Abstract
Mesenchymal stem cells (MSC) inhibit the response of allogeneic T lymphocytes in culture. Because the mechanisms of this effect may differ according to the existence of cell contact, we investigated the differences in gene expression of inhibitory molecules during MSC-T lymphocyte coculture when cell contact does and does not occur. Human MSC and T lymphocytes were cultured together in standard and transwell cultures. MSC gene expression was analyzed by semiquantitative real-time RT-PCR. MSC elicited a high dose-dependent inhibition of T lymphocytes in cultures with cell contact, but inhibition occurred even without cell contact. In both cases, we observed significant upregulation of IDO, LIF, and HLA-G, along with downregulation of HGF and SDF1. In cultures with cell contact, IL-10 and TGF-β transcripts were expressed in a significantly higher level than in cultures without this contact. Furthermore, in the latter, the increased inhibition of T-cell proliferation was positively correlated with IDO gene expression and negatively correlated with SDF1 gene expression. MSC appear to induce T-cell tolerance by two distinct mechanisms. The first of these, which does not require cell contact, induces expression of the tolerogenic genes IDO, LIF, and HLA-G. The second mechanism, which is contact dependent, modulates IL-10 and TGF-β gene expression. These two mechanisms probably play separate roles in MSC-induced tolerance in allogeneic hematopoietic stem cell transplantation.
Key words: Mesenchymal stem cells, T cells, Cell contact, Inhibitory molecules
INTRODUCTION
The immunomodulatory effects of mesenchymal stem cells (MSC) have been demonstrated in various species, including humans, rodents, and primates (4,8,9,17,21,28) and show clinical promise for the treatment of graft versus host disease (GVHD) and graft failure management.
Specifically, MSC mediate the inhibition of T-lymphocyte proliferation, though the mechanisms by which they do so are not fully understood. No consistent results have appeared from studies of various molecules hypothetically involved in this mechanism: transforming growth factor (TGF-β), hepatocyte growth factor (HGF) (8), interleukin-6 (IL-6), prosta-glandin E2 (PGE2), vascular endothelial growth factor (VEGF), IL-8 (1), IL-10 (5), stromal cell derived fac-tor-1 (SDF-1), osteoprotegerin (OPG) (16), indole-amine 2,3-dioxygenase (IDO) (20), and IL-1β (10). The action of these soluble molecules may be either direct or indirect, and the differences between these two mechanisms are not yet clear. Di Nicola et al. reported that MSC-mediated inhibition of T-cell proliferation differed significantly according to whether cell contact was possible [i.e., whether standard or transwell mixed lymphocyte cultures (MLC) are used] (8). Djouad et al., on the other hand, reported equal inhibition in both types of cultures (9). Krampera et al. found no inhibitory effect at all in cultures without cell contact (15), while others have observed that MSC inhibited lymphocyte cytotoxicity in such cultures (3,13,23). The similarity of the inhibitory effect for transwell and cell contact cultures (13,23) suggested that this inhibition was mediated essentially by soluble factors.
When cultured with T lymphocytes MSC are induced to produce inhibitory soluble factors (9,16); such a conditioned medium also acts on T-cell proliferation after mitogenic activation (16). Conditioning is mediated by soluble factors secreted by monocytes (10). Whether this induction differs in cultures with and without cell contact has not been investigated. We studied a pattern of MSC gene activation in MLC during inhibition of T-cell proliferation. Our aim was to analyze the effect of T-cell contact with MSC and to determine whether this contact is needed to induce the specific expression of these inhibitory molecules by MSC. We investigated both the transcript levels of potential inhibitors (IDO, HGF, TGF-β, IL-10, LIF, and HLA-G) and regulation of the gene expression of SDF-1 homing molecules and of VCAM and LFA3 adhesion molecules, which are involved in MSC–T-cell contact. Our results show that expression of a variety of common genes induces these inhibitory mechanisms. Inhibition of T-cell proliferation in the MLC stimulated MSC expression of IDO, LIF, and HLA-G genes; this upregulation did not require MSC–lymphocyte contact. MSC transcription of IL-10 and TGF-β genes, on the other hand, was dependent on cell contact.
MATERIALS AND METHODS
Isolation and Culture of Human Bone Marrow Mesenchymal Stem Cells
Bone marrow (BM) aspirates were collected from patients undergoing total hip replacement surgery, who provided informed consent. The BM aspirate (10–20 ml) was combined with Iscove’s medium (Biochrom, Berlin, Germany) and centrifuged at 200 × g for 10 min at 20°C. The cells were then resuspended and plated at 50,000 cells/cm2 in a-MEM (Invitrogen, Gergy, France), supplemented with 10% fetal calf serum (research grade FCS, Hyclone, Perbio, Bezons, France). The culture was maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2 and subcultured before confluence. Nonadherent cells were removed after 72 h, and the medium was replaced twice weekly. Before confluence, adherent cells were detached with 0.25% trypsin-EDTA IX (Invitrogen), washed in PBS, and replated at 1000 cells/cm2 for passages 1 and 2 (P1, P2). The MSC obtained at the end of P2 were those used for the MLC. The MSC expanded in culture stained for CD14, CD34, CD45, HLA-DR, CD29, CD44, CD49a, CD73, CD90, CD105, and CD166 (BD Biosciences, Le Pont de Claix, France), conjugated with FITC or PE. Data for at least 5000 cells were acquired using a 488-nm laser flow cytometer (FACS calibur, BD Biosciences), and these data were then analyzed with CELL Questpro software (Becton Dickinson). Cells were also cultured, as previously described (11), in the specific media required for osteogenic (mineral deposit identified by positive von Kossa staining), chondrogenic (aggregate cultures), and adipogenic (identified by Oil Red O-staining of lipid-laden fat cells) differentiation. MSC were routinely frozen in a medium containing 20% dimethyl sulfoxide (DMSO) and 80% FCS.
Preparation of Peripheral Blood Mononuclear Cells
Human peripheral blood mononuclear cells (PBMCs) from healthy volunteers were prepared by gradient centrifugation in a Ficoll solution (density 1.077 g/ ml, Biochrom) at 400 × g for 20 min at room temperature. Cell count and viability were assessed by try-pan blue dye exclusion. PBMCs were incubated at 37°C and 5% CO2 for 24 h in Iscove medium, supplemented with 10% FCS, 1% l-glutamine, and 2% antibiotics. They were then washed by centrifugation and resuspended in Iscove medium supplemented with 1% FCS, 1% L-glutamine, and 2% antibiotics at a concentration of 4 × 106 cells/ml. Next, they were treated with mitomycin (Sigma, Isle d’Abeau, France) (25 mg/ml), incubated for 30 min at 37°C, washed three times by centrifugation for 10 min at 400 × g, and resuspended in 2 ml of RPMI medium supplemented with 10% FCS, 1% L-glutamine, and 2% antibiotics. After cell count and viability were assessed by trypan blue dye exclusion, the cells were used directly in MLC.
Immunomagnetic Selection of Responding T Cells
To isolate CD2 T cells, we magnetically labeled the PBMCs with CD2 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and then loaded them onto the column in a magnetic field according to the manufacturer’s instructions. The magnetically labeled CD2+ cells were retained on the column and eluted as the positively selected cell fraction. Cell count and viability were assessed by trypan blue dye exclusion.
Mixed Lymphocyte Cultures (MLC)
Cultures With Cell Contact
Human CD2 (1 × 105) cells and mitomycin-treated PBMCs isolated from two unrelated donors were cocultured separately (CD2 to PBMC ratio 1:1) or in the presence of MSC, in 200 μl of modified RPMI-1640 medium (In-vitrogen), supplemented with 10% FCS, 1% L-glutamine, and 2% antibiotics in V-shaped 96-well plates (BD Biosciences). Human MSC were plated in triplicate onto 96-well plates in decreasing numbers (3 × 104, 1 × 104, 3 × 103, and 1 × 103 cells/well) and allowed to adhere to the plate for 1-2 h. The ratios of mesenchymal stems to T cells were thus 0.3, 0.1, 0.03, and 0.01. The positive control was CD2 + PBMCs treated with mitomycin, and the negative controls were CD2+ cells alone, mitomycin-treated PBMCs alone, and MSC alone.
Cultures Without Cell Contact
The same procedure was followed in transwell chambers (0.2 μm, Nunc, Roskilde, Denmark): MSC were seeded in the lower chamber and allowed to adhere 1-2 h. Equal numbers of CD2 cells and PBMCs were then cultured in the upper transwell chamber. The MSC/T cell ratios were 0.3 and 0.1.On day 4 of the culture, PBMC proliferative response was measured by thymidine incorporation after incubation with 1μCi/well [3H]thymidine ([3H]TdR) (Amersham, Buckingham, UK) for 18 h after a 3-day culture. A liquid scintillation counter (LKB Wallac 1209 RackBeta, Gaithersburg, MD, USA) measured incorporated radioactivity in counts per minute (cpm).
Semiquantitative Real-Time RT-PCR
We compared gene expression by MSC during T-cell inhibition to that by untreated MSC alone in five parallel experiments with MSC derived from the BM of five different donors. On day 4, MSC cocultured at the 0.3 MSC/T cell ratio (corresponding to the highest level of inhibition observed, see Results) were harvested and washed. We used an anti-CD45 antibody and flow cytometric analysis to assess contamination of the MSC harvested from the mixed culture by hematopoietic cells. Total RNA was extracted with a Trizol reagent kit (Invitrogen, Groningen, Netherlands). RNA concentration and purity were estimated by optical density measurement. RNA isolation was followed by DNAase digestion. Total RNA (1 μg) was reverse transcribed to cDNA with 100 ng of random hexamers and 200 U of superscript II (Invitrogen) at 42°C for 1 h. All reactions were performed with the ABI prism 7700 Sequence Detector V 1.7 system (Applera, Foster City, CA, USA). Reverse and forward primers were designed with its Primer Express™ program; these sequences are reported in Table 1.
TABLE 1.
PRIMER SEQUENCES AND ACCESS NUMBERS OF STUDIED GENES
| Gene Name | Access Number | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| GAPDH | NM002046 | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| VCAM1 | M73255 | CACACACAGGTGGGACACAAA | GACCATCTTCCCAGGCATTTT |
| SDF1a | L36033 | GCCTGAGCTACAGATGCCCA | TTCGGGTCAAFGCACACTTGT |
| IL-10 | NM000572 | CTACGGCGCTGTCATCGAT | GGCATTCTTCACCTGCTCCA |
| TGF-β | NM000660 | CTCTCCGACCTGCCACAGAT | TAACCTAGATGGGCGCGATC |
| LIF | M6342 | ACCAGAAAGATCCTCAACCCCCA | ACGTGGTACTTGCTGCACAGG |
| LFA3 | Y14781-82 | GCTCGCAACCTCCTTACAAGC | TGGCAAAAATTCTCGCTCCTT |
| IDO | X17668 | GGCCTGCGGGAAGCTTAT | TTGGCTGCTGGCTTGCA |
| HLA-G | X17273 | ACCATCCCCATCAGGTATC | ACCGCAGCTCCAGTGACTACA |
| HGF | X16323 | CCCACTTGTTTGTGAGCAACA | AGGACGATTTGGAATGGCG |
To determine amplification efficiency, we constructed calibration curves with a 0.99 correlation: efficiency exceeded 94% for both target genes and GAPDH, the reference (housekeeping) gene we measured. Amplification products were detected with Sybr green I, a dye that binds to all double-stranded DNA. The specificity of PCR products was checked with melting temperature dissociation software (Applera). Threshold cycles (Ct) were selected in the lines in which all samples were in logarithmic phase (14). For relative quantification, values for the target gene product in MSC cocultured with lymphocytes were normalized according to target gene and GAPDH values in MSC alone. We used the 2–ΔΔCT formula to determine these values (14): ΔCt of MSC after coculture with LT = mean Ct of target gene -mean Ct of GAPDH gene; ACt of MSC alone = mean Ct of target gene - mean Ct of GAPDH gene; ΔΔCt = ΔCt of MSC after coculture with lymphocytes - ΔCt of MSC alone. 2–ΔΔCT thus designates the variation in expression of the genes in question in MSC cocul-tures, relative to their expression in control (MSC alone). Genes were considered to be upregulated by MSC in MLC compared with MSC alone if 2–ΔΔCT was >1 and downregulated if <1.
Statistical Analysis
The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 10. Analysis of variance with repeated measures (ANOVA) was used to study dose effects and to compare transwell cultures with cultures with cell contact (Dunnett’s test). Statistical significance was set at a value of p < 0.05. The results were normalized by logarithmic transformation. The Mann-Whitney test was used to assess the significance of changes in gene expression and Spearman’s nonpara-metric test to assess the correlation between gene expression and the MSC inhibitory effect.
RESULTS
Characterization of MSC
At the end of passage P2, the cells were bona fide MSC. They were negative for HLA-DR and hematopoietic antigens CD45, CD34, and CD14, and they expressed antigens known to be present in MSC: CD29, CD44, CD49a, CD73, CD90, CD105, and CD166 (Table 2). When cultured in the appropriate specific media, they differentiated into osteogenic, chondrogenic, and adipogenic cells (data not shown).
TABLE 2.
CHARACTERIZATION OF MSC AT PASSAGE 2
| Antigen | Positive Cells (%) | MFI |
|---|---|---|
| CD45 | <1 | 1 |
| CD34 | <1 | 1 |
| CD14 | <1 | 1 |
| CD29 | 99.7 | 171 |
| CD44 | 94 | 262 |
| CD49a | 63.4 | 4 |
| CD73 | 99.4 | 154 |
| CD90 | 99.6 | 544 |
| CD105 | 99.7 | 126 |
| CD106 | 61.4 | 4 |
| CD166 | 98.4 | 26 |
Result of a representative experiment. At least 5000 cells were analyzed for each antigen. Analyses of the percentage of positive cells and the mean fluorescence intensity (MFI).
T-lymphocyte contamination was successfully eliminated in the cultures with cell contact: FACS analysis showed CD45 surface expression in less than 2% of MSC collected from MLC, after two washings.
MSC Elicited Dose-Dependent Inhibition of T-Cell Proliferation in Cultures With Cell Contact (Fig. 1)
Figure 1.
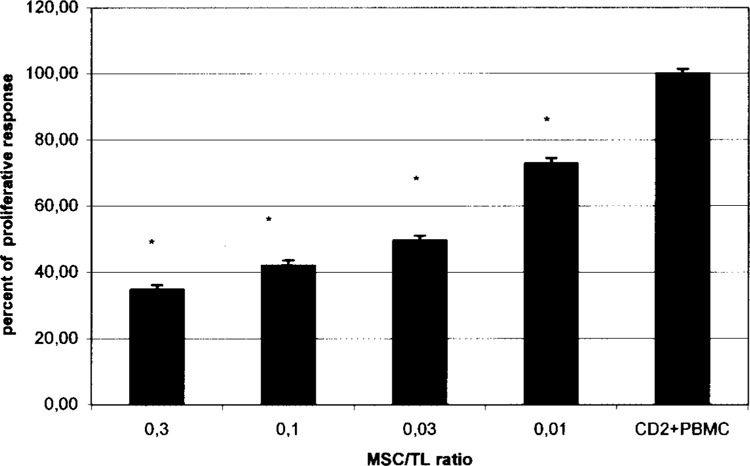
Proliferation of responder CD2+ cells cocultured with allogeneic PBMCs and MSC, with increasing MSC/T cell ratios with cell contact. MSC elicit dose-dependent inhibition of T-cell proliferation in cultures with cell contact. The mean CPM of CD2 + PBMC pairs in the absence of MSC is assigned a 100% value ± SEM. The percentage of proliferative response was the mean of a given MSC/TL ratio, divided by the mean of CD2 + PBMC proliferation. *A statistically significant inhibition of CD2+ cell proliferation at MSC/TL ratios of 0.3. 0.1. 0.03, and 0.01 was observed, compared with the control (CD2 + PBMC), p = 0.058.
Previous studies reported that MSC suppress an ongoing immune response by inhibiting T-cell proliferation stimulated either in MLC or by mitogens (4,8,9,17,21,28). Maccario et al. have compared the effect observed with allogenic MSC to that obtained with autologous MSC. Results showed that more effective suppressive activity on MLC-induced T-cell activation was observed when MSC were allogenic, rather than autologous, with respect to MLC responder cells (19).Accordingly, we examined the third-party human MSC-mediated immunosuppressive effect. The results reported here represent the mean ± SEM of the T-cell proliferation index in seven separate experiments performed in triplicate. Addition of MSC significantly inhibited the proliferative response. The [3H]TdR incorporation test indicates that this inhibition was dose dependent. The percentage of proliferation was 34.84%, 42.63%, 49.56%, and 72.87%, for the 0.3, 0.1, 0.03, and 0.01 ratios, respectively. There was a linear relation between the MSC concentration and inhibition of proliferation (p = 0.058).
MSC Inhibit T-Cell Proliferation More Strongly in Cultures With Cell Contact Than in Cultures Without (Fig. 2)
Figure 2.
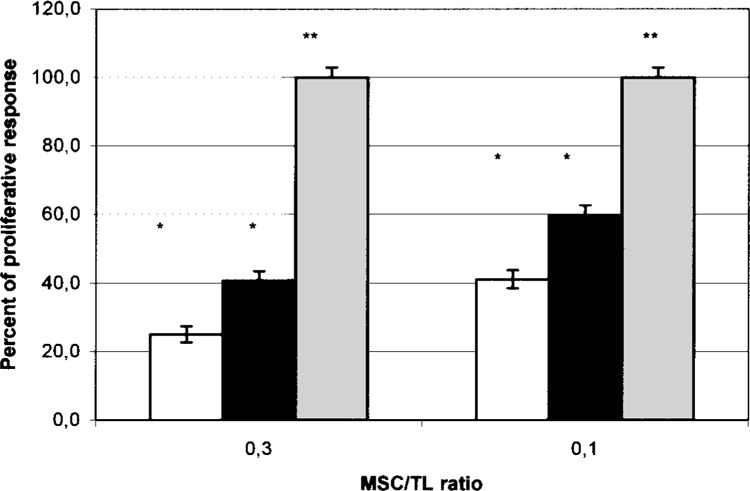
MSC elicit more significant suppression of T-cell proliferation in MLCs with, compared to MSC without, cell contact. Proliferation of responder CD2+ cells with allogeneic PBMCs (gray column) or cocultured with MSC in increasing MSC/T cell ratios with (white column) and without (black column) cell contact. The mean CPM of CD2 + PBMC pairs in the absence of MSC is assigned a 100% value ± SEM (gray columns). The percentage of proliferative response was the mean of a given MSC/TL ratio divided by the mean of CD2 + PBMC proliferation. *A statistically significant inhibition of the proliferation of CD2+ cells was observed, compared with the control (CD2 + PBMC) (p = 0.001). **A statistically significant value was observed when comparing the inhibitory effect between cultures with and without cell contact (p = 0.003 for the MSC/TL ratio 0.3 and p = 0.05 for the MSC/T cell ratio 0.1).
Previous reports of differences in MSC-mediated immunosuppressive effects in cultures with and without direct cell contact were inconsistent (8,15,17). Accordingly, in a separate series of experiments, we created cultures from MSC taken from five separate donors to compare the inhibition of T-lymphocyte proliferation with and without cell contact at ratios of 0.3 and 0.1. Proliferation was lower in the cultures with cell contact compared to cultures without cell contact: 25.1% versus 41% at a ratio of 0.3 (p = 0.003) and 42% versus 60% at a ratio of 0.1 (p = 0.05).
Gene Expression by MSC of Inhibitory Molecules, Adhesion Molecules, and SDF-1 During T-Cell Inhibition (Fig. 3)
Figure 3.
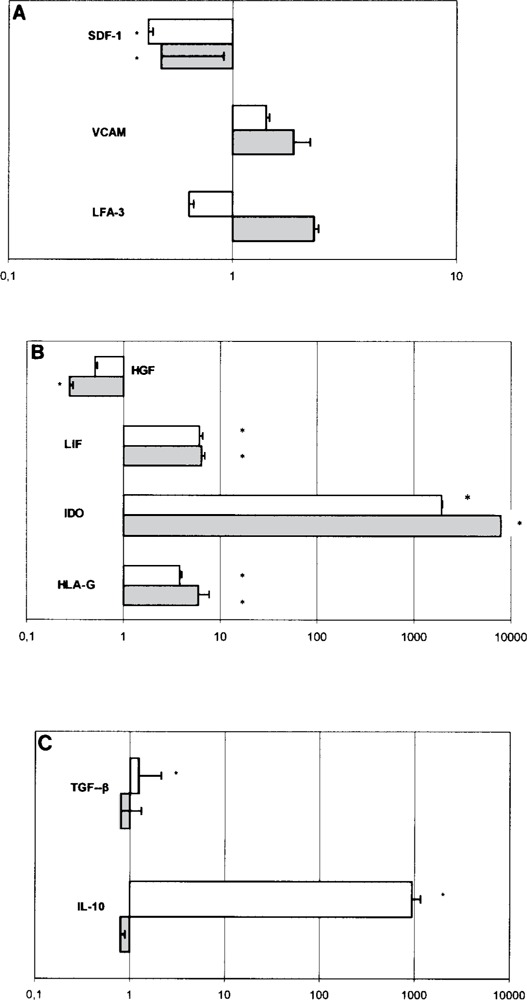
Gene expression of inhibitory molecules, adhesion molecules, and SDF-1 in MSC during T-cell inhibition. Values greater than 1 indicate upregulation of the target genes, while values less than 1 indicate their downregulation. The white columns indicate cultures with cell contact. Gray columns indicate cultures without cell contact. *Statistically significant differences were observed when compared to controls. (A) Adhesion and homing molecules, (B) gene expression of inhibitory molecules modulated with or without contact, and (C) gene expression of inhibitory molecules modulated dependent on contact.
Some studies have demonstrated that MSC use soluble factors to inhibit T-cell functions (3,8,10,13, 23,28) while others have shown the importance of cell contact for this MSC-mediated inhibition (5,15). To clarify these mechanisms, we used semiquantitative real-time RT-PCR to characterize MSC gene expression during modulation of T-cell proliferation in cultures with and without cell contact. The relative quantification of target gene product from MSC alone and to the GAPDH gene product as the internal control. The results were expressed as the increase in the mRNA level of target genes in a coculture of MSC and T cells, with and without cell contact, compared with levels in MSC cultured alone (control).
In MSC cultured with T cells (i.e., in MLC) regardless of cell contact: VCAM expression was upregulated, but not significantly, and SDF-1 expression was significantly downregulated (p < 0.01) (Fig. 3A); HGF expression was significantly downregu-lated (p = 0.001), while expression of IDO (p < 0.001), LIF (p < 0.001), and HLA-G (p < 0.05) was significantly upregulated (Fig. 3B).
Expression of two factors in MLC, on the other hand, appeared to be dependent on contact between MSC and T cells: IL-10 (p = 0.002) and TGF-β (p = 0.003) were expressed in significantly larger quantities in cultures with cell contact (Fig. 3C). LFA-3 was downregulated, but not significantly (Fig. 3A). Significant genes changes are outlined in Table 3.
TABLE 3.
MAIN GENE EXPRESSION CHANGES ACCORDING TO CULTURE CONDITIONS
| Upregulated Genes | Downregulated Genes | |
|---|---|---|
| Without cell contact | VCAM, IDO, HLA-G, LIF | HGF, SDF1 |
| With cell contact | IL-10, TGF-β | LFA3 |
Correlation Between Levels of Immune Suppression and Gene Expression (Fig. 4)
Figure 4.
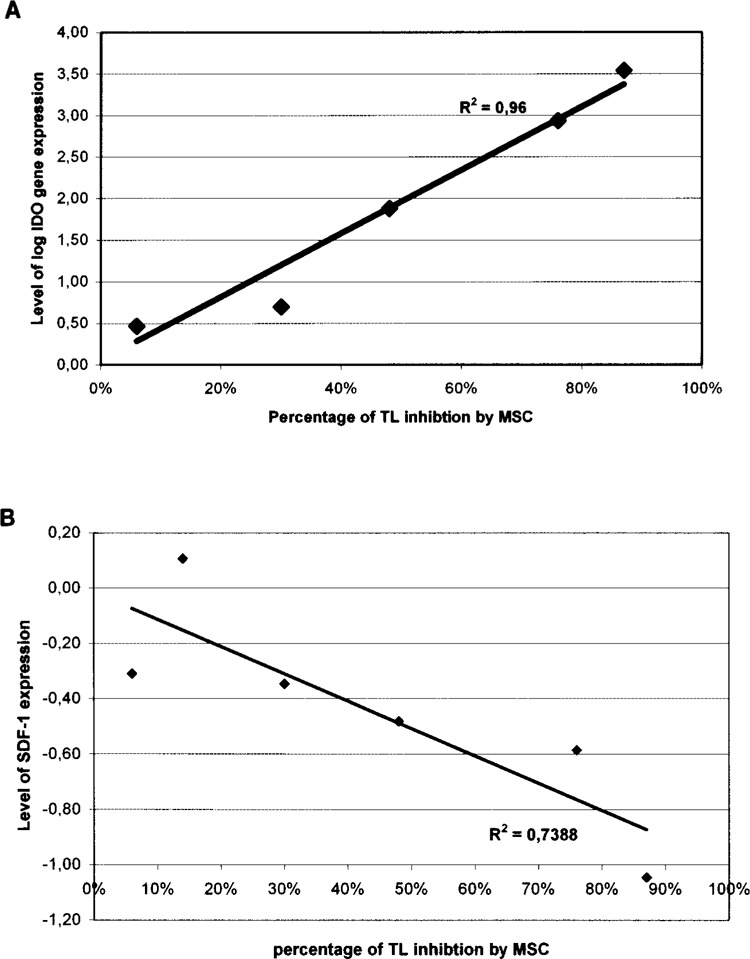
Correlation between MSC inhibitory effects and gene expression. (A) In cultures without cell contact, there was increased IDO gene expression associated with increased inhibition of T-cell proliferation by MSC (R 2 = 0.96). (B) There was decreased SDF-1 gene expression in cultures without cell contact, associated with increased inhibition of TL by MSC (R 2 = 0.73).
To test whether immunosuppression exerted by MSC in cultures without cell contact may be related to up- or downregulation of gene expression, we tested the correlation between transcript levels and percentage inhibition. Without cell contact between MSC and T lymphocytes, the level of MSC-induced immunosuppression was positively correlated with IDO expression (R = 0.96, p = 0.001) and negatively correlated with SDF-1 expression (R = 0.73, p = 0.01). Immunosuppression without cell contact may be related to simultaneous increase of IDO gene expression and decrease of SDF-1 gene expression.
DISCUSSION
MSC-mediated inhibition of immune response is a complex mechanism involving changes in the maturation of antigen-presenting cells (5) and in cytokine secretion profiles (1) as well as the suppression of monocyte differentiation into dendritic cells (12).
Previous studies have shown that MSC in MLC produce TGF-β, HGF, IL-10, and IDO, all of which inhibit T-cell proliferation in vitro (5,8,20). Hypothesizing that several inhibitory factors, operating synergistically, probably mediate this inhibitory mechanism, we investigated whether cell contact between MSC and T lymphocytes is necessary to induce gene expression of the inhibitory factors HLA-G, LIF, and IL-10, the adhesion molecules LFA-3 and VCAM, and the homing factor SDF-1. Our results showed (Table 3) that MSC cocultured with T cells (compared with MSC cultured alone) significantly upregu-lates IDO, HLA-G, and LIF gene expression and downregulates HGF and SDF-1 expression, regardless of any cell contact between MSC and T cells. HLA-G, one of the significantly upregulated genes, modulates the immune system by inhibiting cytolysis mediated by natural killer (NK) cells and cytotoxic T lymphocytes (CTL), CD4+ T-cell alloproliferation (18), and CD8+ T-cell apoptosis (6). HLA-G also has a role in tumor escape and is expressed in melanoma tumors (24).
SDF-1 transcription was downregulated when MSC were cocultured with T cells and in both type of cultures. These results are consistent with those observed by Leblanc et al. (16). The mechanism is probably related to the interaction between SDF-1 and the CXCR-4 receptors in T cells (22).
HGF in our experiments was significantly down-regulated only in the cultures without cell contact. This does not rule out HGF involvement in this inhibition of T-cell proliferation because, as Di Nicola reports, proliferation was partially restored by use of blocking antibodies against HGF and could confirm the presence of statistically significant differences in cultures with and without cell contact (8).
Previous studies have shown that neutralizing antibodies against TGF-β (8) and IL-10 (5) can partially reverse the inhibitory effect of MSC on T-cell proliferation. We showed that IL-10 and TGF-β gene upregulation require MSC–T-cell contact and found statistically significant differences in the inhibition achieved in cultures with and without cell contact. Our findings are consistent with data published by Di Nicola et al. (8), but different from those reported by Krampera et al. (15), which suggested that close contact between MSC and T lymphocytes is necessary for effective inhibition. Our results suggest that IL-10 and TGF-P are responsible for the additional inhibitory effect that occurs with cell contact.
We found a positive correlation between the levels of IDO gene expression and inhibition of T-cell proliferation in cultures without cell contact. This is consistent with the findings of Meisel et al. (20), who showed that MSC use IDO to inhibit T lymphocytes. IL-10, HLA-G, IDO, and LIF are all involved in both feto-maternal (25–27) and organ transplant (2,7,25, 29) tolerance. The upregulation of their genes that we observed here supports the hypothesis that MSC play an important role in allogeneic hematopoietic stem cell transplants because they induce tolerance between donor and recipient, thereby reducing the risk of GVHD and graft failure. We found statistically significant differences between the level of gene expression in cultures with and without cell contact for the following genes: IDO (p = 0.01), IL-10 (p = 0.002), TGF-P (p = 0.003), and HGF (p = 0.001). The differences in the inhibitory effect mediated by MSC in cultures with and without cell contact may be due to the differences in HGF, IDO, IL- 10, and TGF-P transcription in those different conditions.
We hypothesized the following outline to explain the induction of T cells inhibition by MSC. First, MSC are activated by IFN-γ produced by T cells (20). Without cell contact (transwell), MSCs produce IDO, HLA-G, and LIF, which in turn inhibit the proliferation of T cells. A similar mechanism occurs in cell contact through the induction of IDO, HLA-G, and LIF and the additional supplementary cytokines: IL-10 and TGF-β. In cell contact, the adhesion molecules VCAM and LFA-3 interact with their ligands expressed on T cells (VLA-4 and CD2, respectively). These adhesion molecules may be involved in the production of the supplementary cytokines IL-10 and TGF-β (Fig. 5), which allow a more profound inhibitory effect in cell contact cultures. This is supported by our preliminary results: the predominant inhibitory effect of MSC on T-cell proliferation is greatest in cultures with cell contact.
Figure 5.
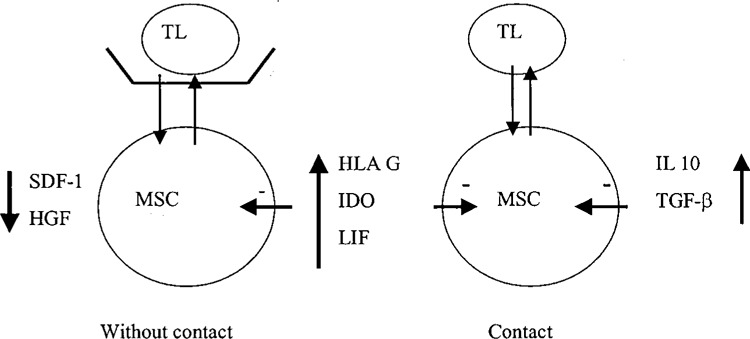
Schema to explain the induction of TL inhibition by MSCMSC are activated by IFN-γ produced by TL. Without cell contact MSC produce IDO, HLA-G, and LIF, which in turn inhibit the proliferation of TL. In cell contact, adhesion molecule VCAM and LFA-3 interact with their ligands expressed on TL (VLA-4 and CD2, respectively). The adhesion molecules might be involved in the production of the supplementary cytokines IL-10 and TGF-β, which allow a more profound inhibitory effect.
We conclude that the inhibition of T-cell proliferation by MSC induces tolerance by several separate mechanisms: those involving IDO, LIF, and HLA-G expression do not require contact, but modulation of IL-10 and TGF-β gene expression depends on it.
ACKNOWLEDGMENTS
We would like to thank Dr. Myriam LAPOBIN for her kind help with the statistical analysis and Mrs. Jo Ann Cahn for her help in improving the writing of this report.
REFERENCES
- 1. Aggarwal S.; Pittenger M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822; 2005. [DOI] [PubMed] [Google Scholar]
- 2. Akita S.; Ishihara H.; Mohammad Abdur R.; Fujii T. Leukemia inhibitory factor gene improves skin allograft survival in the mouse model. Transplantation 70:1026–1031; 2000. [DOI] [PubMed] [Google Scholar]
- 3. Angoulvant D.; Clerc A.; Benchalal S.; Galambrun C.; Farre A.; Bertrand Y.; Eljaafari A. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology 41:469–476; 2004. [PubMed] [Google Scholar]
- 4. Bartholomew A.; Sturgeon C.; Siatskas M.; Ferrer K.; McIntosh K.; Patil S.; Hardy W.; Devine S.; Ucker D.; Deans R.; Moseley A.; Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 30:42–48; 2002. [DOI] [PubMed] [Google Scholar]
- 5. Beyth S.; Borovsky Z.; Mevorach D.; Liebergall M.; Gazit Z.; Aslan H.; Galun E.; Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105:2214–2219; 2005. [DOI] [PubMed] [Google Scholar]
- 6. Contini P.; Ghio M.; Poggi A.; Filaci G.; Indiveri F.; Ferrone S.; Puppo F. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur. J. Immunol. 33:125–134; 2003. [DOI] [PubMed] [Google Scholar]
- 7. Creput C.; Durrbach A.; Charpentier B.; Carosella E. D.; Rouas-Freiss N. HLA G: Immunoregulatory molecule involved in allograft acceptance. Nephrologie 24:451–456; 2003. [PubMed] [Google Scholar]
- 8. Di Nicola M.; Carlo-Stella C.; Magni M.; Milanesi M.; Longoni P. D.; Matteucci P.; Grisanti S.; Gianni A. M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843; 2002. [DOI] [PubMed] [Google Scholar]
- 9. Djouad F.; Plence P.; Bony C.; Tropel P.; Apparailly F.; Sany J.; Noel D.; Jorgensen C. Immuno-suppressive effect of mesenchymal stem cells favors tumor growth in allogenic animals. Blood 102:3837–3844; 2003. [DOI] [PubMed] [Google Scholar]
- 10. Groh M. E.; Maitra B.; Szekely E.; Koç O. N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 33:928–934; 2005. [DOI] [PubMed] [Google Scholar]
- 11. Gronthos S.; Zannettino A. C.; Hay S. J.; Shi S.; Graves S. E.; Kortesidis A.; Simmons P. J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 116:1827–1835; 2003. [DOI] [PubMed] [Google Scholar]
- 12. Jiang X. X.; Zhang Y.; Liu B.; Zhang S. X.; Wu Y.; Yu X. D.; Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105:4120–4126; 2005. [DOI] [PubMed] [Google Scholar]
- 13. Kang H. S.; Habib M.; Ghan G. J.; Abavana, C; Putain J. A.; Ponzio N. M.; Rameshwar P. A paradoxical role for INF-γ in the immune properties of mesenchymal stem cells during viral challenge. Exp. Hematol. 33:796–803; 2005. [DOI] [PubMed] [Google Scholar]
- 14. Kenneth J. L.; Thomas D. S. Analysis of relative gene expression data using relative time quantitative PCR and 2ΔΔCt method. Methods 25:402–408; 2001. [DOI] [PubMed] [Google Scholar]
- 15. Krampera M.; Glennie S.; Dyson J.; Scott D.; Laylor R.; Simpson E.; Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101:3722–3729; 2003. [DOI] [PubMed] [Google Scholar]
- 16. Le Blanc K.; Rasmusson I.; Gotherstrom C.; Seidel C.; Sundberg B.; Sundin M.; Rosendahl K.; Tammik C.; Ringden O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand. J. Immunol. 60:307–315; 2004. [DOI] [PubMed] [Google Scholar]
- 17. Le Blanc K.; Tammik L.; Sundberg B.; Haynesworth S. E.; Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 57:11–20; 2003. [DOI] [PubMed] [Google Scholar]
- 18. Le Rond S.; Le Maoult J.; Creput C.; Menier C.; Deschamps M.; Le Friec G.; Amiol L.; Durrbach A.; Dausset J.; Carosella E. D.; Rouas-Freiss N. Alloreactive CD4+ and CD8+ cells express the immunotolerant HLA-G molecule in mixed lymphocyte reactions: In vivo implications in transplanted patients. Eur. J. Immunol. 34:649–660; 2004. [DOI] [PubMed] [Google Scholar]
- 19. Maccario R.; Podesta M.; Moretta A.; Comoli P.; Montagna D.; Daudt L.; Ibatici A.; Piaggio G.; Pozzi S.; Frassoni F.; Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cells subsets expressing a regulatory/suppressive phenotype. Haematologica 90:516–525; 2005. [PubMed] [Google Scholar]
- 20. Meisel R.; Zibert A.; Laryea M.; Gobel U.; Daubener W.; Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase mediated tryptophan degradation. Blood 103:4619–4621; 2004. [DOI] [PubMed] [Google Scholar]
- 21. Potian J. A.; Aviv H.; Ponzio N. M.; Harrison J. S.; Rameshwar P. Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J. Immunol. 171:3426–3434; 2003. [DOI] [PubMed] [Google Scholar]
- 22. Poznansky M. C.; Olszak I. T.; Foxall R.; Evans R. H.; Luster A. D.; Scadden D. T. Active movement of T-cells away from a chemokine. Nat. Med. 6:543–548; 2000. [DOI] [PubMed] [Google Scholar]
- 23. Rasmusson I.; Ringden O.; Sundberg B.; Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76:1208–1213; 2003. [DOI] [PubMed] [Google Scholar]
- 24. Rebmann V.; Regel J.; Stolke D.; Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Semin. Cancer Biol. 13:371–377; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Roth I.; Corry D. B.; Locksley R. M.; Abrams J. S.; Litton M. J.; Fisher S. J. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184:539–548; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rouas-Freiss N.; Goncalves R. M.; Menier C.; Dausset J.; Carosella E. D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 94:11520–11525; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steckel N. K.; Kuhn U.; Beelen D. W.; Elmaagacli A. H. Indoleamine 2,3-dioxygenase expression in patients with acute graft-versus-host disease after allogeneic stem cell transplantation and in pregnant women: Association with the induction of allogeneic immune tolerance? Scand. J. Immunol. 57:185–191; 2003. [DOI] [PubMed] [Google Scholar]
- 28. Tse W. T.; Pendleton J. D.; Beyer W. M.; Egalka M. C.; Guinan E. C. Suppression of allogenic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 75:389–397; 2003. [DOI] [PubMed] [Google Scholar]
- 29. Wang X. N.; Lange C.; Schulz U.; Sviland L.; Eissner G.; Oliver K. M.; Jackson G. H.; Holler E.; Dickinson A. M. Interleukin-10 modulation of allore-activity and graft-versus-host reactions. Transplantation 74:772–778; 2002. [DOI] [PubMed] [Google Scholar]


