Abstract
Angiotensin II (ANG) stimulates the release of arginine vasopressin (AVP) from the neurohypophysis through activation of the AT1 receptor within the brain, although it remains unclear whether AT1 receptors expressed on AVP-expressing neurons directly mediate this control. We explored the hypothesis that ANG acts through AT1A receptors expressed directly on AVP-producing cells to regulate AVP secretion. In situ hybridization and transgenic mice demonstrated localization of AVP and AT1A mRNA in the supraoptic nucleus (SON) and the paraventricular nucleus (PVN), but coexpression of both AVP and AT1A mRNA was only observed in the SON. Mice harboring a conditional allele for the gene encoding the AT1A receptor (AT1Aflox) were then crossed with AVP-Cre mice to generate mice that lack AT1A in all cells that express the AVP gene (AT1AAVP-KO). AT1AAVP-KO mice exhibited spontaneously increased plasma and serum osmolality but no changes in fluid or salt-intake behaviors, hematocrit, or total body water. AT1AAVP-KO mice exhibited reduced AVP secretion (estimated by measurement of copeptin) in response to osmotic stimuli such as acute hypertonic saline loading and in response to chronic intracerebroventricular ANG infusion. However, the effects of these receptors on AVP release were masked by complex stimuli such as overnight dehydration and DOCA-salt treatment, which simultaneously induce osmotic, volemic, and pressor stresses. Collectively, these data support the expression of AT1A in AVP-producing cells of the SON but not the PVN, and a role for AT1A receptors in these cells in the osmotic regulation of AVP secretion.
Keywords: angiotensin, antidiuretic hormone, osmolality, supraoptic nucleus, vasopressin
INTRODUCTION
Normal cellular function requires a tightly regulated extracellular milieu and can be greatly disturbed when solute concentrations are too low or high. This is especially important in the blood, where changes in osmolality, a measure of solute concentration, can lead to significant fluid imbalance throughout the body (63). For this reason, plasma osmolality is maintained in a very narrow range in humans and other animals. Integral for the maintenance of osmolality is arginine vasopressin (AVP), a neurohypophysial circulating hormone that reabsorbs water from the nephron to decrease solute concentration in the blood, decreasing plasma osmolality. AVP is made in magnocellular neurosecretory neurons of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN), which make and secrete AVP in response to various stimuli (63).
One powerful stimulus for AVP release is angiotensin II (ANG II) (36, 42, 53, 76, 77). We recently found that a unique, double-transgenic mouse model (sRA mice) with brain-specific elevations in renin-angiotensin system (RAS) activity exhibited very large increases in AVP secretion, which caused significant decreases in serum sodium and calcium concentrations via V2 receptor activation (47). However, it was not clear where in the brain ANG was acting to cause these effects. ANG causes most of its effects through the angiotensin type 1 (AT1) receptor, but its specific effects depend on the localization of these receptors. Outside the hypothalamus, murine studies examining the effects of AT1A genetic knockdown in the subfornical organ (SFO) have demonstrated an important role for these receptors in activating neurons in the SON to elevate AVP secretion and also, through separate pathways, increase water intake (82). Alternatively, projections from the SFO can activate neurons in the PVN, but these appear important for sympathetic nerve activity and not AVP production (73, 83). Additionally, the groups of Krause and Leenen (11, 21, 84) have separately demonstrated that specific AT1A deletion from the PVN has blood pressure and energy balance effects, but these are not mediated through AVP. It is known that cells within the SON also express AT1A and respond to ANG by increasing sensitivity to osmotic stimuli, but the physiological role of these AT1A receptors in the control of AVP secretion remains unclear (10, 27, 48, 56, 76, 91).
Collectively, these data support a major stimulatory effect of the brain RAS on AVP release and a physiologically and pathophysiologically relevant effect of this release on blood chemistries. However, the neurocircuitry that mediates angiotensinergic control of AVP release remains unclear. We hypothesized a role for ANG AT1A receptors, localized specifically to AVP-producing neurons, in the physiological control of AVP release. Novel transgenic mouse models were developed to first identify the subset of AVP-producing cells that express the AT1A gene, and additional novel mouse models were developed to specifically disrupt the AT1A gene within AVP-producing cells to examine the physiological relevance of the AT1A receptors within these cells.
MATERIALS AND METHODS
Animals.
All studies complied with the Guide for the Care Use of Laboratory Animals (8th ed.; Ref. 51) and were approved by the University of Iowa Animal Care and Use Committee. Mice expressing Cre-recombinase via the AVP promoter (31) were obtained on the C57BL/6J background from the Jackson Laboratories (AVP-Cre; stock no: 023530). AVP-Cre mice were bred with mice with a conditional allele for the endogenous AT1A gene, originally developed by Alan M. Daugherty (45, 62). Resulting AT1AAVP-KO mice exhibit selective disruption of the conditional AT1A gene in any cells expressing AVP. Adult mice of both sexes, ranging in age from 8 to 20 wk, were obtained from in-house breeding colonies. Animals were housed up to five per cage in conventional forced-air shoebox cages with shredded paper bedding and ad libitum access to standard chow (Teklad 7013) or filtered tap water unless otherwise indicated. Room temperature was maintained at 22–25°C, and light was provided in a 12:12-h light-dark cycle.
Development of triple-transgenic (AVP-Cre x CAG-stopflox-tdTomato x NZ44) mice.
AVP-Cre mice were bred with mice expressing a conditionally activatable red fluorescent reporter construct, with the strong ubiquitous CAG promoter-driving expression of a transcriptional stop cassette flanked by loxP sequences, followed by the tdTomato gene. Excision of the stop cassette within cells with an active AVP promoter results in expression of the tdTomato reporter, and therefore, cells that have expressed AVP at some point in their developmental lineage will exhibit red fluorescence.
These animals were then bred to mice that harbor a BAC transgene including the mouse AT1A gene and ~70 kb of flanking sequence both 5′ and 3′ of the AT1A gene. Within this BAC construct (the NZ44 construct, originally developed by the GenSat project at the Rockefeller University), an eGFP green fluorescent reporter and transcriptional stop sequence have been inserted immediately following the transcriptional start site for the AT1A gene. As a result, mice harboring the NZ44 construct exhibit tightly regulated expression of eGFP in cells with active expression of the AT1A gene, but no additional (transgenic) expression of AT1A mRNA (28). NZ44 mice bred more than seven generations onto the C57BL/6J background strain were obtained from Teresa A. Milner at Cornell University.
Resulting triple-transgenic (AVP-Cre x CAG-stopflox-tdTomato x NZ44) mice exhibited red fluorescence in any cells, which had or continued to exhibit expression of AVP, and green fluorescence in any cells, which exhibited expression of AT1A. Brains were prepared and stained with a GFP antibody as previously (14).
Immunostaining and imaging.
In situ hybridization on brains flash frozen in OCT was performed using RNAscope (ACD) probes: AVP (4013191-C2) and AT1A (481161-C1), as previously (14) using Zeiss LSM710 confocal microscope with Zeiss Zen Imaging Software.
Blood and urine chemistry.
Trunk blood samples were collected after CO2 asphyxiation into tubes coated with EDTA (for plasma) or uncoated tubes (for serum). Samples were then centrifuged (5,000 g for 10 min), and the supernatant was transferred to a separate tube and frozen at −80°C until analysis. Plasma and urine copeptin were assessed using a commercially available EIA (CEA365Mu; Cloud-Clone) (71). Additional fresh blood samples were directly loaded into heparin-coated capillary tubes and centrifuged (13,000 g for 10 min) to manually determine hematocrit. For determination of copeptin after dehydration, blood was collected by submandibular cheek bleed.
Osmolality was measured using freezing-point depression (Fiske), and clinical chemistries were determined using a handheld analyzer (iSTAT, Chem8+ cartridges; Abbott Laboratories,) (47). Plasma sodium, glucose, and blood urea nitrogen (BUN) were used to calculate the estimated plasma osmolality (POsm):
The plasma osmol gap was calculated using measured and calculated plasma osmolalities within individual blood samples:
Ingestive behaviors/metabolic caging.
Food and fluid intake behaviors and urine and fecal outputs were determined, and urine and fecal samples were collected, using custom-modified single-mouse sized metabolic cages (Nalgene/Techniplast) (29). Mice were acclimated to cages for at least 2 days before data collection. For studies examining salt preference, drink placements were switched every 24 h throughout the study to account for side bias.
Intracerebroventricular ANG infusion.
While mice were under ketamine anesthesia, an osmotic minipump (model 1004; ALZET) filled with artificial cerebrospinal fluid (Tocris) or 0.05 or 0.50 mg/ml ANG (A9525; Sigma; to deliver ANG at rates of 5 or 50 ng/h) was implanted subcutaneously and connected to a brain infusion cannula (Brain Infusion Kit 3; ALZET) that was inserted at 1.0 mm lateral, −0.3 mm anterior-posterior, and −2.3 mm dorsal-ventral relative to the bregma (14).
Blood pressure.
Systolic blood pressure (SBP) and heart rate were determined using tail-cuff plethysmography as previously described (47, 71, 86). Animals were trained to the apparatus by daily assessments for 2 wk before the collection of 5 days of daily measures, which were averaged and used for statistical analyses.
DOCA-salt model.
While mice were under isoflurane anesthesia, a 50-mg pellet of DOCA was implanted in the subcutaneous space between the scapulae. Animals were then offered a 0.15 mol/l NaCl drink solution (in addition to standard chow and tap water). Notably, animals were not uninephrecotomized in the current study, as we previously demonstrated that this mild version of the DOCA-salt model produces the hallmark phenotypes of hypertension, polydipsia, and increased resting metabolic rate in wild-type C57BL/6J mice (30).
Statistics.
Differences between two groups were assessed by Student’s t-test. Differences between treated and untreated controls and AT1AAVP-KO mice were assessed by two-way ANOVA using Tukey’s test for multiple comparisons. For time-course studies, area under the curve was calculated and two-way ANOVA was performed, followed by Tukey’s test for multiple comparisons. P < 0.05 was considered significant.
RESULTS
AT1A localizes to AVP-expressing cells within the SON but not PVN.
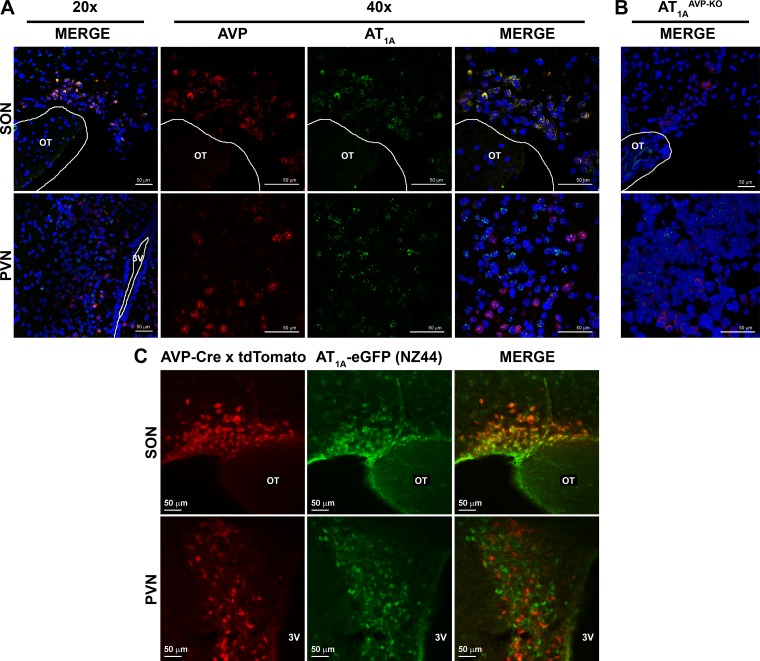
We first set out to determine whether AT1A colocalizes with AVP. In situ hybridization to detect mRNA for AVP and AT1A confirmed the expected expression of both transcripts within the PVN and SON of wild-type C57BL/6J mice, but colocalization in the same cells was only observed within the SON (Fig. 1A). The specific colocalization of AT1A expression within AVP neurons of the SON was supported by in situ hybridization studies in mice lacking AT1A in AVP-producing cells (AT1AAVP-KO mice), as the transcript for AT1A was not detected within the SON of these mice, but the AT1A transcript was still detected within the PVN (Fig. 1B). Importantly, AVP expression was not detected in other relevant brain regions of wild-type mice such as the SFO, and the AT1A transcript appeared unchanged in the SFO of AT1AAVP-KO mice (data not shown).
Fig. 1.
Arginine vasopressin (AVP) and AT1A RNA colocalize within the supraoptic nucleus (SON) but not the paraventricular nucleus (PVN). A and B: fluorescent in situ hybridization detecting mRNA for AVP (red) and AT1A (green) with a DAPI stain (blue) in wild-type (A) and AT1AAVP-KO (B) mice. C: fluorescent reporters in triple-transgenic AVP-Cre x CAG-stopflox-tdTomato x NZ44 mice: tdTomato (red) reporter activated by AVP-Cre and eGFP (green) expressed via the NZ44 AT1A BAC transgene.
Similar localization results were obtained using a genetic reporter mouse. Mice harboring a P1 artificial chromosome transgene encoding the AT1A gene, with an eGFP reporter and stop sequence inserted immediately following the transcriptional start site (NZ44 mice), have previously been demonstrated to faithfully express eGFP in cells that express AT1A within the brain (14, 28). NZ44 mice were bred to AVP-Cre mice described above and to mice harboring a conditionally activatable tdTomato red fluorescent reporter construct, as previously (14). The resulting triple-transgenic mice express the green fluorescent eGFP reporter in cells with current expression of the AT1A promoter and red fluorescent tdTomato reporter in cells with current or historical expression of the AVP promoter. In these mice, red and green fluorescence was detected in both the SON and PVN, but cellular colocalization was once again only observed within the SON, not the PVN (Fig. 1C).
Baseline fluid homeostasis is altered in AT1AAVP-KO mice.
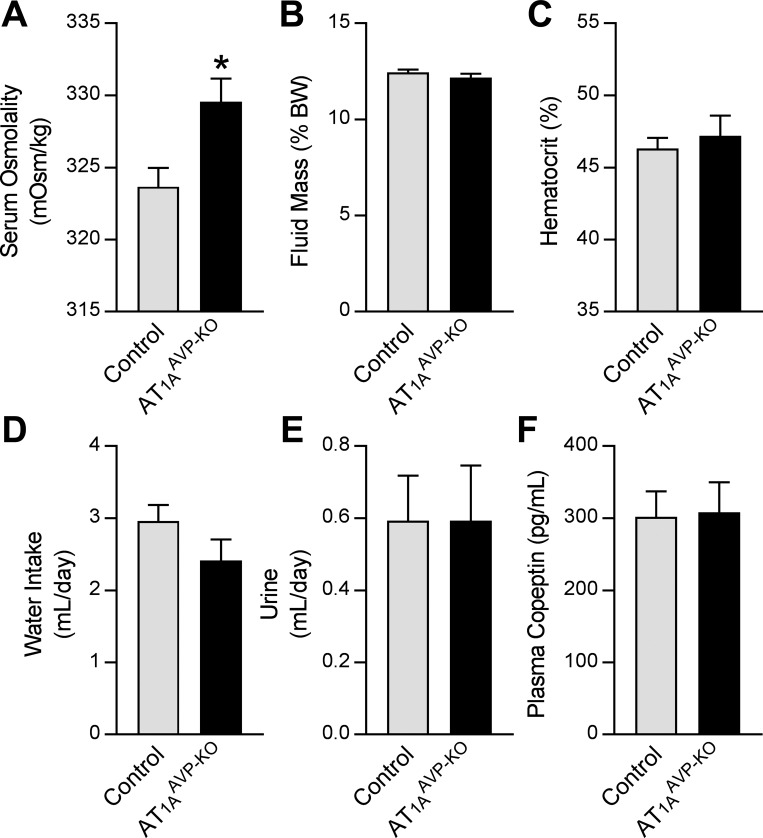
AT1AAVP-KO mice exhibited increased serum (Fig. 2A) and plasma (Table 1) osmolality compared with littermate controls, despite normal free body water by NMR (Fig. 2B), hematocrit (Fig. 2C), water intake (Fig. 2D), and urine production (Fig. 2E). Copeptin is a stable COOH fragment of the AVP proprotein that is secreted in a 1:1 molar ratio with AVP, has a long half-life, is primarily eliminated through the urine, and that has been demonstrated in multiple species as a faithful marker of AVP secretion kinetics (5, 25, 47, 81). Because AVP is rapidly metabolized, we used copeptin as a biomarker of chronic AVP secretion. Like the other measures of fluid status, plasma (Fig. 2F) and urine copeptin (not shown) were unchanged in AT1AAVP-KO mice. These findings are consistent with the hypothesis that AT1A receptors located on AVP-expressing cells are involved in control of the osmotic threshold for AVP release.
Fig. 2.
AT1AAVP-KO mice exhibit altered fluid homeostasis. A: serum osmolality (control: male n = 24, female n = 9; AT1AAVP-KO: male n = 16, female n = 3) at baseline. B–E: measures of body fluid status, including fluid mass (B; control: male n = 9, female n = 9; AT1AAVP-KO: male n = 3, female n = 2), hematocrit (C; control: male n = 8, female n = 6, AT1AAVP-KO: male n = 5, female n = 2), water intake (D; control: male n = 9, female n = 9; AT1AAVP-KO: male n = 13, female n = 9), and urine production (E: control: male n = 9, female n = 9; AT1AAVP-KO: male n = 13, female n = 9). F: plasma copeptin (control: male n = 7; female n = 11; AT1AAVP-KO: male n = 4, female n = 4) at baseline. Data are expressed as means ± SE. *P < 0.05, between genotypes.
Table 1.
Blood chemistries from plasma isolated in lithium heparin-coated tubes
| End Point | Control | AT1AAVP-KO | P Value |
|---|---|---|---|
| Measured plasma osmolality, mosmol/kg H2O | 321 ± 1 | 329 ± 3 | 0.01 |
| Na, mM | 149 ± 0.4 | 149 ± 0.7 | 0.99 |
| Cl, mM | 116 ± 1.2 | 117 ± 1.3 | 0.87 |
| Ionized Ca, mM | 1.2 ± 0.04 | 1.2 ± 0.05 | 0.51 |
| Glucose, mg/dl | 199 ± 3 | 187 ± 8 | 0.19 |
| BUN, mg/dl | 21.1 ± 1.4 | 21.5 ± 1.8 | 0.87 |
| Hematocrit, % | 44.4 ± 1 | 44.1 ± 1 | 0.75 |
| Calculated plasma osmolality, mosmol/kg H2O | 317 ± 1 | 316 ± 2 | 0.72 |
| Osmol gap, mosmol/kg H2O | 3.8 ± 2.1 | 13.5 ± 2.6 | 0.01 |
Values are means ± SE. For controls: n = 4 men and 4 women; for AT1AAVP-KO: n = 5 men and 3 women. BUN, blood urea nitrogen.
To identify the primary osmolyte responsible for the increased serum and plasma osmolalities, we examined an array of blood chemistry end points in a subset of mice. For these and all other studies, both sexes were included. However, although sex had expected effects on specific end points (BUN, hematocrit, and triglycerides), genotype differences or lack genotype differences were consistent regardless of sex for all end points. Moreover, no interactions between sex and genotype were noted for any end point. Thus sexes have been combined for data presentation. No significant differences between AT1AAVP-KO and littermate mice were observed in plasma sodium, chloride, ionized calcium, glucose, BUN, and hematocrit (Table 1). There were also no differences in triglycerides (control n = 10: 93.4 ± 8.8; AT1AAVP-KO n = 10: 102.7 ± 10.2 mg/dl; P = 0.50) or nonesterified fatty acids (control n = 10: 0.677 ± 0.063; AT1AAVP-KO n = 10: 0.682 ± 0.054 mM; P = 0.96). As a result, the calculated plasma osmolality was similar between groups, and a significant difference in the magnitude of the osmol gap was noted in AT1AAVP-KO mice (Table 1). Thus the specific osmolyte that accounts for the observed increase in serum and plasma osmolalities in AT1AAVP-KO mice remains unclear.
Sodium-seeking behavior is unchanged in AT1AAVP-KO mice.
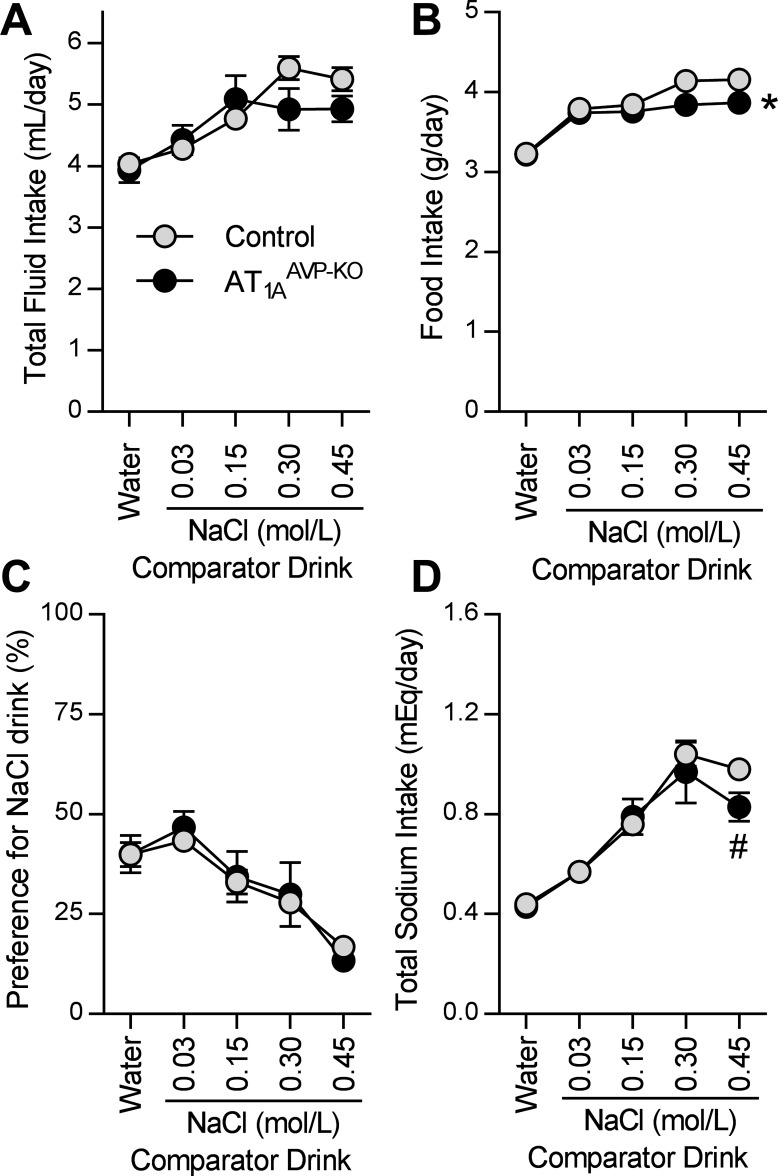
Ingestive behaviors, such as water and sodium intake, are sensitive to plasma osmolality and AVP, and therefore, we explored intake behaviors in AT1AAVP-KO mice. When presented with increasing concentrations of NaCl drink solutions in a two-bottle choice format versus water, AT1AAVP-KO mice exhibited essentially normal total daily intake of total fluid (including water plus comparator drink) (Fig. 3A) and powdered chow food (Fig. 3B). AT1AAVP-KO mice exhibited similar NaCl preference/aversion behaviors including aversions to hypertonic (0.30 and 0.45 mol/l) NaCl (Fig. 3C) and total sodium intakes (including food plus fluid sources) compared with littermate control mice (Fig. 3D). Collectively, these data support the conclusion that AT1A receptors localized to AVP-expressing cells do not critically contribute to fluid, food, or sodium intake, preference for NaCl, or appetite for NaCl under normal conditions.
Fig. 3.
Fluid intake and sodium preference are largely normal in AT1AAVP-KO mice. A–D: fluid intake (A), food intake (B), preference for the NaCl drink (C), and total sodium intake (D) in mice receiving the option to drink water plus an indicated comparator drink with varied concentrations of NaCl dissolved in water in an alternating 2-bottle choice paradigm. Data are expressed as means ± SE. *P < 0.05, overall between genotypes; #P < 0.05 compared with corresponding treatment between genotypes (control: male n = 15, female n = 12; AT1AAVP-KO: male n = 6, female n = 6).
Acute sodium loading unmasks differences in AT1AAVP-KO mice.
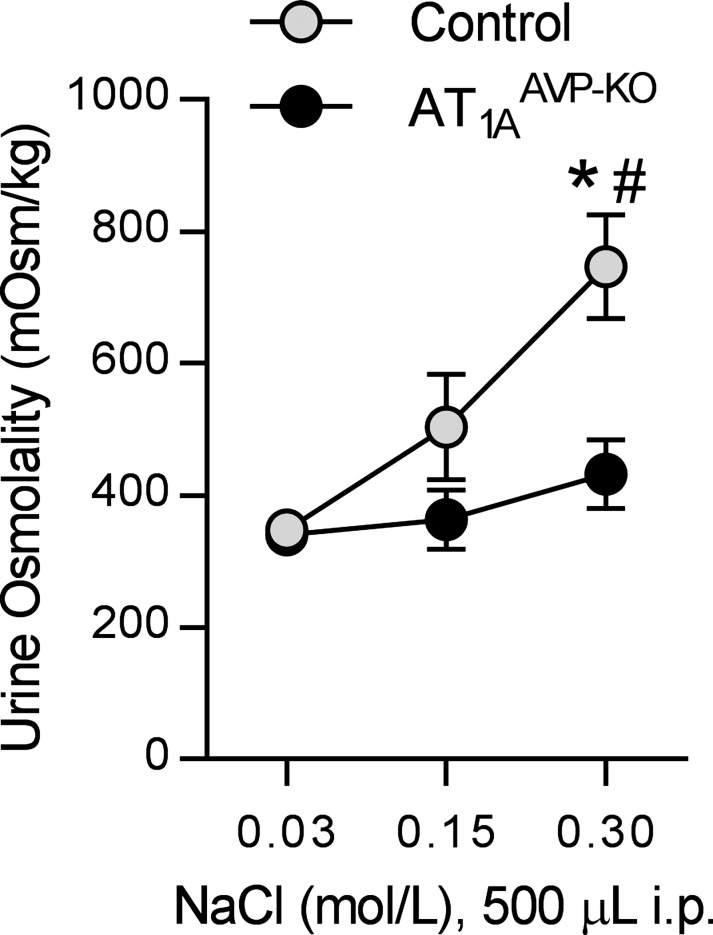
To examine AVP release and action in response to acute osmotic stimuli in AT1AAVP-KO mice, we injected hypo-, iso-, and hypertonic NaCl solutions into the intraperitoneal cavity. This resulted in increased urine osmolality in control littermate mice, but AT1AAVP-KO mice failed to increase urine osmolality responses to hypertonic solutions (Fig. 4). These findings are consistent with reduced AVP release and action with hyperosmotic stimuli in AT1AAVP-KO mice.
Fig. 4.
Renal concentrating response to acute hyperosmotic load is blunted in AT1AAVP-KO mice. Urine osmolality in response to acute intraperitoneal injections of 500 µl of varied concentrations of NaCl solution (control: male n = 4, female n = 2; AT1AAVP-KO: male n = 3, female n = 2). Data are expressed as means ± SE. *P < 0.05 between genotypes; #P < 0.05 compared with 0.03 mol/NaCl within genotype.
Intracerebroventricular ANG does not stimulate copeptin secretion in AT1AAVP-KO mice.
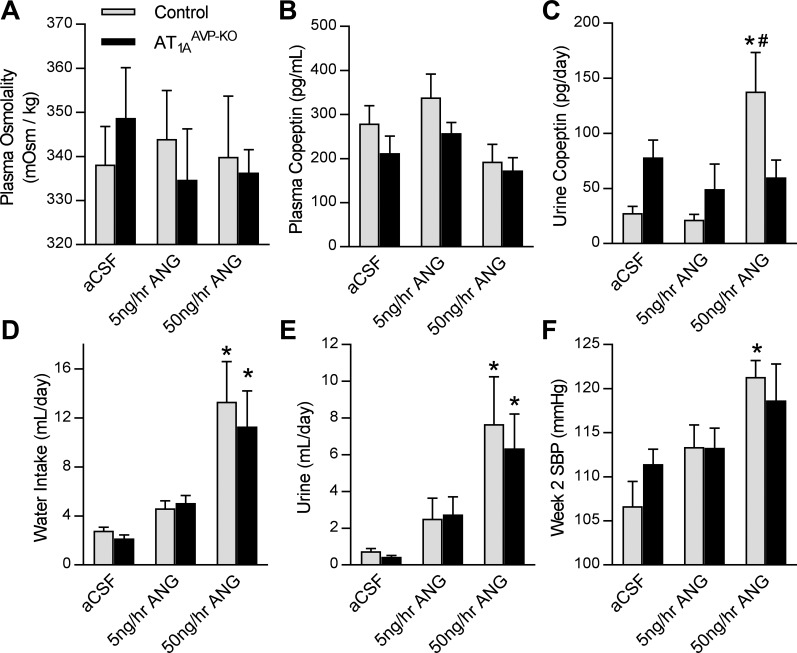
Next, to determine whether disruption of AT1A in AVP-expressing cells modified ANG-mediated stimulation of AVP secretion, AT1AAVP-KO mice underwent chronic intracerebroventricular infusion of ANG. Similar to what we previously observed in mice with transgenic activation of the brain RAS (47), infusion of ANG for 2 wk had no significant effects on plasma osmolality (Fig. 5A) or copeptin (Fig. 5B), but total daily loss of copeptin to the urine, as an index of total daily secretion of AVP, was significantly increased by ANG in control littermate mice but not in AT1AAVP-KO mice (Fig. 5C). The effects of intracerebroventricular ANG action, including polydipsia (Fig. 5D), polyuria (Fig. 5E), and elevated SBP (Fig. 5F) were similar in AT1AAVP-KO mice compared with controls. Collectively, these results support the conclusion that AT1A receptors, localized to AVP-expressing cells, are important for AVP secretion in response to ANG but are largely dispensable for other major physiological effects of ANG such as drinking behavior, urine production, and blood pressure control.
Fig. 5.
Copeptin secretion is not elevated by chronic intracerebroventricular ANG in AT1AAVP-KO mice. A: plasma osmolality (effect of ANG: P = 0.88, effect of genotype: P = 0.94, interaction: P = 0.69) in response to 2 wk of artificial cerebrospinal fluid (aCSF; control: male n = 4, female n = 10; AT1AAVP-KO: male n = 8, female n = 6), 5 ng/h ANG (control: male n = 6, female n = 4; AT1AAVP-KO: male n = 2, female n = 6), and 50 ng/h ANG (control: male n = 3, female n = 2; AT1AAVP-KO: male n = 5, female n = 5). B–F: plasma copeptin (B; effect of ANG: P = 0.04, effect of genotype: P = 0.12, interaction: P = 0.73), total daily loss of copeptin to urine (C: effect of ANG: P < 0.01, effect of genotype: P = 0.98, interaction: P < 0.01), water intake (D; effect of ANG: P < 0.01, effect of genotype: P = 0.55, interaction: P = 0.76), urine output (E; effect of ANG: P < 0.01, effect of genotype: P = 0.62, interaction: P = 0.82), and absolute SBP at week 2 (F; effect of ANG: P < 0.01, effect of genotype: P = 0.77, interaction: P = 0.37) in response to intracerebroventricular ANG. Data are expressed as means ± SE. *P < 0.05 compared with aCSF within genotype; #P < 0.05 compared with aCSF or 5 ng/h ANG within genotype.
Osmotic effects of AT1AAVP-KO are masked by complex stimuli.
AVP secretion is essential for survival during dehydration, and dehydration stimulates AVP secretion through both increased plasma osmolality and decreased blood volume. Therefore, we examined AT1AAVP-KO mice after 24-h dehydration. Plasma osmolality (Fig. 6A), copeptin (Fig. 6B), and hematocrit (Fig. 6C) were similar between AT1AAVP-KO and littermate control mice after 24-h dehydration, supporting the concept that the contribution of AT1A receptors on AVP-expressing cells to AVP release is overwhelmed by stimuli that simultaneously modify both osmolality and blood volume.
Fig. 6.
AT1AAVP-KO mice exhibit normal fluid balance responses to 24-h dehydration. A–C: Plasma osmolality (A; control: male n = 3, female n = 5; AT1AAVP-KO: male n = 2, female n = 3), plasma copeptin (B; control: male n = 4, female n = 5; AT1AAVP-KO: male n = 4, female n = 4), and hematocrit (C) following overnight dehydration. Data are expressed as means ± SE.
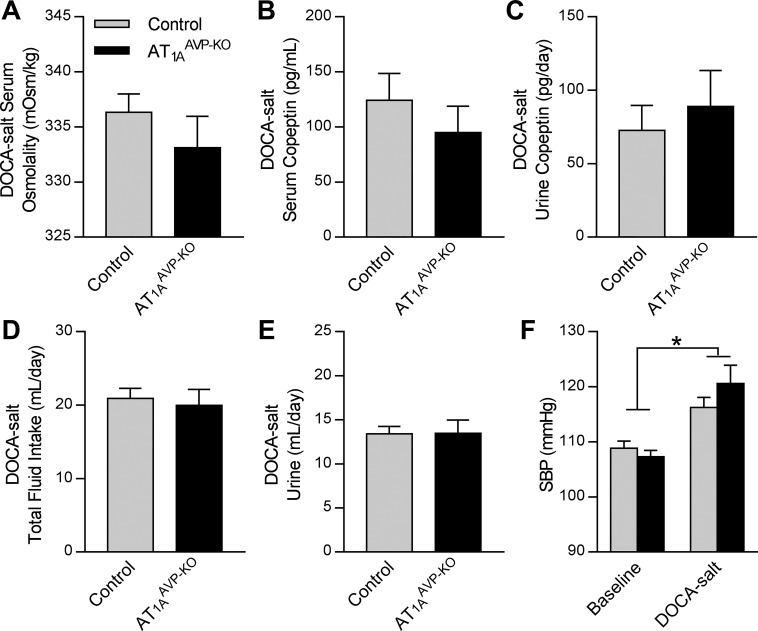
DOCA-salt treatment is known to cause increased brain RAS activity and brain AT1-dependent increases in SBP and AVP secretion (30, 37, 44, 55). This model is also characterized by elevated circulating mineralocorticoids, which influence fluid homeostasis through multiple independent mechanisms. After 3 wk of DOCA-salt treatment, total daily copeptin loss to urine was increased, regardless of group or sex (sham 8.9 ± 25.7 vs. DOCA-salt 81.5 ± 14.1 pg/day, P < 0.05). By three-way ANOVA, the results were as follows: DOCA-salt P = 0.02, Genotype P = 0.81, Sex P = 0.77, Genotype × DOCA-salt P = 0.72, Genotype × Sex P = 0.95, Sex × DOCA-salt P = 0.73, and three-way interaction P = 0.93. AT1AAVP-KO mice exhibited no differences in osmolality (Fig. 7A), serum (Fig. 7B), or urine (Fig. 7C) copeptin, total fluid intake (Fig. 7D), urine production (Fig. 7E), or SBP (Fig. 7F) compared with controls. Collectively, these findings support the conclusion that the fine control of AVP release by osmotic stimuli through AT1A receptors located on AVP-expressing cells is overwhelmed by complex stimuli such as dehydration and DOCA-salt.
Fig. 7.
AT1AAVP-KO mice exhibit normal responses to DOCA-salt treatment. A–E: serum osmolality (A), serum copeptin (B), urine copeptin (C), total fluid intake (D), and urine output (E) in DOCA-salt treated mice. F: baseline SBP and SBP in response to DOCA-salt (effect of DOCA: P < 0.01, effect of genotype: P = 0.56, interaction: P = 0.23). Data are expressed as means ± SE. *P < 0.05 for main effect of DOCA-salt treatment (control: male n = 12, female n = 11; AT1AAVP-KO: male n = 5, female n = 3).
DISCUSSION
AVP is recognized for its critical roles in fluid homeostasis and in cardiovascular disorders, including hypertension (9, 12, 41, 47, 90), sepsis (50, 69), heart failure (38), and preeclampsia (26, 70, 71, 89, 92). As recently and comprehensively reviewed by Brown (8) and Iovino et al. (35), substantial progress has been made in understanding the neurocircuitry, electrophysiology, pharmacology, and many physiological controllers of AVP secretion. Nonetheless, the specific neurocircuitry and molecular mechanisms (receptors, second-messengers, and modulators localized to specific cells within specific nuclei of the brain) that mediate the control of AVP secretion by the renin-angiotensin system remain insufficiently explored. For example, many previous studies have implicated brain AT1 receptors in the control of AVP secretion (2, 24, 32, 40, 49, 54, 57–59, 64, 65, 72, 75, 78–80), and the control and function of AVP-expressing cells of the SON and PVN were assumed to be similar or identical. Here, we demonstrate that AT1A receptors are expressed by AVP-expressing neurons of the SON but not the PVN. Furthermore, genetic disruption of AT1A receptors localized to AVP-expressing neurons resulted in altered fluid balance effects of osmotic stimuli but not other more complex stimuli. Thus we conclude that AT1A on AVP-producing neurons of the SON is important for the control of AVP release specifically in response to osmotic stimuli.
The discovery that AT1A is expressed by AVP-expressing neurons of the SON but not the PVN is intriguing. Previously, we demonstrated that transgenic hyperactivity of the brain RAS (in double-transgenic “sRA” mice) resulted in AVP-dependent hypertension and an induction of AVP protein within the SON but not the PVN (47). Others have demonstrated unique regulation of AVP expression and release in the SON vs. PVN (11, 34, 73, 83), and these data support the concept that direct action of ANG on AVP-expressing cells of the SON vs. polysynaptic communication from ANG-sensitive cells to AVP-expressing neurons of the PVN may help explain this divergence in regional control of AVP. It is important to acknowledge that, although no colocalization of AT1A and AVP was observed by FISH or reporter expression within multiple levels of the PVN, we cannot rule out the possibility that AT1A may be differentially expressed by magnocellular vs. parvocellular neurons of the PVN, and the identity of AT1A-expressing cells within the PVN is not clarified by the current study. Recently, de Kloet et al. (22) developed a novel transgenic mouse that expresses AT1A, Cre recombinase, and tdTomato via the endogenous AT1A locus. The team demonstrated that within the PVN, tdTomato expression was colocalized with corticotrophin-releasing hormone and thyroid-releasing hormone but not with AVP or oxytocin. Together with the study from de Kloet et al. (22), the current findings using two additional unique and complementary localization methods support the concept that AVP-expressing neurons of the PVN do not express AT1A.
Our studies demonstrate a specific role of AT1A on AVP-expressing cells in the control of fluid homeostasis, especially in response to osmotic stimuli. These findings support previous work demonstrating that the AT1 antagonist losartan can partially prevent elevations in plasma AVP caused by intracerebroventricular ANG (58), and also expand on work by Bourque and colleagues, who demonstrated that ANG increases nonselective cation current (10) and mechanosensitivity of the actin cytoskeleton (91) in isolated magnocellular neurosecretory cells of the SON. In addition, our findings build on multiple previous demonstrations by Sladek et al. (78–80) that ANG acts on the SON to modulate osmotic sensitivity. Specifically, our work confirms a role for AT1A receptors on AVP-expressing cells in the SON on fluid balance physiology and further demonstrates a role for these AT1A receptors in modulating responses to osmotic stimuli in vivo.
While we saw important differences in AVP action following an acute osmotic stimulus, we do not see differences following dehydration, which induces AVP release through both osmotic and volemic mechanisms, or DOCA-salt, which represents an even more complex stimulus. DOCA-salt treatment induces a progressive, phasic salt-sensitive hypertension that involves water and sodium retention, autonomic activity, and various hormonal systems including AVP and endothelin (74, 88). Brooks and colleagues (6, 7, 52) demonstrated that DOCA-salt treatment alters osmotic control and simultaneously the hypertension induced by DOCA-salt is dependent on osmotic changes, likely in part through altered AVP control. Thus the current data support a role of AT1A receptors on AVP-expressing cells as important in osmotic but not complex or combinatorial (osmotic, volemic, and pressor) mechanisms of AVP control. Moreover, it has been previously demonstrated that low volume status supersedes osmolality as a stimulus for AVP release (63), which may explain the lack of differences between groups following these treatments. For example, Cowley and colleagues (60, 61) demonstrated that the relationship between AVP release and plasma osmolality is influenced by volume status, as acute changes in volume status affect both the osmotic release threshold for AVP and the slope of the AVP/osmolality relationship. This dominant control of AVP release by volemic stimuli, which supersedes osmotic control mechanisms, presumably exists to accelerate corrections in volume status beyond what would be achieved by osmotic regulation alone. Thus the current findings highlight a specific role for AT1A on AVP-expressing cells specifically in osmotic control of AVP, and it is perhaps not surprising that such fine control of AVP release is masked in the context of more complex stimuli that simultaneously induce osmotic and volemic mechanisms.
To examine the role of AT1A on AVP-expressing neurons in AVP release, fluid balance, and blood pressure in response to brain ANG, we used a chronic intracerebroventricular ANG infusion model. Whereas many other groups have utilized chronic peripheral infusion of ANG in rats as well as acute intracerebroventricular injection of ANG into mice to study rapid blood pressure responses, to the best of our knowledge this is the first report of the blood pressure effects of chronic intracerebroventricular ANG infusion in mice. Notably, in the current study we utilized tail-cuff plethysmography, which should be replicated using more advanced techniques such as direct arterial pressure recordings. Nonetheless, we were surprised to discover that a 2-wk 5 ng/h intracerebroventricular infusion of ANG was minimally effective at increasing SBP, as we recently reported that this same dosing schedule results in a robust elevation in resting metabolic rate in C57BL/6J mice within 10 days (14). In contrast, here we determined that a 10-fold higher dose of intracerebroventricular ANG was required to moderately increase SBP. We posit that this differential effect of intracerebroventricular ANG to stimulate metabolism vs. blood pressure may result from the relative exposure of relevant brain structures to the ventricles. For example, the ependymal layer of the ventricles prevents free diffusion of peptides from the cerebrospinal fluid into the interstitial space of the brain, yet many studies have documented stimulatory effects of intracerebroventricular injection or infusion of ANG on intake behavior, blood pressure, and metabolism. Thus ANG within the ventricles must act at specific cell types that have access or exposure to the ventricular space. Supporting this concept, we recently documented secretion of RAS peptides by the SFO into the ventricles (1). The current study may therefore be limited, in that intracerebroventricular ANG may not reach the SON or PVN and perhaps direct infusion of ANG into the SON may have differential effects in our mice. Site-specific activation of the RAS within the SON (such as we previously performed in SFO; Ref. 16) may conceivably uncover differential effects in AT1AAVP-KO mice; however; this seems unlikely, as the dysfunctions in osmotic control in AT1AAVP-KO mice at baseline support the conclusion that SON AVP neurons are already dysfunctional in these animals without the need for additional local RAS activation. Furthermore, differential sensitivity of C57BL/6J mice to intracerebroventricular ANG may reflect species-specific differences in sensitivity to specific actions of the RAS, as mice are much less sensitive to the blood pressure stimulatory effects of ANG than rats (13). Additionally, secretion of AVP from SON neurons into the circulation occurs at axon terminals located in the posterior pituitary, and it has been established that various circulating hormones (purines, opioids, dynorphin, cholecystokinin, catecholamines, and ANG) act at receptors that can be localized to these terminals to modify secretion kinetics (reviewed in Ref. 8). Thus it is possible that the manipulation of AT1A in AVP neurons may cause differential sensitivity to circulating ANG. Interestingly, while rats exhibit rapid and robust drinking responses to peripherally-administered ANG, mice of various strains are largely resistant to this effect (23, 43, 67, 68). Therefore, future studies of the consequences of AT1A manipulation in AVP neurons on sensitivity to circulating ANG should be performed in multiple species. Lastly, we cannot rule out a compensatory role for AT1B in AT1AAVP-KO mice, which may also explain these findings.
We were surprised to determine that typical major osmolytes (sodium, chloride, glucose, and urea) did not account for the increase in plasma and serum osmolality in AT1AAVP-KO mice. Initial characterization of other potential contributors (potassium, calcium, triglycerides, and nonesterified fatty acids) also failed to identify the osmolyte that mediates the observed difference. Wehner et al. (85) previously reviewed the large array of known inorganic and organic osmolytes, and future studies should be aimed at determining whether polyols such as sorbitol, myo-inositol, methylamines, or amino acid changes mediate the observed increase in the osmol gap in AT1AAVP-KO mice. Identifying the mechanisms that control atypical osmolyte concentrations may inform our understanding of the biology of physiological and pathophysiological states involving altered AVP and osmotic control such as syndrome of inappropriate antidiuretic hormone secretion, hypertension, heart failure, sepsis, pregnancy, and preeclampsia.
Perspectives and Significance
Throughout the current study, we utilized measures of copeptin as a marker for AVP secretion because of the many technical challenges in studying AVP release kinetics in small animals. Copeptin is a stable circulating peptide released in a 1:1 molar ratio with AVP (50). Whereas AVP is enzymatically cleared very rapidly, copeptin is primarily cleared via renal excretion over a matter of hours, making it well suited for studying AVP release kinetics integrated over time (4). Multiple groups have demonstrated that measures of plasma AVP are strongly correlated with plasma copeptin. Morgenthaler et al. (R = 0.78, P < 0.0001) (50), Westermann et al. (R2 = 0.718, P < 0.001) (87), Jochberger et al. (R2 = 0.614, P < 0.001) (39), Balanescu (Spearman’s rank coefficient = 0.8) (3), Hew-Butler et al. (up to R = 0.94, P < 0.05) (33), Roussel et al. (R = 0.686, P < 0.001) (66), and Ettema et al. (25) have all independently demonstrated correlations between plasma copeptin and AVP in humans. Importantly, the strength of the correlation is modified by physiological and pathophysiological divergence in clearance rates of the two peptides. For example, changes in the correlation between copeptin and AVP occur during exercise (33), renal disease (25, 66), and/or hemodialysis (25). Thus measures of copeptin in these animals should be reflective of AVP release rates, and therefore, we conclude that AT1A on AVP-expressing neurons of the SON contribute to the control of AVP release, but we are hesitant to overinterpret effects on acute release kinetics or instantaneous values of plasma AVP. Future, carefully executed longitudinal, kinetic studies are required to empirically establish the strength and modulators of the AVP-copeptin correlation in rodents.
In summary, AVP-expressing neurons of the SON (but not PVN) express the AT1A receptor, and genetic disruption of the AT1A receptor in AVP-expressing cells of mice has major effects on osmotic control physiology. These findings highlight SON-vs.-PVN-specific regulatory mechanisms contributing to the regulation of AVP and fluid balance. Given the important role of AVP release in osmotic control (17), physiological (e.g., pregnancy; Refs. 18–20, 46) and pathophysiological states characterized by altered fluid homeostasis should be examined for changes in AT1A signaling.
GRANTS
We were supported by National Institutes of Health Grants GM-067795, HL-134850, and HL-084207, American Heart Association Grants 13SDG143400012, 14PRE20380401, 15SFRN23480000, 15SFRN23730000, 15SFRN23760002, 15SFRN23860007, 15UFEL25850040, 16PRE30980043, 18PRE33960377, and 18EIA33890055, American Physiological Society (Undergraduate Research Excellence Fellowships and Undergraduate Summer Research Fellowships Programs), University of Iowa Center for Hypertension Research, and Roy J. Carver Trust. The Central Microscopy Research Facility has also received funding for the Zeiss LSM710 microscope (1S10-RR-025439-01).
DISCLOSURES
M. K. Santillan and J. L. Grobe have submitted patent applications PCT/US2014/015327, PCT/US2014/015631, and PCT/US2018/027152 and hold U.S. Patent no. 9,937,182 describing measures and manipulations of the AVP system in the diagnosis and treatment of preeclampsia, and have received pilot grants from ESCO Ventures (Singapore) to pursue that work.
AUTHOR CONTRIBUTIONS
J.A.S., G.L.P., M.K.S., K.N.G.-C., C.D.S., and J.L.G. conceived and designed research; J.A.S., D.W.L., S.Y.Z., S.A.S., K.E.C., N.A.P., M.R.L., K.N.G.-C., and J.L.G. performed experiments; J.A.S., D.W.L., S.Y.Z., S.A.S., K.E.C., N.A.P., M.R.L., K.N.G.-C., and J.L.G. analyzed data; J.A.S., D.W.L., S.Y.Z., S.A.S., K.E.C., N.A.P., G.L.P., M.K.S., K.N.G.-C., C.D.S., and J.L.G. interpreted results of experiments; J.A.S. and K.N.G.-C. prepared figures; J.A.S. drafted manuscript; J.A.S., G.L.P., M.K.S., K.N.G.-C., C.D.S., and J.L.G. edited and revised manuscript; J.A.S., D.W.L., S.Y.Z., S.A.S., K.E.C., N.A.P., M.R.L., G.L.P., M.K.S., K.N.G.-C., C.D.S., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for support from the University of Iowa Medical Scientist Training Program, animal husbandry support provided by the University of Iowa Office of Animal Resources, genotyping support provided by the University of Iowa Genome Editing Facility, histology support provided by the University of Iowa Comparative Pathology Laboratory, and microscopy support from the University of Iowa Central Microscopy Research Facility. NZ44 mice were provided by Dr. Teresa A. Milner (Cornell University) and AT1Aflox mice were provided by Dr. Alan Daugherty (University of Kentucky). We also acknowledge the intellectual and technical insights of Katherine J. Perschbacher, Dr. Guorui Deng, Dr. Sabrina M. Scroggins, Dr. Donna A. Santillan, Dr. Matthew J. Potthoff, and Dr. Kamal Rahmouni.
REFERENCES
- 1.Agassandian K, Grobe JL, Liu X, Agassandian M, Thompson AP, Sigmund CD, Cassell MD. Evidence for intraventricular secretion of angiotensinogen and angiotensin by the subfornical organ using transgenic mice. Am J Physiol Regul Integr Comp Physiol 312: R973–R981, 2017. doi: 10.1152/ajpregu.00511.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 61: 437–444, 1995. doi: 10.1159/000126866. [DOI] [PubMed] [Google Scholar]
- 3.Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab 96: 1046–1052, 2011. doi: 10.1210/jc.2010-2499. [DOI] [PubMed] [Google Scholar]
- 4.Bankir L, Bichet DG, Morgenthaler NG. Vasopressin: physiology, assessment and osmosensation. J Intern Med 282: 284–297, 2017. doi: 10.1111/joim.12645. [DOI] [PubMed] [Google Scholar]
- 5.Bolignano D, Cabassi A, Fiaccadori E, Ghigo E, Pasquali R, Peracino A, Peri A, Plebani M, Santoro A, Settanni F, Zoccali C. Copeptin (CTproAVP), a new tool for understanding the role of vasopressin in pathophysiology. Clin Chem Lab Med 52: 1447–1456, 2014. doi: 10.1515/cclm-2014-0379. [DOI] [PubMed] [Google Scholar]
- 6.Brooks DP, Share L, Crofton JT, Guthe C, Ling WD, Bohr DF. Increased sensitivity of the osmotic control of vasopressin in sheep with deoxycorticosterone acetate-induced hypertension. J Endocrinol 107: 309–315, 1985. doi: 10.1677/joe.0.1070309. [DOI] [PubMed] [Google Scholar]
- 7.Brooks VL, Freeman KL, Qi Y. Time course of synergistic interaction between DOCA and salt on blood pressure: roles of vasopressin and hepatic osmoreceptors. Am J Physiol Regul Integr Comp Physiol 291: R1825–R1834, 2006. doi: 10.1152/ajpregu.00068.2006. [DOI] [PubMed] [Google Scholar]
- 8.Brown CH. Magnocellular neurons and posterior pituitary function. Compr Physiol 6: 1701–1741, 2016. doi: 10.1002/cphy.c150053. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep 17: 39, 2015. doi: 10.1007/s11906-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakfe Y, Bourque CW. Excitatory peptides and osmotic pressure modulate mechanosensitive cation channels in concert. Nat Neurosci 3: 572–579, 2000. doi: 10.1038/75744. [DOI] [PubMed] [Google Scholar]
- 11.Chen A, Huang BS, Wang HW, Ahmad M, Leenen FH. Knockdown of mineralocorticoid or angiotensin II type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin II hypertension in rats. J Physiol 592: 3523–3536, 2014. doi: 10.1113/jphysiol.2014.275560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron 85: 549–560, 2015. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cholewa BC, Meister CJ, Mattson DL. Importance of the renin-angiotensin system in the regulation of arterial blood pressure in conscious mice and rats. Acta Physiol Scand 183: 309–320, 2005. doi: 10.1111/j.1365-201X.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- 14.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coble JP, Cassell MD, Davis DR, Grobe JL, Sigmund CD. Activation of the renin-angiotensin system, specifically in the subfornical organ is sufficient to induce fluid intake. Am J Physiol Regul Integr Comp Physiol 307: R376–R386, 2014. doi: 10.1152/ajpregu.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol 10: 852–862, 2015. doi: 10.2215/CJN.10741013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davison JM, Gilmore EA, Dürr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 246: F105–F109, 1984. doi: 10.1152/ajprenal.1984.246.1.F105. [DOI] [PubMed] [Google Scholar]
- 19.Davison JM, Sheills EA, Philips PR, Barron WM, Lindheimer MD. Metabolic clearance of vasopressin and an analogue resistant to vasopressinase in human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 264: F348–F353, 1993. doi: 10.1152/ajprenal.1993.264.2.F348. [DOI] [PubMed] [Google Scholar]
- 20.Davison JM, Shiells EA, Philips PR, Lindheimer MD. Serial evaluation of vasopressin release and thirst in human pregnancy. Role of human chorionic gonadotrophin in the osmoregulatory changes of gestation. J Clin Invest 81: 798–806, 1988. doi: 10.1172/JCI113386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, Nguyen D, Cahill KM, Sumners C, Stern JE, Krause EG. A unique “angiotensin-sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J Neurosci 37: 3478–3490, 2017. doi: 10.1523/JNEUROSCI.3674-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denton DA, Blair-West JR, McBurnie M, Osborne PG, Tarjan E, Williams RM, Weisinger RS. Angiotensin and salt appetite of BALB/c mice. Am J Physiol Regul Integr Comp Physiol 259: R729–R735, 1990. doi: 10.1152/ajpregu.1990.259.4.R729. [DOI] [PubMed] [Google Scholar]
- 24.Egli M, Laurent JP, Mosimann R, Felix D, Imboden H. Morphological and immunocytochemical characterization of electrophysiologically investigated neurons in the PVN of the rat. J Neurosci Methods 95: 145–150, 2000. doi: 10.1016/S0165-0270(99)00166-1. [DOI] [PubMed] [Google Scholar]
- 25.Ettema EM, Heida J, Casteleijn NF, Boesten L, Westerhuis R, Gaillard C, Gansevoort RT, Franssen CF, Zittema D. . The effect of renal function and hemodialysis treatment on plasma vasopressin and copeptin levels. Kidney Int Rep 2: 410–419, 2017. doi: 10.1016/j.ekir.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foda AA, Abdel Aal IA. Maternal and neonatal copeptin levels at cesarean section and vaginal delivery. Eur J Obstet Gynecol Reprod Biol 165: 215–218, 2012. doi: 10.1016/j.ejogrb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Gagnon DJ, Cousineau D, Boucher PJ. Release of vasopressin by angiotensin II and prostaglandin E2 from the rat neuro-hypophysis in vitro. Life Sci 12: 487–497, 1973. doi: 10.1016/0024-3205(73)90201-4. [DOI] [Google Scholar]
- 28.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226: 489–509, 2012. doi: 10.1016/j.neuroscience.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol 1614: 123–146, 2017. doi: 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, Smith KA, Dang C, Sunkin S, Bernard A, Oh SW, Madisen L, Zeng H. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits 8: 76, 2014. doi: 10.3389/fncir.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hay M, Edwards GL, Lindsley K, Murphy S, Sharma RV, Bhalla RC, Johnson AK. Increases in cytosolic Ca2+ in rat area postrema/mNTS neurons produced by angiotensin II and arginine-vasopressin. Neurosci Lett 151: 121–125, 1993. doi: 10.1016/0304-3940(93)90001-2. [DOI] [PubMed] [Google Scholar]
- 33.Hew-Butler T, Hoffman MD, Stuempfle KJ, Rogers IR, Morgenthaler NG, Verbalis JG. Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med 21: 211–217, 2011. doi: 10.1097/JSM.0b013e31821a62c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-“ouabain” pathway. Am J Physiol Heart Circ Physiol 299: H422–H430, 2010. doi: 10.1152/ajpheart.00256.2010. [DOI] [PubMed] [Google Scholar]
- 35.Iovino M, Giagulli VA, Licchelli B, Iovino E, Guastamacchia E, Triggiani V. Synaptic inputs of neural afferent pathways to vasopressin- and oxytocin-secreting neurons of supraoptic and paraventricular hypothalamic nuclei. Endocr Metab Immune Disord Drug Targets 16: 276–287, 2016. doi: 10.2174/1871530317666170104124229. [DOI] [PubMed] [Google Scholar]
- 36.Iovino M, Steardo L. Vasopressin release to central and peripheral angiotensin II in rats with lesions of the subfornical organ. Brain Res 322: 365–368, 1984. doi: 10.1016/0006-8993(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 37.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- 38.Izumi Y, Miura K, Iwao H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J Pharmacol Sci 124: 1–6, 2014. doi: 10.1254/jphs.13R13CP. [DOI] [PubMed] [Google Scholar]
- 39.Jochberger S, Dörler J, Luckner G, Mayr VD, Wenzel V, Ulmer H, Morgenthaler NG, Hasibeder WR, Dünser MW. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit Care Med 37: 476–482, 2009. doi: 10.1097/CCM.0b013e3181957532. [DOI] [PubMed] [Google Scholar]
- 40.Jöhren O, Imboden H, Häuser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-converting enzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res 757: 218–227, 1997. doi: 10.1016/S0006-8993(97)00220-5. [DOI] [PubMed] [Google Scholar]
- 41.Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ, Kim JS, Han HC, Colwell CS, Kim YI. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res 113: 1296–1307, 2013. doi: 10.1161/CIRCRESAHA.113.301814. [DOI] [PubMed] [Google Scholar]
- 42.Knepel W, Nutto D, Meyer DK. Effect of transection of subfornical organ efferent projections on vasopressin release induced by angiotensin or isoprenaline in the rat. Brain Res 248: 180–184, 1982. doi: 10.1016/0006-8993(82)91161-1. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi H, Uemura H, Wada M, Takei Y. Ecological adaptation of angiotensin-induced thirst mechanism in tetrapods. Gen Comp Endocrinol 38: 93–104, 1979. doi: 10.1016/0016-6480(79)90093-5. [DOI] [PubMed] [Google Scholar]
- 44.Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertens Res 23: 109–118, 2000. doi: 10.1291/hypres.23.109. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol 301: R1067–R1077, 2011. doi: 10.1152/ajpregu.00124.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindheimer MD, Barron WM, Davison JM. Osmoregulation of thirst and vasopressin release in pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 257: F159–F169, 1989. doi: 10.1152/ajprenal.1989.257.2.F159. [DOI] [PubMed] [Google Scholar]
- 47.Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol 304: R818–R828, 2013. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinley MJ, Allen AM, Clevers J, Paxinos G, Mendelsohn FAO. Angiotensin receptor binding in human hypothalamus: autoradiographic localization. Brain Res 420: 375–379, 1987. doi: 10.1016/0006-8993(87)91260-1. [DOI] [PubMed] [Google Scholar]
- 49.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 AND AT2 receptors in the brain. Clin Exp Pharmacol Physiol 23, Suppl 3: S99–S104, 1996. doi: 10.1111/j.1440-1681.1996.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 50.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 51.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals The National Academies Collection: reports funded by National Institutes of Health. In: Guide for the Care and Use of Laboratory Animals. Washington DC: National Academies Press, 2011. [Google Scholar]
- 52.O’Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension 48: 658–663, 2006. doi: 10.1161/01.HYP.0000238140.06251.7a. [DOI] [PubMed] [Google Scholar]
- 53.Okuya S, Inenaga K, Kaneko T, Yamashita H. Angiotensin II sensitive neurons in the supraoptic nucleus, subfornical organ and anteroventral third ventricle of rats in vitro. Brain Res 402: 58–67, 1987. doi: 10.1016/0006-8993(87)91047-X. [DOI] [PubMed] [Google Scholar]
- 54.Ozaki Y, Soya A, Nakamura J, Matsumoto T, Ueta Y. Potentiation by angiotensin II of spontaneous excitatory postsynaptic currents in rat supraoptic magnocellular neurones. J Neuroendocrinol 16: 871–879, 2004. doi: 10.1111/j.1365-2826.2004.01244.x. [DOI] [PubMed] [Google Scholar]
- 55.Park CG, Leenen FH. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci 16: 553–557, 2001. doi: 10.3346/jkms.2001.16.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfister J, Spengler C, Grouzmann E, Raizada MK, Felix D, Imboden H. Intracellular staining of angiotensin receptors in the PVN and SON of the rat. Brain Res 754: 307–310, 1997. doi: 10.1016/S0006-8993(97)00180-7. [DOI] [PubMed] [Google Scholar]
- 57.Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept 78: 1–11, 1998. doi: 10.1016/S0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 58.Qadri F, Culman J, Veltmar A, Maas K, Rascher W, Unger T. Angiotensin II-induced vasopressin release is mediated through alpha-1 adrenoceptors and angiotensin II AT1 receptors in the supraoptic nucleus. J Pharmacol Exp Ther 267: 567–574, 1993. [PubMed] [Google Scholar]
- 59.Qadri F, Waldmann T, Wolf A, Höhle S, Rascher W, Unger T. Differential contribution of angiotensinergic and cholinergic receptors in the hypothalamic paraventricular nucleus to osmotically induced AVP release. J Pharmacol Exp Ther 285: 1012–1018, 1998. [PubMed] [Google Scholar]
- 60.Quillen EW Jr, Cowley AW Jr. Influence of volume changes on osmolality-vasopressin relationships in conscious dogs. Am J Physiol Heart Circ Physiol 244: H73–H79, 1983. doi: 10.1152/ajpheart.1983.244.1.H73. [DOI] [PubMed] [Google Scholar]
- 61.Quillen EW Jr, Skelton MM, Rubin J, Cowley AW Jr. Osmotic control of vasopressin with chronically altered volume states in anephric dogs. Am J Physiol Endocrinol Metab 247: E355–E361, 1984. doi: 10.1152/ajpendo.1984.247.3.E355. [DOI] [PubMed] [Google Scholar]
- 62.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor-/- mice. Circ Res 108: 574–581, 2011. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeves WB, Bichet DG, Andreoli TE. Posterior pituitary and water metabolism. In: Williams Textbook of Endocrinology (9th ed.), edited by Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Philadelphia, PA: Saunders, 1998. [Google Scholar]
- 64.Renaud LP. CNS pathways mediating cardiovascular regulation of vasopressin. Clin Exp Pharmacol Physiol 23: 157–160, 1996. doi: 10.1111/j.1440-1681.1996.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 65.Rossi NF. Dopaminergic control of angiotensin II-induced vasopressin secretion in vitro. Am J Physiol Endocrinol Physiol 275: E687–E693, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Roussel R, Fezeu L, Marre M, Velho G, Fumeron F, Jungers P, Lantieri O, Balkau B, Bouby N, Bankir L, Bichet DG. Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab 99: 4656–4663, 2014. doi: 10.1210/jc.2014-2295. [DOI] [PubMed] [Google Scholar]
- 67.Rowland NE, Fregly MJ. Characteristics of thirst and sodium appetite in mice (Mus musculus). Behav Neurosci 102: 969–974, 1988. doi: 10.1037/0735-7044.102.6.969. [DOI] [PubMed] [Google Scholar]
- 68.Rowland NE, Goldstein BE, Robertson KL. Role of angiotensin in body fluid homeostasis of mice: fluid intake, plasma hormones, and brain Fos. Am J Physiol Regul Integr Comp Physiol 284: R1586–R1594, 2003. doi: 10.1152/ajpregu.00730.2002. [DOI] [PubMed] [Google Scholar]
- 69.Russell JA, Walley KR. Vasopressin and its immune effects in septic shock. J Innate Immun 2: 446–460, 2010. doi: 10.1159/000318531. [DOI] [PubMed] [Google Scholar]
- 70.Sandgren JA, Scroggins SM, Santillan DA, Devor EJ, Gibson-Corley KN, Pierce GL, Sigmund CD, Santillan MK, Grobe JL. Vasopressin: the missing link for preeclampsia? Am J Physiol Regul Integr Comp Physiol 309: R1586–R1594, 2015. doi: 10.1152/ajpregu.00073.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 64: 852–859, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saxena A, Bachelor M, Park YH, Carreno FR, Nedungadi TP, Cunningham JT. Angiotensin II induces membrane trafficking of natively expressed transient receptor potential vanilloid type 4 channels in hypothalamic 4B cells. Am J Physiol Regul Integr Comp Physiol 307: R945–R955, 2014. doi: 10.1152/ajpregu.00224.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saxena A, Little JT, Nedungadi TP, Cunningham JT. Angiotensin II type 1a receptors in subfornical organ contribute towards chronic intermittent hypoxia-associated sustained increase in mean arterial pressure. Am J Physiol Heart Circ Physiol 308: H435–H446, 2015. doi: 10.1152/ajpheart.00747.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schenk J, McNeill JH. The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods 27: 161–170, 1992. doi: 10.1016/1056-8719(92)90036-Z. [DOI] [PubMed] [Google Scholar]
- 75.Shi L, Mao C, Wu J, Morrissey P, Lee J, Xu Z. Effects of i.c.v. losartan on the angiotensin II-mediated vasopressin release and hypothalamic fos expression in near-term ovine fetuses. Peptides 27: 2230–2238, 2006. doi: 10.1016/j.peptides.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu K, Share L, Claybaugh JR. Potentiation by angiotensin II of the vasopressin response to an increasing plasma osmolality. Endocrinology 93: 42–50, 1973. doi: 10.1210/endo-93-1-42. [DOI] [PubMed] [Google Scholar]
- 77.Sklar AH, Schrier RW. Central nervous system mediators of vasopressin release. Physiol Rev 63: 1243–1280, 1983. doi: 10.1152/physrev.1983.63.4.1243. [DOI] [PubMed] [Google Scholar]
- 78.Sladek CD, Blair ML, Ramsay DJ. Further studies on the role of angiotensin in the osmotic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology 111: 599–607, 1982. doi: 10.1210/endo-111-2-599. [DOI] [PubMed] [Google Scholar]
- 79.Sladek CD, Johnson AK. Effect of anteroventral third ventricle lesions on vasopressin release by organ-cultured hypothalamo-neurohypophyseal explants. Neuroendocrinology 37: 78–84, 1983. doi: 10.1159/000123519. [DOI] [PubMed] [Google Scholar]
- 80.Sladek CD, Joynt RJ. Role of angiotensin in the osmotic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology 106: 173–178, 1980. doi: 10.1210/endo-106-1-173. [DOI] [PubMed] [Google Scholar]
- 81.Szczepańska-Sadowska E, Paczwa P, Loń S, Ganten D. Increased pressor function of central vasopressinergic system in hypertensive renin transgenic rats. J Hypertens 16: 1505–1514, 1998. doi: 10.1097/00004872-199816100-00016. [DOI] [PubMed] [Google Scholar]
- 82.Walch JD, Nedungadi TP, Cunningham JT. ANG II receptor subtype 1a gene knockdown in the subfornical organ prevents increased drinking behavior in bile duct-ligated rats. Am J Physiol Regul Integr Comp Physiol 307: R597–R607, 2014. doi: 10.1152/ajpregu.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang HW, Huang BS, White RA, Chen A, Ahmad M, Leenen FH. Mineralocorticoid and angiotensin II type 1 receptors in the subfornical organ mediate angiotensin II - induced hypothalamic reactive oxygen species and hypertension. Neuroscience 329: 112–121, 2016. doi: 10.1016/j.neuroscience.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Hiller H, Smith JA, de Kloet AD, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus control cardiovascular reactivity and anxiety-like behavior in male mice. Physiol Genomics 48: 667–676, 2016. doi: 10.1152/physiolgenomics.00029.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148: 1–80, 2003. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- 86.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, Claflin KE, Burnett CM, Pearson NA, Lutter ML, Grobe JL. Dietary sodium suppresses digestive efficiency via the renin-angiotensin system. Sci Rep 5: 11123, 2015. doi: 10.1038/srep11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westermann I, Dünser MW, Haas T, Jochberger S, Luckner G, Mayr VD, Wenzel V, Stadlbauer KH, Innerhofer P, Morgenthaler N, Hasibeder WR, Voelckel WG. Endogenous vasopressin and copeptin response in multiple trauma patients. Shock 28: 644–649, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Yemane H, Busauskas M, Burris SK, Knuepfer MM. Neurohumoral mechanisms in deoxycorticosterone acetate (DOCA)-salt hypertension in rats. Exp Physiol 95: 51–55, 2010. doi: 10.1113/expphysiol.2008.046334. [DOI] [PubMed] [Google Scholar]
- 89.Yeung EH, Liu A, Mills JL, Zhang C, Männistö T, Lu Z, Tsai MY, Mendola P. Increased levels of copeptin before clinical diagnosis of preeclampsia. Hypertension 64: 1362–1367, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi SS, Kim HJ, Do SG, Lee YB, Ahn HJ, Hwang IK, Yoon YS. Arginine vasopressin (AVP) expressional changes in the hypothalamic paraventricular and supraoptic nuclei of stroke-prone spontaneously hypertensive rats. Anat Cell Biol 45: 114–120, 2012. doi: 10.5115/acb.2012.45.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Z, Bourque CW. Amplification of transducer gain by angiotensin II-mediated enhancement of cortical actin density in osmosensory neurons. J Neurosci 28: 9536–9544, 2008. doi: 10.1523/JNEUROSCI.1495-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, Danisman N. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. J Obstet Gynaecol Res 37: 1198–1202, 2011. doi: 10.1111/j.1447-0756.2010.01498.x. [DOI] [PubMed] [Google Scholar]