Abstract
Several viruses are recognized as the direct or indirect causative agents of human tumors and other severe human diseases. Vascular endothelial growth factor (VEGF) is identified as a principal proangiogenic factor that enhances the production of new blood vessels from existing vascular network. Therefore, oncogenic viruses such as Kaposi’s sarcoma herpesvirus (KSHV) and Epstein-Barr virus (EBV) and non-oncogenic viruses such as herpes simplex virus (HSV-1) and dengue virus, which lack their own angiogenic factors, rely on the recruitment of cellular genes for angiogenesis in tumor progression or disease pathogenesis. This review summarizes how human viruses exploit the cellular signaling machinery to upregulate the expression of VEGF and benefit from its physiological functions for their own pathogenesis. Understanding the interplay between viruses and VEGF upregulation will pave the way to design targeted and effective therapeutic approaches for viral oncogenesis and severe diseases.
Keywords: angiogenesis, vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor (VEGFR), Epstein-Barr virus (EBV), Kaposi’s sarcoma herpesvirus (KSHV), Hepatitis C Viruses (HCV), Hepatitis B Viruses (HBV), Herpes Simplex Virus (HSV), Hantavirus, Dengue fever virus, therapeutics
1. Introduction
Angiogenesis is an important physiologic process which involves formation of new blood vessels from already existing vasculature. It plays a vital role during development and wound healing but also during disease pathology such as tumor growth and progression. Several stimulators are involved in the angiogenesis process including vascular endothelial growth factor (VEGF), which plays a crucial role in activating endothelial cells through binding to receptors on the cell surface called vascular endothelial growth factor receptor (VEGFR) [1]. There are four VEGF isoforms in mammals (VEGF-A,B,C,D) and the placental growth factor (PlGF) encoded by different but related genes [2]. Most of these isoforms express alternative splice variants or proteolytic cleaved proteins to produce a range of functional VEGF isoforms and splice variants. Furthermore, VEGF isoforms signal through different receptors (VEGFR1,R2 and R3) and coreceptors including the neuropilin receptors (Nrp1 and 2), heparan sulfate and integrins [3]. The three VEGFRs are tyrosine kinases and mediate signal transduction upon ligand binding and dimerization [4]. VEGFR1 binds VEGF-A, VEGF-B and PlGF. It is also present in a soluble form and therefore expected to play a negative regulatory role in VEGF signaling [5]. VEGFR2 binds VEGF-A, VEGF-C and VEGF-D and is implicated in most vascular and endothelial biological processes through phosphorylation activities at several tyrosine residues in its cytoplasmic tail [6]. VEGFR3 is the receptor for VEGF-D and VEGF-C and expected to play a role in lymphatic endothelium development [6]. Nonetheless, VEGFR3 was found to additionally play a role through its kinase activity in sprouting of endothelial cells during blood vessels formation [7,8].
VEGF-A is the prototype of all VEGFs and by far the most extensively studied isoform and therefore in some literature is referred to as VEGF. Seven proangiogenic splice variants exist from VEGF-A based on the presence of alternative splice site selection in exons 6 and 7 generating a variety of isoforms partially or completely lacking exons 6, 7 or 8 [9,10]. This family of splice variants is called VEGF-Axxx, where the triple-x indicates the number of amino acids in the protein. Another family of VEGF-A splice variants is produced based on an extra splicing site in exon 8 in addition to the splicing sites in exon 6 and 7. The involvement of the distal exon 8 splicing site in VEGF-A production generates VEGF isoforms similar to their VEGF-Axxx counterparts but with six different amino acids at its carboxy-terminus. This family of splice variants is called VEGF-Axxxb and is believed to have antiangiogenic properties [9,10].
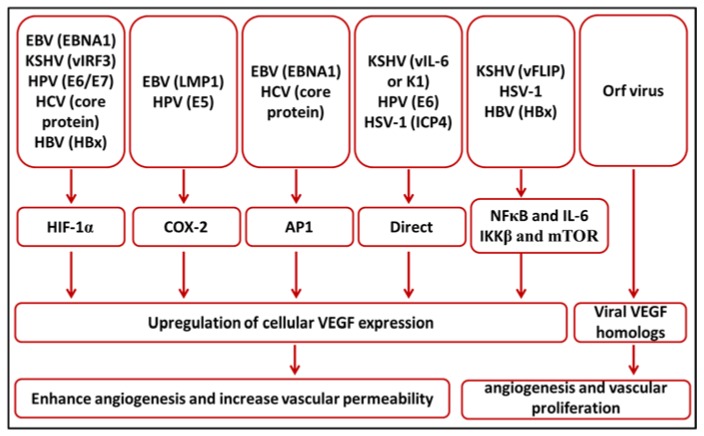
Human viruses cause a variety of diseases where neither specific successful treatment nor safe and effective vaccine are available. VEGFs and their receptors have been implicated in the pathophysiology of many of these diseases. This review discusses the involvement of VEGFs and their receptors in viral diseases and the possible therapeutic applications. Table 1 and Figure 1 summarize common human viruses and their diverse mechanism of upregulation of VEGF expression.
Table 1.
Summary of viruses exploiting vascular endothelial growth factor (VEGF) upregulation in human diseases.
| Virus | Disease | Mechanism of VEGF Upregulation | References |
|---|---|---|---|
| EBV | Nasopharyngeal carcinoma | LMP1 upregulates the expression of VEGF through the phosphorylation of JNKs/c-Jun signaling pathways | [20,21] |
| LMP1 upregulates COX-2 expression which leads to the upregulation of VEGF expression | [22] | ||
| EBNA1 activates the AP-1 transcription factor which enhances the transcription of VEGF | [24] | ||
| Gastric carcinoma | Overexpression of HIF-1α leads to the upregulation of VEGF expression | [26] | |
| KSHV | Kaposi’s sarcoma | vFLIP activates the transcription factor NF-κB which induce inflammatory response leads to upregulation of VEGF | [38,39,40] |
| Viral K1 and vIL-6 directly implicated in expression of VEGF | [41,42] | ||
| vGPCR enhances the upregulation of VEGF | [43] | ||
| vIRF3 stabilizes HIF-1α to enhance VEGF expression | [44] | ||
| HPV | Cervical cancer, head and neck carcinoma | E6 binds to a responsive region consisting of four SP-1 sites in the VEGF promoter region | [58] |
| E7 upregulates VEGF expression through the telomerase reverse-transcriptase (hTERT) and telomerase activity | [59] | ||
| E5 activates the EGFR which in turn leads to the phosphorylation of PI3K and Akt, enhancement of the transcription of COX-2 leading to increase in VEGF expression | [56,60] | ||
| HCV | Hepatocellular carcinoma | Core protein stabilizes HIF-1α which automatically upregulates the expression of VEGF | [67,68,69] |
| Core protein activates the AP-1 transcription factor which potentiates VEGF expression through direct binding to its promoter | [71] | ||
| HBV | Hepatocellular carcinoma | HBx protein stabilizes the HIF-1α and enhances VEGF expression | [79,80,81] |
| HBx induces mTOR and IKKβ which in turn induces VEGF expression | [82] | ||
| Pre-S protein potentiates the expression of VEGF | [83] | ||
| HSV-1 | Herpetic stromal keratitis | ICP4 binds directly to the proximal promoter of VEGF gene and drives its transcription | [90] |
| HSV-1 disturbs the balance between VEGF and its neutralizing receptor sVEGFR1 | [91] | ||
| DENV | Dengue hemorrhagic fever, dengue shock syndrome | Upregulation of Th1 and Th2 cytokines and VEGF | [102,103] |
| Hantaviruses | Hantavirus Pulmonary Syndrome, Hemorrhagic Fever with Renal Syndrome | Mechanism jet to be elucidated | |
| Orf virus | Pustular skin disease | Encodes VEGF homolog called VEGF-E | [113,115] |
Figure 1.
A schematic diagram summarizes how different viruses mediate vascular endothelial growth factor (VEGF) upregulation.
2. Upregulation of VEGF Expression in Viral Oncogenesis
2.1. Epstein-Barr Virus (EBV)
EBV is a human herpesvirus that asymptomatically infects the majority of the human population. EBV infection has been implicated in many diseases such as infectious mononucleosis after primary infection and neoplasia including lymphoproliferative disorders and lymphomas such as Burkitt’s lymphoma, nasal NK/T-cell lymphoma and a subset of Hodgkin’s lymphoma [11]. EBV was also found to be associated with solid tumors such as nasopharyngeal carcinoma (NPC) and small fraction of gastric carcinoma [12,13]. During infection, EBV establishes a state of latency where only few viral proteins are expressed driven by the pressure from the immune system.
VEGF expression and microvascular density were shown to directly correlate with NPC and its metastatic progression, indicating the importance of VEGF for NPC growth and progression [14]. Several other studies found a correlation between the severity of NPC or its metastatic progression and the levels of VEGF in circulation, saliva or in the tumor itself [15,16,17,18]. The EBV latent membrane protein 1 (LMP1) is one of the proteins expressed during the latency state II of EBV and is frequently detected in NPC along with EBNA1, LMP2A and B, and a transcript from BamHI A restriction fragment (BART) [19]. The overexpression of the oncoprotein LMP1 in transgenic mice led to upregulation of VEGF expression early in life accompanied with hyperplasia and increased vascularization which progressed spontaneously later to carcinoma [20]. LMP1 is a transmembrane protein and seems to exert its effect on VEGF expression through other mediators such as the JNKs/c-Jun signaling [21]. Furthermore, LMP1 expression was found to be concomitant with COX-2 expression in NPC. Overexpression of LMP1 in nasopharyngeal epithelial cell lines increased the expression of COX-2, which in turn enhanced VEGF expression [22]. EBV-LMP1 expression was also found to be significantly associated with VEGF expression in diffuse large B cell lymphoma (DLBCL) and their expression was associated with reduced survival rate [23].
EBNA1, an EBV nuclear protein, plays an important role in virus persistence and was demonstrated to be expressed in EBV-associated tumors. EBNA1 seems to play an additional role in EBV-associated malignancies by indirect enhancement of angiogenesis through the activation of AP-1 transcription factor in NPC [24]. AP-1 expression is enhanced by EBNA1 binding and consequently leads to increased expression of its downstream targets such as VEGF and IL-8 [24].
EBV is associated with a small fraction of gastric carcinoma cases and was associated with increased alteration in the PI3K pathway [25]. Several viral sequences were detected in the tumor cells including EBNA1. VEGF expression is upregulated in EBV-associated gastric cancer and influenced by the overexpression of hypoxia-inducible factor-1 alpha (HIF-1α) [26]. LMP1 is known to directly induce the expression of the HIF-1α [27]; however, LMP1 is not expressed in EBV-associated gastric carcinoma. Whether EBV achieves the upregulation of VEGF through EBNA1 in gastric carcinoma needs to be elucidated.
It is obvious that EBV upregulates the expression of VEGF via its own oncoproteins, LMP1 and EBNA1, and exploits the VEGF proangiogenic characteristics to promote the growth and progression of its tumors. EBV is a ubiquitous virus and infects a large proportion of the human population. The oncoproteins, LMP1 and EBNA1, are expressed in most EBV-infected cells yet and luckily only a small number of EBV infections progress to tumor. What are the major factors involved in EBV-associated tumorigenesis are not clearly identified but many host genetic factors and environmental risk factors are expected to play an additional role.
2.2. Kaposi’s Sarcoma-Associated Herpesvirus (KSHV)
Kaposi’s sarcoma (KS) is a malignancy characterized by neoangiogenesis and infiltration of inflammatory cells. It is also characterized by the presence of spindle cells which were all found to be infected by the Kaposi’s sarcoma-associated herpesvirus (KSHV) [28,29]. KSHV was detected in all forms of KS and is associated with two other neoplasms, the primary effusion lymphoma and multicentric Castleman disease. KS lesions express large amounts of VEGF which is important for the tumor growth, while blocking of the VEGF receptors abolishes tumor growth [28,30,31]. Moreover, VEGFR2 was shown to be upregulated in the tumor endothelial cells and the stromal vessels in tumor and tissues surrounding it [32,33]. Animal experiments provided an insight into the role of KSHV genes in VEGF upregulation. KSHV-infected endothelial cells when injected into nude mouse promoted tumor formation with expression of elevated levels of VEGF [34]. In another mouse model, bone marrow-derived endothelial cells were infected with KSHV-genetically engineered in a bacterial artificial chromosome (BAC) and injected into severe combined immunodeficiency (SCID) mice [35]. The mice developed tumor reminiscent of KS and expressed increased levels of VEGF [35].
Similar to EBV, KSHV is present in KS spindle cells in a latent form and only few genes are expressed. The viral FLICE inhibitory protein (vFLIP) encoded by KSHV ORFK13 is one of these genes, which is responsible for the spindling morphology of KS endothelial cells [36,37]. vFLIP activates the canonical and noncanonical NF-κB pathways and hence it is responsible for the inflammatory profile observed in KS lesion [38,39]. The expression of inflammatory mediators and growth factors induced through vFLIP activation of NF-κB attracts the migration and recruitment of inflammatory cells such as monocytes and macrophages, which constitute a generous source of VEGF production [40].
Other KSHV proteins were implicated in VEGF expression directly such as K1 and viral interlukin-6 (vIL-6) [41,42]. The viral G-protein coupled receptor (vGPCR) was reported to produce KS-like lesions with high VEGF levels in transgenic mice [43]. The viral interferon regulatory factor 3 (vIRF3) was found to enhance the stability of HIF-1α to induce VEGF expression [44]. It is worth noting that all of these proteins are considered proteins of the lytic KSHV replication cycle and not expressed in KS lesions where KSHV is mainly latent. However, it was demonstrated that a minor number of KSHV-infected cells undergoes spontaneous virus lytic replication [45]. This small fraction of cells provides continuous virus supply to infect new cells and provides a wide spectrum of viral genes that are involved in inflammatory profile of the KS lesion.
2.3. Human Papillomavirus (HPV)
HPV infects the mucosal and cutaneous tissues and is the common cause of warts on skin and genitalia. In 1970, HPV was identified in cervical cancer and a decade later in a subset of oropharyngeal carcinomas (OPC) [46]. Since then, HPV was implicated in the pathogenesis of many human cancers at variable percentages including cervical cancer, anal cancers, penile cancers, vaginal cancers, vulvar cancers, head and neck carcinoma, and skin cancer [47]. About 179 genotypes of HPV are known so far based on variation in their genome sequence [48]. HPV genotypes can be classified into low risk and high risk based on their malignancy transforming capability. Those genotypes implicated in HPV-associated malignancies are considered high risk and these are mainly HPV16 and HPV18; however, other genotypes such as HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52 and HPV56 are being frequently reported in cancer [48,49,50]. HPV encodes 10 proteins, three of which (E5, E6 and E7) are detected in human tumors and considered to be responsible for oncogenesis [51]. E5 protein on its own has a weak transforming activity in cell culture but it can collaboratively potentiate the transforming activity of both E6 and E7 [52]. The transforming magnitude of HPV lies in E6 and E7 proteins where both are able to enhance cell proliferation, destabilize the genome and more importantly abrogate apoptosis [51]. E6 utilizes the cellular ubiquitin ligase E6AP to target p53 for degradation [53]. E7 activity leads to activation of the elongation factor 2 (E2F) and increased expression of the cellular p16 and ultimately enhance cell proliferation [54]. Further details on the role of E6 and E7 in HPV oncogenesis can be found in [51].
E6 and E7, especially of HPV16 and HPV18 genotypes, also play a role in VEGF upregulation and therefore by establishing the angiogenic structure of HPV-associated cancers. HPV16 E6 and E7 oncoproteins were demonstrated to upregulate the expression of HIF-1α and VEGF in non-small cell lung carcinoma and cervical carcinoma cells [55,56,57]. Using inhibitors that target the ERK1/2 or PI3K pathways leads to complete inhibition of HIF-1α and VEGF expression, suggesting the involvement of these signaling pathways in E6- and E7-mediated angiogenesis [56]. E6 seems to have a direct effect on the VEGF promoter. It binds to a responsive region consisting of four SP-1 sites in the VEGF proximal promoter region [58]. While E7 exerts its effect on upregulation of VEGF expression through the telomerase reverse-transcriptase (hTERT) and telomerase activity [59], E5 also induces the expression of VEGF. E5 activates the EGFR which in turn leads to the phosphorylation of downstream molecules PI3K and Akt. These enhance the transcription of COX-2, through increasing its promoter activity, leading to increase in VEGF expression [60]. This tight controlled upregulation of VEGF by the three oncoproteins of HPV indicates the importance of VEGF for HPV-associated malignancies and makes it an attractive target for therapeutic approaches.
2.4. Hepatitis C Viruses (HCV)
HCV infects a large number of the human population and in the majority of cases the infection is chronic, leading to frequent inflammation which ends up with cirrhosis in about 20% of cases and with hepatocellular carcinoma (HCC) in up to 5% of cases [61]. HCV infection constitutes a risk for development of HCC whose incidence is increasing in many countries [62]. In addition to chronic inflammation, HCV may also directly potentiate the risk of developing HCC by regulating several host pathways including those involved in angiogenesis. HCV proteins (core, NS3, NS5A, NS5B and E) through interference with cellular factors (Rb, Cyclin D, Cyclin E, RAF/MAP/ERK) stimulate cell cycle progression, enhance cellular proliferation, and inhibit apoptosis, which are key players in tumorigenesis [61].
Similar to other tumors, angiogenesis in HCC is critical for tumor growth and progression. HCV was found to be associated with higher microvessel density in HCC [63]. Several studies have shown the upregulation of the proangiogenic factor VEGF in HCV-related HCC tissues, patient’s serum and in cell culture experiments [64,65,66]. Polymorphisms in the VEGF gene were found to increase the VEGF expression levels and to be associated with higher risk of HCC [66]. It is also suggested that VEGF serum levels could be utilized as a prognostic factor of HCC [64,65]. The HCV core protein seems to be the major moderator of HCV-dependent VEGF upregulation in different mechanisms. The core protein stabilizes HIF-1α, which automatically upregulates the expression of VEGF [67,68,69]. Inhibition of the Jak/Stat pathway was found to abrogate the core protein-mediated activation of the androgen receptor and, thereby, the downregulation of VEGF expression, which suggests a role for Jak/Stat signaling pathway in HCV-mediated VEGF expression [70]. Recently it was shown that the core protein also activates the AP-1 transcription factor, which potentiates VEGF expression through direct binding to its promoter [71].
2.5. Hepatitis B Viruses (HBV)
HBV is responsible for about half of HCC cases worldwide [72,73]. Contrary to HCV, HBV is a DNA virus; therefore, it can integrate itself in the cellular genome resulting in deletions and general instability [74]. Additionally, HBV establishes chronic infection in 15–40% of cases leading to continuous cycles of necrotic inflammation leading to cirrhosis and eventually to HCC [72]. Similar to other cancers, VEGF seems be the important angiogenic factor of the HBV-related hepatocarcinogenesis. The gene expression profile in HBV-related HCC mouse model showed clear induction of VEGF- and EGF-mediated pathways [75]. VEGF expression was found to be upregulated along with COX-2 in tissue sections from human HCC with HBV infection and this expression was found to be positively correlated with microvessel density (MVD) [76,77].
The hepatitis B viral protein x (HBx) is the key inducer of VEGF expression in HBV-related HCC. VEGF transcription was found to be induced in HBx stably transfected cells [78]. Similar to the HCV core protein, HBx stabilizes HIF-1α and enhances the increase in VEGF expression [79,80,81]. Overexpression of HBx in hepatoma cells leads to induction of mTOR and IKKβ, which in turn enhance cell proliferation and increase the expression of VEGF [82]. Pre-S protein was also reported to potentiate the expression of VEGF and, by that, augment the angiogenic environment produced by the virus [83].
The availability and accessibility of safe and effective recombinant HBV vaccine will markedly decrease the number of new HBV cases and thereby the HBV-related HCC. According to the World Health Organization (WHO), HBV vaccine was incorporated in the infant immunization programs of 95% of countries and about 50% of countries had adopted the recommended dose at birth [84].
3. Upregulation of VEGF Expression in Non-Oncogenic Viral Infections
3.1. Herpes Simplex Virus-1 (HSV-1)
HSV type 1 infects more than half of the human population and the rate of infection may reach up to 90% in some geographical areas [85]. It causes Herpes labialis (cold sores) after primary infection and then establishes a lifelong infection residing latently in the trigeminal ganglia. HSV-1 may also cause ocular infection called herpetic stromal keratitis (HSK), where the cornea loses transparency due to neovascularization, which results in impaired vision and blindness [86]. VEGF and its VEGFR2 receptor are involved in the pathogenesis of HSK through the induction of lymphatic neoangiogenesis in the cornea and the underlying stromal tissue [87]. HSV-1 infection of the cornea triggers VEGF expression in the cornea and the stromal cells despite the absence of HSV-stromal cells infection [88]. It is believed that VEGF expression in the stroma is mediated by a paracrine effect of the IL-6 cytokine, which is also induced by HSV-1 infection of the cornea [89]. The infection cellular protein-4 (ICP4) seems to be the major player in HSV-mediated VEGF expression. ICP4 binds directly to the proximal promoter of the VEGF gene and drives the transcription of VEGF mRNA in collaboration with other early viral proteins [90]. HSV-1 infection of the cornea does not only upregulate VEGF expression, but also disrupts the balance with its soluble neutralizing receptor (soluble vascular growth factor receptor-1, sVEGFR1) by facilitating the sVEGFR1 degradation via the metalloproteases enzymes which are produced by the infiltrating inflammatory cells as a result of infection [91]. HSV-1 additionally exploits host factors such as the host microRNA-132 and the cytokine IL-17A to disturb the balance between VEGF and its neutralizing receptor sVEGFR1 and render the immune-privileged corneal tissue accessible to inflammatory cells and mediators and induces neovascularization [92,93]. It was recently observed that the fibroblast growth factor-2 (FGF-2), whose expression is also upregulated after HSV-1 infection, sustains the VEGF-mediated neovascularization of the cornea even after resolving of HSV-1 infection [94].
3.2. Dengue Virus (DENV)
Dengue virus is an arthropod-borne virus transmitted to human through the Aedes mosquito vector. There have been four serotypes identified (DENV1 to DENV4). DENV causes a mild feverish disease called Dengue fever (DF). However, the disease may develop into severe complications called dengue hemorrhagic fever (DHF), which is characterized by increased capillary permeability and plasma leakage [95]. Continuous plasma leakage may lead to decreased intravascular volume and hypotensive shock (dengue shock syndrome, DSS). VEGF was previously called vascular permeability factor (VPF) as it caused increase permeability of capillaries [96]. Many studies have shown the presence of elevated serum levels of VEGF in patients with DHF but not DF [97,98,99,100,101]. Other studies have demonstrated that DENV infection of pulmonary endothelial cell lines upregulates the expression of VEGF along with many Th1 and Th2 cytokines [102,103]. For this reason, it is believed that immune preparedness is a major determinant of disease severity [95].
3.3. Hantaviruses
The Hantavirus genus belongs to the family Bunyaviridae and comprises a group of zoonotic viruses (such as Andes virus, Hantaan virus, Seoul virus, and others), which transmit from the primary reservoir to human through inhalation of aerosols from rodents’ feces, urine or saliva [104]. Rodents have been identified as the primary reservoir for several Hantaviruses from different geographical regions [105]. Hantaviruses cause serious diseases called Hantavirus Pulmonary Syndrome (HPS) and Hemorrhagic Fever with Renal Syndrome (HFRS) with fatality rate of up to 40% [104,105]. Disease manifestations are overlapping and characterized by increased permeability and vasodilatation which leads to extravasation of inflammatory mediators and blood in the affected organs. High levels of VEGF were implicated in the pathogenesis of many Hantaviruses [106,107].
Andes virus for example infects pulmonary endothelial cells in vitro and induces VEGF expression which leads to abnormal increased permeability [108]. VEGF expression in Andes-infected endothelial cells was found to be preceded by production of virus progeny [109]. Andes virus and Hantaan virus disturb the assembly of adherence junction of vascular endothelial cells by internalizing cadherins of endothelial cell junction and dysregulating β3-integrin and therefore allow extravasation of blood and inflammatory mediators [107,110]. Several other studies showed the upregulation of the VEGF by one or another virus from the Hantavirus genus [111,112].
4. Viral VEGF Homolog Proteins
Some viruses encode their own proangiogenic homolog such as the VEGF-E encoded by orf virus (ORFV). ORFV belongs to the genus Parapox of the family Poxviridae. It infects keratinocytes and causes pustular skin disease in sheep and goats which may transmit to human through direct contact [113]. Tissue sections from the ORFV skin lesions show high vascularization and infiltration of inflammatory components [114]. Considering the vascularized and edematous nature of the ORFV lesion, it was tempting to imagine the involvement of VEGF in ORFV pathogenesis. In 1994, it was discovered that ORFV encodes a homolog of the human VEGF later called VEGF-E and was found to be responsible for the virus-associated angiogenesis [113,115]. ORFV lacking functional VEGF-E gene causes lesions without dermal swelling and vascular proliferation [116,117]. VEGF-E shares about 25% homology with VEGF-A and binds with strong affinity to VEGFR2 [118,119]. VEGF-E lacks hairpin-binding domain and, therefore, cannot bind and engage the coreceptor heparan sulfate, whereas the VEGF-E variant encoded by the ORFVNZ2 was found to bind to the coreceptor neuropilin-1 (NRP-1) through the RPPR peptide in its carboxyterminus and to induce the assembly of VEGFR2-NRP-1 complex [120,121,122]. VEGF-E can induce a strong angiogenic response comparable to VEGF-A, however, without the hemorrhagic effect and without disturbing the endothelial junctions which are considered adverse side effects of VEGF-A angiogenic properties [118]. This favorable angiogenic property of VEGF-E makes it a good candidate for proangiogenic therapy in clinical practice (discussed below).
Other viruses from the Parapox genus were found also to express VEGF homolog such as the Bovine papular stomatitis virus (BPSV) and Pseudocowpox (PCPV), which may also infect human [123,124]. A VEGF homolog was also detected in the Parapoxviruses of red deer in New Zealand (PVNZ); however, this virus was not reported to infect human [125].
5. Therapeutic Applications of Targeting VEGF in Viral Diseases
Apparently, targeting the molecular modulators of angiogenesis, in particular VEGF, is an attractive area of research and tempting approach for drug design to treat viral oncogenesis and other viral diseases when angiogenesis is involved. Lack of specific treatment and effective vaccination for most virus diseases adds more significance to this approach.
A bulk of evidence shows that the inhibition of VEGF function in viral oncogenesis and viral diseases leads to very promising outcome [126,127]. The encouraging results from inhibition of VEGF-mediated angiogenesis in tumors led to testing anti-VEGF antibody in phase II multicenter clinical trial [128]. It was found that addition of anti-VEGF monoclonal antibody (Bevacizumab) to the traditional chemoradiation treatment of NPC apparently delayed the progression of distant metastasis [128]. The use of rapamycin and other mTOR inhibitors clearly reduced the secretion of VEGF and led to inhibition of KS growth and formation of neovasculature [129]. Recently two clinical phase II trials were performed to test the efficiency of targeting VEGF in cervical neoplasia and NPC. The first study investigated the use of celecoxib on cervical intraepithelial neoplasia 3 (CIN 3) and found that histologic regression rate was only observed in patients with high levels of serum VEGF [130]. On the other hand, testing cetuximab and pemetrexed in combination with radiation therapy showed a promising efficacy with the expected toxicity from these two drug combinations regardless of HPV positivity status [131]. Furthermore, the reduction of VEGF levels by melatonin enhanced the therapeutic potency of the HPV DNA vaccine through potentiating the immune response and production of HPV-E7-specific CD8+ cells [132].
The high vascular nature of HCC made the anti-angiogenic therapies an attractive approach for treatment. Sorafenib, for example, was one of the first anti-angiogenic drugs, which showed improved survival of patients with advanced HCC. Sorafenib is an inhibitor of several tyrosine kinases including the VEGF receptors and was shown to induce apoptosis in cell lines from HCC and inhibit angiogenesis in HCC mouse model [133]. The promising therapeutic effects of sorafenib lead to development of other anti-angiogenic agents that were tested in phase II and III clinical trials alone or in combination and showed promising success in reduction of tumor growth and improving survival in the presence or absence of HCV [134]. Interestingly, sorafenib has an additional inhibitory effect on multiple steps of HCV replication [135,136]. In HBV-related HCC treated with sorafenib, high HBV load was associated with poor prognosis unless an anti-viral therapy was added to the therapy [137].
Targeting VEGF and its receptors in herpetic stromal keratitis by local application of siRNA or with a delivery vehicle in mouse models markedly reduced neovascularization and proved a useful therapy approach for angiogenesis-related ocular diseases [138]. The use of anti-VEGF such as bevacizumab or ranibizumab, along with other therapy techniques including surgery and the use of immunosuppressive drugs, enhanced the restoration of the cornea in herpetic keratitis [139].
The late appearance of the symptoms in Hantavirus Pulmonary Syndrome (HPS) makes antiviral treatment such as interferon and ribavirin ineffective against the disease [140,141]. Therefore, treatment strategies stabilizing endothelial cell permeability and tissue vasculature seem the potential approach to reduce disease severity and mortality [140]. In line with this suggestion, it was observed that the angiopoietin 1 (Ang-1) and sphingosine 1-phosphate (S1P) inhibit endothelial cell permeability induced by Hantavirus [107]. Using the VGEFR2 inhibitor (pazopanib) in addition to the src kinase inhibitor (dasatinib) dramatically inhibited endothelial cell permeability induced by ANDV [142]. Similarly, the use of vandetanib as an inhibitor of VEGFR2 phosphorylation reduced VE-cadherin degradation and modestly increased the survival in HPS animal model [143].
It is worth noting that despite the initial expectation of successful anti-angiogenic therapies, limitation appeared quickly represented by initial response then quick development of resistance. Therefore, and as shown by other studies, anti-angiogenic therapeutic approaches should be used in combination with other drugs that target additional pathway in the disease pathology. Furthermore, studies on therapeutic applications of VEGF and other angiogenic factors in viral malignancies and viral severe diseases should be directed against the viral protein which enhances the production of VEGF rather than cellular VEGF itself and other modulator of angiogenesis. Such viral targets are limited in number, provide good selective targeting approach, and avoid the disturbance of the normal physiologic functions of cellular protein. For example, the use of an LMP1 antibody in combination with the classical chemotherapy showed a marked reduction in VEGF and apoptosis and inhibited NPC xenograft growth in nude mice [144].
VEGF therapeutic applications may extend beyond the inhibition of angiogenesis to promotion of angiogenic effects in clinical practice. The favorable prongiogenic properties of the VEGF-E without enhancement of inflammation and vascular permeability which are common characters of other VEGFs suggest a possible application for VEGF-E in pro-angiogenic therapies [145]. A chimeric protein consisting of VEGF-E and the human PlGF-enhanced vascularization in ischemic tissue [146]. Animal experiments in equine found that the use of Orf virus VEGF-E and IL-10 promotes wound healing and reduces inflammation but has no effect on the speed of wound closure process [147].
6. Summary and Conclusions
VEGF seems to be an important player in the pathogenesis of many viral diseases. Therefore, many viruses seek the upregulation of VEGF by several means and some viruses bring their VEGF homolog with them to the infected host (Figure 1). HIF-1α, COX-2 and AP1 appear to be the most common target pathways for virus-mediated upregulation of VEGF. However, some other viruses activate certain inflammatory mediators which end up by the upregulation of VEGF expression. Other viruses directly activate the VEGF promotor to enhance its expression by their own effector proteins (Figure 1). Therefore, major research efforts are required for a very good understanding of the role of viral gene products in upregulation of VEGF expression and are essential for designing novel therapeutic protocols and discovering new chemicals that selectively target viral genes and spare cellular physiologic functions.
Acknowledgments
The author would like to thank all members of the Department of Microbiology, College of Medicine at Imam Abdulrahman Bin Faisal University for their continuous support and encouragement.
Abbreviations
| AP-1 | Activator protein 1 |
| COX-2 | Cyclooxygenase-2 |
| DENV | Dengue virus |
| E5, E6, E7 | HPV early proteins 5, 6 and 7 |
| EBNA1 | EBV nuclear antigen 1 |
| EBV | Epstein-Barr virus |
| EGFR | Epidermal growth factor receptor |
| HBV | Hepatitis B virus |
| HBx | HBV-x protein |
| HCV | Hepatitis C virus |
| HIF-1α | Hypoxia inducible factor-1 alpha |
| HPV | Human papilloma virus |
| HSV-1 | Herpes simples virus-1 |
| hTERT | Human Telomerase reverse transcriptase |
| ICP4 | Infected cell protein 4 |
| JNK | c-Jun N-terminal kinases |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| LMP1 | latent membrane protein 1 |
| NF-κB | Nuclear factor kappa B |
| NPC | Nasopharyngeal carcinoma |
| Npr | Neuropilin receptor |
| PI3K | Phosphatidylinositol 3-kinase |
| PlGF | Placental growth factor |
| SP-1 | Specificity protein 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| vFLIP | Viral FLICE inhibitory protein |
| vGPCR | Viral G-protein coupled receptor |
| vIRF3 | Viral interferon regulatory factor 3 |
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Evans I. An Overview of VEGF-Mediated Signal Transduction. Methods Mol. Biol. 2015;1332:91–120. doi: 10.1007/978-1-4939-2917-7_7. [DOI] [PubMed] [Google Scholar]
- 3.Koch S., Tugues S., Li X., Gualandi L., Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 4.Roskoski R.J. VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 5.Kendall R.L., Thomas K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claesson-Welsh L. VEGF receptor signal transduction-A brief update. Vasc. Pharmacol. 2016;86:14–17. doi: 10.1016/j.vph.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D., Zheng W., Franco C.A., Murtomaki A., Aranda E., et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedito R., Rocha S.F., Woeste M., Zamykal M., Radtke F., Casanovas O., Duarte A., Pytowski B., Adams R.H. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 9.Harper S.J., Bates D.O. VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat. Rev. Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biselli-Chicote P.M., Oliveira A.R., Pavarino E.C., Goloni-Bertollo E.M. VEGF gene alternative splicing: Pro- and anti-angiogenic isoforms in cancer. J. Cancer Res. Clin. Oncol. 2012;138:363–370. doi: 10.1007/s00432-011-1073-2. [DOI] [PubMed] [Google Scholar]
- 11.Shannon-Lowe C., Rickinson A.B., Bell A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L., Li C., Pan L. Nasopharyngeal carcinoma: A review of current updates. Exp. Ther. Med. 2018;15:3687–3692. doi: 10.3892/etm.2018.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae J.M., Kim E.H. Epstein-Barr Virus and Gastric Cancer Risk: A Meta-analysis With Meta-regression of Case-control Studies. J. Prev. Med. Public Health. 2016;49:97–107. doi: 10.3961/jpmph.15.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang G.W., Sunagawa M., Li J.E., Shimada S., Gang Z., Tokeshi Y., Kosugi T. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110:2066–2069. doi: 10.1097/00005537-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Qian C.N., Zhang C.Q., Guo X., Hong M.H., Cao S.M., Mai W.Y., Min H.Q., Zeng Y.X. Elevation of serum vascular endothelial growth factor in male patients with metastatic nasopharyngeal carcinoma. Cancer. 2000;88:255–261. doi: 10.1002/(SICI)1097-0142(20000115)88:2<255::AID-CNCR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Wakisaka N., Wen Q.H., Yoshizaki T., Nishimura T., Furukawa M., Kawahara E., Nakanishi I. Association of vascular endothelial growth factor expression with angiogenesis and lymph node metastasis in nasopharyngeal carcinoma. Laryngoscope. 1999;109:810–814. doi: 10.1097/00005537-199905000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Krishna S.M., James S., Balaram P. Expression of VEGF as prognosticator in primary nasopharyngeal cancer and its relation to EBV status. Virus Res. 2006;115:85–90. doi: 10.1016/j.virusres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Polz-Dacewicz M., Strycharz-Dudziak M., Dworzanski J., Stec A., Kocot J. Salivary and serum IL-10, TNF-alpha, TGF-beta, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infect. Agent Cancer. 2016;11:45. doi: 10.1186/s13027-016-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H.S., Lu F., Lieberman P.M. Epigenetic regulation of EBV and KSHV latency. Curr. Opin. Virol. 2013;3:251–259. doi: 10.1016/j.coviro.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson D., Charalambous C., Wilson J.B. Epstein-Barr virus latent membrane protein 1 (CAO) up-regulates VEGF and TGF alpha concomitant with hyperlasia, with subsequent up-regulation of p16 and MMP9. Cancer Res. 2005;65:8826–8835. doi: 10.1158/0008-5472.CAN-05-0591. [DOI] [PubMed] [Google Scholar]
- 21.Yang L., Liu L., Xu Z., Liao W., Feng D., Dong X., Xu S., Xiao L., Lu J., Luo X., et al. EBV-LMP1 targeted DNAzyme enhances radiosensitivity by inhibiting tumor angiogenesis via the JNKs/HIF-1 pathway in nasopharyngeal carcinoma. Oncotarget. 2015;6:5804–5817. doi: 10.18632/oncotarget.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murono S., Inoue H., Tanabe T., Joab I., Yoshizaki T., Furukawa M., Pagano J.S. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA. 2001;98:6905–6910. doi: 10.1073/pnas.121016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paydas S., Ergin M., Erdogan S., Seydaoglu G. Prognostic significance of EBV-LMP1 and VEGF-A expressions in non-Hodgkin’s lymphomas. Leuk. Res. 2008;32:1424–1430. doi: 10.1016/j.leukres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 24.O’Neil J.D., Owen T.J., Wood V.H., Date K.L., Valentine R., Chukwuma M.B., Arrand J.R., Dawson C.W., Young L.S. Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitro. J. Gen. Virol. 2008;89:2833–2842. doi: 10.1099/vir.0.2008/003392-0. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Vega F., Mina M., Armenia J., Chatila W.K., Luna A., La K.C., Dimitriadoy S., Liu D.L., Kantheti H.S., Saghafinia S., et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang W., Morgan D.R., Meyers M.O., Dominguez R.L., Martinez E., Kakudo K., Kuan P.F., Banet N., Muallem H., Woodward K., et al. Epstein-barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect. Agent Cancer. 2012;7:21. doi: 10.1186/1750-9378-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung W.W., Chu Y.C., Chen P.R., Liao M.H., Lee J.W. Positive regulation of HIF-1A expression by EBV oncoprotein LMP1 in nasopharyngeal carcinoma cells. Cancer Lett. 2016;382:21–31. doi: 10.1016/j.canlet.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Boshoff C. Kaposi’s sarcoma. Coupling herpesvirus to angiogenesis. Nature. 1998;391:24–25. doi: 10.1038/34054. [DOI] [PubMed] [Google Scholar]
- 29.Ensoli B., Sturzl M. HHV-8 and multistep tumorigenesis. Trends Microbiol. 1999;7:310–312. doi: 10.1016/S0966-842X(99)01544-9. [DOI] [PubMed] [Google Scholar]
- 30.Marchio S., Primo L., Pagano M., Palestro G., Albini A., Veikkola T., Cascone I., Alitalo K., Bussolino F. Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi’s sarcoma cells. J. Biol. Chem. 1999;274:27617–27622. doi: 10.1074/jbc.274.39.27617. [DOI] [PubMed] [Google Scholar]
- 31.Arasteh K., Hannah A. The role of vascular endothelial growth factor (VEGF) in AIDS-related Kaposi’s sarcoma. Oncologist. 2000;5:28–31. doi: 10.1634/theoncologist.5-suppl_1-28. [DOI] [PubMed] [Google Scholar]
- 32.Brown L.F., Tognazzi K., Dvorak H.F., Harrist T.J. Strong expression of kinase insert domain-containing receptor, a vascular permeability factor/vascular endothelial growth factor receptor in AIDS-associated Kaposi’s sarcoma and cutaneous angiosarcoma. Am. J. Pathol. 1996;148:1065–1074. [PMC free article] [PubMed] [Google Scholar]
- 33.Flore O., Rafii S., Ely S., O’Leary J.J., Hyjek E.M., Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 34.An F.Q., Folarin H.M., Compitello N., Roth J., Gerson S.L., McCrae K.R., Fakhari F.D., Dittmer D.P., Renne R. Long-term-infected telomerase-immortalized endothelial cells: A model for Kaposi’s sarcoma-associated herpesvirus latency in vitro and in vivo. J. Virol. 2006;80:4833–4846. doi: 10.1128/JVI.80.10.4833-4846.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutlu A.D., Cavallin L.E., Vincent L., Chiozzini C., Eroles P., Duran E.M., Asgari Z., Hooper A.T., La Perle K.M., Hilsher C., et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: A cell and animal model of virally induced Kaposi’s sarcoma. Cancer Cell. 2007;11:245–258. doi: 10.1016/j.ccr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alkharsah K.R., Singh V.V., Bosco R., Santag S., Grundhoff A., Konrad A., Sturzl M., Wirth D., Dittrich-Breiholz O., Kracht M., et al. Deletion of Kaposi’s sarcoma-associated herpesvirus FLICE inhibitory protein, vFLIP, from the viral genome compromises the activation of STAT1-responsive cellular genes and spindle cell formation in endothelial cells. J. Virol. 2011;85:10375–10388. doi: 10.1128/JVI.00226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grossmann C., Podgrabinska S., Skobe M., Ganem D. Activation of NF-kappaB by the latent vFLIP gene of Kaposi’s sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J. Virol. 2006;80:7179–7185. doi: 10.1128/JVI.01603-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field N., Low W., Daniels M., Howell S., Daviet L., Boshoff C., Collins M. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 2003;116:3721–3728. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- 39.Matta H., Mazzacurati L., Schamus S., Yang T., Sun Q., Chaudhary P.M. Kaposi’s sarcoma-associated herpesvirus (KSHV) oncoprotein K13 bypasses TRAFs and directly interacts with the IkappaB kinase complex to selectively activate NF-kappaB without JNK activation. J. Biol. Chem. 2007;282:24858–24865. doi: 10.1074/jbc.M700118200. [DOI] [PubMed] [Google Scholar]
- 40.Sakakibara S., Pise-Masison C.A., Brady J.N., Tosato G. Gene regulation and functional alterations induced by Kaposi’s sarcoma-associated herpesvirus-encoded ORFK13/vFLIP in endothelial cells. J. Virol. 2009;83:2140–2153. doi: 10.1128/JVI.01871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoki Y., Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999;94:4247–4254. [PubMed] [Google Scholar]
- 42.Wang L., Wakisaka N., Tomlinson C.C., DeWire S.M., Krall S., Pagano J.S., Damania B. The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 2004;64:2774–2781. doi: 10.1158/0008-5472.CAN-03-3653. [DOI] [PubMed] [Google Scholar]
- 43.Yang T.Y., Chen S.C., Leach M.W., Manfra D., Homey B., Wiekowski M., Sullivan L., Jenh C.H., Narula S.K., Chensue S.W., et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J. Exp. Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin Y.C., Joo C.H., Gack M.U., Lee H.R., Jung J.U. Kaposi’s sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res. 2008;68:1751–1759. doi: 10.1158/0008-5472.CAN-07-2766. [DOI] [PubMed] [Google Scholar]
- 45.Vieira J., O’Hearn P.M. Use of the red fluorescent protein as a marker of Kaposi’s sarcoma-associated herpesvirus lytic gene expression. Virology. 2004;325:225–240. doi: 10.1016/j.virol.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Spence T., Bruce J., Yip K.W., Liu F.F. HPV Associated Head and Neck Cancer. Cancers. 2016;8:75. doi: 10.3390/cancers8080075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas T.L. Cancer Prevention: HPV Vaccination. Semin. Oncol. Nurs. 2016;32:273–280. doi: 10.1016/j.soncn.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bratman S.V., Bruce J.P., O’Sullivan B., Pugh T.J., Xu W., Yip K.W., Liu F.F. Human Papillomavirus Genotype Association With Survival in Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016;2:823–826. doi: 10.1001/jamaoncol.2015.6587. [DOI] [PubMed] [Google Scholar]
- 49.Ndiaye C., Mena M., Alemany L., Arbyn M., Castellsague X., Laporte L., Bosch F.X., de Sanjose S., Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 50.Anderson L.A., O’Rorke M.A., Wilson R., Jamison J., Gavin A.T., Northern Ireland HPV Working Group HPV prevalence and type-distribution in cervical cancer and premalignant lesions of the cervix: A population-based study from Northern Ireland. J. Med. Virol. 2016;88:1262–1270. doi: 10.1002/jmv.24447. [DOI] [PubMed] [Google Scholar]
- 51.Moody C.A., Laimins L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 52.DiMaio D., Petti L.M. The E5 proteins. Virology. 2013;445:99–114. doi: 10.1016/j.virol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheffner M., Werness B.A., Huibregtse J.M., Levine A.J., Howley P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 54.Song S., Liem A., Miller J.A., Lambert P.F. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–150. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 55.Li G., He L., Zhang E., Shi J., Zhang Q., Le A.D., Zhou K., Tang X. Overexpression of human papillomavirus (HPV) type 16 oncoproteins promotes angiogenesis via enhancing HIF-1alpha and VEGF expression in non-small cell lung cancer cells. Cancer Lett. 2011;311:160–170. doi: 10.1016/j.canlet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Tang X., Zhang Q., Nishitani J., Brown J., Shi S., Le A.D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin. Cancer Res. 2007;13:2568–2576. doi: 10.1158/1078-0432.CCR-06-2704. [DOI] [PubMed] [Google Scholar]
- 57.Toussaint-Smith E., Donner D.B., Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23:2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Ocejo O., Viloria-Petit A., Bequet-Romero M., Mukhopadhyay D., Rak J., Kerbel R.S. Oncogenes and tumor angiogenesis: The HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19:4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 59.Li F., Cui J. Human telomerase reverse transcriptase regulates vascular endothelial growth factor expression via human papillomavirus oncogene E7 in HPV-18-positive cervical cancer cells. Med. Oncol. 2015;32:199. doi: 10.1007/s12032-015-0649-0. [DOI] [PubMed] [Google Scholar]
- 60.Tilborghs S., Corthouts J., Verhoeven Y., Arias D., Rolfo C., Trinh X.B., van Dam P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017;120:141–150. doi: 10.1016/j.critrevonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Vescovo T., Refolo G., Vitagliano G., Fimia G.M., Piacentini M. Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin. Microbiol. Infect. 2016;22:853–861. doi: 10.1016/j.cmi.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 62.El-Serag H.B., Kramer J.R., Chen G.J., Duan Z., Richardson P.A., Davila J.A. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011;60:92–97. doi: 10.1136/gut.2010.230508. [DOI] [PubMed] [Google Scholar]
- 63.Messerini L., Novelli L., Comin C.E. Microvessel density and clinicopathological characteristics in hepatitis C virus and hepatitis B virus related hepatocellular carcinoma. J. Clin. Pathol. 2004;57:867–871. doi: 10.1136/jcp.2003.015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Llovet J.M., Pena C.E., Lathia C.D., Shan M., Meinhardt G., Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PubMed] [Google Scholar]
- 65.Mukozu T., Nagai H., Matsui D., Kanekawa T., Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013–1021. doi: 10.1016/S0016-5085(12)63799-8. [DOI] [PubMed] [Google Scholar]
- 66.Yvamoto E.Y., Ferreira R.F., Nogueira V., Pinhe M.A., Tenani G.D., Andrade J.G., Baitello M.E., Gregorio M.L., Fucuta P.S., Silva R.F., Souza D.R., et al. Influence of vascular endothelial growth factor and alpha-fetoprotein on hepatocellular carcinoma. Genet. Mol. Res. 2015;14:17453–17462. doi: 10.4238/2015.December.21.16. [DOI] [PubMed] [Google Scholar]
- 67.Shimoda K., Mori M., Shibuta K., Banner B.F., Barnard G.F. Vascular endothelial growth factor/vascular permeability factor mRNA expression in patients with chronic hepatitis C and hepatocellular carcinoma. Int. J. Oncol. 1999;14:353–359. doi: 10.3892/ijo.14.2.353. [DOI] [PubMed] [Google Scholar]
- 68.Abe M., Koga H., Yoshida T., Masuda H., Iwamoto H., Sakata M., Hanada S., Nakamura T., Taniguchi E., Kawaguchi T., et al. Hepatitis C virus core protein upregulates the expression of vascular endothelial growth factor via the nuclear factor-kappaB/hypoxia-inducible factor-1alpha axis under hypoxic conditions. Hepatol. Res. 2012;42:591–600. doi: 10.1111/j.1872-034X.2011.00953.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhu C., Liu X., Wang S., Yan X., Tang Z., Wu K., Li Y., Liu F. Hepatitis C virus core protein induces hypoxia-inducible factor 1alpha-mediated vascular endothelial growth factor expression in Huh7.5.1 cells. Mol. Med. Rep. 2014;9:2010–2014. doi: 10.3892/mmr.2014.2039. [DOI] [PubMed] [Google Scholar]
- 70.Kanda T., Steele R., Ray R., Ray R.B. Hepatitis C virus core protein augments androgen receptor-mediated signaling. J. Virol. 2008;82:11066–11072. doi: 10.1128/JVI.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shao Y.Y., Hsieh M.S., Wang H.Y., Li Y.S., Lin H., Hsu H.W., Huang C.Y., Hsu C.H., Cheng A.L. Hepatitis C virus core protein potentiates proangiogenic activity of hepatocellular carcinoma cells. Oncotarget. 2017;8:86681–86692. doi: 10.18632/oncotarget.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ringelhan M., O’Connor T., Protzer U., Heikenwalder M. The direct and indirect roles of HBV in liver cancer: Prospective markers for HCC screening and potential therapeutic targets. J. Pathol. 2015;235:355–367. doi: 10.1002/path.4434. [DOI] [PubMed] [Google Scholar]
- 73.Ringelhan M., Protzer U. Oncogenic potential of hepatitis B virus encoded proteins. Curr. Opin. Virol. 2015;14:109–115. doi: 10.1016/j.coviro.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Bonilla Guerrero R., Roberts L.R. The role of hepatitis B virus integrations in the pathogenesis of human hepatocellular carcinoma. J. Hepatol. 2005;42:760–777. doi: 10.1016/j.jhep.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Nosaka T., Naito T., Hiramatsu K., Ohtani M., Nemoto T., Marusawa H., Ma N., Hiraku Y., Kawanishi S., Yamashita T., et al. Gene expression profiling of hepatocarcinogenesis in a mouse model of chronic hepatitis B. PLoS ONE. 2017;12:e0185442. doi: 10.1371/journal.pone.0185442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z.B., Shen S.Q., Ding Y.M., Wang W.X., Tao J.P., Liang L.J., Hu W.J. The angiogenic and prognostic implications of VEGF, Ang-1, Ang-2, and MMP-9 for hepatocellular carcinoma with background of hepatitis B virus. Med. Oncol. 2009;26:365–371. doi: 10.1007/s12032-008-9130-7. [DOI] [PubMed] [Google Scholar]
- 77.Cheng A.S., Chan H.L., To K.F., Leung W.K., Chan K.K., Liew C.T., Sung J.J. Cyclooxygenase-2 pathway correlates with vascular endothelial growth factor expression and tumor angiogenesis in hepatitis B virus-associated hepatocellular carcinoma. Int. J. Oncol. 2004;24:853–860. doi: 10.3892/ijo.24.4.853. [DOI] [PubMed] [Google Scholar]
- 78.Lee S.W., Lee Y.M., Bae S.K., Murakami S., Yun Y., Kim K.W. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis. Biochem. Biophys. Res. Commun. 2000;268:456–461. doi: 10.1006/bbrc.2000.2093. [DOI] [PubMed] [Google Scholar]
- 79.Yoo Y.G., Na T.Y., Seo H.W., Seong J.K., Park C.K., Shin Y.K., Lee M.O. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene. 2008;27:3405–3413. doi: 10.1038/sj.onc.1211000. [DOI] [PubMed] [Google Scholar]
- 80.Yoo Y.G., Oh S.H., Park E.S., Cho H., Lee N., Park H., Kim D.K., Yu D.Y., Seong J.K., Lee M.O. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J. Biol. Chem. 2003;278:39076–39084. doi: 10.1074/jbc.M305101200. [DOI] [PubMed] [Google Scholar]
- 81.Liu L.P., Hu B.G., Ye C., Ho R.L., Chen G.G., Lai P.B. HBx mutants differentially affect the activation of hypoxia-inducible factor-1alpha in hepatocellular carcinoma. Br. J. Cancer. 2014;110:1066–1073. doi: 10.1038/bjc.2013.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yen C.J., Lin Y.J., Yen C.S., Tsai H.W., Tsai T.F., Chang K.Y., Huang W.C., Lin P.W., Chiang C.W., Chang T.T. Hepatitis B virus X protein upregulates mTOR signaling through IKKbeta to increase cell proliferation and VEGF production in hepatocellular carcinoma. PLoS ONE. 2012;7:e41931. doi: 10.1371/journal.pone.0041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J.C., Teng C.F., Wu H.C., Tsai H.W., Chuang H.C., Tsai T.F., Hsu Y.H., Huang W., Wu L.W., Su I.J. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology. 2009;49:1962–1971. doi: 10.1002/hep.22889. [DOI] [PubMed] [Google Scholar]
- 84.World Health O. Hepatitis B vaccines: WHO position paper, July 2017-Recommendations. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 85.Smith J.S., Robinson N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002;186:S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 86.Park P.J., Chang M., Garg N., Zhu J., Chang J.H., Shukla D. Corneal lymphangiogenesis in herpetic stromal keratitis. Surv. Ophthalmol. 2015;60:60–71. doi: 10.1016/j.survophthal.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wuest T.R., Carr D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng M., Deshpande S., Lee S., Ferrara N., Rouse B.T. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biswas P.S., Banerjee K., Kinchington P.R., Rouse B.T. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp. Eye Res. 2006;82:46–54. doi: 10.1016/j.exer.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 90.Wuest T., Zheng M., Efstathiou S., Halford W.P., Carr D.J. The herpes simplex virus-1 transactivator infected cell protein-4 drives VEGF-A dependent neovascularization. PLoS Pathog. 2011;7:e1002278. doi: 10.1371/journal.ppat.1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suryawanshi A., Mulik S., Sharma S., Reddy P.B., Sehrawat S., Rouse B.T. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J. Immunol. 2011;186:3653–3665. doi: 10.4049/jimmunol.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suryawanshi A., Veiga-Parga T., Reddy P.B., Rajasagi N.K., Rouse B.T. IL-17A differentially regulates corneal vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J. Immunol. 2012;188:3434–3446. doi: 10.4049/jimmunol.1102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulik S., Xu J., Reddy P.B., Rajasagi N.K., Gimenez F., Sharma S., Lu P.Y., Rouse B.T. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am. J. Pathol. 2012;181:525–534. doi: 10.1016/j.ajpath.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gurung H.R., Carr M.M., Bryant K., Chucair-Elliott A.J., Carr D.J. Fibroblast growth factor-2 drives and maintains progressive corneal neovascularization following HSV-1 infection. Mucosal Immunol. 2018;11:172–185. doi: 10.1038/mi.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Screaton G., Mongkolsapaya J., Yacoub S., Roberts C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015;15:745–759. doi: 10.1038/nri3916. [DOI] [PubMed] [Google Scholar]
- 96.Dvorak H.F. Discovery of vascular permeability factor (VPF) Exp. Cell Res. 2006;312:522–556. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 97.Del Moral-Hernandez O., Martinez-Hernandez N.E., Mosso-Pani M.A., Hernandez-Sotelo D., Illades-Aguiar B., Flores-Alfaro E., Antonio-Vejar V., Leyva-Vazquez M.A. Association DENV1 and DENV2 infection with high serum levels of soluble thrombomodulin and VEGF in patients with dengue fever and dengue hemorrhagic fever. Int. J. Clin. Exp. Med. 2014;7:370–378. [PMC free article] [PubMed] [Google Scholar]
- 98.Furuta T., Murao L.A., Lan N.T., Huy N.T., Huong V.T., Thuy T.T., Tham V.D., Nga C.T., Ha T.T., Ohmoto Y., et al. Association of mast cell-derived VEGF and proteases in Dengue shock syndrome. PLoS Negl. Trop. Dis. 2012;6:e1505. doi: 10.1371/journal.pntd.0001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Srikiatkhachorn A., Ajariyakhajorn C., Endy T.P., Kalayanarooj S., Libraty D.H., Green S., Ennis F.A., Rothman A.L. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J. Virol. 2007;81:1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tseng C.S., Lo H.W., Teng H.C., Lo W.C., Ker C.G. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 2005;43:99–102. doi: 10.1016/j.femsim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Van de Weg C.A., Pannuti C.S., van den Ham H.J., de Araujo E.S., Boas L.S., Felix A.C., Carvalho K.I., Levi J.E., Romano C.M., Centrone C.C., et al. Serum angiopoietin-2 and soluble VEGF receptor 2 are surrogate markers for plasma leakage in patients with acute dengue virus infection. J. Clin. Virol. 2014;60:328–335. doi: 10.1016/j.jcv.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Azizan A., Fitzpatrick K., Signorovitz A., Tanner R., Hernandez H., Stark L., Sweat M. Profile of time-dependent VEGF upregulation in human pulmonary endothelial cells, HPMEC-ST1.6R infected with DENV-1, -2, -3, and -4 viruses. Virol. J. 2009;6:49. doi: 10.1186/1743-422X-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Azizan A., Sweat J., Espino C., Gemmer J., Stark L., Kazanis D. Differential proinflammatory and angiogenesis-specific cytokine production in human pulmonary endothelial cells, HPMEC-ST1.6R infected with dengue-2 and dengue-3 virus. J. Virol. Methods. 2006;138:211–217. doi: 10.1016/j.jviromet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 104.Holmes E.C., Zhang Y.Z. The evolution and emergence of hantaviruses. Curr. Opin. Virol. 2015;10:27–33. doi: 10.1016/j.coviro.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Kruger D.H., Figueiredo L.T., Song J.W., Klempa B. Hantaviruses—Globally emerging pathogens. J. Clin. Virol. 2015;64:128–136. doi: 10.1016/j.jcv.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 106.Li Y., Wang W., Wang J.P., Pan L., Zhang Y., Yu H.T., Jiang W., Wang P.Z., Bai X.F. Elevated vascular endothelial growth factor levels induce hyperpermeability of endothelial cells in hantavirus infection. J. Int. Med. Res. 2012;40:1812–1821. doi: 10.1177/030006051204000519. [DOI] [PubMed] [Google Scholar]
- 107.Gavrilovskaya I.N., Gorbunova E.E., Mackow N.A., Mackow E.R. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 2008;82:5797–5806. doi: 10.1128/JVI.02397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gavrilovskaya I.N., Gorbunova E.E., Mackow E.R. Hypoxia induces permeability and giant cell responses of Andes virus-infected pulmonary endothelial cells by activating the mTOR-S6K signaling pathway. J. Virol. 2013;87:12999–13008. doi: 10.1128/JVI.02103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sundstrom K.B., Nguyen Hoang A.T., Gupta S., Ahlm C., Svensson M., Klingstrom J. Andes Hantavirus-Infection of a 3D Human Lung Tissue Model Reveals a Late Peak in Progeny Virus Production Followed by Increased Levels of Proinflammatory Cytokines and VEGF-A. PLoS ONE. 2016;11:e0149354. doi: 10.1371/journal.pone.0149354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gorbunova E., Gavrilovskaya I.N., Mackow E.R. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J. Virol. 2010;84:7405–7411. doi: 10.1128/JVI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Easterbrook J.D., Klein S.L. Seoul virus enhances regulatory and reduces proinflammatory responses in male Norway rats. J. Med. Virol. 2008;80:1308–1318. doi: 10.1002/jmv.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krautkramer E., Nusshag C., Baumann A., Schafer J., Hofmann J., Schnitzler P., Klempa B., Witkowski P.T., Kruger D.H., Zeier M. Clinical characterization of two severe cases of hemorrhagic fever with renal syndrome (HFRS) caused by hantaviruses Puumala and Dobrava-Belgrade genotype Sochi. BMC Infect. Dis. 2016;16:675. doi: 10.1186/s12879-016-2012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleming S.B., Wise L.M., Mercer A.A. Molecular genetic analysis of orf virus: A poxvirus that has adapted to skin. Viruses. 2015;7:1505–1539. doi: 10.3390/v7031505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogawa S., Oku A., Sawano A., Yamaguchi S., Yazaki Y., Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 115.Lyttle D.J., Fraser K.M., Fleming S.B., Mercer A.A., Robinson A.J. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J. Virol. 1994;68:84–92. doi: 10.1128/jvi.68.1.84-92.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wise L.M., Savory L.J., Dryden N.H., Whelan E.M., Fleming S.B., Mercer A.A. Major amino acid sequence variants of viral vascular endothelial growth factor are functionally equivalent during Orf virus infection of sheep skin. Virus Res. 2007;128:115–125. doi: 10.1016/j.virusres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 117.Savory L.J., Stacker S.A., Fleming S.B., Niven B.E., Mercer A.A. Viral vascular endothelial growth factor plays a critical role in orf virus infection. J. Virol. 2000;74:10699–106706. doi: 10.1128/JVI.74.22.10699-10706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shibuya M. Vascular endothelial growth factor receptor-2: Its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003;94:751–756. doi: 10.1111/j.1349-7006.2003.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meyer M., Clauss M., Lepple-Wienhues A., Waltenberger J., Augustin H.G., Ziche M., Lanz C., Buttner M., Rziha H.J., Dehio C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cebe-Suarez S., Grunewald F.S., Jaussi R., Li X., Claesson-Welsh L., Spillmann D., Mercer A.A., Prota A.E., Ballmer-Hofer K. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J. 2008;22:3078–3086. doi: 10.1096/fj.08-107219. [DOI] [PubMed] [Google Scholar]
- 121.Wise L.M., Veikkola T., Mercer A.A., Savory L.J., Fleming S.B., Caesar C., Vitali A., Makinen T., Alitalo K., Stacker S.A. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc. Natl. Acad. Sci. USA. 1999;96:3071–3076. doi: 10.1073/pnas.96.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tokunaga Y., Yamazaki Y., Morita T. Localization of heparin- and neuropilin-1-recognition sites of viral VEGFs. Biochem. Biophys. Res. Commun. 2006;348:957–962. doi: 10.1016/j.bbrc.2006.07.117. [DOI] [PubMed] [Google Scholar]
- 123.Ueda N., Wise L.M., Stacker S.A., Fleming S.B., Mercer A.A. Pseudocowpox virus encodes a homolog of vascular endothelial growth factor. Virology. 2003;305:298–309. doi: 10.1006/viro.2002.1750. [DOI] [PubMed] [Google Scholar]
- 124.Inder M.K., Ueda N., Mercer A.A., Fleming S.B., Wise L.M. Bovine papular stomatitis virus encodes a functionally distinct VEGF that binds both VEGFR-1 and VEGFR-2. J. Gen. Virol. 2007;88:781–791. doi: 10.1099/vir.0.82582-0. [DOI] [PubMed] [Google Scholar]
- 125.Ueda N., Inder M.K., Wise L.M., Fleming S.B., Mercer A.A. Parapoxvirus of red deer in New Zealand encodes a variant of viral vascular endothelial growth factor. Virus Res. 2007;124:50–58. doi: 10.1016/j.virusres.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 126.Hamden K.E., Whitman A.G., Ford P.W., Shelton J.G., McCubrey J.A., Akula S.M. Raf and VEGF: Emerging therapeutic targets in Kaposi’s sarcoma-associated herpesvirus infection and angiogenesis in hematopoietic and nonhematopoietic tumors. Leukemia. 2005;19:18–26. doi: 10.1038/sj.leu.2403532. [DOI] [PubMed] [Google Scholar]
- 127.Tsang J., Lee V.H., Kwong D.L. Novel therapy for nasopharyngeal carcinoma—Where are we. Oral Oncol. 2014;50:798–801. doi: 10.1016/j.oraloncology.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Lee N.Y., Zhang Q., Pfister D.G., Kim J., Garden A.S., Mechalakos J., Hu K., Le Q.T., Colevas A.D., Glisson B.S., et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roy D., Sin S.H., Lucas A., Venkataramanan R., Wang L., Eason A., Chavakula V., Hilton I.B., Tamburro K.M., Damania B., et al. mTOR inhibitors block Kaposi sarcoma growth by inhibiting essential autocrine growth factors and tumor angiogenesis. Cancer Res. 2013;73:2235–2246. doi: 10.1158/0008-5472.CAN-12-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rader J.S., Sill M.W., Beumer J.H., Lankes H.A., Benbrook D.M., Garcia F., Trimble C., Tate Thigpen J., Lieberman R., Zuna R.E., et al. A stratified randomized double-blind phase II trial of celecoxib for treating patients with cervical intraepithelial neoplasia: The potential predictive value of VEGF serum levels: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;145:291–297. doi: 10.1016/j.ygyno.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Argiris A., Bauman J.E., Ohr J., Gooding W.E., Heron D.E., Duvvuri U., Kubicek G.J., Posluszny D.M., Vassilakopoulou M., Kim S., et al. Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer. Ann. Oncol. 2016;27:1594–1600. doi: 10.1093/annonc/mdw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baghban Rahimi S., Mohebbi A., Vakilzadeh G., Biglari P., Razeghi Jahromi S., Mohebi S.R., Shirian S., Gorji A., Ghaemi A. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018;163:587–597. doi: 10.1007/s00705-017-3647-z. [DOI] [PubMed] [Google Scholar]
- 133.Huynh H., Ngo V.C., Koong H.N., Poon D., Choo S.P., Toh H.C., Thng C.H., Chow P., Ong H.S., Chung A., et al. AZD6244 enhances the anti-tumor activity of sorafenib in ectopic and orthotopic models of human hepatocellular carcinoma (HCC) J. Hepatol. 2010;52:79–87. doi: 10.1016/j.jhep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 134.Sampat K.R., O’Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18:430–438. doi: 10.1634/theoncologist.2012-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Himmelsbach K., Sauter D., Baumert T.F., Ludwig L., Blum H.E., Hildt E. New aspects of an anti-tumour drug: Sorafenib efficiently inhibits HCV replication. Gut. 2009;58:1644–1653. doi: 10.1136/gut.2009.182212. [DOI] [PubMed] [Google Scholar]
- 136.Descamps V., Helle F., Louandre C., Martin E., Brochot E., Izquierdo L., Fournier C., Hoffmann T.W., Castelain S., Duverlie G., et al. The kinase-inhibitor sorafenib inhibits multiple steps of the Hepatitis C Virus infectious cycle in vitro. Antiviral Res. 2015;118:93–102. doi: 10.1016/j.antiviral.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 137.Yang Y., Wen F., Li J., Zhang P., Yan W., Hao P., Xia F., Bi F., Li Q. A high baseline HBV load and antiviral therapy affect the survival of patients with advanced HBV-related HCC treated with sorafenib. Liver Int. 2015;35:2147–2154. doi: 10.1111/liv.12805. [DOI] [PubMed] [Google Scholar]
- 138.Kim B., Tang Q., Biswas P.S., Xu J., Schiffelers R.M., Xie F.Y., Ansari A.M., Scaria P.V., Woodle M.C., Lu P., et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: Therapeutic strategy for herpetic stromal keratitis. Am. J. Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalogeropoulos D., Geka A., Malamos K., Kanari M., Kalogeropoulos C. New Therapeutic Perceptions in a Patient with Complicated Herpes Simplex Virus 1 Keratitis: A Case Report and Review of the Literature. Am. J. Case Rep. 2017;18:1382–1389. doi: 10.12659/AJCR.906506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mackow E.R., Gorbunova E.E., Dalrymple N.A., Gavrilovskaya I.N. Role of vascular and lymphatic endothelial cells in hantavirus pulmonary syndrome suggests targeted therapeutic approaches. Lymphat. Res. Biol. 2013;11:128–135. doi: 10.1089/lrb.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jonsson C.B., Hooper J., Mertz G. Treatment of hantavirus pulmonary syndrome. Antiviral Res. 2008;78:162–169. doi: 10.1016/j.antiviral.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gorbunova E.E., Gavrilovskaya I.N., Pepini T., Mackow E.R. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J. Virol. 2011;85:2296–2303. doi: 10.1128/JVI.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bird B.H., Shrivastava-Ranjan P., Dodd K.A., Erickson B.R., Spiropoulou C.F. Effect of Vandetanib on Andes virus survival in the hamster model of Hantavirus pulmonary syndrome. Antiviral Res. 2016;132:66–69. doi: 10.1016/j.antiviral.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 144.Mao Y., Zhang D.W., Wen J., Cao Q., Chen R.J., Zhu J., Feng Z.Q. A novel LMP1 antibody synergizes with mitomycin C to inhibit nasopharyngeal carcinoma growth in vivo through inducing apoptosis and downregulating vascular endothelial growth factor. Int. J. Mol. Sci. 2012;13:2208–2218. doi: 10.3390/ijms13022208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng Y., Murakami M., Takahashi H., Yamauchi M., Kiba A., Yamaguchi S., Yabana N., Alitalo K., Shibuya M. Chimeric VEGF-E(NZ7)/PlGF promotes angiogenesis via VEGFR-2 without significant enhancement of vascular permeability and inflammation. Arterioscler. Thromb. Vasc. Biol. 2006;26:2019–2026. doi: 10.1161/01.ATV.0000233336.53574.a1. [DOI] [PubMed] [Google Scholar]
- 146.Inoue N., Kondo T., Kobayashi K., Aoki M., Numaguchi Y., Shibuya M., Murohara T. Therapeutic angiogenesis using novel vascular endothelial growth factor-E/human placental growth factor chimera genes. Arterioscler. Thromb. Vasc. Biol. 2007;27:99–105. doi: 10.1161/01.ATV.0000251504.61247.d5. [DOI] [PubMed] [Google Scholar]
- 147.Bodaan C.J., Wise L.M., Wakelin K.A., Stuart G.S., Real N.C., Mercer A.A., Riley C.B., Theoret C. Short-term treatment of equine wounds with orf virus IL-10 and VEGF-E dampens inflammation and promotes repair processes without accelerating closure. Wound Repair Regen. 2016;24:966–980. doi: 10.1111/wrr.12488. [DOI] [PubMed] [Google Scholar]