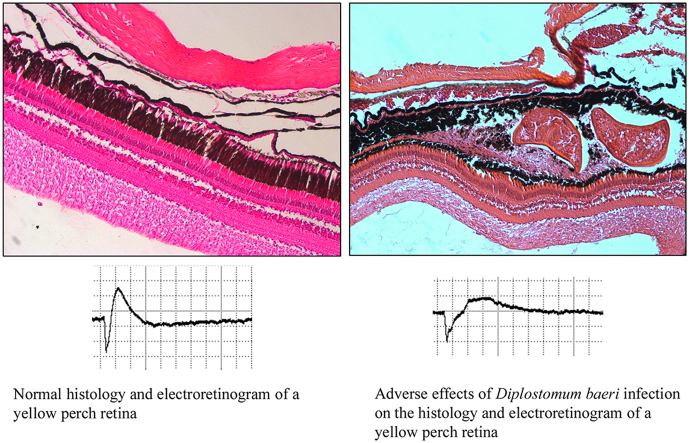
Abstract
Histologic studies of fish from Douglas Lake, Cheboygan County, Michigan, USA show that Diplostomum spp. infect the lens of spottail shiners (Notropis hudsonius) and common shiners (Luxilus cornutus). In contrast, infection was confined to the choroidal vasculature of yellow perch (Perca flavescens), and the morphology of the pigment epithelium and retina in regions adjacent to the metacercariae was abnormal. The difference in location of metacercariae within the host suggested that different Diplostomum species may infect shiners and perch in Douglas Lake. Species diversity was investigated by sequencing the barcode region of the cytochrome oxidase I gene of metacercariae. Four species of Diplostomum were identified, all four of which were present in shiner lenses; however, only Diplostomum baeri was present in the perch choroid. To determine whether infection of perch eyes affects the response of the retina to a light stimulus, electroretinograms (ERG) were recorded. The amplitude of the b-wave of the ERG was reduced and the b-wave latency was increased in infected perch, as compared to uninfected eyes, and the flicker-fusion frequency was also reduced. Infection of the yellow perch choroid by Diplostomum baeri, which shows strong host and tissue specificity, has an adverse effect on retinal function, lending support to the hypothesis that parasite-induced impairment of host vision may afford Diplostomum baeri the evolutionary benefit of increasing the likelihood of transmission, via host fish predation, to its definitive avian host.
Keywords: Diplostomum, Electroretinogram, Lens, Retina, Common shiner (Luxilus cornutus), Spottail shiner (Notropis hudsonius), Yellow perch (Perca flavescens)
Graphical abstract
Highlights
-
•
Diplostomum baeri infects the choroidal vasculature of the yellow perch eye.
-
•
The metacercariae cause extensive damage to the choroid, pigment epeithelium and retina.
-
•
Diplostomum baeri infection adversely affects the response of the perch retina to light.
-
•
Impairment of vision may promote transmission of Diplostomum baeri to its avian host.
1. Introduction
As is typical with most digenetic trematodes, Diplostomum spp. have a 3-host life cycle (La Rue et al., 1926; Chappell et al., 1994). While the miracidial and cercarial larval stages are free-living, the metacercarial stage is an obligatory parasite in the eyes many of different fishes, including percidae (Höglund and Thulin, 1992; Marcogliese et al., 2001a,b), cyprinidae (Höglund and Thulin, 1992; Marcogliese and Compagna, 1999) and salmonidae (Betterton, 1974; Dwyer and Smith, 1989; Shariff et al., 1980; Schwab, 2004; Padros et al., 2018). Adult worms only develop if a fish infected with metacerciae is eaten by suitable definitive host, usually a species of gull (Larus sp.) (Palmieri et al., 1976; 1977).
This communication reports on a study of infection of the eyes of spottail shiners (Notropis hudsonius), common shiners (Luxilus cornutus), yellow perch (Perca flavescens) from Douglas Lake, Cheboygan County, Michigan by metacercariae of Diplostomum spp. in which we observed that infection is limited to the lens of shiners, while in perch the infection is located in the choroidal layer behind the retina. Because the presence of metacercariae in the choroid would likely have a profound adverse effect on retinal function, this study focuses primarily on the perch, documenting pathologic effects of infection by histology. Using the techniques of Locke et al. (2010a,b, 2015) we demonstrate that perch eyes are infected by Diplostomum baeri, and that this infection impairs the electrophysiologic function of the retina.
Impairment of visual function might make fishes more susceptible to predation by the definitive host. Crowden and Broom (1980) were the first to demonstrate that ocular infection in dace (Leuciscus leuciscus) by Diplostomum sp. impairs feeding behavior and renders infected fishes more susceptible to predation by the parasite's definitive host, and others have provided support for this hypothesis with behavioral studies of several species in the laboratory (Brassard et al., 1982; Owen et al., 1993; Seppälä et al., 2004; Seppälä et al., 2006a) and field (Seppälä et al., 2006b).
Of the different parts of the ocular system, Diplostomum spp. most commonly takes up residence in the lens, although retinal infection was also observed in a few studies (Lester and Huizinga, 1977; Höglund and Thulin, 1992; Marcogliese et al., 2001a). Infection of the lens causes in lens opacities, or cataracts. Karvonen et al. (2004) examined lenses of rainbow trout (Oncorhynchus mykiss) infected by Diplostomum spathaceum using a slit-lamp ophthalmic microscope. They scored the extent of cataracts, showing that severity is directly related to the number of metacercariae in the lens. Karvonen and Seppälä (2008) also showed that the size of infected lenses of salmonids is reduced. This is possibly due to loss of lens material, which was previously reported by Shariff et al. (1980). Cataracts interfere with the transmission of light and focusing of an image on the retina. Techniques for measurement of light transmittance by fish lenses have been developed (McCandless et al., 1969; Bassi et al., 1984). Such studies have not yet been conducted on lenses infected by Diplostomum spp., but would be of great interest.
Retinal function can be measured electrophysiologically, allowing direct evaluation of effects of a retinal infection by metacercariae. In the present study we show that in the yellow perch, Diplostomum baeri infection of the choroid layer of the eye, which contains the blood supply for the retina, causes extensive damage to the choroid, pigment epithelium and photoreceptors. It is expected that this would have adverse effects on the response of the retina to light. This hypothesis was tested by recording the electroretinogram (ERG) from normal and infected perch eyes, in vitro.
The ERG is the massed response of the retinal cells to a light stimulus, recorded by placing electrodes in front of and behind the retina. The waveform of the ERG has three major components, the negative a-wave, positive b-wave and the c-wave, which may or may not be present. The a-wave represents the initial response of the photoreceptors to the light stimulus. The b-wave is due to electrical activity of cells post-synaptic to the photoreceptors, including the neural ON bipolar and amacrine cells and possibly the Müller (glial) cells that respond to extracellular potassium fluxes caused by neuronal activity. The c-wave is the response of the pigment epithelial cells to potassium fluxes due to photoreceptor activity (For a review of the ERG, see Perlman, 2017). Changes in a-wave and b-wave amplitude and latency (time to peak) provide information on possible effects of Diplostomum baeri infection on the ability of a fish to detect the presence and activity of predators.
2. Materials and methods
2.1. Fish collection
Fishes were collected under a State of Michigan sport fishing license and a Michigan-Department of Natural Resources Scientific Collecting Permit. The research was approved by the institutional animal care and use committees of Calvin College and the University of Michigan. Yellow perch (Perca flavescens), were taken by hook and line from North Fishtail Bay, Douglas Lake, Cheboygan County, Michigan (45.58, −84.66) and from Carlton Cove, Paradise Lake, Emmett County, Michigan (45.69, −84.77). These lakes were chosen because the populations of Stagnicola emarginata, a snail intermediate host for Diplostomum spp., are commonly found in Douglas Lake but not in Paradise Lake (Blankespoor, C.L., 2012, unpublished data on file at the University of Michigan Biological Station). Common shiners (Luxilus cornutus, also known as Notropus cornutus) were taken by hook and line from North Fishtail Bay, Douglas Lake. Spottail shiners (Notropis hudsonius) were taken by seining along the east shore of the north end of Pells Island, Douglas Lake (45.58, −84.71) and along the east shore of the north end of Grapevine Point, Douglas Lake (45.57, −84.68). Perch were 11.8 ± 1.5 cm long (n = 81), common shiners 11.4 ± 1.2 cm long (n = 9) and spottail shiners 4–5 cm long (n = 72).
2.2. Examination of eyes for infection by Diplostomum spp.
Perch and common shiners were anesthetized with tricaine methane sulphonate (MS-222), 100 mg/l in water buffered to pH 7 with NaHCO3, and doubly pithed. Spottail shiners were decapitated.
To examine spottail shiner lenses for infection, the eyes, which are about 2–3 mm in diameter, were removed from the fish and pierced with a #10 triangular scalpel blade. The lens was expressed into Ringer solution using fine forceps and examined for the presence of metacercariae and lens opacities using a dissecting microscope. Lenses were also macerated in Ringer solution using fine forceps and Vanness scissors to release metacercariae for DNA sequencing.
For examination of perch and common shiner eyes, which are 6–7 mm in diameter, the cornea was removed from the eye. The lens was carefully removed using forceps and examined as described above. The remaining eyecup was examined under a dissecting microscope for the possible presence of metacercariae in the vitreous humor. The retina and choroid were then removed and macerated in Ringer solution using fine forceps and dissecting needles, and the tissue and Ringer solution were examined for the presence of metacercariae. Common shiner and perch lenses were macerated to release metacercariae for DNA sequencing.
2.3. Histology of eyes
Eyes used for histology were from freshly killed fish that had been anesthetized and pithed.
For frozen sectioning, spottail shiner eyes were frozen in liquid nitrogen and embedded in tissue freezing medium. Sections of retina or lens, 6–8 μm thick, were cut in a cryostat and stained with toluidine blue O or hematoxylin and eosin (H & E) by standard methods.
For paraffin sectioning, perch eyes were fixed in 10% neutral buffered formalin. To promote penetration of fixative into the retina, the cornea and lens were removed from the eyes before fixation. The eyes were embedded in paraffin, 6 μm sections were cut and H & E staining was performed by standard methods. Perch retinas were also stained for inflammatory cells using Wright-Giemsa stain by standard methods. Frozen and paraffin sections were imaged with Ziess Axiovision software (Carl Ziess Microscopy, Thornwood, NJ).
2.4. DNA extraction, PCR, and sequencing
To study the diversity of Diplostomum sp. in shiners and perch from Douglas Lake, metacercariae were collected from macerated lenses and retinas under a dissecting microscope with fine forceps, placed in ethanol and stored at 4 °C. DNA extraction from individual metacercariae was conducted by a modification of the method of Truett et al. (2000) using 20 μl of both the alkaline and neutralizing solutions, 5 min of heating at 95 °C, and grinding with a micropestle. PCR amplification of the barcode region of the cytochrome c oxidase I (COI) gene was performed with the primer combinations Dice1F/Dice11R and Dice1F/Dice14R, described by Van Steenkiste et al. (2015), but without added T3 or T7 tails. These PCRs used 10 μl Taq 2X MeanGreen Master Mix (Empirical Biosciences) resulting in 0.2 mM dNTPs, 1.5 mM MgCl2, 0.5 μM each primer, in a total volume of 20 μl. Thermocycling conditions followed Van Steenkiste et al. (2015): 94 °C for 2 min; 3 cycles of 94 °C for 40 s, 51 °C for 40 s, 72 °C for 1 min; 5 ‘touchdown’ cycles of 94 °C for 40 s, 50 °C–46 °C for 40 s (dropping 1 °C per cycle), 72 °C for 1 min; 35 cycles of 94 °C for 40 s, 45 °C for 40 s, 72 °C for 1 min; and a final extension at 72 °C for 5 min. PCR products were visualized on 1.0% TBE agarose gels stained with SYBR″ Safe (Invitrogen). Successful PCR amplicons were enzymatically purified using ExoSAP-IT (Affymetrix, Santa Clara, CA).
DNA sequencing was completed by the Research Technology Support Facility Genomics Core at Michigan State University, East Lansing, MI, USA, or at the Genomic Sciences Laboratory at North Carolina State University, Raleigh, NC, USA. Forward and reverse DNA traces provided easy alignment and required only minor editing using Sequencher 5.4.6 (2016, Gene Codes, Ann Arbor, MI, USA). Representatives of COI sequences from all clusters were submitted to BLAST searches in GenBank, to find matches with sequences published in other studies of diplostomids. Sequences were aligned in Sequencher. Neighbor-joining and maximum likelihood trees (maximum composite likelihood) were calculated in MEGA 7.0 (Tamura et al., 2013).
2.5. Recording of electroretinograms
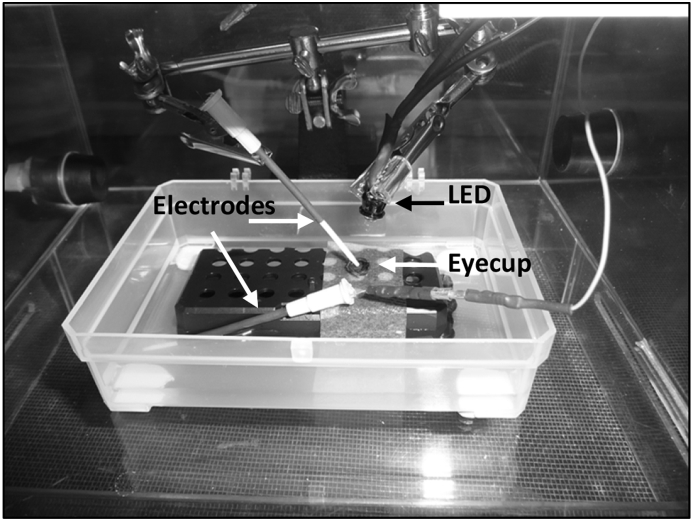
The procedure for recording ERGs was based on previously reported methods (Hoffert and Ubels, 1979a; Ubels et al., 1977, 1984). Electrodes were constructed from 16 gauge peripheral venous catheters (BD Angiocath, Becton, Dickinson, Franklin Lakes, NJ) filled with Ringer solution/4% agarose and connected to a preamplifier via a silver/silver chloride wire that was inserted into the agarose. The wire was shielded from light by black heat-shrink tubing placed around the catheter to prevent photoelectric artifacts. ERGs were recorded and analyzed using a Windac/Pro physiologic data acquisition system (DATAQ Instruments, Akron, OH).
Perch were euthanized as described above, the eyes were enucleated and the cornea and lens were removed to form an eyecup. The eyecup was placed in a custom-built plastic recording chamber partly filled with Ringer solution so that the back of the eye was in contact with the solution. The active electrode was placed into the vitreous humor of the eye and a reference electrode was placed in the solution in the recording chamber (Fig. 1). The contralateral eye was dissected as described above to determine whether metacercariae were present.
Fig. 1.
ERG recording chamber. The eyecup sits on filter paper saturated with Ringer solution making contact with the reference electrode. The recording electrode is in the vitreous humor of the eyecup. The LED was placed 4 cm from the eyecup.
The light source was a 87K7113 white LED (Newark Electronics, Chicago, IL) placed 4 cm from the eye and activated by a Grass SD9 stimulator (Grass Instrument Co., West Warwick, RI). A 300-ohm resistor was placed in the circuit between the LED and the stimulator. The output of the LED was measured using a Perkin-Elmer VTB-6061 silicon photodiode (Perkin Elmer, Waltham, MA). At a 100 V, 1 msec output from the stimulator the energy of the light stimulus at 4 cm was 1.5 μJ/cm2. This was the maximum stimulus delivered to the eye in any recording protocol and always yielded a maximal electrical response from the eye.
The eyecup in its recording chamber along with the electrodes and LED was placed in a sealed Lucite box that was continuously flushed with 100% O2 during the recording session. This was necessary because of the high O2 demands of the fish retina (Hoffert and Ubels, 1979a; Ubels et al., 1977, 1984). This box was placed in a darkened Faraday cage to reduce electrical interference.
Immediately after completing the recording setup, a single 1.5 μJ/cm2 stimulus was delivered to the eye to confirm that the retina was responsive. The eye was then dark adapted for 30 min. The following protocol was then followed. Three 0.3 μJ/cm2 stimuli were delivered at 5 sec intervals. After a 2 min interval, three 0.45 μJ/cm2 stimuli were delivered, followed by stimuli of 0.6, 0.9, 1.2 and 1.5 μJ/cm2 using the same protocol. It was determined that the ERGs in each group of three did not differ, so the first recording at each stimulus intensity was used for data analysis.
At the end of the above recording protocol, the eye was stimulated at 1.5 μJ/cm2, 1 msec duration at 5 Hz for a 2 s interval. After 2 min, the frequency was increased to 10 Hz and the eye was again stimulated. This was repeated at 5 Hz increments until a stimulation frequency was reached at which the retina no longer responded to individual stimuli. This is known as the flicker fusion frequency, which gives an indication of the eye's ability to respond to rapid movements. When the recording session was complete, the eyecup was either examined for the presence of metacercaria or prepared for histology.
3. Results
3.1. Location of ocular infections and histology
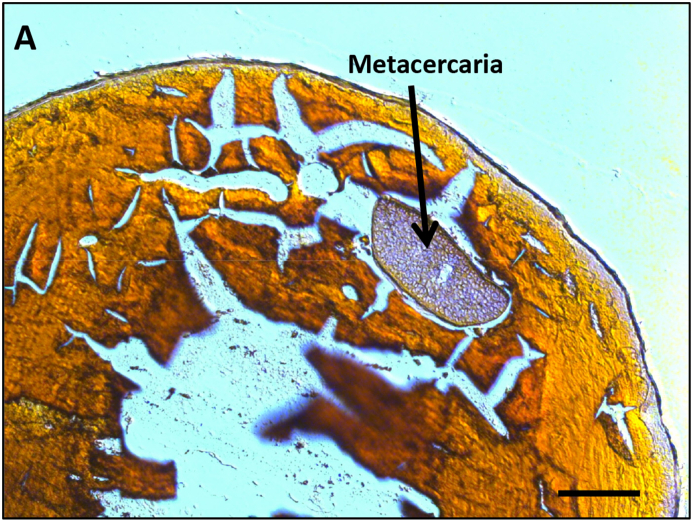
Metacercariae were nearly always located in the lens of spottail and common shiners. Examination of fresh lenses and frozen sections of spottail shiner eyes (Fig. 2) showed that the metacercaria were in the soft lens cortex rather than in the rather than in the nucleus. Metacercaria were absent from the vitreous humor, although a single metacercaria was observed in the retina on a frozen section of one shiner eye (data not shown).
Fig. 2.
Frozen section of spottail shiner (Notropis hudsonius) lens, stained with H&E, showing presence of a Diplostomum sp. metacercaria in the lens cortex. The lens nucleus was lost during sectioning of the frozen tissue. (Bar = 100 μm).
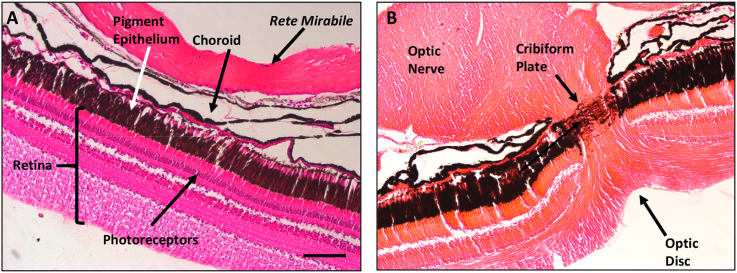
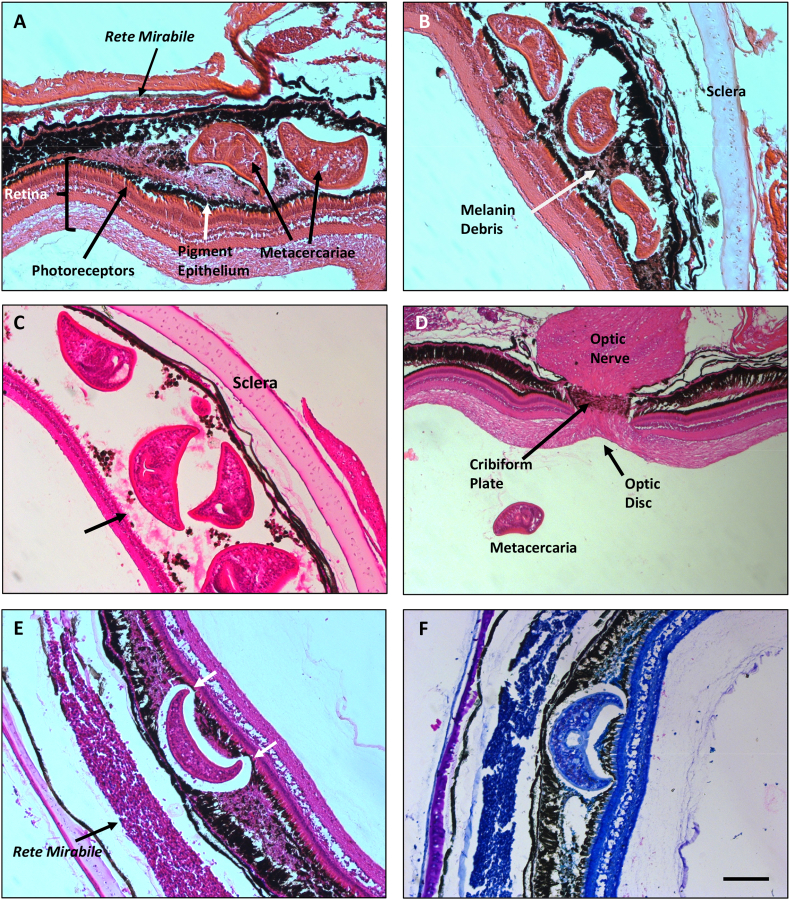
Metacercaria were never observed in the vitreous humor of freshly dissected perch eyes, however, maceration of the retina and choroid in Ringer solution released numerous metacercaria. This suggested location of the infection in or behind the retina. Histological studies were conducted so that the precise location of the metacercariae and nature of the damage caused by the infection could be observed with greater resolution. For reference, images of normal retina, pigment epithelium, choroid and optic nerve of uninfected perch are provided (Fig. 3). The proximity of the rete mirabile to the pigment epithelium and retina is also noted in the normal eye (Fig. 3A).
Fig. 3.
Paraffin sections of normal yellow perch (Perca flavescens) retinas stained with H&E. A. Structure of the retina, photoreceptor layer, pigment epithelium, choroid and the rete mirabile which supplies high levels of O2 to the retina. B. Normal structure of the optic nerve, optic disc and cribiform plate. Bar = 100 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Images from infected eyes show that the metacercaria are in the choroidal layer behind the retina, rather than within the retina itself (Fig. 4). Depending on the extent of the infection, damage involves 1) primarily the choroid, 2) thinning or loss of the pigment epithelium and 3) in more extreme cases, loss of photoreceptor outer segments. The presence of metacercariae caused damage to pigmented cells in the choroid and lysis of pigment epithelial cells resulting in an accumulation of numerous melanin granules from cells that had been destroyed (Fig. 4A and B). Where large pockets of numerous metacercariae were present the pigment epithelium and photoreceptor layer of the retina were completely obliterated (Fig. 4C). In contrast, regions of infected eyes that had no metacercariae had normal retinal, pigment epithelial and optic nerve structure (Fig. 4 A and D). The proximity of the retina and rete mirabile was maintained in these uninfected regions (Fig. 4A), while the presence of metacercariae increases the distance between these tissues (Fig. 4E). It is also noted in Fig. 4E that the pigment epithelium and photoreceptors are damaged in locations that are in contact with the metacercaria.
Fig. 4.
Paraffin sections of yellow perch (Perca flavescens) retinas and choroid infected with Diplostomum baeri, stained with H&E. A. Metacercariae in the choroid layer with thinning of the pigment epithelium. Retina in the unifected region on the far left of the image is in proximity to the rete mirabile. B. Pocket of metacercariae with melanin debris due to extensive damage to the choroid and pigment epithelium. C. Large pocket of metacercariae with melanin debris and loss of the pigment epithelium and photoreceptor layer (arrow). D. Uninfected region of the same eye shown in image C with normal retina, pigment epithelium and optic nerve. This was the only metacercaria seen in the vitreous humor of a perch on a histological section. E. Presence of the metacercaria increases the diffusion distance for O2 from the rete mirabile to the retina. Note damage to the pigment epithelium in locations of contact with the metacercaria. F. Wright-Giemsa stain of the same region shown in image E. No inflammatory cells were detected. Bar = 100 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To determine whether areas of the choroidal tissue that contain metacercariae are infiltrated with inflammatory cells, sections were stained with Wright-Giemsa. Results were negative (Fig. 4F).
While the choroidal tissue of all perch from Douglas Lake that were examined (n = 50) contained metacercariae, infection of the lens in perch from Douglas Lake was rare. Only 10 of the 100 lenses from these fish were infected, with only 1 or 2 metacercariae per lens.
3.2. Identification of Diplostomum species by DNA sequencing
Sequences from the barcode region of COI were obtained from 66 individual metacercariae (GenBank MF142160, MF142162 - MF142201 and MF142204 - MF142228). The length of quality sequences obtained varied from 523 nt to 781 nt. Of the 66 metacercariae that were successfully sequenced, 17 were from spottail shiners (6 host fish), 7 from common shiners (3 host fish), and 42 from perch (16 host fish). Sequence and phylogenetic analyses revealed that the choroid of perch was infected only with Diplostomum baeri and that the metacercariae from one perch lens were of this species. There were also two instances of Diplostomum baeri infecting shiners, but otherwise the shiners were infected with the following species known only from studies employing DNA sequencing: Diplostomum sp.1, Diplostomum sp.3, and Diplostomum sp.4 (Désilets et al., 2013; Locke et al., 2010b; Locke et al., 2015) (see Supplementary Fig. 1 for phylogenetic results).
3.3. Effect of Diplostomum baeri infection on the yellow perch electroretinogram
To determine whether infection of the choroid of the perch eye with Diplostomum baeri, resulting in localized damage to the pigment epithelium and photoreceptor layer, affects visual function, the electroretinogram was recorded from infected eyes of fish from Douglas Lake. These eyes were heavily infected, with the average number of metacercariae in retinas used for ERG recordings 89 ± 43 (range 30–180, n = 9 fishes). Controls were uninfected eyes of perch from Paradise Lake.
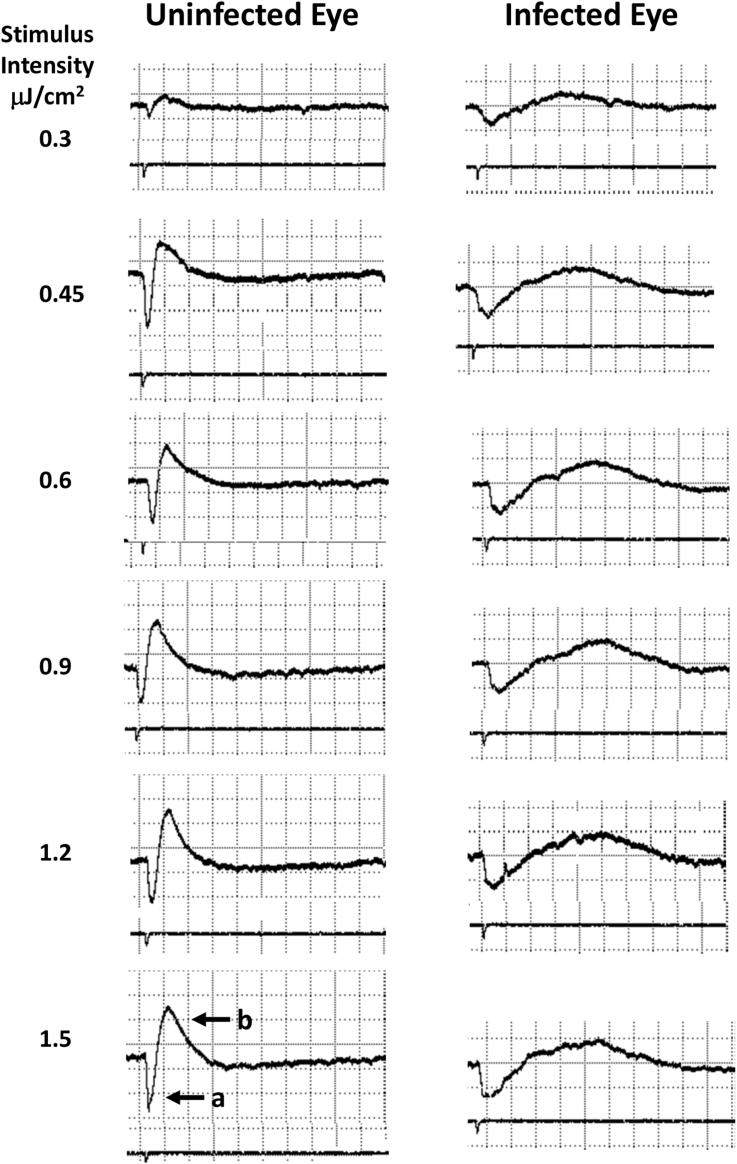
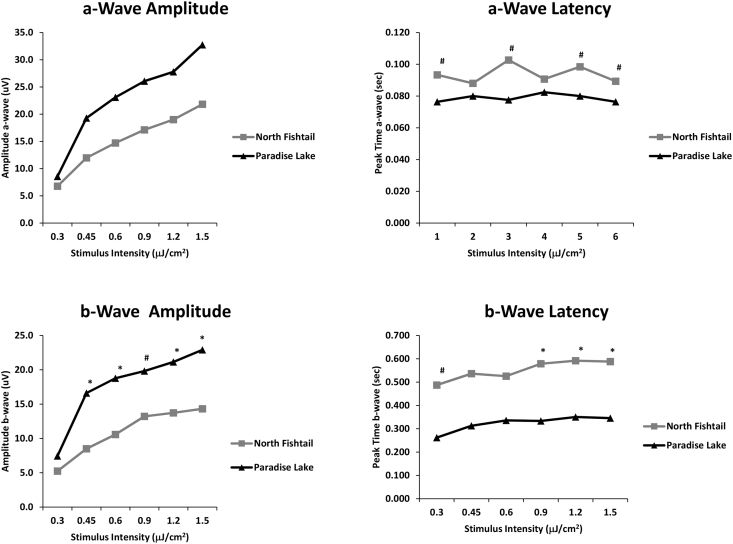
Uninfected perch had typical ERGs with a negative a-wave and rapidly rising b-wave (Fig. 5). In contrast, the ERG waveforms from infected fish were markedly altered, with attenuation of b-wave amplitude and increased latency (Fig. 5). Statistical analysis showed that infection did not have a significant effect on the a-wave amplitude (Fig. 6), perhaps due to variability related to severity of infection, a variable that cannot be controlled. However, as discussed below, this apparent lack of an effect on the a-wave must be interpreted with caution. The amplitude of the b-wave was significantly attenuated in infected eyes as compared to eyes with normal retinas (Fig. 6). There was a strong trend toward an increase in a-wave latency in infected eyes (p = 0.05 < p ≤ 0.06) (Curran-Everett and Benos, 2004) while the b-wave latency was significantly increased (Fig. 6). (see Supplementary Table 1 for detailed statistical information).
Fig. 5.
Representative electroretinograms from eyes of an uninfected yellow perch (Perca flavescens) and a perch infected with Diplostomum baeri. Arrows indicate the a-wave and the b-wave. The ERG waves were analyzed for amplitude and latency (time to peak). Note reduction in ERG b-wave amplitude and increase in b-wave latency recorded from the infected eye. Stimulus intensity units = μJ/cm2, time base = 0.27 s/div, amplitude = 15.5 mV/div.
Fig. 6.
Effect of Diplostomum baeri infection on the electroretinogram of yellow perch (Perca flavescens) as compared to recordings from normal fish. Infection had no effect on the a-wave but significantly reduced b-wave amplitude. Infection caused a strong trend toward an increase in latency (time to peak) of the a-wave and significantly increased the latency of the b-wave. (*p ≤ 0.05; # 0.05 < p ≤ 0.06; t-test, n = 10).
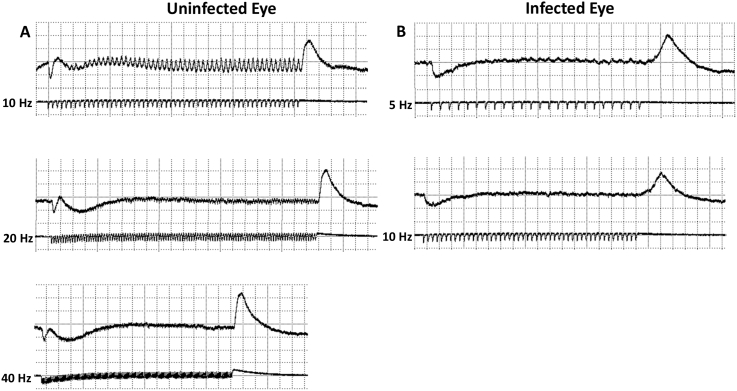
The flicker fusion frequency of normal retinas was as high as 40 Hz and as low as 5–10 Hz in perch eyes infected by Diplostomum baeri (Fig. 7). The mean flicker fusion frequency of infected retinas was 15.8 ± 3.8 Hz (n = 7) as compared to 23.0 ± 9.2 Hz (n = 10) for normal retinas This difference was statistically significant (t-test, p < 0.05).
Fig. 7.
Flicker fusion frequency of the electroretinogram is reduced by Diplostomum baeri infection. A. Responses to stimulation at 10 Hz, 20 Hz and 40 Hz in an uninfected yellow perch (Perca flavescens). Flicker fusion is reached at 40 Hz. B. Responses to stimulation at 5 Hz and 10 Hz in an infected perch. Flicker fusion is reached at 10 Hz. Time base = 0.27 s/div, amplitude = 15.5 mV/div.
4. Discussion
4.1. Summary
The DNA sequence data in this study show that the shiners and perch in Douglas Lake are infected by four species of Diplostomum. Three parasite species (Diplostomum sp.1, Diplostomum sp.3, and Diplostomum sp.4) infected only shiners and almost always infect the lens of the eye.
By contrast, yellow perch are only infected by Diplostomum baeri, and the infection in most fishes is confined to the choroidal layer behind the retina. The infection causes severe damage to the choroid, pigment epithelium and retina. Electrophysiological recordings show that this damage results in significant reduction in retinal function, supporting our hypothesis that vision of perch is impaired by the metacercarial infection.
4.2. Diplostomum species in Douglas Lake
In recent years several investigators have used DNA sequencing techniques to investigate Diplostomum species diversity in various aquatic systems in (Behrmann-Godel, 2013; Désilets et al., 2013, Locke et al., 2010b; Locke et al., 2015). The species identified in the lens in our study agree with reports by Désilets et al. (2013), Locke et al. (2010a,b) and Locke et al. (2015) that Diplostomum sp.1, sp.3, and sp.4 appear to be generalists, infecting multiple species and choosing the lens, an immunologically-safe site. Our data also update previous reports that the species inhabiting Douglas Lake is Diplostomum flexicaudum (Cort et al., 1937; Keas and Blankespoor, 1997), a name not found in more recent publications.
The identification of Diplostomum baeri as the only species infecting perch in Douglas Lake and strongly preferring the choroid, is consistent with previous observations that Diplostomum baeri is a specialist in yellow perch and targets a specific tissue type (Désilets et al., 2013; Locke et al., 2010a,b; Locke et al., 2015). Our identification, using molecular techniques, of Diplostomum baeri in tissues associated with the yellow perch retina also updates a previous report, based only on morphology, that the species infecting the retinas of yellow perch is Diplostomum adamsi (Lester and Huizinga, 1977). The report that cutthroat trout (Salmo clarki) retinas are infected by Diplostomum baeri (Heckmann and Ching, 1987; Dwyer and Smith, 1989) has not been confirmed by DNA sequencing. It has also been reported by Höglund and Thulin (1992) and Behrmann-Godel, 2013 that Diplostomum baeri infects the eye of the European perch (Perca fluviatalis) which is closely related to the North American yellow perch. This identification of Diplostomum baeri must be approached with caution, since Georgieva et al. (2013) have shown that the European Diplostomum baeri is a species complex and is different than the North American species (Locke et al., 2015). None the less, it is interesting that the European Diplostomum baeri is also confined, according to Höglund and Thulin (1992), to the retinal tissue of a perch.
4.3. Pathology associated with Diplostomum baeri infection
Most studies of eye infection by Diplostomum spp. report metacercariae in the lens and vitreous humor. In the present study the only metacercaria that was observed in the vitreous humor of a yellow perch is seen in Fig. 4D, and is possibly an artifact of dissection.
There are only a few reports of retinal infection that illustrate location and pathology. Marcogliese et al. (2001a) observed Diplostomum sp. in the retina of yellow perch, and Höglund and Thulin (1992) reported that metacercariae are in the choroid of the European perch, but provided no histologic evidence. Lester and Huizinga (1977) published photomicrographs of metacercaria between the pigment epithelium and photoreceptors of yellow perch. Shariff et al. (1980) and Heckmann and Ching (1987) published single images of metacercaria behind the retinas of cutthroat trout (Salmo clarki) and rainbow trout (Oncorhynchus mykiss), respectively. A very recent report by Padros et al. (2018, published while this communication was in revision) shows that Diplostomum sp. is located between the pigment and photoreceptors of arctic charr (Savelinus alpinus) causing extensive damage to both the pigment epithelial cells and photoreceptor outer segments.
In the yellow perch, although Diplostomum baeri were occasionally observed in contact with the photoreceptors (Fig. 4E), we interpret the location of the metacercariae to be primarily within the choroidal layer. This location is of advantage to the parasite in that this would provide an abundant supply of oxygen and nutrients. As discussed in more detail below, the presence of metacercariae in the choroid is expected to be a distinct disadvantage to the fish due to impairment of oxygen delivery to the retina.
Thinning and loss of the pigment epithelium was observed whether metacercaria were behind this tissue in the choroid or within it. This is of profound importance for retinal function because of the importance of pigment epithelial cells in support of retinal function. Spent photoreceptor outer segment discs are phagocytosed by the pigment epithelium, and these cells are essential in the vitamin A cycle which supplies the chromophore, cis-retinal, to the photoreceptors (Bok, 1990; Saari, 2016). As such, damage to the pigment epithelium will adversely affect the ability of the retina to respond to light. Of note, in both perch (Fig. 4C) and arctic charr (Palmieri et al., 1977) damage to photoreceptors is greatest in areas where the pigment epithelium is lost.
As discussed above, Diplostomum sp. is commonly located in the lens. This provides the metacercariae with a rich source of protein and protects them from the immune system of the fish. In contrast, location in or near the choroid, while providing nutrients, would be expected to render the metacercariae highly vulnerable to attack by the immune system. It is therefore of interest that no inflammatory cells are observed in the choroid of perch in the present study or in infected arctic charr (Palmieri et al., 1977). In contrast to other Diplostomum spp., Diplostomum baeri and the species that infects arctic charr, which was not identified, have apparently adapted in ways that render them unrecognizable or protect them from attack by the immune system.
4.4. Effect of infection on retinal function
In contrast to most mammals, the retina of teleost fishes has no blood vessels and is therefore dependent on diffusion of O2 from the choroidal circulation that lies behind the retina. In many species, including the yellow perch, the choroid includes a network of capillaries called the rete mirabile (Fig. 3A; 4A and E), which is a counter-current O2 multiplier that generates a PO2 in excess of 400 mm Hg, promoting diffusion of O2 to the inner retinal layers (Wittenberg and Wittenberg, 1962; Fairbanks et al., 1969). Disruption of the function of this system results in attenuation of the electroretinogram (Fonner et al., 1973; Hoffert and Ubels, 1979b). Based on these observations, it would be expected that infection by Diplostomum baeri that damages the choroid (Fig. 4) would reduce O2 delivery to the retinal cells. The physical presence of metacercariae between the choroid and photoreceptors would also result in a diffusion barrier between the rete mirabile and the retina (Fig. 4E). Impairment of O2 diffusion would also presumably affect the inner retina, the location of the bipolar cells and Müller cells that are responsible for generation of the b-wave.
The response of the retina to light begins in the photoreceptors, which are the source of the a-wave of the ERG. While there was a strong trend towards attenuation of the a-wave, the decrease was not statistically significant, due to a high level of variability among fish. This was unexpected based on the degree of damage seen in infected eyes, but can be explained on several levels. First, the ERG is the sum of the response to light of the entire retina. Therefore, as the positive b-wave amplitude decreases the negative a-wave amplitude can increase, masking an adverse effect on photoreceptor function. Second, the photoreceptors are close to the choroidal blood vessels and infected fish might obtain O2 from adjacent undamaged regions. Third, the degree of anatomically detectable damage to the photoreceptors varied within retinas and from fish to fish (Fig. 4), leading to variability in effects of Diplostomum baeri infection on the a-wave. In spite of this variability in regional damage to photoreceptors and in a-wave amplitude, the b-wave can still be significantly attenuated because signals from photoreceptors diverge widely in the inner retina and because of the O2 delivery problem discussed above.
In infected fish there was significant attenuation of the amplitude of the b-wave, as compared to normal fish at all but the lowest stimulus intensity. This decrease in the response to light by cells of the inner retina would also inhibit transmission of signals to the ganglion cells, which due to the infection would also be O2 deficient. In turn, transmission of action potentials by the ganglion cells to the visual cortex would be inhibited. The decrease in b-wave amplitude would result in a decrease in sensitively to light, impairing vision, especially in dim conditions. This would result in decreased ability of a sight feeder like the perch to see prey. Perhaps more importantly, impaired vision will also increase vulnerability of infected fish to predation.
The latency of the ERG waves was also increased in infected perch, which would lead to a delayed response to visual signals. The decrease in flicker fusion frequency of infected retinas would decrease the ability of the fish to detect and respond to rapid movements. Both of these effects of Diplostomum baeri infection on the ERG could result in greater susceptibility to predation, thereby increasing the chances of transmission of metacercariae to the definitive host.
Note that perch infected with Diplostomum baeri are not blind. Large regions of the choroid and retina have normal morphology, the retina does respond to light and the optic nerve is intact. The fishes are able to feed and apparently can reproduce. The average size of the perch in this study, 11.7 cm (range = 8.4–14.1) is within the range that has been reported for sexual maturity of yellow perch in Michigan lakes (Schneider, 1984). Therefore, although the infected fish are at increased risk for predation, the population can be maintained, which is beneficial to hosts and parasites.
5. Conclusion
Parasites with complex, multi-host life cycles often alter the physiology or behavior of a host in a manner that facilitates transmission to its subsequent host (Blankespoor et al., 1997; Holmes and Bethel, 1972; Moore, 2002; Poulin, 2010). Studies of rainbow trout by Seppälä et al. (2006b) and round gobies (Neogobius melanostomus) by Flink et al. (2017) show that cataracts induced by Diplostomum spp. infection cause increased risk of avian predation. We have now demonstrated that Diplostomum baeri infection of the yellow perch choroid causes impairment of retinal function. This may have an adverse effect on vision severe enough to increase risk of predation. All of these studies lend support to Crowden and Broom's (1980) hypothesis that parasite-induced impairment of host vision affords the potential evolutionary benefit of increasing the likelihood of transmission of Diplostomum spp. to their definitive hosts. It has also been suggested that other piscivorous avian species that are not definitive hosts for Diplostomum spp. may benefit from the presence of visually impaired prey (Gopko et al., 2017). Given the cosmopolitan distribution of eye flukes and the various roles fish play in freshwater ecosystems, the ecological implications of visual impairment in this parasite-host system are far-reaching.
Acknowledgements
This study was supported by the University of Michigan Biological Station, the Calvin College Fund for Eye Research, and the Calvin College Department of Biology, none of which were involved in the design or conduct of the study. The authors thank Dr. Uko Zylstra, Department of Biology, Calvin College, for assistance with histology of the retina.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.05.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Bassi C.J., Williams R.C., Powers R.K. Light transmittance by goldfish eyes of different sizes. Vis. Res. 1984;10:1415–1419. doi: 10.1016/0042-6989(84)90197-4. [DOI] [PubMed] [Google Scholar]
- Behrmann-Godel J. Parasite identification, succession and infection pathways in perch fry (Perca fluviatilis): new insights through a combined morphological and genetic approach. Parasitology. 2013;140:509–520. doi: 10.1017/S0031182012001989. [DOI] [PubMed] [Google Scholar]
- Betterton C. Studies on the host specificity of the eye fluke, Diplostomum spathaceum, in brown and rainbow trout. Parasitology. 1974;69:11–29. doi: 10.1017/s003118200004614x. [DOI] [PubMed] [Google Scholar]
- Blankespoor C.L., Pappas P.W., Eisner T. Impairment of the chemical defense of the beetle, Tenebrio molitor, by metacestodes (cysticercoids) of the tapeworm, Hymenolepis diminuta. Parasitology. 1997;115:105–110. doi: 10.1017/s0031182097008901. [DOI] [PubMed] [Google Scholar]
- Bok D. Processing and transport of retinoids by the retinal pigment epithelium. Eye. 1990;4:326–332. doi: 10.1038/eye.1990.44. [DOI] [PubMed] [Google Scholar]
- Brassard P., Rau M.E., Curtis M.A. Parasite-induced host susceptibility to predation in diplostomiasis. Parasitology. 1982;85:495–501. [Google Scholar]
- Chappell L.H., Hardie L.J., Secombes C.J. Diplostomiasis: the disease and host-parasite interactions. In: Pike A.W., Lewis J.W., editors. Parasitic Diseases of Fish. Samara Publishing Limited; Dyfed, Wales, UK: 1994. pp. 59–86. [Google Scholar]
- Cort W., McMullen D., Brackett S. Ecological studies on the cercariae in Stagnicola emarginata angulata (Sowerby) in the Douglas Lake region. Michigan. J. Parasitol. 1937;23:504–532. [Google Scholar]
- Crowden A.E., Broom D.M. Effects of the eyefluke, Diplostomum spathaceum, on the behaviour of dace (Leuciscus leuciscus) Anim. Behav. 1980;28:287–294. [Google Scholar]
- Curran-Everett D., Benos D.J. Guidelines for reporting statistics in journals published by the American Physiological Society. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R247–R249. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- Désilets H.D., Locke S.A., McLaughlin J.D., Marcogliese D.J. Community structure of Diplostomum spp. (Digenea: Diplostomidae) in eyes of fish: main determinants and potential interspecific interactions. Int. J. Parasitol. 2013;43:929–939. doi: 10.1016/j.ijpara.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Dwyer W.P., Smith C.E. Metacercariae of Diplostomum spathaceum in the eyes of fishes from yellowstone lake. Wyoming. J. Wildl. Dis. 1989;25:126–129. doi: 10.7589/0090-3558-25.1.126. [DOI] [PubMed] [Google Scholar]
- Fairbanks M.B., Hoffert J.R., Fromm P.O. The dependence of the oxygen-concentration system of the teleost eye (Salmo gairdneri) on the enzyme carbonic anhydrase. J. Gen. Physiol. 1969;54:203–211. doi: 10.1085/jgp.54.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flink H., Behrens J.W., Svensson P.A. Consequences of eye fluke infection on anti-predator behaviours in invasive round gobies in Kalmar Sound. Parasitol. Res. 2017;116:1653–1663. doi: 10.1007/s00436-017-5439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonner D.B., Hoffert J.R., Fromm P.O. The importance of the counter current oxygen multiplier mechanism in maintaining retinal function in the teleost. Comp. Biochem. Physiol., A. 1973;46A:559–567. doi: 10.1016/0300-9629(73)90107-2. [DOI] [PubMed] [Google Scholar]
- Georgieva S., Soldánová M., Pérez-del-Olmo A., Dangel D.R., Sitko J., Sures B., Kostadinova A. Molecular prospecting for european Diplostomum (Digenea: Diplostomidae) reveals cryptic diversity. Int. J. Parasitol. 2013;43:57–72. doi: 10.1016/j.ijpara.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Gopko M., Mikheev V.N., Taskinen J. Deterioration of basic components of the anti-predator behavior in fish harboring eye fluke larvae. Behav. Ecol. Sociobiol. 2017;71:1–10. [Google Scholar]
- Heckmann R.A., Ching H.L. Parasites of the cutthroat trout, Salmo clarki, and longnose suckers, Catostomus catostomus, from Yellowstone Lake, Wyoming. Great Basin Nat. 1987;47:259–275. [Google Scholar]
- Hoffert J.R., Ubels J.L. Electrophysiological and metabolic responses of the isolated teleost retina to changes in PO2 and temperature. Comp. Biochem. Physiol., A. 1979;62A:309–316. [Google Scholar]
- Hoffert J.R., Ubels J.L. The intraocular PO2 and electroretinogram of the trout as affected by temperature and ventilatory flow. Comp. Biochem. Physiol. 1979;62A:563–568. [Google Scholar]
- Höglund J., Thulin J. Identification of Diplostomum spp. in the retina of perch Perca fluviatalis and the lens of roach Rutilus rutilus from the Baltic Sea – an experimental study. Syst. Parasitol. 1992;21:1–19. [Google Scholar]
- Holmes J.C., Bethel W.M. Modification of intermediate host behaviour by parasites. In: Canning E.U., Wright C.A., editors. Behavioural Aspects of Parasite Transmission. vol. 51. 1972. pp. 123–149. (Zool. J. Linnean Soc.). (Suppl. 1) [Google Scholar]
- Karvonen A., Seppälä O. Eye fluke infection and lens size reduction in fish: a quantitative analysis. Dis. Aquat. Org. 2008;80:21–26. doi: 10.3354/dao01918. [DOI] [PubMed] [Google Scholar]
- Karvonen A., Seppälä O., Valtonen E.T. Eye fluke-induced cataract formation in fish: quantitative analysis using an ophthalmological microscope. Parasitology. 2004;129:473–478. doi: 10.1017/s0031182004006006. [DOI] [PubMed] [Google Scholar]
- Keas B., Blankespoor H. The prevalence of cercariae from Stagnicola emarginata (Lymnaeidae) over 50 Years in northern Michigan. J. Parasitol. 1997;83:536–540. [PubMed] [Google Scholar]
- La Rue G.R., Butler E.P., Berkhout P.G. Studies on the trematode family Strigeidae (Holostomidae): no. IV. The eye of fishes, an important habitat for larval Strigeidae. Trans. Am. Microsc. Soc. 1926;45:282–288. [Google Scholar]
- Lester R.J.G., Huizinga H.W. Diplostomum adamsi sp.n.: description, life cycle, and pathogenesis in the retina of Perca flavescens. Can. J. Zool. 1977;55:64–73. doi: 10.1139/z77-007. [DOI] [PubMed] [Google Scholar]
- Locke S.A., McLaughlin J.D., Marcogliese D.J. DNA barcodes show cryptic diversity and a potential physiologic basis for host specificity among Diplostomoida (Platyhelminthes: Dgenea) parasitizing freshwater fishes in the St. Lawrence River, Canada. Mol. Ecol. 2010;19:2813–2827. doi: 10.1111/j.1365-294X.2010.04713.x. [DOI] [PubMed] [Google Scholar]
- Locke S.A., McLaughlin J.D., Dayanandan S., Marcogliese D.J. Diversity and specificity in Diplostomum spp. metacercariae in freshwater fishes revealed by cytochrome c oxidase I and internal transcribed spacer sequences. Int. J. Parasitol. 2010;40:333–343. doi: 10.1016/j.ijpara.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Locke S.A., Al-Nasiri F.S., Caffara M., Drago F., Kalbe M., Lapierre A.R., McLaughlin J.D., Nie P., Overstreet R.M., Souza G.T.R., Takemoto R.M., Marcogliese D.J. Diversity, specificity and speciation in larval Diplostomidae (Platyhelminthes: Digenea) in the eyes of freshwater fish, as revealed by DNA barcodes. Int. J. Parasitol. 2015;45:841–855. doi: 10.1016/j.ijpara.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Marcogliese D.J., Compagna S. Diplostomatid eye flukes in young-of-the-year and forage fishes in the St. Lawrence River. Quebec. J. Aquat. Anim. Health. 1999;11:275–282. [Google Scholar]
- Marcogliese D.J., Compagna S., Bergeron E., McLaughlin J.D. Population biology of eye flukes in fish from a large fluvial ecosystem: the importance of gulls and habitat characteristics. Can. J. Zool. 2001;79:1102–1113. [Google Scholar]
- Marcogliese D.J., Dumont P., Gendron A.D., Mailhot Y., Bergeron E., McLaughlin J.D. Spatial and temporal variation in Diplostomum spp. in walleye (Stizostedion vitreum) and white suckers (Catostomus commersoni) from the St. Lawrence River. Can. J. Zool. 2001;79:355–369. [Google Scholar]
- McCandless R.L., Hoffert J.R., Fromm P.O. Light transmission by corneas, aqueous humor and crystalline lenses of fishes. Vis. Res. 1969;9:223–232. doi: 10.1016/0042-6989(69)90002-9. [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford University Press; New York: 2002. Parasites and the Behavior of Animals; pp. 1–315. [Google Scholar]
- Owen S.F., Barber I., Hart P.J.B. Low level infection by eye fluke, Diplostomum spp., affects the vision of three-spined sticklebacks, Gasterosteus aculeatus. J. Fish. Biol. 1993;43:803–806. [Google Scholar]
- Padros F., Knuden R., Blasco-Costa I. Histopathological characterisation of retinal lesions associated to Diplostomum species (Platyhelminthes: Trematoda) infection in polymorphic Arctic charr Salvelinus alpinus. Int. J. Parasito. - Parasites Wildl. 2018;7:68–74. doi: 10.1016/j.ijppaw.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri J.R., Heckmann R.A., Evans R.S. Life cycle and incidence of Diplostomum spathaceum Rudolphi (1819) (Trematoda: Diplostomatidae) in Utah. West. N. Am. Nat. 1976;36:86–96. [Google Scholar]
- Palmieri J.R., Heckmann R.A., Evans R.S. Life history and habitat analysis of the eye fluke Diplostomum spathaceum (Trematoda: Diplostomatidae) in Utah. J. Parasitol. 1977;63:427–429. [PubMed] [Google Scholar]
- Perlman I. 2017. The Electroretinogram: ERG. Webvision. Moran Eye Center Web.http://webvision.med.utah.edu/book/electrophysiology/the-electroretinogram-erg/ [Google Scholar]
- Poulin R. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Stud. Behav. 2010;41:151–186. [Google Scholar]
- Saari J.C. Vitamin A and vision. Subcell. Biochem. 2016;81:231–259. doi: 10.1007/978-94-024-0945-1_9. [DOI] [PubMed] [Google Scholar]
- Schneider J.C. 1984. Yellow Perch Maturity and Fecundity as a Function of Age and Growth. Michigan Department of Natural Resources Fisheries Division, Fisheries Research Report No. 1915.http://quod.lib.umich.edu/f/fishery/AAG2862.1915.001?rgn=main;view=fulltext [Google Scholar]
- Schwab I.R. Everyone wants a window seat. Br. J. Ophthalmol. 2004;88:455. doi: 10.1136/bjo.2003.041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä O., Karvonen A., Valtonen E.T. Parasite-induced change in host behaviour and susceptibility to predation in an eye fluke–fish interaction. Anim. Behav. 2004;68:257–263. [Google Scholar]
- Seppälä O., Karvonen A., Valtonen E.T. Host manipulation by parasites and risk of non-host predation: is manipulation costly in an eye fluke–fish interaction? Evol. Ecol. Res. 2006;8:871–879. [Google Scholar]
- Seppälä O., Karvonen A., Valtonen E.T. Susceptibility of eye fluke-infected fish to predation by bird hosts. Parasitology. 2006;132:575–579. doi: 10.1017/S0031182005009431. [DOI] [PubMed] [Google Scholar]
- Shariff M., Richards R.R., Sommerville C. The histopathology of acute and chronic infections of rainbow trout Salmo gairdneri Richardson with eye flukes, Diplostomum spp. J. Fish. Dis. 1980;3:455–465. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett G.E., Heeger P., Mynatt R.L., Truett A.A., Walker J.A., Warman M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29(52):54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Ubels J.L., Hoffert J.R., Fromm P.O. Ocular oxygen toxicity: the effect of hyperbaric oxygen on the in vitro electroretinogram. Comp. Biochem. Physiol., A. 1977;57A:29–32. [Google Scholar]
- Ubels J.L., Edelhauser H.F., Antoine M.E. Choroidal rete mirabile function and resistance to oxygen toxicity in fish. Exp. Eye Res. 1984;38:353–362. doi: 10.1016/0014-4835(84)90190-8. [DOI] [PubMed] [Google Scholar]
- Van Steenkiste N., Locke S.A., Castelin M., Marcogliese D.J., Abbott C.L. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes) Mol. Ecol. Res. 2015;15:945–952. doi: 10.1111/1755-0998.12358. [DOI] [PubMed] [Google Scholar]
- Wittenberg J.B., Wittenberg B.A. Active secretion of oxygen into the eye of the fish. Nature, Lond. 1962;194:106–107. doi: 10.1038/194106a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.