Abstract
Cutaneous melanoma is a complex disorder characterized by an elevated degree of heterogeneity, features that place it among the most aggressive types of cancer. Although significant progress was recorded in both the understanding of melanoma biology and genetics, and in therapeutic approaches, this malignancy still represents a major problem worldwide due to its high incidence and the lack of a curative treatment for advanced stages. This review offers a survey of the most recent information available regarding the melanoma epidemiology, etiology, and genetic profile. Also discussed was the topic of cutaneous melanoma murine models outlining the role of these models in understanding the molecular pathways involved in melanoma initiation, progression, and metastasis.
Keywords: cutaneous melanoma, incidence, etiology, animal models, genetic profile
1. Introduction
Melanoma, the malignancy that is referred to as “the cancer that rises with the Sun” [1], originates from melanocytes that switched to cancerous cells as a consequence of aberrant changes at molecular and biochemical levels [2]. Albeit melanoma adds up the smallest number of skin cancer cases (<10%) [3], its aggressiveness and its high mortality rate make it the deadliest type of skin cancer. In the last four to five decades, a constant increase of melanoma incidence was recorded, placing this malignant disorder on the 19th position worldwide among the most common cancer types [1], whereas when referring to individual countries, the highest incidence was recorded in Australia (fourth place) [4], New Zealand, United States (Northern Region), and European countries (Northern and Western regions), and a lower incidence in South-Eastern Asia and South-Central Asia [5,6].
Melanoma is characterized by an extensive degree of heterogeneity in terms of clinical, dermatological, and histopathological presentation [4], genomic profile [7,8], and risk factors (skin type, exposure to sun radiation, number of nevi, age, gender, immune status, family history or former removed melanomas) [9], which awards this disorder as a significant public health issue and an important matter of concern for the scientists in the field of cancer. The impressive number of results achieved at a simple search in PubMed database for “melanoma” (over 8800 papers were published since 1 January 2017 up to present, whereas all-time publications exceeded 119,000 articles) witnesses the magnitude of this concern.
This paper offers a concise overview of the latest data concerning the epidemiology, etiology, genetic profile, and the state of the art of murine models of cutaneous melanoma, highlighting the contribution of these models in understanding the molecular mechanisms of melanoma.
2. Epidemiology and Etiology of Cutaneous Melanoma
Early stage diagnosed melanoma that was surgically removed is considered a curable pathology with a good prognosis, whereas the treatment options for advanced/metastatic melanoma remain poor [4]. On this basis, many efforts were done worldwide in the last decades for the prevention and early diagnosis of melanoma, as follows: campaigns to reduce hazardous sun exposure, sun protection policies [10,11], national primary prevention efforts in the 1990s and the SunSmart Campaign—2003 (in United Kingdom), primary prevention messages communicated by the national cancer societies and radiation safety agencies (in Norway and Sweden), mass-media campaigns in the early 1980s (in Australia and New Zealand) [11], collection of the melanoma data in population-based European cancer registries [12], Euromelanoma campaign and EUROCARE 5 [13], and web platforms—The Virtual Melanoma Cell—used to enable the disease-specific mining of high-throughput data [14]. Moreover, in order to improve the early detection of melanoma (which is associated with a reduced mortality), a novel approach was suggested: the use of smartphones apps and the store-and-forward teledermatology via a smartphone [15]. Another tailored prevention form consisted of the development of a telephone communication protocol for disclosing melanoma genomic risk information to the asymptomatic general population after a sample of their saliva was tested for melanoma [16].
Given the estimates of GLOBOCAN and WHO concerning the future burden of melanoma, a high interest was assigned to the trends of melanoma incidence and mortality rates taking into consideration parameters as age, gender, geographical localization, ultraviolet (UV) exposure, and tumor thickness and invasiveness, which are topics described in numerous epidemiological studies and meta-analyses [11,12,13,17,18,19]. In a study that included the data collected from 11 countries (Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Malta, Romania, Serbia, Slovakia, Slovenia, and Turkey) from South-Eastern Europe, it was analyzed the age-specific incidence and mortality trends of melanoma and were drawn the following conclusions: (i) the incidence rates augmented uniformly over the 2000–2010 period, but at a lower extent as compared to North-Western Europe; (ii) the incidence was higher in men as compared to women at middle (50–69 years) and older (70+ years) ages in most countries; and (iii) the mortality trends were less favorable than in North-Western Europe [13]. Whiteman and collaborators analyzed recent trends and estimated future melanoma incidence (a projection until 2031) in six populations with European heritage in terms of UV exposure patterns and different approaches to melanoma control and found an increasing trend of melanoma incidence in United States white population, in United Kingdom, Swedish, and Norwegian populations, a steady incidence in New Zealand, and a decreasing one in Australia [11]. A very recent article investigated melanoma incidence and mortality in 13 European countries (during 1995–2012 period) by country, age, sex, and Breslow thickness, and the results showed that the incidence of invasive melanoma continues to increase, mainly due to thin lesions, with the greatest increase in the Netherlands, the mortality trend being also an ascending one in most countries [12].
In spite of all the preventive measures, informative campaigns for early detection of melanoma and novel targeted therapies, the incidence of melanoma still keeps an ascending trend worldwide, and the causal factors of melanomagenesis remain under debate.
Cutaneous melanoma, which arises from epidermal melanocytes, is described as a disorder of people that present a fair-skinned phenotype, a family history of melanoma, and erratic genetic risk factors [20]. The melanocytes genitors are considered to be the neural crest progenitor cells that differentiate during embryonic development via “dorsolateral” pathway into melanoblasts which migrate to dermis where the differentiation process results in melanocytes that will further migrate to the epidermis [2]. Melanocytes are responsible for pigment production—melanins (black eumelanin and pheomelanin) that play key roles in offering protection against DNA damage induced by UV radiation [21], thus recent studies assert the duality of these molecules (mainly for pheomelanin) in melanomagenesis, the photodamage exceeding the photoprotection [22,23,24]. The synthesis of melanin (melanogenesis) is regulated by a multitude of agents (including hormones) that interact via pathways triggered by receptor-dependent and independent mechanisms, in hormonal, auto-, para-, or intracrine manner (the positive regulators of melanogenesis: MC1 receptor, melanocortins, ACTH—adrenocorticotropic hormone, l-tyrosine and l-dihydroxyphenylalanine—l-DOPA) [25,26]. Moreover, it was proven that melanogenesis influences melanoma behavior [27] and its response to therapy [28,29], and it also exerts an immunosuppressive effect [30,31]. The regulation of melanin transport following ultraviolet exposure together with the control of melanocytes growth and melanin synthesis is accomplished by keratinocytes. Once the keratinocytes lose control over melanocytes, the latter start to grow in an uncontrolled manner and acquire the ability to migrate out of skin, processes that indicate the development of melanoma [32].
The role of ultraviolet (UV) radiation in the melanoma occurrence is not fully elucidated and seems to be complex (since only a reduced number of UV signature mutations were detected in melanoma patients as compared to the ones diagnosed with non-melanoma skin cancers), but it was stated that intermittent UV exposure is linked to melanoma development [18]. In the case of cutaneous melanoma development, the risk is higher after a number of episodes of intense UV exposure (sunburn) during childhood (before the age of 10), as compared to the other skin malignancies when the risk of development is correlated to lifetime exposure to UV radiation [33]. Genomic and next generation sequencing studies attested the role of UV radiation as the main mutagen in cutaneous melanoma. Moreover, it was demonstrated that in invasive melanoma the number of UV-induced mutations is higher than the number of nevi (being included here also the matched precursor nevi), and the consequences are increased somatic mutation burdens [33,34].
Other genetic and phenotype factors were discussed to be involved in melanoma development, like: gender, age, skin type, number of nevi (>50 moles—high risk), family history, immune status, etc. [9,21]. There were also reported gender disparities concerning the incidence of melanoma in women versus men (with an increasing trend in men), referring to physiologic differences in skin structure, baseline differences in immune systems, the influence of sex hormone levels, and estrogen receptor expression [18,35].
Cutaneous melanoma was described as one of the most immunogenic cancers with heterogeneous histological and clinical features, and a significant number of mutations, which explains the low rate of tumor regression, multi-drug resistance to targeted therapies, and reduced survival rate [21,36]. The aggressiveness of melanoma could be explained by the ability of melanoma cells to escape apoptosis by overexpressing the apoptosis-inhibitory genes (as survivin and other inhibitory apoptosis proteins—IAPs) or by reducing the apoptosis-stimulatory genes expressions what leads to apoptosis failure and an augmented risk of metastasis [37]. In order to have a clear picture of melanoma initiation, progression, and metastasis, it is imperative to have the basic knowledge about these processes stages and the underlying mechanisms involved.
The development of melanoma occurs in a stepwise manner from benign nevus to invasive melanoma, a model proposed by Wallace Clark and collaborators that includes six steps very well defined in clinical and histopathological terms [33,38]. The starting point is a benign nevus composed of a clonal population of melanocytes that were aberrantly transformed into a hyperplastic lesion which will not advance due to cellular senescence. The following step is represented by the radial growth phase (RGP) that consists of nevus transformation into a dysplastic lesion that will evolve to a superficial spreading stage, confined to epidermis with low invasive potential. In the final step, also known as the vertical growth phase, the cells from the radial growth phase acquire the capacity to invade the dermis and metastasize [39,40]. Another aspect that should be kept in mind is the tumor vascularization—the supplier of the nutrients which, until the tumor reaches the size of 2–3 mm, occurs naturally by passive diffusion. After the tumor becomes bigger than 2–3 mm, the angiogenesis process, the new blood vessel formation, is initiated in order to sustain the needs of the melanoma cells. Once the tumor is amply vascularized, the mass of the tumor augments [36]. Some studies assert the idea that melanoma development is accompanied by an epithelial-to-mesenchymal (EMT) switch characterized by the melanocytes loss of E-cadherin expression and acquisition of some mesenchymal markers as SNAIL (transcription factor of zinc-finger family), SLUG (transcriptional repressor of E-cadherin), TWIST (Twist-related protein 1), and ZEB1 (Zinc finger E-box binding homeobox 1 transcription factor) [32,41].
A number of histological subtypes was described for cutaneous melanoma, like: superficial spreading melanoma, nodular melanoma, polypoid melanoma, acral lentiginous melanoma, lentigo maligna melanoma, and some uncommon forms: desmoplastic melanoma, nevoid melanoma, amelanotic melanoma, and verrucous melanoma [4,7,21]. The variability of melanoma in both clinical presentation and dermatoscopical features, and sometimes the lack of these specific features, becomes a challenge in establishing the diagnosis and even brought it the name of “the great imitator” [4].
When it comes to predict the outcome of primary tumors, it is applied the 2009 American Joint Committee on Cancer (AJCC) Melanoma Staging and Classification System that evaluates: tumor thickness, ulceration, mitotic figures, and microscopic satellites [42,43]. The number of mitoses (established by histopathological measurements) is an important prognostic factor for thin melanomas (Breslow thickness < 0.75 mm) and was included in the 7th classification of AJCC. The dermoscopic features like black color and peripheral streaks are positively correlated with thin melanomas with mitoses, while brown color and atypical pigment network are associated with a less aggressive phenotype [44]. According to the National Comprehensive Cancer Network guidelines, the patients who have a Breslow index of 0.76–1.0 mm with no ulceration or mitotic rate are not subjected for sentinel lymph node biopsy. Based on the 2009 AJCC staging system, mitotic rate higher than or equal to 1 mitosis/mm2 was correlated with poor disease-specific survival, especially in patients with a melanoma thickness less than or equal to 1.0 mm thick [45,46].
3. Cutaneous Melanoma Genetic Profile
One of the features that places melanoma in the top of the most aggressive type of cancer is represented by its heterogeneous nature. The intratumor and intertumor heterogeneity in melanoma were explained very comprehensively in an excellent recent review [45]. According to Grzywa et al, the intratumor heterogeneity is characterized by genomic instability (having as result the acquisition of common mutations—which occur early in tumor evolution and are found in all regions, of shared/branch mutations—that occur later and were detected only in some regions, and of private mutations—that occur in tumor progression phase, present in a single compartment), genomic and epigenomic alterations (having as consequence heterogeneous genes expression), and epigenetic dysregulation [45]. The complexity of melanoma is also conferred by the myriad of signaling pathways involved in melanoma development, pathways that coincide with the ones required for melanocytes development, like: Notch, Wnt, endothelins, SOX (sex-determining region Y–like–SRY high-mobility group—HMG box) proteins, mitogen-activated protein kinase (MAPK) signaling pathway, phosphatidylinositol-3-kinase (PI3K) pathway, G-protein-coupled receptor (GPCR) family, and epithelial-to-mesenchymal transition [40,46].
A considerable progress was registered in the genomic field of melanoma in recent years, a major role being played by the novel techniques like: next-generation sequencing and large-scale expression analyses of tumors which offer a landscape of the mutations existent in melanoma, the mutation rate in melanoma exceeding all the other cancers rates (the number of mutations per Mb ranged from 0.1 to 100 with an average value of 16.8 mutations/Mb according to The Cancer Genome Atlas (TCGA) data) [45,47].
The molecular pathways and the genes involved in melanomagenesis were discussed extensively in retrospective [2,39,40,48,49] and recent studies [26,45,50,51,52,53], this being the reason why the present review will discuss only the genes involved in the development of cutaneous melanoma in a concise manner.
The multitude mutations discovered to be engaged in melanoma increases the difficulty in identifying which are the “driver” (causative) mutations and the “passenger” (bystander) mutations [47,53]. A very detailed description of the genes known to be altered in melanoma, together with their impact in melanomagenesis and their potential to become targets for therapy, was compiled by Shtivelman and coauthors [47]. Based on the results of a whole-genome sequencing analysis, the genes susceptible to mutations in cutaneous melanoma are: BRAF, cyclin-dependent kinase N2A (CDKN2A), NRAS, and TP53 [53].
BRAF is a serine-threonine kinase involved in cell proliferation that triggers MAPK (mitogen-activated protein kinase) signaling pathway after its activation by RAS family of proteins. MAPK pathway controls important cellular processes, as: cell cycle progression, differentiation, and upregulation of transcription, and the existence of BRAF mutations will determine impairment of these processes, the end-point being oncogenesis [8,50,52]. BRAF mutations are very common in cutaneous melanoma and trigger MAPK pathway activation (60% of the cutaneous melanomas exhibit MAPK activating mutations), whereas in other types of melanoma, such as acral, mucosal, conjunctival, and uveal, its incidence is quite low [50,53].
Valine-to-glutamic acid substitution at codon 600 (BRAF (V600E)) is the most prevalent mutation in melanoma (detected in approximately 50% of melanomas) and might be the repercussion of a secondary effect of UV damage, like a nonclassic DNA mutation induced by UV radiation or the synthesis of reactive oxygen species [7,53]. This mutation was reported in melanomas and melanocytic nevi, leading to activation of RAS/RAF/MEK/ERK pathway, a key player in the initiation of melanocytic tumors [7,8]. A novel BRAF mutation (an aminoacidic insertion in codon 599) was identified in a melanoma patient in the P-loop activating site, mutation that was not discovered before in melanoma, but was detected rarely in Papillary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma, which highlights the heterogeneity of this disease [54].
NRAS mutations represent the second most frequent cause of altered signaling via MAPK pathway. This type of mutations was identified in 15–30% of melanomas and were found at codon 12, 13, or 61. Of note, NRAS and BRAF mutations are mutually exclusive, the presence of co-mutations was rarely observed, and in order to trigger malignant transformation, additional mutations are required, such as loss of tumor suppressors p16INK4A (Cyclin-dependent kinase inhibitor 2A) or PTEN (phosphatase and tensin homolog protein) [51,52]. The consequences of activated BRAF or NRAS mutations consist of aberrant cell growth, followed by premature growth arrest via oncogene-induced senescence, the resulted lesions remain benign and do not switch to malignancy in the absence of other mutations [55].
NF1 protein, also known as neurofibromin 1, is considered a “driver” mutation in a subset of melanoma. NF1 mutations were associated with initiation of melanoma and are prevalent in chronically sun-exposed skin. In addition, regulates negatively RAS family leading to RAF inhibitor resistance. To note, NF1 mutations or suppression might appear in parallel to BRAF mutations [51,52,53,54].
The cyclin-dependent kinase inhibitor 2A gene (CDKN2A) is the familial melanoma locus (located on the short arm of chromosome 9) that controls two tumor suppressor proteins (p14-ARF and p16-INK4A) with major roles in cell proliferation and senescence [7,51]. CDKN2A mutations were reported in approximatively 15% of familial melanomas, the somatic defects happened as a result of an impaired or loss of function (mutations, homozygous deletions or DNA methylation-induced epigenetic silencing) and are correlated with an invasive potential. These mutations are prevalent in melanomas (in 90%) and in dysplastic nevi (10%), and are not expressed in common melanocytic nevi [2,51,53,55].
4. Murine Models of Melanoma
The information acquired to offer a complete picture of melanoma etiology and progression was relied on melanoma models. According to Herlyn and Fukunaga-Kalabis, in 2010, it was estimated a number of 5000 cell lines developed by different laboratories, and over 200 of these melanoma cell lines were characterized in terms of genetic aberrations, gene expressions patterns, and biological properties (in vitro invasion ability, tumor development, and metastasis in immunodeficient mice) [56]. Despite the considerable amount of data that was provided by the use of melanoma cell lines, these models present several limitations, such as: a different behavior of cells in culture conditions as the ones in a patient’s body, the interactions with the tumor environment cannot be recreated in vitro, and each melanoma cell behaves as a stem cell due to its capacity of self-renewal and to develop tumors [56]. Other approach designed to overcome the limitations of the in vitro melanoma models and of the xenograft mouse models (differences between human and murine skin architecture, disparities in histopathological features, incapacity to recreate the initial events involved in the early invasion through the basement membrane) was a fully humanized 3D skin equivalent to early melanoma invasion model [57]. The number of relevant animal melanoma models available in the literature in the last decades have hampered the paucity concerning the cellular processes involved in melanoma initiation, progression, and metastasis, and also the key genes related to tumorigenesis [20]. An ideal model of melanoma would be considered the model that recreates human disease, having an UV-based etiology, the histopathological features of cutaneous melanoma and its molecular genetic fingerprint, and can be subjected to genetic and immunologic manipulation [58]. Although there were obtained multiple animal models of melanoma using large animals (horses, dogs (reviewed in [20]), Sinclair swine [21,59]) and small animals (zebrafish [60,61], opossum—Monodelphis domestica—[58], gerbils and hamsters [62,63,64], and mice [2,20,21,39,50,58,65]), the ideal melanoma model that accomplishes all the requirements mentioned above was not developed yet.
Experimental animal models also proved to be reliable sources of data concerning (i) the screening for novel antimelanoma agents: temozolomide (B16F10 metastatic melanoma model) [66,67], thymoquinone (B16F10 intracerebral melanoma model using C57BL/6J mice as host) [68], oncolytic herpes simplex virus HF10, and dacarbazine combined therapy (DBA/2 mice subcutaneously inoculated with clone M3 mouse melanoma cells) [69], gliotoxin (a xenograft mouse model using athymic mice) [70], recombinant methioninase [71], cancer vaccines [72], natural compounds [73,74]; (ii) molecular discovery—the comprehension of melanoma metastatic pathway involving microvascular environment [75]; and (iii) in vivo tracing of melanocytic lineage cells [76].
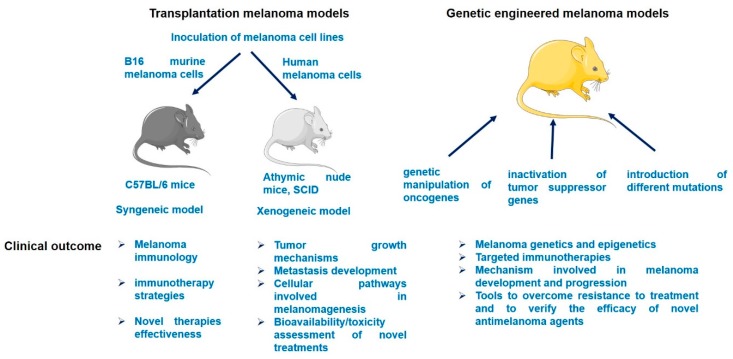
Due to the considerable number of published melanoma models, in this review only the murine melanoma models will be briefly discussed: xenograft mouse models, genetic engineered models (GEM), and UV-induced models (Figure 1).
Figure 1.
A schematic overview of murine melanoma models and their clinical outcomes.
4.1. Xenograft Models
Mouse models applied for melanoma study have a long history [77] and exhibit several advantages compared to other animal models (fish, opossum, horse, dogs, pigs), such as: relevant known data concerning the genetic background which allows the possibility of genetic manipulation, easy breeding and handling, multiple studies about molecular pathways dissection, and representing appropriate hosts for patient-derived xenografts (PDXs) to develop semblable human disease and to establish personalized antimelanoma therapies [21,78].
The xenograft melanoma mouse models were obtained by inoculation of different melanoma cell lines into immunocompromised mice (summarized in Table 1) [50,79].
Table 1.
Several examples of xenotransplanted (xenogeneic and syngeneic) melanoma mouse models.
| Model Type | Cell Line Inoculated | Strain of Mice | Type of Melanoma Developed | Clinical Outcome | References |
|---|---|---|---|---|---|
| Xenogeneic | MV3—melanoma cell line derived from transplanted fragments of a fresh human melanoma metastasis subcutaneously (s.c.) into a nude mouse | Nude mice | Metastatic melanoma in lungs | Useful tool to evaluate the antimetastatic potential of different agents | [85] |
| Xenogeneic | A375—human melanoma cell line | Balb/c nude mice | Cutaneous melanoma | To assess the metastatic potential of the cells in vivo and as further tool for testing novel melanoma agents | [81] |
| Xenogeneic | A375 human melanoma cells (s.c. inoculum) | NOD/SCID mice | Cutaneous melanoma | To test the efficiency of the oncolytic virus VSV-GP against metastatic melanoma | [86] |
| Xenogeneic | 518A2 melanoma cells with BRAF V600E mutation and CDKN2A exon 2 deletion (s.c. inoculum) | Athymic nude mice (Harlan Winkelmann, Germany) | Cutaneous melanoma | To elucidate the mechanism of action of gliotoxin, an inhibitor of canonical NOTCH2/CSL transactivation (a signaling pathway detected in multiple human neoplasms) | [70] |
| Xenogeneic | A375 human melanoma cells (s.c. inoculum) | Athymic nude mice | Cutaneous melanoma | To evaluate itraconazole as possible inhibitor in melanoma and to establish its mechanism of action | [87] |
| Xenogeneic | UACC 903 and 1205 Lu melanoma cells | Athymic-Foxn1nu nude mice | Melanoma | To verify the efficacy/toxicity of a combination of drugs (Celecoxib and Plumbagin) formulated as nano-delivery system against melanoma | [88] |
| Syngeneic | Harding-Passey melanoma cells | Balb/c and DBA/2F1 mice | Intracranial tumors | To study or modulate immune responses—Th2 response | [79,80] |
| Syngeneic | Cloudman S91 melanoma | DBA/2 mice | Melanoma | To evaluate the effectiveness of novel anticancer therapies and drug delivery platforms | [79,80] |
| Syngeneic | B16 melanoma cell lines | C57BL/6 mice | Melanoma | To produce tumor line variants with organs preferences and to test the efficacy of immunotherapy (cytokines, vaccines) | [79,80,89,90] |
| Syngeneic | B164A5 melanoma cell line (s.c. inoculated) | C57BL/6J mice | Melanoma | To assess the antimelanoma effects of betulinic acid, a natural compound | [73] |
| Syngeneic | B164A5 melanoma cell line intraperitoneally (i.p. inoculated) | C57BL/6 | Metastatic melanoma | To gather data regarding tumor progression and metastasis | [83] |
| Syngeneic | B16-OVA melanoma cells (s.c. inoculum) | C57BL/6J | Cutaneous melanoma | To test the efficiency of the oncolytic virus VSV-GP against melanoma | [86] |
| Syngeneic | B16-OVA melanoma cells intravenously (i.v. injection of cells suspension) | C57BL/6J | Lung metastatic melanoma | To test the efficiency of the oncolytic virus VSV-GP against metastatic melanoma | [86] |
| Syngeneic | B164A5, B16F10, B16GMCSF, B16FLT3 melanoma cells | C57Bl/6J | Metastatic melanoma | To verify the metastatic potential of the cells | [84] |
| Syngeneic | B16.OVA melanoma cells (s.c. inoculum) | C57BL/6J | Cutaneous melanoma | To check the antitumor potential of dasatinib, a specific BCR/ABL and SRC-family tyrosine kinase inhibitor | [91] |
The xenograft models generated in immunocompromised mice by injection of human melanoma cells inoculum subcutaneously, exhibit a pathology semblable to human disease (after inoculation melanocytes proliferate and metastasize via lymphatic vessels and blood) and are frequently used to acquire data concerning the tumor growth mechanisms, the main cellular pathways involved in tumorigenesis, the bioavailability/toxicity associated to novel treatments [50].
This type of models proved to be successful in imitating the advanced metastatic melanoma, but was also employed to critically assess the behavior of melanoma cells per se, in terms of invasiveness, metastatic potential, and the role of stem cells [79]. The first immunocompromised mouse model built was an athymic nude/nude mouse in 1969, which allowed the growth of solid human tumors [80], followed by the CB17-SCID mice (which possessed natural killer (NK) cells and supported the xenograft of the human cells, but the tumor growth was limited) and NK-deficient NOD (Non-Obese Diabetic)/SCID (Severe Combined Immunodeficient) mice (which accepted the growth of most of the melanoma cells inoculated) [79].
In one of our recent studies, was proved that Balb/c athymic mice represent an eligible host for A375 achromic/amelanotic human melanoma cells, the primary tumors became well-defined at day 20 post-inoculation. Furthermore, lung metastases were detected at day 30 post-inoculation while by monitoring the survival time, the longer the survival, the lower the number of metastasis was recorded (an increased number of mast cells around the tumor was notified) [81]. As presented in the case of syngeneic models, there were some limitations observed for these models, like: the cultured melanoma cells lines are purified and show some differences compared to parent cells; during culture the cells might lose some metastatic promoting markers and the clinical outcome might be irrelevant [50].
4.2. Syngeneic Models
Syngeneic mouse models were obtained by inoculation of melanoma cells into mice that present the same genetic background (examples resumed in Table 1) [43]. The mice used for syngeneic allograft models are immunocompetent and these models are preferred to gather insights into the melanoma microenvironment by allowing inherent interactions between melanocytes and the immune cells [50].
One of the first syngeneic melanoma models was realized by Fidler and Kripke [77], which generated individual sublines of B16 mouse melanoma, the suspension of cells being thereafter administered intravenously to syngeneic C57BL/6 mice to verify their ability to form secondary tumors in lungs [38]. The B16 sublines remained the most common used cell lines for syngeneic transplantation, two of them, B16F1 (low metastatic potential—used for primary tumors development) and B16F10 (high metastatic potential—lung metastases) being well-established sub-clones and reliable platforms of data about melanoma immunology and immunotherapy strategies [50].
Another B16 subline described as a useful tool for in vivo melanoma models is B16 melanoma 4A5, a line derived from a cutaneous melanoma aroused in C57BL/6 mice that presents fibroblastic-like shape and ability to produce melanin, a feature that ceases after many passages in vitro [82]. Inoculation of B16 melanoma 4A5 cells intraperitoneally to C57BL/6 mice led to the development of lung and spleen metastases in less than 30 days post-administration [83], whereas subcutaneously injection of cells into C57BL/6 mice offers the possibility to assess the evolution of primary tumors and to test the effectiveness of different antimelanoma agents, the survival time being longer than in the case of intravenous or intraperitoneally inoculum [73,84]. The drawbacks of the syngeneic models consist of: (i) a short time frame between the appearance of the primary tumors and the metastasis occurrence, which impairs the pursuit of potential antimelanoma agents efficiency; and (ii) the murine origin of the cells that makes difficult the translation of the data obtained and might become inconsequent for human pathology [50].
4.3. Patient-Derived Tumor Xenografts (PDXs)
Another preclinical model of melanoma is represented by the patient-derived tumor xenografts model. This type of model consists of fresh tumor grafts collected from patients (under ethical approval) implantation into immunodeficient mice (athymic nude or NSG (NOD/SCID γ) mice) [20,50,80]. The use of human biopsies as xenografts offers several benefits as compared to cell lines, including: preservation of the clinical characteristics of parent tumor in terms of histology, transcriptome, polymorphism, DNA expression and sequence, and chromosomal architecture [20,80]. The heterogeneity of PDXs models and their ability to resemble the initial tumor made them suitable tools for the characterization of metastatic melanoma behavior, drug discovery, clinical response studies, identification of drug resistance and combined therapy effects, guidance in clinical management of melanoma patients, target identification, and validation strategies [20,67,71,80,92]. Besides the notable progress in melanoma preclinical and translational research field offered by PDXs models, there were also some drawbacks stated, such as: it is a time-consuming process that requires technical skills, a long-term experiment (three to nine months for melanoma development without a 100% rate of success), the lack of a fully functional immune system (the use of immunocompromised mice), inconveniencies for genetic manipulation, and high costs [20,50,80].
4.4. Genetically Engineered Models (GEM)
It is well-known that the transformation of a normal cell into a cancerous one involves a series of genetic and epigenetic changes, changes that were also described in melanoma [79]. In order to have a clear picture and to understand the role of these changes, many efforts were channeled to the development of genetic engineered melanoma mouse models. The genetic engineered models (GEMs) were performed using different approaches, such as: genetic manipulation of the ectopic expression of oncogenes, inactivation of tumor suppressor genes, and introduction of different mutations [39,79]. These models contributed significantly to depict the cells of origin by lineage tracing approaches (consists in labeling a single cell with a marker that offers visibility into the mechanisms involved in tumor initiation and progression of pre-neoplastic lesions) and to observe the results of the treatment administered, to identify the genes responsible for melanoma progression, and the molecular mechanisms associated with melanoma late stages [39,79,93]. In addition, GEMs proved to be reliable and reproducible models (present the same basic mutations) for evaluating the role of impaired genes/pathways and of the immune system cells in melanoma biology and treatment resistance. Other important features of GEMs consist of: the capacity to develop spontaneous melanoma tumors at their inherent site (spontaneous melanoma rarely occurs in adult mice), the ability to generate other mutations in order to verify their potential susceptibility or resistance to therapy, and the presence of a fully functional immune system that influences the tumor growth [39,94]. Besides the multiple applications of these models, there were also some limitations described: high costs and effort, the latency period for tumors appearance is long (9–12 months), and most frequently the mutagenic load is not similar with the one described in human tumors [20,50,94].
In order to obtain a relevant genetic engineered melanoma mouse model, there are some factors that should be considered, like: skin morphology (distinct anatomic features between human and murine skin), melanocytic lineage (the promoter used to drive expression to a specific cell type), carcinogenic agents (for the initiation or the enhancement of melanoma development), mice age (adult mice are more resistant at developing spontaneous melanoma), and skin microenvironment [94,95].
The first genetic engineered melanoma mouse model was the transgenic mouse model—Tyr-SV40, that exhibited overexpression of SV40 T antigen (Tag) under the control of melanocyte-specific tyrosinase (Tyr) gene promoter and developed melanoma spontaneously or after UV irradiation [2,94,95]. Linda Chin and her collaborators established the first mouse model—Tyr-HRAS by knocking out melanoma specific genes, Cdkn2a−/−, and developed spontaneous melanoma that do not metastasize [2,48,94]. The group of Dhomen built the BRAFV600E melanoma model that proved the necessity of additional genetic alterations to develop melanoma [94,96]. Subsequent studies showed that silencing PTEN gene in BRAFV600E melanoma model led to the development of metastatic melanoma [50,94,97]. Other genes that were manipulated to develop genetic mouse models of melanoma were: RET—a proto-oncogene that enciphers glial cell-derived neurotrophic factor-specific receptor tyrosine kinase and interferes in the progression stage of melanoma [50]; Mt1-hepatocyte growth factor/scatter factor—Mt1-HGF/SF [94,98]; G-protein-coupled receptor GRM-1 (metabotropic glutamate receptor-1) [79]; guanine nucleotide-binding protein G(q) subunit α (GNAQ) [80]; and cyclin-dependent kinase 4 (CDK4) [2,20,95]. These basic genetic engineered models represented the starting point for more complex mouse models by induction of additional mutations and were clearly described in several studies [20,49,80,95].
Some excellent reviews of recent date have addressed the available genetic engineered mouse models of melanoma and their role in the advancement of human melanoma initiation and progression [20,21,95], thus this paper will offer a summarized version (see Table 2) of the described models obtained by overexpression of genes, in terms of: the gene mutation, animal strain, signaling pathways altered, and the promoter involved in melanoma development.
Table 2.
Representative examples of genetic engineered mouse (GEM) models of melanoma.
| GEM Name | Gene Mutation | Animal Strain/Background | Signaling Pathways Altered | Promoter ± Carcinogen | References |
|---|---|---|---|---|---|
| Tyr-SV40 Tag (high expression) | SV40 T antigen (Tag)-overexpression | C57/BL6 | pRB (p16)/p53 (ARF) | Tyr | [2,80,99] |
| Tyr-SV40 Tag (low expression) | SV40 T antigen (Tag)-overexpression | C57/BL6 | pRB (p16)/p53 (ARF) | Tyr + UV radiation | |
| (MT1)-Ret TRP1-Ret (G12V) | Ret proto-oncogen-overexpression | NMRI C3H | MAPK (Ras)/MAPK (Raf)/PTEN/Akt Ras and PI3K | Mt1 + UV radiation | [2,100,101] |
| Mt1-HGF/SF | HGF/SF-overexpression | FVB | MAPK (p38MAPK) MAPK (Ras)/MAPK (Raf)/PTEN/Akt | Mt1 + UV radiation | [2,20,98,102,103] |
| Krt4-Scf | Scf-overexpression | C57/BL | Kit receptor, MAPK | Krt4 | [2,104] |
| Tyr-Hras (G12V) | Hras (G12V)-oncogene overexpression | Mixed | MAPK (Ras)/MAPK (Raf)/PTEN/Akt | Tyr + DMBA or UV | [105] |
| Tyr::NRASQ61K | NRAS (NRASQ61K)-overexpression | Tyr::N-RasQ61K transgenic mice | pRB (p16)/p53 (ARF)/MAPK (Ras)/MAPK (Raf)/PTEN/Akt | Tyr | [20,106] |
| Hgf-Cdk4R24C | Overexpression of HGF and an oncogenic mutation CDK4R24C | HGF × CDK4R24C C57BL/6 mice | pRB (p16) | MT1+ DMBA and TPA | [20,107] |
| BrafCA | BrafV600E from the endogenous Braf gene | Tyr::CreER; BRafCA/+; Ptenlox5/lox5 mouse | BRAF→MEK1/2→ERK1/2 MAPK PTEN→INK4A and/or ARF | Tyr::CreERT2 + UVB | [97] |
| Hairless RFP–RET-transgenic mice of line 304-hr/hr-HL-RET mice | Crossings of RET-mice with HL-mice (Hos:HRM) under C57BL/6J background | MAPK (Ras)/MAPK (Raf)/PTEN/Akt | [65] |
4.5. UV-Induced Mouse Melanoma Models
Ultraviolet radiation exposure is considered one of the main etiological factors involved in melanoma development, but until present a clear link between UV-induced DNA damage and melanoma initiation has not been established. Recent studies suggested that DNA impairment requires the presence of other dysregulated genes (via epigenetic events) to initiate UV-induced melanomagenesis [39].
To elucidate the UV radiation role in melanoma development, several animal models were proposed but some important setbacks were encountered, including: the localization of melanocytes in human skin (at epidermal–dermal junctions and within hair follicles) versus mice skin (most melanocytes are located in hair follicles, only few are found in epidermis, mainly in non-hairy skin—ears, footpad) [2,58,79], the diversity of human melanoma genetic, histopathological, and clinical features (numerous genes and signaling pathways) which hinders the development of a model that mirrors the characteristics of human melanoma [79].
The mice characteristics regarding the epidermal melanocytes (their number augments in the first 2 weeks after birth and starts to decline during hair follicles formation process when they cross the basement membrane) were thought to be responsible for the inability to develop spontaneous melanoma in adult mice after UV exposure (acute—intense, short-term or chronic—low doses) [2]. Still, there were developed melanoma models using hairless mice subjected to DMBA (7,12-dimethylbenzanthracene)—as initiator agent and UV irradiation promoter, and transgenic mice carrying BRAF mutations (increased risk to develop melanoma when UV irradiation was added) [33].
There were also recorded differences regarding melanocytes location in murine skin dependent on age: in newborn mice are located at the epidermal–dermal junction, whereas in adolescent and adult mice, melanocytes are restricted to hair follicles in corporal skin. Melanocytes and hair pigmentation are two connected processes since melanocytes play not only a pigmentary role, but also a hair growth-regulatory one being strictly coupled to the anagen phase of hair cycle [108,109].
The most successful protocols to develop melanoma in mice after UV exposure consisted of: exposure of genetic engineered mice overexpressing SV40 Tag and HGF/SF starting with day 4 after birth; these data being somehow in accordance with the results of multiple human epidemiological studies which assert that an increased exposure to sun in the childhood augments the risk for melanoma [2].
A relevant model of melanoma was considered to be the one initiated in neonatal transgenic for hepatocyte growth factor/scatter factor (HGF/SF) mice (after a single neonatal dose of mild erythemal UVB radiation), that presents melanocytes localized in the epidermis and the tumor arose in different phases similar with the ones described for human melanoma: benign nevus, radial growth phase, vertical growth phase, and late metastatic spread to other organs [58,80]. The mouse melanoma model obtained by UV irradiation is considered to be the most reliable model to clinically characterize melanomagenesis [80]. Other approaches to initiate melanomagenesis involved the application of DMBA, a polycyclic aromatic hydrocarbon with immunosuppressive effects and TPA (13-O-tetradecanoyl phobol acetate), a phorbol ester responsible for skin irritation and black lesions (that will transform to melanoma) after topical administration [88].
Our research group developed a photo-chemically induced skin carcinogenesis model using SKH-1 hairless mice and the association of UV irradiation and topical application of DMBA and TPA solutions, and the tumors resulted were non-melanoma skin cancer type with a high resemblance to human pathology (the incidence was higher in male mice) [110]. Even though the chemically induced melanoma models are frequently used for the evaluation of immune therapies effects on tumor growth, one of the main drawbacks is represented by the fact that the cells arising from the lesions induced are nonpigmented and do not recreate precisely the human pathology [80].
5. Conclusions
The impressive advances in melanoma biology, immunology, genetics, and epigenetics comprehension represent palpable reasons for an optimistic future for targeted treatments and immunotherapy. The information provided by mouse models managed to fill some of the gaps existent in melanoma knowledge and offer an arsenal to prevail the new challenges and setbacks associated to heterogeneity of melanoma; still, further considerable efforts are required to conceal melanoma complexity. Nonetheless, melanoma remains a very aggressive type of cancer with a high mortality rate, particularly in advanced stages, and a central topic for prospective cancer research.
Acknowledgments
This study was supported by the intern grant PII-C2-TC-2014-16498-10 offered by the “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania.
Abbreviations
| A375 | Human melanoma cells |
| ACTH | Adrenocorticotropic hormone |
| AJCC | American Joint Committee on Cancer |
| B16.OVA | Murine melanoma cell line |
| B164A5 | Murine melanoma cell line |
| B16F10 | Murine metastatic melanoma cells |
| B16FLT3 | Murine melanoma cell line |
| B16GMCSF | Murine melanoma cell line |
| BRAF | Human gene that encodes B-Raf protein |
| CDK4 | Cyclin-dependent kinase 4 |
| CDKN2A | Cyclin-dependent kinase N2A |
| DMBA | 7,12-dimethylbenzanthracene |
| DNA | Deoxyribonucleic acid |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| EUROCARE 5 | European Cancer Registry based Study on Survival and Care of Cancer Patients |
| GEM | Genetically engineered models |
| GLOBOCAN | Global Cancer Incidence, Mortality and Prevalence |
| GNAQ | Guanine nucleotide-binding protein G(q) subunit α |
| GPCR | G-protein-coupled receptor |
| GRM-1 | Metabotropic glutamate receptor-1 |
| HGF/SF | Hepatocyte growth factor/scatter factor |
| i.p. | Intraperitoneally |
| i.v. | Intravenously |
| IAPs | Inhibitory apoptosis proteins |
| l-DOPA | l-dihydroxyphenylalanine |
| MAPK | Mitogen-activated protein kinase |
| Mb | Megabase |
| MC1 receptor | Melanocortin 1 receptor |
| Mt1 | Metallothionein |
| Mt1-HGF/SF | Mt1-hepatocyte growth factor/scatter factor |
| NF1 | Neurofibromin 1 |
| NK | Natural killer cells |
| NRAS | Neuroblastoma RAS viral oncogene homolog |
| p14-ARF | Tumor suppressor protein |
| p16-INK4A | Tumor suppressor protein |
| PDXs | Patient-derived tumor xenografts |
| PI3K | Phosphatidylinositol-3-kinase pathway |
| PTEN | Phosphatase and tensin homolog protein |
| RGP | Radial growth phase |
| s.c. | Subcutaneously |
| SLUG | Transcriptional repressor of E-cadherin |
| SNAIL | Transcription factor |
| TCGA | The Cancer Genome Atlas |
| TP53 | Tumor protein p53 |
| TPA | 13-O-tetradecanoyl phobol acetate |
| TWIST | Twist-related protein 1 |
| Tyr | Melanocyte-specific tyrosinase gene promoter |
| UV | Ultraviolet |
| WHO | World Health Organization |
| ZEB1 | Zinc finger E-box binding homeobox 1 transcription factor |
Author Contributions
D.C., C.D., E.-A.M, I.P., T.B., D.N. and O.B. searched and selected the literature. D.C. and C.D. wrote the paper and realized the figure and tables. E.-A.M., I.P. and D.N. corrected the manuscript. C.D., T.B., D.N. and O.B have been equally involved in revising the manuscript critically. All authors read and approved the final manuscript that was critically edited by the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Holmes D. The cancer that rises with the Sun. Nature. 2014;515:S110–S111. doi: 10.1038/515S110a. [DOI] [PubMed] [Google Scholar]
- 2.Walker G.J., Hayward N.K. Pathways to melanoma development: Lessons from the mouse. J. Investig. Dermatol. 2002;119:783–792. doi: 10.1046/j.1523-1747.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Haridas P., McGovern J.A., McElwain S.D.L., Simpson M.J. Quantitative comparison of the spreading and invasion of radial growth phase and metastatic melanoma cells in a three-dimensional human skin equivalent model. PeerJ. 2017;5:e3754. doi: 10.7717/peerj.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Šitum M., Buljan M., Kolić M., Vučić M. Melanoma-clinical, dermatoscopical, and histopathological morphological characteristics. Acta Dermatovenerol. Croat. 2014;22:1–12. [PubMed] [Google Scholar]
- 5.GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. [(accessed on 15 March 2018)]; Available online: http://globocan.iarc.fr.
- 6.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 7.Tsao H., Chin L., Garraway L.A., Fisher D.E. Melanoma: From mutations to medicine. Genes Dev. 2012;26:1131–1155. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roh M.R., Eliades P., Gupta S., Tsao H. Genetics of melanocytic nevi. Pigment Cell Melanoma Res. 2015;28:661–672. doi: 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orthaber K., Pristovnik M., Skok K., Perić B., Maver U. Skin Cancer and its Treatment: Novel Treatment Approaches with Emphasis on Nanotechnology. J. Nanomater. 2017:2606271. doi: 10.1155/2017/2606271. [DOI] [Google Scholar]
- 10.US Department of Health and Human Services . The Surgeon General’s Call to Action to Prevent Skin Cancer. US Department of Health and Human Services, Office of the Surgeon General; Washington, DC, USA: 2014. [Google Scholar]
- 11.Whiteman D.C., Green A.C., Olsen C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations though 2031. J. Investig. Dermatol. 2016;136:1161–1171. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Sacchetto L., Zanetti R., Comber H., Bouchardy C., Brewster D.H., Broganelli P., Chirlaque M.D., Coza D., Galceran J., Gavin A., et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur. J. Cancer. 2018;92:108–118. doi: 10.1016/j.ejca.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Barbaric J., Sekerija M., Agius D., Coza D., Dimitrova N., Demetriou A., Safaei Diba C., Eser S., Gavric Z., Primic-Zakelj M., et al. Disparities in melanoma incidence and mortality in South-Eastern Europe: Increasing incidence and divergent mortality patterns. Is progress around the corner? Eur. J. Cancer. 2016;55:47–55. doi: 10.1016/j.ejca.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer F.S., Cantone M., Eberhardt M., Jaitly T., Walter L., Wittmann J., Gupta S.K., Khan F.M., Wolkenhauer O., Pützer B.M., et al. A web platform for the network analysis of high-throughput data in melanoma and its use to investigate mechanisms of resistance to anti-PD1 immunotherapy. Biochim. Biophys. Acta. 2018 doi: 10.1016/j.bbadis.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Rat C., Hild S., Rault Sérandour J., Gaultier A., Quereux G., Dreno B., Nguyen J.M. Use of Smartphones for Early Detection of Melanoma: Systematic Review. J. Med. Internet Res. 2018;20:e135. doi: 10.2196/jmir.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton G.L., Smit A.K., Freeman L., Badcock C., Dunlop K., Butow P.N., Kirk J., Cust A.E. Development and Evaluation of a Telephone Communication Protocol for the Delivery of Personalized Melanoma Genomic Risk to the General Population. J. Genet. Couns. 2018;27:370–380. doi: 10.1007/s10897-017-0183-7. [DOI] [PubMed] [Google Scholar]
- 17.Strömberg U., Holmén A., Peterson S. Spatial disparities in melanoma incidence and prognosis with consideration to stage at diagnosis, gender and marital status. Spat. Spatiotemporal Epidemiol. 2016;19:21–27. doi: 10.1016/j.sste.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Liu-Smith F., Farhat A.M., Arce A., Ziogas A., Taylor T., Wang Z., Yourk V., Liu J., Wu J., McEligot A.J., et al. Sex differences in the association of cutaneous melanoma incidence rates and geographic ultraviolet light exposure. J. Am. Acad. Dermatol. 2017;76:499–505.e3. doi: 10.1016/j.jaad.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karimkhani C., Green A.C., Nijsten T., Weinstock M.A., Dellavalle R.P., Naghavi M., Fitzmaurice C. The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017;177:134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Weyden L., Patton E.E., Wood G.A., Foote A.K., Brenn T., Arends M.J., Adams D.J. Cross-species models of human melanoma. J. Pathol. 2016;238:152–165. doi: 10.1002/path.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourneuf E. The MeLiM Minipig: An Original Spontaneous Model to Explore Cutaneous Melanoma Genetic Basis. Front. Genet. 2017;8:146. doi: 10.3389/fgene.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitra D., Luo X., Morgan A., Wang J., Hoang M.P., Lo J., Guerrero C.R., Lennerz J.K., Mihm M.C., Wargo J.A., et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panzella L., Leone L., Greco G., Vitiello G., D’Errico G., Napolitano A., d’Ischia M. Red human hair pheomelanin is a potent pro-oxidant mediating UV-independent contributory mechanisms of melanomagenesis. Pigment Cell Melanoma Res. 2014;27:244–252. doi: 10.1111/pcmr.12199. [DOI] [PubMed] [Google Scholar]
- 24.Solano F. Photoprotection versus photodamage: Updating an old but still unsolved controversy about melanin. Polym. Int. 2016;65:1276–1287. doi: 10.1002/pi.5117. [DOI] [Google Scholar]
- 25.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 26.Slominski A., Zmijewski M.A., Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski A., Kim T.K., Brożyna A.A., Janjetovic Z., Brooks D.L., Schwab L.P., Skobowiat C., Jóźwicki W., Seagroves T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slominski R.M., Zmijewski M.A., Slominski A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015;24:258–259. doi: 10.1111/exd.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brożyna A.A., Jóźwicki W., Roszkowski K., Filipiak J., Slominski A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7:17844–17853. doi: 10.18632/oncotarget.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski A., Paus R., Mihm M.C. Inhibition of Melanomagenesis as an Adjuvant Strategy in the Treatment of Melanotic Melanomas: Selective Review and Hypothesis. Anticancer Res. 1998;18:3709–3716. [PubMed] [Google Scholar]
- 31.Slominski A., Zbytek B., Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer. 2009;124:1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faião-Flores F., Smalley K.S.M. Get with the Program! Stemness and Reprogramming in Melanoma Metastasis. J. Investig. Dermatol. 2018;138:10–13. doi: 10.1016/j.jid.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Colebatch A.J., Scolyer R.A. Trajectories of premalignancy during the journey from melanocyte to melanoma. Pathology. 2018;50:16–23. doi: 10.1016/j.pathol.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Melamed R.D., Aydin I.T., Rajan G.S., Phelps R., Silvers D.N., Emmett K.J., Brunner G., Rabadan R., Celebi J.T. Genomic characterization of dysplastic nevi unveils implications for diagnosis of melanoma. J. Investig. Dermatol. 2017;137:905–909. doi: 10.1016/j.jid.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Roh M.R., Eliades P., Gupta S., Grant-Kels J.M., Tsao H. Cutaneous melanoma in women. Int. J. Women’s Dermatol. 2017;3:S11–S15. doi: 10.1016/j.ijwd.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naves L.B., Dhand C., Venugopal J.R., Rajamani L., Ramakrishna S., Almeida L. Nanotechnology for the treatment of melanoma skin cancer. Prog. Biomater. 2017;6:13–26. doi: 10.1007/s40204-017-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigon R.B., Oyafuso M.H., Fujimura A.T., Gonçalez M.L., do Prado A.H., Gremião M.P., Chorilli M. Nanotechnology-Based Drug Delivery Systems for Melanoma Antitumoral Therapy: A Review. Biomed. Res. Int. 2015:841817. doi: 10.1155/2015/841817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller A.J., Mihm M.C., Jr. Melanoma. N. Engl. J. Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 39.Zaidi M.R., Day C.P., Merlino G. From UVs to metastases: Modeling melanoma initiation and progression in the mouse. J. Investig. Dermatol. 2008;128:2381–2391. doi: 10.1038/jid.2008.177. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Fukunaga-Kalabis M., Li L., Herlyn M. Developmental pathways activated in melanocytes and melanoma. Arch. Biochem. Biophys. 2014;563:13–21. doi: 10.1016/j.abb.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caramel J., Papadogeorgakis E., Hill L., Browne G.J., Richard G., Wierinckx A., Saldanha G., Osborne J., Hutchinson P., Tse G., et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell. 2013;24:466e80. doi: 10.1016/j.ccr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Balch C.M., Gershenwald J.E., Soong S.J., Thompson J.F., Atkins M.B., Byrd D.R., Buzaid A.C., Cochran A.J., Coit D.G., Ding S., et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrawala S., Maley A., Greskovich C., Stuart L., Parker D., Swerlick R., Stoff B. Discordance of histopathologic parameters in cutaneous melanoma: Clinical implications. J. Am. Acad. Dermatol. 2016;74:75–80. doi: 10.1016/j.jaad.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Ribero S., Argenziano G., Lallas A., Moscarella E., Benati E., Raucci M., Piana S., Longo C. Dermoscopic features predicting the presence of mitoses in thin melanoma. J. Dermatol. Sci. 2017;86:158–161. doi: 10.1016/j.jdermsci.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Grzywa T.M., Paskal W., Włodarski P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017;10:956–975. doi: 10.1016/j.tranon.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paluncic J., Kovacevic Z., Jansson P.J., Kalinowski D., Merlot A.M., Huang M.L., Lok H.C., Sahni S., Lane D.J., Richardson D. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta. 2016;1863:770–784. doi: 10.1016/j.bbamcr.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Shtivelman E., Davies M.Q., Hwu P., Yang J., Lotem M., Oren M., Flaherty K.T., Fisher D.E. Pathways and therapeutic targets in melanoma. Oncotarget. 2014;5:1701–1752. doi: 10.18632/oncotarget.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin L. Modeling malignant melanoma in mice: Pathogenesis and maintenance. Oncogene. 1999;18:5304–5310. doi: 10.1038/sj.onc.1203106. [DOI] [PubMed] [Google Scholar]
- 49.McKinney A.J., Holmen S.L. Animal models of melanoma: A somatic cell gene delivery mouse model allows rapid evaluation of genes implicated in human melanoma. Chin. J. Cancer. 2011;30:153–162. doi: 10.5732/cjc.011.10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Q., Das M., Liu Y., Huang L. Targeted drug delivery to melanoma. Adv. Drug Deliv. Rev. 2017 doi: 10.1016/j.addr.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Martín-Gorgojo A., Nagore E. Melanoma Arising in a Melanocytic Nevus. Actas Dermosifiliogr. 2018;109:123–132. doi: 10.1016/j.ad.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Leonardi G.C., Falzone L., Salemi R., Zanghì A., Spandidos D.A., Mccubrey J.A., Candido S., Libra M. Cutaneous melanoma: From pathogenesis to therapy (Review) Int. J. Oncol. 2018;52:1071–1080. doi: 10.3892/ijo.2018.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erlich T.H., Fisher D.E. Pathways in melanoma development. G. Ital. Dermatol. Venereol. 2018;153:68–76. doi: 10.23736/S0392-0488.17.05795-9. [DOI] [PubMed] [Google Scholar]
- 54.Trubini S., Ubiali A., Paties C.T., Cavanna L. Novel BRAF mutation in melanoma: A case report. Mol. Clin. Oncol. 2018;8:460–462. doi: 10.3892/mco.2018.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conde-Perez A., Larue L. Human relevance of NRAS/BRAF mouse melanoma models. Eur. J. Cell Biol. 2014;93:82–86. doi: 10.1016/j.ejcb.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Herlyn M., Fukunaga-Kalabis M. What is a good model for melanoma? J. Investig. Dermatol. 2010;130:911–912. doi: 10.1038/jid.2009.441. [DOI] [PubMed] [Google Scholar]
- 57.Hill D.S., Robinson N.D.P., Caley M.P., Chen M., O’Toole E.A., Armstrong J.L., Przyborski S., Lovat P.E. A novel fully-humanised 3D skin equivalent to model early melanoma invasion. Mol. Cancer Ther. 2015;14:2665–2673. doi: 10.1158/1535-7163.MCT-15-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ha L., Noonan F.P., De Fabo E.C., Merlino G. Animal models of melanoma. J. Investig. Dermatol. Symp. Proc. 2005;10:86–88. doi: 10.1111/j.1087-0024.2005.200409.x. [DOI] [PubMed] [Google Scholar]
- 59.Schook L.B., Collares T.V., Hu W., Liang Y., Rodrigues F.M., Rund L.A., Schachtschneider K.M., Seixas F.K., Singh K., Wells K.D., et al. A genetic porcine model of cancer. PLoS ONE. 2015;10:e0128864. doi: 10.1371/journal.pone.0128864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Idilli A.I., Precazzini F., Mione M.C., Anelli V. Zebrafish in Translational Cancer Research: Insight into Leukemia, Melanoma, Glioma and Endocrine Tumor Biology. Genes (Basel) 2017;8:E236. doi: 10.3390/genes8090236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bootorabi F., Manouchehri H., Changizi R., Barker H., Palazzo E., Saltari A., Parikka M., Pincelli C., Aspatwar A. Zebrafish as a Model Organism for the Development of Drugs for Skin Cancer. Int. J. Mol. Sci. 2017;18:E1550. doi: 10.3390/ijms18071550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Śniegocka M., Podgórska E., Płonka P.M., Elas M., Romanowska-Dixon B., Szczygieł M., Żmijewski M.A., Cichorek M., Markiewicz A., Brożyna A.A., et al. Transplantable Melanomas in Hamsters and Gerbils as Models for Human Melanoma. Sensitization in Melanoma Radiotherapy-From Animal Models to Clinical Trials. Int. J. Mol. Sci. 2018;19:1048. doi: 10.3390/ijms19041048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slominski A., Paus R. Bomirski melanomas—A versatile and powerful model for pigment cell and melanoma research (review) Int. J. Oncol. 1993;2:221–228. doi: 10.3892/ijo.2.2.221. [DOI] [PubMed] [Google Scholar]
- 64.Bomirski A., Słominski A., Bigda J. The natural history of a family of transplantable melanomas in hamsters. Cancer Metastasis Rev. 1988;7:95–118. doi: 10.1007/BF00046481. [DOI] [PubMed] [Google Scholar]
- 65.Thang N.D., Yajima I., Nakagawa K., Tsuzuki T., Kumasaka M.Y., Ohgami N., Ly T.B., Iwamoto T., Watanabe D., Kato M. A novel hairless mouse model for malignant melanoma. J. Dermatol. Sci. 2012;65:207–212. doi: 10.1016/j.jdermsci.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Mathieu V., Le Mercier M., De Neve N., Sauvage S., Gras T., Roland I., Lefranc F., Kiss R. Galectin-1 knockdown increases sensitivity to temozolomide in a B16F10 mouse metastatic melanoma model. J. Investig. Dermatol. 2007;127:2399–2410. doi: 10.1038/sj.jid.5700869. [DOI] [PubMed] [Google Scholar]
- 67.Kawaguchi K., Igarashi K., Li S., Han Q., Tan Y., Kiyuna T., Miyake K., Murakami T., Chmielowski B., Nelson S.D., et al. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2017;8:85516–85525. doi: 10.18632/oncotarget.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatiboglu M.A., Kocyigit A., Guler E.M., Akdur K., Nalli A., Karatas E., Tuzgen S. Thymoquinone Induces Apoptosis in B16-F10 Melanoma Cell Through Inhibition of p-STAT3 and Inhibits Tumor Growth in a Murine Intracerebral Melanoma Model. World Neurosurg. 2018 doi: 10.1016/j.wneu.2018.02.136. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka R., Goshima F., Esaki S., Sato Y., Murata T., Nishiyama Y., Watanabe D., Kimura H. The efficacy of combination therapy with oncolytic herpes simplex virus HF10 and dacarbazine in a mouse melanoma model. Am. J. Cancer Res. 2017;7:1693–1703. [PMC free article] [PubMed] [Google Scholar]
- 70.Hubmann R., Sieghart W., Schnabl S., Araghi M., Hilgarth M., Reiter M., Demirtas D., Valent P., Zielinski C., Jäger U., et al. Gliotoxin Targets Nuclear NOTCH2 in Human Solid Tumor Derived Cell Lines In Vitro and Inhibits Melanoma Growth in Xenograft Mouse Model. Front. Pharmacol. 2017;8:319. doi: 10.3389/fphar.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawaguchi K., Igarashi K., Li S., Han Q., Tan Y., Miyake K., Kiyuna T., Miyake M., Murakami T., Chmielowski B., et al. Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as -positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget. 2018;9:915–923. doi: 10.18632/oncotarget.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fotaki G., Jin C., Kerzeli I.K., Ramachandran M., Martikainen M.M., Karlsson-Parra A., Yu D., Essand M. Cancer vaccine based on a combination of an infection-enhanced adenoviral vector and pro-inflammatory allogeneic DCs leads to sustained antigen-specific immune responses in three melanoma models. Oncoimmunology. 2018;7:e1397250. doi: 10.1080/2162402X.2017.1397250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soica C., Danciu C., Savoiu-Balint G., Borcan F., Ambrus R., Zupko I., Bojin F., Coricovac D., Ciurlea S., Avram S., et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014;15:8235–8255. doi: 10.3390/ijms15058235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soica C., Oprean C., Borcan F., Danciu C., Trandafirescu C., Coricovac D., Crăiniceanu Z., Dehelean C.A., Munteanu M. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules. 2014;19:4924–4940. doi: 10.3390/molecules19044924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang R., Andersen L.M.K., Rofstad E.K. Metastatic pathway and the microvascular and physicochemical microenvironments of human melanoma xenografts. J. Transl. Med. 2017;15:203. doi: 10.1186/s12967-017-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crawford M., Leclerc V., Dagnino L. A reporter mouse model for in vivo tracing and in vitro molecular studies of melanocytic lineage cells and their diseases. Biol. Open. 2017;6:1219–1228. doi: 10.1242/bio.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fidler I.J., Kripke M.L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 78.Hartsough E.J., Aplin A.E. Of mice and melanoma: PDX System for modeling personalized medicine. Clin. Cancer Res. 2016;22:1550–1552. doi: 10.1158/1078-0432.CCR-15-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Becker J.C., Houben R., Schrama D., Voigt H., Ugurel S., Reisfeld R.A. Mouse models for melanoma: A personal perspective. Exp. Dermatol. 2010;19:157–164. doi: 10.1111/j.1600-0625.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 80.Kuzu O.F., Nguyen F.D., Noory M.A., Sharma A. Current State of Animal (Mouse) Modeling in Melanoma Research. Cancer Growth Metastasis. 2015;8:81–94. doi: 10.4137/CGM.S21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avram S., Coricovac D.E., Pavel I.Z., Pinzaru I., Ghiulai R., Baderca F., Soica C., Muntean D., Branisteanu D.E., Spandidos D.A., et al. Standardization of A375 human melanoma models on chicken embryo chorioallantoic membrane and Balb/c nude mice. Oncol. Rep. 2017;38:89–99. doi: 10.3892/or.2017.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pfarr K., Danciu C., Arlt O., Neske C., Dehelean C., Pfeilschifter J.M., Radeke H.H. Simultaneous and Dose Dependent Melanoma Cytotoxic and Immune Stimulatory Activity of Betulin. PLoS ONE. 2015;10:e0118802. doi: 10.1371/journal.pone.0118802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gheorgheosu D., Dehelean C., Cristea M., Muntean D. Development of the B16 Murine Melanoma Model. Ann. Rom. Soc. Cell Biol. 2011;16:148–156. [Google Scholar]
- 84.Danciu C., Oprean C., Coricovac D.E., Cioca A., Cimpean A., Radeke H., Soica C., Dehelean C. Behaviour of four different B16 murine melanoma cell sublines: C57BL/6J skin. Int. J. Exp. Pathol. 2015;96:73–80. doi: 10.1111/iep.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Muijen G.N., Jansen K.F., Cornelissen I.M., Smeets D.F., Beck J.L., Ruiter D.J. Establishment and characterization of a human melanoma cell line (MV3) which is highly metastatic in nude mice. Int. J. Cancer. 1991;48:85–91. doi: 10.1002/ijc.2910480116. [DOI] [PubMed] [Google Scholar]
- 86.Kimpel J., Urbiola C., Koske I., Tober R., Banki Z., Wollmann G., von Laer D. The Oncolytic Virus VSV-GP Is Effective against Malignant Melanoma. Viruses. 2018;10:E108. doi: 10.3390/v10030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang G., Liu M., Wang Q., Shen Y., Mei H., Li D., Liu W. Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget. 2017;8:28510–28525. doi: 10.18632/oncotarget.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gowda R., Kardos G., Sharma A., Singh S., Robertson G.P. Nanoparticle-Based Celecoxib and Plumbagin for the Synergistic Treatment of Melanoma. Mol. Cancer Ther. 2017;16:440–452. doi: 10.1158/1535-7163.MCT-16-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidler I.J., Nicolson G.L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J. Natl. Cancer Inst. 1976;57:1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 90.Kochenderfer J.N., Gress R.E. A comparison and critical analysis of preclinical anticancer vaccination strategies. Exp. Biol. Med. (Maywood) 2007;232:1130–1141. doi: 10.3181/0702-MR-42. [DOI] [PubMed] [Google Scholar]
- 91.Hekim C., Ilander M., Yan J., Michaud E., Smykla R., Vähä-Koskela M., Savola P., Tähtinen S., Saikko L., Hemminki A., et al. Dasatinib Changes Immune Cell Profiles Concomitant with Reduced Tumor Growth in Several Murine Solid Tumor Models. Cancer Immunol. Res. 2017;5:157–169. doi: 10.1158/2326-6066.CIR-16-0061-T. [DOI] [PubMed] [Google Scholar]
- 92.Hoffman R.M. Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma. Int. J. Mol. Sci. 2017;18:1875. doi: 10.3390/ijms18091875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alladin A., Jechlinger M. Towards a holistic and mechanistic understanding of tumourigenesis via genetically engineered mouse models. Curr. Opin. Syst. Biol. 2017;6:74–79. doi: 10.1016/j.coisb.2017.10.004. [DOI] [Google Scholar]
- 94.Beaumont K.A., Mohana-Kumaran N., Haass N.K. Modeling Melanoma in vitro and in vivo. Healthcare. 2013;2:27–46. doi: 10.3390/healthcare2010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pérez-Guijarro E., Day C.P., Merlino G., Zaidi M.R. Genetically engineered mouse models of melanoma. Cancer. 2017;123:2089–2103. doi: 10.1002/cncr.30684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dhomen N., Reis-Filho J.S., da Rocha Dias S., Hayward R., Savage K., Delmas V., Larue L., Pritchard C., Marais R. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 97.Dankort D., Curley D.P., Cartlidge R.A., Nelson B., Karnezis A.N., Damsky W.E., Jr., You M.J., DePinho R.A., McMahon M., Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noonan F.P., Otsuka T., Bang S., Anver M., Merlino G. Accelerated ultraviolet radiation-induced carcinogenesis in hepatocyte growth factor/scatter factor transgenic mice. Cancer Res. 2000;60:3738–3743. [PubMed] [Google Scholar]
- 99.Mintz B., Silvers W.K. Transgenic mouse model of malignant skin melanoma. Proc. Natl. Acad. Sci. USA. 1993;90:8817–8821. doi: 10.1073/pnas.90.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iwamoto T., Takahashi M., Ito M., Hamatani K., Ohbayashi M., Wajjwalku W., Isobe K., Nakashima I. Aberrant melanogenesis and melanocytic tumour development in transgenic mice that carry a metallothionein/ret fusion gene. EMBO J. 1991;10:3167–3175. doi: 10.1002/j.1460-2075.1991.tb04878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kato M., Takahashi M., Akhand A.A., Liu W., Dai Y., Shimizu S., Iwamoto T., Suzuki H., Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 102.Noonan F., Recio J., Takayama H., Duray P., Anver M.R., Rush W.L., De Fabo E.C., Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- 103.Leonard M.K., Pamidimukkala N., Puts G.S., Snyder D.E., Slominski A.T., Kaetzel D.M. The HGF/SF Mouse Model of UV-Induced Melanoma as an In Vivo Sensor for Metastasis-Regulating Gene. Int. J. Mol. Sci. 2017;18:1647. doi: 10.3390/ijms18081647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kunisada T., Lu S.Z., Yoshida H., Nishikawa S., Mizoguchi M., Hayashi S., Tyrrell L., Williams D.A., Wang X., Longley B.J. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J. Exp. Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chin L., Pomerantz J., Polsky D., Jacobson M., Cohen C., Cordon-Cardo C., Horner J.W., DePinho R.A. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ackermann J., Frutschi M., Kaloulis K., McKee T., Trumpp A., Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 107.Tormo D., Ferrer A., Bosch P., Gaffal E., Kohlmeyer J., Landsberg J., Tueeting T. Therapeutic efficacy of antigen-specific vaccination and toll-like receptor stimulation against established transplanted and autochthonous melanoma in mice. Cancer Res. 2006;66:5427–5435. doi: 10.1158/0008-5472.CAN-06-0399. [DOI] [PubMed] [Google Scholar]
- 108.Slominski A., Paus R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J. Investig. Dermatol. 1993;101(Suppl. 1):90S–97S. doi: 10.1016/0022-202X(93)90507-E. [DOI] [PubMed] [Google Scholar]
- 109.Slominski A., Wortsman J., Plonka P.M., Schallreuter K.U., Paus R., Tobin D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dehelean C.A., Soica C., Pinzaru I., Coricovac D., Danciu C., Pavel I., Borcan F., Spandidos D.A., Tsatsakis A.M., Baderca F. Sex differences and pathology status correlated to the toxicity of some common carcinogens in experimental skin carcinoma. Food Chem. Toxicol. 2016;95:149–158. doi: 10.1016/j.fct.2016.07.007. [DOI] [PubMed] [Google Scholar]