Abstract
Amyloid diseases are characterized by the deposition of proteins in the form of amyloid fibrils, in organs that eventually fail. The development of effective drug candidates follows from the understanding of the molecular processes that lead to protein aggregation. Here, we study amyloidogenic segments of transthyretin (TTR). TTR is a transporter of thyroxine and retinol in the blood and cerebrospinal fluid. When mutated and/or as a result of aging, TTR aggregates into amyloid fibrils that accumulate in organs such as the heart. Recently, we reported two amyloidogenic segments that drive amyloid aggregation. Here, we report the crystal structure of another six amyloidogenic segments of TTR. We found that the segments from the C‐terminal region of TTR form in‐register steric‐zippers with highly‐interdigitated, wet interfaces, whereas the β‐strand B from the N‐terminal region of TTR forms an out‐of‐register assembly, previously associated with oligomeric formation. Our results contribute fundamental information for understanding the mechanism of aggregation of TTR.
Keywords: amyloid, transthyretin, steric zipper, out‐of‐register, amyloidogenic segments, crystallography
Short abstract
PDB Code(s): http://firstglance.jmol.org/fg.htm?mol=6C3F; http://firstglance.jmol.org/fg.htm?mol=6C3G; http://firstglance.jmol.org/fg.htm?mol=6C3S; http://firstglance.jmol.org/fg.htm?mol=6C3T; http://firstglance.jmol.org/fg.htm?mol=6C4O; http://firstglance.jmol.org/fg.htm?mol=6C88
Abbreviations
- ATTR
transthyretin amyloidosis;
- TTR
transthyretin.
Introduction
Several pathological conditions, including Alzheimer's disease, Parkinson's diseases, type II diabetes, cardiac amyloidosis, and prion diseases, are associated with the formation of amyloid fibrils and other protein aggregate deposits that result in cytotoxicity and/or mechanical tissue damage.1 Each amyloid disease is associated with the deposition of particular proteins in the form of elongated, unbranched amyloid fibers. For a long time, treatments for amyloid diseases have been held back by limited information on the structures of the amyloid state of proteins and the causes of aggregation of these disease agents. In recent years, scientists have made incredible progress in this regard. Together with NMR and Cryo‐EM reconstruction methods, x‐ray crystallography has proven to be a powerful tool for structure determination of proteins in their amyloid state and have notably contributed to the understanding of the molecular architecture of the amyloid spine.
The in‐register steric zipper is one of the two main structural motifs that have been identified in amyloid proteins. The spine of amyloid fibrils is made up of pairs of β‐sheets, each of which is composed of hundreds of thousands of β‐strands stacked along the fibril axis by hydrogen bonding.2 This structural arrangement is responsible for the cross‐β diffraction pattern that amyloid fibrils display when subjected to an x‐ray beam.3 Overall, the spine of the amyloid fibril is made of specific protein segments that are the adhesive parts of amyloid proteins.4 The molecular architecture of the amyloid spine has been reproduced by determining the crystal structures of these adhesive segments in their amyloid state.5 These structures reveal a motif of a pair of in‐register β‐sheets whose side chains interdigitate into a steric zipper, forming a dry interface between the two β‐sheets.4 To date, more than 100 atomic‐resolution crystal structures of in‐register steric zippers have been determined from over 15 disease‐associated amyloid proteins.2
The out‐of‐register β‐sheet is the second structural motif that has been associated with amyloid polymers, and more specifically with oligomeric assemblies. In‐register sheets (parallel or antiparallel) are easily recognized in fibrillar assemblies because their strands run perpendicular to the fibril axis. Correspondingly, out‐of‐register β‐sheets are easily recognized in fibrillar assemblies because their strands tilt away from the fibril axis perpendicular, as a result of shearing between strands. Structural details of out‐of‐register sheets were first revealed for an 11‐residue segment of the nonpathogenic amyloid‐forming protein, αB‐crystallin.6 Six copies of the segment stacked in an antiparallel out‐of‐register β‐sheet, which curved into a closed β‐barrel with shear number, S = 6. Since then, several other out‐of‐register structures of toxic segments of amyloid proteins have been identified.7, 8, 9
Transthyretin (TTR) is a 55‐kDa tetrameric protein associated with systemic amyloidoses. In healthy individuals, TTR functions as a hormone transporter that travels in the blood and cerebrospinal fluid.10 The x‐ray structure of the TTR tetramer shows that each identical 127‐residue subunit is made of eight β‐strands (named A through H) and one α‐helix.11 Under pathological conditions, TTR tetramers dissociate and monomers unravel to form amyloid fibrils that deposit in virtually every organ leading to tissue damage, organ failure, and eventual death.12 Amyloid deposition of mutant and wild‐type protein causes hereditary and wild‐type TTR amyloidoses (ATTR), respectively. ATTR fibrils comprise full‐length TTR and/or C‐terminal fragments, indicating that the C‐terminus of TTR is essential for amyloid formation.13 In a previous study, we showed that the self‐association of two segments of TTR from the β strands F and H drive amyloid fibril formation; however several other segments have the capacity to form amyloid‐like fibrils in isolation.14 We determined the crystal structures of the two amyloid‐driving segments in their amyloid states forming in‐register steric zippers.14 Here, we report the crystal structures of six new amyloidogenic segments of TTR in their amyloid state, including a segment that contains a familial mutation associated with early‐onset ATTR. In this study, we found the two amyloid structural motifs among them: in‐register steric zippers formed by segments from the C‐terminal end, and one out‐of‐register steric zipper formed by an N‐terminal segment.
Results
Overall, we have analyzed the amyloidogenicity of thirteen TTR segments predicted to form steric zippers (Fig. 1). These are 12LMVKVL17, 25AINVAV27, 30NVAVHV28, 32VAVHVF33, 37AADTWE42 (carrying the familial mutation ATTR‐D38A), 47GTKSES52, 65VEGIYK70, 68IYKVEI73, 80KALGIS85, 91AEVVFT96, 105YTIAAL110, 106TIAALLS112, and 119TAVVTN124. In a previous study, we showed that the segments 12LMVKVL17, 25AINVAV28, 30VAVHVF33, 80KALGIS85, 91AEVVFT96, 105YTIAAL110, 106TIAALLS112, and 119TAVVTN124 can form amyloid‐like fibrils in solution, which display a cross‐β diffraction pattern when subjected to x‐ray.14 In that study, we also showed that the segments 91AEVVFT96 and 119TAVVTN124 drive transthyretin aggregation in vitro by self‐association and formation of steric zipper spines of amyloid fibrils. 91AEVVFT96 forms a Class‐7 in‐register steric zipper in which the β‐strands stack in antiparallel equifacial β‐sheets, and these sheets in turn pack side‐by‐side, related by translation. 119TAVVTN124 forms a Class‐2 in‐register steric zipper in which the β‐strands stack into parallel β‐sheets and the sheets pack face‐to‐back. Here, we expand our previous work and report the crystal structures of six additional amyloidogenic segments of TTR forming five in‐register and one out‐of‐register steric zippers. Data collection and refinement statistics can be found in Table 1. Several distance measurements, buried surface area, shape complementarity, and solvation energy were calculated and can be found in Table 2.
Figure 1.
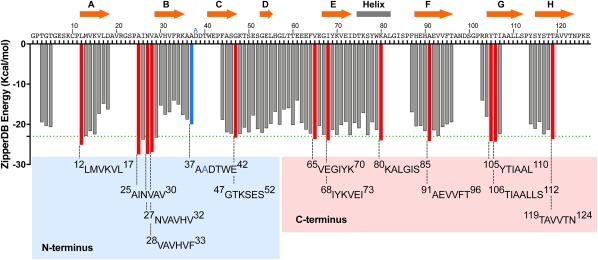
Propensities of steric zipper formation of each 6‐residue segment within the TTR sequence. Amyloidogenic propensities were calculated using the 3D profiling method ZipperDB.15 Segments having energies of −23 kcal mol − 1 (green dashed line) or lower are predicted to form fibrils.16 Segments that we selected for crystallization are represented with red bars. The segment containing the familial mutation ATTR‐D38A is represented with a blue bar. The ZipperDB Rosetta energy prediction of the wild‐type sequence 37ADDTWE42 is −18.0 kcal mol−1 compared to −20.0 kcal mol−1 of the mutant sequence 37AADTWE42. The schematic of the secondary structure of native TTR is shown on top of the sequence. The blue and pink boxes highlight the TTR segment sequences of the N‐terminal and C‐terminal regions, respectively, that we described in this study. Only two segments of the N‐terminus, 28VAVHVF33 and 37AADTWE42, resulted in the formation of diffracting crystals. From the C‐terminus, only the segment 65VEGIYK70 did not result in a crystal structure.
Table 1.
Statistics of Structure Determination of TTR Amyloidogenic Segments
| 28VAVHVF33 | 37AADTWE42 | 68IYKVEI73 | 80KALGIS85 | 105YTIAAL110 | 106TIAALLS112 | |
|---|---|---|---|---|---|---|
| Protein Data Bank Code | ||||||
| Data collection | ||||||
| Resolution (Å) | 18.37–1.85 | 21.58–1.00 | 15.96–1.50 | 12.57–1.60 | 22.40–1.60 | 14.21–1.79 |
| Space group | P212121 | P21 | P21 | P1 | I222 | P21 |
| Unit cell dimensions (Å) | ||||||
| a | 11.53 | 9.02 | 9.60 | 8.06 | 18.75 | 9.60 |
| b | 20.36 | 43.16 | 29.45 | 9.48 | 9.56 | 17.30 |
| c | 36.73 | 9.392 | 16.03 | 25.66 | 44.80 | 25.00 |
| Unit cell angles (°) | ||||||
| α | 90.00 | 90.00 | 90.00 | 96.35 | 90.00 | 90.00 |
| β | 90.00 | 102.84 | 95.66 | 96.35 | 90.00 | 95.76 |
| γ | 90.00 | 90.00 | 90.00 | 110.05 | 90.00 | 90.00 |
| Measured reflections | 2808 | 44658 | 3865 | 2536 | 3501 | 2757 |
| Unique reflections | 907 | 3204 | 1413 | 871 | 625 | 2692 |
| completeness (%) | 82.4 (82.8) | 83.5 (46.8) | 96.3 (96.7) | 95.6 (93.5) | 98.9 (99.1) | 97.5 (93.4) |
| Rmerge (%)a | 27.8 (43.1) | 12.8 (3.97) | 15.4 (51.7) | 13.8 (67.5) | 10.3 (31.8) | 21.7 (55.6) |
| <I/σI> | 5.23 (2.79) | 18.43 (5.57) | 5.06 (2.08) | 5.72 (1.41) | 14.22 (5.24) | 5.5 (1.8) |
| Refinement | ||||||
| Final Rwork (%)b | 18.5 | 11.1 | 20.5 | 18.1 | 20.0 | 18.7 |
| Final Rfree (%)c | 19.7 | 12.4 | 26.2 | 21.2 | 24.4 | 22.8 |
| r.m.s.d. bond length (Å) | 0.006 | 0.005 | 0.008 | 0.006 | 0.007 | 0.014 |
| r.m.s.d. bond angle (°) | 0.896 | 1.264 | 1.144 | 1.170 | 0.896 | 1.641 |
| Number of protein atoms | 102 | 98 | 108 | 82 | 46 | 96 |
| Number of ligand/ion atoms | 0 | 0 | 7 | 11 | 0 | 0 |
| Number of water atoms | 4 | 5 | 4 | 2 | 4 | 5 |
| Mean B value (Å2) | 5.35 | 3.311 | 5.02 | 9.92 | 10.22 | 6.636 |
Values in parentheses correspond to the highest resolution shell. a R merge = Σ I j − <l>/Σ I. b R work = Σ |F o – F c|j/Σ F o. c R free = Σ |F o – F c|j/Σ F o calculated using a random set containing 10% reflections that were not included throughout structure refinement.
Table 2.
Structural Analytics of TTR Steric Zippers
| Peptide sequence | A. Rosetta relax energy (kcal/mol) | B. Distance sheet‐sheet ± S.D. (Å) | C. Distance strand‐strand (Å) | D. Area buried (Å2) | E. Shape Complementarity | F. Solvation Energy |
|---|---|---|---|---|---|---|
| 28VAVHVF33 | −17.6 (–2.93) | 9.7 ± 0.2 | 4.82 | 89 | 0.57 | 1602 |
| 37AADTWE42 | −16.6 (–2.77) | 8.8 ± 0.1 | 4.70 | 150 | 0.75 | 264 |
| 68IYKVEI73 | −23.6 (–3.93) | 8.0 ± 0.1 | 4.80 | 114 | 0.77 | 1839 |
| 80KALGIS85 | −14.3 (–2.38) | 7.7 ± 0.1 | 4.74 | 152 | 0.71 | 1669 |
| 91AEVVFT96 | −21.4 (–3.57) | 9.3 ± 0.5 | 4.77 | 131 | 0.82 | 1651 |
| 105YTIAAL110 | −21.2 (–3.53) | 9.4 ± 1.0 | 4.79 | 131 | 0.74 | 1683 |
| 106TIAALLS112 | −25.8 (–3.69) | 8.7 ± 0.6 | 4.80 | 152 | 0.67 | 2170 |
| 119TAVVTN124 | −17.0 (–2.83) | 8.2 ± 0.8 | 4.75 | 129 | 0.87 | 917 |
A. Rosetta relax energies calculated from the reported structures by each strand. In parenthesis, average Rosetta relax energies by residue. B. Sheet‐to‐sheet distances are calculated as the average distance between third degree polynomial fits to backbone atoms of opposite β‐sheets, which have been projected down the “fibril” axis. The standard deviation is also reported. C. Strand‐to‐strand distance of parallel sheets is given by the corresponding unit cell length. For in‐register antiparallel sheets, it is calculated as this unit cell length divided by two. For out‐of‐register antiparallel sheets, it is taken as an average over stacked backbone atoms of strand n and n + 2. D. Area buried is calculated as the difference between the solvent accessible surface area of one β‐sheet alone and the same β‐sheet when is in contact with the opposite β‐sheet17. The average area buried per β‐strand is reported. E. Shape complementarity values are calculated for interfaces between opposing sheets of ten β‐strands each.18
The hexameric segment 68IYKVEI73 forms a Class 7 steric zipper, in which β‐strands stack into antiparallel, equifacial β‐sheets, and these sheets pack side‐by‐side, related by translation [Fig. 2(A)]. The interface is hydrophobic and lacks tight interdigitation. In addition, this steric zipper presents the shortest interface among TTR amyloid structures [Fig. 2(Aii)]; only three side chains (68isoleucine, 71valine, and 73isoleucine) participate in the formation of the hydrophobic interface.
Figure 2.
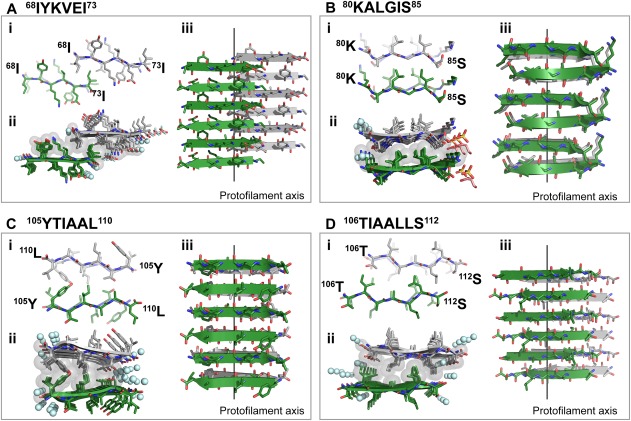
Crystal structures of the amyloidogenic C‐terminal segments 68IYKVEI73 (A), 80KALGIS85 (B), 105YTIAAL110 (C), and 106TIAALLS112 (D) forming in‐register steric zippers. One sheet is shown as dark green; the other is shown as gray. i. View down the fibril axis showing two β‐strands of each β‐sheet in projection. First and last residues of the two top β‐strands are labeled. ii. View down the fibril axis showing two β‐sheets in projection. Water molecules are shown as aquamarine spheres. Gray spheres represent the van der Waals radii of the side chain atoms of the tightly packed fibril core. Other ions present in the crystal are shown as sticks. iii. Lateral view of the fibril with the fibril axis indicated by the black line. i and iii. Waters and other ligands are not shown for clarity.
The segment 80KALGIS85 forms a Class 6 steric zipper, in which β‐strands stacked into antiparallel, antifacial β‐sheets, and these stack face‐to‐back [Fig. 2(B)]. The 80KALGIS85 steric zipper contains a highly hydrophobic interface in which leucines and isoleucines of one β‐sheet interact with alanines and glycines from the opposite β‐sheet [Fig. 2(Bii)]. As a result of the structural packing and the short or absent side chains of alanines and glycines, this steric zipper shows limited interdigitation.
The segment 105YTIAAL110 forms a Class 8 steric zipper, in which β‐strands stack into antiparallel, equifacial β‐sheets, and these sheets stack side‐by‐side, related by a screw axis normal to the sheet face [Fig. 2(C)]. The average distance between opposing sheets, 9.4 Å, has a large standard deviation due to the disparate pairing of tyrosines at one edge of the zipper, reaching a maximum distance of 12.6 Å, and alanines at the other edge. Notably, the overlapping segment 106TIAALLS112 forms a steric zipper with different symmetry—class 6 [Fig. 2(D)].
We also determined the structure of the segment 37AADTWE42, containing the familial ATTR‐D38A mutation associated with neuropathic and cardiomyopathic ATTR19 (Fig. 3). This segment forms a class 6 steric zipper in which β‐strands stack into antiparallel, antifacial β‐sheets, and these sheets stack face‐to‐back [Fig. 3(A–C)]. In contrast to the other amyloid structures, the steric zipper of 37AADTWE42 contains a highly‐hydrated interface that includes the polar residues threonine and aspartate [Fig. 3(B)]. We found that the presence of a wild‐type aspartate in position 38 would be incompatible with the steric zipper formation [Fig. 3(D)].
Figure 3.
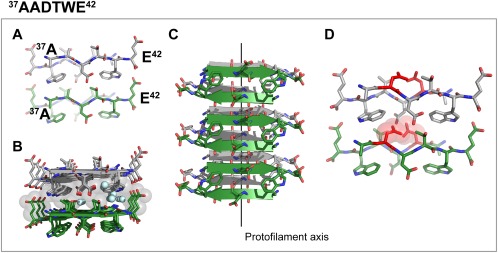
Crystal structure of the in‐register steric zipper formed by the amyloidogenic segment 37AADTWE42, which carries the familial mutation ATTR‐D38A. One sheet is shown as dark green; the other is shown as gray. (A) View down the fibril axis showing two β‐strands of each β‐sheet in projection. First and last residues of the two top β‐strands are labeled. (B) View down the fibril axis showing two β‐sheets in projection. Water molecules are shown as aquamarine spheres. Gray spheres represent the van der Waals radii of the side chain atoms of the tightly packed fibril core. (C) Lateral view of the fibril with the fibril axis indicated by the black line. (D) View down of the fibril axis showing that the wild‐type sequence 37ADDTWE42 would introduce severe steric clashes with 38Aspartate, preventing the formation of this steric zipper. Spheres represent the van der Waals radii of the side chain atoms of 38Aspartate. A, C, and D. Waters molecules are not shown for clarity.
Finally, we obtained the structure of 28VAVHVF33, which corresponds to the B β‐strand of TTR (Fig. 4). This segment forms an out‐of‐register class‐5 steric zipper in which β‐strands stack into antiparallel, antifacial, out‐of‐register β‐sheets, and these sheets pack face‐to‐face, through a highly hydrophobic interface exclusively made of valines and no ordered water molecules [Fig. 4(A,B)]. The strands from opposing β‐sheets cross at an angle of 115° and β‐strands do not run perpendicular to the fibril axis as in in‐register steric zippers; instead, the β‐strands cross the fibril axis at an angle of 65° from the fibril axis [Fig. 4(C)]. As a result, each strand within each sheet of 28VAVHVF33 is out of register by one residue, as shown in Figure 4(D). In contrast to previously reported out of register steric zipper structures8, 9, 28, 28VAVHVF33 does not display alternating weak and strong hydrogen‐bonding interfaces. Instead, every two strands connect throughout the sheet with the same number of hydrogen bonds [Fig. 4(D)].
Figure 4.
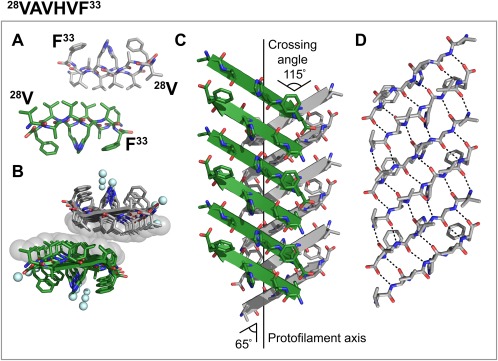
Crystal structure of the out‐of‐register steric zipper formed by the amyloidogenic segment 28VAVHVF33. One sheet is shown as dark green; the other is shown as gray. (A) View down the fibril axis showing two β‐strands of each β‐sheet in projection. First and last residues of the two top β‐strands are labeled. (B) View down the fibril axis showing two β‐sheets in projection. Water molecules are shown as aquamarine spheres. Spheres represent the van der Waals radii of the side chain atoms of the tightly packed fibril core. (C) Lateral view of the fibril with the fibril axis indicated by the black line. The strands of opposing β‐sheets cross at an angle of 115°, 65° to the fibril axis. (D) Intra‐sheet hydrogen bonding along one β‐sheet. A, C, and D. Waters molecules are not shown for clarity.
Discussion
TTR is an amyloid protein whose aggregation causes transthyretin amyloidosis (ATTR).12 We have previously shown that TTR contains several amyloidogenic segments that can form amyloid fibrils when incubated in isolation.14 Here, we expand our earlier efforts with a further structural characterization of amyloidogenic segments of TTR.
Steric zippers from the C‐terminus of TTR
Our study provides structural information of the amyloidogenic C‐terminal region of TTR, found in the protein deposits of all ATTR patients. Some studies suggest that the spine of an amyloid fibril is made of a single, short, specific adhesive segment of the parental amyloid protein.2 However, this is not the case in TTR deposits, which consist of long protein stretches with lengths and composition that depend on pathology.13 Westermark and colleagues have identified two types of fibrils among ATTR patients.13 Type B fibrils contain full‐length TTR and are long, extracellular and highly congophilic. Type A fibrils contain a mixture of full‐length and truncated C‐terminal TTR fragments, and are short and less congophilic. Although we showed that two segments drive TTR aggregation,14 the presence of C‐terminal residues 50 to 127 in both type A and type B ATTR fibrils indicates that this region may be part of the amyloid fibril spine. Our efforts to crystallize TTR segments predicted to form amyloid fibrils included thirteen segments in total, twelve wild‐type and one carrying the familial mutation ATTR‐D38A. Six of thirteen, including the mutant segment 37AADTWE42, are from the N‐terminal region of TTR (residues 1 to 50) and seven are from the C‐terminal region (residues 50 to 127). We obtained crystal structures from only two segments of the N‐terminal region (33%), and one of them was the mutant segment 37AADTWE42. In contrast, six C‐terminal segments resulted in crystal structures of amyloid‐like fibrils (86%), including the two amyloid‐driving segments 91AEVVFT96 and 119TAVVTN124.14 They all display the distinctive in‐register steric zipper assembly14 (Fig. 2). The only C‐terminal segment that did not crystallize was 65VEGIYK70, which is the first segment predicted to form amyloid fibrils within the C‐terminal stretch and overlaps with 68IYKVEI73 that displays a class 7 in‐register steric zipper [Fig. 2(A)]. Altogether, 32 residues of the 77 residues of the C‐terminus can form amyloid steric zippers, denoting the potential of this region to be involved in the amyloid spine.
Although the sequences of the two segments 105YTIAAL110 and 106TIAALLS112 overlap, their amyloid structures reveal different packing and interfaces (Fig. 2). A previous study of this region by NMR described a third structural assembly of a class 4 steric zipper, distinct from class 8 zippers (105YTIAAL110) and 6 (106TIAALLS112) observed by crystallography.20 This structural variability is known as “packing polymorphism,” where the same segment can adopt different steric zipper assemblies.21 Similar examples are found in IAPP, associated with type II diabetes,21 and amyloid‐β and tau, associated with Alzheimer's disease.22, 23 The high structural variability of an amyloidogenic segment, such as 105YTIAALLS112, may be connected to the formation of distinct aggregate subpopulations, and perhaps may play a role in the formation of Type A and Type B fibril subtypes.
Steric zippers from the N‐terminus of TTR
We determined the structure of a steric zipper formed by the segment 37AADTWE42, containing the familial ATTR‐D38A mutation. We have recently found that the amyloid fibrils extracted from the heart of an ATTR‐D38A patient were categorized as type B and, therefore, comprise full‐length TTR (data not shown). We hypothesize that the mutation ATTR‐D38A may create a new steric zipper forming segment since we found that the presence of the wild‐type aspartate in position 38 would be incompatible with the steric zipper formation [Fig. 2(D)]. However, despite its high shape complementarity and large amount of surface area buried, two factors indicative of a strong assembly (Table 2), the highly hydrated interface of the steric zipper formed by 37AADTWE42 (Fig. 3) and its low solvation energy (Table 2) may suggest a weak overall assembly, thereby questioning its implication in fibril formation.
The structural screening of TTR amyloidogenic segments revealed an out‐of‐register structure formed by 28VAVHVF33 (Fig. 4). The 28VAVHVF33 structure differs from the amyloid‐like in‐register highly‐stable structures; it displays the lowest shape complementarity and area buried among the structures of TTR amyloidogenic segments (Table 2). Similar to previously determined out‐of‐register structures, the β‐strands of 28VAVHVF33 cross the protofilament axis at an acute angle.8, 9 However, in contrast to previous out‐of‐register structures, each strand of 28VAVHVF33 participates in equal number of hydrogen bonds (n = 6) with strands neighboring either side [Fig. 4(D)], therefore indicating a stable in‐sheet packing.
Out‐of‐register assemblies are associated with amyloid oligomer formation.6, 7, 8 These sheets show a propensity to curve, likely hindering their ability to form long fibrils, and limiting their growth to oligomeric assemblies. The structure of 28VAVHVF33 presented here suggests that TTR may be also capable of forming out‐of‐register oligomers. Although the pathological implications of oligomers in ATTR remains unclear,24 a recent report shows the detection of TTR oligomers in plasma of ATTR patients.25 In that study, Kelly and colleagues generated peptide‐based probes that selectively bind TTR oligomers circulating in plasma of polyneuropathic ATTR patients. Interestingly, the probe contains the amyloidogenic segment TTR(25–34) from the β‐strand B, with the segment 28VAVHVF33 as the minimal binding competent sequence. However, they showed that the probe binds C‐terminal fragments of TTR that lack the TTR(25–34) segment, indicating that binding of the probe to TTR oligomers must occur through a different region of TTR. Perhaps other segments from the C‐terminus may form a similar out‐of‐register oligomeric structure that the 28VAVHVF33 has the ability to recognize.
Conclusions
The present study represents an expansion of the current knowledge on transthyretin aggregation. We have previously shown that the strands F and H drive aggregation of transthyretin by self‐association.14 Here, we show the crystal structures of six additional segments in their amyloid state and describe an antiparallel out‐of‐register zipper that may be involved in TTR oligomer formation.
Material and Methods
Sample preparation and crystallization conditions
Peptides were synthesized at > 97% purity from GenScript (Piscataway, New Jersey) and GL Biochem (Shanghai, China). Peptides were dissolved in water or 10% acetonitrile at a concentration that depended on the solubility of the peptide (Table 3). All peptide solutions were spin filtered (0.22 μm) prior to crystallization experiments at 18°C via hanging‐drop vapor diffusion. Crystallizations conditions are detailed in Table 3.
Table 3.
Sample Preparation and Crystallization Conditions
| TTR segment | Sample preparation | Crystallization conditions |
|---|---|---|
| 28VAVHVF33 | 10 mg/mL in water | 0.2 M Magnesium chloride hexahydrate, 0.1 M HEPES sodium pH 7.5, and 30% v/v 2‐Propanol |
| 37AADTWE42 | 100 mg/mL in water | 0.2 M Ammonium phosphate monobasic, 0.1 M Tris pH 8.5, and 50% v/v MPD |
| 68IYKVEI73 | 10 mg/mL in water | 1.5 M NaCl, and 10% v/v ethanol |
| 80KALGIS85 | 10 mg/mL in water | 0.1 M Citric acid pH 3.5, and 2.0 M Ammonium sulfate |
| 105YTIAAL110 | 10 mg/mL in water | 100 mM Bis‐Tris pH 5.5 and 3 M sodium chloride |
| 106TIAALLS112 | 5 mg/mL in 10% acetonitrile | 100 mM Tris pH 8.5 and 0.3 M magnesium formate dihydrate |
Data collection and structure refinement
Data collection and refinement statistics of crystal structures are detailed in Table 1. X‐ray diffraction data were collected at the Advanced Photon Source beamline 24‐ID‐E. Molecular replacement was performed with the program Phaser26 using as search models an idealized polyalanine β‐strand. Crystallographic refinement was performed using PHENIX,27 REFMAC,28 and BUSTER.29 Model building was performed with Coot30 and illustrated with PyMOL.31 The coordinates have been deposited in the Protein Data Bank with accession codes 6C3F, 6C3G, 6C3S, 6C3T, 6C4O, and 6C88.
Structure calculations
Overall energies were calculated from the steric zipper structures by Rosetta Relax.32 Calculations of the area buried and shape complementarity were performed with AREAIMOL17 and SC,18, 33 respectively. Area buried was calculated as the difference in solvent accessible surface area of a central β‐strand within the context of a ten‐stranded β‐sheet and the solvent accessible surface area of the same β‐strand in the same ten‐stranded sheet in contact with the opposite ten‐stranded β‐sheet in the zipper. Rosetta energies and shape complementarity were calculated from an assembly made of two opposed β‐sheets with ten β‐strands each, in the absence of waters or ions. Sheet‐to‐sheet distances were calculated as the average distance between third degree polynomial fits to backbone atoms of opposing β‐sheets, which had been projected down the “fibril” axis. Strand‐to‐strand distance of parallel sheets is given by the corresponding unit cell length. For in‐register antiparallel sheets, it is calculated as this unit cell length divided by two. For out‐of‐register antiparallel sheets, it is taken as an average over stacked backbone atoms of strand n and n + 2.
Conflict of Interests
D.S.E. is an advisor and equity holder of ADRx, Inc. L.S. is a consultant of ADRx, Inc.
Acknowledgments
We thank Glyn Devlin for providing TIAALLS peptide. We thank Duilio Cascio for his assistance with data collection, Michael Collazo of the UCLA Crystallization Facility, the UCLA‐Department of Energy (DOE) X‐ray Crystallography Core Facility, and M. Capel, K. Rajashankar, N. Sukumar, J. Schuermann, I. Kourinov, and F. Murphy at Northeastern Collaborative Access Team beamline 24‐ID‐E at the Advanced Photon Source (APS). Data and materials availability: The authors declare that all data generated or analyzed during this study that support the findings are available within this published article and its supplementary information files.
References
- 1. Eisenberg D, Jucker M (2012) The amyloid state of proteins in human diseases. Cell 148:1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eisenberg DS, Sawaya MR (2017) Structural studies of amyloid proteins at the molecular level. Annu Rev Biochem 86:69–95. [DOI] [PubMed] [Google Scholar]
- 3. Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC (1997) Common core structure of amyloid fibrils by synchrotron X‐ray diffraction. J Mol Biol 273:729–739. [DOI] [PubMed] [Google Scholar]
- 4. Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D (2005) Structure of the cross‐beta spine of amyloid‐like fibrils. Nature 435:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJ, McFarlane HT, Madsen AO, Riekel C, Eisenberg D (2007) Atomic structures of amyloid cross‐beta spines reveal varied steric zippers. Nature 447:453–457. [DOI] [PubMed] [Google Scholar]
- 6. Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D (2012) Atomic view of a toxic amyloid small oligomer. Science 335:1228–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sangwan S, Zhao A, Adams KL, Jayson CK, Sawaya MR, Guenther EL, Pan AC, Ngo J, Moore DM, Soriaga AB, Do TD, Goldschmidt L, Nelson R, Bowers MT, Koehler CM, Shaw DE, Novitch BG, Eisenberg DS (2017) Atomic structure of a toxic, oligomeric segment of SOD1 linked to amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci U S A 114:8770–8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu C, Zhao M, Jiang L, Cheng PN, Park J, Sawaya MR, Pensalfini A, Gou D, Berk AJ, Glabe CG, Nowick J, Eisenberg D (2012) Out‐of‐register β‐sheets suggest a pathway to toxic amyloid aggregates. Proc Natl Acad Sci U S A 109:20913–20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soriaga AB, Sangwan S, Macdonald R, Sawaya MR, Eisenberg D (2016) Crystal structures of IAPP amyloidogenic segments reveal a novel packing motif of out‐of‐register beta sheets. J Phys Chem B 120:5810–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingenbleek Y, Young V (1994) Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr 14:495–533. [DOI] [PubMed] [Google Scholar]
- 11. Blake CC, Geisow MJ, Oatley SJ, Rerat B, Rerat C (1978) Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol 121:339–356. [DOI] [PubMed] [Google Scholar]
- 12. Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, Berk JL, Plante‐Bordeneuve V, Schmidt HH, Merlini G (2015) Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 66:2451–2466. [DOI] [PubMed] [Google Scholar]
- 13. Bergstrom J, Gustavsson A, Hellman U, Sletten K, Murphy CL, Weiss DT, Solomon A, Olofsson BO, Westermark P (2005) Amyloid deposits in transthyretin‐derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. J Pathol 206:224–232. [DOI] [PubMed] [Google Scholar]
- 14. Saelices L, Johnson LM, Liang WY, Sawaya MR, Cascio D, Ruchala P, Whitelegge J, Jiang L, Riek R, Eisenberg DS (2015) Uncovering the mechanism of aggregation of human transthyretin. J Biol Chem 290:28932–28943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson MJ, Sievers SA, Karanicolas J, Ivanova MI, Baker D, Eisenberg D (2006) The 3D profile method for identifying fibril‐forming segments of proteins. Proc Natl Acad Sci U S A 103:4074–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldschmidt L, Teng PK, Riek R, Eisenberg D (2010) Identifying the amylome, proteins capable of forming amyloid‐like fibrils. Proc Natl Acad Sci U S A 107:3487–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee B, Richards FM (1971) The interpretation of protein structures: estimation of static accessibility. J Mol Biol 55:379–400. [DOI] [PubMed] [Google Scholar]
- 18. Lawrence MC, Colman PM (1993) Shape complementarity at protein/protein interfaces. J Mol Biol 234:946–950. [DOI] [PubMed] [Google Scholar]
- 19. Kishikawa M, Nakanishi T, Miyazaki A, Shimizu A, Kusaka H, Fukui M, Nishiue T (1999) A new amyloidogenic transthyretin variant, [D38A], detected by electrospray ionization/mass spectrometry. Amyloid 6:278–281. [DOI] [PubMed] [Google Scholar]
- 20. Fitzpatrick AW, Debelouchina GT, Bayro MJ, Clare DK, Caporini MA, Bajaj VS, Jaroniec CP, Wang L, Ladizhansky V, Müller SA, MacPhee CE, Waudby CA, Mott HR, De Simone A, Knowles TP, Saibil HR, Vendruscolo M, Orlova EV, Griffin RG, Dobson CM (2013) Atomic structure and hierarchical assembly of a cross‐β amyloid fibril. Proc Natl Acad Sci U S A 110:5468–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiltzius JJ, Landau M, Nelson R, Sawaya MR, Apostol MI, Goldschmidt L, Soriaga AB, Cascio D, Rajashankar K, Eisenberg D (2009) Molecular mechanisms for protein‐encoded inheritance. Nat Struct Mol Biol 16:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colletier JP, Laganowsky A, Landau M, Zhao M, Soriaga AB, Goldschmidt L, Flot D, Cascio D, Sawaya MR, Eisenberg D, (2011) Molecular basis for amyloid‐beta polymorphism. Proc Natl Acad Sci U S A 108:16938–16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seidler PM, Boyer DR, Rodriguez JA, Sawaya MR, Cascio D, Murray K, Gonen T, Eisenberg DS (2018) Structure‐based inhibitors of tau aggregation. Nat Chem 10:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manral P, Reixach N (2015) Amyloidogenic and non‐amyloidogenic transthyretin variants interact differently with human cardiomyocytes: insights into early events of non‐fibrillar tissue damage. Biosci Rep 35:e00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schonhoft JD, Monteiro C, Plate L, Eisele YS, Kelly JM, Boland D, Parker CG, Cravatt BF, Teruya S, Helmke S, Maurer M, Berk J, Sekijima Y, Novais M, Coelho T, Powers ET, Kelly JW (2017) Peptide probes detect misfolded transthyretin oligomers in plasma of hereditary amyloidosis patients. Sci Transl Med 9:eaam7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCoy AJ, Grosse‐Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) Phaser crystallographic software. J Appl Cryst 40:658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zwart PH, Afonine PV, Grosse‐Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, McKee E, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Storoni LC, Terwilliger TC, Adams PD (2008) Automated structure solution with the PHENIX suite. Methods Mol Biol 426:419–435. [DOI] [PubMed] [Google Scholar]
- 28. Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum‐likelihood method. Acta Cryst D 53:240–255. [DOI] [PubMed] [Google Scholar]
- 29. Blanc E, Roversi P, Vonrhein C, Flensburg C, Lea SM, Bricogne G (2004) Refinement of severely incomplete structures with maximum likelihood in BUSTER‐TNT. Acta Cryst 60:2210–2221. [DOI] [PubMed] [Google Scholar]
- 30. Emsley P, Cowtan K (2004) Coot: model‐building tools for molecular graphics. Acta Cryst 60:2126–2132. [DOI] [PubMed] [Google Scholar]
- 31. DeLano WL (2002) PyMOL Molecular Viewer. DeLano Scientific, San Carlos, CA, 700.
- 32. Conway P, Tyka MD, DiMaio F, Konerding DE, Baker D (2014) Relaxation of backbone bond geometry improves protein energy landscape modeling. Protein Sci 23:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connolly ML (1983) Solvent‐accessible surfaces of proteins and nucleic acids. Science 221:709–713. [DOI] [PubMed] [Google Scholar]


