Abstract
Mitochondrial bioenergetic contributions to sex differences in human skeletal muscle metabolism remain poorly defined. The primary aim of this study was to determine whether mitochondrial respiratory kinetics differed between healthy young men and women in permeabilized skeletal muscle fibers. While men and women displayed similar (P > 0.05) maximal respiration rates and abundance of mitochondrial/adenosine diphosphate (ADP) transport proteins, women had lower (P < 0.05) mitochondrial ADP sensitivity (+30% apparent Km) and absolute respiration rates at a physiologically relevant ADP concentration (100 μM). Moreover, although men and women exhibited similar carnitine palmitoyl transferase-I protein content- and palmitoyl-CoA-supported respiration, women displayed greater sensitivity to malonyl-CoA-mediated respiratory inhibition. These data establish baseline sex differences in mitochondrial bioenergetics and provide the foundation for studying mitochondrial function within the context of metabolic perturbations and diseases that affect men and women differently.
Keywords: ADP sensitivity, aerobic metabolism, lipid metabolism, mitochondria, sex differences, skeletal muscle
INTRODUCTION
Humans display sex differences in skeletal muscle metabolism that may be due to genetic (34), environmental, and/or hormonal influences (37). While an explanation for these differences remains poorly defined, elevated 17-β estradiol in women is believed to promote the induction of genes regulating lipid metabolism and mitochondrial function. In support of this, women have a greater proportion of type I muscle fibers (4, 8, 9, 35, 43), higher intramuscular lipid stores (33), and greater mRNA expression of genes associated with fatty acid metabolism, including cluster of differentiation-36 (CD36)/fatty acid binding protein (FABPpm) (19), hormone-sensitive lipase (33), carnitine palmitoyl transferase-I (CPT-I), β-hydroxyacyl-CoA dehydrogenase (β-HAD) (23), and citrate synthase (23), while short-term supplementation of 17-β estradiol in men increases peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and the expression of proteins involved in mitochondrial β-oxidation (22). Moreover, lower 17-β estradiol in aging women is associated with a greater risk of developing chronic diseases linked with impaired mitochondrial metabolism, including heart disease and Type 2 diabetes (11). Altogether, these data implicate alterations in mitochondrial bioenergetics as a contributor to metabolic sex differences.
Although 17-β estradiol has the potential to influence mitochondrial gene transcription, men and women do not display differences in mitochondrial enzymatic function, density, or morphology (38), suggesting an alternative explanation. Instead, this hormone may influence the intrinsic function of the mitochondria. In this regard, 17-β estradiol has recently been shown in rodents to positively regulate mitochondrial bioenergetics, independent of mitochondrial content or maximal enzymatic rates, by directly influencing mitochondrial membrane viscosity (39). Therefore, sex differences in mitochondrial bioenergetics in humans may exist despite similar mitochondrial protein expression and enzymatic capacity.
A prominent control point for regulating aerobic metabolism involves the transport of ADP and lipids through the mitochondrial voltage-dependent anion channel (VDAC)/adenine-nucleotide translocase (ANT) axis (12) and CPT-I (24), respectively. Given that membrane fluidity is believed to influence the structure/function of integral membrane proteins, and 17-β estradiol has been linked to mitochondrial membrane viscosity (39), we hypothesized that women would exhibit respiratory substrate kinetics conducive toward greater mitochondrial substrate transport/metabolism. To examine this, we determined muscle mitochondrial and substrate transporter protein expression, mitochondrial respiration, and substrate sensitivity between healthy young men and women.
METHODS
Participants and skeletal muscle biopsy.
Sixteen healthy moderately trained men (n = 8) and women (n = 8) that reported to be recreationally active 2–3 times per week were recruited for the study. Individuals arrived at the laboratory in the fasted state, body mass and height were recorded, and a dual-energy X-ray absorptiometry scan was performed. Thereafter, participants were rested in a semi-supine position on a bed, the skin surrounding the vastus lateralis muscle was cleaned, local anesthetic was applied (2% lidocaine, 0.005 mg/ml epinephrine), and an incision was made to the skin and fascia. A sample of vastus lateralis (~30 mg) was extracted using a Bergström needle and immediately placed in ice-cold BIOPS for preparation of permeabilized muscle fibers (described below). All participants provided informed written and oral consent before commencing the study, and all procedures were approved by the Hamilton Integrated Research Ethics Board at McMaster University, and conformed to the most recent version of the Declaration of Helsinki.
Preparation of permeabilized muscle fibers.
As previously validated (40), permeabilized muscle fibers were separated under a microscope in preservation buffer (BIOPS, in mM: 50 MES, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.77 ATP, 15 PCr, and 6.56 MgCl2·H2O; pH 7.1). Fibers were treated with saponin (30 μg/ml) (18) to permeabilize the sarcolemma and were then washed in MIRO5 (0.5 mM EGTA, 3 mM MgCl2·6H2O, 60 mM potassium lactobionate, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 1 g/l fatty acid-free BSA; pH 7.1) until respiration analyses.
Mitochondrial respiration in permeabilized muscle fibers.
Mitochondrial respiration measurements were performed in MIRO5 using high-resolution respirometry (Oroboros Oxygraph-2K, Innsbruck, Austria) at 37°C. All experiments were conducted between 200 and 180 μM of oxygen, and the buffer was equilibrated to room air after the addition of each substrate. All experiments were performed in the presence of 5 µM blebbistatin, a myosin II inhibitor. ADP-stimulated respiration was determined by titrating ADP (0, 100, 250, 500, 1,000, 2,000, 4,000, 6,000, 8,000, 10,000, 12,000, 16,000, 18,000 μM) until a plateau was attained in the presence of saturating pyruvate (5 mM) and malate (5 mM). Subsequently, glutamate (10 mM) and succinate (10 mM) were added at the end of experiments to determine maximal complex I- and II-supported respiration, respectively. In separate fiber experiments, palmitoyl-CoA (P-CoA)-stimulated respiration (25 and 50 μM) was determined in the presence of malate (5 mM), ADP (20 mM), and l-carnitine (2 mM). To determine percent inhibition as a surrogate of CPT-I sensitivity to malonyl-CoA (M-CoA), M-CoA (7 μM) was added on the back end of experiments. Respiratory control ratios (RCRs) were calculated by dividing state 3 (+ADP)/state 2 (−ADP) respiration. Cytochrome c (10 μM) was added at the end of all experiments to ensure mitochondrial integrity and did not stimulate mitochondrial respiration (data not shown). All fiber bundles were recovered and freeze dried, and data were normalized to fiber weight.
Fiber digestion and Western blot analysis.
Western blot analysis was performed on remaining nonpermeabilized skeletal muscle from the biopsy. Muscle was freeze-dried, weighed, and digested (5 μl/μg) in a buffer containing glycerol (10%), β-mercaptoethanol (5%), SDS (2.3%), and Tris·HCl (62.5 mM; pH 6.8) (13). Samples were then loaded equally for OXPHOS (1:500, cat. no. ab110413; Abcam), pyruvate-dehydrogenase-subunit E1α (PDHE1α, 1:5,000, cat no. 459400; Invitrogen), pyruvate dehydrogenase kinase-4 (PDK4, 1:1,000, cat. no. ab38242; Abcam), PGC-1α (1:1,000, cat. no. 516557; Calbiochem), peroxisome proliferator-activated receptor γ coactivator-β (PGC-1β, 1:1,000, cat. no. NB110–58858; Novus), sirtuin-1 (SIRT1, 1:1,000, cat. no. 2493; Cell Signaling), histone acetyltransferase-5 (GCN5, 1:1,000, cat. no. 607202; Biolegend), VDAC (1:5,000, cat. no. ab14734; Abcam), mitochondrial creatine-kinase (Mi-CK, 1:5,000, cat. no. ab131188; Abcam), ANT1 (1:1,000, cat. no. ab110322; Abcam), FABPpm, (1:30,000), CD36 (1:1,000, sc13572; Santa Cruz), adipose-triglyceride-lipase (ATGL, 1:1,000, cat. no. 10006409; Cayman), Lipin 1 (1:1,000, cat. no. ab84950; Abcam), glycerol-3-phosphate acyltransferase (GPAT; 1:1,000; Santa Cruz), diglyceride acyltransferase (DGAT; 1:1,000, cat. no. IMG-30279; Imgenex), CPT-I (1:1,000, cat. no. CPT1M11-A; Alpha-diagnostics), and β-HAD (1:1,000, cat. no. ab154088; Abcam). CIV is absent from OXPHOS images as a result of the digestion protocol, as previously reported (13). Antibody validation for these targets have been previously reported (1, 3, 5, 6, 14, 25–29, 31, 32, 44). Proteins were separated using SDS-PAGE, blocked, and incubated with the appropriate primary and secondary antibodies. All membranes were detected using enhanced chemiluminescence (ChemiGenius2 Bioimaging system; SynGene).
Statistical analyses.
Michaelis-Menten kinetics were determined using GraphPad Prism 7 software. Sex differences were determined using Student’s independent samples t-test (SigmaPlot 11.0, Systat Software) between men and women. The P values were two-tailed for all parameters with the exception of CD36 that has been previously reported to exhibit sex differences (one-tailed). Associations between fat mass and mitochondrial bioenergetics were performed using Pearson r correlational analyses. Significance was determined as P < 0.05. Data are expressed as means ± SE.
RESULTS
Participant characteristics.
Men and women were matched for age and body mass index, and therefore, there were no significant differences in these parameters (Table 1). As expected, women had a lower height, lower body mass, and lean mass, and greater fat mass than men (P = 0.09 for absolute fat mass; P = 0.002 relative fat mass (Table 1).
Table 1.
Participant characteristics
| Men | Women | P Value | |
|---|---|---|---|
| Age, yr | 23 ± 1 | 21 ± 1 | 0.193 |
| Height, m | 1.8 ± 0.02 | 1.6 ± 0.02 | <0.001 |
| Total mass, kg | 76.1 ± 2.0 | 63.2 ± 3.5 | 0.006 |
| BMI | 23.8 ± 0.62 | 24.1 ± 1.6 | 0.865 |
| Body fat, % | 19 ± 2 | 32 ± 3 | 0.002 |
| Body fat, kg | 14.0 ± 1.4 | 20.0 ± 3.1 | 0.09 |
| Lean mass, kg | 59.2 ± 1.9 | 40.5 ± 1.5 | <0.001 |
Data are expressed as means ± SE. BMI, body mass index.
Women have lower ADP sensitivity than men independent of maximal respiration rates and mitochondrial protein expression in skeletal muscle.
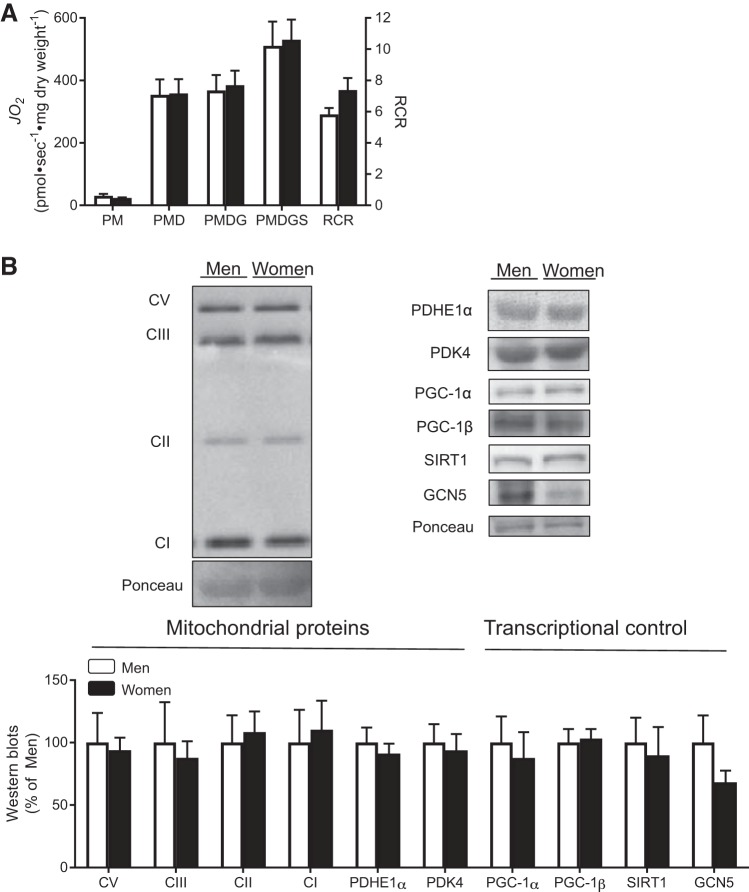
Given that 17-β estradiol has recently been shown to regulate mitochondrial respiration in rodents (39), we first assessed maximal respiration rates in men and women. RCR values above 4 confirm viable fiber preparations in both men and women (Fig. 1A). Men and women had similar mitochondrial respiration rates in the presence of complex I (pyruvate, glutamate)- and II (succinate)-linked substrates, as well as maximal ADP-stimulated respiration (Fig. 1A). In addition, men and women displayed similar abundance of mitochondrial proteins, including various electron transport chain complexes (Fig. 1B), and similar levels of key proteins involved in transcriptional regulation of mitochondrial biogenesis, including PGC-1α, PGC-1β, SIRT1, and GCN5 (Fig. 1B).
Fig. 1.
Maximal mitochondrial respiration and markers of mitochondrial content. Rates of oxygen consumption (JO2; A) in permeabilized muscle fibers were determined in men and women with the sequential addition of pyruvate+malate (PM, Complex I), ADP (PMD), glutamate (PMDG, maximal Complex I), and succinate (PMDGS, Complex I+II). Respiratory control ratio (RCR) is complex I respiration in the presence and absence of ADP. B: Western blots were used to estimate the abundance of mitochondrial proteins, as well as proteins involved in regulating mitochondrial biogenesis. GCN5, histone acetyltransferase-5; PDHE1α, pyruvate-dehydrogenase-subunit E1α; PDK4, pyruvate dehydrogenase kinase-4; PGC-1α, peroxisome proliferator-activated receptor γ-coactivator 1-α; PGC-1β, peroxisome proliferator-activated receptor-γ coactivator 1-β; SIRT1, sirtuin-1. C denotes the complex of various subunits of the electron transport chain (CI, CII, CIII, and CV). *Significant difference between men and women (P < 0.05). Data are expressed as means ± SE; n = 7 or 8 participants for each sex.
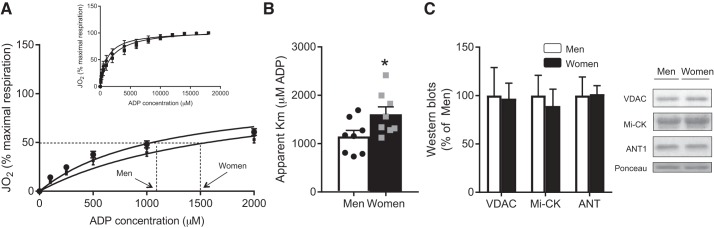
We next examined mitochondrial ADP sensitivity, as we reasoned this could be regulated independent of mitochondrial content. While men had comparable apparent Km values as the existing literature for ADP (13, 21, 30), women had significantly lower ADP sensitivity (~30%; greater apparent Km) (Fig. 2, A and B) and lower absolute respiration at a physiological (100 μM) concentration of ADP (men: 82.53 ± 17.69; women: 47.50 ± 2.56, pmol·s−1·mg dry wt−1, P < 0.05) than men. This occurred independent of alterations in the content of ADP transport proteins VDAC, Mi-CK, and ANT1 (Fig. 2C). Altogether, these data highlight sex differences in the regulation of the VDAC/ANT axis, independent of mitochondrial capacity measurements.
Fig. 2.
Mitochondrial ADP respiratory sensitivity. Rates of oxygen consumption (JO2) in permeabilized muscle fibers were determined in men and women in the presence of pyruvate+malate and various concentrations of ADP. These values followed a Michaelis-Menten kinetic curve (A; inset shows the full kinetic curve; circles denote men and squares denote women), which was used to estimate ADP sensitivity (apparent ADP Km; B). Western blots were utilized to estimate the abundance of mitochondrial proteins involved in ADP transport (C). ANT1, adenine nucleotide translocase-1; Mi-CK, mitochondrial creatine-kinase; VDAC, voltage-dependent anion channel. *Significant difference between men and women (P < 0.05). Data are expressed as means ± SE; n = 8 (respiration analyses) and n = 7 or 8 (Western blots) participants for each sex.
Women have similar P-CoA-stimulated respiration rates and greater sensitivity to M-CoA than men in skeletal muscle.
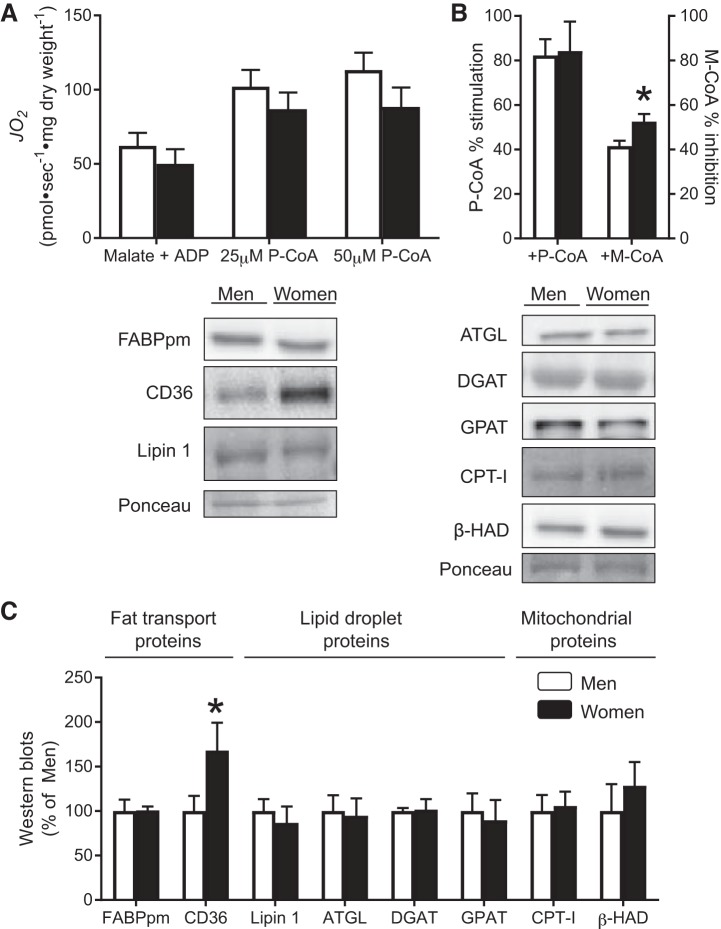
We next examined indexes of mitochondrial fatty acid oxidation (e.g., CPT-I-dependent respiration) and protein content of markers involved in lipid handling between men and women. There was no difference in absolute P-CoA-stimulated respiration rates in the presence of P-CoA (25 or 50 μM) between men and women (Fig. 3A). Given the observation that women had slightly lower respiration rates compared with men before the addition of P-CoA, we also expressed the data as percent stimulation to examine the direct ability of P-CoA to stimulate respiration. When we used this approach, there were still no differences in percent stimulation on P-CoA respiration between men and women (Fig. 3B), further suggesting a similar capacity for lipid-supported respiration. In contrast, the ability for M-CoA to inhibit P-CoA-stimulated respiration was greater in women (Fig. 3B), implicating a greater sensitivity of CPT-I to M-CoA inhibition in human skeletal muscle of women. While this response would predict lower CPT-I flux in women, in vivo, fatty acid oxidation is also influenced by substrate availability. Therefore, we next examined markers of lipid transport, synthesis/lipolysis, and oxidation. Supporting the P-CoA respiration data, CPT-I and β-HAD protein content were similar between sexes (Fig. 3C). While there were no differences in proteins involved in lipid synthesis/lipolysis (ATGL, Lipin 1, GPAT, DGAT) or FABPpm, a key plasma membrane lipid transporter (CD36) was higher in women (Fig. 3C), which could contribute to sex differences in intramuscular lipid metabolism by increasing substrate availability.
Fig. 3.
Mitochondrial lipid supported respiration and proteins involved in fatty acid metabolism. A: rates of oxygen consumption (JO2) in permeabilized muscle fibers were determined in men and women in the presence of 25 and 50 μM palmitoyl-CoA (P-CoA). B: ability of P-CoA to stimulate respiration and malonyl-CoA (M-CoA) to inhibit respiration was also determined. C: Western blots were used to estimate the abundance of proteins involved in fatty acid metabolism. ATGL, adipose-triglyceride-lipase; CD36, cluster of differentiation-36; CPT-I, carnitine palmitoyl transferase-I; DGAT, diacylglycerol acyltransferase; FABPpm, fatty acid binding protein; GPAT, glycerol-3-phosphate acyltransferase; β-HAD, β-hydroxyacyl-CoA dehydrogenase. *Significant difference between men and women (P < 0.05). Data represent means ± SE; n = 5–8 participants for each sex in A and B due to an inability to recover some fibers to obtain accurate weights after respiration experiments were performed; n = 7 or 8 participants for each sex in C.
Association between body fat mass and mitochondrial bioenergetics.
Since the women in the current study had greater fat mass than men, and previous work has highlighted a relationship between fat mass and muscle mitochondrial capacity (16), we next used Pearson r correlational analyses to determine the relationship between mitochondrial kinetics and fat mass. There was a trending positive association between percent fat mass and apparent Km for ADP (r = 0.477; P = 0.06). Similarly, there was a trend for a positive relationship between percent fat mass and M-CoA inhibition (r = 0.348; P = 0.1).
DISCUSSION
Our data provide novel insight that women have moderately lower mitochondrial ADP sensitivity and greater M-CoA sensitivity than men. This occurred independent of maximal respiration and abundance of mitochondrial proteins or substrate transporters in resting skeletal muscle. Altogether, these data do not support the supposition that women display higher mitochondrial membrane substrate transport.
Sex differences in skeletal muscle ADP sensitivity.
Although men and women are known to have different skeletal muscle metabolic properties (4, 8, 9, 35, 43), whether this divergence extends to mitochondrial function and substrate transport capacity remains poorly defined. However, this may be an important consideration for understanding general skeletal muscle metabolism, given that the transport of ADP through the VDAC/ANT axis could modulate cytosolic ADP concentrations and glycolytic flux (42), and it is already well established that men and women have different fuel reliance depending on energy demand (4, 10, 15). In our current study, the similar maximal respiration rates suggest any potential hormonal differences between men and women do not alter mitochondrial capacity, which is further supported by the similar protein expression of electron transport chain complexes. While previous work in mice has shown that ablation of circulating 17-β estradiol was detrimental for maximal respiratory flux (39), our current data suggest that women, who typically have two- to threefold higher 17-β estradiol levels (17), do not display different respiratory capacity than men.
Another major aim of the present study was to examine potential sex differences in mitochondrial ADP sensitivity. Although we hypothesized that women would have greater respiratory kinetics in response to ADP, women, in fact, displayed lower ADP sensitivity. The lower mitochondrial ADP sensitivity and respiration rate at 100 μM ADP in women occurred in the presence of similar levels of VDAC, Mi-CK, and ANT1, suggesting a possible sex-specific regulation of ADP sensitivity. Although the functional role for the lower ADP sensitivity remains to be determined and may be influenced by a potential relationship with fat mass, this lower ADP sensitivity does not likely contribute to the improvement in aerobic metabolism or fatty acid oxidation observed in women compared with men (7).
Sex differences in CPT-I regulation by M-CoA in skeletal muscle.
It is well established that women, or men supplementing with 17-β estradiol, rely more heavily on lipid oxidation during exercise (37). Although the mechanisms of action for these sex differences in lipid metabolism remain poorly defined, an increase in lipid delivery to muscle has been proposed as a contributing factor. In addition to women displaying higher lipoprotein lipase (19) and intramuscular lipid content (33), our data demonstrate that women have higher CD36 protein expression, as observed previously (19). Surprisingly, despite fatty acid metabolism being a mitochondrial localized process, direct comparisons between mitochondrial respiratory function between sexes have been lacking. In the present study, we show that men and women display similar CPT-I content and P-CoA-stimulated respiration rates. Importantly, we used submaximal concentrations of P-CoA that reflect physiological muscle concentrations (41) and revealed similar responses among men and women, supporting previous findings of unaltered maximal CPT-I activity (2). In addition, we present a novel finding that women have greater sensitivity for M-CoA inhibition of P-CoA-stimulated respiration. Although speculative, the greater M-CoA sensitivity in women may be required to sustain low-energy demands at rest and turn down mitochondrial substrate flux, especially given that women in the current study had greater percent fat mass, a trending relationship between percent fat mass and M-CoA sensitivity, and, typically have greater intramuscular lipid stores (33). Regardless, the present finding of greater M-CoA sensitivity and similar P-CoA sensitivity suggest greater CPT-I flux may not contribute to sex differences in humans.
Perspectives and Significance
Overall, we provide novel insight that men and women have similar maximal respiration rates and different substrate sensitivity independent of protein expression of substrate transporters, lipid handling, or mitochondrial capacity in resting skeletal muscle. Within this context, an important consideration is participant training status, as exercise training can decrease ADP sensitivity (21). While women displayed lower ADP sensitivity indicative of greater physical fitness, training can also decrease M-CoA sensitivity (36), which is the opposite response that would be expected due to the observed sex differences. Therefore, it is unlikely that training status can fully explain the present data, especially considering both sexes had similar maximal respiration and mitochondrial content, parameters that typically increase together with training and positively correlate with maximal mitochondrial oxygen consumption in humans (20). Another potential confounding variable relates to skeletal muscle fiber type, as women are known to have a greater abundance of type I muscle fibers (4, 8, 9, 35, 43), and in rodents, oxidative muscles display lower mitochondrial ADP sensitivity (45). It remains unknown what accounts for this fiber type difference, and future work should determine whether type I fibers display different isoforms of ADP transport proteins (i.e., ANT/VDAC). Nevertheless, the present data provide compelling evidence that women display lower mitochondrial ADP sensitivity and greater M-CoA sensitivity, and given the relationship between mitochondrial content and function and various chronic diseases (11), these baseline sex differences provide the foundation for studying the role of mitochondrial bioenergetics within the context of metabolic perturbations and diseased states.
GRANTS
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada (to G. P. Holloway and S. M. Phillips), and infrastructure was purchased with assistance from the Canadian Foundation for Innovation/Ontario Research Fund (to G. P. Holloway). P. M. Miotto is supported by an Ontario Women’s Health Scholars Doctoral Award. C. McGlory is supported by a Diabetes Canada Fellowship Award. T. M. Holloway is supported by an NSERC Postdoctoral Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M.M., S.M.P., and G.P.H. conceived and designed research; P.M.M. performed experiments; P.M.M. analyzed data; P.M.M., C.M., T.M.H., S.M.P., and G.P.H. interpreted results of experiments; P.M.M. and G.P.H. prepared figures; P.M.M. and G.P.H. drafted manuscript; P.M.M., C.M., T.M.H., S.M.P., and G.P.H. edited and revised manuscript; P.M.M., C.M., T.M.H., S.M.P., and G.P.H. approved final version of manuscript.
REFERENCES
- 1.Beaudoin MS, Snook LA, Arkell AM, Simpson JA, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol 305: R542–R551, 2013. doi: 10.1152/ajpregu.00200.2013. [DOI] [PubMed] [Google Scholar]
- 2.Berthon PM, Howlett RA, Heigenhauser GJ, Spriet LL. Human skeletal muscle carnitine palmitoyltransferase I activity determined in isolated intact mitochondria. J Appl Physiol (1985) 85: 148–153, 1998. doi: 10.1152/jappl.1998.85.1.148. [DOI] [PubMed] [Google Scholar]
- 3.Bruce CR, Brolin C, Turner N, Cleasby ME, van der Leij FR, Cooney GJ, Kraegen EW. Overexpression of carnitine palmitoyltransferase I in skeletal muscle in vivo increases fatty acid oxidation and reduces triacylglycerol esterification. Am J Physiol Endocrinol Metab 292: E1231–E1237, 2007. doi: 10.1152/ajpendo.00561.2006. [DOI] [PubMed] [Google Scholar]
- 4.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 280: E898–E907, 2001. doi: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- 5.Chacko AD, Liberante F, Paul I, Longley DB, Fennell DA. Voltage-dependent anion channel-1 regulates death receptor mediated apoptosis by enabling cleavage of caspase-8. BMC Cancer 10: 380, 2010. doi: 10.1186/1471-2407-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent JR, Martins VF, Svensson K, LaBarge SA, Schlenk NC, Esparza MC, Buckner EH, Meyer GA, Hamilton DL, Schenk S, Philp A. Muscle-specific knockout of general control of amino acid synthesis 5 (GCN5) does not enhance basal or endurance exercise-induced mitochondrial adaptation. Mol Metab 6: 1574–1584, 2017. doi: 10.1016/j.molmet.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devries MC. Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol 101: 243–249, 2016. doi: 10.1113/EP085369. [DOI] [PubMed] [Google Scholar]
- 8.Esbjörnsson-Liljedahl M, Sundberg CJ, Norman B, Jansson E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol (1985) 87: 1326–1332, 1999. doi: 10.1152/jappl.1999.87.4.1326. [DOI] [PubMed] [Google Scholar]
- 9.Esbjörnsson M, Sylvén C, Holm I, Jansson E. Fast twitch fibres may predict anaerobic performance in both females and males. Int J Sports Med 14: 257–263, 1993. doi: 10.1055/s-2007-1021174. [DOI] [PubMed] [Google Scholar]
- 10.Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini MF, Trimmer JK, Brooks GA. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J Appl Physiol (1985) 85: 1175–1186, 1998. doi: 10.1152/jappl.1998.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 11.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med 89: 157–161, 1978. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 12.Guzun R, Gonzalez-Granillo M, Karu-Varikmaa M, Grichine A, Usson Y, Kaambre T, Guerrero-Roesch K, Kuznetsov A, Schlattner U, Saks V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within mitochondrial interactosome. Biochim Biophys Acta 1818: 1545–1554, 2012. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Herbst EA, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski A, Heigenhauser GJ, Spriet LL, Holloway GP. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol 592: 1341–1352, 2014. doi: 10.1113/jphysiol.2013.267336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway GP, Gurd BJ, Snook LA, Lally J, Bonen A. Compensatory increases in nuclear PGC1α protein are primarily associated with subsarcolemmal mitochondrial adaptations in ZDF rats. Diabetes 59: 819–828, 2010. doi: 10.2337/db09-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol (1985) 85: 1823–1832, 1998. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 16.Hütter E, Skovbro M, Lener B, Prats C, Rabøl R, Dela F, Jansen-Dürr P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 6: 245–256, 2007. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 17.Ichii S, Forchielli E, Perloff WH, Dorfman RI. Determination of plasma estrone and estradiol-17β. Anal Biochem 5: 422–425, 1963. doi: 10.1016/0003-2697(63)90043-5. [DOI] [PubMed] [Google Scholar]
- 18.Kane DA, Lin CT, Anderson EJ, Kwak HB, Cox JH, Brophy PM, Hickner RC, Neufer PD, Cortright RN. Progesterone increases skeletal muscle mitochondrial H2O2 emission in nonmenopausal women. Am J Physiol Endocrinol Metab 300: E528–E535, 2011. doi: 10.1152/ajpendo.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol (1985) 97: 1209–1218, 2004. doi: 10.1152/japplphysiol.01278.2003. [DOI] [PubMed] [Google Scholar]
- 20.Lee HK. Method of proof and evidences for the concept that mitochondrial genome is a thrifty genome. Diabetes Res Clin Pract 54, Suppl 2: S57–S63, 2001. doi: 10.1016/S0168-8227(01)00336-9. [DOI] [PubMed] [Google Scholar]
- 21.Ludzki A, Paglialunga S, Smith BK, Herbst EA, Allison MK, Heigenhauser GJ, Neufer PD, Holloway GP. Rapid repression of ADP transport by palmitoyl-CoA is attenuated by exercise training in humans: a potential mechanism to decrease oxidative stress and improve skeletal muscle insulin signaling. Diabetes 64: 2769–2779, 2015. doi: 10.2337/db14-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17β-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics 42: 342–347, 2010. doi: 10.1152/physiolgenomics.00016.2010. [DOI] [PubMed] [Google Scholar]
- 23.Maher AC, Fu MH, Isfort RJ, Varbanov AR, Qu XA, Tarnopolsky MA. Sex differences in global mRNA content of human skeletal muscle. PLoS One 4: e6335, 2009. doi: 10.1371/journal.pone.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244: 1–14, 1997. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 25.Miotto PM, Frendo-Cumbo S, Sacco SM, Wright DC, Ward WE, Holloway GP. Combined high-fat-resveratrol diet and RIP140 knockout mice reveal a novel relationship between elevated bone mitochondrial content and compromised bone microarchitecture, bone mineral mass, and bone strength in the tibia. Mol Nutr Food Res 60: 1994–2007, 2016. doi: 10.1002/mnfr.201500870. [DOI] [PubMed] [Google Scholar]
- 26.Miotto PM, Holloway GP. In the absence of phosphate shuttling, exercise reveals the in vivo importance of creatine-independent mitochondrial ADP transport. Biochem J 473: 2831–2843, 2016. doi: 10.1042/BCJ20160373. [DOI] [PubMed] [Google Scholar]
- 27.Molven A, Hollister-Lock J, Hu J, Martinez R, Njølstad PR, Liew CW, Weir G, Kulkarni RN. The hypoglycemic phenotype is islet cell-autonomous in short-chain hydroxyacyl-CoA dehydrogenase-deficient mice. Diabetes 65: 1672–1678, 2016. doi: 10.2337/db15-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57: 1166–1175, 2008. doi: 10.2337/db07-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickerson JG, Alkhateeb H, Benton CR, Lally J, Nickerson J, Han XX, Wilson MH, Jain SS, Snook LA, Glatz JF, Chabowski A, Luiken JJ, Bonen A. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem 284: 16522–16530, 2009. doi: 10.1074/jbc.M109.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, Heigenhauser GJ, Neufer PD, Spriet LL, Holloway GP. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol 590: 5475–5486, 2012. doi: 10.1113/jphysiol.2012.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC Jr, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem 283: 13087–13099, 2008. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren NSX, Ji M, Tokar EJ, Busch EL, Xu X, Lewis D, Li X, Jin A, Zhang Y, Wu WKK, Huang W, Li L, Fargo DC, Keku TO, Sandler RS, Li X. Haploinsufficiency of SIRT1 enhances glutamine metabolism and promotes cancer development. Curr Biol 27: 483–494, 2017. doi: 10.1016/j.cub.2016.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roepstorff C, Donsmark M, Thiele M, Vistisen B, Stewart G, Vissing K, Schjerling P, Hardie DG, Galbo H, Kiens B. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol Endocrinol Metab 291: E1106–E1114, 2006. doi: 10.1152/ajpendo.00097.2006. [DOI] [PubMed] [Google Scholar]
- 34.Roth SM, Ferrell RE, Peters DG, Metter EJ, Hurley BF, Rogers MA. Influence of age, sex, and strength training on human muscle gene expression determined by microarray. Physiol Genomics 10: 181–190, 2002. doi: 10.1152/physiolgenomics.00028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC, Bouchard C. Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol Occup Physiol 54: 250–253, 1985. doi: 10.1007/BF00426141. [DOI] [PubMed] [Google Scholar]
- 36.Starritt EC, Howlett RA, Heigenhauser GJ, Spriet LL. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am J Physiol Endocrinol Metab 278: E462–E468, 2000. doi: 10.1152/ajpendo.2000.278.3.E462. [DOI] [PubMed] [Google Scholar]
- 37.Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc 40: 648–654, 2008. doi: 10.1249/MSS.0b013e31816212ff. [DOI] [PubMed] [Google Scholar]
- 38.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol 292: R1271–R1278, 2007. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 39.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab 27: 167–179.e7, 2018. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta 892: 191–196, 1987. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 41.Watt MJ, Heigenhauser GJ, O’Neill M, Spriet LL. Hormone-sensitive lipase activity and fatty acyl-CoA content in human skeletal muscle during prolonged exercise. J Appl Physiol (1985) 95: 314–321, 2003. doi: 10.1152/japplphysiol.01181.2002. [DOI] [PubMed] [Google Scholar]
- 42.Wu TF, Davis EJ. Regulation of glycolytic flux in an energetically controlled cell-free system: the effects of adenine nucleotide ratios, inorganic phosphate, pH, and citrate. Arch Biochem Biophys 209: 85–99, 1981. doi: 10.1016/0003-9861(81)90260-5. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol (1985) 99: 1085–1092, 2005. doi: 10.1152/japplphysiol.00247.2005. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Zhong W, Sun Q, Sun X, Zhou Z. Adipose-specific lipin1 overexpression in mice protects against alcohol-induced liver injury. Sci Rep 8: 408, 2018. doi: 10.1038/s41598-017-18837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoll J, Koulmann N, Bahi L, Ventura-Clapier R, Bigard AX. Quantitative and qualitative adaptation of skeletal muscle mitochondria to increased physical activity. J Cell Physiol 194: 186–193, 2003. doi: 10.1002/jcp.10224. [DOI] [PubMed] [Google Scholar]