Abstract
Electronic (e)-cigarettes theoretically may be safer than conventional tobacco. However, our prior studies demonstrated direct adverse effects of e-cigarette vapor (EV) on airway cells, including decreased viability and function. We hypothesize that repetitive, chronic inhalation of EV will diminish airway barrier function, leading to inflammatory protein release into circulation, creating a systemic inflammatory state, ultimately leading to distant organ injury and dysfunction. C57BL/6 and CD-1 mice underwent nose only EV exposure daily for 3–6 mo, followed by cardiorenal physiological testing. Primary human bronchial epithelial cells were grown at an air-liquid interface and exposed to EV for 15 min daily for 3–5 days before functional testing. Daily inhalation of EV increased circulating proinflammatory and profibrotic proteins in both C57BL/6 and CD-1 mice: the greatest increases observed were in angiopoietin-1 (31-fold) and EGF (25-fold). Proinflammatory responses were recapitulated by daily EV exposures in vitro of human airway epithelium, with EV epithelium secreting higher IL-8 in response to infection (227 vs. 37 pg/ml, respectively; P < 0.05). Chronic EV inhalation in vivo reduced renal filtration by 20% (P = 0.017). Fibrosis, assessed by Masson’s trichrome and Picrosirius red staining, was increased in EV kidneys (1.86-fold, C57BL/6; 3.2-fold, CD-1; P < 0.05), heart (2.75-fold, C57BL/6 mice; P < 0.05), and liver (1.77-fold in CD-1; P < 0.0001). Gene expression changes demonstrated profibrotic pathway activation. EV inhalation altered cardiovascular function, with decreased heart rate (P < 0.01), and elevated blood pressure (P = 0.016). These data demonstrate that chronic inhalation of EV may lead to increased inflammation, organ damage, and cardiorenal and hepatic disease.
Keywords: cardiorenal dysfunction, e-cigarette, electronic cigarette, fibrosis, nicotine, systemic inflammation
INTRODUCTION
Electronic cigarettes (e-cigs) became widely available in 2004–2007 (12). They are the newest tobacco products on the market and work by heating and aerosolizing propylene glycol (PG; 1,2-propanediol), glycerin (Gly; 1,2,3-propanetriol), and nicotine. The product inhaled is commonly referred to as e-cig vapor (EV). EV has components in common with cigarette smoke, chief among these nicotine, which can directly cause endothelial dysfunction (56). Some EV also contains acrolein, formaldehyde, and nitrosamines, which are also commonly found in cigarette smoke. This commonality raises the concern of shared toxicities between cigarettes and e-cigs (17, 19). The finding that low-tar and smokeless tobacco products may be linked to systemic inflammation and increased cardiovascular disease further suggests that some of the components of tobacco do not need to undergo combustion to be damaging to human health (9, 58). While the lungs are one of the primary sites of ill effects of cigarette smoke (emphysema and lung cancer), tobacco smoke has significant effects on many other organs, including kidneys, heart, brain, and gastrointestinal tract, via induction of endothelial damage and systemic inflammation (2, 56, 60).
Many human e-cig users pick up the vaping habit as an attempt to help them quit smoking; however, some studies and meta analyses to date suggest that e-cig use reinforces the nicotine addiction and decreases the odds of quitting (25, 39). Our own work has demonstrated that chronic inhalation of EV leads to activation of classic nicotine addiction pathways in the central nervous system (1), which suggests that e-cig users will likely continue using these nicotine delivery devices for years to come. Clinical signals of the adverse effects on human health due to long-term use by e-cig users, such as emphysema, cardiovascular disease, and renal dysfunction, may not, however, be evident for 20–50 yr or until they are exacerbated by pathophysiological challenges.
One mechanism by which chronic inhalation of chemicals causes disease is through disruption of the airway epithelial barrier (13, 18, 59, 68). Normal airways have a solid barrier facilitated through the existence of tight junctions between epithelial cells. Isolated cases of eosinophilic pneumonia (76), lipoid pneumonia (54), acute lung injury, and acute respiratory distress syndrome (personal communication with Jennifer McCallister, Ohio State University) have been reported in e-cig users, demonstrating that short-term exposure to EV may lead to acute epithelial damage and proinflammatory responses within the lungs. We hypothesized that chronic EV inhalation would alter the permeability of epithelial surfaces and increase exposure of parenchymal cells to EV components, leading to damage and inflammation, that promote acute and chronic diseases by recurrent inflammatory signaling driving a systemic proinflammatory state.
Our prior studies with EV utilized established in vitro models and demonstrated negative effects on antimicrobial function of lung cells, alveolar macrophages, epithelial cells, and neutrophils (32). Other groups have also found adverse effects on airway cells in vitro (79) and lung function in vivo (51) and increased susceptibility to infection in vivo (75). In our laboratory, we also found significant cell death (cytotoxicity) of EV on all mammalian cell lines evaluated (32). Cell death commonly activates inflammatory pathways (and vice versa) and can produce tissue changes leading to pathology. Finally, we have published that EV exposure in vitro can induce double-stranded DNA breaks, a serious effect that can lead to malignant conversion (81).
In the current studies, we explored the hypothesis that exposure to the most common components of EV (PG, Gly, and nicotine) alters barrier function of airway epithelium, leading to release of inflammatory proteins into the systemic circulation. Using our in vivo animal model of chronic EV inhalation (32), we assayed serum for evidence of proinflammatory effects of EV inhalation and organs for downstream effects of these proinflammatory signals. We specifically evaluated cardiorenal function, as it is known that inhalation of combustible cigarette smoke is detrimental to both cardiac and renal function (30, 63, 74). One group recently published adverse effects of intraperitoneal injection of e-liquid daily for 1 mo on rat kidney function (28). We sought to confirm these findings using a physiologic exposure to EV, in which e-liquid is placed into a tank and attached to a battery, and the e-liquid is heated and vaporized, producing EV, which is inhaled through our nose only system into the airways. The reason for using this more complex type of exposure is that heating and vaporization of e-liquid alter the chemical composition and can create toxins such as formaldehyde and acrolein; these toxins may cause adverse effects directly on airway and endothelial cells which the other components of EV do not (34, 72, 77).
The following in vivo and in vitro studies were designed and undertaken to evaluate whether e-cig use leads to inflammation. We present here the effects of daily, chronic inhalation of EV containing nicotine on airway permeability, airway inflammatory response to bacterial infection, the systemic inflammatory milieu, downstream organ function, and tissue fibrosis. Informing e-cig users, physicians, businesses, and policy makers of the potential risks of these new devices may lead to the production of safer devices, new policies to limit access to adults, and safer use patterns by the vaping community, and thus decreased adverse effects on human health overall.
MATERIALS AND METHODS
E-cigarettes.
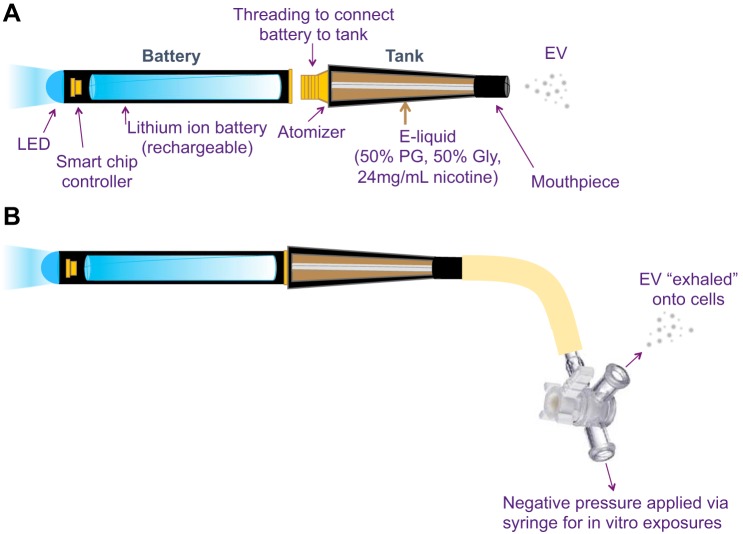
E-liquid consisting of 50% propylene glycol (PG), 50% glycerin (Gly; also referred to as vegetable glycerin), and 24 mg/ml nicotine was used, as it was a common solution used by the general population in 2014, at the time of study design. All e-liquids were mixed in the laboratory after purchase from a popular online vendor (Xtreme Vaping). E-liquid was placed in a standard tank (1.8 Ω) and was attached to a rechargeable lithium ion battery (3.4 V). All e-cig components were purchased from commercial vendors (FastTech, Vapor Authority, and Xtreme Vaping) to maintain relevance to human e-cig users. All exposures were designed to model firsthand EV exposure, with animals and human cells exposed to EV generated from devices used by actual e-cig users (Fig. 1A), and generated with the same pneumatic pressure and puff topography as human e-cig vapers.
Fig. 1.
Diagram of an electronic (e)-cigarette (A). For our in vitro model of firsthand e-cigarette vapor (EV) exposure (B), the e-cigarette hooked up to rubber tubing and a 3-way stopcock, such that negative pressure is applied to the mouthpiece via pulling back the plunger on a 60-ml syringe, generating fresh EV. The syringe is filled with 50 ml of EV each time, and the EV is subsequently exhaled through the side port of the 3-way stopcock onto primary human airway epithelial cells. MPO, myeloperoxidase; RAGE, receptor for advanced glycation end product; M-CSF, macrophage colony-stimulating factor; RBP4, retinol-binding protein 4: LIF, leukemia inhibitory factor; WISP-1, WNT1-inducible signaling pathway; MMP-3, matrix metalloprotease-3.
Primary human bronchial epithelial cell permeability assay.
Primary normal human bronchial epithelial cells (NHBEs) were purchased from Lonza (donors have no reported history of smoking or known lung conditions). The cells were grown according to the manufacturer’s established protocols as mentioned previously (52). In brief, the cells were resuspended in growth media (Lonza) and seeded onto cell culture inserts (0.4-μm pore size; Costar), coated with type I rat tail collagen (BD Biosciences). Cells were maintained in B-ALI differentiation media (Lonza) with inducers for 3 wk in the basal chamber for differentiation into mucociliary cells. Transepithelial electrical resistance (TEER) was measured using the Voltohmeter on days 14 and 21 and demonstrated epithelial confluency via increasing electrical transmittance in all wells. When the TEER values reached a plateau, starting on day 21, NHBE cells were transferred to an exposure chamber and EV or Air was introduced for 15 min daily for 2–5 days. Each EV breath (50 ml) was produced by pneumatic activation of the e-cig via a 60-ml syringe (Fig. 1B), with exhalation of the EV onto the apical surface of the NHBE cells, followed by two air breaths (50 ml apiece), to mimic the act of breathing. Because EV leaves a greasy residue on exposed surfaces, after exposure, Transwells were gently transferred to new wells containing fresh media (380 µl) at the basal interface and were placed back at 37°C with 5% CO2. NHBE barrier function, and specifically permeability due to interruptions in the junctional complex, were evaluated with and without infection with Pseudomonas aeruginosa (PSA; 1 × 106 colony-forming units/well in 50 µl) PAO1 (16) by application of FITC-dextran, molecular weight 3–5,000 (Sigma), to the apical surface for 15 min, followed by transfer of 50 µL from the lower Transwell chamber to a flat-bottom 96-well plate. The amount of paracellular permeability was measured using a fluorescence plate reader with excitation of 490 nm and emission at 520 nm. NHBE cells were either kept uninfected or were infected with PSA PAO1 (1 × 106 colony-forming units/well in 50 µl) (5). After 2 h of infection, basolateral supernatant was collected. NHBE cells were harvested either with RLT buffer (Qiagen) or with RIPA buffer and stored at −80°C for RNA and protein studies.
ELISA and Western blot.
The supernatants collected from NHBE cells were measured for IL-8/LIX using the Human IL-8/CXCL8 Quantikine ELISA Kit (R&D Systems) following the manufacturer supplied protocol. For Western blots, cell lysates were prepared from NHBE cells with ice-cold RIPA buffer containing protease inhibitor (Roche) and phosphatase inhibitor (Sigma) cocktails. An equal amount of protein (50 μg) was loaded in each lane of 8% SDS-PAGE and transferred onto nitrocellulose membrane (Bio-Rad). The membrane was blocked in 5% nonfat milk in Tris-buffered saline with 0.05% Tween-20 (TBST) and incubated with the tight junction protein antibody for zona occludins (ZO1) (Proteintech; 1:1,000) and tubulin (Proteintech; 1:10,000) in 5% milk-TBST for incubation overnight at 4°C. ZO1 was detected at a molecular mass of 195 kDa, while tubulin was detected at a molecular mass of 55 kDa. After imaging was completed, blots were opened in ImageJ and converted to eight-bit files, the background was removed via adjustment of the threshold to Huang, and the integrated density of each band was measured.
Animals.
Six- to eight-week-old female C57BL/6 and CD-1 (ICR) mice were purchased from Harlan (Envigo). Inbred C57BL/6 mice are known to be susceptible to emphysema and oxidative stress (29), while outbred CD-1 mice are resistant (14). Mice were acclimated to the individual, soft mesh restraints (SciReq) for 30 min daily for 2 days. Mice were then exposed to EV daily, for 5 days per week, for 3–6 mo, using the nose-only InExpose system (SciReq) as we previously described (32), using a flow rate of 2 l/min and exposure time of 4 s of EV every 20 s for 60 min daily (7). Because C57BL/6 mice are more susceptible to pathologic effects of smoke inhalation (emphysema), and more susceptible to disease in general, the duration of their chronic EV exposure was set at 3 mo, while the hardy, emphysema-resistant CD-1 strain was exposed to EV for 6 mo. Mice in the air control group were placed in the same restraints but inhaled room air only. Cheek bleeds were performed 30 min postexposure, and serum cotinine concentration was determined via ELISA (Calbiotech). Serum cotinine levels in C57BL/6 mice were 269 ± 15.6 ng/ml and in CD-1 mice were 288 ± 39 ng/ml, post-EV exposure for 60 min. C57BL/6 mice were exposed to EV or Air daily for 3 mo. CD-1 mice were exposed to EV or Air daily for 6 mo. All mice were placed in prewarmed cages for 30 min to recover after restraint. The last exposure was done the day of harvest, with mice being placed on a warming pad for heart rate (HR) and blood pressure (BP) measurements (15–30 min postexposure), followed by application of anesthesia, terminal intracardiac bleed, and organ harvest. Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committees at the University of California San Diego and the Veterans Affairs San Diego Healthcare System.
Circulating proinflammatory cytokines.
Blood was placed at 4°C for 15 min and spun at 3,000 rpm for 15 min at 4°C, and plasma was stored at −80°C for measurement of total protein (BCA Total Protein Assay Kit; Pierce) and inflammatory cytokines via Mouse XL Cytokine Array (Proteome Profiler by R&D Systems), according to the manufacturer’s instructions. In brief, for CD-1 mice, plasma samples were individually checked for protein concentration before pooling for proteome array, with equal volumes of plasma from individual mice combined into one sample (n = 6 for both groups). Individual plasma samples from C57BL/6 mice were run (n = 3 for both groups). Proteome films were blinded, scanned, and uploaded to ImageJ, background was removed via threshold adjustment, and pixel density for each pair of cytokine dots was quantified. Data are presented as a ratio of EV to Air.
Renal function.
To determine glomerular filtration rate, the week before harvest, mice were exposed to EV or Air for 60 min and allowed to recover for 60 min. Mice were placed under inhaled isoflurane anesthesia, and FITC-sinistrin (2 µl/g body wt) was injected retro-orbitally with a 30-gauge needle. Subsequently, the anesthetized mouse fully regained consciousness. Tail vein puncture was utilized to collect blood in 10 µl Na-heparin Minicaps at the following times after injection: 3, 5, 7, 10, 15, 35, 56, and 75 min. Samples were analyzed for FITC-Sinistrin concentration using a NanoDrop 3300.
Cardiac function.
For the week before harvest, EV and Air mice underwent HR and BP monitoring for 30 min after EV or Air exposure (n = 6 for all groups). HR and BP were obtained using a CODA Monitor, noninvasive BP system (Kent Scientific) as described previously (23, 42, 43). We concomitantly checked HR via pulse oximeter by PhysioSuite (Kent Scientific).
Renal, cardiac, and liver fibrosis evaluation.
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/ml) and euthanized, by a terminal intracardiac bleed. The right kidney, one lobe of liver, and the base of the heart were then immediately dissected and placed in Z-fix at 4°C. After 48 h, all organs were moved to 75% ethanol and submitted to the University of California, San Diego histology core for paraffin embedding. Collagen was detected in 5-μm sections first by Masson’s trichrome stain. All histology slides underwent quantification of fibrosis by calculating the mean percent fibrotic area in >30 randomly acquired ×20 images using computer-aided morphometry performed using a macro in ImageJ as previously described (23, 31, 42, 43). Collagen deposition was also detected by 5-μm sections on Picrosirius red staining. All histology slides were blinded and underwent quantification under bright-field microscopy by calculating a relative area of brightness at a set threshold (157,255), in relation to the area of six randomly acquired ×10 images, computer-aided morphometry performed using an ImageJ macro (https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html). All slides underwent these computer analyses in an identical fashion. Fibrotic area is presented relative to that of air controls.
Quantification of fibrosis markers in renal and cardiac parenchyma.
After daily exposure to EV for 5 days per week, for 4 wk (1 mo), the left kidney and the apex of the heart from both CD-1 and C57BL/6 Air and EV mice were snap frozen and stored at −80°C. Thirty milligrams of frozen left ventricular or renal tissues were homogenized in TRIzol, and total RNA was isolated using the RNeasy kit (Qiagen), followed immediately by cDNA synthesis using the First Strand cDNA synthesis kit (Qiagen), according to the manufacturer’s protocol. One microgram of total RNA was used for the initial reaction. cDNA was stored at −20°C and was used for quantitative real-time polymerase chain reaction (qPCR) within 2 wk.
To quantify extracellular matrix gene expression in murine cardiac and renal tissues, species specific primers were purchased from Qiagen for collagen 1a1 (Col1a1; PPM03845F), collagen 3a1 (Col3a1; PPM04784B), collagen 4a1 (Col4a1; PPM05145A), matrix metalloprotease-2 (Mmp2; PPM03642C), integrin β1 (Itgb1; PPM03668D), fibrillin 1 (Fbn; PPM36411E), and elastin (Eln; PPM36834B), in addition to GAPDH (PPM02946E) as a control. RT2 SYBR Green qPCR reaction mix (Qiagen) was used according to the manufacturer’s protocol, with an ABI 7500 Fast platform (Life Technologies). Determination of mRNA expression was computed by comparing the relative change in cycle threshold value of the target (ΔCt) from the internal control GAPDH. Fold change in expression in EV tissues versus air controls was then calculated for each mRNA in each sample using expression = 2−ΔΔCt methodology (36).
Statistical analyses.
Data are presented as means ± SE. Data obtained were analyzed by t-test or by two-way ANOVA, where appropriate. Analyses were conducted using Graph Pad Prism 6 software.
RESULTS
E-cig vapor inhalation increased the levels of circulating inflammatory cytokines.
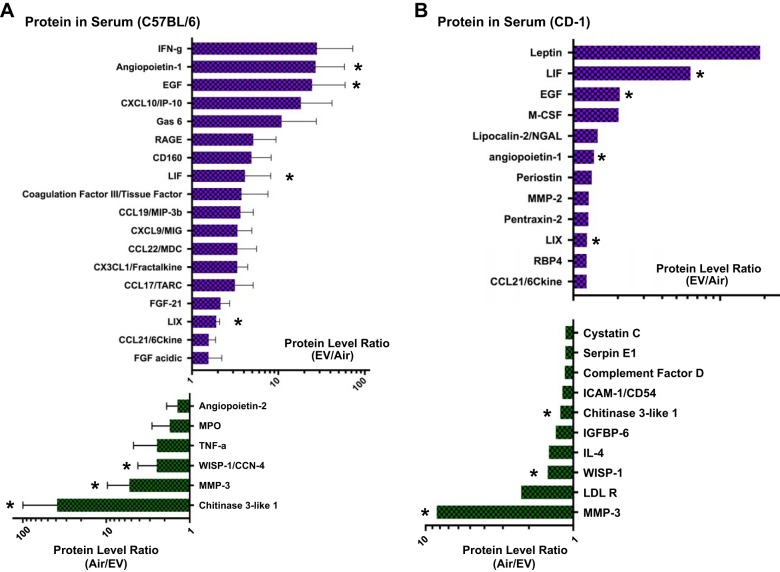
We hypothesized that repetitive inhalation of EV leads to stress in or damage to pulmonary epithelial cells, which leads to the release of factors into systemic circulation. In mice inhaling 1 h of EV daily, for 5 days per week, we found higher levels of several inflammatory cytokines in the circulation (significance was defined as a 20% change compared with Air control mice) and lower levels as well (Fig. 2, A and B). Proteins that changed in both strains of mice (*) are of particular interest since these changes were induced by chronic EV inhalation across genetically different backgrounds (Table 1). Overall, the finding of changes in expression, production, or secretion of multiple inflammatory protein levels, both increases and decreases, suggests that chronic EV inhalation causes systemic immunomodulation.
Fig. 2.
C57BL/6 (A) and CD-1 (B) mice exposed daily to EV for 3 and 6 mo, respectively, had modulated levels of inflammatory proteins in the serum, consistent with an altered systemic inflammatory state. Sera were evaluated by 111-cytokine antibody array (Proteome Profiler Mouse XL Array; R&D Systems), and graphed as a ratio of EV/Air for proteins that increased with EV exposure and Air/EV for proteins that decreased with EV exposure. A: changes in C57BL/6 serum protein levels caused by EV exposure are shown, with a 20% threshold in either direction, including large rises in angiopoietin-1 and EGF in EV mice, and much decreased Chitinase 3-like 1 and MMP-3 in EV mice (n = 3 per group). B: serum protein changes in CD-1 mice, including large increases in LIF (murine equivalent of IL-8) and EGF, and large decreases in MMP-3 and WISP-1 (n = 6 per group, pooled). *Protein changes occurred in both CD1 and C57BL/6 mice.
Table 1.
Circulating inflammatory proteins that changed in both C57BL/6 and CD-1 mice chronically exposed to EV
| Protein | Fold Change in C57BL/6 | Fold Change in CD-1 |
|---|---|---|
| LIF* | 30.6 | 6.29 |
| EGF* | 24.6 | 2.06 |
| Angiopoietin-1* | 27 | 1.38 |
| LIX* | 1.92 | 1.24 |
| MMP-3† | −5.29 | −8.42 |
| Chitinase 3-like 1† | −38.9 | −1.22 |
| WISP-1† | −2.15 | −1.49 |
LIF, leukemia inhibitory factor; MMP-3, matrix metalloprotease-3; WISP-1, WNT1-inducible signaling pathway.
Proteins that were elevated in the plasma of e-cigarette vapor (EV) mice.
Proteins that were decreased in the plasma of EV mice.
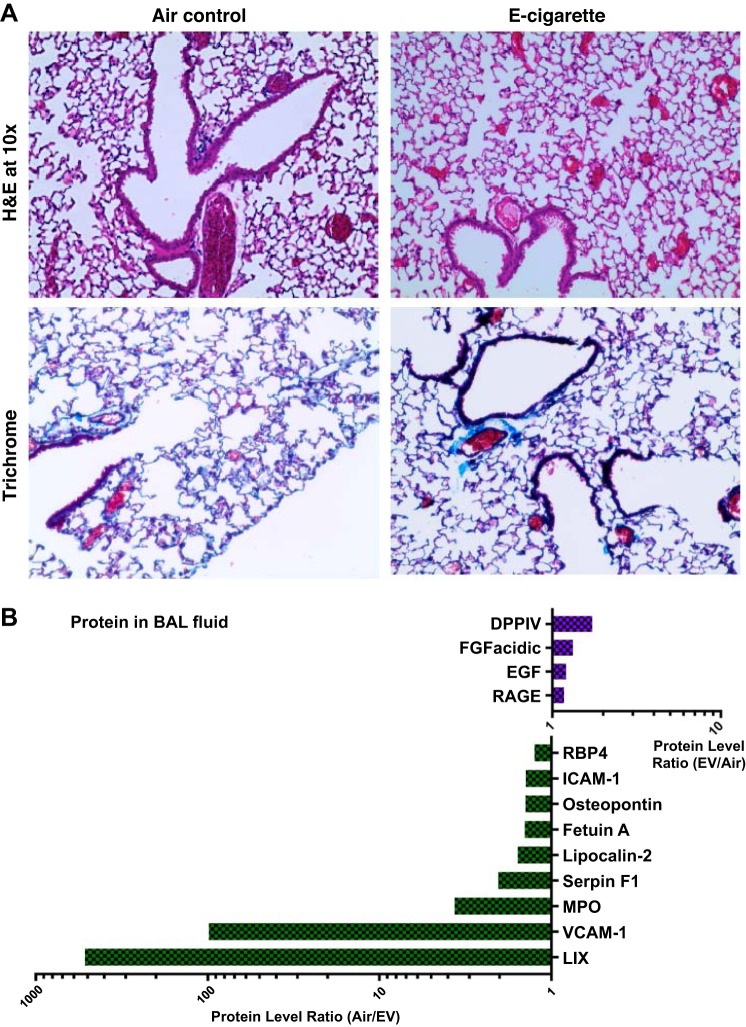
To begin to understand where the immunomodulatory signals originate, we evaluated the inflammatory state of the lungs. Lungs from CD-1 mice were examined and had normal histopathology (Fig. 3A). Within the bronchoalveolar lavage, dipeptidyl peptidase-4 (DPPIV/CD26) was elevated 1.7-fold in EV mice versus controls (Fig. 3B). DPPIV is an enzyme expressed on the surface of cells. It is an intrinsic membrane glycoprotein and has general immune regulation and signal transduction functions. DPPIV has been shown to modulate macrophage M1/M2 polarization (86) and modulate T-cell recruitment to the lungs (49, 71), while inhibition of DPPIV decreases T-cell-mediated inflammation (73). DPPIV is thought to play a pathological role in the development of liver, cardiac, and kidney fibrosis (3, 37, 40, 69).
Fig. 3.
Mice that inhaled EV for an hour daily had inflammatory changes only at the protein level. A: lung parenchyma was stained with hemotoxylin and eosin (H&E) and Masson’s trichrome stains. One lung slice per mouse, including large, medium, and small airways, was evaluated by a blinded pathologist, and no pulmonary inflammation, emphysema or fibrosis was found in EV mice relative to Air controls (n = 6 per group). B: the airways of mice, as measured through bronchoalveolar lavage (BAL), had alterations in the inflammatory cytokine profile. BAL was pooled within EV and Air control groups (n = 6 within groups) and was evaluated by 111-cytokine antibody array (Proteome Profiler Mouse XL Array; R&D Systems) and graphed as a ratio of EV/Air. BAL from EV mice had decreased levels of LIX (murine version of IL-8; 519-fold lower or ~0.2% of Air levels) and VCAM-1 (99-fold lower or 1% of Air levels). EV BAL had increased levels of DPPIV (1.7-fold or 58% higher than Air levels).
Nicotine itself has effects on endothelial cell function. The nicotine metabolite cotinine was measured in all mice immediately after the final 60-min EV or air exposure. C57BL/6 mice exposed to EV had an average plasma cotinine level of 268.8 versus 25.6 ng/ml in Air controls, while CD-1 female mice exposed to EV had an average of 287.9 versus 3.1 ng/ml plasma cotininein Air controls.
Short-term daily exposure to e-cig vapor weakened human airway epithelium barrier function in vitro.
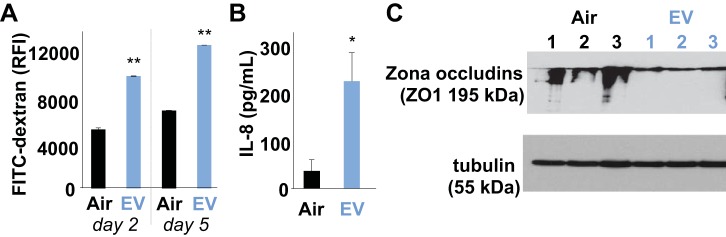
One mechanism by which chronic inhalation of EV may lead to systemic inflammatory changes is by inducing inflammatory signals at the level of the airways. Exposure of the apical surface of confluent NHBEs to 15 min daily of EV for 2 and 5 days led to diminished barrier function with the passage of 48 and 46% more FITC-dextran from the apical surface down through the basal surface, respectively (8,070 vs. 4,200 RFI at day 2, and 10,300 vs. 5,600 RFI on day 5; P < 0.01; Fig. 4A). Further increase in permeability was observed in NHBEs following infection with Pseudomonas aeruginosa. Secretion of the proinflammatory neutrophil chemokine IL-8 by NHBEs increased sixfold by ELISA quantification, following EV exposure, in the setting of bacterial superinfection (P < 0.05; Fig. 4B). Tight junction proteins that function to keep the epithelium impermeable were evaluated by Western blot. The level of ZO1 was lower in NHBEs after acute EV exposure (P = 0.024), suggesting changes in tight junctions induced by EV exposure (Fig. 4C). Decreased tight junction proteins suggest that EV leads to reduced barrier function in the lungs, which in turn can allow greater passage of external factors (antigens and chemicals) into the lung parenchyma and bloodstream.
Fig. 4.
Primary normal human bronchial epithelial cells (NHBEs) became leaky and proinflammatory with daily short 15 min EV exposures for 2–5 days. A: EV-treated NHBE cells tested for permeability with FITC-dextran had greater passage of small molecules, compared with controls exposed to Air only, on both days 2 and 5 (P < 0.01; means ± SE; wells were run in triplicate). B: EV-exposed NHBEs secreted more IL-8 than Air controls in response to bacterial infection (37 vs. 227 pg/ml, respectively; P < 0.05; wells were run in triplicate). C: protein quantification of Western blots of the tight junction protein zona occludins (ZO1) found 3.3-fold lower quantities in NHBEs after EV exposure, as compared with Air controls (P = 0.024; n = 3). Levels of loading control tubulin were similar across samples (P = 0.99).
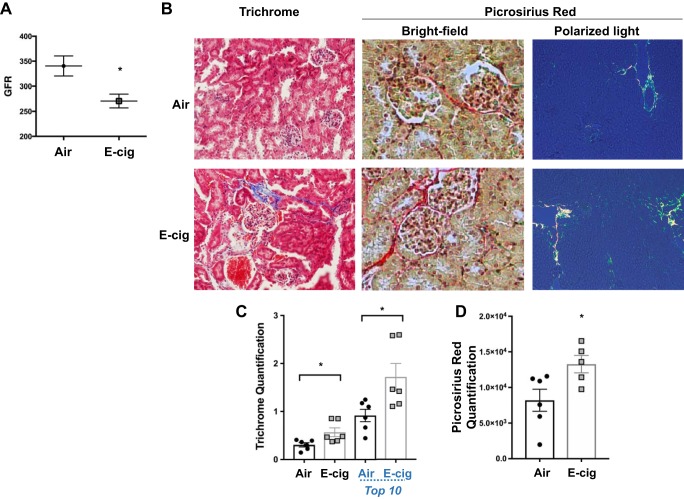
Chronic exposure to daily e-cig vapor induced renal dysfunction and fibrosis in C57BL/6 mice.
To determine whether the circulating protein changes demonstrated above are associated with organ dysfunction we first evaluated renal function. Three months of daily EV inhalation induced a 20% reduction in glomerular filtration rate in C57BL/6 mice as compared with experimental controls (P = 0.017; Fig. 5A), as measured by FITC-sinistrin clearance. With the use of blinded evaluation of Masson’s trichrome-stained renal parenchyma (Fig. 5B), kidneys from EV-exposed mice were found to have 87% more collagen in their parenchyma (evidence of renal fibrosis) than did air controls (1.88-fold increase, P < 0.05; Fig. 5C). Blinded evaluation of Picrosirius red-stained renal parenchyma (Fig. 5B) confirmed higher deposition of collagen in EV kidneys compared with Air controls (1.62-fold increase, P = 0.034; Fig. 5D). These data suggest that regular EV inhalation may have profibrotic effects on kidney parenchyma, which could lead to decreased renal function after a relatively short duration of exposure.
Fig. 5.
Chronic EV inhalation diminished cardiorenal function and induced renal fibrosis in C57BL/6 mice exposed to EV for 3 mo. A: EV induced a 20% reduction in glomerular filtration rate (GFR) as compared with experimental controls (P = 0.017). B: representative ×20 Masson’s trichrome, ×20 Picrosirius red bright-field, and ×10 polarized light photomicrographs of renal tissue fibrosis. C: fibrosis was quantified in kidneys from EV and Air mice, by blinded grading of kidney sections. EV kidneys had 87% (1.86-fold increase) more collagen vs. experimental controls. When only the 10 sections with the highest levels of collagen staining were compared, EV kidneys still had 1.88-fold higher levels of fibrosis, compared with controls. D: Picrosirius red staining also demonstrated higher collagen content in EV-exposed mice, relative to Air controls (1.62-fold increase, P = 0.034). Means ± SE are shown; n = 5–6 per group; *P < 0.05.
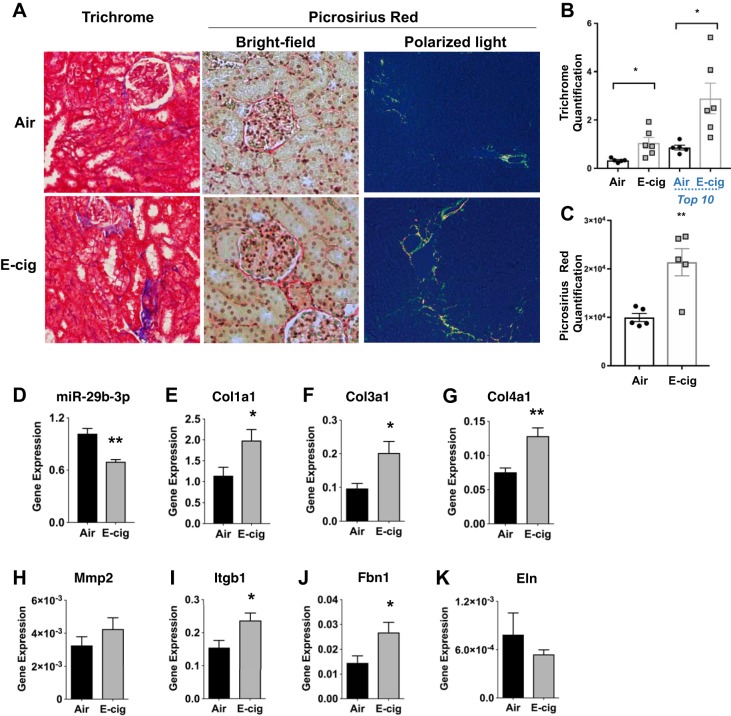
Long-term e-cig exposure in outbred CD-1 mice also induced renal fibrosis.
When genetically diverse, and thus hardier, CD-1 mice were exposed to 1 h daily of EV for 6 mo, they also developed renal fibrosis (Fig. 6A). By trichrome staining, EV kidney parenchyma had a 3.2-fold increase in fibrosis compared with experimental controls (P = 0.022; Fig. 6B). By Picrosirius red staining, EV kidney parenchyma had 2.14-fold higher collagen deposition compared with air controls (P < 0.01; Fig. 6C). These data suggest that chronic inhalation of EV leads to activation of profibrotic pathways systemically, impacting nonpulmonary organs. Changes in profibrotic gene expression were also observed at earlier times (Fig. 6, D–K). These data add confidence to our findings that fibrosis is stimulated by EV exposure since outbred CD-1 mice are genetically diverse, with higher likelihood of results being translatable to human subjects.
Fig. 6.
Induction of kidney fibrosis also occurred in CD-1 mice exposed to EV for 6 mo. A: kidney parenchyma stained with Masson’s trichrome and Picrosirius red stains. B: in CD-1 mice, daily EV inhalation for 6 mo led to a 3.2-fold increase in renal fibrosis, assessed by Masson’s trichrome stain, relative to Air controls (means ± SE are shown; P = 0.022). C: Picrosirius red staining also demonstrated 2.14-fold higher collagen content in EV-exposed mice, relative to Air controls (means ± SE are shown; P < 0.01). D–K: to assess for the origin of fibrosis, genes associated with fibrosis and extracellular matrix pathways were evaluated after only 4 wk of EV or Air exposure. Lower expression of the antifibrotic miRNA miR-29b-3p (D) and higher expression of collagen-1 within CD-1 renal parenchyma (E), suggest that fibrosis begins early in the course of daily EV inhalation. The expression of additional profibrotic factors, Col3a1 (F), Col4a1 (G), Itgb1 (I), and Fbn1 (J), were all significantly increased in renal tissues from e-cig-exposed animals (P < 0.05). Extracellular matrix remodeling factor Mmp2 trended up but not significantly (H). However, the fibrosis component Eln was not significantly different (K). *P < 0.05; n = 5–6 for all groups.
Recent work in our laboratory has revealed that decreased tissue expression of miR-29b-3p is a mechanism of cardiorenal toxicity and organ fibrosis in chronic kidney disease and in response to combustible cigarette exposure (21, 22, 48). We found molecular evidence of early profibrotic changes in renal tissues of EV exposed mice at 1 mo, lower expression of the antifibrotic miRNA miR-29b-3p (Fig. 6D), and higher expression of collagen-1 within CD-1 renal parenchyma (Fig. 6E). The expression of additional profibrotic factors, Col3a1, Col4a1, Itgb1, and Fbn1, was all significantly increased in renal tissues from e-cig-exposed animals (P < 0.05; Fig. 6, F, G, I, and J). Extracellular matrix remodeling factor Mmp2 trended up but not significantly (Fig. 6H). The fibrosis component Eln trended down (Fig. 6K). When we completed similar renal studies with our collaborators studying renal fibrosis in a cigarette smoke inhalation model, we found a similar fibrosis pattern (21). These data suggest that a shared component of EV and cigarette smoke, such as nicotine, may be the etiologic agent in distant organ injury and fibrosis.
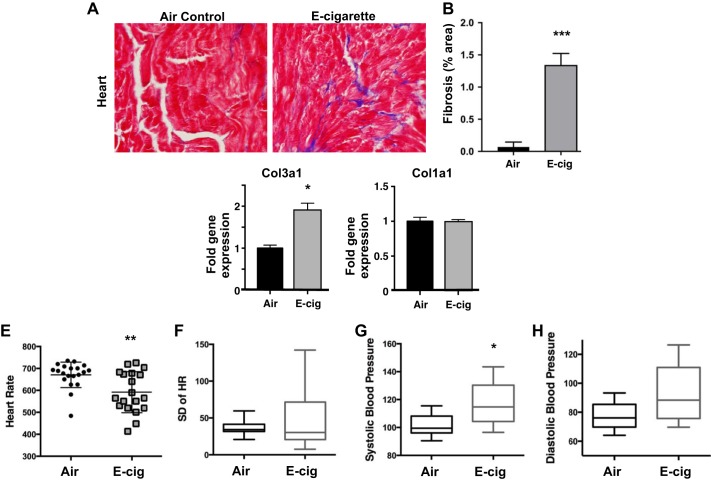
Chronic e-cig vapor inhalation induced cardiac fibrosis and altered cardiovascular function.
Examination of cardiac tissue revealed a 2.75-fold greater level of fibrosis in hearts from CD-1 mice exposed to EV for 6 mo compared with controls (P < 0.001; Figs. 7, A and B). When we examined hearts after only 4 wk of daily EV exposure, we found increased expression of collagen-3 (P < 0.05; Fig. 7C), although the levels of collagen-1 were not elevated (Fig. 7D). These data extend our findings from kidney to heart and thus suggest global profibrotic signaling induced by chronic EV inhalation.
Fig. 7.
Chronic inhalation of EV induced cardiac fibrosis and altered cardiovascular function. A: Masson’s trichrome stain of fixed cardiac ventricular tissue from CD-1 mice exposed to EV daily for 6 mo. B: quantitative analysis of EV relative to control determined that EV hearts had 2.75-fold greater level of collagen staining in ventricular tissue compared with controls (***P < 0.001). C and D: when tissues were harvested after only 4 wk of EV exposure, cardiac tissues were found to have higher expression of collagen-3 mRNA (C) but normal expression levels of collagen-1 mRNA (D) (**P < 0.05). For A–D, n = 6 per group. E: in C57BL/6 mice, EV daily for 3 mo led to decreased heart rates (HR), as compared with Air controls (*P < 0.01). F: heart rates (HRs) were more variable in EV exposed mice, as indicated by greater SD within beat-to-beat measurements of each mouse. G: systolic blood pressure was increased in e-cigarette-exposed mice (P = 0.016). H: diastolic blood pressure trended up in EV mice (*P = 0.050). For E–H, n = 19 for EV and n = 20 for Air controls.
We evaluated cardiovascular function in the C57BL/6 mice exposed to EV for 3 mo and found decreased HRs as compared with air controls (P < 0.01; Fig. 7E). EV mice also tended to have more HR variability (oscillations between consecutive instantaneous HR; Fig. 7F). Systolic BP was increased in e-cig-exposed mice (P = 0.016; Fig. 7G) and diastolic BP trended up (P = 0.050; Fig. 7H). These data demonstrate the effects of daily EV inhalation on cardiac function, including BP and HR, which could have a long-term impact on cardiac hypertrophy and function.
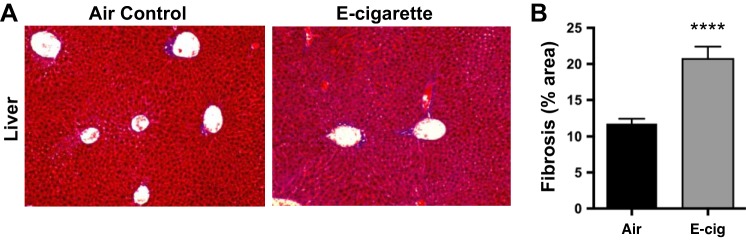
Chronic e-cig vapor inhalation induced hepatic fibrosis.
The finding of multiorgan fibrosis suggests the presence of a circulating profibrotic signal and the possibility that other susceptible organs may be affected. Livers from CD-1 mice exposed to EV for 6 mo were examined in a blinded fashion and consistently shown to have higher levels of fibrosis by collagen staining (Fig. 8, A and B).
Fig. 8.
Chronic inhalation of EV led to hepatic fibrosis in CD-1 mice exposed to EV for 6 mo. A: representative Masson’s trichrome photomicrographs of fixed hepatic tissue. B: quantitative analysis of EV relative to control determined that EV livers exposed to EV daily for 6 mo had 1.9-fold higher collagen deposition, relative to Air controls. Means ± SE are shown; n = 6 per group. ****P < 0.0001.
DISCUSSION
E-cigs are considered by many to be safer than conventional cigarettes. While this is most likely the case in terms of carcinogenesis (81), conclusions as to their general safety have yet to be made. We present here, for the first time, evidence that chronic e-cig use negatively impacts multiple organs in mammals of different genetic backgrounds. Daily inhalation of EV made from PG, Gly, and 24 mg/ml nicotine for 3 or 6 mo led to fibrosis in heart, kidney, and liver tissues, with concomitant changes in cardiac and renal function. In vitro data suggest that toxic components of EV may be disrupting airway epithelium, triggering cells to secrete profibrotic proteins into circulating blood, leading to damage to multiple organs. Although all three organs affected in our studies had a similar pattern of fibrosis, gene expression changes were not identical. Kidney parenchyma had elevations in Col1a1 and Col3a1 at 1 mo, while cardiac tissue only had elevation of Col3a1. The regulation of these two collagens in the myocardium is known to be via complex and diverse mechanisms (8, 57). Different organ systems are known to respond to stress and inflammation in different ways and over different time frames (24). Myofibroblasts arise from stromal cells within each organ and are the primary sources of extracellular matrix protein production. Because myofibroblasts have organ-environment-based differences in gene expression, which leads to functional heterogeneity, there are differences in the types of collagen deposited, and the timing thereof, for myofibroblasts from cardiac, renal and hepatic tissues. Although extensive research has been done on fibrosis, there is still much unknown about the multitude of cellular and molecular pathways in fibrosis induction, progression, and termination.
Increased levels of circulating inflammatory proteins due to chronic EV exposure.
To determine potential pathways through which multiorgan fibrosis is incurred, inflammatory protein profiles were examined using plasma from two mouse strains (C57BL/6: individual samples; CD-1: pooled samples). We report here only proteins that were different by 20% or more in EV mice relative to Air controls, as changes of that magnitude have a greater likelihood of potential biological effects. We focused on changes in circulating inflammatory proteins that occurred in both C57BL/6 and CD-1 strains, as activation of inflammatory pathways in disparate genetic backgrounds is more likely to be associated with the downstream organ damage and fibrosis that was found in our models.
Leukemia inhibitory factor (LIF) is a member of the IL-6 cytokine family and is commonly systemically elevated in the setting of inflammation (62). LIF was elevated in the circulation of EV mice, as compared with Air, with a 30.6-fold increase in C57BL/6 mice and a 6.3-fold increase in CD-1 mice (Table 1). LIF is involved with regulation of cell differentiation, proliferation, and survival, via activation of both the JAK/STAT3 and MAPK pathways, which increase the ability of tumor cells to invade. More importantly, LIF is produced by pulmonary cells, including epithelial, smooth muscle, and innate immune cells, in response to stressful stimuli, including inhalation of air pollution and endotoxin (47), and is thought to confer protection (78), even in the setting of acute respiratory distress syndrome. Elevations of circulating LIF in our models suggest that inhalation of EV induces stress on pulmonary cells, leading to production and release of LIF as a protective response. Increased LIF also suggests increased autophagy, which may be an adaptive response to stress or lead to cell death, and LIF is known to increase EGF expression, which experienced a 24.6-fold increase in the plasma of C57BL/6 EV mice, and a 2.1-fold increase in the plasma of CD-1 EV mice (Table 1). Elevated LIF and EGF suggest that EV mice may have systemic increases in cell-proliferation signals.
Angiopoietin 1 (Ang-1) was increased in EV plasma from C57BL/6 and CD-1 mice, compared with experimental controls (27-fold and 1.38-fold higher in EV vs. Air, respectively). In human smokers, Ang-1 has been found to be elevated in the blood, before mild, moderate, or severe chronic obstructive pulmonary disease develops (45). This may be due to ongoing vascular remodeling secondary to damage by cigarette smoke inhalation. In a C57BL/6 mouse model of renal injury, Ang-1 was shown to be elevated in the setting of increased fibrosis (84). It has been demonstrated that increased production of Ang-1 by kidney cells can protect against further fibrosis (46, 70). In addition, other data show that the production of Ang-1 can decrease cardiac fibrosis in the setting of myocyte injury (15, 83). Thus increased release of Ang-1 may also indicate that defensive, protective mechanisms are triggered as a result of renal or cardiac fibrosis in EV mice. Further studies are needed to determine the tissues from which Ang-1 is originating during EV inhalation.
LIX was elevated 1.92-fold (92% increase) in C57BL/6, and 1.24-fold (24% increase) in CD-1 mice. CXCL5 (LIX) is secreted by alveolar epithelial type II cells, and plays a role in recruitment of neutrophils and macrophages into the lungs (4, 35). LIX is known to be elevated in the setting of chronic obstructive pulmonary disease, smoke exposure, and atherosclerosis (61). Thus the increase in LIX may contribute to an influx of immune cells into the lung parenchyma and suggests that further inhalation of EV may lead to disease.
Decreased levels of circulating inflammatory proteins due to chronic EV exposure.
MMP-3 has the capacity to degrade multiple components of the extracellular matrix. MMP-3 can degrade collagen III, IV, V, IX, elastin, laminin, and fibronectin and thus participates in clearance of fibrosis (tissue remodeling). MMPs in general are known to participate in numerous healing and pathologic processes, and changes in plasma levels have been correlated with disease progression and mortality. For example, MMP-3 is often elevated in the circulation of subjects with rheumatologic disease (44), diabetes (82), and cancer (38) but has been found to be lower in the setting of acute myocardial infarct, when activation of fibrosis helps stabilize infarcted cardiac tissue (64, 65). Increased levels of MMP-3 after acute MI are associated with increased adverse cardiac remodeling and death (41). Chronic inhalation of EV led to diminished circulating levels of MMP-3, with a 5.29-fold decrease in C57BL/6 mice, and an 8.42-fold decrease in CD-1 mice (Table 1). Studies have found that knocking out MMP-3 leads to increased tumor growth and metastases, with reduced tumor infiltration of innate immune cells (55). Thus diminished circulating MMP-3 in EV mice may indicate that organ injury due to chronic EV inhalation is occurring and is leading to activation of fibrosis pathways. However, these lower levels of MMP-3 may also be evidence of increased risk of carcinogenesis.
Chitinase 3-like 1 (YKL-40) was decreased 38.9-fold in C57BL/6 and 1.22-fold decreased in CD-1 mice. Chitinase 3-like 1 is also elevated in neutrophilic inflammation and is thought to be secreted more by proinflammatory macrophage phenotypes and less so by anti-inflammatory macrophages (50). One hypothesis of why YKL-40 is diminished in the setting of chronic EV inhalation is that the nicotine within the EV activated anti-inflammatory pathways (11), leading to a shift to anti-inflammatory monocytes in the circulation.
WNT1-inducible signaling pathway (WISP-1) is associated with pathological processes including inflammation, tissue repair, and cancer (26). WISP-1 was decreased 2.15-fold in C57BL/6, and 1.49-fold decreased in CD-1 mice. However, the significance of these changes is unclear, as elevated circulating WISP-1 has been associated with renal fibrosis (85), and in cancer studies, WISP-1 levels tend to be elevated as well (53, 80).
One of the limitations of our studies was the use of pooled plasma samples from CD-1 mice for the proteome array studies, as pooling of samples can mask biological variance. We focused on changes only found in both strains, to increase the likelihood of detecting changes associated with the organ fibrosis seen in both strains. Further studies will be needed to assess the biological importance of the differences found, and determine the mechanistic underpinnings.
Relevance of our data to human e-cig vapers.
These studies were done with commercially purchased e-cig batteries, tanks, and e-liquids, with puff topography for in vivo studies based off of current use patterns to best mimic human use. We included the most common ingredients found in e-liquids: PG, Gly, and nicotine. Thus, humans using the same or similar devices (Vape pens) and e-liquids could be at risk for the effects seen in our models. However, because of the wide variety of e-cig devices and e-liquids on the market, and the variability across batches, our findings from one brand may not be relevant to e-cig users (vapers) of other types of e-cigs, e-cigs from other batches or other sources, or even the same devices but at different resistance or voltage (10). E-cig researchers across the world are working to develop guidelines to increase consistency across studies and increase our ability to compare e-cig studies to one another (33).
Many investigators work primarily with male mice, as they tend to be more susceptible to organ damage. Therefore, the finding of multiorgan fibrosis in female mice could represent an important preclinical signal. Because there are many sex-related disparities in biomedical research, and in murine research in particular, the studies presented here will need to be replicated in male mice to determine whether the findings are relevant across sexes. C57BL/6 mice are the most commonly used strain for basic science research, and many cigarette smoke exposure studies have been completed in this emphysema-susceptible strain (6, 67). Outbred CD-1 mice are more genetically diverse and thus are a hardier strain. Because of their added genetic diversity, significant findings in CD-1 mice may be more relevant to human pathophysiology (Figs. 1A, 4, and 5). The fact that we found organ fibrosis in both, genetically disparate, strains of mice, suggests that our results may have a greater likelihood of translatability to humans.
The studies discussed here are limited in that they were done in mice, and there are many known disparities between murine and human inflammatory responses and disease pathology (66). Exposures were done for 1 h daily, which is a limited pattern of e-cig use compared with that of humans, who more commonly inhale EV for short periods of time throughout the day (20). Nonetheless, the fact that changes were observed with only once daily exposure suggests that even larger changes in the same parameters might accompany multiple daily dosing. Finally, this work was done with e-liquid containing all three of the ingredients found in most e-liquids, PG, Gly, and nicotine, and thus we cannot discriminate between them in terms of which may be driving the pathology seen. Further research is needed to evaluate the potential effects of each individual component.
Role of airway epithelium in systemic effects of e-cigs.
Airway cells, including bronchial epithelial cells, are the first line of defense and protect the host from toxic inhalants. Epithelial permeability is critical for tissue homeostasis (27). The use of e-cigs causes modulation of innate immune homeostasis and alters inflammatory cytokine expression. The decreased expression of tight junction protein ZO1 and increased permeability of bronchial epithelial cells can give components of EV access to the systemic circulation, by which they can interact with other tissues to generate fibrosis, as observed clearly here. In addition, the decreased barrier function may allow greater passage of external antigens and inhaled chemicals into the body, increasing inflammation both locally in the lungs and systemically. The further worsening of EV-exposed airway epithelial barrier function in the setting of infection suggests that vaping may allow easier entry for pathogens into the lung parenchyma and circulation. This may lead to increased rates of invasive bacterial infections in e-cig users. These studies were limited by the relatively acute ex vivo exposure over 3–5 days. Further studies are needed to determine whether tight junction and permeability changes persist in the chronic setting, in vivo.
Perspectives and Significance
Our findings of multiorgan dysfunction and fibrosis induced by regular inhalation of EV produced by vape pens illustrate the need to expand clinical, epidemiological, and basic science research studies to include possible effects on organ systems outside of the pulmonary system. Our findings of significant pathophysiologic affects caused by inhalation of nonflavored EV give credence to the belief that there are toxic effects of EV components, beyond those of flavorings alone. The data presented here highlight the need to devote more resources to study these increasingly popular nicotine delivery devices.
GRANTS
This work was funded by an American Heart Association Beginning Grant-in-Aid [16BGIA27790079; Principal Investigator (PI): L. Crotty Alexander], University of Alabama at Birmingham-University of California, San Diego O’Brien Center Daniel O’Connor Scholar Award [National Institutes of Health (NIH) Grant P30-DK079337; PI: L. Crotty Alexander], Veterans Affairs (VA) Biomedical Laboratory Research & Development Service Career Development Award (1IK2BX001313; PI; L. Crotty Alexander), American Thoracic Society Foundation Award (PI: Crotty Alexander), VA Merit Award (BX002175; PI: P. Singh), NIH Grants R01-DK-107852 and R03-DK-101841 (PI: P. Singh), R01-HL13705201 (PI: L. Crotty Alexander), R37-HL-028143 and P01-HL-080101 (PI: J. H. Brown), a F32-DK-104615 (to C. Drummond), and from the Tobacco-Related Disease Research Program Grant (Trdrp) 26IP-0033 (to E. C. Breen).
The funding sources had no role in experimental design, collection, analysis, or interpretation of the data, in the writing of the manuscript, or in the decision to submit the paper for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.E.C.A., S.D., J.T., and J.H.B. conceived and designed research; L.E.C.A., C.A.D., M.H., D.P.M., A.M., A.W., S.D., Z.Y., J.H.L., K.V., A.D., J.S., C.J., and E.C.B. performed experiments; L.E.C.A., C.A.D., M.H., D.P.M., A.M., A.W., S.D., Z.Y., J.H.L., K.V., A.D., J.S., C.J., J.T., and E.C.B. analyzed data; L.E.C.A., C.A.D., M.H., A.M., A.W., S.D., P.S., Z.Y., J.H.L., A.D., J.S., C.J., J.T., J.H.B., and E.C.B. interpreted results of experiments; L.E.C.A., C.A.D., A.W., P.S., A.D., C.J., and E.C.B. prepared figures; L.E.C.A. and C.A.D. drafted manuscript; L.E.C.A., C.A.D., M.H., A.M., S.D., P.S., A.D., J.T., J.H.B., and E.C.B. edited and revised manuscript; L.E.C.A., C.A.D., M.H., D.P.M., A.M., A.W., S.D., P.S., Z.Y., J.H.L., K.V., A.D., J.S., C.J., J.T., J.H.B., and E.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lauren Ma, Rita Al-kolla, Albert Tran, and Stefanie Ung for help and dedication in exposing mice daily. We also thank Miriam Scadeng, who contributed significant in vivo MRI imaging time and effort, and Atul Malhotra for support and mentorship.
REFERENCES
- 1.Alasmari F, Crotty Alexander LE, Nelson JA, Schiefer IT, Breen E, Drummond CA, Sari Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry 77: 1–8, 2017. doi: 10.1016/j.pnpbp.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737, 2004. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Apaijai N, Inthachai T, Lekawanvijit S, Chattipakorn SC, Chattipakorn N. Effects of dipeptidyl peptidase-4 inhibitor in insulin-resistant rats with myocardial infarction. J Endocrinol 229: 245–258, 2016. doi: 10.1530/JOE-16-0096. [DOI] [PubMed] [Google Scholar]
- 4.Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, Jeyaseelan S. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol 47: 104–111, 2012. doi: 10.1165/rcmb.2011-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balloy V, Varet H, Dillies MA, Proux C, Jagla B, Coppée JY, Tabary O, Corvol H, Chignard M, Guillot L. Normal and cystic fibrosis human bronchial epithelial cells infected with Pseudomonas aeruginosa exhibit distinct gene activation patterns. PLoS One 10: e0140979, 2015. doi: 10.1371/journal.pone.0140979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartalesi B, Cavarra E, Fineschi S, Lucattelli M, Lunghi B, Martorana PA, Lungarella G. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur Respir J 25: 15–22, 2005. doi: 10.1183/09031936.04.00067204. [DOI] [PubMed] [Google Scholar]
- 7.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One 10: e0117222, 2015. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop JE, Lindahl G. Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc Res 42: 27–44, 1999. doi: 10.1016/S0008-6363(99)00021-8. [DOI] [PubMed] [Google Scholar]
- 9.Bolinder G, Alfredsson L, Englund A, de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am J Public Health 84: 399–404, 1994. doi: 10.2105/AJPH.84.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control 23, Suppl 2: ii4–ii10, 2014. doi: 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Café-Mendes CC, Garay-Malpartida HM, Malta MB, de Sá Lima L, Scavone C, Ferreira ZS, Markus RP, Marcourakis T. Chronic nicotine treatment decreases LPS signaling through NF-κB and TLR-4 modulation in the hippocampus. Neurosci Lett 636: 218–224, 2017. doi: 10.1016/j.neulet.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 12.Caponnetto P, Campagna D, Papale G, Russo C, Polosa R. The emerging phenomenon of electronic cigarettes. Expert Rev Respir Med 6: 63–74, 2012. doi: 10.1586/ers.11.92. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Brighton LE, Collier AM, Bromberg PA. Correlative ultrastructural investigations of airway epithelium following experimental exposure to defined air pollutants and lifestyle exposure to tobacco smoke. Inhal Toxicol 25: 134–140, 2013. doi: 10.3109/08958378.2013.763314. [DOI] [PubMed] [Google Scholar]
- 14.Cavarra E, Bartalesi B, Lucattelli M, Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA, Lungarella G. Effects of cigarette smoke in mice with different levels of alpha(1)-proteinase inhibitor and sensitivity to oxidants. Am J Respir Crit Care Med 164: 886–890, 2001. doi: 10.1164/ajrccm.164.5.2010032. [DOI] [PubMed] [Google Scholar]
- 15.Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes 57: 3335–3343, 2008. doi: 10.2337/db08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark CA, Thomas LK, Azghani AO. Inhibition of protein kinase C attenuates Pseudomonas aeruginosa elastase-induced epithelial barrier disruption. Am J Respir Cell Mol Biol 45: 1263–1271, 2011. doi: 10.1165/rcmb.2010-0459OC. [DOI] [PubMed] [Google Scholar]
- 17.Crotty Alexander L, Fuster M, Montgrain P, Malhotra A. The need for more e-cigarette data: a call to action. Am J Respir Crit Care Med 192: 275–276, 2015. doi: 10.1164/rccm.201505-0915ED. [DOI] [PubMed] [Google Scholar]
- 18.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory diseases of the lung induced by conventional cigarette smoke: a review. Chest 148: 1307–1322, 2015. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 19.Crotty Alexander LE, Vyas A, Schraufnagel DE, Malhotra A. Electronic cigarettes: the new face of nicotine delivery and addiction. J Thorac Dis 7: E248–E251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawkins L, Turner J, Roberts A, Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction 108: 1115–1125, 2013. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 21.Drummond CA, Crotty Alexander LE, Haller ST, Fan X, Xie JX, Kennedy DJ, Liu J, Yan Y, Hernandez DA, Mathew DP, Cooper CJ, Shapiro JI, Tian J. Cigarette smoking causes epigenetic changes associated with cardiorenal fibrosis. Physiol Genomics 48: 950–960, 2016. doi: 10.1152/physiolgenomics.00070.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond CA, Hill MC, Shi H, Fan X, Xie JX, Haller ST, Kennedy DJ, Liu J, Garrett MR, Xie Z, Cooper CJ, Shapiro JI, Tian J. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol Genomics 48: 220–229, 2016. doi: 10.1152/physiolgenomics.00116.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond CA, Sayed M, Evans KL, Shi H, Wang X, Haller ST, Liu J, Cooper CJ, Xie Z, Shapiro JI, Tian J. Reduction of Na/K-ATPase affects cardiac remodeling and increases c-kit cell abundance in partial nephrectomized mice. Am J Physiol Heart Circ Physiol 306: H1631–H1643, 2014. doi: 10.1152/ajpheart.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eddy AA. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl 4: 2–8, 2014. doi: 10.1038/kisup.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend 160: 218–221, 2016. doi: 10.1016/j.drugalcdep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Feng M, Jia S. Dual effect of WISP-1 in diverse pathological processes. Chin J Cancer Res 28: 553–560, 2016. doi: 10.21147/j.issn.1000-9604.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers 1: e24997, 2013. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golli NE, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, Dallagi Y, El May MV, El Fazaa S. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol 77: 109–116, 2016. doi: 10.1016/j.yrtph.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 30.Gupta RK, Gupta R, Maheshwari VD, Mawliya M. Impact of smoking on microalbuminuria and urinary albumin creatinine ratio in non-diabetic normotensive smokers. Indian J Nephrol 24: 92–96, 2014. doi: 10.4103/0971-4065.127893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haller ST, Kennedy DJ, Shidyak A, Budny GV, Malhotra D, Fedorova OV, Shapiro JI, Bagrov AY. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am J Hypertens 25: 690–696, 2012. doi: 10.1038/ajh.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 94: 667–679, 2016. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 33.Iskandar AR, Gonzalez-Suarez I, Majeed S, Marescotti D, Sewer A, Xiang Y, Leroy P, Guedj E, Mathis C, Schaller JP, Vanscheeuwijck P, Frentzel S, Martin F, Ivanov NV, Peitsch MC, Hoeng J. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol Mech Methods 26: 389–413, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372: 392–394, 2015. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 35.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 32: 531–539, 2005. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JA, Stroud RE, O’Quinn EC, Black LE, Barth JL, Elefteriades JA, Bavaria JE, Gorman JH 3rd, Gorman RC, Spinale FG, Ikonomidis JS. Selective microRNA suppression in human thoracic aneurysms: relationship of miR-29a to aortic size and proteolytic induction. Circ Cardiovasc Genet 4: 605–613, 2011. doi: 10.1161/CIRCGENETICS.111.960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung GS, Jeon JH, Choe MS, Kim SW, Lee IK, Kim MK, Park KG. Renoprotective effect of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in streptozotocin-induced type 1 diabetic mice. Diabetes Metab J 40: 211–221, 2016. doi: 10.4093/dmj.2016.40.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung K, Nowak L, Lein M, Priem F, Schnorr D, Loening SA. Matrix metalloproteinases 1 and 3, tissue inhibitor of metalloproteinase-1 and the complex of metalloproteinase-1/tissue inhibitor in plasma of patients with prostate cancer. Int J Cancer 74: 220–223, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 4: 116–128, 2016. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 63: 2120–2131, 2014. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 41.Kelly D, Khan S, Cockerill G, Ng LL, Thompson M, Samani NJ, Squire IB. Circulating stromelysin-1 (MMP-3): a novel predictor of LV dysfunction, remodelling and all-cause mortality after acute myocardial infarction. Eur J Heart Fail 10: 133–139, 2008. doi: 10.1016/j.ejheart.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy DJ, Elkareh J, Shidyak A, Shapiro AP, Smaili S, Mutgi K, Gupta S, Tian J, Morgan E, Khouri S, Cooper CJ, Periyasamy SM, Xie Z, Malhotra D, Fedorova OV, Bagrov AY, Shapiro JI. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol 294: F450–F454, 2008. doi: 10.1152/ajprenal.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 47: 488–495, 2006. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 44.Keyszer G, Lambiri I, Nagel R, Keysser C, Keysser M, Gromnica-Ihle E, Franz J, Burmester GR, Jung K. Circulating levels of matrix metalloproteinases MMP-3 and MMP-1, tissue inhibitor of metalloproteinases 1 (TIMP-1), and MMP-1/TIMP-1 complex in rheumatic disease. Correlation with clinical activity of rheumatoid arthritis versus other surrogate markers. J Rheumatol 26: 251–258, 1999. [PubMed] [Google Scholar]
- 45.Kierszniewska-Stępień D, Pietras T, Ciebiada M, Górski P, Stępień H. Concentration of angiopoietins 1 and 2 and their receptor Tie-2 in peripheral blood in patients with chronic obstructive pulmonary disease. Postepy Dermatol Alergol 32: 443–448, 2015. doi: 10.5114/pdia.2014.44008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim W, Moon SO, Lee SY, Jang KY, Cho CH, Koh GY, Choi KS, Yoon KH, Sung MJ, Kim DH, Lee S, Kang KP, Park SK. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol 17: 2474–2483, 2006. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- 47.Knight DA, Lydell CP, Zhou D, Weir TD, Robert Schellenberg R, Bai TR. Leukemia inhibitory factor (LIF) and LIF receptor in human lung. Distribution and regulation of LIF release. Am J Respir Cell Mol Biol 20: 834–841, 1999. doi: 10.1165/ajrcmb.20.4.3429. [DOI] [PubMed] [Google Scholar]
- 48.Kriegel AJ, Liu Y, Cohen B, Usa K, Liu Y, Liang M. MiR-382 targeting of kallikrein 5 contributes to renal inner medullary interstitial fibrosis. Physiol Genomics 44: 259–267, 2012. doi: 10.1152/physiolgenomics.00173.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kruschinski C, Skripuletz T, Bedoui S, Tschernig T, Pabst R, Nassenstein C, Braun A, von Hörsten S. CD26 (dipeptidyl-peptidase IV)-dependent recruitment of T cells in a rat asthma model. Clin Exp Immunol 139: 17–24, 2005. doi: 10.1111/j.1365-2249.2005.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunz LI, van’t Wout EF, van Schadewijk A, Postma DS, Kerstjens HA, Sterk PJ, Hiemstra PS. Regulation of YKL-40 expression by corticosteroids: effect on proinflammatory macrophages in vitro and its modulation in COPD in vivo. Respir Res 16: 154, 2015. doi: 10.1186/s12931-015-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larcombe AN, Janka MA, Mullins BJ, Berry LJ, Bredin A, Franklin PJ. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am J Physiol Lung Cell Mol Physiol 313: L67–L79, 2017. doi: 10.1152/ajplung.00203.2016. [DOI] [PubMed] [Google Scholar]
- 52.Lever AR, Park H, Mulhern TJ, Jackson GR, Comolli JC, Borenstein JT, Hayden PJ, Prantil-Baun R. Comprehensive evaluation of poly(I:C) induced inflammatory response in an airway epithelial model. Physiol Rep 3: e12334, 2015. doi: 10.14814/phy2.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li FJ, Wang XJ, Zhou XL. WISP-1 overexpression upregulates cell proliferation in human salivary gland carcinomas via regulating MMP-2 expression. Onco Targets Ther 9: 6539–6548, 2016. doi: 10.2147/OTT.S107166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest 141: 1110–1113, 2012. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 55.McCawley LJ, Crawford HC, King LE Jr, Mudgett J, Matrisian LM. A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res 64: 6965–6972, 2004. doi: 10.1158/0008-5472.CAN-04-0910. [DOI] [PubMed] [Google Scholar]
- 56.Mercado C, Jaimes EA. Cigarette smoking as a risk factor for atherosclerosis and renal disease: novel pathogenic insights. Curr Hypertens Rep 9: 66–72, 2007. doi: 10.1007/s11906-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 88: 1141–1146, 1991. doi: 10.1172/JCI115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Negri E, Franzosi MG, La Vecchia C, Santoro L, Nobili A, Tognoni G; GISSI-EFRIM Investigators . Tar yield of cigarettes and risk of acute myocardial infarction. BMJ 306: 1567–1570, 1993. doi: 10.1136/bmj.306.6892.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivera DS, Boggs SE, Beenhouwer C, Aden J, Knall C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhal Toxicol 19: 13–22, 2007. doi: 10.1080/08958370600985768. [DOI] [PubMed] [Google Scholar]
- 60.Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol 292: H130–H139, 2007. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- 61.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 968–975, 2003. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 62.Ren SG, Seliktar J, Li X, Braunstein GD, Melmed S. Measurement of leukemia inhibitory factor in biological fluids by radioimmunoassay. J Clin Endocrinol Metab 83: 1275–1283, 1998. [DOI] [PubMed] [Google Scholar]
- 63.Righetti M, Sessa A. Cigarette smoking and kidney involvement. J Nephrol 14: 3–6, 2001. [PubMed] [Google Scholar]
- 64.Samnegård A, Silveira A, Lundman P, Boquist S, Odeberg J, Hulthe J, McPheat W, Tornvall P, Bergstrand L, Ericsson CG, Hamsten A, Eriksson P. Serum matrix metalloproteinase-3 concentration is influenced by MMP-3 -1612 5A/6A promoter genotype and associated with myocardial infarction. J Intern Med 258: 411–419, 2005. doi: 10.1111/j.1365-2796.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 65.Samnegård A, Silveira A, Tornvall P, Hamsten A, Ericsson CG, Eriksson P. Lower serum concentration of matrix metalloproteinase-3 in the acute stage of myocardial infarction. J Intern Med 259: 530–536, 2006. doi: 10.1111/j.1365-2796.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 66.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program . Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 163: 2329–2335, 2003. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, Salit J, Harvey BG, Crystal RG. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci 68: 877–892, 2011. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi S, Koya D, Kanasaki K. Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogenesis Tissue Repair 9: 1, 2016. doi: 10.1186/s13069-016-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh S, Manson SR, Lee H, Kim Y, Liu T, Guo Q, Geminiani JJ, Austin PF, Chen YM. Tubular overexpression of angiopoietin-1 attenuates renal fibrosis. PLoS One 11: e0158908, 2016. doi: 10.1371/journal.pone.0158908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skripuletz T, Schmiedl A, Schade J, Bedoui S, Glaab T, Pabst R, von Hörsten S, Stephan M. Dose-dependent recruitment of CD25+ and CD26+ T cells in a novel F344 rat model of asthma. Am J Physiol Lung Cell Mol Physiol 292: L1564–L1571, 2007. doi: 10.1152/ajplung.00273.2006. [DOI] [PubMed] [Google Scholar]
- 72.Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol 50: 9644–9651, 2016. doi: 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- 73.Steinbrecher A, Reinhold D, Quigley L, Gado A, Tresser N, Izikson L, Born I, Faust J, Neubert K, Martin R, Ansorge S, Brocke S. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J Immunol 166: 2041–2048, 2001. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- 74.Sukhija R, Bursac Z, Kakar P, Fink L, Fort C, Satwani S, Aronow WS, Bansal D, Mehta JL. Effect of statins on the development of renal dysfunction. Am J Cardiol 101: 975–979, 2008. doi: 10.1016/j.amjcard.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 75.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10: e0116861, 2015. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med 47: 15–17, 2014. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 77.Uchiyama S, Senoo Y, Hayashida H, Inaba Y, Nakagome H, Kunugita N. Determination of chemical compounds generated from second-generation e-cigarettes using a sorbent cartridge followed by a two-step elution method. Anal Sci 32: 549–555, 2016. doi: 10.2116/analsci.32.549. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Chen Q, Corne J, Zhu Z, Lee CG, Bhandari V, Homer RJ, Elias JA. Pulmonary expression of leukemia inhibitory factor induces B cell hyperplasia and confers protection in hyperoxia. J Biol Chem 278: 31226–31232, 2003. doi: 10.1074/jbc.M301820200. [DOI] [PubMed] [Google Scholar]
- 79.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 9: e108342, 2014. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang JY, Yang MW, Huo YM, Liu W, Liu DJ, Li J, Zhang JF, Hua R, Sun YW. High expression of WISP-1 correlates with poor prognosis in pancreatic ductal adenocarcinoma. Am J Transl Res 7: 1621–1628, 2015. [PMC free article] [PubMed] [Google Scholar]
- 81.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE, Brumund KT, Wang-Rodriguez J, Ongkeko WM. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol 52: 58–65, 2016. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zákovičová E, Charvat J, Kukacka J, Chlumsky J, Svab P, Kvapil M. Circulating serum matrix metalloproteinase-3 and metalloproteinase-9 are not associated with echocardiographic parameters of diastolic function in asymptomatic type 2 diabetic patients. J Int Med Res 38: 2093–2099, 2010. doi: 10.1177/147323001003800625. [DOI] [PubMed] [Google Scholar]
- 83.Zeng H, Li L, Chen JX. Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardial apoptosis in db/db mouse infarcted hearts. PLoS One 7: e35905, 2012. doi: 10.1371/journal.pone.0035905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci 91: 199–206, 2012. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong X, Tu YJ, Li Y, Zhang P, Wang W, Chen SS, Li L, Chung AC, Lan HY, Chen HY, Li GS, Wang L. Serum levels of WNT1-inducible signaling pathway protein-1 (WISP-1): a noninvasive biomarker of renal fibrosis in subjects with chronic kidney disease. Am J Transl Res 9: 2920–2932, 2017. [PMC free article] [PubMed] [Google Scholar]
- 86.Zhuge F, Ni Y, Nagashimada M, Nagata N, Xu L, Mukaida N, Kaneko S, Ota T. DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes 65: 2966–2979, 2016. doi: 10.2337/db16-0317. [DOI] [PubMed] [Google Scholar]