Abstract
Insulin-like growth factor 2 (IGF2), a small, secreted protein, is critical for fetal and prenatal growth in humans and other mammals. The IGF2 gene and its mouse homolog comprise part of a conserved linkage group that is regulated by parental imprinting, with IGF2/Igf2 being expressed from the paternal chromosome, and the adjacent H19 gene from the maternal chromosome. By using information extracted from public genomic and gene expression databases, I have now analyzed this locus in nine nonhuman primate species representing over 60 million years of evolutionary divergence from a common progenitor. Both IGF2 and H19 genes and the entire locus have been conserved among these primates. Each primate IGF2 gene except for gibbon and marmoset is composed of 10 exons and contains five potential promoters, each with distinctive 5′-untranslated exons. Similarly, except for marmoset and mouse lemur, H19 consists of six exons and has two promoters. DNA sequence conservation is high, not only in orthologous exons and promoters, but also in a putative imprinting control region located 5′ to H19 and in multiple potential distal enhancer elements found 3′ to H19. Collectively, these results support the hypothesis that common regulatory processes shaped the IGF2 - H19 locus before the onset of primate speciation more than 85 million years ago. This study also leads to the conclusion that inaccuracies in data presentation in genetic repositories could limit our ability to develop novel insights about roles of individual genes and multigene loci in mammalian physiology and disease.
Keywords: gene transcription, genomics, H19, IGF2, parental imprinting, primate evolution
INTRODUCTION
Insulin-like growth factor 2 (IGF2) is a 67-residue secreted protein that plays a key role in fetal development and growth and in a variety of physiological and pathophysiological processes in humans and other mammals (22, 36, 37, 52). Collectively with IGF1 and insulin (INS), IGF2 comprises a gene family that is present in many mammals and nonmammalian vertebrates (13, 62, 63, 67, 76).
In humans, alterations in IGF2 protein expression have been linked to several developmental growth syndromes (3, 20). In Beckwith-Wiedemann syndrome, asymmetric overgrowth is thought to be caused by focal overexpression of IGF2 (3, 20), while in Silver-Russell syndrome, reduced levels of IGF2 are associated with diminished pre- and postnatal somatic growth and dysmorphic features and bodily asymmetry (3, 20). IGF2 also controls aspects of growth in other mammals, including mice (16) and pigs (8, 40, 70). In the latter species, increased expression of IGF2 found in more heavily muscled animals was linked to a single nucleotide polymorphism (SNP) in an IGF2 promoter (70) that interfered with binding of a transcriptional repressor (8, 40).
IGF2 appears to be moderately well conserved among human, mouse, and rat, the few mammals in which the gene has been studied in detail (7, 12, 25, 26, 42, 45, 61, 62, 64, 67). Human IGF2 and mouse Igf2 also comprise a conserved linkage group on chromosome 11p15.5 and chromosome 7, respectively, that additionally includes tyrosine hydroxylase (TH/Th), INS (Ins2 in mice), and the gene encoding the long noncoding RNA, H19. Parental imprinting regulates both IGF2/Igf2 and H19 genes, with H19 mRNA being expressed from the maternally derived chromosome, and IGF2/Igf2 primarily from the paternal chromosome (42, 45). DNA sequences termed an imprinting control region (ICR, Fig. 1A) mediate this chromosome-of origin-specific gene activity (19). The ICR contains recognition sites for the regulatory molecule, CCTC binding factor (CTCF) (19, 50, 75). In maternal chromatin CTCF binds to the ICR and thereby directs interactions of distal enhancers to the H19 promoter while preventing their binding to IGF2/Igf2 promoters (24, 50, 75). In contrast, in paternal chromatin DNA in the ICR becomes methylated, which blocks CTCF access, and allows the same enhancers to activate IGF2/Igf2 (24, 50, 75).
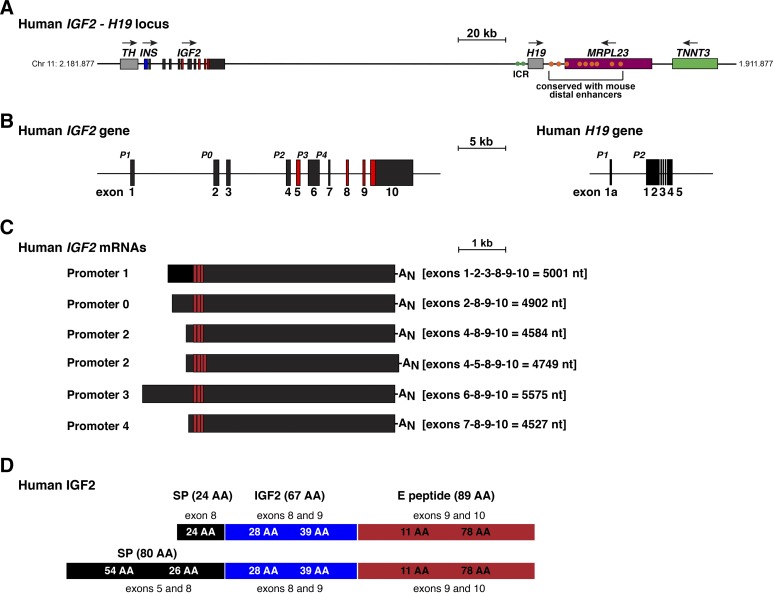
Fig. 1.
The human IGF2 - H19 locus and genes. A: map of the human IGF2 - H19 locus on chromosome 11p15.5 with chromosomal coordinates listed. For IGF2, exons are depicted as boxes, and introns as horizontal lines. Other genes are illustrated as single boxes and include tyrosine hydroxylase (TH), insulin (INS), H19, mitochondrial ribosomal protein L23 (MRPL23), and troponin T3, fast skeletal type (TNNT3). Horizontal arrows show the direction of gene transcription. A scale bar is indicated. Green circles represent the imprinting control region (ICR), which is located adjacent to H19 (19, 50, 75), and orange ovals depict the 9 human homologs of the 10 distal enhancers that were identified and functionally mapped in the mouse genome (30, 60). B: detailed view of human IGF2 and H19 genes, with exons as boxes (10 for IGF2 and 6 for H19), and introns and flanking DNA as horizontal lines. The letter ‘P’ indicates gene promoters (P0 and P1–P4 for IGF2, and P1 and P2 for H19), and a scale bar is shown. For IGF2, noncoding exons are in black or dark gray and coding exons are colored red. For H19, all exons are in black. C: illustration of the 6 major types of human IGF2 mRNAs. The responsible promoters are noted, the exons found in each transcript type are listed, and the length of each mRNA is stated in nucleotides (nt). AN depicts the polyadenylic acid tail found at the 3′ end of mRNAs. D: illustration of human IGF2 protein precursors, showing the derivation of each segment from different IGF2 exons. Mature, 67-amino acid IGF2 is in blue; signal peptides (SP) are in black, and the 89-amino acid E peptide is in red. AA, amino acid.
The mouse Igf2 gene consists of eight exons, with gene transcription being controlled by four promoters, termed P0–P3 (12, 43, 61). The human IGF2 gene contains 10 exons and has a fifth promoter regulating its own noncoding exon, plus an additional coding exon (Fig. 1, A and B) (42, 45, 66, 67). Since IGF2 gene expression and protein production are maintained throughout life in humans (14, 66) but disappear during the early postnatal period in mice (16, 35, 42), it had been thought that this fifth human IGF2 promoter was responsible for sustained gene activity in adults (56). As this promoter is expressed from both alleles in some human tissues and thus appears not to be imprinted (74), it also had been postulated that lack or loss of imprinting control governed maintenance of IGF2 activity in adult humans (74). Recent observations have demonstrated that this idea appears to be incorrect, as several IGF2 gene promoters are active in multiple adult human tissues (60). Thus, the molecular mechanisms responsible for maintaining IGF2 mRNA biosynthesis and protein production throughout life in humans or attenuating it in mice have not been established.
Recent advances in molecular genetics present opportunities for gaining new insights into human physiology, disease predisposition, and human origins and evolution (1, 34, 53) through analysis of information found in genomic databases (39). Yet understanding these data has been challenging, in part because of problems of annotation and difficulties in extracting and verifying accuracy and completeness of the information, as was shown recently for human IGF2 (60). The present analysis was undertaken to learn more about the IGF2 - H19 locus in primates, the evolutionarily closest human relatives (58), with the goal of gaining preliminary insight into processes that may have imposed or limited constraints on locus regulation and gene function during primate speciation.
MATERIALS AND METHODS
Genome database searches and analysis.
Mammalian genes and loci were accessed in the Ensembl Genome Browser (https://www.ensembl.org/index.html) and the UCSC Genome Browser (https://genome.ucsc.edu). Searches were performed with BlastN under normal sensitivity (maximum e-value of 10; mismatch scores: 1,-3; gap penalties: opening 5, extension, 2; filtered low complexity regions, and repeat sequences masked) using as queries human IGF2 or H19 DNA segments or other nearby genomic regions, as obtained from Homo sapiens genome assembly GRCh38,. The following genomes were searched: bonobo (Pan paniscus, Bonobo panpan1.1), chimpanzee (Pan troglodytes, Pan_tro_3.0), gibbon (Nomascus leucogenys, Nlue_3.0), gorilla (Gorilla gorilla, gorGor4), macaque (Macaca mulatta, Mmul_8.0.1), marmoset (Callithrix jacchus, C_jacchus3.2.1), mouse (Mus musculus, genome assembly GRCm38), mouse lemur (Microcebus murinus, Mmur_3.0), olive baboon (Papio anubis, PapAnu3.0), and orangutan (Pongo abelii, PPYG2). The highest scoring results in all cases mapped to the IGF2 gene, the H19 gene, or the IGF2 - H19 locus. Data on human H19 gene expression were extracted from the Portal for the Genotype-Expression Project (GTEx v7, https://www.gtexportal.org/home/), using the exon expression module and analyzing alternatively spliced transcripts, which were categorized by the presence of specific exons or exon segments. Analysis of human IGF2 gene expression with the same database has been reported recently (60). Examination of H19 gene expression in different primates was conducted using the Sequence Read Archive of the National Center for Biotechnology Information (SRA NCBI; https://www.ncbi.nlm.nih.gov/sra), the NCBI AceView program (https://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/) (68), and the Nonhuman Primate Reference (http://www.nhprtr.org/) (48, 51). Sources for IGF2 protein sequences included GENCODE/Ensemble databases, the NCBI Consensus CDS Protein Set (https://www.ncbi.nlm.nih.gov/CCDS/), and the Uniprot browser (http://www.uniprot.org/); when unavailable, DNA sequences from IGF2 exons were translated with assistance of Serial Cloner 1.3. Phylogenetic relationships among IGF2 protein domains were determined using the MUSCLE 3.8.31 and PhyML 3.1/3.0 aLRT programs from Phylogeny.fr (http://www.phylogeny.fr/index.cgi) (17). Data are presented in text and tables as percent identity over the entire query region, unless specified otherwise.
RESULTS
Human IGF2 - H19 locus.
The human IGF2 - H19 locus on chromosome 11p15.5 contains five protein-coding genes and several genes expressing noncoding RNAs (60) (Fig. 1A). The locus also comprises a conserved linkage group with a portion of mouse chromosome 7 (45), as noted in the Introduction. In both species, IGF2/Igf2 and H19 gene regulation are influenced by parental imprinting, in which H19 mRNA is expressed in most tissues from the maternally derived chromosome, and IGF2/Igf2 from the paternal chromosome, through regulation by CTCF at an ICR found 5′ to H19 in both species (Fig. 1A) (19, 24, 50, 75). Experiments in mice have demonstrated specifically that CTCF binding to the ICR in maternal chromatin blocks access of distal enhancers to Igf2 promoters (50, 75). Ten distal enhancer elements have been mapped structurally and functionally in the mouse genome to a region 3′ to H19 (30). DNA homology searches revealed sequences similar to nine of these 10 elements in analogous locations in the human genome (Fig. 1A), although DNA identity was limited in all but one of them (60). Moreover, in the human genome, seven of the nine identified elements are found within the MRPL23 gene (Fig. 1A) (60), while in the mouse all of the enhancers map to intergenic DNA (30) (see Fig. 9).
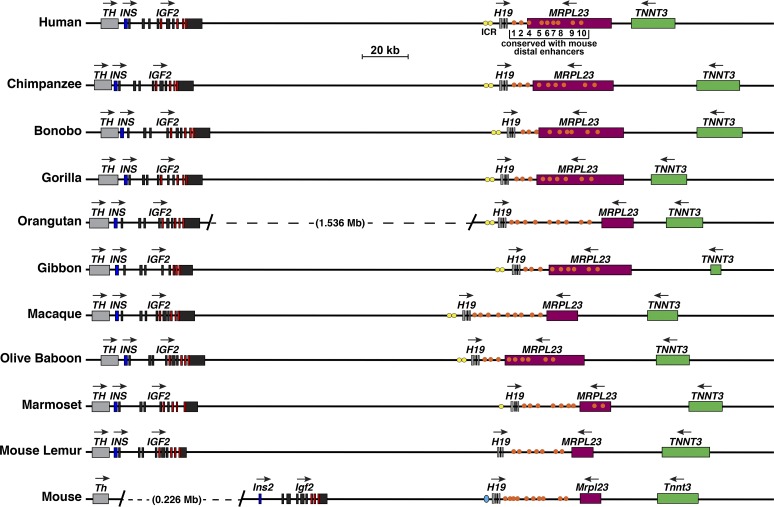
Fig. 9.
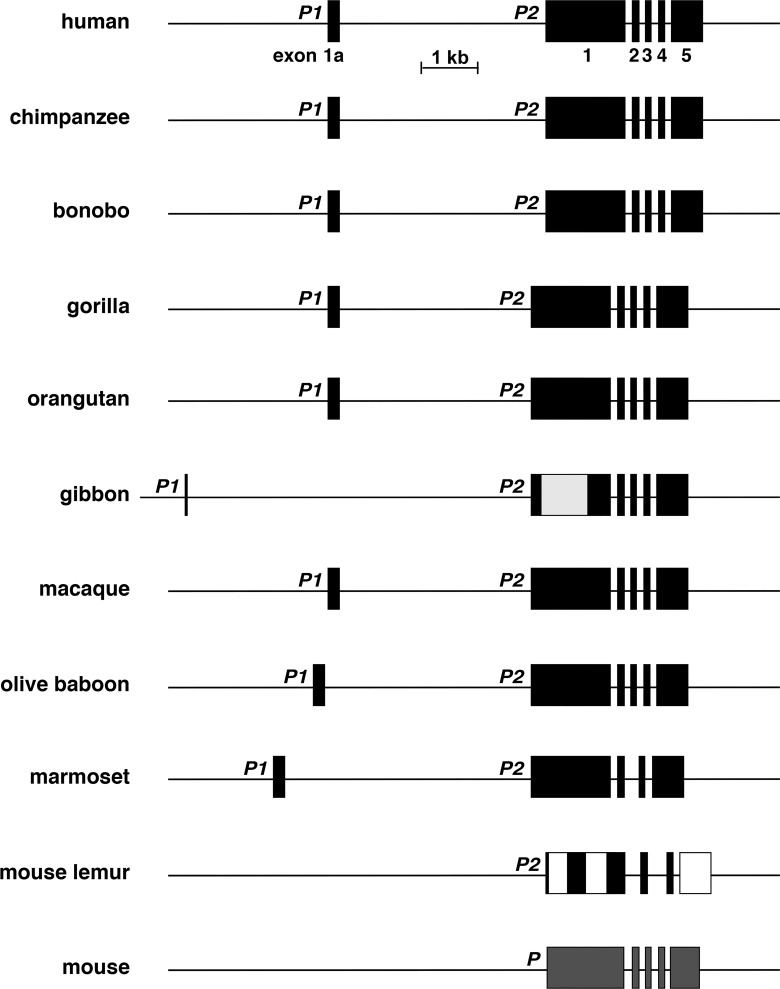
Comparison of IGF2 - H19 locus and genes in primates and in the mouse. Schematics of human IGF2 - H19, other primate IGF2 - H19, and mouse Igf2 - H19 genes and loci are shown. For IGF2/Igf2 and H19, individual exons are indicated as boxes. Other genes are shown as single boxes, and include tyrosine hydroxylase (TH/Th), insulin (INS/Ins2), mitochondrial ribosomal protein L23 (MRPL23/Mrlp23), and troponin T3, fast skeletal type (TNNT3/Tnnt3). A horizontal arrow indicates the direction of transcription for each gene. Yellow or aqua ovals (primate or mouse, respectively) depict the imprinting control region (ICR) 5′ to H19, and orange circles indicate the 10 distal enhancers that were identified and functionally mapped in the mouse genome (30, 60) and their primate homologs. A scale bar is also shown. In the orangutan genome, there is a larger distance between IGF2 and H19 than in the other species, as indicated; in the mouse genome Th and Ins2 are separated by ~226 kilobases.
IGF2 gene in primates.
As assessed by analysis of primary research publications, IGF2 is a 10-exon, 5-promoter gene in humans (Fig. 1B) (42, 45, 66, 67), yet in both the Ensembl and UCSC Genome Browsers this information is not available. As of February 2018, Ensembl continued to report that human IGF2 contains nine exons and four promoters, while in the UCSC browser it is described as consisting of seven exons and three promoters. The reasons for these discrepancies and for the incompleteness of the information in the two databases are unknown, since in both the same version of the human genome (GRCh38/hg38) has been used for annotation. In addition, all of the DNA sequences that comprise human IGF2, as based on primary research publications (42, 45, 66, 67), can be mapped to the IGF2 locus by BlastN searches (60) and are thus present in GRCh38/hg38 (Fig. 1B). Each of the five human IGF2 promoters governs the expression of distinctive noncoding exons, but all share the exons that contain the IGF2 protein coding region and 3′ nontranslated DNA (Fig. 1, B and C). The exception to this model of ‘one promoter-one transcript” is human promoter 2 (P2), which is responsible for two IGF2 mRNAs that differ by the presence or absence of alternatively spliced exon 5. The presence of exon 5 in some IGF2 transcripts leads to a second predicted IGF2 protein precursor of 236 residues, although it contains the same 67-amino acid mature IGF2 (Fig. 1, C and D).
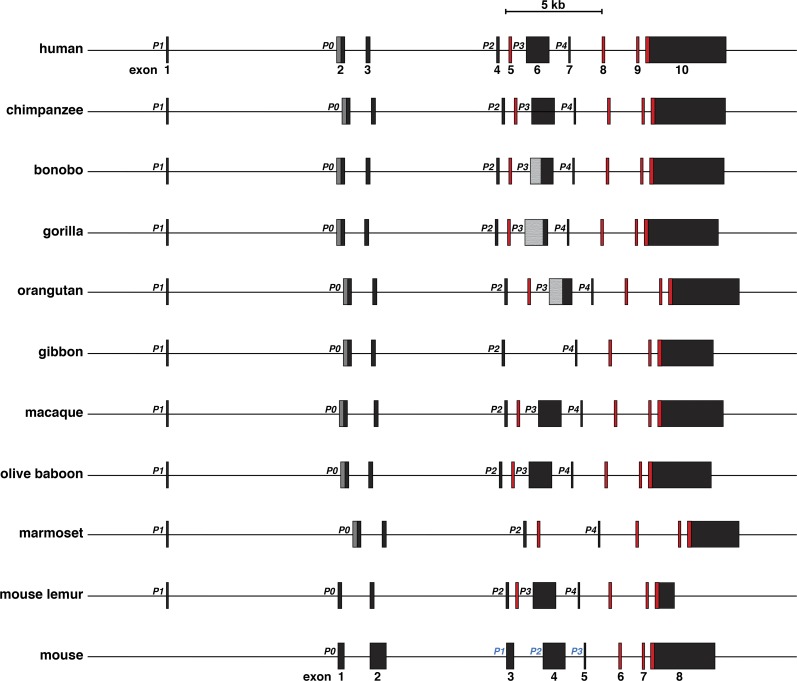
With human IGF2 exon and promoter segments as queries, IGF2 also appears to be a 10-exon, 9-intron, and 5-promoter gene in most of the primates analyzed here (Fig. 2, Tables 1 and 2), and the overall structure of each gene closely resembles that of human IGF2 (Fig. 2, Table 1), even though in all cases, the information annotated in the respective genomic databases for each of the nonhuman primates was incomplete (e.g., one promoter mapped in orangutan, macaque, and olive baboon, two in chimpanzee, bonobo, and gibbon, three in gorilla and mouse lemur, and four in marmoset). When all of the newly defined data was considered, there was remarkable congruence with human IGF2 in gene structure among seven of nine nonhuman primate species, and all nine were within 10% of human IGF2 (29.2 kb) in length, ranging from 26.4 kb in mouse lemur to 31.8 kb in marmoset (Fig. 2).
Fig. 2.
Comparison of IGF2 genes in primates and in the mouse. Schematics of human IGF2, other primate IGF2, and mouse Igf2 genes are shown. Promoters are labeled; the different terminology employed for the mouse promoters (in blue text) derives from genomic databases. All exons are indicated as boxes, with noncoding exons in black or gray and coding exons in red. The dark gray region in exon 2 represents the additional part of the exon that is transcribed when P0 is active (exon 2 large in Table 2). The smaller black segment is transcribed when P1 is active. The lighter gray portion of exon 6 depicts areas that have not been characterized because of poor quality DNA sequence in bonobo, gorilla, and orangutan (see Table 1). A scale bar is also shown.
Table 1.
Percent nucleotide identity with human IGF2 exons
| Species | Exon 1, 115 bp | Exon 2, 220 bp | Exon 2 lg, 478 bp | Exon 3, 242 bp | Exon 4, 160 bp | Exon 5, 165 bp | Exon 6, 1161 bp | Exon 7, bp | Exon 8, 163 bp | Exon 9, 149 bp | Exon 10, 4112 bp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chimpanzee | 99 | 99 | 99 | 98 | 99 | 99 | 99 | 100 | 100 | 100 | 98 (3804) |
| Bonobo | 99 | 98 | 99 | 98 | 99 | 99 | 99* (619) | 100 | 100 | 100 | 97* (3658) |
| Gorilla | 99 | 99 | 99 | 99 | 98 | 99 | 98* (229) | 100 | 100 | 100 | 97 (3789) |
| Orangutan | 100 | 97 | 97 | 94 | 98 | 98 | 98* (490) | 94 | 100 | 100 | 94* (3904) |
| Gibbon | 100 | 96 | 97 | 95 | 97 | no match | no match | 100 | 100 | 100 | 94 (2690) |
| Macaque | 97 | 95 (204) | 95 | 90 | 96 (156) | 98 | 95 (1145) | 98 | 99 | 100 | 92 (3613) |
| Olive baboon | 97 | 95 (204) | 94 | 90 | 96 (156) | 96 | 98 | 97 | 99 | 99 | 92 (3514) |
| Marmoset | 91 (111) | 89 (164) | 84 (411) | 87 (201) | 94 (151) | 90 | no match | 92 (99) | 98 (160) | 96 | 89 (2972) |
| Mouse lemur | 91 (35) | 85 (204) | 85 (204) | 86 (131) | 89 (101) | 90 | 89 | 86 (69) | 95 (160) | 95 | 87 (1427) |
| Mouse | no match | 86 (166) | 86 (166) | 91 (45) | 91 (85) | no match | 86 (1033) | 100 (28) | 88 (161) | 89 | 87 (865) |
Number of base pairs aligned is in parentheses if less than length of human exon.
Poor-quality DNA sequence.No match, no exon detected.
Table 2.
Percent nucleotide identity with human IGF2 promoters
| Species | Promoter 1, 600 bp | Promoter 0, 600 bp | Promoter 2, 1000 bp | Promoter 3, 1000 bp | Promoter 4, 1000 bp |
|---|---|---|---|---|---|
| Chimpanzee | 98 | 97 | 99 | 99 | 98 |
| Bonobo | 98 | 97 | 99 | 99 (470)* | 98 (799)* |
| Gorilla | 99 | 97 | 98 | 99 (762)* | 97 |
| Orangutan | 98 | 96 | 95 | 99 (794) | 96 (881)* |
| Gibbon | 97 | 96 (522)* | 97 | no match | 97 (497)* |
| Macaque | 93 (581) | 90 (590) | 93 (982) | 94 (868) | 96 |
| Olive Baboon | 94 (580) | 90 (534) | 95 (896) | 95 | 96 |
| Marmoset | 88 (485) | 89 (362) | 89 (822) | 91 (801) | 89 (109)* |
| Mouse lemur | 81 (138) | 88 (48) | 80 (399) | 86 (346) | 88 (278) |
| Mouse | no match | 83 (115) | no match | no match | 88 (156) |
Number of base pairs aligned is in parentheses if less than length of human promoter.
Poor quality DNA sequence. No match, no promoter detected.
DNA sequence identity was 94% or more for all 10 IGF2 exons in the human, chimpanzee, bonobo, gorilla, and orangutan genes, was 90% and above for macaque and olive baboon, and was 95% or more in all species for exons 8 and 9, the two wholly coding exons (Table 1). Nucleotide identity was nearly as high for the five IGF2 promoters, being at least 90% in all primates except for marmoset and mouse lemur (88–91% and 80–88%, respectively, Table 2). As might be anticipated, these analyses revealed a hierarchy of gene similarity and nucleotide sequence identity that was greatest in those primate species evolutionarily closer to humans. In chimpanzees and gorillas, where the overall DNA match with the human genome is >98.5% (49, 58), nucleotide sequence identity ranged from 97–100% for all five IGF2 promoters and all 10 exons. Sequence similarities were lower in the macaque, where homology with the human genome is ~93.5%, and in olive baboon (90–100%), and were even less in more distantly related marmoset and mouse lemur (80–98%, Tables 1 and 2). Of note, the eight mouse Igf2 exons were as similar to their human counterparts as were mouse lemur and human exons (Table 1).
There are limited published data regarding potential molecular mechanisms controlling the five human IGF2 promoters (29, 42, 54, 69). Several transcription factors, including C/EBPα, C/EBPβ, and SP1, have been shown to activate P1 through proximal promoter control elements (56, 57). AP2 had been found to stimulate P3 (55), and WT1, the Wilms tumor repressor protein, was shown to inhibit P3 (18). Limited in vivo analyses have demonstrated that P0 is active in human fetal tissues and in adult skeletal muscle (15, 26, 28, 29, 42, 45); other promoters appear to be expressed in different organs and tissues (15, 26, 28, 29, 42, 45, 60). There are minimal data on IGF2 promoter function in nonhuman primates, although the high level of DNA sequence conservation with the orthologous human promoters suggests that similar mechanisms may be involved in IGF2 gene regulation in these latter species (Table 2).
H19 gene in primates.
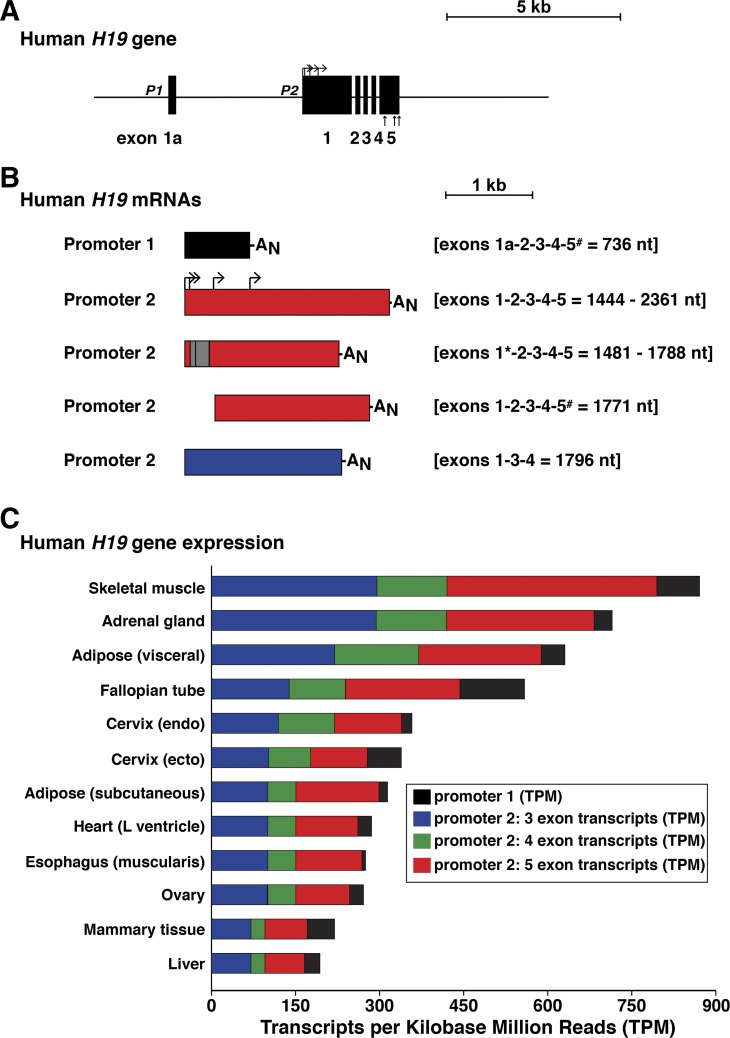
Human H19 is a 6-exon, 2-promoter gene (Fig. 3A). There are multiple H19 mRNAs as a result of transcription from each promoter, exon skipping, alternative transcription start sites, and intraexonic alternative splicing (Fig. 3B). Analysis of information from GTEx (release 7), which has obtained gene expression data from many human tissues by RNA sequencing (5, 65a, 77), reveals that the vast majority of H19 transcripts derive from promoter 2, with ~40% containing the RNA representations of five exons and 40% three exons (Fig. 3C).
Fig. 3.
The human H19 gene and its expression. A: detailed view of the human H19 gene, with exons as boxes and introns and flanking DNA as horizontal lines. P1 and P2 depict the 2 gene promoters. Bent arrows indicate different transcription start sites directed by P2 and straight vertical arrows denote the locations of alternative polyadenylation sites. A scale bar is shown. B: illustration of major classes of human H19 mRNAs. The responsible promoters are listed, exons found in each transcript type are indicated, and the length of each mRNA is stated in nucleotides (nt). Gray boxes define areas of exons involved in alternative RNA splicing (also indicated by *), and bent arrows indicate different transcription start sites; AN depicts the polyadenylic acid tail found at the 3′ end of mRNAs (alternative polyadenylation is indicated by #). C: organ- and tissue-specific human H19 gene expression was obtained from the Genotype-Tissue Expression (GTEx) portal, and graphed as transcripts per kilobase million reads (TPM). The results represent tissue samples from 714 donor individuals (66% male, 34% female; age range: 20–79 yr (for details see: https://www.gtexportal.org/home/tissueSummaryPage#sex). The 12 organs and tissues with the highest levels of H19 mRNAs are depicted. Color-coding represents the major types of transcripts detected in each tissue or organ, and is matched with B, although the 4-exon mRNAs detected in GTEx data do not appear to correspond to a previously described H19 transcript. The vast majority of H19 mRNAs use promoter 2.
By using human H19 exon and promoter DNA sequences as queries, H19 also appears to be a 6-exon, 2-promoter gene in most of the primates analyzed here (Fig. 4, Tables 3 and 4), and in the majority their individual structures resemble that of human H19 (Fig. 4, Table 3), even though the annotated data found in the respective genome databases for each of these species except for macaque were incomplete (e.g., one apparent H19 exon in chimpanzee, bonobo, gorilla, gibbon, olive baboon, marmoset, and mouse lemur, and none in orangutan). When all of the newly mapped data were analyzed, there was overall congruence in H19 gene organization in seven of nine nonhuman primates (2 promoters and 6 exons, Fig. 4). In addition, the total lengths of seven genes were within 14% of human H19 (6,307 base pairs), and ranged from 5,989 base pairs in olive baboon to 7,203 base pairs in marmoset (gibbon and mouse lemur were outliers, as the number of exons and overall DNA sequence identity were less than the other species; Fig. 4).
Fig. 4.
Comparison of H19 genes among primates and in the mouse. Schematics of human H19, other primate H19, and mouse H19 genes are shown. Promoters are labeled and exons are indicated as boxes. Gray regions depict areas of exons that have not been characterized fully in some species. The lighter gray portion of exon 1 in gibbon depicts an area that has not been characterized because of poor quality DNA sequence, and the white portions of exons 1 and 5 in mouse lemur represent regions of low similarity with other primates (see Table 3). Mouse H19 has a single promoter (labeled as P). A scale bar is also shown.
Table 3.
Percent nucleotide identity with human H19 exons
| Species | Exon 1a, 253 bp | Exon 1, 1358 bp | Exon 2, 135 bp | Exon 3, 113 bp | Exon 4, 123 bp | Exon 5, 632 bp |
|---|---|---|---|---|---|---|
| Chimpanzee | 98 | 99 | 98 | 100 | 99 | 98 |
| Bonobo | 98 | 99 | 98 | 100 | 99 | 98 |
| Gorilla | 98 | 99 | 96 | 98 | 100 (120) | 98 |
| Orangutan | 98 | 98 | 96 | 98 | 99 | 97 |
| Gibbon | 93 (43) | 98* (686) | 96 | 99 | 99 | 97 |
| Macaque | 94 | 96 | 92 | 96 | 95 (116) | 95 |
| Olive Baboon | 94 | 96 | 91 | 96 (106) | 94 | 96 |
| Marmoset | 83 (245) | 89 (1323) | 91 | no match | 92 (70) | 87 (629) |
| Mouse lemur | no match | 89 (672) | 94 (35) | no match | 97 (32) | 92 (77) |
| Mouse | no match | 92 (354) | 94 (35) | no match | 95 (41) | 92 (62) |
Number of base pairs aligned is in parentheses if less than length of human exon.
Poor DNA sequence quality. No match, no exon detected.
Table 4.
Percent nucleotide identity with human H19 promoter
| Species | Promoter 1, 1000 bp | Promoter 2, 1000 bp |
|---|---|---|
| Chimpanzee | 99 | 99 |
| Bonobo | 99 | 99 |
| Gorilla | 97 | 99 |
| Orangutan | 95 | 95 |
| Gibbon | 85 (820) | 95 |
| Macaque | 91 (962) | 92 (989) |
| Olive Baboon | 92 | 92 (985) |
| Marmoset | 81 (948) | 86 (763) |
| Mouse lemur | 97 (77) | 87 (87) |
| Mouse | no match | 89 (107) |
Number of base pairs aligned is in parentheses if less than length of human promoter.
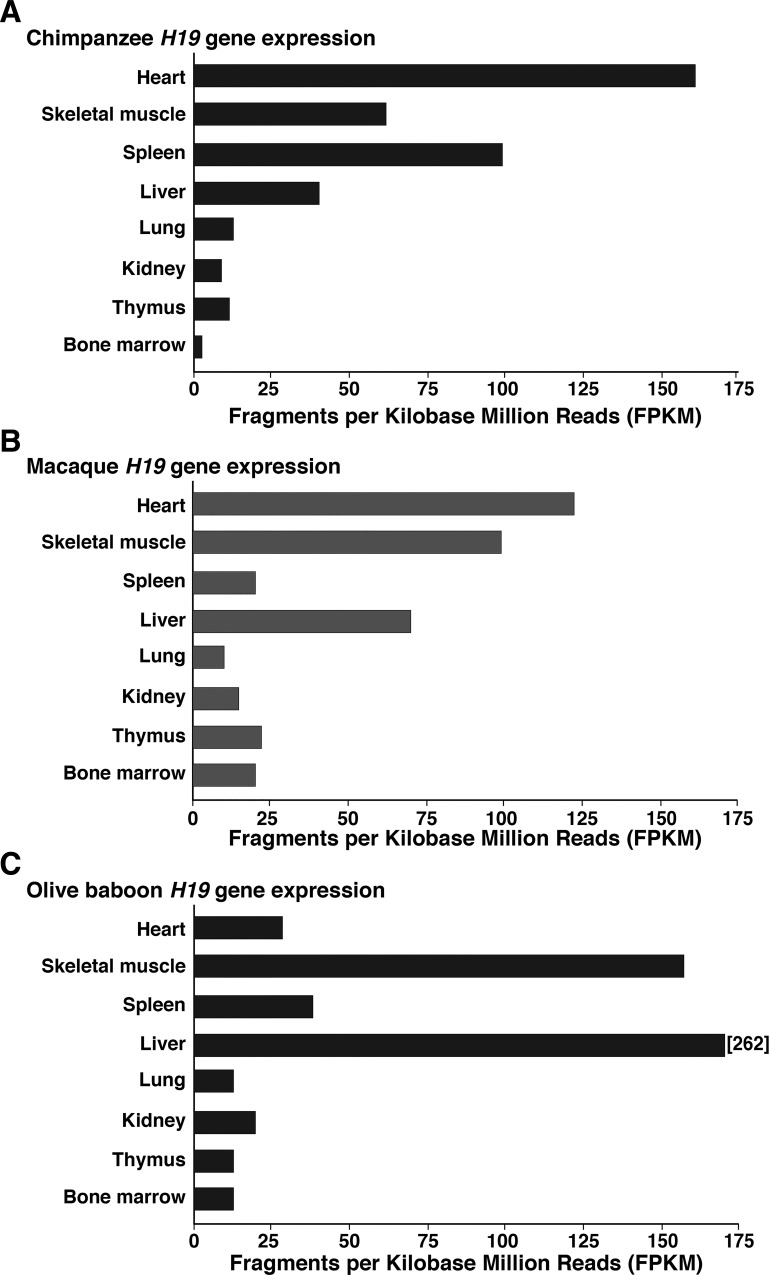
Analysis of information in the SRA NCBI data resource, in NCBI AceView, and in the Nonhuman Primate Reference revealed that H19 transcripts were expressed at varying levels in a variety of adult primate tissues, with the most complete data found for chimpanzee, macaque, and olive baboon (Fig. 5). There is limited information on promoter specificity, being available only for macaque, and demonstrating that both H19 promoters are active in heart, skeletal muscle, and bone marrow, but that promoter 2 predominates in thymus.
Fig. 5.
Relative expression of H19 mRNAs in primates. Results were obtained from the SRA NCBI (Sequence Read Archive of the National Center for Biotechnology Information), from NCBI AceView, and from the Nonhuman Primate Reference, and were derived from RNA sequencing experiments. Expression data are presented as sequenced fragments per kilobase million reads (FPKM) for different tissues from chimpanzee (A), macaque (B), and olive baboon (C). Tissue samples all were obtained from individual adult females (51). Little information was available for other species.
Conserved IGF2 protein sequences in primates.
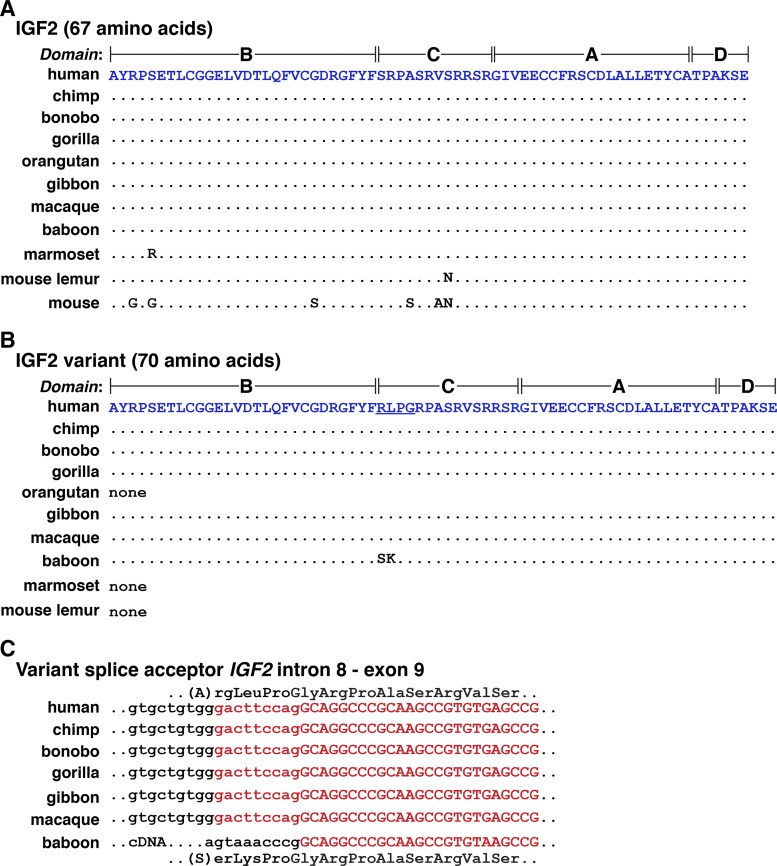
In humans, single-chain 67-residue IGF2, consisting of four domains, termed B, C, A, and D (6), is encoded within two different types of protein precursors that differ at the NH2 terminus because of mechanisms that include or exclude exon 5 in several classes of IGF2 mRNAs (Fig. 1, C and D). Among the nine nonhuman primates evaluated, mature IGF2 was identical to the human protein in seven species (chimpanzee, bonobo, gorilla, orangutan, gibbon, macaque, olive baboon), and there were single amino acid substitutions in the other two, marmoset (Ser5 to Arg) and mouse lemur (Ser36 to Asn) (Table 5, Fig. 6A). Of note, for marmoset and mouse lemur, no IGF2 was found in UniProt or other databases, and the predicted sequence of the protein was derived by translation of exons [also see (76)].
Table 5.
Percent amino acid identity with human IGF2
| Species | Signal Peptide (24 AA) | Signal Peptide 2 (80 AA) | Mature IGF2 (67 AA) | E Peptide (89 AA) |
|---|---|---|---|---|
| Chimpanzee | 100 | 100 | 100 | 100 |
| Bonobo | 100 | 100 | 100 | 100 |
| Gorilla | 100 | 100 | 100 | 98 |
| Orangutan | 100 | 96 | 100 | 97 |
| Gibbon | 100 | no exon 5 | 100 | 99 |
| Macaque | 100 | 95 | 100 | 94 |
| Baboon | 100 | none | 100 | 94 |
| Marmoset | 96 | none | 99 | 85 |
| Mouse lemur | 79 | 80 | 99 | 83* |
| Mouse | 80 | none | 91 | 82 |
86 amino acids (AA).
Fig. 6.
Alignments of mature IGF2 proteins. A: amino acid sequences of 67-amino acid IGF2 from 10 primates and the mouse are illustrated in single letter code. Dots depict identities, and differences among species are indicated. B: amino acid sequences of an IGF2 variant with 70 amino acids from different primates are depicted in single letter code. The altered residues are underlined for human IGF2. Dots depict identities, and differences among species are indicated. “None” indicates that the variant has not been identified or predicted in the given primate. C: the molecular basis for variant 70-amino acid IGF2 is a consequence of alternative splicing into IGF2 exon 9, which adds an additional 9 nucleotides (in lowercase and in red) to the 5′ end of the exon, and changes a serine codon into arginine-leucine-proline-glycine in all species listed except for olive baboon. In the latter, as based on a cDNA sequence deposited in GenBank, a different 5′ end of exon 9 is proposed (in black), which results in serine-lysine-proline-glycine codons. This sequence cannot be identified at the 3′ end of IGF2 intron 8 in the olive baboon genome, and thus the cDNA cannot be verified.
A variant 70-residue human IGF2 had been described previously based on cDNA sequencing results (31), in which the amino acids arginine, leucine, proline, and glycine replaced serine29 in the C domain of the protein (Fig. 6B). Subsequent studies demonstrated that this protein comprised ~25% of serum IGF2 in humans, and bound with lower affinity than 67-amino acid IGF2 to the IGF1 receptor (27). An IGF2 mRNA encoding this same protein was identified in chimpanzee, bonobo, gorilla, gibbon, and macaque (Fig. 6B). In these species and in humans, use of an alternative upstream splice acceptor site is responsible for adding nine nucleotides to the 5′ end of exon 9 that encode the extra amino acids (Fig. 6C). In olive baboon, a different 70-amino acid variant IGF2 has been predicted based on a cDNA sequence in GenBank, but the additional nucleotides 5′ to exon 9 (Fig. 6C) do not correspond to those found in the olive baboon genome, so the existence of this latter variant protein must be considered unproven. Similarly, a 69-residue human IGF2 has been described in which serine33 was replaced by cysteine, glycine, and aspartic acid in the C domain (79), but the corresponding nucleotide changes could not be found in the human genome.
Two different signal peptides are predicted for the human IGF2 protein precursor. One contains 24 amino acids and begins with a methionine codon near the 5′ end of IGF2 exon 8, and the other is predicted to have 80 residues and is encoded by exons 5 (54 codons) and 8 (26 codons) (Fig. 1, C and D; Fig. 7). Both appear to be highly conserved among the primates studied, with the only exception being mouse lemur (the short signal peptide differs by 5/24 amino acids, and the longer sequence by 16/80 residues; Table 5, Fig. 7). However, the potential 80-amino acid signal peptide could not be detected in gibbon, olive baboon, or marmoset (Table 5, Fig. 7B), and as it is far longer than other described mammalian signal sequences (72, 73), it is not known if mature IGF2 can be generated from this type of protein precursor.
Fig. 7.
Alignments of IGF2 signal peptides. Amino acid sequences of IGF2 signal peptides from 10 primates are shown in single letter code. A: 24-amino acid IGF2 signal peptide 1. B: 80-amino acid IGF2 signal peptide 2. The last 24 residues are identical to signal peptide 1. Dots depict identities, and differences among species are indicated.
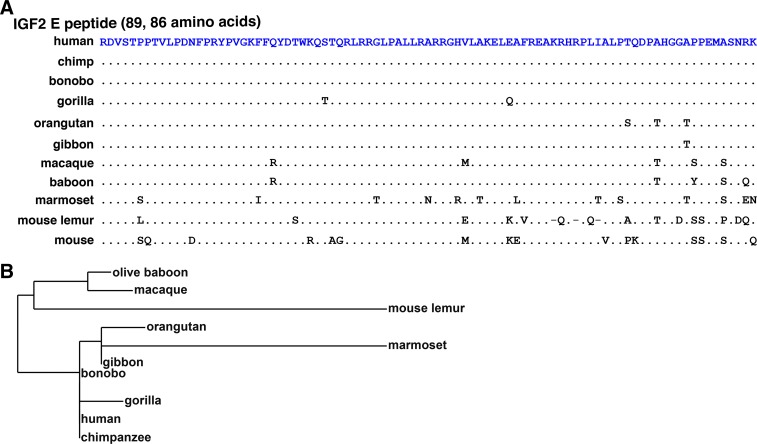
The E peptide is located at the COOH-terminal end of the IGF2 protein progenitor (Fig. 1D) and consists of 89 amino acids in all primates studied except for mouse lemur (86 residues; Table 5, Fig. 8A). The E region was conserved in all species except marmoset and mouse lemur, with no more than five amino acid differences from the human E domain being detected in seven other primate species (Table 5; Fig. 8, A and B).
Fig. 8.
Alignments of IGF2 E peptides. A: amino acid sequences of COOH-terminal IGF2 E peptides from 10 primates are depicted in single letter code. Dots indicate identities, dashes depict no residues, and differences among species are shown. The IGF2 E domain comprises 89 amino acids in all species except for mouse lemur (86 residues). B: phylogram of the IGF2 E peptide among primates. The length of each branch approximates the evolutionary distance based on these amino acid sequences.
IGF2 - H19 locus organization and regulation in primates.
The IGF2 - H19 locus in primates is illustrated in Fig. 9. The loci are very similar in most of these species in terms of gene order, length, and intergenic distance, particularly among human, chimpanzee, bonobo, and gorilla genomes. However, in orangutan, there has been a >10-fold increase in the distance between IGF2 and H19 compared with the other primates, and in a few species, the length of TNNT3 or MRPL23, their distance from each other, or the distance between H19 and MRPL23 has changed. Other features to note include the presence of a recognizable ICR in all nonhuman primates except for mouse lemur, as determined by DNA sequence similarity with the human ICR (65), although one-half is not detected in marmoset (Fig. 9, Table 6). Putative enhancer elements located 3′ to H19 also are conserved, with >88% identity being found for all nine elements in all species except for mouse lemur, where several segments are either not detectable or appear to be truncated (Table 7). Together, there is congruence in the overall structure of this locus and in the respective genes and regulatory elements among humans and nonhuman primates.
Table 6.
Percent nucleotide identity with human IGF2 - H19 ICR
| Species | Human ICR, 4260 bp |
|---|---|
| Chimpanzee | 99 |
| Bonobo | 99 |
| Gorilla | 97 |
| Orangutan | 95 |
| Gibbon | 94 (3388) |
| Macaque | 90 (4112) |
| Olive baboon | 90 (3704) |
| Marmoset | 82 (1590) |
| Mouse lemur | no match |
Number of base pairs aligned is in parentheses if less than length of human segment.
Table 7.
Percent nucleotide identity with human IGF2 - H19 locus enhancers
| Species | CS 1, 240 bp | CS 2, 472 bp | CS 4, 360 bp | CS 5, 360 bp | CS 6, 360 bp | CS 7, 240 bp | CS 8, 300 bp | CS 9, 486 bp | CS 10, 300 bp |
|---|---|---|---|---|---|---|---|---|---|
| Chimpanzee | 98 | 98 | 99 | 99 | 99 | 98 | 99 | 99 | 99 |
| Bonobo | 98 | 98 | 99 | 99 | 99 | 99 | 99 | 99 | 98 |
| Gorilla | 99 | 99 | 99 | 99 | 99 | 98 | 98 | 98 | 98 |
| Orangutan | 99 | 98 | 96 | 98 | 99 | 97 | 96 | 99 | 96 |
| Gibbon | 98 | 99 | 98 | 98 | 99 | 94 | 95 | 98 | 99 |
| Macaque | 95 | 96 | 97 | 96 | 96 | 93 | 93 | 97 | 92 |
| Olive baboon | 94 | 96 | 97 | 95 | 95 | 93 | 93 | 97 | 92 |
| Marmoset | 88 | 94 | 94 | 93 | 92 | 90 | 90 | 91 | 93 |
| Mouse lemur | no match | no match | 87 (128) | 88 (44) | 86 (91) | 87 (100) | 85 (200) | 91 (92) | 93 (214) |
DISCUSSION
Human IGF2 is a complicated gene that encodes a small and relatively simple 67-residue secreted single-chain protein (42, 45, 60, 66, 67). Five distinct gene promoters govern the expression of six different classes of IGF2 mRNAs that are translated into two different protein precursors (Fig. 1) (60). The primates examined here all encode single-copy IGF2 genes that share features with human IGF2, such as gene length and overall structure, including the presence of five promoters (except for gibbon, Fig. 2 and Table 2) and 10 exons (except for marmoset and gibbon, Fig. 2 and Table 1). The encoded protein precursors also are highly similar (Figs. 6–8, Table 5) (76), in agreement with previous observations that examined the coding regions of 15,027 orthologous genes in five primate species (human, colobus monkey, rhesus macaque, tamarin, vervet), and identified IGF2 as being in the top 17% of conserved predicted proteins (23).
Human IGF2 resides in a locus with several additional nearby genes, including TH, INS, H19, and others (Fig. 1A), that are conserved in both chromosomal order and transcriptional orientation with their mouse homologs (45). All of these genes are present and arranged in identical order, although the distance between IGF2 and H19 was far greater in orangutan than in the other nonhuman primates analyzed here (Fig. 9). Like IGF2, H19 also is conserved in the 10 species studied and contains two promoters and six exons, except for marmoset (2 promoters, 5 exons) and mouse lemur (1 promoter, 4 exons) (Fig. 4, Table 3). In both mice and humans, parental imprinting appears to be a key mechanism of gene regulation for IGF2/Igf2 and H19, with an ICR located just 5′ to H19 playing a critical role in controlling chromosome of origin-specific gene activity through the actions of the transcription factor, CTCF at the ICR (19, 50, 75), as noted in the introduction. Although most of the studies defining CTCF-mediated mechanisms of imprinting control have been performed in mice, in humans, “experiments of nature” also have demonstrated its role IGF2 gene regulation. Rare individuals have been shown to have ICR deletion mutations and corresponding biallelic expression of IGF2 and silencing of H19 (65). While no comparable studies have been described in nonhuman primates, the ~4,260 base pair bipartite human ICR (65) is conserved among most of the species studied here (90–99% identity, Table 6), the exception being mouse lemur, although in marmoset only one of the two segments could be identified (Table 6, Fig. 9). In addition, homologs of the nine putative distal enhancers identified previously by DNA sequence similarity and syntenic locations between human IGF2 - H19 and mouse Igf2 - H19 loci (60), also are present in eight of nine nonhuman primate genomes, the exception again being mouse lemur in which only seven of the elements could be detected (Table 7, Fig. 9).
There also is substantial DNA sequence identity for each of the five human IGF2 promoters among the nonhuman primates studied here (Table 2), most notably for P1, in which C/EBPα, C/EBPβ, and SP1 transcription factors have been shown to stimulate promoter activity through proximal control elements (56, 57), but less so for P3, where AP2 has been found to be stimulatory (55) and the WT1 Wilms tumor repressor to be inhibitory (18). At present, very preliminary gene expression data show that in chimpanzee, macaque, and marmoset, P1 or P3 are more active in the liver than other IGF2 promoters (data not shown). In contrast, more extensive studies have found that P4 is most active in human liver (60).
Somatic growth in humans and other species is controlled by both genetic and environmental factors (4). For example, major alterations in IGF2 expression have been causally linked to the overgrowth and undergrowth disorders Beckwith-Wiedemann and Silver-Russell syndromes, respectively (3, 20). More broadly, many classes of genes have an impact on pre- and postnatal growth rates and duration and thus on final adult height (4, 44). Some of these genetic influences may be traced to our ancestors, not only from extinct human populations, such as Neanderthals and Denisovans (4, 33, 71), but also from common primate progenitors. In this context, recent observations addressing genetic influences on human height have identified a small number of uncommon single gene variants with large potential effects (41). The protein product of STC2, one of these rare modified genes, potentially can influence the bioavailability of IGF2 (41). Each of the nucleotide variants identified (see SNPs rs148833559 and rs146441603) predicts an amino acid substitution in the encoded protein, stanniocalcin 2 (STC2; R44 > L, and M86 > I, respectively) (41). STC2 inhibits the actions of PAPP-A (32), a secreted metalloproteinase that is highly expressed during human pregnancy (9, 11). PAPP-A cleaves IGF binding protein-4 (IGFBP-4), which when intact binds to and limits the biological actions of IGF1 and IGF2 (9, 32). Mice lacking the Papp-a gene show diminished somatic growth, presumably because of unrestrained activity of IGFBP-4 (10). Humans with one of these uncommon STC2 gene variants are 1–2 cm taller than otherwise predicted, potentially because of reduced inhibitory activity toward PAPP-A that leads to a decline in steady-state abundance of IGFBP-4 and thus more available IGF1 and IGF2. A multiple sequence alignment of STC2 in humans and seven other primates demonstrates that the proteins are 88% identical and reveals that R44 and M86 are conserved in all of these species (data not shown), suggesting that comparable mechanisms could be at play in these organisms should similar variants be present in specific individuals.
Every individual human genome contains millions of DNA sequence polymorphisms (46, 47), and many of these have the potential to modify protein function or gene expression because of their location within coding regions, promoters, enhancers, or other regulatory DNA elements (2). The IGF2 gene is no exception, and nonsynonymous SNPs have been identified within the part of exon 10 encoding the E domain, changing R156 to H [see rs61732764; found in ~0.4% of human genomes (59)], at the splice acceptor site in the intron 4-exon 5 junction [see rs149483638; found in ~2% of the population (59)], and in many other sites in the gene and locus. Comparable data addressing genomic variability are limited in nonhuman primates, most likely because of the far smaller number of genomes that have been sequenced to date. In the most recent update of the macaque genome (Mmul_8.0.1, release 91), over 90 SNPs have been mapped to the IGF2 gene and more to 5′ flanking DNA. Two of these SNPs potentially cause amino acid substitutions: rs295002480 predicts a change of T30 to A in the alternative signal peptide, and rs311303169 predicts the modification of the penultimate residue of the E domain from W179 to A [also see (78) for more general aspects of DNA polymorphisms in macaques]. Similar types of polymorphic variants most likely exist in other primates as well, although relatively small population sizes compared with humans will limit their potential to provide insights into primate speciation or other aspects of their growth biology. For example, nearly 10 million SNPs have been identified in both Sumatran and Bornean orangutans (38) and potentially could be used to determine if the >1.5 Mb distance between orangutan IGF2 and H19 genes (see Fig. 9) precludes or otherwise modifies their chromosome of origin-specific imprinting.
The essential roles of IGF2 in growth and other aspects of human physiology are potentially reflected by its complex gene organization and mechanisms of regulation. The extensive structural and DNA sequence similarity in the IGF2 and H19 genes and within the IGF2 - H19 locus among the 10 species studied here, and the high level of amino acid conservation in the IGF2 protein, gives support to the provisional idea that functional mechanisms have maintained common regulatory processes since primate speciation began over 85 million years ago (49, 58). Detailed study of other individual genes and loci using careful analysis of data embedded in genomic repositories has the potential to provide new insights into the evolutionary origins of different physiological and pathological functions in humans and other primate lineages.
GRANTS
These studies were supported in part by National Institutes of Health Research Grant R01 DK-042748-28 (to P. Rotwein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
P.R. conceived and designed research; performed experiments; analyzed data; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Acuna-Hidalgo R, Veltman JA, Hoischen A. New insights into the generation and role of de novo mutations in health and disease. Genome Biol 17: 241, 2016. doi: 10.1186/s13059-016-1110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet 16: 197–212, 2015. doi: 10.1038/nrg3891. [DOI] [PubMed] [Google Scholar]
- 3.Azzi S, Abi Habib W, Netchine I. Beckwith-Wiedemann and Russell-Silver Syndromes: from new molecular insights to the comprehension of imprinting regulation. Curr Opin Endocrinol Diabetes Obes 21: 30–38, 2014. doi: 10.1097/MED.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 4.Baron J, Sävendahl L, De Luca F, Dauber A, Phillip M, Wit JM, Nilsson O. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol 11: 735–746, 2015. doi: 10.1038/nrendo.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battle A, Brown CD, Engelhardt BE, Montgomery SB; GTEx Consortium, Laboratory, Data Analysis &Coordinating Center (LDACC)—Analysis Working Group, Statistical Methods groups—Analysis Working Group, Enhancing GTEx (eGTEx) groups, NIH Common Fund, NIH/NCI, NIH/NHGRI, NIH/NIMH, NIH/NIDA, Biospecimen Collection Source Site—NDRI, Biospecimen Collection Source Site—RPCI, Biospecimen Core Resource—VARI, Brain Bank Repository—University of Miami Brain Endowment Bank, Leidos Biomedical—Project Management, ELSI Study, Genome Browser Data Integration &Visualization—EBI, Genome Browser Data Integration &Visualization—UCSC Genomics Institute, University of California Santa Cruz, Lead analysts, Laboratory, Data Analysis &Coordinating Center (LDACC), NIH program management, Biospecimen collection, Pathology; eQTL manuscript working group . Genetic effects on gene expression across human tissues. Nature 550: 204–213, 2017. doi: 10.1038/nature24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blundell TL, Humbel RE. Hormone families: pancreatic hormones and homologous growth factors. Nature 287: 781–787, 1980. doi: 10.1038/287781a0. [DOI] [PubMed] [Google Scholar]
- 7.Brown AL, Graham DE, Nissley SP, Hill DJ, Strain AJ, Rechler MM. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem 261: 13144–13150, 1986. [PubMed] [Google Scholar]
- 8.Butter F, Kappei D, Buchholz F, Vermeulen M, Mann M. A domesticated transposon mediates the effects of a single-nucleotide polymorphism responsible for enhanced muscle growth. EMBO Rep 11: 305–311, 2010. doi: 10.1038/embor.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab 23: 242–249, 2012. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131: 1187–1194, 2004. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 11.Conover CA, Oxvig C. PAPP-A: a promising therapeutic target for healthy longevity. Aging Cell 16: 205–209, 2017. doi: 10.1111/acel.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417: 945–948, 2002. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 13.Das R, Dobens LL. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochem Soc Trans 43: 1057–1062, 2015. doi: 10.1042/BST20150078. [DOI] [PubMed] [Google Scholar]
- 14.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10: 68–91, 1989. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 15.de Pagter-Holthuizen P, Jansen M, van Schaik FM, van der Kammen R, Oosterwijk C, Van den Brande JL, Sussenbach JS. The human insulin-like growth factor II gene contains two development-specific promoters. FEBS Lett 214: 259–264, 1987. doi: 10.1016/0014-5793(87)80066-2. [DOI] [PubMed] [Google Scholar]
- 16.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64: 849–859, 1991. doi: 10.1016/0092-8674(91)90513-X. [DOI] [PubMed] [Google Scholar]
- 17.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36, Web Server: W465–W469, 2008. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond IA, Madden SL, Rohwer-Nutter P, Bell GI, Sukhatme VP, Rauscher FJ III. Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science 257: 674–678, 1992. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- 19.Edwards CA, Ferguson-Smith AC. Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol 19: 281–289, 2007. doi: 10.1016/j.ceb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Eggermann T, Begemann M, Spengler S, Schröder C, Kordass U, Binder G. Genetic and epigenetic findings in Silver-Russell syndrome. Pediatr Endocrinol Rev 8: 86–93, 2010. [PubMed] [Google Scholar]
- 22.Gems D, Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 75: 621–644, 2013. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- 23.George RD, McVicker G, Diederich R, Ng SB, MacKenzie AP, Swanson WJ, Shendure J, Thomas JH. Trans genomic capture and sequencing of primate exomes reveals new targets of positive selection. Genome Res 21: 1686–1694, 2011. doi: 10.1101/gr.121327.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannoukakis N, Deal C, Paquette J, Goodyer CG, Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat Genet 4: 98–101, 1993. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- 25.Graham DE, Rechler MM, Brown AL, Frunzio R, Romanus JA, Bruni CB, Whitfield HJ, Nissley SP, Seelig S, Berry S. Coordinate developmental regulation of high and low molecular weight mRNAs for rat insulin-like growth factor II. Proc Natl Acad Sci USA 83: 4519–4523, 1986. doi: 10.1073/pnas.83.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray A, Tam AW, Dull TJ, Hayflick J, Pintar J, Cavenee WK, Koufos A, Ullrich A. Tissue-specific and developmentally regulated transcription of the insulin-like growth factor 2 gene. DNA 6: 283–295, 1987. doi: 10.1089/dna.1987.6.283. [DOI] [PubMed] [Google Scholar]
- 27.Hampton B, Burgess WH, Marshak DR, Cullen KJ, Perdue JF. Purification and characterization of an insulin-like growth factor II variant from human plasma. J Biol Chem 264: 19155–19160, 1989. [PubMed] [Google Scholar]
- 28.Holthuizen P, van der Lee FM, Ikejiri K, Yamamoto M, Sussenbach JS. Identification and initial characterization of a fourth leader exon and promoter of the human IGF-II gene. Biochim Biophys Acta 1087: 341–343, 1990. doi: 10.1016/0167-4781(90)90010-Y. [DOI] [PubMed] [Google Scholar]
- 29.Ikejiri K, Wasada T, Haruki K, Hizuka N, Hirata Y, Yamamoto M. Identification of a novel transcription unit in the human insulin-like growth factor-II gene. Biochem J 280: 439–444, 1991. doi: 10.1042/bj2800439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishihara K, Hatano N, Furuumi H, Kato R, Iwaki T, Miura K, Jinno Y, Sasaki H. Comparative genomic sequencing identifies novel tissue-specific enhancers and sequence elements for methylation-sensitive factors implicated in Igf2/H19 imprinting. Genome Res 10: 664–671, 2000. doi: 10.1101/gr.10.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen M, van Schaik FM, van Tol H, Van den Brande JL, Sussenbach JS. Nucleotide sequences of cDNAs encoding precursors of human insulin-like growth factor II (IGF-II) and an IGF-II variant. FEBS Lett 179: 243–246, 1985. doi: 10.1016/0014-5793(85)80527-5. [DOI] [PubMed] [Google Scholar]
- 32.Jepsen MR, Kløverpris S, Mikkelsen JH, Pedersen JH, Füchtbauer EM, Laursen LS, Oxvig C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem 290: 3430–3439, 2015. doi: 10.1074/jbc.M114.611665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, McLaughlin RL, Gallego Llorente M, Cassidy LM, Gamba C, Meshveliani T, Bar-Yosef O, Müller W, Belfer-Cohen A, Matskevich Z, Jakeli N, Higham TF, Currat M, Lordkipanidze D, Hofreiter M, Manica A, Pinhasi R, Bradley DG. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat Commun 6: 8912, 2015. doi: 10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsanis N. The continuum of causality in human genetic disorders. Genome Biol 17: 233, 2016. doi: 10.1186/s13059-016-1107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JE, Pintar J, Efstratiadis A. Pattern of the insulin-like growth factor II gene expression during early mouse embryogenesis. Development 110: 151–159, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Livingstone C. IGF2 and cancer. Endocr Relat Cancer 20: R321–R339, 2013. doi: 10.1530/ERC-13-0231. [DOI] [PubMed] [Google Scholar]
- 37.Livingstone C, Borai A. Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin Endocrinol (Oxf) 80: 773–781, 2014. doi: 10.1111/cen.12446. [DOI] [PubMed] [Google Scholar]
- 38.Locke DP, Hillier LW, Warren WC, Worley KC, Nazareth LV, Muzny DM, Yang SP, Wang Z, Chinwalla AT, Minx P, Mitreva M, Cook L, Delehaunty KD, Fronick C, Schmidt H, Fulton LA, Fulton RS, Nelson JO, Magrini V, Pohl C, Graves TA, Markovic C, Cree A, Dinh HH, Hume J, Kovar CL, Fowler GR, Lunter G, Meader S, Heger A, Ponting CP, Marques-Bonet T, Alkan C, Chen L, Cheng Z, Kidd JM, Eichler EE, White S, Searle S, Vilella AJ, Chen Y, Flicek P, Ma J, Raney B, Suh B, Burhans R, Herrero J, Haussler D, Faria R, Fernando O, Darré F, Farré D, Gazave E, Oliva M, Navarro A, Roberto R, Capozzi O, Archidiacono N, Della Valle G, Purgato S, Rocchi M, Konkel MK, Walker JA, Ullmer B, Batzer MA, Smit AF, Hubley R, Casola C, Schrider DR, Hahn MW, Quesada V, Puente XS, Ordoñez GR, López-Otín C, Vinar T, Brejova B, Ratan A, Harris RS, Miller W, Kosiol C, Lawson HA, Taliwal V, Martins AL, Siepel A, Roychoudhury A, Ma X, Degenhardt J, Bustamante CD, Gutenkunst RN, Mailund T, Dutheil JY, Hobolth A, Schierup MH, Ryder OA, Yoshinaga Y, de Jong PJ, Weinstock GM, Rogers J, Mardis ER, Gibbs RA, Wilson RK. Comparative and demographic analysis of orang-utan genomes. Nature 469: 529–533, 2011. doi: 10.1038/nature09687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manolio TA, Fowler DM, Starita LM, Haendel MA, MacArthur DG, Biesecker LG, Worthey E, Chisholm RL, Green ED, Jacob HJ, McLeod HL, Roden D, Rodriguez LL, Williams MS, Cooper GM, Cox NJ, Herman GE, Kingsmore S, Lo C, Lutz C, MacRae CA, Nussbaum RL, Ordovas JM, Ramos EM, Robinson PN, Rubinstein WS, Seidman C, Stranger BE, Wang H, Westerfield M, Bult C. Bedside back to bench: building bridges between basic and clinical genomic research. Cell 169: 6–12, 2017. doi: 10.1016/j.cell.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markljung E, Jiang L, Jaffe JD, Mikkelsen TS, Wallerman O, Larhammar M, Zhang X, Wang L, Saenz-Vash V, Gnirke A, Lindroth AM, Barrés R, Yan J, Strömberg S, De S, Pontén F, Lander ES, Carr SA, Zierath JR, Kullander K, Wadelius C, Lindblad-Toh K, Andersson G, Hjälm G, Andersson L. ZBED6, a novel transcription factor derived from a domesticated DNA transposon regulates IGF2 expression and muscle growth. PLoS Biol 7: e1000256, 2009. doi: 10.1371/journal.pbio.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR, Fine RS, Lu Y, Schurmann C, Highland HM, Rüeger S, Thorleifsson G, Justice AE, Lamparter D, Stirrups KE, Turcot V, Young KL, Winkler TW, Esko T, Karaderi T, Locke AE, Masca NG, Ng MC, Mudgal P, Rivas MA, Vedantam S, Mahajan A, Guo X, Abecasis G, Aben KK, Adair LS, Alam DS, Albrecht E, Allin KH, Allison M, Amouyel P, Appel EV, Arveiler D, Asselbergs FW, Auer PL, Balkau B, Banas B, Bang LE, Benn M, Bergmann S, Bielak LF, Blüher M, Boeing H, Boerwinkle E, Böger CA, Bonnycastle LL, Bork-Jensen J, Bots ML, Bottinger EP, Bowden DW, Brandslund I, Breen G, Brilliant MH, Broer L, Burt AA, Butterworth AS, Carey DJ, Caulfield MJ, Chambers JC, Chasman DI, Chen YI, Chowdhury R, Christensen C, Chu AY, Cocca M, Collins FS, Cook JP, Corley J, Galbany JC, Cox AJ, Cuellar-Partida G, Danesh J, Davies G, de Bakker PI, de Borst GJ, de Denus S, de Groot MC, de Mutsert R, Deary IJ, Dedoussis G, Demerath EW, den Hollander AI, Dennis JG, Di Angelantonio E, Drenos F, Du M, Dunning AM, Easton DF, Ebeling T, Edwards TL, Ellinor PT, Elliott P, Evangelou E, Farmaki AE, Faul JD, Feitosa MF, Feng S, Ferrannini E, Ferrario MM, Ferrieres J, Florez JC, Ford I, Fornage M, Franks PW, Frikke-Schmidt R, Galesloot TE, Gan W, Gandin I, Gasparini P, Giedraitis V, Giri A, Girotto G, Gordon SD, Gordon-Larsen P, Gorski M, Grarup N, Grove ML, Gudnason V, Gustafsson S, Hansen T, Harris KM, Harris TB, Hattersley AT, Hayward C, He L, Heid IM, Heikkilä K, Helgeland Ø, Hernesniemi J, Hewitt AW, Hocking LJ, Hollensted M, Holmen OL, Hovingh GK, Howson JM, Hoyng CB, Huang PL, Hveem K, Ikram MA, Ingelsson E, Jackson AU, Jansson JH, Jarvik GP, Jensen GB, Jhun MA, Jia Y, Jiang X, Johansson S, Jørgensen ME, Jørgensen T, Jousilahti P, Jukema JW, Kahali B, Kahn RS, Kähönen M, Kamstrup PR, Kanoni S, Kaprio J, Karaleftheri M, Kardia SL, Karpe F, Kee F, Keeman R, Kiemeney LA, Kitajima H, Kluivers KB, Kocher T, Komulainen P, Kontto J, Kooner JS, Kooperberg C, Kovacs P, Kriebel J, Kuivaniemi H, Küry S, Kuusisto J, La Bianca M, Laakso M, Lakka TA, Lange EM, Lange LA, Langefeld CD, Langenberg C, Larson EB, Lee IT, Lehtimäki T, Lewis CE, Li H, Li J, Li-Gao R, Lin H, Lin LA, Lin X, Lind L, Lindström J, Linneberg A, Liu Y, Liu Y, Lophatananon A, Luan J, Lubitz SA, Lyytikäinen LP, Mackey DA, Madden PA, Manning AK, Männistö S, Marenne G, Marten J, Martin NG, Mazul AL, Meidtner K, Metspalu A, Mitchell P, Mohlke KL, Mook-Kanamori DO, Morgan A, Morris AD, Morris AP, Müller-Nurasyid M, Munroe PB, Nalls MA, Nauck M, Nelson CP, Neville M, Nielsen SF, Nikus K, Njølstad PR, Nordestgaard BG, Ntalla I, O’Connel JR, Oksa H, Loohuis LM, Ophoff RA, Owen KR, Packard CJ, Padmanabhan S, Palmer CN, Pasterkamp G, Patel AP, Pattie A, Pedersen O, Peissig PL, Peloso GM, Pennell CE, Perola M, Perry JA, Perry JR, Person TN, Pirie A, Polasek O, Posthuma D, Raitakari OT, Rasheed A, Rauramaa R, Reilly DF, Reiner AP, Renström F, Ridker PM, Rioux JD, Robertson N, Robino A, Rolandsson O, Rudan I, Ruth KS, Saleheen D, Salomaa V, Samani NJ, Sandow K, Sapkota Y, Sattar N, Schmidt MK, Schreiner PJ, Schulze MB, Scott RA, Segura-Lepe MP, Shah S, Sim X, Sivapalaratnam S, Small KS, Smith AV, Smith JA, Southam L, Spector TD, Speliotes EK, Starr JM, Steinthorsdottir V, Stringham HM, Stumvoll M, Surendran P, ’t Hart LM, Tansey KE, Tardif JC, Taylor KD, Teumer A, Thompson DJ, Thorsteinsdottir U, Thuesen BH, Tönjes A, Tromp G, Trompet S, Tsafantakis E, Tuomilehto J, Tybjaerg-Hansen A, Tyrer JP, Uher R, Uitterlinden AG, Ulivi S, van der Laan SW, Van Der Leij AR, van Duijn CM, van Schoor NM, van Setten J, Varbo A, Varga TV, Varma R, Edwards DR, Vermeulen SH, Vestergaard H, Vitart V, Vogt TF, Vozzi D, Walker M, Wang F, Wang CA, Wang S, Wang Y, Wareham NJ, Warren HR, Wessel J, Willems SM, Wilson JG, Witte DR, Woods MO, Wu Y, Yaghootkar H, Yao J, Yao P, Yerges-Armstrong LM, Young R, Zeggini E, Zhan X, Zhang W, Zhao JH, Zhao W, Zhao W, Zheng H, Zhou W, Rotter JI, Boehnke M, Kathiresan S, McCarthy MI, Willer CJ, Stefansson K, Borecki IB, Liu DJ, North KE, Heard-Costa NL, Pers TH, Lindgren CM, Oxvig C, Kutalik Z, Rivadeneira F, Loos RJ, Frayling TM, Hirschhorn JN, Deloukas P, Lettre G; EPIC-InterAct Consortium; CHD Exome+ Consortium; ExomeBP Consortium; T2D-Genes Consortium; GoT2D Genes Consortium; Global Lipids Genetics Consortium; ReproGen Consortium; MAGIC Investigators . Rare and low-frequency coding variants alter human adult height. Nature 542: 186–190, 2017. doi: 10.1038/nature21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monk D, Sanches R, Arnaud P, Apostolidou S, Hills FA, Abu-Amero S, Murrell A, Friess H, Reik W, Stanier P, Constância M, Moore GE. Imprinting of IGF2 P0 transcript and novel alternatively spliced INS-IGF2 isoforms show differences between mouse and human. Hum Mol Genet 15: 1259–1269, 2006. doi: 10.1093/hmg/ddl041. [DOI] [PubMed] [Google Scholar]
- 43.Moore T, Constancia M, Zubair M, Bailleul B, Feil R, Sasaki H, Reik W. Multiple imprinted sense and antisense transcripts, differential methylation and tandem repeats in a putative imprinting control region upstream of mouse Igf2. Proc Natl Acad Sci USA 94: 12509–12514, 1997. doi: 10.1073/pnas.94.23.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narasimhan S. Matters of size. Cell 163: 1043–1045, 2015. doi: 10.1016/j.cell.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Nordin M, Bergman D, Halje M, Engström W, Ward A. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Prolif 47: 189–199, 2014. doi: 10.1111/cpr.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuttle X, Giannuzzi G, Duyzend MH, Schraiber JG, Narvaiza I, Sudmant PH, Penn O, Chiatante G, Malig M, Huddleston J, Benner C, Camponeschi F, Ciofi-Baffoni S, Stessman HA, Marchetto MC, Denman L, Harshman L, Baker C, Raja A, Penewit K, Janke N, Tang WJ, Ventura M, Banci L, Antonacci F, Akey JM, Amemiya CT, Gage FH, Reymond A, Eichler EE. Emergence of a Homo sapiens-specific gene family and chromosome 16p11.2 CNV susceptibility. Nature 536: 205–209, 2016. doi: 10.1038/nature19075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nat Rev Genet 16: 275–284, 2015. doi: 10.1038/nrg3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng X, Thierry-Mieg J, Thierry-Mieg D, Nishida A, Pipes L, Bozinoski M, Thomas MJ, Kelly S, Weiss JM, Raveendran M, Muzny D, Gibbs RA, Rogers J, Schroth GP, Katze MG, Mason CE. Tissue-specific transcriptome sequencing analysis expands the non-human primate reference transcriptome resource (NHPRTR). Nucleic Acids Res 43, D1: D737–D742, 2015. doi: 10.1093/nar/gku1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MA, Kessing B, Pontius J, Roelke M, Rumpler Y, Schneider MP, Silva A, O’Brien SJ, Pecon-Slattery J. A molecular phylogeny of living primates. PLoS Genet 7: e1001342, 2011. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell 137: 1194–1211, 2009. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pipes L, Li S, Bozinoski M, Palermo R, Peng X, Blood P, Kelly S, Weiss JM, Thierry-Mieg J, Thierry-Mieg D, Zumbo P, Chen R, Schroth GP, Mason CE, Katze MG. The non-human primate reference transcriptome resource (NHPRTR) for comparative functional genomics. Nucleic Acids Res 41, D1: D906–D914, 2013. doi: 10.1093/nar/gks1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159–169, 2012. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 53.Quintana-Murci L. Understanding rare and common diseases in the context of human evolution. Genome Biol 17: 225, 2016. doi: 10.1186/s13059-016-1093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raizis AM, Eccles MR, Reeve AE. Structural analysis of the human insulin-like growth factor-II P3 promoter. Biochem J 289: 133–139, 1993. doi: 10.1042/bj2890133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rietveld LE, Koonen-Reemst AM, Sussenbach JS, Holthuizen PE. Dual role for transcription factor AP-2 in the regulation of the major fetal promoter P3 of the gene for human insulin-like growth factor II. Biochem J 338: 799–806, 1999. doi: 10.1042/bj3380799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodenburg RJ, Holthuizen PE, Sussenbach JS. A functional Sp1 binding site is essential for the activity of the adult liver-specific human insulin-like growth factor II promoter. Mol Endocrinol 11: 237–250, 1997. doi: 10.1210/mend.11.2.9888. [DOI] [PubMed] [Google Scholar]
- 57.Rodenburg RJ, Teertstra W, Holthuizen PE, Sussenbach JS. Postnatal liver-specific expression of human insulin-like growth factor-II is highly stimulated by the transcriptional activators liver-enriched activating protein and CCAAT/enhancer binding protein-alpha. Mol Endocrinol 9: 424–434, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Rogers J, Gibbs RA. Comparative primate genomics: emerging patterns of genome content and dynamics. Nat Rev Genet 15: 347–359, 2014. doi: 10.1038/nrg3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rotwein P. Large scale analysis of variation in the insulin-like growth factor family in humans reveals rare disease links and common polymorphisms. J Biol Chem, 292: 19608, 2017. doi: 10.1074/jbc.AAC117.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rotwein P. The complex genetics of human insulin-like growth factor 2 are not reflected in public databases. J Biol Chem 293: 4324–4333, 2018. doi: 10.1074/jbc.RA117.001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rotwein P, Hall LJ. Evolution of insulin-like growth factor II: characterization of the mouse IGF-II gene and identification of two pseudo-exons. DNA Cell Biol 9: 725–735, 1990. doi: 10.1089/dna.1990.9.725. [DOI] [PubMed] [Google Scholar]
- 62.Rotwein P. Molecular biology of IGF-I and IGF-II, in The IGF System (Rosenfeld RG, Roberts CT Jr, editors). Totowa, NH: Humana Press, 1999, p. 19–35. doi: 10.1007/978-1-59259-712-3_2. [DOI] [Google Scholar]
- 63.Schwartz TS, Bronikowski AM. Evolution and function of the insulin and insulin-like signaling network in ectothermic reptiles: some answers and more questions. Integr Comp Biol 56: 171–184, 2016. doi: 10.1093/icb/icw046. [DOI] [PubMed] [Google Scholar]
- 64.Soares MB, Ishii DN, Efstratiadis A. Developmental and tissue-specific expression of a family of transcripts related to rat insulin-like growth factor II mRNA. Nucleic Acids Res 13: 1119–1134, 1985. doi: 10.1093/nar/13.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sparago A, Cerrato F, Vernucci M, Ferrero GB, Silengo MC, Riccio A. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat Genet 36: 958–960, 2004. doi: 10.1038/ng1410. [DOI] [PubMed] [Google Scholar]
- 65a.Stranger BE, Brigham LE, Hasz R, Hunter M, Johns C, Johnson M, Kopen G, Leinweber WF, Lonsdale JT, McDonald A, Mestichelli B, Myer K, Roe B, Salvatore M, Shad S, Thomas JA, Walters G, Washington M, Wheeler J, Bridge J, Foster BA, Gillard BM, Karasik E, Kumar R, Miklos M, Moser MT, Jewell SD, Montroy RG, Rohrer DC, Valley DR, Davis DA, Mash DC, Gould SE, Guan P, Koester S, Little AR, Martin C, Moore HM, Rao A, Struewing JP, Volpi S, Hansen KD, Hickey PF, Rizzardi LF, Hou L, Liu Y, Molinie B, Park Y, Rinaldi N, Wang L, Van Wittenberghe N, Claussnitzer M, Gelfand ET, Li Q, Linder S, Zhang R, Smith KS, Tsang EK, Chen LS, Demanelis K, Doherty JA, Jasmine F, Kibriya MG, Jiang L, Lin S, Wang M, Jian R, Li X, Chan J, Bates D, Diegel M, Halow J, Haugen E, Johnson A, Kaul R, Lee K, Maurano MT, Nelson J, Neri FJ, Sandstrom R, Fernando MS, Linke C, Oliva M, Skol A, Wu F, Akey JM, Feinberg AP, Li JB, Pierce BL, Stamatoyannopoulos JA, Tang H, Ardlie KG, Kellis M, Snyder MP, Montgomery SB; eGTEx Project . Enhancing GTEx by bridging the gaps between genotype, gene expression, and disease. Nat Genet 49: 1664–1670, 2017. doi: 10.1038/ng.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sussenbach JS, Rodenburg RJ, Scheper W, Holthuizen P. Transcriptional and post-transcriptional regulation of the human IGF-II gene expression. Adv Exp Med Biol 343: 63–71, 1993. doi: 10.1007/978-1-4615-2988-0_7. [DOI] [PubMed] [Google Scholar]
- 67.Sussenbach JS, Steenbergh PH, Holthuizen P. Structure and expression of the human insulin-like growth factor genes. Growth Regul 2: 1–9, 1992. [PubMed] [Google Scholar]
- 68.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol 7, Suppl 1: S12.1–S14, 2006. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Dijk MA, van Schaik FM, Bootsma HJ, Holthuizen P, Sussenbach JS. Initial characterization of the four promoters of the human insulin-like growth factor II gene. Mol Cell Endocrinol 81: 81–94, 1991. doi: 10.1016/0303-7207(91)90207-9. [DOI] [PubMed] [Google Scholar]
- 70.Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, Archibald AL, Haley CS, Buys N, Tally M, Andersson G, Georges M, Andersson L. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature 425: 832–836, 2003. doi: 10.1038/nature02064. [DOI] [PubMed] [Google Scholar]
- 71.Vattathil S, Akey JM. Small amounts of archaic admixture provide big insights into human history. Cell 163: 281–284, 2015. doi: 10.1016/j.cell.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 72.von Heijne G. Signal sequences. The limits of variation. J Mol Biol 184: 99–105, 1985. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 73.von Heijne G. The signal peptide. J Membr Biol 115: 195–201, 1990. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]
- 74.Vu TH, Hoffman AR. Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature 371: 714–717, 1994. doi: 10.1038/371714a0. [DOI] [PubMed] [Google Scholar]
- 75.Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev 17: 400–407, 2007. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallis M. New insulin-like growth factor (IGF)-precursor sequences from mammalian genomes: the molecular evolution of IGFs and associated peptides in primates. Growth Horm IGF Res 19: 12–23, 2009. doi: 10.1016/j.ghir.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Ward MC, Gilad Y. Human genomics: Cracking the regulatory code. Nature 550: 190–191, 2017. doi: 10.1038/550190a. [DOI] [PubMed] [Google Scholar]
- 78.Xue C, Raveendran M, Harris RA, Fawcett GL, Liu X, White S, Dahdouli M, Rio Deiros D, Below JE, Salerno W, Cox L, Fan G, Ferguson B, Horvath J, Johnson Z, Kanthaswamy S, Kubisch HM, Liu D, Platt M, Smith DG, Sun B, Vallender EJ, Wang F, Wiseman RW, Chen R, Muzny DM, Gibbs RA, Yu F, Rogers J. The population genomics of rhesus macaques (Macaca mulatta) based on whole-genome sequences. Genome Res 26: 1651–1662, 2016. doi: 10.1101/gr.204255.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zumstein PP, Lüthi C, Humbel RE. Amino acid sequence of a variant pro-form of insulin-like growth factor II. Proc Natl Acad Sci USA 82: 3169–3172, 1985. doi: 10.1073/pnas.82.10.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]