Abstract
Recent preclinical studies show renal denervation (RDNx) may be an effective treatment for hypertension; however, the mechanism remains unknown. We have recently reported total RDNx (TRDNx) and afferent-selective RDNx (ARDNx) similarly attenuated the development of deoxycorticosterone acetate (DOCA)-salt hypertension. Whereas TRDNx abolished renal inflammation, ARDNx had a minimal effect despite an identical antihypertensive effect. Although this study established that ARDNx attenuates the development of DOCA-salt hypertension, it is unknown whether this mechanism remains operative once hypertension is established. The current study tested the hypothesis that TRDNx and ARDNx would similarly decrease mean arterial pressure (MAP) in the DOCA-salt hypertensive rat, and only TRDNx would mitigate renal inflammation. After 21 days of DOCA-salt treatment, male Sprague-Dawley rats underwent TRDNx (n = 16), ARDNx (n = 16), or Sham (n = 14) treatment and were monitored for 14 days. Compared with baseline, TRDNx and ARDNx decreased MAP similarly (TRDNx −14 ± 4 and ARDNx −15 ± 6 mmHg). After analysis of diurnal rhythm, rhythm-adjusted mean and amplitude of night/day cycle were also reduced in TRDNx and ARDNx groups compared with Sham. Notably, no change in renal inflammation, injury, or function was detected with either treatment. We conclude from these findings that: 1) RDNx mitigates established DOCA-salt hypertension; 2) the MAP responses to RDNx are primarily mediated by ablation of afferent renal nerves; and 3) renal nerves do not contribute to the maintenance of renal inflammation in DOCA-salt hypertension.
Keywords: deoxycorticosterone acetate-salt, renal nerves, sympathetic nervous system
INTRODUCTION
Cardiovascular disease (CVD) remains the most pervasive cause of morbidity and mortality in adults worldwide (25, 54), and hypertension is the single greatest risk factor for CVD (2). While the etiology of hypertension is multifaceted, a principal role for increased sympathetic nerve activity (SNA) has been well documented in both clinical and experimental settings (10, 11, 47). In recent years, particular attention has been focused on the renal sympathetic nerves, since renal denervation (RDNx) attenuates hypertension in several experimental models (11). These findings were translated to the first clinical trials (13, 30), where a catheter-based method for RDNx to treat drug-resistant hypertension resulted in a sustained decrease in arterial pressure. Unsurprisingly, these trials generated a large amount of interest for this catheter-based RDNx approach to treat drug-resistant hypertension. However, the recent failure of the SYMPLICITY-3 trial to meet its efficacy end point after 6 mo (6) has forced a reexamination of the conditions under which RDNx is an effective treatment for hypertension and its mechanism of action. The failure of this trial was likely because of several methodological shortcomings, including improper training and variation of intraprocedural efficacy (12, 42). It is noteworthy that more recent clinical trials using different catheter technology and highly trained experienced interventionists have observed an antihypertensive response to RDNx (40, 43, 55, 56), including the early results from the SPYRAL HTN-OFF MED trial (50).
Our laboratory and others have investigated the separate roles of renal efferent (brain→kidney) and afferent (kidney→brain) nerves to cause hypertension in several preclinical models (3, 15, 16, 29, 58). We recently reported that selective ablation of afferent renal nerves attenuates the deoxycorticosterone acetate-salt (DOCA-salt) rat model of hypertension by the same magnitude as total RDNx (TRDNx) (3, 16), suggesting ablation of afferent nerves mediates the antihypertensive effects of RDNx in this model. This is consistent with our finding that resting afferent renal nerve activity (ARNA) is 2.5-fold higher in DOCA-salt rats compared with normotensive controls. Furthermore, this increase in ARNA was associated with increased renal tissue levels of several proinflammatory cytokines (3). We hypothesize that renal inflammation in this model is responsible for the increase in ARNA, as a result, increased activity of the sympathetic nervous system and, in turn, hypertension.
Recent reports suggest renal nerves directly contribute to renal inflammation through the trafficking and activation of inflammatory cells and cytokine production (3, 26, 51, 58). We and others have reported several proinflammatory renal cytokines [e.g., interleukin (IL)-1β, IL-2, IL-6, monocyte chemoattractant protein-1 (MCP-1), and growth-related oncogene/keratinocyte chemoattractant (GRO/KC)] are increased in animal models of hypertension, and RDNx prevents this increase (3, 58). Interestingly, in our recent study, afferent-selective RDNx (ARDNx) did not abolish the renal inflammation to the extent TRDNx did, despite the identical antihypertensive response to both treatments (3). These findings suggest afferent renal nerves mediate the hypertensive response to the development of DOCA-salt hypertension secondary to renal inflammation, which we hypothesized was dependent on efferent renal sympathetic nerves.
It is important to note that, at the present time, there is no clear evidence that these relationships between renal nerves, renal inflammation, and hypertension remain operative once hypertension is established. Indeed, the theoretical foundation for RDNx as a hypertension therapy is based almost exclusively on reports that RDNx attenuates the development of hypertension in animal models (3, 15, 16, 19, 35, 58). However, there are far fewer preclinical studies on the efficacy and mechanism of RDNx in treating preexistent hypertension (5, 15, 32, 36) [see Tables 2 and 3 in Osborn and Foss (39)]. Although understanding the role of renal nerves in the pathogenesis of hypertension is important, it has little clinical application since RDNx is not used as a prophylactic therapy. In contrast, the ability of RDNx to reverse hypertension is highly important since it has direct applications to current catheter-based RDNx therapies in hypertensive humans.
Therefore, the present study was conducted to establish whether the cardiovascular and renal inflammatory responses to TRDNx and ARDNx observed during the development of DOCA-salt hypertension (3) translate to the reversal of the preexistent hypertension in this model. More specifically, we tested the hypotheses that 1) TRDNx and ARDNx would similarly reverse DOCA-salt hypertension; 2) TRDNx and ARDNx would decrease sympathetic tone; and 3) TRDNx would reduce renal inflammation and improve renal function, whereas ARDNx would have no effect.
METHODS
Experimental design and treatments.
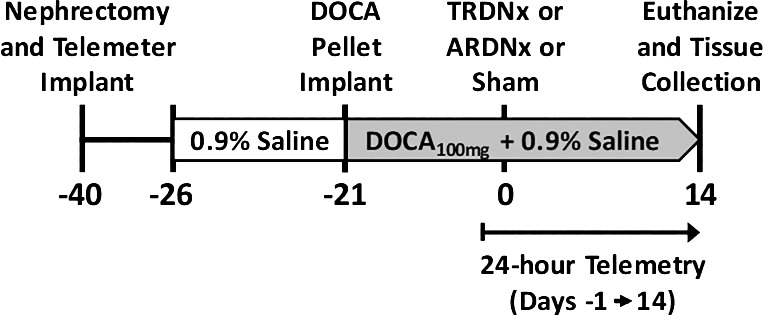
All procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were housed in a temperature-controlled room with a 12:12-h on-off light schedule (on: 0700–1900). These experiments were conducted using the DOCA-salt rat model, a well-established preclinical model that recapitulates moderate to severe salt-sensitive hypertension and renal dysfunction (18). An overview of the experimental protocol is depicted in Fig. 1. Forty-six uninephrectomized male Sprague-Dawley rats (weight: 275–300 g; age: 10–12 wk) underwent a 3-wk DOCA-salt treatment (100 mg DOCA sc; 0.9% saline ad libitum) as we previously have published (3). After the 3-wk DOCA-salt treatment, rats were anesthetized and randomly assigned to one of the following three treatment groups: 1) DOCA-salt + total renal denervation (TRDNx; n = 16); 2) DOCA-salt + selective afferent ablation (ARDNx; n = 16); or 3) DOCA-salt + sham denervation (Sham; n = 14). TRDNx was achieved by surgical ablation of renal nerves paired with periaxonal application of 10% phenol (in 100% ethanol). With the use of our previously published technique to ablate renal afferent nerves (16), ARDNx was achieved by periaxonal application of 33 mM capsaicin (in 5% Tween 80, 5% ethanol, and 90% 150 mM NaCl). For all surgical procedures, rats were anesthetized with 2–3% isoflurane (Phoenix Pharmaceutical, St. Joseph, MO). Atropine (0.2 mg/kg ip; West-Ward Pharmaceuticals, Eatontown, NJ), ketoprofen (5 mg/kg sc; Fort Dodge Animal Health, Overland Park, KS), and gentamicin (2.5 mg/kg im; Hospira, Lake Forest, IL) were administered before surgery. All animals were instrumented with radiotelemeters (model HD-S10; Data Sciences) at the time of nephrectomy. Cardiovascular parameters (24-h arterial pressure, heart rate) were monitored continuously by radiotelemetry from the day before treatment surgery (protocol day −1) through 14 days posttreatment (protocol day 14).
Fig. 1.
Experiment protocol timeline. Cardiovascular parameters were monitored daily from protocol day −1 (baseline) through day 14 (end of protocol). DOCA, deoxycorticosterone acetate; TRDNx, total renal denervation; ARDNx, afferent-selective renal denervation.
Neurogenic pressor activity.
Neurogenic pressor activity, an estimate of peripheral sympathetic tone, was assessed on protocol days −1 (before surgery) and 14 (end of protocol) by measuring the acute depressor response to ganglionic blockade (30 mg/kg hexamethonium bromide ip) before and at the end of the protocol (45). At the end of the protocol, animals were euthanized the following day by decapitation. Allometric data were recorded, and tissue samples were dissected and snap-frozen for further analysis.
Biochemical and histological analysis of renal inflammation.
The renal inflammatory profiles of each rat were determined by measuring several proinflammatory chemo- and cytokines in renal tissue. Specifically, we measured renal content of GRO/KC, MCP-1, IL-1β, IL-2, and IL-6, which were the analytes most influenced by DOCA-salt and denervation treatment in our recent study in the development of DOCA-salt hypertension (3). Renal tissue samples (containing cortex and medulla) were homogenized in phosphate-buffered saline containing EDTA-free protease inhibitor cocktail (cOmplete Mini, item no. 11836170001; Roche Diagnostics), and inflammatory analytes were measured in homogenate solutions according to the manufacturer’s instructions (MILLIPLEX Custom Assay; MilliporeSigma). Data are expressed in picograms of analyte per milligram of total protein.
Additional renal histological analysis was performed in a subset of animals (n = 6/group) to quantify glomerular injury, as well as tissue T cell and macrophage infiltration. Kidneys were collected at necropsy and bisected. Tissue was fixed in 10% formalin and embedded in paraffin. Tissues were processed and stained by the University of Minnesota Veterinary Diagnostic Laboratory. Transverse sections (4 µm thickness) of renal tissue were cut and stained with hematoxylin and eosin (H&E). Single-blinded assessment of renal injury was scored by a board-certified pathologist, in which glomerular injury was scored on an ordinal scale of 0 to 4, as defined by Raij and colleagues (41a).
Additional immunohistochemistry analysis was employed to measure renal T cell and macrophage infiltration. After slide preparation, tissues were deparaffinized and underwent antigen retrieval. Primary polyclonal antibodies were used to detect T cells (anti-CD3, no. CP215; Biocare Medical) and macrophage (anti-Iba1, no. CP290; Biocare Medical). A peroxidase-labeled polymer conjugated to goat antirabbit Ig secondary antibody (EnVision + HRP; Agilent/Dako) was used as the detection reagent and 3-amino-9-ethylcarbazole as the chromogen. Last, tissues were counterstained with Mayer’s hematoxylin and photographed using a Nikon Eclipse outfitted with a Nikon DS-Fi1 digital color camera. Signal was quantified automatically as percent area from scanned slides at ×10 magnification using methods outlined by Ruifrok and Johnston (44) using ImageJ Software (46). No differences were detected between cortical and medullary staining. As such, renal immune cell infiltration is expressed as percent area of renal parenchyma.
Renal function was estimated after necropsy by measurement of serum creatinine, blood urea nitrogen, and urinary protein-to-creatinine ratio using commercial kits (Arbor Assays). All assays were performed to the manufacturer’s instructions.
Confirmation of denervation efficacy.
Renal norepinephrine (NE) content was measured to assess efficacy of efferent nerve ablation. Protein homogenates were assayed by high-performance liquid chromatography analysis with electrochemical detection (3, 16). Efficacy of afferent nerve ablation was assessed through the enzyme-linked immunosorbent assay detection of calcitonin gene-related peptide (CGRP) in renal tissue content as recently described (16). In isolated renal pelvic samples, CGRP tissue content was assayed according to the assay’s manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI). Data are expressed as picograms of analyte per milligrams of total protein. Homogenate total protein concentration was measured by Bradford assay.
Statistical analysis.
Repeated measures of 24-h telemetry data (protocol days 0–14) were analyzed by a two-way ANOVA with a Bonferroni post hoc test, where data sphericity was not assumed and a Greenhouse-Geiser correction was applied to reduce risk of type I error (33). To assess diurnal rhythm of mean arterial pressure before and after treatment, hourly averages of a 24-h cycle (protocol days −1 and 14) were analyzed using a single-component cosinor regression (9). MESOR (a rhythm-adjusted mean), amplitude of day/night cycle, and acrophase (time-to-peak value) were derived from hourly averages of 24-h recordings of mean arterial pressure using the following equation:
All nonrepeated measures data were analyzed by one-way ANOVA with a Bonferroni post hoc test. Statistical analyses were performed with GraphPad Prism 7.0 software. Statistical significance was accepted at P < 0.05 (2-tailed). Data are presented as means ± SE.
RESULTS
Denervation efficacy.
TRDNx significantly reduced renal NE content to <5% of Sham values [1.8 ± 0.5 (P < 0.05) vs. 34.1 ± 4.3 ng/g tissue], and NE content in ARDNx kidneys (31.1.2 ± 3.8 ng/g tissue) was not statistically different from Sham. Regarding afferent nerve ablation, both TRDNx and ARDNx significantly reduced CGRP content compared with Sham [TRDNx 22.9 ± 5.7 (P < 0.05) vs. ARDNx 29.6 ± 7.4 (P < 0.05) vs. Sham 477.4 ± 41.2 pg/mg protein], indicating a similar efficacy was achieved. Together, these data indicate TRDNx and ARDNx techniques effectively ablated >90% of the targeted nerves.
Cardiovascular and allometric responses.
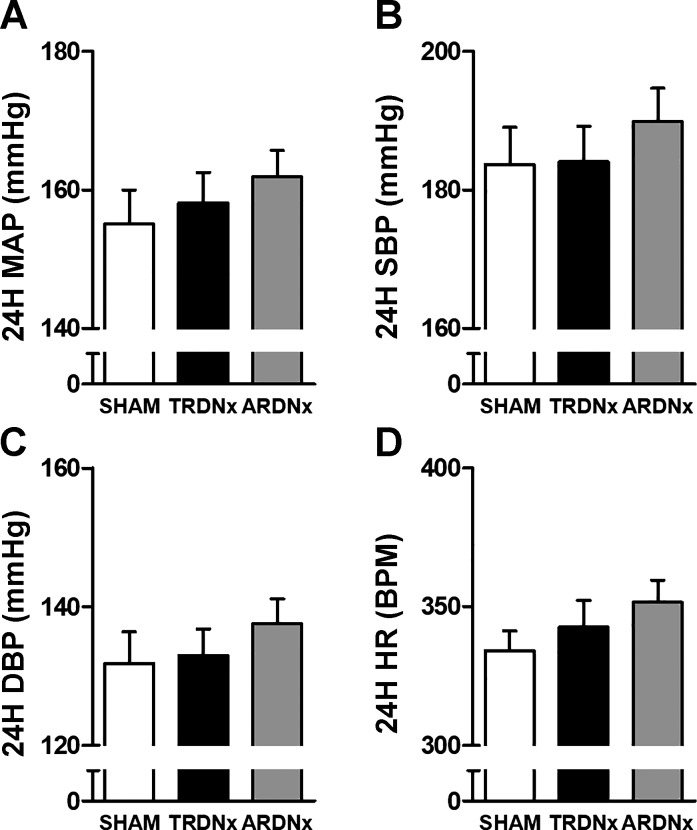
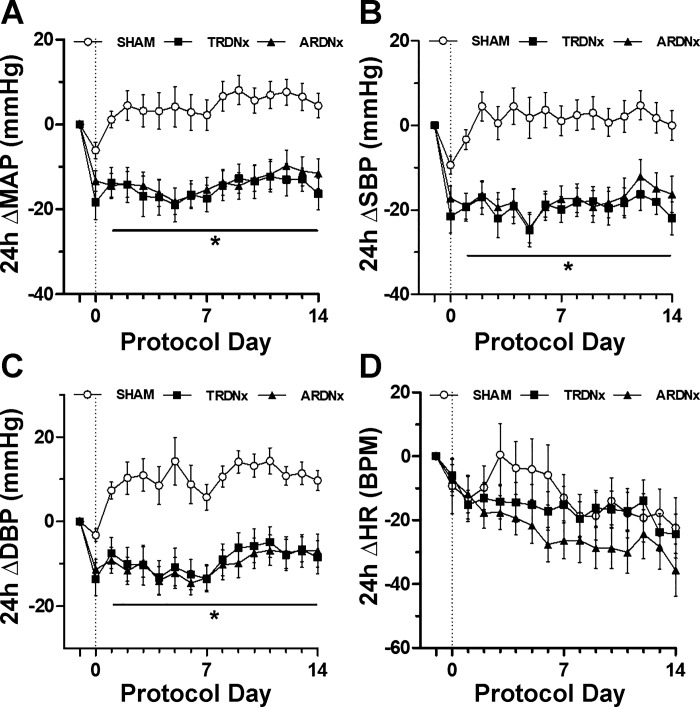
Before treatment, no significant difference was detected between treatment groups regarding cardiovascular parameters (Fig. 2, A–D), including 24-h mean arterial pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR). Over the following 2 wk, MAP, SBP, and DBP temporal responses were significantly lower (P < 0.05) following treatment compared with Sham controls (Fig. 3, A–C), but there was no significant effect of treatment on HR (Fig. 3D). There was no significant effect of treatment on average daily sodium intake over the 14-day posttreatment period (Sham 16.8 ± 1.8, TRDNx 16.7 ± 1.9, and ARDNx 17.1 ± 2.6 mmol/day). Regarding allometric measurements at necropsy, no significant differences in body weight or heart weight were detected between groups; however, renal weight was significantly reduced in TRDNx and ARDNx groups vs. Sham (Table 1).
Fig. 2.
Baseline cardiovascular parameters. After 3 wk of deoxycorticosterone acetate (DOCA)-salt, no difference in 24-h averages of mean arterial pressure (MAP, A), systolic blood pressure (SBP, B), diastolic blood pressure (DBP, C), or heart rate (HR, D) were detected at baseline (protocol day −1). Data are presented as means ± SE. Sham: n = 14; TRDNx: n = 16; ARDNx: n = 16.
Fig. 3.
Temporal hemodynamic response. Over the 14 days after treatment, change in (Δ) mean arterial pressure (MAP), systolic blood pressure (ΔSBP), and diastolic blood pressure (ΔDBP) were significantly lower (P < 0.05, protocol days 2–14) in both total renal denervation (TRDNx) and afferent-selective renal denervation (ARDNx) groups vs. Sham. Data are presented as means ± SE. *P < 0.05 vs. Sham; repeated-measures 2-way ANOVA with Bonferroni post hoc test. Sham: n = 14; TRDNx: n = 16; ARDNx: n = 16.
Table 1.
Allometric and biochemical analysis following necropsy
| Allometric/Biochemical Measurement | Sham (n = 14) | TRDNx (n = 16) | ARDNx (n = 16) |
|---|---|---|---|
| Body wt, g | 517 ± 22 | 522 ± 20 | 517 ± 19 |
| Heart wt, g/kg body wt | 3.45 ± 0.08 | 3.48 ± 0.09 | 3.39 ± 0.06 |
| Kidney wt, g/kg body wt | 7.56 ± 0.28 | 6.64 ± 0.14* | 6.71 ± 0.17* |
| CreatininePlasma, mg/dl | 2.62 ± 0.18 | 2.53 ± 0.26 | 2.51 ± 0.18 |
| BUN, mg/dl | 19.53 ± 1.57 | 17.07 ± 0.72 | 18.36 ± 1.13 |
| UPCR, AU | 2.92 ± 0.46 | 2.17 ± 0.61 | 2.74 ± 0.63 |
Data are presented at means ± SE; n, no. of rats. TRDNx, total renal denervation; ARDNx, afferent-selective renal denervation; BUN, blood urea nitrogen; UPCR, urinary protein-to-creatinine ratio; AU, arbitrary units.
P < 0.05 vs. Sham; one-way ANOVA with Bonferroni post hoc test.
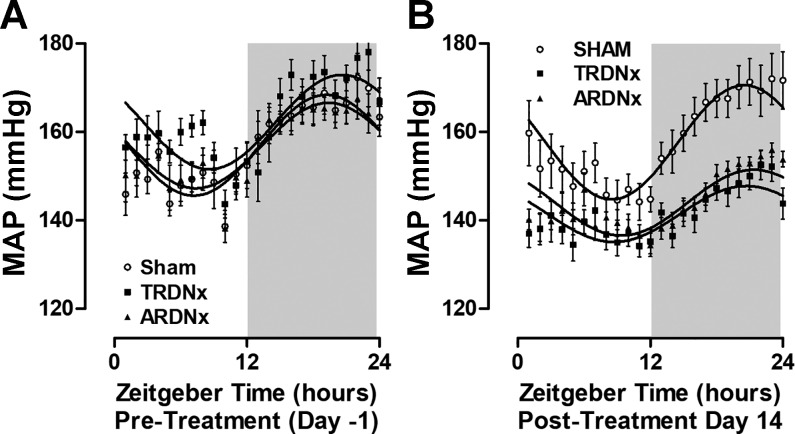
Cosinor-based rhythmometry was used to measure the MESOR, amplitude, and acrophase of MAP diurnal rhythm before and after treatment (Table 2). At baseline (treatment day −1), no significant difference was detected in MESOR (rhythm-adjusted mean) or AMP (amplitude of rhythm) between all treatment groups before treatment (Fig. 4A). Two weeks following treatment (treatment day 14), both MESOR and AMP of MAP diurnal rhythm were significantly reduced in both TRDNx and ARDNx animals compared with Sham controls (Fig. 4B). No significant effect of either treatment on MAP acrophase was detected. Cosinor regression analysis data are presented in Table 2.
Table 2.
Mean arterial pressure cosinor regression analysis
| Cosinor Regression Statistic | Sham (n = 14) | TRDNx (n = 16) | ARDNx (n = 16) |
|---|---|---|---|
| Protocol day −1 | |||
| MESOR, mmHg | 157.0 ± 0.9 | 162.3 ± 0.8 | 156.9 ± 0.8 |
| Amplitude, mmHg | 11.4 ± 1.3 | 10.7 ± 0.9 | 9.7 ± 1.1 |
| Acrophase, h | 19.3 ± 0.3 | 19.8 ± 0.6 | 19.3 ± 0.3 |
| Protocol day 14 | |||
| MESOR, mmHg | 154.4 ± 0.8 | 141.4 ± 0.6* | 144.0 ± 0.6* |
| Amplitude, mmHg | 13.6 ± 1.1 | 6.3 ± 0.9* | 7.0 ± 0.8* |
| Acrophase, h | 20.5 ± 0.6 | 20.2 ± 0.4 | 21.7 ± 0.5 |
Data are presented at means ± SE; n, no. of rats. MESOR, amplitude of day/night cycle, and acrophase were derived from hourly averages of 24-h recordings of mean arterial pressure at baseline (protocol day −1) 2 wk after treatment (protocol day 14).
P < 0.05 vs. time-matched Sham; one-way ANOVA with Bonferroni post hoc test.
Fig. 4.
Diurnal rhythm of mean arterial pressure (MAP). Cosinor regression analysis of 24-h MAP at baseline (protocol day −1, A) and after treatment (protocol day 14, B) was employed to assess the effects of treatment on MAP diurnal rhythm. Hourly averages of MAP are presented as means ± SE and fit with a cosinor regression curve. Cosinor regression analysis data are presented in Table 2. TRDNx, total renal denervation; ARDNx, afferent-selective renal denervation. Sham: n = 14; TRDNx: n = 16; ARDNx: n = 16.
Neurogenic pressor activity.
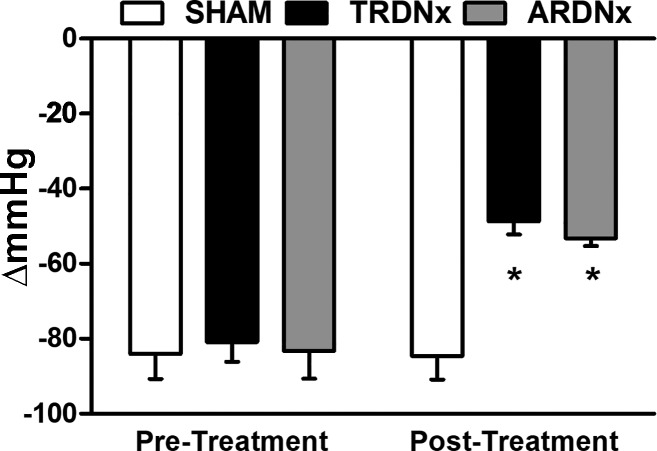
The peak acute depressor response to acute ganglionic blockade, termed as neurogenic pressor activity (45), was measured before and 2 wk after denervation and Sham treatments. Whereas Sham treatment had no significant effect on neurogenic pressor activity over time, both TRDNx and ARDNx decreased this measure of peripheral sympathetic tone by ~40% (Fig. 5).
Fig. 5.
Neurogenic pressor activity. Measured by recording the acute depressor response to ganglionic blockade (30 mg/kg hexamethonium bromide), neurogenic pressure activity was measured before and 14 days after denervation treatment. TRDNx, total renal denervation; ARDNx, afferent-selective renal denervation. Pressor activity was similar across all groups at baseline (Sham −84 ± 7 vs. TRDNx −81 ± 5 vs. ARDNx −83 ± 7 mmHg). After treatment (2 wk), pressor activity was reduced in TRDNx and ARDNx compared with the time-matched Sham group and their respective baseline values [Sham −85 ± 6 vs. TRDNx −49 ± 4 (P < 0.05) vs. ARDNx −53 ± 2 mmHg (P < 0.05)]. Data are presented as means ± SE. *P < .05 vs. time-matched Sham; one-way ANOVA with Bonferroni post hoc test. Sham: n = 14; TRDNx: n = 16; ARDNx: n = 16.
Renal inflammation and renal function.
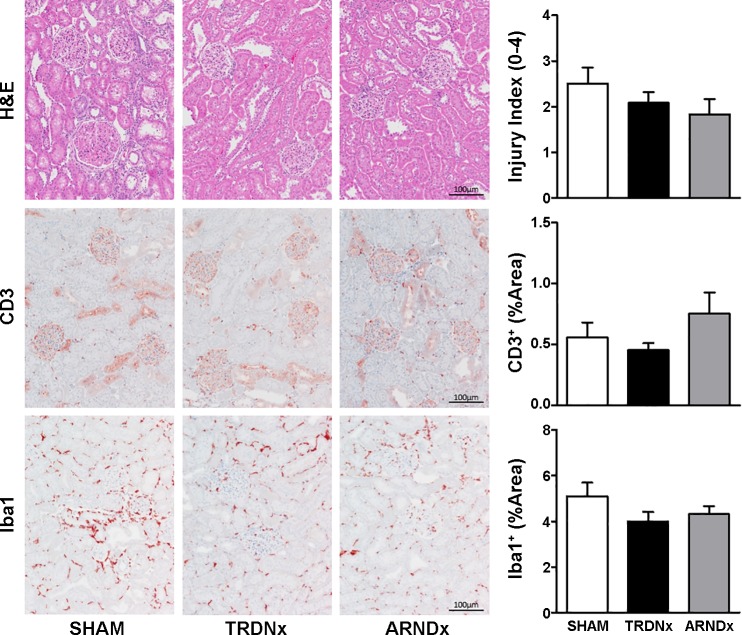
Renal inflammation was assessed biochemically and histologically. First, proinflammatory cytokines in renal parenchyma were measured 14 days after treatment (Table 3). The cytokines measured (GRO/KC, MCP-1, IL-1β, IL-2, IL-6) were similar across all groups, where no significant effect of either TRDNx or ARDNx was detected. Furthermore, histological analysis of renal injury and immune cells (e.g., macrophage, T cells) was quantified in a subset of animals from each group (n = 6/group). As depicted in Fig. 6, no significant effect of either TRDNx or ARDNx treatment was observed in renal injury (H&E stain), T cell infiltration (CD3+ stain), or macrophage infiltration (Iba1+ stain) analyses. Finally, estimates of renal function (serum creatinine, blood urea nitrogen, urine protein-to-creatinine ratio) were not significantly different between any groups 2 wk after treatment (Table 1).
Table 3.
Renal inflammatory cytokine content
| Inflammatory Cytokine, pg/mg protein | Sham (n = 14) | TRDNx (n = 16) | ARDNx (n = 16) |
|---|---|---|---|
| IL-1β | 31.9 ± 6.8 | 31.3 ± 5.8 | 35.6 ± 6.7 |
| IL-2 | 4.5 ± 0.7 | 4.5 ± 0.7 | 4.7 ± 0.7 |
| IL-6 | 187.8 ± 52.7 | 214.8 ± 57.2 | 222.6 ± 52.2 |
| MCP-1 | 17.9 ± 1.4 | 19.2 ± 1.2 | 20.9 ± 1.4 |
| GRO/KC | 10.3 ± 3.4 | 11.8 ± 3.9 | 11.4 ± 3.3 |
Data are presented at means ± SE; n, no. of rats. IL-1β, interleukin-1β; IL-2, interleukin-2; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; GRO/KC, growth-related oncogene/keratinocyte chemoattractant. Cytokine content was normalized to total protein (pg/mg).
Fig. 6.
Histological assessment of renal injury and immune cell infiltration. In a subset of animals in each group (n = 6/group), renal injury, T cell infiltration, and macrophage infiltration were assessed by histological analysis. Hematoxylin and eosin (H&E)-stained tissues were scored by a blinded investigator for injury. Immunohistochemical staining for CD3 and Iba1 was employed to quantify T cell and macrophage density in renal parenchyma, respectively. Together, no effect of total renal denervation (TRDNx) or afferent-selective renal denervation (ARDNx)was detected in any histological measurement. Representative images of cortical tissue are presented at ×10 magnification. Scale bar = 100 µm. Data are presented as means ± SE.
DISCUSSION
The objective of this study was to investigate whether renal denervation can reverse hypertension and renal inflammation in preexistent DOCA-salt hypertension and determine the individual contribution of afferent and efferent renal nerves to these effects. There were four primary findings. First, TRDNx and ARDNx both reduced MAP by nearly 15 mmHg 2 wk after treatment compared with Sham. Second, MAP diurnal rhythm (MESOR, amplitude) was significantly lower in TRDNx and ARDNx versus Sham. Third, neurogenic pressor activity was reduced 2 wk after treatment in both TRDNx and ARDNx compared with Sham. Finally, based on proinflammatory cytokine and histological analyses, neither TRDNx nor ARDNx reversed preexistent renal inflammation.
These findings are consistent with our hypothesis that renal denervation lowers MAP in this hypertensive rat model, as well as lowers peripheral sympathetic tone, and these effects are mediated through the ablation of afferent, and not efferent, renal nerves. In contrast to this hypothesis, neither renal denervation treatment reversed renal inflammation or improved renal function. A detailed discussion of these findings and relation to previous studies is presented in the following sections.
Role of afferent and efferent renal nerves in mediating the cardiovascular and neural responses to renal denervation in DOCA-salt hypertension.
Since the original reports of Katholi and colleagues (24), the sympathetic nervous system, particularly the renal sympathetic nerves, has been known to contribute to DOCA-salt hypertension development and maintenance (3, 7, 16, 19, 22–24, 49). We recently expanded these studies by investigating the individual contributions of efferent and afferent renal nerves during the development of DOCA-salt hypertension (3, 16) by comparing the effects of targeted ablation of renal afferent nerves with those observed with total (afferent + efferent) denervation. The effect of TRDNx and ARDNx on the development of DOCA-salt hypertension was identical in that both attenuated the steady-state increase in MAP by ~50% with the same temporal profile over 3 wk of DOCA-salt (3, 16). Moreover, resting afferent renal nerve activity was directly recorded and found to be ~2.5-fold higher in hypertensive DOCA-salt rats compared with normotensive controls (3). Based on these findings, we concluded that renal nerves account for one-half of the developmental phase of DOCA-salt hypertension, and this effect is primarily mediated by elevated afferent renal nerve activity.
Similar to previous studies of the development of DOCA-salt hypertension (3, 16), we found that TRDNx lowered arterial pressure by ~15mmHg in rats with preexisting DOCA-salt hypertension in the present study. This is comparable in magnitude to the degree TRDNx attenuated the development of DOCA-salt hypertension in two studies from our laboratory (3, 16). This response to TRDNx in established DOCA-salt hypertension is similar to that reported in the established phase of other preclinical models (38). We also reported TRDNx mitigates hypertension in the late phase of Dahl-S hypertensive rats (15); however, it is important to note that, in contrast to the present study, ARDNx had no effect on MAP in the Dahl-S model, suggesting the response was mediated entirely by efferent renal nerve ablation. In another study Becker and colleagues reported that TRDNx lowers MAP by ~10–15 mmHg in the hypertensive endothelin receptor type B-deficient rat; however, the role of afferent innervation remains unknown in this model (5). Interestingly, there were no significant effects noted on the diurnal rhythm variables following total renal denervation (5). Moreover, the blood pressure-lowering effect of TRDNx is not specific to hypertensive conditions, since TRDNx lowers arterial pressure in normotensive Sprague-Dawley rats (3, 16). In contrast, the blood pressure-lowering effect of ARDNx is disease specific, since ARDNx has no effect on blood pressure in the normotensive rat (3, 15). Altogether, these findings strongly indicate that the contribution of efferent vs. afferent nerves in mediating the response to renal denervation is model dependent.
Although we have not fully defined the mechanism by which ARDNx decreases MAP in the DOCA-salt model, we hypothesize the antihypertensive response to both TRDNx and ARDNx is mediated by a reduction of peripheral SNA through the ablation of afferent renal nerves. Because of the current methodological limitations of measuring SNA chronically, we estimated peripheral sympathetic tone indirectly by the acute depressor response to acute ganglionic blockade (45). Compared with Sham controls, in which the depressor response was ~80 mmHg, TRDNx and ARDNx both suppressed this response to ~50 mmHg, nearly a 40% reduction. Furthermore, the significant reduction in the MESOR and amplitude of mean arterial pressure diurnal rhythm may be indicative of a suppressed sympathetic tone (17), since sympathetic activity is highest during the active phase. A similar mechanism was posited in the recent clinical studies from Persu et al. (41) and Ewen et al. (14) in which reduced blood pressure variability following catheter-based RDNx in hypertensive patients was hypothesized to be a result of sympathetic suppression. Our data support this hypothesis in that sympathetic tone assessed by ganglionic blockade was reduced by TRDNx and ARDNx. Importantly, we note that these findings are limited to the indirect methodology used to measure sympathetic tone in this study, and studies using chronic direct sympathetic nerve recordings are required to confirm our findings.
Nonetheless, this putative sympathoinhibitory effect of TRDNx and ARDNx is consistent with recent findings in humans where catheter-based RDNx markedly reduced arterial pressure and muscle SNA (48, 59). Both reports concluded that the suppression of muscle SNA may represent an overall reduction of peripheral SNA, which likely mediates the antihypertensive effect of RDNx. Because both treatments destroy afferent renal nerves, and afferent renal nerve discharge is elevated in the hypertensive DOCA-salt rat (3), we hypothesize that the attenuation of neurogenic pressor activity is because of interruption of sympathoexcitatory afferent renal nerve activity. This hypothesis is consistent with reports (4, 8) that afferent renal nerves modulate neuronal activity in brain regions known to regulate sympathetic activity, such as the rostral ventrolateral medulla and paraventricular nucleus, pathways that are both activated and contribute to DOCA-salt hypertension (1, 31, 37). It remains unknown whether this afferent-mediated signaling and circuitry remain operative in this model, and further mechanistic studies are required to elucidate this mechanism.
We also note that the current study did not investigate the extent to which ARDNx affects “organ-specific” versus “global” sympathetic nerve activity in DOCA-salt rats. Based on the role of the reno-renal reflex in arterial pressure control (20, 28), it is logical that ARDNx influences both systemic and renal sympathetic (efferent) nerve activity. As such, the antihypertensive response to ARDNx may be mediated exclusively by a reduction in SNA to the kidney. However, it is also possible that ARDNx and TRDNx both decrease SNA to other vascular beds, as recently reported by Veiga and colleagues (52) in a rodent model of chronic kidney disease. Nevertheless, the current data support our initial hypothesis that renal denervation reverses DOCA-salt hypertension and lowers sympathetic tone, and these effects are primarily dependent on afferent nerve ablation.
Renal nerves and inflammation: a causative or associative relationship?
Recent studies suggest that renal nerves are implicated in trafficking immune cells to the kidney. Studies by our group and others have found TRDNx abrogates the influx of T cells and inflammatory cytokines in the kidney during the development of experimental hypertension (3, 58). Specifically, Xiao et al. reported that TRDNx prevented T cell infiltration and attenuated angiotensin II-induced hypertension in the mouse. Interestingly, ARDNx had no effect on renal inflammation or arterial pressure, leading the authors to conclude renal efferent, and not afferent, nerves regulate the inflammatory response and hypertension in this model. Studies from our laboratory suggest that afferent nerves contribute to renal inflammation, but perhaps to a lesser extent than efferent renal nerves (3). Specifically, TRDNx abolished the renal inflammation during the development of DOCA-salt hypertension, whereas ARDNx had a lesser effect. From these previous reports, we hypothesized that TRDNx would also reduce preexisting renal inflammation in established DOCA-salt hypertension in the rat.
Based on the assessment of 1) renal proinflammatory cytokines, 2) histological analysis of renal parenchymal injury, and 3) immune cell infiltration, neither treatment had a significant effect on renal inflammation or injury in this model of established hypertension despite the antihypertensive response to both treatments. We conclude that renal nerves contribute to the development of renal inflammation in DOCA-salt hypertension but play no discernable role in the maintenance of inflammation once it is established.
Although there is a dearth of clinical studies directly measuring renal inflammation following renal denervation, there are several studies in which peripheral inflammation was assessed. Kampmann et al. reported similar cardiovascular and inflammatory responses in hypertensive humans that underwent catheter-based RDNx, where all measured circulating inflammatory cytokines (TNF-α, IL-6, and IL-1β) remained unaffected 6 mo posttreatment, despite a significant decrease in arterial pressure (21). Notably, the magnitude of hypertension before treatment, as well as the antihypertensive response, in this clinical study was remarkably similar to this current study’s results. In contrast to these results, a recent clinical report from Zaldivia and colleagues (59) reported a significant reduction in circulating inflammatory cytokines (MCP-1, IL-1β, TNF-α, and IL-12) several months after catheter-based RDNx in hypertensive patients. Indeed, additional clinical and preclinical investigations are required to elucidate the anti-inflammatory effect following renal denervation, since the recent literature remains inconclusive.
Similar to the inflammation results, we did not observe any significant improvement in estimates of renal function (i.e., plasma creatinine, blood urea nitrogen, and urine protein-to-creatinine ratio) with either TRDNx or ARDNx. These findings are comparable to those observed in hypertensive humans treated with catheter-based RDNx (34), where biochemical markers of renal function were unaffected by catheter-based RDNx. It is important to note that the current study is limited in its conclusions regarding the role of renal function; we did not directly measure glomerular filtration rate or renal blood flow. Therefore, although our estimates of renal function do not explain the hemodynamic responses to TRDNx or ARDNx, it remains possible that undetected changes in glomerular filtration or renal vascular resistance contributed to the antihypertensive responses in the current study. Mahfoud and colleagues reported that renal resistance index was significantly reduced in patients following catheter-based RDNx despite no detectable effect in the patients’ biochemical estimates of renal function (34). Nevertheless, further studies directly measuring glomerular filtration rate and renal blood flow following TRDNx and ARDNx are required to address these potential mechanisms.
Nonetheless, from the currently available data, we conclude the arterial pressure and sympathoinhibitory effects of ARDNx and TRDNx in this model occur independent of significant effects on renal injury, inflammation, and function. Although our study does not support the hypothesis that renal nerves contribute to the maintenance of inflammation, we speculate that renal inflammation contributes to the hypertension by driving the activity of sympathoexcitatory renal afferent nerves.
Perspectives and Significance
The vast majority of preclinical studies on the antihypertensive effects of RDNx have investigated the developmental phase of hypertension (39). Although these studies are critical to our understanding of the role of renal sympathetic nerves in the pathogenesis of hypertension, the ability of RDNx to reverse preexistent hypertension is more clinically relevant. In light of the recent clinical trials for catheter-based renal nerve ablation (40, 43, 50, 55, 56), it is clear that additional preclinical studies are needed to advance development of this novel therapy and refine its application.
Overall, our findings support the application of RDNx to treat established hypertension in humans. The present study, combined with our previous studies, supports the hypothesis that the development and maintenance of hypertension in this preclinical model is mediated by afferent renal nerves. Although our study suggests that renal denervation does not reverse preexisting renal inflammation, the fact that it markedly reduces mean arterial pressure implies it will nonetheless reduce further development of cardiovascular disease (57). Moreover, we predict that development of novel therapies for targeted ablation or neuromodulation of afferent renal nerves may be beneficial in diseases in which inflammatory cells in the kidney chronically drive overactivity of the sympathetic nervous system through activation of these sensory nerves.
Finally, it is important to recognize that the mechanism by which RDNx decreases arterial pressure is model dependent. Our laboratory and others have reported disparate effects and mechanisms of RDNx treatment in numerous animal models of hypertension (3, 5, 7, 15, 16, 19, 22–24, 27, 53, 58). In light of the fact that there is currently no diagnostic test to predict patients who would benefit from RDNx, it is important to further investigate the mechanisms by which renal nerves cause and maintain hypertension, with the goal of developing novel effective treatments and diagnostics that are useful in the clinic.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) R01 Grant HL-116476 (J. W. Osborn and G. D. Fink). C. T. Banek was supported by NHLBI T32 Training Grant 2T32-HL-7741–21 (D. Ingbar) and by American Heart Association Postdoctoral Fellowship 17POST33661003 (C.T. Banek).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.T.B. and J.W.O. conceived and designed research; C.T.B., M.M.G., D.C.B., D.V.H., and N.A.-J. performed experiments; C.T.B., M.M.G., D.C.B., D.V.H., N.A.-J., and A.P.-M. analyzed data; C.T.B., A.P.-M., G.D.F., and J.W.O. interpreted results of experiments; C.T.B. prepared figures; C.T.B. drafted manuscript; C.T.B., G.D.F., and J.W.O. edited and revised manuscript; C.T.B., M.M.G., D.C.B., D.V.H., N.A.-J., A.P.-M., G.D.F., and J.W.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the assistance from the University of Minnesota’s Cytokine Reference Laboratory in the measurement of tissue cytokines. Also, we appreciate the University of Minnesota’s Veterinary Diagnostic Laboratory for histological services. Finally, we thank Robert Burnett of Michigan State University for technical assistance in the renal norepinephrine quantification.
REFERENCES
- 1.Abrams JM, Engeland WC, Osborn JW. Effect of intracerebroventricular benzamil on cardiovascular and central autonomic responses to DOCA-salt treatment. Am J Physiol Regul Integr Comp Physiol 299: R1500–R1510, 2010. doi: 10.1152/ajpregu.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 121: 293–298, 1991. doi: 10.1016/0002-8703(91)90861-B. [DOI] [PubMed] [Google Scholar]
- 3.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bealer SL. Hypothalamic knife cuts attenuate maintenance of deoxycorticosterone acetate-salt induced hypertension. Brain Res 309: 192–195, 1984. doi: 10.1016/0006-8993(84)91029-1. [DOI] [PubMed] [Google Scholar]
- 5.Becker BK, Feagans AC, Chen D, Kasztan M, Jin C, Speed JS, Pollock JS, Pollock DM. Renal denervation attenuates hypertension but not salt sensitivity in ETB receptor-deficient rats. Am J Physiol Regul Integr Comp Physiol 313: R425–R437, 2017. doi: 10.1152/ajpregu.00174.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370: 1393–1401, 2014. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 7.Cabral AM, Silva IF, Gardioli CR, Mauad H, Vasquez EC. Diverse effects of renal denervation on ventricular hypertrophy and blood pressure in DOCA-salt hypertensive rats. Braz J Med Biol Res 31: 587–590, 1998. doi: 10.1590/S0100-879X1998000400018. [DOI] [PubMed] [Google Scholar]
- 8.Ciriello J. Contribution of forebrain mechanisms in the maintenance of deoxycorticosterone acetate-salt hypertension. Clin Exp Hypertens A 10, Suppl 1: 169–178, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model 11: 16, 2014. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, Joyal D. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol 62: 231–241, 2013. doi: 10.1016/j.jacc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 11.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol 298: R245–R253, 2010. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 12.Donazzan L, Ewen S, Böhm M. Until questions on intraprocedural efficacy control, renal nerve distribution and predictors of BP response are answered, the interpretation of clinical trials on renal denervation will remain uncertain. Evid Based Med 19: 227–228, 2014. doi: 10.1136/ebmed-2014-110028. [DOI] [PubMed] [Google Scholar]
- 13.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators . Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903–1909, 2010. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 14.Ewen S, Dörr O, Ukena C, Linz D, Cremers B, Laufs U, Hamm C, Nef H, Bauer A, Mancia G, Böhm M, Mahfoud F. Blood pressure variability after catheter-based renal sympathetic denervation in patients with resistant hypertension. J Hypertens 33: 2512–2518, 2015. doi: 10.1097/HJH.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 15.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol 310: R262–R267, 2016. doi: 10.1152/ajpregu.00408.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension 52: 925–931, 2008. doi: 10.1161/HYPERTENSIONAHA.108.116368. [DOI] [PubMed] [Google Scholar]
- 18.Iyer A, Chan V, Brown L. The doca-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev 6: 291–297, 2010. doi: 10.2174/157340310793566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 20.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Kampmann U, Mathiassen ON, Christensen KL, Buus NH, Bjerre M, Vase H, Møller N, Kaltoft A, Poulsen PL. Effects of renal denervation on insulin sensitivity and inflammatory markers in nondiabetic patients with treatment-resistant hypertension. J Diabetes Res 2017: 6915310, 2017. doi: 10.1155/2017/6915310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandlikar SS, Fink GD. Mild DOCA-salt hypertension: sympathetic system and role of renal nerves. Am J Physiol Heart Circ Physiol 300: H1781–H1787, 2011. doi: 10.1152/ajpheart.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katholi RE, Naftilan AJ, Bishop SP, Oparil S. Role of the renal nerves in the maintenance of DOCA-salt hypertension in the rat. Influence on the renal vasculature and sodium excretion. Hypertension 5: 427–435, 1983. doi: 10.1161/01.HYP.5.4.427. [DOI] [PubMed] [Google Scholar]
- 24.Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 2: 266–273, 1980. doi: 10.1161/01.HYP.2.3.266. [DOI] [PubMed] [Google Scholar]
- 25.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int 87: 350–358, 2015. doi: 10.1038/ki.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline RL, Kelton PM, Mercer PF. Effect of renal denervation on the development of hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol 56: 818–822, 1978. doi: 10.1139/y78-128. [DOI] [PubMed] [Google Scholar]
- 28.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 308: R79–R95, 2015. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 42: 968–973, 2003. doi: 10.1161/01.HYP.0000097549.70134.D8. [DOI] [PubMed] [Google Scholar]
- 30.Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383: 622–629, 2014. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 31.Kubo T, Fukumori R, Kobayashi M, Yamaguchi H. Altered cholinergic mechanisms and blood pressure regulation in the rostral ventrolateral medulla of DOCA-salt hypertensive rats. Brain Res Bull 45: 327–332, 1998. doi: 10.1016/S0361-9230(97)00380-8. [DOI] [PubMed] [Google Scholar]
- 32.Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D, Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension 65: 1223–1230, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res 28: 303–311, 1994. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- 34.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Böhm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60: 419–424, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- 35.Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regul Integr Comp Physiol 308: R708–R713, 2015. doi: 10.1152/ajpregu.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathis KW, Venegas-Pont M, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, Ryan MJ. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol 305: R711–R719, 2013. doi: 10.1152/ajpregu.00602.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakata T, Takeda K, Itho H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Paraventricular nucleus lesions attenuate the development of hypertension in DOCA/salt-treated rats. Am J Hypertens 2: 625–630, 1989. doi: 10.1093/ajh/2.8.625. [DOI] [PubMed] [Google Scholar]
- 38.Osborn JW, Banek CT. Catheter-based renal nerve ablation as a novel hypertension therapy: lost, and then found, in translation. Hypertension 71: 383–388, 2018. doi: 10.1161/HYPERTENSIONAHA.117.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol 7: 263–320, 2017. doi: 10.1002/cphy.c150047. [DOI] [PubMed] [Google Scholar]
- 40.Papademetriou V, Tsioufis CP, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Worthley MI, Worthley SG. Catheter-based renal denervation for resistant hypertension: 12-month results of the EnligHTN I first-in-human study using a multielectrode ablation system. Hypertension 64: 565–572, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03605. [DOI] [PubMed] [Google Scholar]
- 41.Persu A, Gordin D, Jacobs L, Thijs L, Bots ML, Spiering W, Miroslawska A, Spaak J, Rosa J, de Jong MR, Berra E, Fadl Elmula FEM, Wuerzner G, Taylor AHM, Olszanecka A, Czarnecka D, Mark PB, Burnier M, Renkin J, Kjeldsen SE, Widimsky J, Elvan A, Kahan T, Steigen TK, Blankestijn PJ, Tikkanen I, Staessen JA; European Network COordinating research on Renal Denervation (ENCOReD) . Blood pressure response to renal denervation is correlated with baseline blood pressure variability: a patient-level meta-analysis. J Hypertens, 36: 221–229, 2018. doi: 10.1097/HJH.0000000000001582. [DOI] [PubMed] [Google Scholar]
- 41a.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Leor O, Bonet J, Bayes-Genis A. Renal denervation for resistant hypertension. N Engl J Med 371: 182–183, 2014. doi: 10.1056/NEJMc1405677. [DOI] [PubMed] [Google Scholar]
- 43.Rosa J, Widimský P, Toušek P, Petrák O, Čurila K, Waldauf P, Bednář F, Zelinka T, Holaj R, Štrauch B, Šomlóová Z, Táborský M, Václavík J, Kociánová E, Branny M, Nykl I, Jiravský O, Widimský J Jr. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 65: 407–413, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04019. [DOI] [PubMed] [Google Scholar]
- 44.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291–299, 2001. [PubMed] [Google Scholar]
- 45.Santajuliana D, Hornfeldt BJ, Osborn JW. Use of ganglionic blockers to assess neurogenic pressor activity in conscious rats. J Pharmacol Toxicol Methods 35: 45–54, 1996. doi: 10.1016/1056-8719(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 46.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: mechanisms, current findings, and future prospects. Curr Hypertens Rep 14: 247–253, 2012. doi: 10.1007/s11906-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 48.Seravalle G, D’Arrigo G, Tripepi G, Mallamaci F, Brambilla G, Mancia G, Grassi G, Zoccali C. Sympathetic nerve traffic and blood pressure changes after bilateral renal denervation in resistant hypertension: a time-integrated analysis. Nephrol Dial Transplant 32: 1351–1356, 2017. doi: 10.1093/ndt/gfx200. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Iyoda I, Yamasaki H, Takeda K, Okajima H, Sasaki S, Yoshimura M, Nakagawa M, Ijichi H. Retardation of the development of hypertension in DOCA-salt rats by renal denervation. Jpn Circ J 48: 567–574, 1984. doi: 10.1253/jcj.48.567. [DOI] [PubMed] [Google Scholar]
- 50.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M, Aoki J, Batson B, Böhm M, Choi JW, Cohen DL, Dangas G, David S, Davies J, Devireddy CM, Kandzari D, Kario K, Lee DP, Lurz PC, Patel M, Patel K, Schmieder RE, Sharp ASP, Singh J, Tsioufis K, Walton A, Weber T, Weil J, Zeller T, Ziada K, Tanabe K, Wilkins R, Mahfoud F, East C, Wilensky R, Contreras J, Steigerwalt S, Chapman N, Lea JP, Reedus D, Hoshide S, Ma A, Fengler K, Svetkey L, Rao A, Schmid A, Watkinson AF, Brown A, Tousoulis D, Hopper I, Suppan M, Agdirlioglu T, Noory E, Chasen C; SPYRAL HTN-OFF MED trial investigators . Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390: 2160–2170, 2017. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 51.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, Neuhuber W, Tiegs G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol 19: 1371–1378, 2008. doi: 10.1681/ASN.2007050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veiga GL, Nishi EE, Estrela HF, Lincevicius GS, Gomes GN, Simões Sato AY, Campos RR, Bergamaschi CT. Total renal denervation reduces sympathoexcitation to different target organs in a model of chronic kidney disease. Auton Neurosci 204: 81–87, 2017. doi: 10.1016/j.autneu.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Villarreal D, Freeman RH, Davis JO, Garoutte G, Sweet WD. Pathogenesis of one-kidney, one-clip hypertension in rats after renal denervation. Am J Physiol Heart Circ Physiol 247: H61–H66, 1984. doi: 10.1152/ajpheart.1984.247.1.H61. [DOI] [PubMed] [Google Scholar]
- 54.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension 71: 1–481, 2018. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 55.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J 34: 2132–2140, 2013. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worthley SG, Wilkins GT, Webster MW, Montarello JK, Delacroix S, Whitbourn RJ, Warren RJ. Safety and performance of the second generation EnligHTN™ Renal Denervation System in patients with drug-resistant, uncontrolled hypertension. Atherosclerosis 262: 94–100, 2017. doi: 10.1016/j.atherosclerosis.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J II, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaldivia MT, Rivera J, Hering D, Marusic P, Sata Y, Lim B, Eikelis N, Lee R, Lambert GW, Esler MD, Htun NM, Duval J, Hammond L, Eisenhardt SU, Flierl U, Schlaich MP, Peter K. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 69: 323–331, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08373. [DOI] [PubMed] [Google Scholar]