Abstract
The Fasciclin 1 (FAS1) domain is an ancient structural motif in extracellular proteins present in all kingdoms of life and particularly abundant in plants. The FAS1 domain accommodates multiple interaction surfaces, enabling it to bind different ligands. The frequently observed tandem FAS1 arrangement might both positively and negatively regulate ligand binding. Additional protein domains and post-translational modifications are partially conserved between different evolutionary clades. Human FAS1 family members are associated with multiple aspects of health and disease. At the cellular level, mammalian FAS1 proteins are implicated in extracellular matrix structure, cell to extracellular matrix and cell to cell adhesion, paracrine signaling, intracellular trafficking and endocytosis. Mammalian FAS1 proteins bind to the integrin family of receptors and to protein and carbohydrate components of the extracellular matrix. FAS1 protein encoding plant genes exert effects on cellulosic and non-cellulosic cell wall structure and cellular signaling but to establish the modes of action for any plant FAS1 protein still requires biochemical experimentation. In fungi, eubacteria and archaea, the differential presence of FAS1 proteins in closely related organisms and isolated biochemical data suggest functions in pathogenicity and symbiosis. The inter-kingdom comparison of FAS1 proteins suggests that molecular mechanisms mediating interactions between cells and their environment may have evolved at the earliest known stages of evolution.
Keywords: arabinogalactan proteins, cellulose, pectin, matricellular proteins, SOS5, periostin, Mpb83
1. A Brief History of FAS1 Domain Proteins
The first FAS1 protein was identified in an insect model for central nervous system development, the grasshopper Schistocerca americana. In order to identify cell surface molecules potentially involved in the formation of axon bundles (fascicles), monoclonal antibodies (mAbs) recognizing cell surface antigens on specific fascicles were characterized. One of these antibodies recognized a 70 kDa glycoprotein named Fasciclin 1 (SaFas1 (Appendix A)) [1]. The genes coding for grasshopper SaFas1 and Drosophila melanogaster DmFas1 were cloned soon afterwards [2] and a homologous fruit fly gene called Midline fasciclin (DmMfas) was identified later [3]. In the fruit fly, a DmFas1 knockout affected neuronal branching as well as synaptic function [4] and laser ablation of the grasshopper ortholog SaFas1 led to disrupted cell adhesion of pioneer axons [5]. The crystal structure of DmFas1 provided the prototype for the structurally novel FAS1 domain [6]. In the meantime, molecular techniques and sequence comparison tools revealed the widespread occurrence of homologous proteins defined by the FAS1 domain (IPR000782; PF02469). The Homo sapiens genome encodes four FAS1 domain proteins named transforming growth factor-β induced protein (HsTgfbi), Periostin (HsPn), Stabilin-1 (HsStab1) and Stabilin-2 (HsStab2). The HsTgfbi gene (Appendix B) was identified in human adenocarcinoma cells as a transcript that was induced 20-fold by transforming growth factor-β [7]. Likewise, HsPn, was cloned based on its expression in an osteoblast cell line [8] and subsequently found to be enriched in the periosteum [9]. Finally, HsStab1 and HsStab2 were identified using two different antibodies binding to a subpopulation of endothelial cells and to the hyaluronic acid (HA) clearance receptor, respectively [10]. Due to their association with a multitude of clinical conditions, human and mammalian FAS1 proteins have been the focus of numerous detailed studies that are instructive for a general understanding of FAS1 domain proteins. Unexpectedly for proteins associated with cell adhesion, FAS1 domain proteins exist not only in animals but also in plants, fungi and prokaryotes. The first plant FAS1 protein was discovered using antibody interference in the alga Volvox carteri [11], a simple model for multi-cellularity consisting of just two cell types. When specific mAbs raised against a crude membrane preparation were added to volvox cultures they inhibited embryo development. The cognate protein was named algal cell adhesion molecule (CAM) based on its apparent role in the formation of intercellular contacts during early embryogenesis. The existence and physiological role of algal-CAM, which contains two FAS1 domains, raised the exciting possibility of a cell adhesion mechanism conserved between animals and plants. In higher plants FAS1 domain proteins were also identified by the biochemical and bioinformatic analysis of a group of highly O-glycosylated hydroxyproline-rich glycoproteins called arabinogalactan-proteins (AGPs) [12,13]. The bioinformatic investigation of the Arabidopsis thaliana genome revealed the existence of many fasciclin-like AGPs (FLAs) in plants [12,13,14]. At the same time a different investigation mapped one of several Arabidopsis thaliana salt overly sensitive (sos) mutations to the Salt overly sensitive 5 (AtSos5) gene encoding the AtFla4 protein [15]. In a mutant screen in a crop plant, the rice locus Microspore and tapetum regulator 1 (OsMtr1) was found to be required for male reproductive development and to encode a tandem FAS1 glycoprotein [16]. In fungi, FAS1 proteins have been identified in transcriptomics or proteomics studies in the Shiitake mushroom Lentinula edodes [17] and the rice pathogen Magnaporthe oryzae [18], while in the fission yeast Schizosaccharomyces pombe the FAS1 domain protein SpFsc1 was identified in a screen for autophagy related loci [19]. Apparently, FAS1 proteins already existed before the evolution of eukaryotes. The best-known eubacterial FAS1 proteins are Mpb70 and Mpb83, which were identified in Mycobacterium bovis culture filtrates [8,20,21,22,23,24]. Database queries reveal FAS1 proteins in both eubacteria and archaea, suggesting the inception of the domain preceded the existence of last universal common ancestor (LUCA) [25]. FAS1 proteins are often implicated in the interaction between the cell and the extracellular matrix (ECM). Considering the diversity of ECM architectures and compositions FAS1 domain proteins are surprisingly widespread between different kingdoms of uni- and multicellular life. However, despite their seemingly boundless presence throughout the tree of life, FAS1 proteins are not ubiquitous, especially in microbes whose genomes rapidly adapt to differing life styles. This suggests that FAS1 domain proteins are not essential for life per se but are suited for specialized cellular interactions that for some organisms are not required. I will next describe what is known about the structure of the FAS1 domain itself and discuss diverse additional structural features of FAS1 proteins in various kingdoms. This will be followed by a review of the biological roles of mammalian and plant FAS1 domain proteins, including the relationship of structure to function, which should help elucidate the mechanisms of FAS1 proteins in plant development.
2. The Structure of the Fasciclin 1 Domain
2.1. The Fasciclin 1 Domain
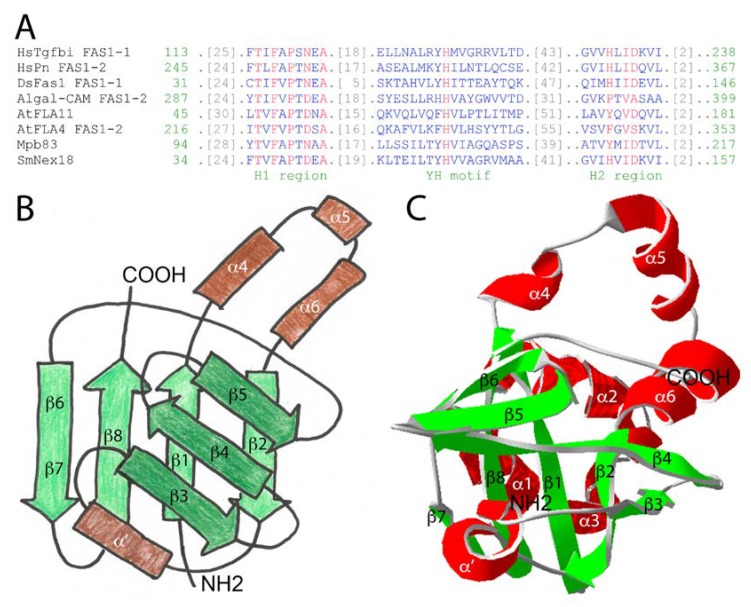
The FAS1 domain extends to approximately 140 amino acids. Although sequence conservation between different FAS1 proteins can be quite low, there exist two more highly conserved sequence stretches of around 15 residues called H1 and H2 and a conserved central YH motif (Figure 1A). Therefore, to identify FAS1 domain proteins in sequence databases, domain enhanced lookup time accelerated BLAST (DELTA-BLAST) should be used [26]. Using X-ray crystallography and NMR spectroscopy, several studies have elucidated the structures of isolated FAS1 domains or of entire FAS1 proteins [27,28,29,30,31,32,33]. The FAS1 domain is globular and contains a central structural fold of two β-sheets oriented at an almost perpendicular angle, varyingly described as β-wedge or β-sandwich (Figure 1B).
Figure 1.
The FAS1 domain across kingdoms of life. (A) Delta-BLAST alignment of some FAS1 domains mentioned in this article. Note the conservation of the N and C-proximal H1 and the H2 region as well as the central YH motif. The sequences used by Delta-BLAST were HsTgfbi (NP_000349.1), HsPn (gi 93138709), DsFas1 FAS1-3 (1O70_A), Algal-CAM (gi 75282282), AtFLA11 (gi 116247778), AtFLA4 (gi 75206907), MbMpb83 (gi 614094354), and SmNex18 (gi 81635876). Red/blue indicates highly/not conserved residues; color bits threshold set to 2.5; (B) The general topology of the FAS1 domain features is reminiscent of the “thumbs-up” gesture. The secondary structure of HsTgfbi FAS1-1 is annotated omitting the three N-terminal helices for clarity; (C) The crystal structure of HsTgfbi FAS1-1 [33] in ribbon display.
In the human HsTgfbi structure, the first β-sheet encompasses strands β1–β2–β8–β6/7 [33] (Figure 1C). The two inner strands are oriented parallel and the two outer ones antiparallel. The second β-sheet consists of β3–β4–β5. There are three α-helices at the N-terminus (not shown in Figure 1B), three more (α4 to α6) between β1 and β2 and a less highly conserved α-helix (α′) between β2 and β3. The FAS1 domain (Figure 1B, redrawn from [25]) is a member of the “β-grasp fold” superfamily [25] and may be imagined as a “thumbs-up” gesture of the right hand with the palm representing the first β-sheet (light green in Figure 1B), the bent index, middle and ring fingers symbolizing the second β-sheet (dark green in Figure 1B) and the thumb and pinkie resembling α4 to α6 (light brown in Figure 1B) and α′ (dark brown in Figure 1B), respectively. Among all FAS1 proteins, structure to function relations have been most intensely studied for HsTgfbi (reviewed in [34]). Therefore, the elucidation of the entire HsTgfbi crystal structure [33] could be seen as the “Rosetta stone” for a better mechanistic understanding of the many biological roles of FAS1 proteins in different organisms including plants. Several studies identified individual regions and amino acid residues on the four Tgfbi FAS1 domains that are critical for function. The main approach was to use in vitro cell adhesion as a functional assay (Figure 2).
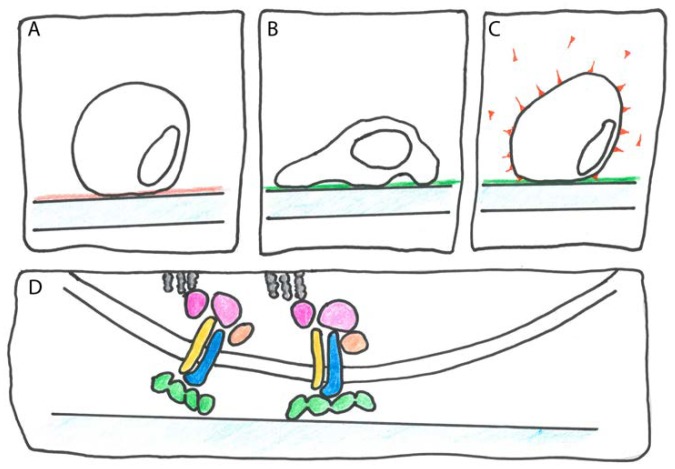
Figure 2.
Cell adhesion assay. (A) On an uncoated or control-coated (red) plastic substrate, cell adhesion is inefficient; (B) cells adhere rapidly when plastic is coated with an adhesion protein such as Tgfbi or Pn (green); (C) to identify the receptor for the adhesion protein, integrin isotype specific antibodies (red) are co-incubated; (D) mammalian FAS1 proteins are thought to bind to different types of dimeric integrins (blue and yellow) that mediate mechanical contact between the cytoskeleton (grey) and the ECM as well as transduce intracellular signals using numerous associated proteins (pink and nude).
Briefly, cells more efficiently adhere to surfaces coated with adhesion proteins such as Tgfbi than to control-coated surfaces (Figure 2A,B). That this adhesion is dependent on the family of ECM receptors called integrins can be tested by adding integrin antibodies that block the Tgfbi-stimulated cell adhesion (Figure 2C). To identify sites on Tgfbi that might mediate cell adhesion, peptides corresponding to conserved Tgfbi sequence motifs were added. For instance, the NKDIL and the EPDIM peptides that are part of the FAS1-2 and the FAS1-4 (see following section) domains, respectively, both interfered with Tgfbi stimulated cell adhesion. By contrast, the KADHH peptide in the corresponding region of FAS1-1 had no effect [35]. In a study of a different cell type expressing a different integrin, the NKDIL and EPDIM peptides did not interfere with adhesion but an 18-amino acid peptide that covered the YH motif did [36]. A HsPn specific mAb identified a corresponding integrin interaction region on FAS1-2 [37]. The cell adhesion assay is too crude to demonstrate direct binding between FAS1 proteins and integrin; however, in combination with the crystal structure of HsTgfbi it showed that different integrins interact with different surface regions of the FAS1 domains [33]. Although integrins are not known in plants, given the complex biological roles of some FAS1 proteins of plants, this insight should be valuable for the prediction of their molecular function.
2.2. Single and Tandem Fasciclin 1 Domains
While prokaryotic representatives typically contain a single FAS1 domain, many eukaryotic family members contain two or more arranged in tandem (numbered FAS1-1, FAS1-2 etc. from N- to C-terminus). The two insect family members Fas1 and Mfas, and mammalian Tgfbi and Pn each contain four FAS1 domains, while there are seven in the two mammalian stabilins (Figure 3).
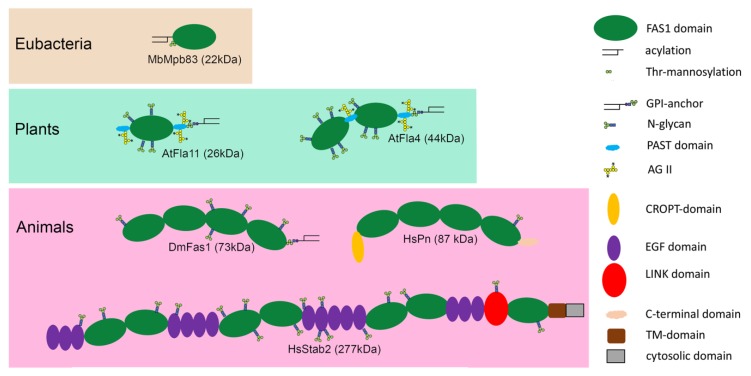
Figure 3.
Domain organization and post translational modifications of six representative FAS1 proteins discussed in this article. Proteins are shown with their N-termini on the left. As opposed to the C-terminally attached GPI-anchors Mpb83 is acylated near its N-terminus. Note that the annotation of AG II glycosylation is tentative and that the annotation of N-glycans is based on bioinformatic predictions. Abbreviations used in this Figure, GPI: glycosylphosphoinositidyl; TM: transmembrane; AG II: arabinogalactan type II; CROPT: Cysteine-Rich domain of Pn and TGFBIp; EGF: epidermal growth factor; PAST: domain rich in Pro, Ala, Ser and Thr.
In plants, there exist both single FAS1 FLAs as well as family members with two domains in tandem. FAS1 tandem proteins also exist in fungi, where dual and five-fold tandems have been reported [18,19]. The tandem FAS1 domains structurally interact with each other, as was found in a structure of DmFas1 domains FAS1-3 an FAS1-4 [27] and of whole HsTgfbi [33]. It was suggested that the C-proximal domain in a tandem pair might bind to interaction partners such as integrins while the N-proximal domains might compete with this binding acting either as negative regulators or as safeguards against unwanted ligand binding [28]. A recent structure to function study of AtFla4 in Arabidopsis thaliana showed that the C-proximal FAS1 domain was necessary and sufficient for genetic function [38]. By contrast, some studies showed cooperativity between all FAS1 domains [39]. To which degree the intramolecular interaction between neighboring FAS1 domains might affect the intermolecular interaction with other binding partners in vivo remains an open question.
2.3. Membrane Association
Most FAS1 domain proteins contain additional structural features and post-translational modifications some of which are shared between phylogenetically distinct family members. Practically all FAS1 proteins have an N-terminal secretion peptide; however, the final localization is determined by other signals. Mycobacterial Mpb83 is lipid-anchored to the plasma membrane by Cys acylation of the LAGC motif close to the N-terminus [40,41]. In plants, most FAS1 proteins are anchored to the outer sheet of the plasma membrane by a glycosylphosphoinositidyl (GPI) lipid anchor [14]. This modification is attached to proteins in the endoplasmic reticulum in exchange of a C-terminal signal peptide and exists in FAS1 proteins of plants, insects and fungi [17,18,42]. However, only one of the two D. melanogaster FAS1 proteins, DmFas1, is GPI-anchored [43]. The two mammalian stabilins and SpFsc1 from fission yeast are type 1 transmembrane proteins with cytosolic tails at their C-termini [10,19]. By contrast, Mpb70 from mycobacteria, mammalian Tgfbi and Pn, DmMfas and a group of FLAs are soluble proteins without any membrane anchoring motif [14,34]. Hence most FAS1 proteins are either bound to the plasma membrane or are soluble. In case of Mpb83 both surface anchored and soluble forms exist. Likewise, GPI-anchored proteins can be released from the plasma membrane by the action of phospholipases. The GPI anchor is probably needed for efficient transport of GPI-anchored proteins to the plasma membrane and potentially for their controlled release. In plants the GPI-anchor acts as a sorting signal for plasmodesmata [44]. Whether in non-animal eukaryotes such as plants and fungi that lack a cholesterol-rich plasma membrane, the GPI modification functions similar to animals to sequester proteins into membrane microdomains is under debate [45].
2.4. Glycosylation
Secreted proteins are often glycosylated. This is also the case for many FAS1 proteins, but not for all of them. For instance, although Mpb70 and Mpb83 share 75% sequence identity, but the latter is O-glycosylated close to its N-terminus with three threonine-linked mannose residues [46]. Another example is the N-linked glycosylation of four animal FAS1 proteins, mammalian Tgfbi and Pn and insect Fas1 and Mfas that are similar in size and all have four FAS1 domains, however, they carry zero, one, five and eight N-glycans, respectively. Likewise, in plant FAS1 proteins, N-glycosylation of FAS1 domains ranges from two to eight N-glycans [14,16]. A distinguishing feature of FAS1 proteins in plants is the presence of regions rich in proline, alanine, serine and threonine (PAST). Especially sequences containing the repetitive pattern (P-A/S/T)n, whose Pro residues are post-translationally converted to Hyp and galactosylated are typical for AGPs [47]. Indeed, the first FLAs were identified in precipitates obtained with β-glucosyl Yariv reagent [12,13], which binds to β(1→3) galactose chains present on many AGPs [48,49]. However, the peptide patterns for O-glycosylation are not as precisely defined as the N-X-T/S N-glycosylation motif [50], and biochemical identification of O-glycosylation on defined proteins is not as straightforward as the molecular weight shift caused by enzymatic removal of N-glycans. Hence one should be aware of potential discrepancies as to which FAS1 proteins are indeed FLAs, e.g., Algal-CAM contains an extensive PAST-rich N-terminal region and so does OsMtr1, and after removing N-glycans the apparent molecular weights of both proteins were larger than expected for the apoprotein [11,16]. Therefore, both proteins might well be members of the FLA family. By contrast, other FAS1 proteins such as the wheat locus named TaFLA9 lack any PAST-rich regions [51] and might be wrongly annotated as FLAs. However, the AtFla4 protein is decorated with AGP-like glycan epitopes despite the absence of canonical (P-A/S/T)n repeats [38]. Clearly, the simple presence or absence of PAST-rich regions is an inaccurate predictor for AGP-like O-glycosylation. The O-glycans of FLAs and other AGPs are known as type II arabinogalactans (AG II). They consist of Hyp-linked galactose that is the basis of a (1–3) β-linked galactan backbone with (1–6) β-linked galactose branches and kinks. AG II contains terminal modifications, mainly by l-arabinofuranose, l-rhamnose, l-fucose, d-glucuronic acid, 4-methyl-d-glucuronic acid and d-xylose [52,53,54]. Although pharmacological and genetic interference with AG II function has revealed many biological roles for these carbohydrates [55,56], models of how AGPs function remain sparse. An AGP of low abundance was shown to form a covalent link with both hemicellulose and pectin [57]. In fractionated cell walls, AGP-associated carbohydrate epitopes are abundant in pectic and hemicellulosic fractions and some are even present in the cellulose fraction [58]. Moreover, AGPs are self-adhesive in a calcium-dependent fashion [59], and can bind to pectin via carboxyl–calcium ion bridges [60]. Such results indirectly suggest that AG II-modified proteins FLAs might at least partially be linked to cell wall polysaccharides via their O-glycans.
2.5. Additional Domains
For human FAS1 proteins, O-glycosylation has not been reported [43]; however, they contain additional domains that expand their spectrum of molecular interactions. The N-terminal region of mature HsTgfbi, HsPn and DsMfas folds into a structure called Cysteine-Rich domain of Pn and TGFBIp (CROPT) [33], that mediates the interaction with various forms of collagen, fibronectin and the heterophilic binding between HsPn and HsTgfbi (reviewed in [43,61]). The human stabilins contain three dual FAS1 domain tandems and one isolated FAS1 domain, that each are separated by a varying number of Epidermal Growth Factor (EGF) repeats and an X-link domain. These domains are thought to be involved in phosphatidylserine and hyaluronic acid binding, respectively, but are not present in FAS1 proteins other than mammalian stabilins [62,63]. The C-terminal domain (CTD) of HsTgfbi contains the RGD motif that, although crucial for integrin binding of many adhesion proteins, is not required for the adhesion stimulating properties of HsTgfbi [64,65]. However, the RGD motif might modulate binding of HsTgfbi to specific integrins [39]. The CTD of HsPn, displays a high degree of intrinsic disorder and is both differentially spliced [66] and proteolytically regulated [67]. Interestingly, compared to full-length forms, HsPn forms lacking the CTD show relatively enhanced binding to ECM proteins [68]. The extreme C-terminus is highly basic and is thought to bind to heparin. Differential splicing and proteolysis might switch between forms of HsPn that more readily binds to HS and forms that more strongly interact with matrix proteins [61,67]. Compared to vertebrates, differential splicing is less frequent in plants; however, a great degree of regulatory fine-tuning might be provided by differential gene expression of the large number of plant genes encoding FAS1 proteins.
2.6. Groups of Fasciclin 1 Proteins in Plants
Compared to other organisms, plants contain the greatest number of FAS1 protein encoding genes. The majority of them are annotated as FLAs. The numbers of FLA encoding loci vary considerably between different plant genomes such as cotton (17 and 19 loci in Gossypium barbadense and G. hirsutum, respectively [69,70]), Eucalyptus grandis (18 [71]), Arabidopsis thaliana (21 [14]), Cannabis sativa (23 [72]), Brassica rapa (33 [73]), wheat Triticum aestivum (34 [51]), rice Oryza sativa (27 [74]) and cottonwood Populus trichocarpa (35 [75]). Apparently, diversity in this gene family varies even between closely related species (e.g., between the two Gossypium species or between Arabidopsis thaliana and Brassica rapa). An important step to understand the diversity of this complex gene family was sub-classification according to the presence of one or two FAS1 domains, their sequence similarity, the presence of GPI-anchors and the organization of PAST-regions [14]. Inclusion of FAS1 domain proteins that are not yet annotated as AGPs, such as OsMtr1 and Algal-CAM, might slightly enlarge the FLA family. The present FLA sub-classification has four groups: group A members are GPI-anchored and contain a single FAS1 domain flanked by two PAST-regions, group B are not GPI-anchored and sport a tandem of two FAS1 domains separated by a PAST-region. Group C contains GPI-anchored, single and tandem FAS1 proteins, with the single FAS1 domains phylogenetically closest to the N-proximal FAS1 domains of that group. Moreover, group C has PAST regions inserted close to their C-termini and between FAS1 domains. Lastly, group D contains FLAs that are not closely related to any other group and might represent separate branches of diversification.
3. Biological Aspects of FAS1 Domain Proteins across the Tree of Life
Human and Mammalian Fasciclin 1 Proteins
FAS1 proteins have been the subject of experimentation in a variety of species; however, by far the most work has been published about the four human representatives [reviewed in [34,43,76,77,78,79,80]]. Particularly HsPn, HsTgfbi and HsStab2 have been implicated in a plethora of medically relevant conditions ranging from normal development [76,81] to wound healing [77], bone regeneration [82,83] and cancer [67]. A Medline search shows 624, 1229, 105 and 718 entries for Tgfbi, Pn, Stab1 and Stab2, respectively (2 March 2018, Title/Abstract, synonyms see Appendix B). A detailed medical discussion is beyond the scope of this article; however, selected highlights are presented to provide a critical viewpoint on the molecular function of FAS1 domain proteins in plants. Missense mutations in the HsTgfbi locus are known to cause different types of corneal dystrophy [84]. In many cases of this disease, mutated HsTgfbi forms aggregates in the corneal stroma. This is due to the thermal and pH instability of the protein and to its high abundance in the cornea [85]; however, the condition might not help us to understand HsTgfbi function. A complete Tgfbi gene knockout in mice caused neither corneal abnormalities nor any other major defects [86], suggesting compensation by other ECM molecules. Although Pn knock-out mice showed that this locus is essential for normal osteogenesis and dental development [87,88], the two closely-related Pn and Tgfbi loci function identically in many ways [43]. The most intriguing common property of mammalian FAS1 proteins is their interaction with the integrin family of ECM receptors (Table 1). Hetero-dimeric integrins are expressed in a cell-type-specific fashion and bind to many different ECM proteins such as fibronectin, laminin and fibrinogen. The cell adhesion assay described above (Figure 2) suggests that FAS1 domains might bind to integrins; however, to establish the mode of binding, alternative assays such as co-immunoprecipitation of recombinant proteins and Foerster resonance energy transfer are required (Table 1).
Table 1.
Mammalian Fas1 proteins interact with integrin receptors.
| Integrin Type | TGFBI | PN | STAB-2 |
|---|---|---|---|
| α1β1 | [64] I,A | ||
| α2β1 | [89] C,F | ||
| α3β1 | [35] A,I,P [90] A,I [91] A,I |
||
| α4 | [92] I | ||
| α5 | [92] I | ||
| α5β1 | [93] A,I,G | ||
| α6β4 | [94] A,I | [95] I | |
| α7β1 | [96] A,I | ||
| αvβ3 | [97] A,I,P [98] A,I [99] C,F |
[100] I,A [101] I [88] I [102] A,D [103] G [104] I [105] I |
|
| αvβ5 | [106] A,I,P [36] A,I,P |
[100] I,A [101] I [102] A,D [104] I |
[107] C,T |
| β3 | [108] G,A | ||
| αMβ2 | [65] A,C,I | [109] A,I | [110] I,G |
Assay type: G: genetic interference (siRNA, transfection, mutants & epistasis analysis etc.); A: cell adhesion (coating of plastic surfaces); I: immuno-interference (e.g., anti-integrin antibodies inhibit PN mediated responses); P: peptide interference (peptide of Fas1 domain interferes with integrin dependent binding); C: Co-IP (endogenous or added recombinant Fas1 protein); F: immunofluorescence co-localization; D: DNA-aptamer inhibition; T: FRET.
The integrin complexes are hubs of mechano-chemical signaling and interact with the cytoskeleton and with numerous signaling pathways including focal adhesion kinase (FAK), mitogen activated protein kinase (MAPK), Target of rapamycin (mTOR), and β-catenin that influence cell division and migration, differentiation and programmed cell death [76]. FAS1 proteins not only bind and influence integrins as a stationary component of the ECM but also act as mobile paracrine factors. An example for the latter is the role of HsPn in glioblastoma. In this invasive brain cancer, glioblastoma stem cells (GSCs) secrete HsPn that acts as chemoattractant for circulatory monocytes (MCs)—precursors of macrophages (Figure 4A).
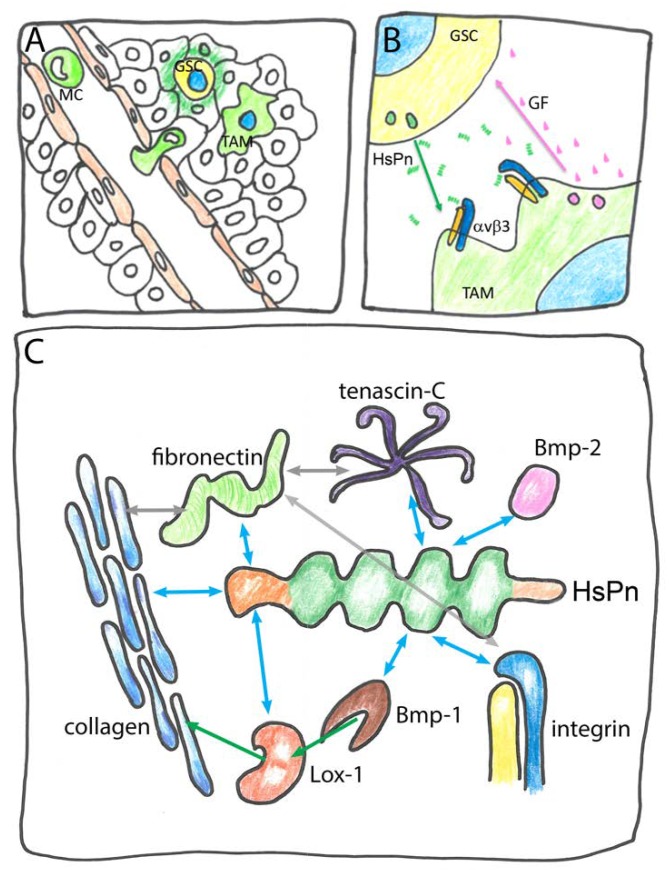
Figure 4.
Multiple roles of HsPn: (A) Circulatory monocytes (MC) are attracted by glioblastoma stem cells (GSC) that secrete HsPn (dark green) to invade the site of the primary tumor and differentiate into tumor associated macrophages (TAM); (B) GSC-secreted Pn via integrin αvβ3 signaling triggers the release of growth factors (GF) that establish a metastatic niche; (C) molecular interactions of Pn in the ECM. Blue double arrows indicate binding of HsPn. Grey double arrows indicate binding of other components. Green arrows indicate activating reactions: the metalloprotease Bone morphogenetic protein Bmp-1 proteolytically activates Lysine oxidase 1 (Lox-1) in turn enhancing collagen crosslinking.
MCs are induced to invade the site of the primary tumor and to differentiate into tumor associated macrophages (TAMs) by HsPn binding to MC-expressed integrin. In turn, TAMs secrete growth factors that further stimulate cell division in GSCs generating a metastatic niche [105] (Figure 4B). A similar model for Pn function has previously been proposed for other malignancies such as cholonangiocarcinoma and renal carcinoma [93,104]. Both HsPn and HsTgfbi directly bind to several ECM components and modulate the biosynthesis of the ECM proteins (Figure 4C). Owing to its versatile binding properties, mammalian Tgfbi was previously known as collagen-associated protein containing the RGD sequence (RGD-CAP) [111], while HsPn is known to bind to collagen and fibronectin [61]. The latter interaction affects the correct secretion and deposition of these fibrous proteins [112] and additional interactions facilitate the intramolecular crosslinking of collagen in the ECM. Via its FAS1-1 and FAS1-3 domain Pn binds to Bone morphogenetic protein-1 (Bmp-1), a metalloprotease that proteolytically activates lysine oxidase-1 (Lox-1), which, in turn, enhances collagen crosslinking [113,114]. The peptide growth factor Bone morphogenetic protein-2 (Bmp-2) that is not related to Bmp-1 also binds to Pn albeit at a different site [113]. The physiological role of this interaction remains unknown. Another matrix protein interacting with Pn is tenascin-C that forms a remarkable six-armed structure named hexabrachion and it was suggested that the link between tenascin-C and collagen formed by Pn might adapt the ECM to mechanical stress [68]. While some of the ligands of HsPn and HsTgfbi bind to the CROPT domain or the C-terminal domain, the interaction with integrin occurs via FAS1 domains. In overview, the two secreted FAS1 proteins HsTgfbi and HsPn exemplify how FAS1 domains accommodate a variety of protein–protein interactions enabling a single protein to act both in ECM integration and in intercellular signaling. By contrast, the membrane anchored stabilins mostly act as receptors for self ligands and as cell–cell interaction signals. One of their biological functions is in the removal of unwanted material from extracellular spaces and circulation by professional scavenger cells. Despite their similarity at the domain and sequence level, HsStab1 and HsStab2 are associated with distinct ligands and processes. HsStab1 acts as an intracellular sorting receptor as well as an endocytotic cell surface receptor for ligands such as acetylated low-density lipoprotein (acLDL) [115], secreted protein acidic and rich in cysteine (SPARC) [116,117], placental lactogen (PL) [118] and a chitinase-like protein called SI-CLP [119,120]. The FAS1-7 domain of HsStab1 binds to PL and SI-CLP while acLDL and SPARC interact with domains other than FAS1. HsStab1 can guide its various ligands on the secretory route from the Golgi into the lysosomes after endocytosis and through transcytosis (reviewed in [79]). HsStab2 binds ligands such as acLDL and advanced glycation products as well as various glycosaminoglycans such as HA, chondroitin sulfate and dermatan sulfate as well as the anticoagulant heparin and is required for the physiological clearance of these materials from circulation [121]. The polysaccharides bind to the X-link domain on Stab2 [122]. Both HsStab1 and HsStab2 have also been implicated in the phagocytosis of unwanted cells [123]. With their EGF domains, the stabilins recognize the “eat-me!” signal phosphatidylserine (PS) present at the surface of apoptotic cells and aged erythrocytes. HsStab2 utilizes its FAS1 domain in this process to bind to and cooperate with αvβ5 integrin [107]. The interaction between HsStab2 and PS is important for the fusion of uninucleate myoblasts during myofiber formation with PS functioning as a “fuse-me!” signal [124,125]. Finally, the interaction of HsStab2 FAS1 domains with integrins mediated both homotypic and heterotypic cell to cell adhesion [110,126]. In summary, each of the four human FAS1 proteins fulfils a variety of different biological functions. While secreted HsTgfbi and HsPn act in cell–ECM adhesion, ECM structuring and intercellular signaling, the membrane-anchored HsStab1 and HsStab2 proteins act in endocytosis, intracellular trafficking and cell to cell recognition.
4. Biological Functions of Plant Fasciclin 1 Proteins
The varying gene numbers for FLAs in different plant species shows that gene duplication and gene loss are common. On the one hand, this suggests that the large repertoire of FAS1 proteins might be fine-tuned at the level of differential gene expression analogous to differential mRNA splicing in vertebrates. On the other hand, gene redundancy makes the investigation of FLA function more cumbersome. However, the non-redundant AtFla1, AtFla3, AtFla4, OsMtr1 and the pair of AtFla11 and -12 provide a glimpse of the role of FAS1 proteins in plants.
4.1. The AtFla4 AtFei Pathway
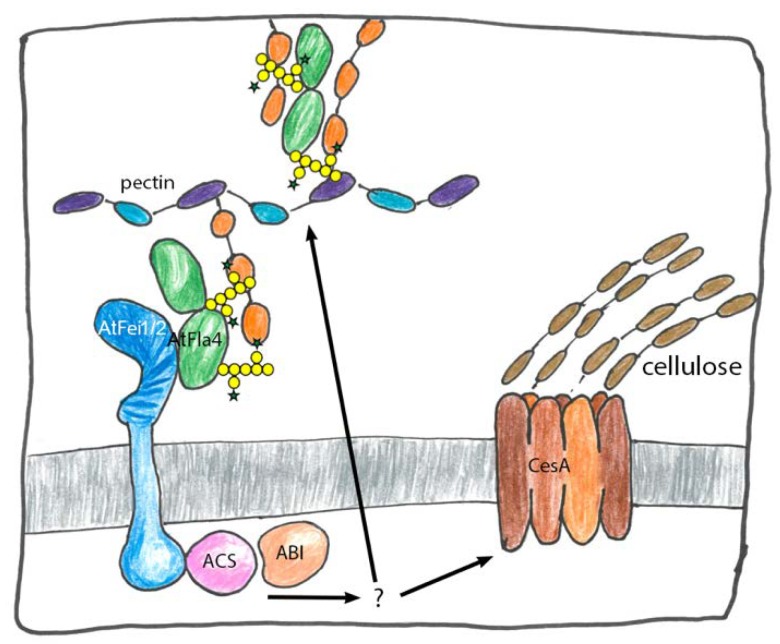
A mutant screen in Arabidopsis thaliana that discovered several key regulators of salt stress response [127] also identified a mutant that carried a missense mutation in the AtFla4 locus [15] often called atsos5 and here referred to as atfla4 for consistency. In atfla4 root elongation was almost completely stalled after transfer to growth media containing moderate levels of NaCl and the mutant root developed a characteristic radially swollen tip. Likewise, elevated levels of sucrose induced this phenotype in atfla4 [128], which is reminiscent of many conditional mutants defective in cell wall polysaccharide biosynthesis [129]. Although conditional root swelling is the most dramatic aspect of the atfla4 phenotype, the mutant’s abnormally thickened hypocotyls and inflorescence stems, its fat roots under salt-free conditions, larger leaves, shorter siliques and its abnormal seed coat mucilage indicate that AtFla4 acts non-conditionally throughout the plant [15,128,130,131,132,133,134]. Cell walls in atfla4 roots were shown to lack the pectin-rich middle lamella that is essential for intercellular adhesion, suggesting a potential function of AtFla4 in pectin biosynthesis or structure [15]. However, double knockouts in the two similar leucine-rich receptor-like kinases (LRR-RLKs) AtFei1 and AtFei2 were not only phenotypically identical to atfla4 but also non-additively interacted with it [128]. This suggested that AtFla4 and the two AtFei loci act in a linear genetic pathway. The atfei1 atfei2 double mutants were hypersensitive to cellulose biosynthesis inhibition and showed a decrease in crystalline cellulose production under restrictive conditions. As these phenotypic features were reminiscent of cellulose biosynthetic mutants it was proposed that the Fei-RLKs and by association AtFla4, might function in the biosynthesis of this important polymer [128]. The phenotype of both atfla4 and atfei1 atfei2 was modulated by variety of growth regulators. Firstly, the atfei1 atfei2 root phenotype was suppressed by inhibition of the biosynthesis and oxidation of ACC but not of the perception and signaling of its product ethylene [128]. This suggested that, in line with AtFla4, the AtFei loci might negatively regulate a novel ethylene-independent ACC signaling pathway that is activated by cell wall defects [135]. Both AtFei proteins interacted with ACC synthase in yeast two hybrid assays but did not phosphorylate them in vitro [128]. Secondly, abscisic acid (ABA) signaling acted synergistically with AtFla4 to regulate root elongation and abiotic stress response as the atfla4 phenotype was suppressed by exogenous ABA and by ABA-oversensitive mutants and a atfla4-like phenotype was induced by ABA inhibition [130]. Interestingly, AtFla4 did not interact with two NADPH respiratory burst oxidases that are required for stress responses triggered by cellulose synthase inhibition [136,137]. Finally, both the atfei1 atfei2 double and the atfla4 single mutants non-additively interacted with the IAA-alanine resistant 4 (AtIar4) locus [138]. These studies implicate the AtFla4 AtFei pathway with a variety of intracellular signaling pathways; however, they do not provide a model for the relation between the cell wall and AtFla4. Insights into this problem were stimulated by the characterization of the seed coat mucilage phenotype of atfla4 and atfei2. During seed maturation, pectic mucilage polymers are secreted into a pocket between the plasma membrane and the primary cell wall. Upon hydration of the mature seed the mucilage polymers rapidly swell and rupture the primary walls. However, the mucilage normally adheres to the ruptured primary cell wall [139]. There exist numerous mutants affected in aspects of seed coat mucilage biosynthesis and maturation. They are instructive models for cell wall biosynthesis throughout the plant [140]. Because in atfla4 and atfei2 mutants and in the cellulose synthase mutant atcesA5 seed coat mucilage adhesion was found to be defective it was initially suggested that AtFla4 and AtFei2 might act in cellulose biosynthesis [131,132]. However, the subsequent comparative analysis of atfla4, atfei2, atcesA5 and other mucilage adherence mutants showed distinct roles of AtFla4 and AtFei2 on the one hand and AtCesA5 on the other hand [133,134]. Rather than influencing cellulose or hemicellulose structure, AtFla4 acted on the pectin network, which was clearly distinct from the genetic actions of AtCesA5 and of the xylan biosynthetic locus Mucilage-modified5 (AtMum5) [133,141,142]. In contrast to mutants in the latter genes, atfla4 non-additively interacted with two pectin-related loci. Firstly, Mucilage-modified2 (AtMum2) encodes a β-galactosidase acting on galactan side chains of pectic rhamnogalacturonan type I (RG I) and its loss of function prevents the extrusion of mucilage upon hydration [143]. The extrusion defect of atmum2 was suppressed in the atfla4 atmum2 double mutant [133]. Secondly, Flying saucer 1 (AtFly1) encodes a transmembrane E3 ubiquitin ligase thought to regulate the degree of pectin methyl esterification. Loss of AtFly1 function led to ectopic adhesion between primary cell walls and mucilage [144] and atfla4 suppressed this phenotype [133,134]. A possible explanation for these observations is that AtFla4 might physically interact with galactan side chains on rhamnogalacturonan type I (RGI) pectin structures that are normally remodeled by the β-galactosidase encoded by AtMum2, to control timely mucilage maturation and adherence. This hypothesis is consistent with the localization of AtFla4 in the mucilage pocket during seed coat development [133,134]. How does AtFei2 fit into this scenario, and can the characterization of the seed coat mucilage phenotype be extrapolated to the whole plant? In the case of seed coat mucilage, AtFei2 might attach AtFla4 to the plasma membrane and thereby contribute to the architecture of the network—an entirely structural explanation. However, it cannot be ruled out that the interaction between the hypothetical AtFla4-pectin matrix and AtFei2 might also convey signals to the cell interior [133,134]. While AtFla4 localized to the developing mucilage pocket and the adjacent plasma membrane of seed coat epidermal cells, AtFla10 and AtFla17 but not AtFla4 were identified in a proteome of mature mucilage [145]. Whatever the biological function of these FLAs in mature mucilage, due to the atfla4 single mutant phenotype they apparently are not functionally equivalent to AtFla4. It is unclear whether the mode of action of AtFla4 in seed coat mucilage is identical to its mechanism throughout the plant; however, the overlapping genetic role with the AtFei genes in both tissues suggests a common mechanism. The AtFla4 protein might interact with various cellular components. It was hypothesized that AtFla4 might bind to the AtFei RLKs [128,146]. The localization of the majority of yellow fluorescent protein-tagged AtFla4 at the plasma membrane is compatible with this possibility [38]. However, AtFla4 was also released into the apoplast, where it might interact with cell wall carbohydrates [38,133]. The interaction of AtFla4 with cell wall polysaccharides remains to be investigated; however, both covalent and non-covalent interactions between pectic polymers and AG II glycan have previously been demonstrated (see above). In fact, it was shown that protein O-galactosylation is required for the function of AtFla4. Several galactosyl transferases (GALTs) mediate the initial glycosylation of hydroxyl proline residues of AGPs. Two of them called AtGalt2 and AtGalt5 together are responsible for the biosynthesis of the bulk of AGPs and their mutation caused a root and seed phenotype identical to atfla4 [147]. Intriguingly, the phenotypes of atgalt2 atgalt5 atfla4 atfei1 atfei2 quintuple and the atfla4 atfei1 atfei2 triple mutants were identical [146]. It was hypothesized that AtFla4 might physically interact with the Fei RLKs via its O-glycans. This hypothesis was seemingly contradicted by a structure to function study where the deletion of potential O-glycosylation sites on AtFla4 did not interfere with genetic complementation of atfla4 [38]. However, the AtFla4 level in the transgenic plants might have been higher than normal or cryptic O-glycosylation sites might still have been present. How AtFla4 and AtFei interact and if O-linked glycosylation plays a direct or indirect role for AtFla4 and AtFei function remains to be tested. Taken together, AtFla4 is a unique genetic paradigm for FAS1 domain proteins in plants. A speculative model (Figure 5) combined from previous studies proposes that after its release from the plasma membrane and via its O-glycan AtFla4 possibly interacts with pectic polymers and contributes to biophysical properties such as swelling and interpolymer connectivity.
Figure 5.
Hypothetical roles of AtFla4 in cell wall structure and signaling. AtFla4 might interact with the pectic network with covalent or non-covalent interactions of its glycans (yellow discs and green stars: O-glycans which are yet to be precisely defined, N-glycans are omitted). It might mechanically link pectin with AtFei1 and AtFei2 receptor kinases and the plasma membrane. The signals transduced by the receptor kinases might involve ACC synthase (ACS) and Abcisic Acid Insensitive 1 (ABI1). How signaling connects the kinases with cellulose synthase (CesA) and pectin biosyn thesis is unclear.
In parallel AtFla4 might also interact with AtFei1 and AtFei2 via its FAS1 domain to link pectin with AtFei1 and -2 and indirectly to ACC synthase. The access of the C-terminal Fas1 domain to the AtFei receptor domain might be modulated by the interaction to the cell wall. Under standard conditions relatively firm binding of AtFla4 to AtFei might suppress ACS. Under cell wall stress conditions binding of AtFla4 to AtFei might be reduced and the ACS suppression by AtFei might be relaxed leading to ACC production and decreased cellulose production and other responses. However, any postulated physical interactions of AtFla4 remain to be demonstrated.
4.2. A Conserved Function of AtFla11 and AtFla12 in Secondary Cell Walls
One of the functionally and economically most interesting aspects of some FLAs is their role for secondary cell wall formation and structure. Secondary cell walls are by far the most abundant re-growing fiber and energy feedstock on this planet and its major constituents—cellulose, xylans and lignin—are among the most abundant biopolymers. Their sustainable and socially compatible production is thought crucial in the global transition to a carbon neutral economy. AtFla11 and AtFla12 (group A; [14]) and their orthologs (Appendix C) from diverse fiber crops and model plants were noted in Arabidopsis thaliana for their expression during the secondary thickening of stems [148], their co-expression with secondary cell–wall–specific cellulose synthase loci [149] and their expression in sclerenchyma cells [150]. A correlative and causative association of AtFla11 and AtFla12 orthologs with secondary cell wall biosynthesis has been observed in many plant species. The Zinnia elegans ZeFla11 gene was found to be expressed in trans-differentiating cell cultures and in a specific subset of secondary cell wall forming vessel cells and adjacent parenchyma cells [151]. AtFla11 and -12 orthologs from poplar, eucalyptus and willow were highly expressed in tension wood (TW), a reaction wood forming at the upper side of branches and stems growing sideways. TW consists of almost pure cellulose deposited in the gelatinous secondary cell wall layer (G-layer) in elongated G-fiber cells and shows a characteristically low-cellulose microfibril angle (MFA, with respect to the growth axis), which entails superior tensile strength [152,153,154]. The three AtFla11/12 eucalyptus orthologs EgFla1, -2 and -3 were highly expressed in stems correlating with secondary cell wall formation [71]. Also in flax and hemp AtFla11/12 orthologs were highly expressed in fiber forming tissues [72,155]. A cotton AtFla12 ortholog was expressed at a higher level and for a longer time period in Gossypum barbadense compared to G. hirsutum correlating with greater fiber strength and length of the former [69]. Hence, AtFla11 orthologs might be natural genetic determinants for fiber crops. So, throughout the higher plants, AtFla11/12 and their orthologs correlate with secondary cell wall formation, specifically the deposition of cellulose microfibrils aligned along the axis of mechanical stress. What is the role of FLAs in this process?
In Arabidopsis thaliana there exists a genetic redundancy between AtFla11 and -12; however, the atfla11 atfla12 double mutant was affected in the mechanical properties of its secondary cell–wall–rich stems [156]. In atfla11 atfla12 tensile strength and stiffness were reduced and MFA was increased. Moreover, the chemical composition and cell wall structure showed a reduction of galactose, arabinose and cellulose and a concomitant increase in lignin content. The authors speculated that the FLAs via their FAS1 domains might form a heteromeric higher-order network strengthening the interaction between cellulose microfibrils [156]. The most highly expressed hybrid aspen locus called PtFLA6 was silenced with antisense RNA. This also reduced the transcript level of many other PtFla genes [157,158]. Although no apparent morphological phenotype was observed, the PtFla6 knockdown resulted in reduced flexural strength and stiffness in stems of transgenic plants. Moreover, the content of AGPs, cellulose and lignin was reduced alongside a reduction of transcript levels for numerous genes involved in cellulose and lignin formation [157]. When TW formation was stimulated in the PtFla6 knockdown plant, the relative frequency of abnormal G-fibers increased, but no other changes were observed [158]. Taken together, the complex response to PtFla6 interference is more compatible with a regulatory role than it is with a direct structural role in the cellulose layer. In Eucalyptus grandis, EgFla1, -2 and -3 were experimentally overexpressed in cambial sectors or in whole tobacco plants [71]. Despite their sequence similarity, each transgene exerted a specific effect on phenotypic parameters such as MFA, cell wall thickness, cell wall composition and xylem cell type specification. This meant that structurally even very similar AtFla11/12 orthologs exerted different effects in the same cellular context [71]. The function of AtFla11/12 orthologs has also been investigated during cotton fiber formation. In this system, trichome cells are initiated at the outer integument of the ovule during anthesis (2 d before to 5 d after anthesis; dpa). Then the trichomes undergo a period of rapid cell elongation (3–20 dpa), which is followed by secondary wall formation (16–40 dpa) and maturation (40–60 dpa). Hence, each cotton fiber is a single trichome cell made up of almost pure cellulose [159]. Its formation is the result of three distinct developmental processes: (1) specification and initiation, (2) expansion and (3) cell wall thickening and maturation. Two AtFla11/12 orthologs in the cotton species Gossypium hirsutum named GhAgp4 (Appendix D) and GhFla1 were suppressed using RNAi [159,160]. GhFla1 was specifically expressed in elongating fibers with peak expression at 10 dpa [70] and indeed, fibers of RNAi lines were much shorter than controls [159]. However, GhFla1 RNAi suppression also resulted in delayed and reduced trichome initiation and, moreover, resulted in decreased RNA levels of other fiber-expressed FLAs and of numerous genes implicated with cell wall biosynthesis. The complex alterations at the transcript level correlated with alterations in cell wall composition including decreased cellulose formation. By contrast, the overexpression of GhFla1 resulted in increased initiation and elongation of cotton fibers as well as upregulation of other FLAs and cell wall biosynthetic loci and in an increased cellulose content [159]. These data confirmed and extended a previous study where the GhAGP4 locus was silenced by RNAi. Like the GhFla1 silencing, this reduced fiber initiation, elongation and quality, and affected the level of transcripts related to cell wall biosynthesis [160]. The observations made in cotton are difficult to reconcile with a biomechanical role of GhFla1 and GhAgp4 as an adhesive between secondary cell wall cellulose microfibrils because not only secondary cell walls were affected by the interference but also the fundamentally different processes of initiation and elongation that happen before. The apparent involvement of these genes in all three phases of fiber formation and their control of mRNA levels for an entire battery of other genes rather suggests that this class of FAS1 proteins fulfils a role in developmental control comparable to a peptide hormone such as the proteoglycan xylogen [161].
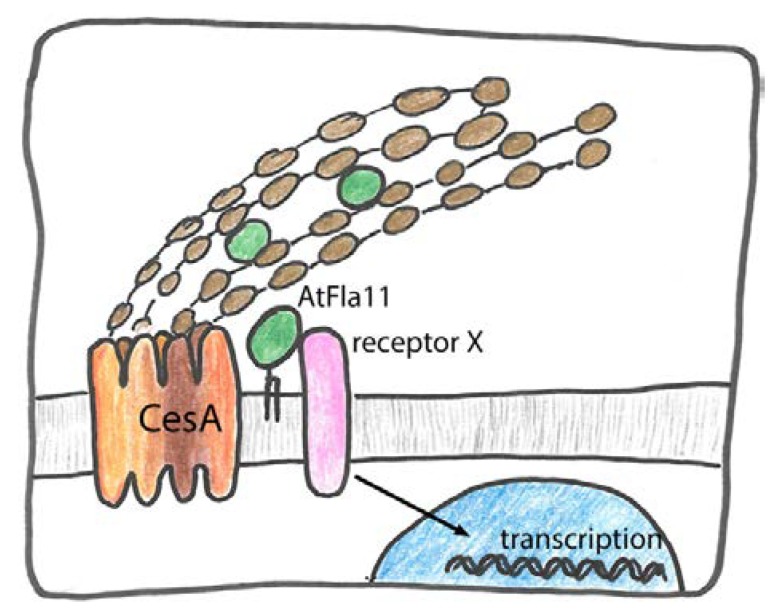
What do we know about possible molecular interactors and subcellular localization of AtFla11/AtFla12 orthologs? Intriguingly, cotton orthologs of AtFla7 and AtFla11 were co-precipitated with active secondary cellulose synthase GhCesA8 that was effectively extracted by inclusion of cellulase in the extraction buffer [162]. This supports the hypothesis that group A FLAs physically interact with the cellulose synthase complex. This hypothesis offers an attractive explanation for co-expression between group A FLAs and secondary cell wall formation related genes and for changes in MFA and mechanical properties in group A FLA loss of function genotypes. If one assumes that nascent cellulose fibers in combination with cellulose synthase are both bound by FLAs, this model could also explain the localization of PtFLA6 in secondary cell walls [157,158]. However, this model offers no simple explanation for the observation that the transcriptional program of secondary cell wall formation as well as cellular differentiation was heavily influenced by the experimental manipulation of FLAs [157], or for the many effects of FLA overexpression. This problem might be solved with a role of FLAs in signaling. So far, there has been one report that suggested that a RLK might physically interact with FLAs. A pull-down experiment using the Arabidopsis thaliana sucrose-induced receptor kinase (AtSirk1) as bait identified AtFla8 and AtFla9 among the 16 interaction partners. Interestingly, among several potential substrates of AtSirk1, there was the primary cell wall specific cellulose synthase catalytic subunit 3 (AtCesA3) [163]. At present, there is no genetic evidence linking AtFla8 and AtFla9 with primary cell wall cellulose synthesis; however, the evidence for their physical interaction with an RLK that acts on cellulose synthase offers an interesting alternative mechanism for how FLAs might influence cell wall biosynthesis. Using their potential to interact with multiple binding partners they might combine receptor kinases with substrates such as cellulose synthase. Taken together, several modes of action can be envisaged for AtFla11/12 and its orthologs (Figure 6).
Figure 6.
Putative role of AtFla11, AtFla12 and their orthologs in secondary cell wall formation. Firstly they might act as secreted structural proteins in secondary cell walls binding to cellulose or other glycans. Secondly, they might be part of outside–in signaling, in connection with an unknown receptor that leads to complex changes of gene expression. Thirdly, they might bind to cellulose synthase (CesA) and directly influence or stabilize its action. Finally, they might act as adaptors between CesA and receptor kinases to modulate CesA phosphorylation.
Firstly, AtFla11/12 might be directly secreted to the cell wall and bind to polymers and cross-link them. Secondly these FAS1 proteins might act as extracellular signals or be part of a receptor complex to influence transcriptional events downstream. Thirdly, they might augment cellulose deposition by acting as a physical part of the cellulose synthase machinery. Fourth, by interacting with both cellulose synthase and receptor kinase they might facilitate or modulate post-translational regulation of cellulose biosynthesis (Figure 6).
4.3. A Cascade of FLAs Acts in Male Gametophyte Development
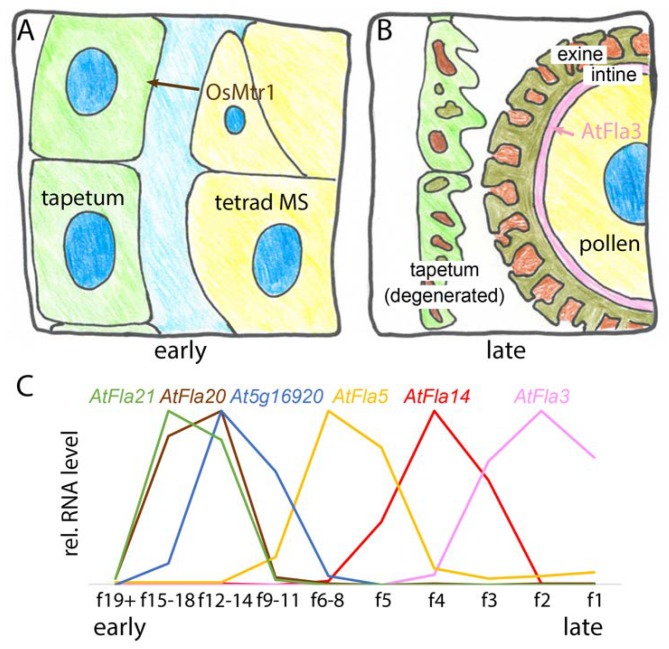
The FAS1 proteins encoded by the AtFla3 and the OsMtr1 genes, respectively, are required for male gametophyte development; however, the mRNA pattern in Arabidopsis thaliana suggests that several FLAs might sequentially act in this process (Figure 7).
Figure 7.
FAS1 proteins act sequentially in male gametophyte development: (A) OsMtr1 acts in early stage of tapetum development. It is specifically expressed and secreted in microspore cells (MS) and influences the development of tapetum cells; (B) AtFla3 acts at the late stage of the formation of the cellulose- and pectin-rich intine layer of pollen cell walls; (C) RNA-Seq data of Arabidopsis thaliana at different floral stages [164,165] show that six FAS1 genes are specifically expressed in anthers at different stages of floral development. f1 indicates the first open flower. The expression patterns of AtFla20 that might be the ortholog of OsMtr1 and of AtFla3 are consistent with roles in early and late pollen development, respectively.
No functional pollen was found in the rice microspore and tapetum regulator 1 (osmtr1) mutant [16]. OsMtr1 expression was specifically detected in early microspore development during meiosis and tetrad formation and during the release of young microspores (Figure 7A). However, despite OsMtr1 never being expressed in the tapetum, a nutritive maternal tissue that surrounds microspores, the osmtr1 mutation dramatically affected tapetum development. This suggested that OsMtr1 might act non-cell autonomously. The osmtr1 mutant tapetum failed to undergo programmed cell death, which normally supports pollen wall formation in developing microspores. Tetrads formed normally in osmtr1 yet, mutant microspores showed defective development as soon as they were released possibly due to the lack of cell wall material contributed by the adjacent tapetum. The localization of OsMtr1 in the microspores and the extracellular space between them and the tapetum suggested that OsMtr1 might act as a secreted signal to coordinate microspore development with tapetum degeneration (Figure 7A). Molecular interactors such as a hypothetical OsMtr1 receptor on tapetum cells remain to be identified [16]. The expression of AtFla3 in pollen during anthesis and pollen germination combined with the high level of expression in anthers is unique among all FLAs and AtFla3 function was investigated using RNAi and overexpression [166]. The AtFla3 RNAi lines were partially male-sterile due to a reduced ability to form functional microspores. Histological analysis showed that AtFla3 RNAi affected the formation of intine, the innermost layer of the pollen wall, which consists mainly of cellulose and pectin. The earliest time point at which intine abnormalities were observed was the late uninucleate stage, suggesting that AtFla3 might act in the formation of intine in microspore development. Abnormal AtFla3 RNAi pollen showed defective cellulose staining, suggesting that AtFlaA3 was required for cellulose deposition in the intine layer. More detailed analyses are required to test whether any other cell wall polymers are affected and if full loss of function of the AtFla3 locus causes a more complete defect of pollen function. During Arabidopsis thaliana floral development at least six FAS1 domain loci are specifically expressed in anthers during separate developmental stages [164,165]. Like its closest rice homologue OsMtr1, AtFla20 is expressed at an early stage, where it overlaps with AtFLA21 and At5g16920—an FAS1 protein not annotated as FLA (Figure 7C). The mRNA levels of AtFla14 and AtFla5 sequentially peak at intermediate stages and AtFla3 is expressed immediately before and during flower opening (anthesis) in mature and germinating pollen, as previously reported [166]. How most of these FAS1 domain proteins are involved in normal pollen wall formation remains to be investigated. Nonetheless, their expression pattern during pollen formation appears to reflect the complex development and composition of the pollen wall.
4.4. Plant FAS1 Proteins Have Many Potential Functions
Several other FLAs are known to perform at least partially non-redundant genetic roles. Firstly, the loss of function mutation of AtFla1 caused a phenotype in the growth regulator induced formation of shoots and roots from the callus [167]. Two alleles in two different wild-type backgrounds were isolated and the wild-type background on its own not only had a dramatic influence on callus derived organ formation but it essentially inverted the genetic effect of AtFla1 on the process. By contrast, the negative effect of the AtFla1 locus on root length and lateral root number was constant between the two tested wild-type backgrounds. It is presently unclear how AtFla1 might influence organ formation but initial promoter studies showed expression of AtFla1 early during the formation of organized tissue from callus. The protein might therefore be required for pattern formation [167]. Secondly, in a gene-editing study in the non-model plant Brassica carinata, BcFla1, a group A FLA that is more similar to AtFla13 than to AtFla11, was implicated in root hair growth, where tip-focused deposition of primary cell wall materials occurs [168]. In a third example, in maize the expression level of group A ZmFla genes was inversely correlated with seed abortion. Consistently, two different mutations in the group A AtFla9 locus that showed abnormally low and high AtFla9 RNA levels displayed increased and decreased seed abortion, respectively [169]. Lastly, a comparative study of 30 different wheat (Triticum aestivum) varieties found an association between the expression of a FAS1 protein and grain milling properties [170]. According to this report, the locus TaFla8 [51] coding for a FAS1 domain that does not contain any PAST-rich domains, is specifically expressed in grain. Interestingly, its level was relatively low in varieties displaying beneficial milling properties compared to varieties scoring poorly with respect to milling efficiency. Hence, human selection of wheat varieties with improved milling properties might have inadvertently reduced the basal expression level of TaFla8, which might normally strengthen cell walls.
5. Fungal FAS1 Proteins
In a screen for genes specifically expressed in fruiting bodies of the Shiitake mushroom Lentinula edodes the LeFlp1 locus coding for a GPI-anchored single FAS1 domain protein was identified and its specific expression during fruiting body formation suggested a developmental role [17]. In another study the MoFlp1 locus of the rice blast fungus Magnaporthe oryzae was shown to encode a GPI-anchored vacuolar tandem FAS1 protein [18]. Its disruption caused defects in the formation of conidia, which, according to the authors, hinted at a potential role of MoFlp1 in autophagy. Interestingly, the fission yeast Schizosaccharomyces pombe FAS1 domain protein SpFsc1 was identified in a genetic screen for autophagy-related loci [19]. SpFsc1 consists of five FAS1 domains in tandem and a transmembrane domain. The protein was detected in the vacuolar membrane and was shown to specifically act in a late step in autophagy, the fusion between the phagosomes with the vacuolar membrane. The mechanistic details including the role of the FAS1 domains in this process remain to be elucidated. In summary, studies on FAS1 domain proteins in fungi are sparse; however, they provide a stimulating variation of the hypothetical modes of action of this protein family.
6. Bacterial FAS1 Proteins
The most thoroughly studied eubacterial FAS1 proteins are the homologues Mpb70 and Mpb83 from Mycobacterium (reviewed in [171]). In various genomes of obligate pathogenic, opportunistic and non-pathogenic mycobacteria [172], both non-pathogenic and pathogenic species contained Mpb70/Mpb83 homologues, while the genomes of opportunistic mycobacteria did not. This suggests a relation of Mpb70/Mpb83 to mycobacterial lifestyle and pathogenesis. While Mpb83/Mpb70 was hypothesized to undergo “homophilic” interactions with mammalian Pn thereby interfering with cell adhesion in bones [171] this idea is not compatible with the secreted nature of Pn and is derived from cell aggregation data [8,173], which could have alternative explanations. Moreover, no intermolecular interactions between Fas1 domains have been reported. However, both host cell to ECM adhesion and host cell to mycobacterium adhesion might be modulated by binding of Mpb70/Mpb83 to molecules presented at the host cell surface. Indeed, Mpb83 was demonstrated to bind to Toll-like receptors 1 and -2 (HsTlr1 and -2), two LRR-RLKs present on human monocytes [41]. The binding of recombinant Mpb83 to HsTlr2 triggered mitogen activated protein kinase (MAPK) signaling and as a consequence induced the production of matrix metalloprotease-9 (MMP-9) and cytokines [174]. The direct interaction between Tlr2 and Mpb83, as shown by surface plasmon resonance [41], is an intriguing finding, given the presence of more than 200 LRR-RLKs in plant genomes [175]. A role of bacterial FAS1 proteins in the interaction between bacteria and their eukaryotic hosts is also observed in symbiotic communities. In the first example, the FAS1 protein SmNex18 (locus SMa1077) of the nitrogen-fixing legume symbiont Sinorhizobium meliloti was shown to be expressed specifically during nodulation, the initial stage of symbiosis between bacterium and plant [176,177]. Secondly, a FAS1 protein was found in Nostonoc cyanobacteria that form symbiotic bacterial–fungal communities called lichen. Nostonoc punctiforme isolates living in lichen highly expressed an unnamed FAS1 protein (locus ACC81089) that was only a minor compound in a closely related free living strain [178]. Moreover, FAS1 proteins featured prominently in the metaproteome of phyllospheric (i.e., plant leaf dwelling) bacteria [179] but were absent from rhizospheric (root-dwelling) bacteria [180]. Likewise, while present in many different genera of archaea, the FAS1 domain is not necessarily ubiquitous within the same genus. To give an example, among the 13 completely sequenced Methanobacterium isolates, only M. paludis isolated from peatland contained FAS1 domain protein sequences [181]. On the one hand, such circumstantial observations support the hypothesis that FAS1 proteins are important for the interaction between prokaryotic colonizers and eukaryotic hosts; on the other hand, they also show that FAS1 proteins are not essential.
7. Concluding Remarks
Can we define a common denominator for the function of FAS1 proteins? The characterization of HsTgfbi structure and function demonstrates the versatility of the FAS1 domain in binding multiple ligands, which might be a conserved feature. The frequent assumption that FAS1 proteins are involved in “cell adhesion,” however, is more context-dependent. It is derived from insightful experiments performed with animal cell cultures; however, it might be misleading for “hard-shelled” cells such as bacteria, fungi and plants that usually adhere to their ECM as a result of turgor pressure and build up adhesive materials such as pectin. By contrast, the designation of Tgfbi and Pn as matricellular proteins, defined as non-structural ECM-components that interact with cell surface receptors as mediators between the cell and its microenvironment [77,182], seems a more productive concept for approaching the common function of FAS1 proteins in all kingdoms of life including plants. The crucial challenge for future work on plant FAS1 proteins will be the identification of molecular interactors both of the FAS1 domain and of their abundant but enigmatic glycan modifications.
Appendix A
Since many genes and proteins from different organisms are discussed in this review, I adopted universal nomenclature rules according to a highly popular textbook [183]. Gene symbols are written in italics, with the first letter in uppercase indicating a wild-type allele and in lowercase indicating a mutant allele. Proteins are written in non-italics. If the text relates to genes or proteins from a certain species, a two-letter prefix (Hs for Homo sapiens, At for Arabidopsis thaliana etc.) is used.
Appendix B
Because many human proteins were independently discovered several times, and maybe also for asserting the status of discovery, there is an abundance of synonyms. Tgfbi is also known as β-ig H3, βIG, BIGH3, kerato-epithelin, RGD-CAP and MP78/70. Pn is synonymous with Osteoblast specific factor-2 (Osf-2). Stab1 has also been named Common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1) and FEEL-1 is an acronym for fasciclin, EGF-like, laminin-type EGF-like, and link domain-containing scavenger receptor-1. Frequent Stab2 synonyms are lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and hyaluronic acid receptor (HARE).
Appendix C
The term “AtFla11/12 ortholog” is here used to designate FLAs from other plant species that are more similar in protein sequence to AtFla11 or AtFla12 than to any other Arabidopsis thaliana protein.
Appendix D
The nomenclature in the cotton literature is somewhat confusing. Some FLAs are named AGPs and GhFla1 refers to two entirely different protein sequences. The GhFla1 that was mentioned by Li et al. (2010) [160] being most similar to AtFla9/6/13 and the GhFla1 silenced by Huang et al. (2013) [159] is an ortholog of AtFla11/12. The GhAgp4 locus (gb EF470295) silenced by Li (2010) [160] is identical to the sequence annotated as GhFla12.
Funding
This research was funded by the Austrian Science Fund FWF (Grant number I1182-B22).
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Bastiani M.J., Harrelson A.L., Snow P.M., Goodman C.S. Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell. 1987;48:745–755. doi: 10.1016/0092-8674(87)90072-9. [DOI] [PubMed] [Google Scholar]
- 2.Snow P.M., Zinn K., Harrelson A.L., McAllister L., Schilling J., Bastiani M.J., Makk G., Goodman C.S. Characterization and cloning of fasciclin I and fasciclin II glycoproteins in the grasshopper. Proc. Natl. Acad. Sci. USA. 1988;85:5291–5295. doi: 10.1073/pnas.85.14.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu S., Sonnenfeld M., Stahl S., Crews S.T. Midline fasciclin: A drosophila fasciclin-I-related membrane protein localized to the CNS midline cells and trachea. J. Neurobiol. 1998;35:77–93. doi: 10.1002/(SICI)1097-4695(199804)35:1<77::AID-NEU7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Y., Shanley J. Altered nerve terminal arborization and synaptic transmission in drosophila mutants of cell adhesion molecule fasciclin I. J. Neurosci. 1995;15:6679–6687. doi: 10.1523/JNEUROSCI.15-10-06679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jay D.G., Keshishian H. Laser inactivation of fasciclin I disrupts axon adhesion of grasshopper pioneer neurons. Nature. 1990;348:548–550. doi: 10.1038/348548a0. [DOI] [PubMed] [Google Scholar]
- 6.Clout N.J., Tisi D., Hohenester E. Novel fold revealed by the structure of a FAS1 domain pair from the insect cell adhesion molecule fasciclin I. Structure. 2003;11:197–203. doi: 10.1016/S0969-2126(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 7.Skonier J., Neubauer M., Madisen L., Bennett K., Plowman G.D., Purchio A.F. cDNA cloning and sequence analysis of βig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-β. DNA Cell Biol. 1992;11:511–522. doi: 10.1089/dna.1992.11.511. [DOI] [PubMed] [Google Scholar]
- 8.Takeshita S., Kikuno R., Tezuka K., Amann E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993;294:271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiuchi K., Amizuka N., Takeshita S., Takamatsu H., Katsuura M., Ozawa H., Toyama Y., Bonewald L.F., Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J. Bone Miner. Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 10.Politz O., Gratchev A., McCourt P.A., Schledzewski K., Guillot P., Johansson S., Svineng G., Franke P., Kannicht C., Kzhyshkowska J., et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem. J. 2002;362:155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber O., Sumper M. Algal-CAMS: Isoforms of a cell adhesion molecule in embryos of the alga Volvox with homology to Drosophila fasciclin I. EMBO J. 1994;13:4212–4222. doi: 10.1002/j.1460-2075.1994.tb06741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz C.J., Johnson K.L., Currie G., Bacic A. The classical arabinogalactan protein gene family of arabidopsis. Plant Cell. 2000;12:1751–1768. doi: 10.1105/tpc.12.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz C.J., Rumsewicz M.P., Johnson K.L., Jones B.J., Gaspar Y.M., Bacic A. Using genomic resources to guide research directions. The arabinogalactan protein gene family as a test case. Plant Physiol. 2002;129:1448–1463. doi: 10.1104/pp.003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson K.L., Jones B.J., Bacic A., Schultz C.J. The fasciclin-like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiol. 2003;133:1911–1925. doi: 10.1104/pp.103.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi H., Kim Y., Guo Y., Stevenson B., Zhu J.K. The Arabidopsis Sos5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell. 2003;15:19–32. doi: 10.1105/tpc.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan H., Liang W., Hu J., Zhang D. MTR1 encodes a secretory fasciclin glycoprotein required for male reproductive development in rice. Dev. Cell. 2012;22:1127–1137. doi: 10.1016/j.devcel.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki Y., Kaneko S., Sunagawa M., Shishido K., Yamazaki T., Nakamura M., Babasaki K. The fruiting-specific LeFlp1 gene, encoding a novel fungal fasciclin-like protein, of the basidiomycetous mushroom Lentinula edodes. Curr. Genet. 2007;51:367–375. doi: 10.1007/s00294-007-0133-2. [DOI] [PubMed] [Google Scholar]
- 18.Liu T.B., Chen G.Q., Min H., Lin F.C. MoFlp1, encoding a novel fungal fasciclin-like protein, is involved in conidiation and pathogenicity in Magnaporthe oryzae. J. Zhejiang Univ. Sci. B. 2009;10:434–444. doi: 10.1631/jzus.B0920017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L.L., Li M., Suo F., Liu X.M., Shen E.Z., Yang B., Dong M.Q., He W.Z., Du L.L. Global analysis of fission yeast mating genes reveals new autophagy factors. PLoS Genet. 2013;9:e1003715. doi: 10.1371/journal.pgen.1003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai S., Matsumoto J., Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect. Immun. 1981;31:1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai S., Miura K., Tokunaga T., Harboe M. Mpb70, a unique antigenic protein isolated from the culture filtrate of BCG substrain Tokyo. Dev. Biol. Stand. 1986;58:511–516. [PubMed] [Google Scholar]
- 22.Terasaka K., Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Yamada T. Complete nucleotide sequence of immunogenic protein Mpb70 from Mycobacterium bovis BCG. FEMS Microbiol. Lett. 1989;49:273–276. doi: 10.1111/j.1574-6968.1989.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 23.Radford A.J., Wood P.R., Billman-Jacobe H., Geysen H.M., Mason T.J., Tribbick G. Epitope mapping of the Mycobacterium bovis secretory protein Mpb70 using overlapping peptide analysis. J. Gen. Microbiol. 1990;136:265–272. doi: 10.1099/00221287-136-2-265. [DOI] [PubMed] [Google Scholar]
- 24.Ulstrup J.C., Jeansson S., Wiker H.G., Harboe M. Relationship of secretion pattern and Mpb70 homology with osteoblast-specific factor 2 to osteitis following Mycobacterium bovis BCG vaccination. Infect. Immun. 1995;63:672–675. doi: 10.1128/iai.63.2.672-675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burroughs A.M., Balaji S., Iyer L.M., Aravind L. Small but versatile: The extraordinary functional and structural diversity of the β-grasp fold. Biol. Dir. 2007;2:18. doi: 10.1186/1745-6150-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boratyn G.M., Schaffer A.A., Agarwala R., Altschul S.F., Lipman D.J., Madden T.L. Domain enhanced lookup time accelerated blast. Biol. Dir. 2012;7:12. doi: 10.1186/1745-6150-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clout N.J., Hohenester E. A model of FAS1 domain 4 of the corneal protein βig-h3 gives a clearer view on corneal dystrophies. Mol. Vis. 2003;9:440–448. [PubMed] [Google Scholar]
- 28.Moody R.G., Williamson M.P. Structure and function of a bacterial fasciclin I domain protein elucidates function of related cell adhesion proteins such as TGFBIp and periostin. FEBS Open Biol. 2013;3:71–77. doi: 10.1016/j.fob.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr M.D., Bloemink M.J., Dentten E., Whelan A.O., Gordon S.V., Kelly G., Frenkiel T.A., Hewinson R.G., Williamson R.A. Solution structure of the Mycobacterium tuberculosis complex protein Mpb70: From tuberculosis pathogenesis to inherited human corneal desease. J. Biol. Chem. 2003;278:43736–43743. doi: 10.1074/jbc.M307235200. [DOI] [PubMed] [Google Scholar]
- 30.Basaiawmoit R.V., Oliveira C.L., Runager K., Sorensen C.S., Behrens M.A., Jonsson B.H., Kristensen T., Klintworth G.K., Enghild J.J., Pedersen J.S., et al. SAXS models of TGFBIp reveal a trimeric structure and show that the overall shape is not affected by the Arg124His mutation. J. Mol. Biol. 2011;408:503–513. doi: 10.1016/j.jmb.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 31.Yun H., Kim E.H., Lee C.W. 1H, 13C, and 15N resonance assignments of FAS1-IV domain of human periostin, a component of extracellular matrix proteins. Biomol. NMR Assign. 2017;12:95–98. doi: 10.1007/s12104-017-9786-z. [DOI] [PubMed] [Google Scholar]
- 32.Underhaug J., Koldso H., Runager K., Nielsen J.T., Sorensen C.S., Kristensen T., Otzen D.E., Karring H., Malmendal A., Schiott B., et al. Mutation in transforming growth factor β induced protein associated with granular corneal dystrophy type 1 reduces the proteolytic susceptibility through local structural stabilization. Biochim. Biophys. Acta. 2013;1834:2812–2822. doi: 10.1016/j.bbapap.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Castellanos R., Nielsen N.S., Runager K., Thogersen I.B., Lukassen M.V., Poulsen E.T., Goulas T., Enghild J.J., Gomis-Ruth F.X. Structural and functional implications of human transforming growth factor β-induced protein, TGFBIp, in corneal dystrophies. Structure. 2017;25:1740–1750. doi: 10.1016/j.str.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Thapa N., Lee B.H., Kim I.S. TGFBIp/βig-h3 protein: A versatile matrix molecule induced by TGF-β. Intern. J. Biochem. Cell. Biol. 2007;39:2183–2194. doi: 10.1016/j.biocel.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Kim J.E., Kim S.J., Lee B.H., Park R.W., Kim K.S., Kim I.S. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-β-induced gene, βig-h3. J. Biol. Chem. 2000;275:30907–30915. doi: 10.1074/jbc.M002752200. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.E., Jeong H.W., Nam J.O., Lee B.H., Choi J.Y., Park R.W., Park J.Y., Kim I.S. Identification of motifs in the fasciclin domains of the transforming growth factor-β-induced matrix protein βig-h3 that interact with the αvβ5 integrin. J. Biol. Chem. 2002;277:46159–46165. doi: 10.1074/jbc.M207055200. [DOI] [PubMed] [Google Scholar]
- 37.Orecchia P., Conte R., Balza E., Castellani P., Borsi L., Zardi L., Mingari M.C., Carnemolla B. Identification of a novel cell binding site of periostin involved in tumour growth. Eur. J. Cancer. 2011;47:2221–2229. doi: 10.1016/j.ejca.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Xue H., Veit C., Abas L., Tryfona T., Maresch D., Ricardi M.M., Estevez J.M., Strasser R., Seifert G.J. Arabidopsis thaliana FLA4 functions as a glycan-stabilized soluble factor via its carboxy-proximal fasciclin 1 domain. Plant J. 2017;91:613–630. doi: 10.1111/tpj.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Son H.N., Nam J.O., Kim S., Kim I.S. Multiple FAS1 domains and the RGD motif of TGFBI act cooperatively to bind αvβ3 integrin, leading to anti-angiogenic and anti-tumor effects. Biochim. Biophys. Acta. 2013;1833:2378–2388. doi: 10.1016/j.bbamcr.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Vosloo W., Tippoo P., Hughes J.E., Harriman N., Emms M., Beatty D.W., Zappe H., Steyn L.M. Characterisation of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene. 1997;188:123–128. doi: 10.1016/S0378-1119(96)00806-2. [DOI] [PubMed] [Google Scholar]
- 41.Chambers M.A., Whelan A.O., Spallek R., Singh M., Coddeville B., Guerardel Y., Elass E. Non-acylated Mycobacterium bovis glycoprotein MPB83 binds to TLR1/2 and stimulates production of matrix metalloproteinase 9. Biochem. Biophys. Res. Commun. 2010;400:403–408. doi: 10.1016/j.bbrc.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 42.Rittenour W.R., Harris S.D. Glycosylphosphatidylinositol-anchored proteins in Fusarium graminearum: Inventory, variability, and virulence. PLoS ONE. 2013;8:e81603. doi: 10.1371/journal.pone.0081603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosher D.F., Johansson M.W., Gillis M.E., Annis D.S. Periostin and TGF-β-induced protein: Two peas in a pod? Crit. Rev. Biochem Mol. Biol. 2015;50:1–13. doi: 10.3109/10409238.2015.1069791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zavaliev R., Dong X., Epel B.L. Glycosylphosphatidylinositol (GPI) modification serves as a primary plasmodesmal sorting signal. Plant Physiol. 2016;172:1061–1073. doi: 10.1104/pp.16.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tapken W., Murphy A.S. Membrane nanodomains in plants: Capturing form, function, and movement. J. Exp. Bot. 2015;66:1573–1586. doi: 10.1093/jxb/erv054. [DOI] [PubMed] [Google Scholar]
- 46.Michell S.L., Whelan A.O., Wheeler P.R., Panico M., Easton R.L., Etienne A.T., Haslam S.M., Dell A., Morris H.R., Reason A.J., et al. The mpb83 antigen from Mycobacterium bovis contains O-linked mannose and (1→3)-mannobiose moieties. J. Biol. Chem. 2003;278:16423–16432. doi: 10.1074/jbc.M207959200. [DOI] [PubMed] [Google Scholar]
- 47.Kieliszewski M.J. The latest hype on Hyp-O-glycosylation codes. Phytochemistry. 2001;57:319–323. doi: 10.1016/S0031-9422(01)00029-2. [DOI] [PubMed] [Google Scholar]
- 48.Kitazawa K., Tryfona T., Yoshimi Y., Hayashi Y., Kawauchi S., Antonov L., Tanaka H., Takahashi T., Kaneko S., Dupree P., et al. β-galactosyl Yariv reagent binds to the β-1,3-galactan of arabinogalactan proteins. Plant Physiol. 2013;161:1117–1126. doi: 10.1104/pp.112.211722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paulsen B.S., Craik D.J., Dunstan D.E., Stone B.A., Bacic A. The Yariv reagent: Behaviour in different solvents and interaction with a gum arabic arabinogalactan-protein. Carbohydr. Pol. 2014;106:460–468. doi: 10.1016/j.carbpol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu M., Igasaki T., Yamada M., Yuasa K., Hasegawa J., Kato T., Tsukagoshi H., Nakamura K., Fukuda H., Matsuoka K. Experimental determination of proline hydroxylation and hydroxyproline arabinogalactosylation motifs in secretory proteins. Plant J. 2005;42:877–889. doi: 10.1111/j.1365-313X.2005.02419.x. [DOI] [PubMed] [Google Scholar]
- 51.Faik A., Abouzouhair J., Sarhan F. Putative fasciclin-like arabinogalactan-proteins (FLA) in wheat (Triticum aestivum) and rice (Oryza sativa): Identification and bioinformatic analyses. Mol. Genet Genom. 2006;276:478–494. doi: 10.1007/s00438-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 52.Tan L., Qiu F., Lamport D.T., Kieliszewski M.J. Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. J. Biol. Chem. 2004;279:13156–13165. doi: 10.1074/jbc.M311864200. [DOI] [PubMed] [Google Scholar]
- 53.Tryfona T., Liang H.C., Kotake T., Tsumuraya Y., Stephens E., Dupree P. Structural characterization of Arabidopsis leaf arabinogalactan polysaccharides. Plant Physiol. 2012;160:653–666. doi: 10.1104/pp.112.202309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tryfona T., Theys T.E., Wagner T., Stott K., Keegstra K., Dupree P. Characterisation of FUT4 and FUT6 α-(1→2)-fucosyltransferases reveals that absence of root arabinogalactan fucosylation increases Arabidopsis root growth salt sensitivity. PLoS ONE. 2014;9:e93291. doi: 10.1371/journal.pone.0093291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seifert G.J., Roberts K. The biology of arabinogalactan proteins. Ann. Rev. Plant Biol. 2007;58:137–161. doi: 10.1146/annurev.arplant.58.032806.103801. [DOI] [PubMed] [Google Scholar]
- 56.Tan L., Showalter A.M., Egelund J., Hernandez-Sanchez A., Doblin M.S., Bacic A. Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Front. Plant Sci. 2012;3:140. doi: 10.3389/fpls.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan L., Eberhard S., Pattathil S., Warder C., Glushka J., Yuan C., Hao Z., Zhu X., Avci U., Miller J.S., et al. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 2013;25:270–287. doi: 10.1105/tpc.112.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moller I., Sorensen I., Bernal A.J., Blaukopf C., Lee K., Obro J., Pettolino F., Roberts A., Mikkelsen J.D., Knox J.P., et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007;50:1118–1128. doi: 10.1111/j.1365-313X.2007.03114.x. [DOI] [PubMed] [Google Scholar]
- 59.Tan L., Tees D., Qian J., Kareem S., Kieliszewski M.J. Intermolecular interactions between glycomodules of plant cell wall arabinogalactan-proteins and extensins. Cell Surf. 2018;1:25–33. doi: 10.1016/j.tcsw.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y., Wang Y., Tan L., Sun L., Petrosino J., Cui M.Z., Hao F., Zhang M. Nanospherical arabinogalactan proteins are a key component of the high-strength adhesive secreted by English ivy. Proc. Natl. Acad. Sci. USA. 2016;113:E3193–3202. doi: 10.1073/pnas.1600406113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kii I., Ito H. Periostin and its interacting proteins in the construction of extracellular architectures. Cell Mol. Life Sci. 2017;74:4269–4277. doi: 10.1007/s00018-017-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park S.Y., Jung M.Y., Lee S.J., Kang K.B., Gratchev A., Riabov V., Kzhyshkowska J., Kim I.S. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J. Cell Sci. 2009;122:3365–3373. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 63.Harris E.N., Parry S., Sutton-Smith M., Pandey M.S., Panico M., Morris H.R., Haslam S.M., Dell A., Weigel P.H. N-glycans on the link domain of human HARE/Stabilin-2 are needed for hyaluronan binding to purified ecto-domain, but not for cellular endocytosis of hyaluronan. Glycobiology. 2010;20:991–1001. doi: 10.1093/glycob/cwq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohno S., Noshiro M., Makihira S., Kawamoto T., Shen M., Yan W., Kawashima-Ohya Y., Fujimoto K., Tanne K., Kato Y. RGD-CAP (βig-h3) enhances the spreading of chondrocytes and fibroblasts via integrin α1β1. Biochim. Biophys. Acta. 1999;1451:196–205. doi: 10.1016/S0167-4889(99)00093-2. [DOI] [PubMed] [Google Scholar]
- 65.Kim H.J., Kim I.S. Transforming growth factor-β-induced gene product, as a novel ligand of integrin αMβ2, promotes monocytes adhesion, migration and chemotaxis. Int. J. Biochem. Cell Biol. 2008;40:991–1004. doi: 10.1016/j.biocel.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Hoersch S., Andrade-Navarro M.A. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol. Biol. 2010;10:30. doi: 10.1186/1471-2148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kudo A. Introductory review: Periostin-gene and protein structure. Cell. Mol. Life Sci. 2017;74:4259–4268. doi: 10.1007/s00018-017-2643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kii I., Nishiyama T., Li M., Matsumoto K., Saito M., Amizuka N., Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J. Biol. Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H., Shi R., Wang X., Pan Y., Li Z., Yang X., Zhang G., Ma Z. Characterization and expression analysis of a fiber differentially expressed fasciclin-like arabinogalactan protein gene in sea island cotton fibers. PLoS ONE. 2013;8:e70185. doi: 10.1371/journal.pone.0070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang G.Q., Xu W.L., Gong S.Y., Li B., Wang X.L., Xu D., Li X.B. Characterization of 19 novel cotton FLA genes and their expression profiling in fiber development and in response to phytohormones and salt stress. Physiol. Plant. 2008;134:348–359. doi: 10.1111/j.1399-3054.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 71.MacMillan C.P., Taylor L., Bi Y., Southerton S.G., Evans R., Spokevicius A. The fasciclin-like arabinogalactan protein family of Eucalyptus grandis contains members that impact wood biology and biomechanics. New Phytol. 2015;206:1314–1327. doi: 10.1111/nph.13320. [DOI] [PubMed] [Google Scholar]
- 72.Guerriero G., Mangeot-Peter L., Legay S., Behr M., Lutts S., Siddiqui K.S., Hausman J.F. Identification of fasciclin-like arabinogalactan proteins in textile hemp (Cannabis sativa L.): In silico analyses and gene expression patterns in different tissues. BMC Genom. 2017;18:741. doi: 10.1186/s12864-017-3970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J., Wu W.G. Genome-wide identification, classification and expression analysis of genes encoding putative fasciclin-like arabinogalactan proteins in Chinese cabbage (Brassica rapa L.) Mol. Biol. Rep. 2012;39:1573–4978. doi: 10.1007/s11033-012-1940-1. [DOI] [PubMed] [Google Scholar]
- 74.Ma H., Zhao J. Genome-wide identification, classification, and expression analysis of the arabinogalactan protein gene family in rice (Oryza sativa L.) J. Exp. Bot. 2010;61:2647–2668. doi: 10.1093/jxb/erq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zang L., Zheng T., Chu Y., Ding C., Zhang W., Huang Q., Su X. Genome-wide analysis of the fasciclin-like arabinogalactan protein gene family reveals differential expression patterns, localization, and salt stress response in Populus. Front. Plant Sci. 2015;6:1140. doi: 10.3389/fpls.2015.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonnet N., Garnero P., Ferrari S. Periostin action in bone. Mol. Cell. Endocrinol. 2016;432:75–82. doi: 10.1016/j.mce.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Walker J.T., McLeod K., Kim S., Conway S.J., Hamilton D.W. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res. 2016;365:453–465. doi: 10.1007/s00441-016-2426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kzhyshkowska J., Gratchev A., Goerdt S. Stabilin-1, a homeostatic scavenger receptor with multiple functions. J. Cell. Mol. Med. 2006;10:635–649. doi: 10.1111/j.1582-4934.2006.tb00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. Sci. World J. 2010;10:2039–2053. doi: 10.1100/tsw.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conway S.J., Izuhara K., Kudo Y., Litvin J., Markwald R., Ouyang G., Arron J.R., Holweg C.T., Kudo A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014;71:1279–1288. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J.E., Kim E.H., Han E.H., Park R.W., Park I.H., Jun S.H., Kim J.C., Young M.F., Kim I.S. A TGF-β-inducible cell adhesion molecule, βig-h3, is downregulated in melorheostosis and involved in osteogenesis. J. Cell. Biochem. 2000;77:169–178. doi: 10.1002/(SICI)1097-4644(20000501)77:2<169::AID-JCB1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 82.Klamer S., Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell. Adh. Migr. 2014;8:563–577. doi: 10.4161/19336918.2014.968501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klamer S.E., Kuijk C.G., Hordijk P.L., van der Schoot C.E., von Lindern M., van Hennik P.B., Voermans C. BIGH3 modulates adhesion and migration of hematopoietic stem and progenitor cells. Cell. Adh. Migr. 2013;7:434–449. doi: 10.4161/cam.26596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han K.E., Choi S.I., Kim T.I., Maeng Y.S., Stulting R.D., Ji Y.W., Kim E.K. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog. Retin. Eye Res. 2016;50:67–88. doi: 10.1016/j.preteyeres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Murugan E., Venkatraman A., Lei Z., Mouvet V., Rui Yi Lim R., Muruganantham N., Goh E., Swee Lim Peh G., Beuerman R.W., Chaurasia S.S., et al. pH induced conformational transitions in the transforming growth factor β-induced protein (TGFβIp) associated corneal dystrophy mutants. Sci. Rep. 2016;6:23836. doi: 10.1038/srep23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poulsen E.T., Runager K., Nielsen N.S., Lukassen M.V., Thomsen K., Snider P., Simmons O., Vorum H., Conway S.J., Enghild J.J. Proteomic profiling of TGFBI-null mouse corneas reveals only minor changes in matrix composition supportive of TGFBI knockdown as therapy against TGFBI-linked corneal dystrophies. FEBS J. 2017;285:101–114. doi: 10.1111/febs.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rios H., Koushik S.V., Wang H., Wang J., Zhou H.M., Lindsley A., Rogers R., Chen Z., Maeda M., Kruzynska-Frejtag A., et al. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol. Cell. Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonnet N., Conway S.J., Ferrari S.L. Regulation of β catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc. Natl. Acad. Sci. USA. 2012;109:15048–15053. doi: 10.1073/pnas.1203085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo Y.S., Zhao R., Ma J., Cui W., Sun Z., Gao B., He S., Han Y.H., Fan J., Yang L., et al. βig-h3 promotes human osteosarcoma cells metastasis by interacting with integrin α2β1 and activating PI3K signaling pathway. PLoS ONE. 2014;9:e90220. doi: 10.1371/journal.pone.0090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bae J.S., Lee S.H., Kim J.E., Choi J.Y., Park R.W., Yong Park J., Park H.S., Sohn Y.S., Lee D.S., Bae Lee E., et al. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–948. doi: 10.1016/S0006-291X(02)00576-4. [DOI] [PubMed] [Google Scholar]
- 91.Park S.W., Bae J.S., Kim K.S., Park S.H., Lee B.H., Choi J.Y., Park J.Y., Ha S.W., Kim Y.L., Kwon T.H., et al. βig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with α3β1 integrin. Exp. Mol. Med. 2004;36:211–219. doi: 10.1038/emm.2004.29. [DOI] [PubMed] [Google Scholar]
- 92.Maeng Y.S., Choi Y.J., Kim E.K. TGFBIp regulates differentiation of EPC (CD133+ c-kit+ lin− cells) to EC through activation of the notch signaling pathway. Stem Cells. 2015;33:2052–2062. doi: 10.1002/stem.2003. [DOI] [PubMed] [Google Scholar]
- 93.Utispan K., Sonongbua J., Thuwajit P., Chau-In S., Pairojkul C., Wongkham S., Thuwajit C. Periostin activates integrin α5β1 through a PI3K/AKT-dependent pathway in invasion of cholangiocarcinoma. Int. J. Oncol. 2012;41:1110–1118. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]
- 94.Kim M.O., Yun S.J., Kim I.S., Sohn S., Lee E.H. Transforming growth factor-β-inducible gene-h3 (βig-h3) promotes cell adhesion of human astrocytoma cells in vitro: Implication of α6β4 integrin. Neurosci. Lett. 2003;336:93–96. doi: 10.1016/S0304-3940(02)01260-0. [DOI] [PubMed] [Google Scholar]
- 95.Lu Y., Liu X., Jiao Y., Xiong X., Wang E., Wang X., Zhang Z., Zhang H., Pan L., Guan Y., et al. Periostin promotes liver steatosis and hypertriglyceridemia through downregulation of PPARα. J. Clin. Investig. 2014;124:3501–3513. doi: 10.1172/JCI74438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson J.W., Thoma B.S., Mikesh M.F., Kramer R.H., Bennett K.L., Purchio A., Bellard B.J., LeBaron R.G. The extracellular matrix protein βig-h3 is expressed at myotendinous junctions and supports muscle cell adhesion. Cell Tissue Res. 2003;313:93–105. doi: 10.1007/s00441-003-0743-z. [DOI] [PubMed] [Google Scholar]
- 97.Nam J.O., Kim J.E., Jeong H.W., Lee S.J., Lee B.H., Choi J.Y., Park R.W., Park J.Y., Kim I.S. Identification of the αvβ3 integrin-interacting motif of βig-h3 and its anti-angiogenic effect. J. Biol. Chem. 2003;278:25902–25909. doi: 10.1074/jbc.M300358200. [DOI] [PubMed] [Google Scholar]
- 98.Thapa N., Kang K.B., Kim I.S. βig-h3 mediates osteoblast adhesion and inhibits differentiation. Bone. 2005;36:232–242. doi: 10.1016/j.bone.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Choi S.I., Maeng Y.S., Kim T.I., Lee Y., Kim Y.S., Kim E.K. Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis. PLoS ONE. 2015;10:e0119561. doi: 10.1371/journal.pone.0119561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gillan L., Matei D., Fishman D.A., Gerbin C.S., Karlan B.Y., Chang D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αvβ3 and αvβ5 integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- 101.Li G., Jin R., Norris R.A., Zhang L., Yu S., Wu F., Markwald R.R., Nanda A., Conway S.J., Smyth S.S., et al. Periostin mediates vascular smooth muscle cell migration through the integrins αvβ3 and αvβ5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee Y.J., Kim I.S., Park S.A., Kim Y., Lee J.E., Noh D.Y., Kim K.T., Ryu S.H., Suh P.G. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol. Ther. 2013;21:1004–1013. doi: 10.1038/mt.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsuzawa M., Arai C., Nomura Y., Murata T., Yamakoshi Y., Oida S., Hanada N., Nakamura Y. Periostin of human periodontal ligament fibroblasts promotes migration of human mesenchymal stem cell through the αvβ3 integrin/FAK/PI3K/Akt pathway. J. Period. Res. 2015;50:855–863. doi: 10.1111/jre.12277. [DOI] [PubMed] [Google Scholar]
- 104.Chuanyu S., Yuqing Z., Chong X., Guowei X., Xiaojun Z. Periostin promotes migration and invasion of renal cell carcinoma through the integrin/focal adhesion kinase/c-Jun N-terminal kinase pathway. Tumour Biol. 2017;39 doi: 10.1177/1010428317694549. [DOI] [PubMed] [Google Scholar]
- 105.Zhou W., Ke S.Q., Huang Z., Flavahan W., Fang X., Paul J., Wu L., Sloan A.E., McLendon R.E., Li X., et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee B.H., Bae J.S., Park R.W., Kim J.E., Park J.Y., Kim I.S. βig-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through αvβ5 integrin. Exp. Mol. Med. 2006;38:153–161. doi: 10.1038/emm.2006.19. [DOI] [PubMed] [Google Scholar]
- 107.Kim S., Park S.Y., Kim S.Y., Bae D.J., Pyo J.H., Hong M., Kim I.S. Cross talk between engulfment receptors stabilin-2 and integrin αvβ5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol. Cell. Biol. 2012;32:2698–2708. doi: 10.1128/MCB.06743-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghatak S., Misra S., Norris R.A., Moreno-Rodriguez R.A., Hoffman S., Levine R.A., Hascall V.C., Markwald R.R. Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J. Biol. Chem. 2014;289:8545–8561. doi: 10.1074/jbc.M113.539882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johansson M.W., Annis D.S., Mosher D.F. αMβ2 integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am. J. Respir. Cel.l Mol. Biol. 2013;48:503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jung M.Y., Park S.Y., Kim I.S. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with αMβ2 integrin. J. Leukoc. Biol. 2007;82:1156–1165. doi: 10.1189/jlb.0107052. [DOI] [PubMed] [Google Scholar]
- 111.Hashimoto K., Noshiro M., Ohno S., Kawamoto T., Satakeda H., Akagawa Y., Nakashima K., Okimura A., Ishida H., Okamoto T., et al. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/βig-h3) that binds to collagen. Biochim. Biophys. Acta. 1997;1355:303–314. doi: 10.1016/S0167-4889(96)00147-4. [DOI] [PubMed] [Google Scholar]
- 112.Kii I., Nishiyama T., Kudo A. Periostin promotes secretion of fibronectin from the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2016;470:888–893. doi: 10.1016/j.bbrc.2016.01.139. [DOI] [PubMed] [Google Scholar]
- 113.Hwang E.Y., Jeong M.S., Park E.K., Kim J.H., Jang S.B. Structural characterization and interaction of periostin and bone morphogenetic protein for regulation of collagen cross-linking. Biochem. Biophys. Res. Commun. 2014;449:425–431. doi: 10.1016/j.bbrc.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 114.Maruhashi T., Kii I., Saito M., Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J. Biol. Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kzhyshkowska J., Gratchev A., Brundiers H., Mamidi S., Krusell L., Goerdt S. Phosphatidylinositide 3-kinase activity is required for stabilin-1-mediated endosomal transport of acLDL. Immunobiology. 2005;210:161–173. doi: 10.1016/j.imbio.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 116.Kzhyshkowska J., Workman G., Cardo-Vila M., Arap W., Pasqualini R., Gratchev A., Krusell L., Goerdt S., Sage E.H. Novel function of alternatively activated macrophages: Stabilin-1-mediated clearance of SPARC. J. Immunol. 2006;176:5825–5832. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 117.Workman G., Sage E.H. Identification of a sequence in the matricellular protein SPARC that interacts with the scavenger receptor stabilin-1. J. Cell. Biochem. 2011;112:1003–1008. doi: 10.1002/jcb.23015. [DOI] [PubMed] [Google Scholar]
- 118.Kzhyshkowska J., Gratchev A., Schmuttermaier C., Brundiers H., Krusell L., Mamidi S., Zhang J., Workman G., Sage E.H., Anderle C., et al. Alternatively activated macrophages regulate extracellular levels of the hormone placental lactogen via receptor-mediated uptake and transcytosis. J. Immunol. 2008;180:3028–3037. doi: 10.4049/jimmunol.180.5.3028. [DOI] [PubMed] [Google Scholar]
- 119.Kzhyshkowska J., Mamidi S., Gratchev A., Kremmer E., Schmuttermaier C., Krusell L., Haus G., Utikal J., Schledzewski K., Scholtze J., et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 120.Meng G., Zhao Y., Bai X., Liu Y., Green T.J., Luo M., Zheng X. Structure of human stabilin-1 interacting chitinase-like protein (SI-CLP) reveals a saccharide-binding cleft with lower sugar-binding selectivity. J. Biol. Chem. 2010;285:39898–39904. doi: 10.1074/jbc.M110.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harris E.N., Weigel J.A., Weigel P.H. The human hyaluronan receptor for endocytosis (HARE/stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 2008;283:17341–17350. doi: 10.1074/jbc.M710360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pandey M.S., Weigel P.H. A hyaluronan receptor for endocytosis (HARE) link domain N-glycan is required for extracellular signal-regulated kinase (ERK) and nuclear factor-κB (Nf-κB) signaling in response to the uptake of hyaluronan but not heparin, dermatan sulfate, or acetylated low density lipoprotein (LDL) J. Biol. Chem. 2014;289:21807–21817. doi: 10.1074/jbc.M114.565846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Penberthy K.K., Ravichandran K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016;269:44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park S.Y., Yun Y., Lim J.S., Kim M.J., Kim S.Y., Kim J.E., Kim I.S. Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nat. Commun. 2016;7:10871. doi: 10.1038/ncomms10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim G.W., Park S.Y., Kim I.S. Novel function of stabilin-2 in myoblast fusion: The recognition of extracellular phosphatidylserine as a “fuse-me” signal. BMB Rep. 2016;49:303–304. doi: 10.5483/BMBRep.2016.49.6.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park S.Y., Jung M.Y., Kim I.S. Stabilin-2 mediates homophilic cell–cell interactions via its FAS1 domains. FEBS Lett. 2009;583:1375–1380. doi: 10.1016/j.febslet.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 127.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu S.L., Rahman A., Baskin T.I., Kieber J.J. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell. 2008;20:3065–3079. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hauser M.T., Morikami A., Benfey P.N. Conditional root expansion mutants of Arabidopsis. Development. 1995;121:1237–1252. doi: 10.1242/dev.121.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seifert G.J., Xue H., Acet T. The Arabidopsis thaliana fasciclin like arabinogalactan protein 4 gene acts synergistically with abscisic acid signalling to control root growth. Ann. Bot. 2014;114:1125–1133. doi: 10.1093/aob/mcu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harpaz-Saad S., McFarlane H.E., Xu S., Divi U.K., Forward B., Western T.L., Kieber J.J. Cellulose synthesis via the FEI2 rlk/Sos5 pathway and cellulose synthase 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J. 2011;68:941–953. doi: 10.1111/j.1365-313X.2011.04760.x. [DOI] [PubMed] [Google Scholar]
- 132.Harpaz-Saad S., Western T.L., Kieber J.J. The FEI2-SOS5 pathway and CELLULOSE SYNTHASE 5 are required for cellulose biosynthesis in the Arabidopsis seed coat and affect pectin mucilage structure. Plant Signal. Behav. 2012;7:285–288. doi: 10.4161/psb.18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Griffiths J.S., Crepeau M.J., Ralet M.C., Seifert G.J., North H.M. Dissecting seed mucilage adherence mediated by FEI2 and Sos5. Front. Plant Sci. 2016;7:1073. doi: 10.3389/fpls.2016.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Griffiths J.S., Tsai A.Y., Xue H., Voiniciuc C., Sola K., Seifert G.J., Mansfield S.D., Haughn G.W. Salt-overly sensitive5 mediates Arabidopsis seed coat mucilage adherence and organization through pectins. Plant Physiol. 2014;165:991–1004. doi: 10.1104/pp.114.239400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsang D.L., Edmond C., Harrington J.L., Nuhse T.S. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol. 2011;156:596–604. doi: 10.1104/pp.111.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xue H., Seifert G.J. Fasciclin like arabinogalactan protein 4 and respiratory burst oxidase homolog d and F independently modulate abscisic acid signaling. Plant Signal. Behav. 2015;10:e989064. doi: 10.4161/15592324.2014.989064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wormit A., Butt S.M., Chairam I., McKenna J.F., Nunes-Nesi A., Kjaer L., O'Donnelly K., Fernie A.R., Woscholski R., Barter M.C., et al. Osmosensitive changes of carbohydrate metabolism in response to cellulose biosynthesis inhibition. Plant Physiol. 2012;159:105–117. doi: 10.1104/pp.112.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Steinwand B.J., Xu S., Polko J.K., Doctor S.M., Westafer M., Kieber J.J. Alterations in auxin homeostasis suppress defects in cell wall function. PLoS ONE. 2014;9:e98193. doi: 10.1371/journal.pone.0098193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.North H.M., Berger A., Saez-Aguayo S., Ralet M.C. Understanding polysaccharide production and properties using seed coat mutants: Future perspectives for the exploitation of natural variants. Ann. Bot. 2014;114:1251–1263. doi: 10.1093/aob/mcu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voiniciuc C., Yang B., Schmidt M.H., Gunl M., Usadel B. Starting to gel: How Arabidopsis seed coat epidermal cells produce specialized secondary cell walls. Int. J. Mol. Sci. 2015;16:3452–3473. doi: 10.3390/ijms16023452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ralet M.C., Crepeau M.J., Vigouroux J., Tran J., Berger A., Salle C., Granier F., Botran L., North H.M. Xylans provide the structural driving force for mucilage adhesion to the Arabidopsis seed coat. Plant Physiol. 2016;171:165–178. doi: 10.1104/pp.16.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Voiniciuc C., Gunl M., Schmidt M.H., Usadel B. Highly branched xylan made by IRX14 and MUCI21 links mucilage to Arabidopsis seeds. Plant Physiol. 2015;169:2481–2495. doi: 10.1104/pp.15.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dean G.H., Zheng H., Tewari J., Huang J., Young D.S., Hwang Y.T., Western T.L., Carpita N.C., McCann M.C., Mansfield S.D., et al. The Arabidopsis Mum2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell. 2007;19:4007–4021. doi: 10.1105/tpc.107.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Voiniciuc C., Dean G.H., Griffiths J.S., Kirchsteiger K., Hwang Y.T., Gillett A., Dow G., Western T.L., Estelle M., Haughn G.W. Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell. 2013;25:944–959. doi: 10.1105/tpc.112.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsai A.Y., Kunieda T., Rogalski J., Foster L.J., Ellis B.E., Haughn G.W. Identification and characterization of Arabidopsis seed coat mucilage proteins. Plant Physiol. 2017;173:1059–1074. doi: 10.1104/pp.16.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Basu D., Tian L., Debrosse T., Poirier E., Emch K., Herock H., Travers A., Showalter A.M. Glycosylation of a fasciclin-like arabinogalactan-protein (SOS5) mediates root growth and seed mucilage adherence via a cell wall receptor-like kinase (FEI1/FEI2) pathway in Arabidopsis. PLoS ONE. 2016;11:e0145092. doi: 10.1371/journal.pone.0145092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Basu D., Wang W., Ma S., DeBrosse T., Poirier E., Emch K., Soukup E., Tian L., Showalter A.M. Two hydroxyproline galactosyltransferases, Galt5 and Galt2, function in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. PLoS ONE. 2015;10:e0125624. doi: 10.1371/journal.pone.0125624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brown D.M., Zeef L.A., Ellis J., Goodacre R., Turner S.R. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell. 2005;17:2281–2295. doi: 10.1105/tpc.105.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Persson S., Wei H., Milne J., Page G.P., Somerville C.R. Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proc. Natl. Acad. Sci. USA. 2005;102:8633–8638. doi: 10.1073/pnas.0503392102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ito S., Suzuki Y., Miyamoto K., Ueda J., Yamaguchi I. AtFLA11, a fasciclin-like arabinogalactan-protein, specifically localized in sclerenchyma cells. Biosci. Biotechnol. Biochem. 2005;69:1963–1969. doi: 10.1271/bbb.69.1963. [DOI] [PubMed] [Google Scholar]
- 151.Dahiya P., Findlay K., Roberts K., McCann M.C. A fasciclin-domain containing gene, ZeFla11, is expressed exclusively in xylem elements that have reticulate wall thickenings in the stem vascular system of Zinnia elegans cv Envy. Planta. 2006;223:1281–1291. doi: 10.1007/s00425-005-0177-9. [DOI] [PubMed] [Google Scholar]
- 152.Lafarguette F., Leplé J.-C., Déjardin A., Laurans F., Costa G., Lesage-Descauses M.-C., Pilate G. Poplar genes encoding fasciclin-like arabinogalactan proteins are highly expressed in tension wood. New Phytol. 2004;164:107–121. doi: 10.1111/j.1469-8137.2004.01175.x. [DOI] [PubMed] [Google Scholar]
- 153.Gritsch C., Wan Y., Mitchell R.A., Shewry P.R., Hanley S.J., Karp A. G-fibre cell wall development in willow stems during tension wood induction. J. Exp. Bot. 2015;66:6447–6459. doi: 10.1093/jxb/erv358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qiu D., Wilson I.W., Gan S., Washusen R., Moran G.F., Southerton S.G. Gene expression in eucalyptus branch wood with marked variation in cellulose microfibril orientation and lacking G-layers. New Phytol. 2008;179:94–103. doi: 10.1111/j.1469-8137.2008.02439.x. [DOI] [PubMed] [Google Scholar]
- 155.Hobson N., Deyholos M.K. LuFLA1PRO and LuBGAL1PRO promote gene expression in the phloem fibres of flax (Linum usitatissimum) Plant Cell Rep. 2013;32:517–528. doi: 10.1007/s00299-013-1383-8. [DOI] [PubMed] [Google Scholar]
- 156.MacMillan C.P., Mansfield S.D., Stachurski Z.H., Evans R., Southerton S.G. Fasciclin-like arabinogalactan proteins: Specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J. 2010;62:689–703. doi: 10.1111/j.1365-313X.2010.04181.x. [DOI] [PubMed] [Google Scholar]
- 157.Wang H., Jiang C., Wang C., Yang Y., Yang L., Gao X., Zhang H. Antisense expression of the fasciclin-like arabinogalactan protein FLA6 gene in Populus inhibits expression of its homologous genes and alters stem biomechanics and cell wall composition in transgenic trees. J. Exp. Bot. 2015;66:1291–1302. doi: 10.1093/jxb/eru479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang H., Jin Y., Wang C., Li B., Jiang C., Sun Z., Zhang Z., Kong F., Zhang H. Fasciclin-like arabinogalactan proteins, PtFLAs, play important roles in GA-mediated tension wood formation in Populus. Sci. Rep. 2017;7:6182. doi: 10.1038/s41598-017-06473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang G.Q., Gong S.Y., Xu W.L., Li W., Li P., Zhang C.J., Li D.D., Zheng Y., Li F.G., Li X.B. A fasciclin-like arabinogalactan protein, GhFLA1, is involved in fiber initiation and elongation of cotton. Plant Physiol. 2013;161:1278–1290. doi: 10.1104/pp.112.203760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Y., Liu D., Tu L., Zhang X., Wang L., Zhu L., Tan J., Deng F. Suppression of GhAgp4 gene expression repressed the initiation and elongation of cotton fiber. Plant Cell Rep. 2010;29:193–202. doi: 10.1007/s00299-009-0812-1. [DOI] [PubMed] [Google Scholar]
- 161.Motose H., Sugiyama M., Fukuda H. A proteoglycan mediates inductive interaction during plant vascular development. Nature. 2004;429:873–878. doi: 10.1038/nature02613. [DOI] [PubMed] [Google Scholar]
- 162.Li A., Wang R., Li X., Liu M., Fan J., Guo K., Luo B., Chen T., Feng S., Wang Y., et al. Proteomic profiling of cellulase-aid-extracted membrane proteins for functional identification of cellulose synthase complexes and their potential associated- components in cotton fibers. Sci. Rep. 2016;6:26356. doi: 10.1038/srep26356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu X.N., Sanchez Rodriguez C., Pertl-Obermeyer H., Obermeyer G., Schulze W.X. Sucrose-induced receptor kinase Sirk1 regulates a plasma membrane aquaporin in Arabidopsis. Mol. Cell. Proteom. 2013;12:2856–2873. doi: 10.1074/mcp.M113.029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klepikova A.V., Logacheva M.D., Dmitriev S.E., Penin A.A. RNA-seq analysis of an apical meristem time series reveals a critical point in Arabidopsis thaliana flower initiation. BMC Genom. 2015;16:466. doi: 10.1186/s12864-015-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Klepikova A.V., Kasianov A.S., Gerasimov E.S., Logacheva M.D., Penin A.A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 2016;88:1058–1070. doi: 10.1111/tpj.13312. [DOI] [PubMed] [Google Scholar]
- 166.Li J., Yu M., Geng L.L., Zhao J. The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. Plant J. 2010;64:482–497. doi: 10.1111/j.1365-313X.2010.04344.x. [DOI] [PubMed] [Google Scholar]
- 167.Johnson K.L., Kibble N.A., Bacic A., Schultz C.J. A fasciclin-like arabinogalactan-protein (FLA) mutant of Arabidopsis thaliana, FLA1, shows defects in shoot regeneration. PLoS ONE. 2011;6:e25154. doi: 10.1371/journal.pone.0025154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kirchner T.W., Niehaus M., Debener T., Schenk M.K., Herde M. Efficient generation of mutations mediated by CRISPR/Cas9 in the hairy root transformation system of Brassica carinata. PLoS ONE. 2017;12:e0185429. doi: 10.1371/journal.pone.0185429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cagnola J.I., de Chassart G.J.D., Ibarra S.E., Chimenti C., Ricardi M.M., Delzer B., Ghiglione H., Zhu T., Otegui M.E., Estevez J.M., et al. Reduced expression of selected fasciclin-like arabinogalactan protein genes associates with the abortion of kernels in field crops of Zea mays (maize) and of Arabidopsis seeds. Plant Cell Environ. 2018 doi: 10.1111/pce.13136. [DOI] [PubMed] [Google Scholar]
- 170.Nirmal R.C., Furtado A., Rangan P., Henry R.J. Fasciclin-like arabinogalactan protein gene expression is associated with yield of flour in the milling of wheat. Sci. Rep. 2017;7:12539. doi: 10.1038/s41598-017-12845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wiker H.G. MPB70 and MPB83—Major antigens of Mycobacterium bovis. Scand. J. Immunol. 2009;69:492–499. doi: 10.1111/j.1365-3083.2009.02256.x. [DOI] [PubMed] [Google Scholar]
- 172.Rahman S.A., Singh Y., Kohli S., Ahmad J., Ehtesham N.Z., Tyagi A.K., Hasnain S.E. Comparative analyses of nonpathogenic, opportunistic, and totally pathogenic mycobacteria reveal genomic and biochemical variabilities and highlight the survival attributes of Mycobacterium tuberculosis. MBio. 2014;5:e02020. doi: 10.1128/mBio.02020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Elkins T., Hortsch M., Bieber A.J., Snow P.M., Goodman C.S. Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. J. Cell Biol. 1990;110:1825–1832. doi: 10.1083/jcb.110.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen S.T., Li J.Y., Zhang Y., Gao X., Cai H. Recombinant MPT83 derived from Mycobacterium tuberculosis induces cytokine production and upregulates the function of mouse macrophages through TLR2. J. Immunol. 2012;188:668–677. doi: 10.4049/jimmunol.1102177. [DOI] [PubMed] [Google Scholar]
- 175.Shiu S.H., Bleecker A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oke V., Long S.R. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 1999;2:641–646. doi: 10.1016/S1369-5274(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 177.Oke V., Long S.R. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol. Microbiol. 1999;32:837–849. doi: 10.1046/j.1365-2958.1999.01402.x. [DOI] [PubMed] [Google Scholar]
- 178.Paulsrud P., Lindblad P. Fasciclin domain proteins are present in Nostoc symbionts of lichens. Appl. Environ. Microb. 2002;68:2036–2039. doi: 10.1128/AEM.68.4.2036-2039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Delmotte N., Knief C., Chaffron S., Innerebner G., Roschitzki B., Schlapbach R., von Mering C., Vorholt J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA. 2009;106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Knief C., Ramette A., Frances L., Alonso-Blanco C., Vorholt J.A. Site and plant species are important determinants of the methylobacterium community composition in the plant phyllosphere. ISME J. 2010;4:719–728. doi: 10.1038/ismej.2010.9. [DOI] [PubMed] [Google Scholar]
- 181.Tejerizo G.T., Kim Y.S., Maus I., Wibberg D., Winkler A., Off S., Puhler A., Scherer P., Schluter A. Genome sequence of Methanobacterium congolense strain buetzberg, a hydrogenotrophic, methanogenic archaeon, isolated from a mesophilic industrial-scale biogas plant utilizing bio-waste. J. Biotechnol. 2017;247:1–5. doi: 10.1016/j.jbiotec.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 182.Bornstein P. Diversity of function is inherent in matricellular proteins: An appraisal of thrombospondin 1. J. Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. 6th ed. Garland Science; New York, NY, USA: 2015. [Google Scholar]