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Hocker AD, Stokes JA, Powell FL, Huxtable AG. The impact of inflammation on respiratory plasticity. Exp Neurol 287: 243–253, 2017. doi: 10.1016/j.expneurol.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS. Intermittent hypoxia-induced spinal inflammation impairs respiratory motor plasticity by a spinal p38 MAP kinase-dependent mechanism. J Neurosci 35: 6871–6880, 2015. doi: 10.1523/JNEUROSCI.4539-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Smith SM, Vinit S, Watters JJ, Mitchell GS. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol (1985) 114: 879–887, 2013. doi: 10.1152/japplphysiol.01347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol 178: 482–489, 2011. doi: 10.1016/j.resp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 13: 533–548, 2014. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Smith MT. Neurotrophins and neuropathic pain: role in pathobiology. Molecules 20: 10657–10688, 2015. doi: 10.3390/molecules200610657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F. Systemic interleukin1B decreases BDNF mRNA expression in the rat hippocampal formation. Neuroscience 53: 297–301, 1993. doi: 10.1016/0306-4522(93)90196-M. [DOI] [PubMed] [Google Scholar]
- Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res 1621: 82–101, 2015. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Lin YT, Ro LS, Wang HL, Chen JC. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation 8: 126, 2011. doi: 10.1186/1742-2094-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151, 2008. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36: 320–328, 2011. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SS, Quan HY, Ma J, Han JS, Jeon BH, Seol GH. Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area. Neurosci Lett 456: 20–24, 2009. doi: 10.1016/j.neulet.2009.03.079. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10: 850–860, 2009. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Niebert M, Vogelgesang S, Koch UR, Bischoff AM, Kron M, Bock N, Manzke T. Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network. PLoS One 6: e21395, 2011. doi: 10.1371/journal.pone.0021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Tang S, Machaalani R, Waters KA. Immunolocalization of pro- and mature-brain derived neurotrophic factor (BDNF) and receptor TrkB in the human brainstem and hippocampus. Brain Res 1354: 1–14, 2010. doi: 10.1016/j.brainres.2010.07.051. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Cauli B, Champagnat J, Fortin G, Katz DM. Expression of functional tyrosine kinase B receptors by rhythmically active respiratory neurons in the pre-Bötzinger complex of neonatal mice. J Neurosci 23: 7685–7689, 2003. doi: 10.1523/JNEUROSCI.23-20-07685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Kramár EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosci 32: 17714–17724, 2012. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. LPS recognition-CD14,TRLs and LPS activation cluster. Trends Immunol 23: 301–304, 2002. doi: 10.1016/S1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 176: 130–135, 2011. doi: 10.1016/j.resp.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Fong AY, Bautista TG, Pilowsky PM. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin Exp Pharmacol Physiol 40: 602–609, 2013. doi: 10.1111/1440-1681.12129. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181–213, 2011. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 70: 304–322, 2010. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]