Abstract
Vertebrates are hosts to numerous parasites, belonging to many different taxa. These parasites differ in transmission, being through either direct contact, a faecal-oral route, ingestion of particular food items, vertical or sexual transmission, or by a vector. Assessing the impact of diet on parasitism can be difficult because analysis of faecal and stomach content are uncertain and labourious; and as with molecular methods, do not provide diet information over a longer period of time. We here explored whether the analysis of stable isotopes in hair provides insight into the impact of diet and the presence of parasites in the rodent Myodes glareolus. Twenty-one animals were examined for parasites and their hair analysed for stable isotopes (C and N). A positive correlation between δ15N and one species of intestinal parasite was observed in females. Furthermore, several ectoparasites were negatively correlated with δ15N, indicating that infections are further associated with foraging habits (size and layout of the home range, length and timing of foraging, interaction with other rodents, etc.) that set the rodents in direct contact with infected hosts. Although a limited number of animals were included, it seemed that the isotope values allowed for identification of the association between diet and parasite occurrence in this rodent. We therefore propose that this method is useful in providing further insight into host biology, feeding preferences and potential exposure to parasites species, contributing to the understanding of the complex relationship between hosts and parasites.
Keywords: δ13C, δ15N, Isotope, Diet, Bank vole, Hair, Parasitism
Graphical abstract
Highlights
-
•
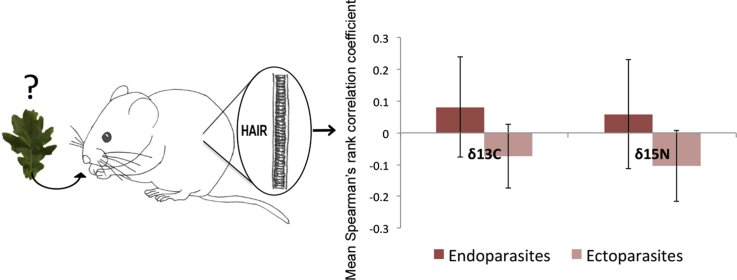
Endoparasites were positively related to δ15N in contrast to ectoparasites.
-
•
Besides isotopes, some parasites were correlated with season and host's gender.
-
•
Isotope analysis in hair exposes associations between parasites and host diet.
1. Introduction
Complex living environments define the selective pressures for animals and in turn shape their life history development by regulating population size and distribution (Vaughan et al., 2011). Although our insight into the relationship between parasites and hosts is steadily growing, the complexity of multiple infections (polyparasitism) is still not fully understood (Bordes and Morand, 2011) and nor is it quite understood why some host species might harbour more parasites species compared to others (Bordes and Morand, 2015). The nutritional status plays an important role in the outcome of infections because malnutrition will reduce the immune response due to a competition for nutrients and energy between the parasite, host and host defences (Bush et al., 2001). Hosts with better nutrition can better invest in immune responses and thus nutrition profiles can determine parasite prevalence (Neve et al., 2007).
Diet composition can be monitored through a number of methods; they range from visual analyses of stomach content (Andreasen et al., 2017), molecular analysis of faecal samples (Deagle et al., 2009), to assessment of stable isotopes (Panarello and Fernández, 2002; Hobbie et al., 2017; Reid and Koch, 2017). The latter can be used to trace the transfer of energy and matter through the foodweb because heavy isotopes accumulate at increasing trophic levels (Vaughan et al., 2011; Galetti et al., 2016). Isotope analysis is often preferred because it enables diet assessment over a substantial time period, for example covering the entire period of hair growth (Panarello and Fernández, 2002; Hobbie et al., 2017). Hair stable isotope composition reflects the isotope composition of the food eaten by the animal (Russo et al., 2017). For example, analysis of C stable isotopes enables distinction between consumed plants with different photosynthetic pathways (e.g. CAM, C3 or C4 plants) (O'Leary, 1988) and thus carried over to the next trophic level (Jensen et al., 2012). More importantly, it has been shown that δ13C and δ15N values increase through the food chain i.e. is positively correlated with ingestion of animal protein (Petzke et al., 2005; Roth and Hobson, 2000), such that herbivorous animals in a C3-system often have δ15N values of ca. 2‰ while (top) predators have values of ca. 6‰ (Ben-David and Flaherty, 2012). Several studies have investigated trophic interactions by the analysis of stable isotope values and the presence of parasites. Although this has been studied in birds (Robinson et al., 2009) and mammals (rabbits: Boag et al., 1998; seals: Sinisalo et al., 2006; Vega et al., 2018), the majority of research has focused on aquatic environments and especially on fish (Johnson et al., 2004; Bertrand et al., 2011; Locke et al., 2013; Nachev et al., 2017).
Rodentia and Chiroptera are known to be the mammalian orders with the highest parasite diversity (Krasnov et al., 2006). The bank vole, Myodes glareolus (formerly Clethrionomys glareolus, Schreber 1780), is a small member of the Cricetidae family that inhabits Paleartic forests (Wilson and Reeder, 2005). M. glareolus has a particularly wide and presumably plastic diet preferences (Sadowska et al., 2008), although it is generally considered to be herbivorous with a high preference for woody plants (Watts, 1968).
The aim of this study is to assess the association between parasite abundance and C and N stable isotopes in hair of M. glareolus. This approach could potentially provide a simple method to study the importance of a systematic diet preference in acquiring different parasites in free-ranging rodents.
2. Material and methods
2.1. Sample collection
Female (n = 13) and male (n = 8) Myodes glareolus specimens were captured in Kongelunden, Denmark (55°34′14.2″N, 12°35′12.7″E). Trapping was conducted in two sampling rounds (September (S1) and October (S2), 2014) with a total of 44 and 80 traps, respectively. Pairs of Ugglan special and Ugglan lemming (Grahnab AB, Hillerstop, Sweden) were placed approximately 10 m distance apart in ca. 200 m long transects. The traps were prebaited with oatflakes and apples for 48 h and were left overnight during trapping.
2.2. Parasitological examination
The bank voles were transported to the University of Copenhagen, anesthetized by isoflourane inhalation and sacrificed by cervical dislocation. Immediately following euthanasia each animal was sexed, measured and weighed intact; then the liver and spleen were weighed separately. Shortly after, the liver, spleen, stomach and intestines were examined for helminths under a dissection stereomicroscope. The thoracic area and subdermis were also examined. The coat, ears, nostrils and perianal region were inspected for ectoparasites. The conjuctival sacs were rinsed with physiological saline solution and examined for nematodes. The recovered parasites were preserved in 70% ethanol for morphological examination and DNA analysis. For microscopic identification purposes, parasites were cleared in Amman's lactophenol and examined under an optical microscope. Eight liver lesions/white spots were subjected to DNA extraction after homogenization by bead-beating (FastPrep®-24, MP Biomedicals Inc., France). The samples were first mixed with magnetic beads (2 mm in diameter) and subsequently beaten six times for 60 s at 0.5 m/s. The homogenate was immediately subjected to DNA extraction using a commercial kit (QIAmp DNA Stool Mini Kit®, Qiagen GmbH, Hilden, Germany). For species-specific identification of Taeniid infection, a 267 base pair (bp) region of the mitochondrial small subunit ribosomal RNA gene (ssu rRNA) was amplified using the previously described Cest3-Cest5 primers (Trachsel et al., 2007). Resulting amplicons were sequenced on both directions using a commercial kit (ABI Prism Big Dye Terminator v 3.1 Sequencing Kit, Applied Biosystems, Foster City, CA). Establishing the consensus sequences was done using the software MEGA6 (Tamura et al., 2013) and comparison to the available sequences in GenBank was done using the online BLASTn analysis (http://blast.ncbi.nlm.nih.gov).
2.3. Isotope analysis
The animals were shaved and the hair collected. Hair samples were then washed twice with 5 ml isopropanol and left in a protected fume hood at room temperature overnight. The hair was grinded to a fine powder using a Retsch Ball mill (mixer mill MM 200; 10 ml stainless steel grinding jars; single 12 mm stainless steel grinding balls) for 5 min at 30 Hz. Pulverized hair (10 mg) of each individual was sent to the Stable Isotope Facility at UC Davis (University of California) for C and N analyses. The samples were analysed using a PDZ Europa ANCA-GSL elemental analyser interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon LTD., Cheshire, UK). For more details see http://stableisotopefacility.ucdavis.edu.
2.4. Statistical analysis
A generalised linear model was used to examine the relationship between isotope ratios (δ13C and δ15N, ‰) and body attributes (sex (male, female), liver weight (g), spleen weight (g), body weight (g) and body length (cm)) and sampling rounds (S1 and S2) (PROC GENMOD; dist = normal, SAS 9.4, SAS Institute, Cary, NC, USA). The associations were further examined for simple one-to-one correlations between isotope ratios and each individual body attribute, to assess the risk of confounding (PROC CORR).
The prevalence rate (number of individuals infected/total number) was calculated (PROC FREQ; with EXACT CI-estimation under the binomial distribution) and the range of intensity was given as minimum and maximum parasite count. Mean abundance and intensity 95% bias-corrected and accelerated (BCa) bootstrap confidence intervals were calculated for each parasite species as suggested by Rózsa et al. (2000). The confidence intervals were calculated using the web tool Quantitative Parasitology, QPweb version 1.0.1.3 (Reiczigel et al., 2013), with the number of bootstrap replications set to 10000. The correlation between parasite abundance was characterised by rank-correlation (PROC CORR Spearman), in order to assess parasite interactions. This was done only for parasite species with prevalence rates ≥10% to avoid potentially weakly supported correlation (Bush et al., 1990).
Generalised linear models (PROC GENMOD, Dist = Poisson, log-linkfunction; and controlling for data heterogeneity by the pscale-function; SAS 9.4, SAS institute) were used for assessment of the association between the abundance for each parasite that occurred on more than 5 hosts (n = 13), the independent effect of body attributes (sex, liver weight, spleen weight, body weight and body length), sampling round and stable isotopes (C and N), in order to assess whether the isotope values provided information beyond traditional simple morphological proxies of body condition and immune response. The models were reduced by stepwise exclusion of insignificant variables (p > 0.05). Arguably, a comparison of parasite intensity – rather than abundance would have been more appropriate for assessing the relationship between parasite infection and other variables, since parasite free individulas might never had been exposed. Similarly, a negative-binomial distribution would have been more approapiate for some parasites, but they were here assessed in identical models using parasite abundance in order to include all individuals and thereby allow direct comparisons.
Given the considerable biological differences between parasites, it was expected that there would be no consistent association between their parasite abundance and hosts diets as assessed from δ13C and δ15N. To assess and illustrate such potential heterogenity, Pearsons Correlation Coefficient (PROC CORR; SAS 9.4) was used to obtain information about the magnitude of association between isotope values and parasite abundance in each sex. The average Correlation Coefficient was calculated from parasites that occurred on at least five individuals (n = 13). The host's body measurements and isotope values are expressed as the range, followed by mean and the S.E.M. in parenthesis.
3. Results
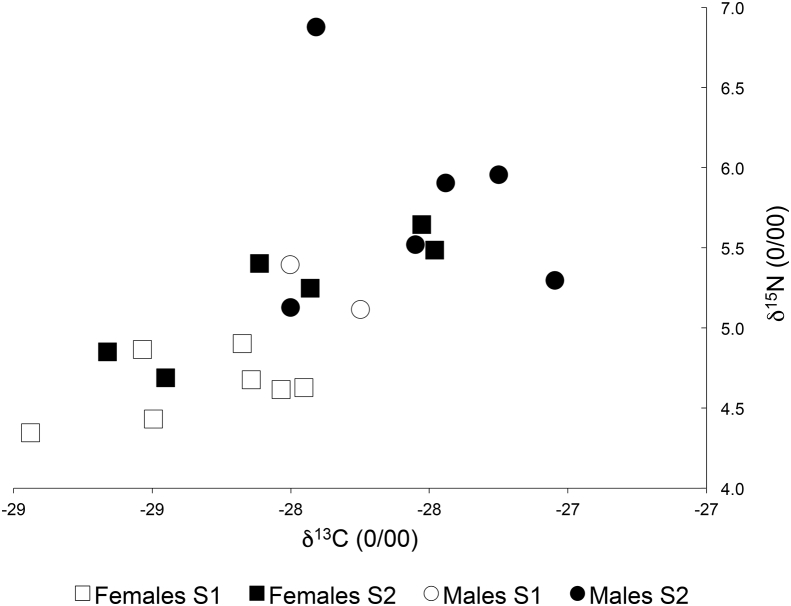
Rodent body length ranged from 8 to 11.2 (9.57 ± 0.19 cm) and the body weight from 17.7 to 34.5 (23.23 ± 1.09 g). The liver and spleen weighed 0.8 to 4.4 (1.65 ± 0.17 g) and 0.04 to 1.9 (0.38 ± 0.11 g), respectively. The values of δ13C and δ15N ranged from −28.94 to −27.05 (−27.97 ± 0.11‰) and 4.34 to 6.88 (5.19 ± 0.13‰), respectively (Fig. 1). There were significant differences in δ15N and δ13C values between females and males; δ15N values were also related to season (Table 1). There were no apparent correlation between isotope ratio variation and body attributes (liver weight, spleen weight, body weight and body length) in the generalised linear models and nor in simpler one-to-one correlation analyses (8 analyses, p > 0.22).
Fig. 1.
The relationship between δ13C and δ15N values for the 21 Myodes glareolus sampled from Kongelunden Denmark in September (S1) and October (S2). The rodents present great variation in isotope values.
Table 1.
Significant association between δ13C and δ15N values and body attributes (sex, liver weight, spleen weight, body weight and body length) and sampling round (S1 or S2) in 21 Myodes glareolus. Insignificant variables (p < 0.05) were step-wise removed.
| Parameter | Estimate | SE | Wald 95% Conf. Limits | Wald Chi-Sq | Pr > ChiSq | AIC | ||
|---|---|---|---|---|---|---|---|---|
| δ15N | Intercept | 5.79 | 0.15 | 5.50 | 6.09 | 1462.89 | <.0001 | 24.8 |
| S1 vs S2 | −0.56 | 0.19 | −0.93 | −0.20 | 9.07 | 0.0026 | ||
| Females vs. Males | −0.58 | 0.19 | −0.96 | −0.21 | 9.30 | 0.0023 | ||
| δ13C | Intercept | −27.62 | 0.14 | −27.90 | −27.34 | 38040.70 | <.0001 | 23.1 |
| Females vs. Males | −0.57 | 0.18 | −0.92 | −0.22 | 9.99 | 0.0016 | ||
| Scale | 0.40 | 0.00 | 0.40 | 0.40 | ||||
All voles had ectoparasites and 90% were infected with endoparasites. A total of 699 endoparasites and 2753 ectoparasites were collected (Table 2). Ectoparasites such as Laelaps hilaris and Hyperlaelaps microti were the most prevalent (95% CI: 76–99 and 90% CI: 69–98, respectively) and Listrophorus brevipes had the highest mean abundance (97.9 CI: 42.8–216) per host. Among the endoparasites, Heligmosomoides glareoli had the highest prevalence (52% CI: 29–74) and Rhabditis orbitalis the highest mean intensity and abundance (97.5 CI: 16.3–213 and 27.8 CI: 4.48–79, respectively) per host.
Table 2.
Parasite infracomunity structure of 21 Myodes glareolus collected in September and October.
| Parasites | Prevalence % (CI 95%) | Range of intensity | Mean intensity (CI 95%) | Mean abundance (CI 95%) | Infection site | Transmission route |
|---|---|---|---|---|---|---|
| ENDOPARASITES | ||||||
| Nematodes | ||||||
| Heligmosomoides glareoli | 52 (29–74) | 1–24 | 4.18 (1.91–11.2) | 2.19 (0.86–6.09) | Intestine | Faecal-Oral |
| Rhabditis orbitalis | 28 (11–52) | 2–275 | 97.5 (16.3–213) | 27.9 (4.48–79) | Eye | Contact |
| Trichuris sp. | 33 (14–56) | 1–4 | 2 (1.29–2.86) | 0.67 (0.29–1.24) | Caecum | Faecal-Oral |
| Trematodes | ||||||
| Corrigia vitta | 19 (5–41) | 3–14 | 8 (4.25–12.2) | 1.52 (0.38–3.95) | Pancreatic ducts | I. H. |
| Cestodes | ||||||
| Paranoplocephala omphalodes | 19 (2–35) | 1–3 | 1.75 (1–2.5) | 0.33 (0.05–0.76) | Intestine | I. H. |
| Hydatigena taeniaeformis | 23 (8–47) | 1–7 | 3 (1–5) | 0.71 (1.43–1.9) | Liver (cysts) | Oral |
| ECTOPARASITES | ||||||
| Fleas | ||||||
| Ctenophtalmus agyrtes | 57 (35–78) | 1–10 | 2.58 (1.58–4.83) | 1.48 (0.76–2.95) | Body surface | Contact |
| Megabothris walkeri | 33 (13–53) | 1–3 | 1.86 (1.14–2.43) | 0.62 (0.24–1.1) | Body surface | Contact |
| Catallagia dacenkoi dacenkoi | 4 (0–23) | 1 | 1 (n/a) | 0.05 (0–0.14) | Body surface | Contact |
| Hystricopsylla orientalis | 4 (0–23) | 1 | 1 (n/a) | 0.05 (0–0.14) | Body surface | Contact |
| Mites | ||||||
| Ixodes ricinus | 47 (25–70) | 1–3 | 1.5 (1.1–1.9) | 0.71 (0.38–1.1) | Body surface | Contact |
| Dermacarus hypudaei | 33 (13–53) | 1–14 | 3.43 (1.43–8.71) | 1.14 (0.33–3.76) | Body surface | Contact |
| Myocoptes japonensis | 61 (38–81) | 1–27 | 5.15 (2.85–11.7) | 3.19 (1.57–7.65) | Body surface | Contact |
| Trichoecious tenax | 38 (18–61) | 1–72 | 16.6 (2.88–42.1) | 6.33 (1–18.7) | Body surface | Contact |
| Radfordia lemnina | 42 (21–65) | 1–17 | 3.89 (1.89–9.11) | 1.67 (0.67–4.48) | Body surface | Contact |
| Listrophorus brevipes | 80 (58–94) | 1–768 | 121 (53.9–267) | 97.9 (42.8–216) | Body surface | Contact |
| Laelaps hilaris | 95 (76–99) | 1–28 | 7.4 (5–11.3) | 7.05 (4.62–10.9) | Body surface | Contact |
| Hyperlaelaps microti | 90 (69–98) | 1–29 | 11.2 (8.14–15.1) | 10.1 (6.95–13.9) | Body surface | Contact |
| Cheyletidae | 4 (0–23) | 1 | 1 (n/a) | 0.05 (0–0.14) | Body surface | Contact |
| Lice | ||||||
| Hoplopleura acanthopus | 4 (0–23) | 1 | 1 (n/a) | 0.05 (0–0.14) | Body surface | Contact |
Note: I.H. refers to the ingestion of an intermediate host; contact refers to direct contact between rodents.
Spearman's rank correlation coefficient (rs) showed that the common parasite species had significant correlations with up to 3 other species of parasites (Table 3), which did not indicate widespread interactions between parasites.
Table 3.
Output from Spearman's rank correlation test between parasite species with prevalence ≥10% in 21 Myodes glareolus.
| H. g. | R. o. | T. sp. | C. v. | P. o. | C. a. | C. d. | D. h. | M. j. | T. t. | R. l. | L. h. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nematodes | ||||||||||||
| H. glareoli (H. g.) | 1.00 | |||||||||||
| R. orbitalis (R. o.) | −0.14 | |||||||||||
| Trichuris sp. (T. sp.) | −0.15 | −0.03 | ||||||||||
| Trematodes | ||||||||||||
| C. vitta (C. v.) | −0.1 | 0.3 | 0.49* | |||||||||
| Cestodes | ||||||||||||
| P. omphalodes (P. o.) | −0.47* | −0.03 | 0.17 | 0 | ||||||||
| Fleas | ||||||||||||
| C. agyrtes (C. a.) | 0.42 | −0.37 | −0.15 | −0.12 | −0.34 | |||||||
| C. dacenkoi (C. d.) | −0.21 | −0.14 | 0.33 | 0.54* | −0.11 | −0.23 | ||||||
| Mites | ||||||||||||
| D. hypudaei (D. h.) | −0.2 | −0.05 | −0.13 | −0.16 | 0.15 | −0.07 | −0.15 | |||||
| M. japonensis (M. j.) | 0.55* | 0.15 | −0.04 | 0.04 | −0.33 | 0.56** | −0.25 | −0.13 | ||||
| T. tenax (T. t.) | 0.19 | 0.54* | 0.09 | 0.04 | −0.19 | −0.02 | −0.17 | −0.26 | 0.44* | |||
| R. lemnina (R. l.) | 0.02 | −0.08 | 0.58** | 0.44* | 0.04 | 0.15 | 0.29 | −0.12 | 0.19 | 0.16 | ||
| L. hilaris (L. h.) | −0.55 | 0.10 | 0.21 | 0.21 | 0.49* | −0.49* | 0.11 | 0.21 | −0.45* | −0.09 | −0.04 | |
| H. microti (H. m.) | −0.31 | 0.25 | −0.33 | −0.15 | 0.23 | −0.31 | −0.13 | −0.01 | −0.41 | −0.06 | −0.55** | 0.09 |
Note: An asterisk indicates the significance level (p < 0.05) and two asterisks (p < 0.01).
The Generalised Linear Model of parasite abundance (Table 4) showed that the load of only one parasite was correlated with host sex. Seasonal variation was indicated for several ectoparasites (Ctenophtalmus agyrtes, Myocoptes japonensis, L. brevipes and H. microti) and one endoparasite (H. glareoli). Rodent's body weight was correlated with Trichuris sp., Dermacarus hypudaei, Radfordia lemnina and L. hilaris; body size was correlated with ectoparasites D. hypudaei and L. brevipes (negative and positive, respectively). The nematode R. orbitalis, as well as the mites M. japonensis and L. brevipes were significantly correlated with liver weight. Spleen weight was found positively correlated with R. orbitalis and negatively with D. hypudaei and R. lemnina. Three species of ectoparasites (Trichoecious tenax, R. lemnina and L. brevipes) and one endoparasite (H. glareoli) were correlated to either δ13C or δ15N. Notably, the parasite with the largest range in intensity showed clear effect of δ15N and sampling round (Fig. 2).
Table 4.
Output from analysis of parasite abundance in 21 Myodes glareolus that included the independent effects of body attributes (sex, liver weight, spleen weight, body weight and body length), sampling round (S1 or S2) and hair δ13C and δ15N values. In all analyses, more than five individuals were infected with the given parasite. Insignificant variables (p < 0.05) were step-wise removed.
| Taxa (no of non-zero values) | Parameter | Estimate | SE | Wald 95% Conf. Limits | Wald Chi-Sq | Pr > ChiSq | AIC | |
|---|---|---|---|---|---|---|---|---|
| Heligmosomoides glareoli | Intercept | −24.81 | 4.42 | −33.47 | −16.15 | 31.50 | <.0001 | 57.8 |
| (11) | S1 vs. S2 | 4.35 | 0.91 | 2.57 | 6.12 | 23.05 | <.0001 | |
| d15N | 4.06 | 0.65 | 2.78 | 5.33 | 38.85 | <.0001 | ||
| Female vs. males | 2.08 | 0.68 | 0.75 | 3.42 | 9.30 | 0.0023 | ||
| Rhabditis orbitalis | Intercept | −1.76 | 2.29 | −6.25 | 2.72 | 0.59 | 0.4410 | 864.6 |
| (6) | Liver weight | 40.68 | 18.13 | 5.15 | 76.22 | 5.04 | 0.0248 | |
| Spleen weight | 57.18 | 23.66 | 10.81 | 103.56 | 5.84 | 0.0157 | ||
| Trichuris sp. | Intercept | −5.25 | 1.74 | −8.66 | −1.85 | 9.17 | 0.0025 | 41.8 |
| (7) | Body weight | 0.19 | 0.06 | 0.07 | 0.31 | 9.84 | 0.0017 | |
| Ctenophthalmus agyrtes | Intercept | −0.54 | 0.58 | −1.67 | 0.60 | 0.87 | 0.3516 | 75.3 |
| (12) | S1 vs. S2 | 1.52 | 0.66 | 0.23 | 2.81 | 5.34 | 0.0208 | |
| Dermacarus hypudaei | Intercept | 9.15 | 4.34 | 0.65 | 17.66 | 4.45 | 0.0349 | 58.6 |
| (7) | Spleen weight | −138.72 | 48.28 | −233.35 | −44.08 | 8.25 | 0.0041 | |
| Body weight | 0.53 | 0.14 | 0.27 | 0.80 | 15.49 | <.0001 | ||
| Body length | −2.19 | 0.72 | −3.60 | −0.78 | 9.30 | 0.0023 | ||
| Myocoptes japonensis | Intercept | −2.77 | 1.85 | −6.39 | 0.85 | 2.25 | 0.1335 | 129 |
| (13) | S1 vs. S2 | 2.02 | 0.90 | 0.25 | 3.79 | 4.98 | 0.0256 | |
| Liver weight | 38.16 | 18.03 | 2.83 | 73.50 | 4.48 | 0.0343 | ||
| Trichoecious tenax | Intercept | −103.05 | 32.89 | −167.52 | −38.58 | 9.82 | 0.0017 | 237.4 |
| (8) | d13C | −3.70 | 1.15 | −5.95 | −1.45 | 10.39 | 0.0013 | |
| Radfordia lemnina | Intercept | 7.19 | 3.03 | 1.25 | 13.13 | 5.63 | 0.0177 | 56.8 |
| (9) | d15N | −2.32 | 0.60 | −3.49 | −1.15 | 15.11 | 0.0001 | |
| Spleen weight | −39.43 | 16.63 | −72.02 | −6.84 | 5.62 | 0.0177 | ||
| Body weight | 0.21 | 0.04 | 0.13 | 0.29 | 27.93 | <.0001 | ||
| Listrophorus brevis | Intercept | 14.76 | 3.75 | 7.42 | 22.11 | 15.52 | <.0001 | 1475.1 |
| (17) | S1 vs. S2 | −5.34 | 1.26 | −7.81 | −2.88 | 18.03 | <.0001 | |
| d15N | −2.32 | 0.52 | −3.35 | −1.30 | 19.66 | <.0001 | ||
| Liver weight | −42.60 | 19.72 | −81.24 | −3.95 | 4.67 | 0.0308 | ||
| Body weight | 0.59 | 0.28 | 0.05 | 1.13 | 4.64 | 0.0312 | ||
| Laelaps hilaris | Intercept | −0.24 | 0.88 | −1.96 | 1.49 | 0.07 | 0.7890 | 164.8 |
| (20) | Body weight | 0.09 | 0.03 | 0.02 | 0.16 | 7.15 | 0.0075 | |
| Hyperlaelaps microti | Intercept | 2.58 | 0.19 | 2.22 | 2.95 | 194.56 | <.0001 | 194 |
| (19) | S1 vs. S2 | −0.81 | 0.37 | −1.54 | −0.08 | 4.79 | 0.0286 | |
Note: No significant correlation was observed for Megabothris walker (7) or Ixodes ricinus (10).
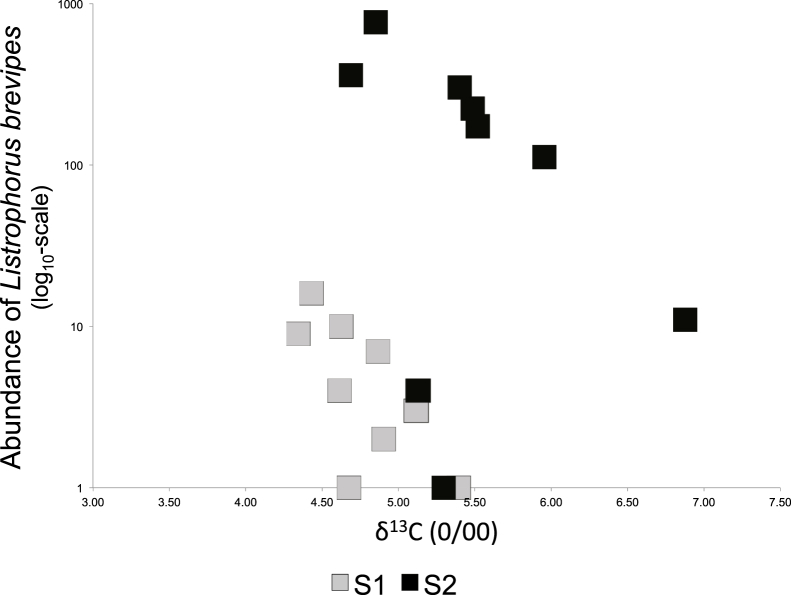
Fig. 2.
The relationship between parasite intensity and δ15N values for the mite Listrophorus brevipes in September (S1) and October (S2) samples of Myodes glareolus. Note the log-scale for parasite abundance.
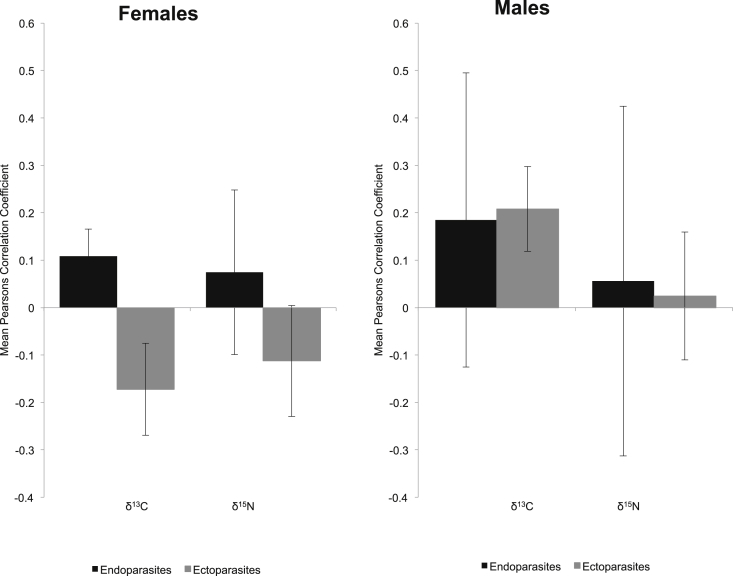
The association between isotopes and parasite abundance included both positive and negative associations, indicating that the correlations across parasites are quite variable. For simple one-to-one correlation between endoparasites and δ13C and δ15N the average Pearsons Correlation Coefficients ranged from −0.20 to 0.34 (0.08 ± 0.16) and 0.01 to 0.39 (0.06 ± 0.17) respectively. For ectoparasites it ranged from −0.04 to 0.36 (−0.07 ± 0.10) for δ13C and −0.001 to 0.43 (−0.10 ± 0.11) for δ15N. The average Pearsons Correlation Coefficient for correlations between δ15N and endoparasites were positive for both males and females, but not significantly (Fig. 3). There was no consistent correlation for δ13C (Fig. 3). Here females with lower δ13C levels had negative correlations, while males with higher δ13C levels had positive correlations.
Fig. 3.
The average Pearsons Correlation Coefficient (error bars: SE) between abundance and δ13C and δ15N for the two parasite groups: ecto and endoparasite for females (left) and males (right). The data includes 13 parasites occurring on 5 or more individuals and indicate consistent correlations for endoparasites and δ15N, while this is not the case for δ13C.
4. Discussion
The average of the δ13C values (−27.97 ± 0.11‰) indicates that the rodents fed mainly on C3 plants (O'Leary, 1988), while the range indicates that they all ingested food items at a higher trophic level. Large ranges in isotope levels have been found in other rodent species (Baltensperger et al., 2015) and have also been previously found in M. glareolus (Balciauskas et al., 2016). The variation in the isotope values between sexes might indicate differences in foraging habits in this species. Moreover, values of δ15N related to sampling round indicate a seasonal change in diet (Fig. 1), but there was no apparent correlation to other host attributes such as body weight and length. This suggests that the isotope ratios carry added information on the individual hosts.
It should be noted that there were positive correlations between some ectoparasites (M. japonensis with C. agyrtes and T. tenax), but most of the correlations between members of this group were negative (Table 3). Negative correlations between ectoparasite species could indicate competition or interference due to their shared distribution on the host's body. A negative correlation was also present between the intestinal helminths H. glareoli and Paranoplocephala omphalodes.
From the 13 species of parasites analysed under the generalised linear model (Table 4), four were significantly correlated with δ13C or δ15N. The results indicate that the abundance of the endoparasite H. glareoli is higher in rodents foraging in a higher trophic level and the abundance of the ectoparasites T. tenax, R. lemnina and L. brevipes is higher in rodents feeding on a lower trophic level. The positive correlation found between H. glareoli with sex, sample round and δ15N indicate that this nematode is present in a higher number in females with a Nitrogen enriched diet in September (S1). Female-biased parasitism has been previously reported in bank voles (Grzybek et al., 2015), and as in our study, foraging habits of females were related to the exposure to an endoparasite.
Another parasite significantly correlated with sample round and isotope levels is L. brevipes. The results for this particular mite translates into large sized rodents feeding mainly in a low Nitrogen enriched diet present a high number of this mite, which further increases later in the season (Fig. 2). A possible explanation for this correlation is that older rodents, which have larger bodies, offer more host area for L. brevipes and have different foraging habits than younger rodents.
It should be noted that while we found significant correlation for three out of the six mite species present on more than 5 of the rodents, the association across parasite groups were quite variable (Fig. 3). The variable association can be due to the diversity of parasite biology and the varying impact of other important factors, which clouds the impact of diets and foraging habits.
Seasonal variation in the parasitic numbers can be related to abiotic factors that allow better development of the parasite as well as to changes in the bank vole's population. Such seasonal effects have already been documented for mites (Zhang et al., 2010) and for helminths (Abu-Madi et al., 2000) and is usually explained by differences in environmental factors and increasing host densities that affect parasite transmission. This could explain why H. glareoli, C. agyrtes and M. japonensis were more abundant in September (S1) and L. brevipes and H. microti in October (S2).
As stated previously, parasites and hosts have a complex association and therefore differences in the host's characteristics and behaviour have an impact on the exposure to parasites and similarly, parasite characteristics will have an influence on the host (Krasnov et al., 2006). Accordingly, in this study parasite abundance was found to be correlated to host's traits such as body weight, size and also liver and spleen weight. Although these traits have been previously found correlated to parasitism (Cowan et al., 2009; Hayward et al., 2017), some studies have not found enough evidence for these correlations (Schulte-Hostedde and Elsasser, 2011; Sackett, 2018). In accordance with Sackett (2018) we consider these associations challenging to explain in detail, because of the complexity of the factors contributing to these correlations, especially in free-ranging rodents.
The interpretation of the correlations between stable isotopes and parasite infection must be considered with caution because a number of relevant factors, such as the occurrence of other parasites were excluded from the analysis. Still, the method allowed the identification of associations between parasitic occurrences and foraging habits of this rodent. The advantages of this method are: the use of a model animal with a broad diet, easy sample collection, assessment of diet and foraging habits over a long period of time and the tracing of energy transfers throughout the foodweb, - which has been previously studied with stable isotopes (Panarello and Fernández, 2002; Vaughan et al., 2011; Bovendrop et al., 2017; Russo et al., 2017). Additionally, this method can provide an insight into host biology by capturing the difference in foraging habits of rodents and its association with potential exposure to parasite species. Thus, it would contribute further to the understanding of the complex relationship between hosts and parasites (Bordes and Morand, 2015). We here noted that M. glareolus has a great variation in foraging habits even within a small sample and that when a comparable variation was found in mean parasite intensity then it resulted in clear identification of relevant variables. Similar results may be achieved for other parasites, but larger sample size is needed to show clearer associations between the foraging habits and the intensity of infection of less common parasites.
To conclude, this method allows for correlation of infection with certain parasites to the bank vole's foraging habits, exposing the expected links to differences in foraging habits. Here it is suggested that, rodents with low Nitrogen in their diet presented more ectoparasites and in contrast, feeding at higher trophic levels was associated with endoparasitic infection. Additional contributions to these associations could arise from other mechanisms i.e., for ectoparasites it can also indicate an energetic impact from lack of protein and from decreased grooming. While such uncertainties remain, this study proves that the analysis of stable isotopes in hair of M. glareolus shows clear association with the abundance of several parasites.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
Acknowledgments
This work was supported by the University of Copenhagen, Denmark. The authors would like to acknowledge the kind assistance of C. Fisher for assisting with the laboratory work. Conflict of interest: none. All applicable institutional and national guidelines for the care and use of animals were followed.
References
- Abu-Madi M.A., Behnke J.M., Lewis J.W., Gilbert F.S. Seasonal and site specific variation in the component community structure of intestinal helminths in Apodemus sylvaticus from three contrasting habitats in south-east England. J. Helminthol. 2000;74:7–15. doi: 10.1017/S0022149X00000020. [DOI] [PubMed] [Google Scholar]
- Andreasen H., Ross S.D., Siebert U., Andersen N.G., Ronnenberg K., Gilles A. Diet composition and food consumption rate of harbor porpoises (Phocoena phocoena) in the western Baltic Sea. Mar. Mamm. Sci. 2017;33:1053–1079. doi: 10.1111/mms.12421. [DOI] [Google Scholar]
- Balciauskas L., Skipityte R., Jasiulionis M., Trakimas G., Balciauskiene L., Remeikis V. The impact of Great Cormorants on biogenic pollution of land ecosystems: stable isotope signatures in small mammals. Sci. Total Environ. 2016;565:376–383. doi: 10.1016/j.scitotenv.2016.04.185. [DOI] [PubMed] [Google Scholar]
- Baltensperger A.P., Huettmann F., Hagelin J.C., Welker J.M. Quantifying trophic niche spaces of small mammals using stable isotopes (δ15N and δ13C) at two scales across Alaska. Can. J. Zool. 2015;93:579–588. doi: 10.1139/cjz-2015-0025. [DOI] [Google Scholar]
- Ben-David M., Flaherty E.A. Stable isotopes in mammalian research: a beginner's guide. J. Mammal. 2012;93:312–328. doi: 10.1644/11-MAMM-S-166.1. [DOI] [Google Scholar]
- Bertrand M., Cabana G., Marcogliese D.J. Estimating the feeding range of a mobile consumer in a river-flood plain system using δ13C gradients and parasites. J. Anim. Ecol. 2011;80:1313–1323. doi: 10.1111/j.1365-2656.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- Boag B., Neilson R., Robinson D., Scrimgeour C.M., Handley L.L. Wild rabbit host and some parasites show trophic-level relationship for delta 13C and delta 15N: a first report. Isot. Environ. Health Stud. 1998;34:81–85. doi: 10.1080/10256019708036335. [DOI] [PubMed] [Google Scholar]
- Bordes F., Morand S. Impacts of parasite diversity on wild vertebrates: limited knowledge but important perspectives. In: Morand S., Krasnov B.R., Littlewood D.T.J., editors. Parasite Diversity and Diversification. Cambridge University Press; UK: 2015. pp. 77–92. [Google Scholar]
- Bordes F., Morand S. The impact of multiple infections on wild animal hosts: a review. Infect. Ecol. Epidemiol. 2011;1(7346) doi: 10.3402/iee.v1i0.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovendrop R.S., Libardi G.S., Sarmento M.M., Camargo P.B., Percequillo A.R. Age and habitat matters: isotopic variation of two sympatric species of rodents in Neotropical Forest. Hystrix. 2017;28:214–221. doi: 10.4404/hystrix%5f28.2-12521. [DOI] [Google Scholar]
- Bush A.O., Aho J.M., Kennedy C.R. Ecological versus philogenetic determinants of helminth parasite species richness. Evol. Ecol. 1990;4:1–20. [Google Scholar]
- Bush A.O., Fernández J.C., Esch G.W. Cambridge University Press; UK: 2001. Parasitism: the Diversity and Ecology of Animal Parasites. [Google Scholar]
- Cowan K.M., Shutler D., Herman T.B., Stewart D.T. Splenic mass of masked shrews, Sorex cinereus, in relation to body mass, sex, age, day of the year, and bladder nematode, Liniscus (Capillaria) maseri, infection. J. Parasitol. 2009;95:228–230. doi: 10.1645/GE-1566.1. [DOI] [PubMed] [Google Scholar]
- Deagle B.E., Kirkwood R., Jarman S.N. Analysis of Australian Fur seal diet by pyrosequencing prey DNA in faeces. Mol. Ecol. 2009;18:2022–2038. doi: 10.1111/j.1365-294X.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- Galetti M., Rodarte R.R., Neves C.L., Moreira M., Costa-Pereira R. Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0152494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzybek M., Bajer A., Behnke-Borowczyk J., Al-Sarraf M., Behnke J.M. Female host sex-biased parasitism with the rodent stomach nematode Mastophorus muris in wild bank voles (Myodes glareolus) Parasitol. Res. 2015;114:523–533. doi: 10.1007/s00436-014-4214-0. [DOI] [PubMed] [Google Scholar]
- Hobbie E.A., Shamhart J., Sheriff M., Ouimette A.P., Trappe M., Schuur E.A.G., Hobbie J.E., Boonstra R., Barnes B.M. Stable isotopes and radiocarbon assess variable importance of plants and fungi in diets of arctic ground squirrels. Arctic. Antarct. Alp. Res. 2017;49:487–500. doi: 10.1657/AAAR0016-062. [DOI] [Google Scholar]
- Hayward A., Tsuboi M., Owusu C., Kotrschal A., Buechel S.D., Zidar J., Cornwallis C.K., Løvlie H., Kolm N. Evolutionary associations between host traits and parasite load: insights from Lake Tanganyika cichlids. J. Evol. Biol. 2017;30:1056–1067. doi: 10.1111/jeb.13053. [DOI] [PubMed] [Google Scholar]
- Jensen P.M., Madsen P., Stoumann Jensen L., Pipper C.B. Differences in carbon and nitrogen stable isotope signatures amongst wild and released pheasant populations. Eur. J. Wildl. Res. 2012:755–760. doi: 10.1007/s10344-012-0609-3. [DOI] [Google Scholar]
- Johnson M.W., Hesslein R.H., Dick T.A. Host length, age, diet, parasites and stable isotopes as predictors of yellow perch (Perca flavescens Mitchill), trophic status in nutrient poor Canadian Shield lakes. Environ. Biol. Fishes. 2004;71:379–388. doi: 10.1007/s10641-004-4189-2. [DOI] [Google Scholar]
- Krasnov B.R., Poulin R., Morand S. Patterns of macroparasite diversity in small mammals. In: Morand S., Krasnov B.R., R P., editors. Micromammals and Macroparasites, from Evolutionary Ecology to Managment. Springer; USA: 2006. pp. 197–232. [Google Scholar]
- Locke S.A., Bulté G., Forbes M.R., Marcogliese D.J. Estimating diet in individual pumpkinseed sunfish Lepomis gibbosus using stomach contents, stable isotopes and parasites. J. Fish Biol. 2013;82:522–537. doi: 10.1111/j.1095-8649.2012.03497.x. [DOI] [PubMed] [Google Scholar]
- Nachev M., Jochmann M.A., Walter F., Wolbert J.B., Schulte S.M., Schmidt T.C., Sures B. Understanding trophic interactions in host-parasite associations using stable isotopes of carbon and nitrogen. Parasites Vectors. 2017;10(90) doi: 10.1186/s13071-017-2030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve L. De, Soler J.J., Ruiz-Rodríguez M., Martín-Gálvez D., Pérez-Contreras T., Soler M. Habitat-specific effects of a food supplementation experiment on immunocompetence in Eurasian Magpie Pica pica nestlings. Ibis. 2007;149:763–773. doi: 10.1111/j.1474-919X.2007.00708.x. [DOI] [Google Scholar]
- O'Leary M.H. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. doi: 10.2307/1310735. [DOI] [Google Scholar]
- Panarello H., Fernández J. Stable carbon isotope measurements on hair from wild animals from altiplanic environments of Jujuy, Argentina. Radiocarbon. 2002;44:709–716. [Google Scholar]
- Petzke K.J., Boeing H., Klaus S., Metges C.C. Carbon and Nitrogen stable isotopic composition of hair Protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. Nutr. Methodol. 2005:1515–1520. doi: 10.1093/jn/135.6.1515. [DOI] [PubMed] [Google Scholar]
- Reiczigel J., Rozsa L., Reiczigel A., Fabian I. 2013. Quantitative Parasitology (QPweb)http://www2.univet.hu/qpweb [Google Scholar]
- Reid R.E.B., Koch P.L. Isotopic ecology of coyotes from scat and road kill carcasses: a complementary approach to feeding experiments. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0174897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.A., Forbes M.R., Herbert C.E. Parasitism, mercury contamination and stable isotopes in fish-eating double crested cormorants: no support for the co-ingestion hypothesis. Can. J. Zool. 2009;87:740–747. doi: 10.1139/Z09-062. [DOI] [Google Scholar]
- Roth J.D., Hobson K.A. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: implications for dietary reconstruction. Can. J. Zool. 2000;78:848–852. doi: 10.1139/z00-008. [DOI] [Google Scholar]
- Rózsa L., Reiczigel J., Majoros G. Quantifying parasites in samples of hosts. J. Parasitol. 2000;86:228–232. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Russo G., Danieli P.P., Primi R., Amici A., Lauteri M. Stable isotopes in tissues discriminate the diet of free-living wild boar from different areas of central Italy. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0183333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett L.C. Does the host matter? Variable influence of host traits on parasitism rates. Int. J. Parasitol. 2018;48:27–39. doi: 10.1016/j.ijpara.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Sadowska E.T., Baliga-Klimczyk K., Chrzaścik K.M., Koteja P. Laboratory model of adaptive radiation: a selection experiment in the bank vole. Physiol. Biochem. Zool. 2008;81:627–640. doi: 10.1086/590164. [DOI] [PubMed] [Google Scholar]
- Schulte-Hostedde A., Elsasser S.C. Spleen mass, body condition, and parasite load in male American mink (Neovison vison) J. Mammal. 2011;92:221–226. [Google Scholar]
- Sinisalo T., Valtonen E.T., Helle E., Jones R.I. Combining stable isotope and intestinal parasite information to evaluate dietary differences between individual ringed seals (Phoca hispida botnica) Can. J. Zool. 2006;84:823–831. doi: 10.1139/z06-067. [DOI] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134(911) doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- Vaughan T.A., Ryan J.M., Czaplewski N.J. Jones and Berlett Publishers; USA: 2011. Mammalogy. [Google Scholar]
- Vega C., Lebreton B., Lehnert K., Asmus R., Siebert U., Asmus H. Stable isotope composition and parasitic infections of harbor seal young-of-the-year used as prey-based diet indicators. Mar. Mamm. Sci. 2018;34:7–26. doi: 10.1111/mms.12433. [DOI] [Google Scholar]
- Watts C.H.S. The foods eaten by wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) in wytham woods, berkshire. J. Anim. Ecol. 1968;37(25) doi: 10.2307/2709. [DOI] [Google Scholar]
- Wilson D.E., Reeder D.A. third ed. John Hopkins University Press; USA: 2005. Mammal Species of the World, a Taxonomic and Geographic Reference. [Google Scholar]
- Zhang L.-B.L., Parsons S., Daszak P., Wei L., Zhu G.-J., Zhang S.-Y. Variation in the abundance of ectoparasitic mites of flat-headed bats. J. Mammal. 2010;91:136–143. doi: 10.1644/08-MAMM-A-306R2.1. [DOI] [Google Scholar]