Abstract
Nonalcoholic steatohepatitis (NASH) is currently the third most common cause of end stage liver disease necessitating transplantation. The question remains how inflammation and NASH develop in the setting of nonalcoholic fatty liver disease (NAFLD) and steatosis. Understand the roles of toll-like receptor 4 (TLR4) and dietary fats in the development of hepatic inflammation. Wild-type and TLR4 KO mice were fed a standard high fat diet (LD), a high saturated fat diet (MD), or an isocaloric control diet (CD). Sera and tissue were analyzed for development of hepatic steatosis, inflammation, and injury. MD induced features of hepatic steatosis and inflammation in wild-type, but not in TLR4 KO, mice. TLR4 KO prevented MD induced increases in NAFLD activity scores, serum alanine aminotransferase levels, and inflammatory cytokine expression. Inflammatory cell infiltration and cytokine expression were also lower in the TLR4 KO mice livers than wild-type mice fed MD. Hepatic expression of Collagen I transcripts and collagen deposition were also decreased in the TLR4 KO MD animals. Results show that TLR4 plays a critical role in the effects of dietary fat composition on the development of hepatic steatosis, inflammation, and injury consistent with nonalcoholic steatohepatitis.
Keywords: NASH, STEATOSIS, DIET, TLR4, LIVER
The prevalence of Non-alcoholic fatty liver disease (NAFLD) within the general population is currently 30% in the US, and 20% globally [Vernon et al., 2011]. Often indolent, active NAFLD may progress to the inflammatory non-alcoholic steatohepatitis (NASH) in about 10–20% of people [Schattenberg and Schuppan, 2011]. Chronic inflammation and healing during NASH can result in fibrosis and eventual cirrhosis [Harrison et al., 2003]. NASH is now thought to underlie 80% of “cryptogenic” cirrhosis [Cuadrado et al., 2005]. According to the “two hit” theory of NASH development [Weinstein et al., 2011], the first “hit” is the development of steatosis and the second “hit” has been speculated to involve activation of the innate immune system by inflammatory agents such as bacterial products translocated across the gut wall, endogenously derived toll-like receptor (TLR), and inflammasome ligands, reactive oxygen species, and/or lipotoxicity. In livers sensitized by steatosis, these factors may elicit excessive proinflammatory cytokine production, inflammatory cell infiltration, and hepatocellular injury. However, the mechanisms underlying NAFLD progression, or why some patients develop stable NAFLD uncomplicated by inflammation while others go on to NASH, are unknown.
No current animal model robustly mimics human NAFLD and NASH [Diehl, 2005]. Widely used models of NASH involve either genetic mutations or the use of diets deficient in essential nutrients such as methionine and choline (MCD diet) or with multiple additives such as trans-fats and cholesterol. Caveats to these models include derangements in liver and serum lipid and cholesterol not characteristic of the conditions seen in human NAFLD and NASH, with a natural history that does not reflect the clinical paradigm [Larter and Yeh, 2008]. For example, the MCD diet is associated with weight loss, whole-body insulin sensitivity, early onset of mitochondrial and oxidative stress, and fibrosis without waning steatosis early in the disease course.
It is not fully known how TLR4 and dietary fat composition are associated in the development of steatohepatitis. Although toll like receptor 4 (TLR4) has been implicated in the above models, it has not been studied in a model of hepatic inflammation mediated by saturated fat [Rivera et al., 2007]. Furthermore, the complex metabolic derangements present in previously used models confound interpretation of results. Recent data suggested that high saturated fat feeding promoted NASH symptoms in mice, which were also obese and insulin resistant [Geng et al., 2013]. This high saturated fat feeding also recapitulated hepatic inflammation in an appropriate etiological context and thus may serve as a model for studying NASH (Manuscript under review).
Increases in dietary fat result in the accumulation of triacylglycerols (TAG) within hepatocytes [Westerbacka et al., 2005]. However, it is unlikely that high cellular TAG alone, in the absence of aberrant lipid composition or metabolism, promotes NASH, as the inhibition of TAG synthesis at the level of diacylglycerol acyltransferase-2 results in decreased steatosis, but increased lobular inflammation and fibrosis in a MCD model [Yamaguchi et al., 2007]. Medium to long chain SFA such as myristate and palmitate, respectively, have been shown to activate TLR4 [Huang et al., 2012]. TLRs recognize conserved molecular motifs of damage and pathogen associated molecular patterns, initiating signaling involved in cytokine expression, immune cell activation, and cell death/survival [Boros and Bromberg, 2006]. TLR4 activation leads to signaling through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interferon regulatory transcription factor 3 (IRF3) and the subsequent production of proinflammatory cytokines such as TNF-α [Yang et al., 1997].
The prototypical ligand of TLR4 is the gram-negative bacterial cell wall component lipopolysaccharide (LPS). The lipid component of LPS, lipid A, is primarily responsible for the immunopathogenesis of LPS via its activation of TLR4. Lipid A contains long- and very long-chain fatty acyl moieties which interact with the hydrophobic ligand binding pocket of TLR4, and recent studies have demonstrated that free SFA, but not unsaturated fatty acids (USFA), can also induce TLR4 activation and the production of inflammatory mediators [Lee et al., 2003]. Moreover, TLR4 activation by SFA has been proposed as a mechanism for the development of insulin resistance and obesity [Holland et al., 2011]. As NASH occurs in this metabolic context, we hypothesized that TLR4 may mediate saturated fat diet-induced hepatic inflammation and hepatocellular injury.
METHODS
ANIMALS
Six-week-old male wild-type C57BL/10J (control) and TLR4 knockout C57BL/10ScNJ (TLR4 KO) mice were purchased from Jackson Laboratories. After 1 week equilibration on normal chow, mice were placed on either a lard-based or milkfat-based high fat diet containing 60% kCal from fat (TD.06414, or TD.09766, respectively) or an isocaloric control diet (TD.08810, 10% kCal from fat), all from Harlan Laboratories. The fatty acid compositions of the diets have been published by our collaborators and co-authors: Geng et al. [2013]. Special diet feeding was continued for 16 weeks at which time mice were sacrificed and tissue and sera collected. At least five animals were assigned for each diet feedings. This study was reviewed and approved by the Medical University of South Carolina’s IACUC (AR# 3003: The Effects of Steatosis on Ischemia/Reperfusion and Liver Regeneration in Mice).
PATHOLOGICAL ANALYSIS OF NAFLD ACTIVITY, OIL RED-O (ORO) STAINING, SERUM TRANSAMINASE MEASUREMENT, AND SERUM ENDOTOXIN
Formalin fixed, paraffin embedded (FFPE) sections were stained with Hematoxylin and Eosin (H&E) and slides graded by an experienced liver pathologist according to the semi-quantitative schema outlined by Kleiner et al. [2005]. Oil Red-O, transaminase, and serum endotoxin measurements were done according to standard protocols. At least five animals per group are for used for analysis.
CYTOCHEMICAL STAINING AND IMMUNOHISTOCHEMISTRY
Neutrophils were identified by Leder stain (specific esterase or naphthol AS-D chloroacetate esterase, Sigma–Aldrich). F4/80 staining was performed by antigen retrieval with proteinase K followed by incubation with primary antibody (rat anti-F4/80, clone CI:A3-1, Abcam) and immunostaining with Vectastain ABC kit (Vector Laboratories) according to manufacturer’s protocol. Positive cells were counted as a ratio of total cells in 10 high-powered fields (HPF) per section. At least five animals per group are used for analysis.
COLLAGEN STAINING
FFPE sections were deparaffinized and rehydrated, followed by incubation for 30 min in a solution containing 0.1% Sirius red (Direct red 80, Sigma) and saturated picric acid (picric acid 6.0 g, 200 ml H2O). Sections were then washed in 0.003 N HCl and cover slipped with Cytoseal 60. Stained slides were analyzed imaged under polarized light with a Olympus Bx50WI scope. At least five animals per group are used for analysis.
QUANTITATIVE REAL TIME RT-PCR
Gene expression was quantified by TaqMan Fast Virus 1-step Master Mix Cat # 4444434 (Applied Biosystems) and TaqMan gene expression assays (Applied Biosystems) according to manufactures protocol. A total of 200 ng of RNA was used. Quantification of a given gene, expressed as relative mRNA level, was calculated after normalization to the housekeeping gene HPRT1 and calculated relative to baseline control using the comparative ΔΔCt method. HPRT1 was chosen based on its stable expression relative to other potential controls such as GAPDH. At least five animals per group are used for analysis.
STATISTICAL ANALYSIS
All values here are expressed as mean ± standard error of the mean. Statistical significance was chosen a priori as α ≤ 0.05. For single, pairwise, comparisons of normally distributed data sets, a Student’s t-test was used. For multiple comparisons of means, a one-way analysis of variance with Tukey–Kramer post hoc tests was used. For non-normally distributed data, complimentary non-parametric methods were applied: Mann–Whitney U test and Kruskal–Wallis one-way analysis of variance with Dunn’s post hoc test. Hypothesis testing was performed using GraphPad PRISM version 5 for Windows (GraphPad Software).
RESULTS
DIETARY FATTY ACID COMPOSITION DETERMINES THE DEVELOPMENT OF HEPATIC STEATOSIS, INFLAMMATION, AND HEPATOCELLULAR INJURY THROUGH TLR4
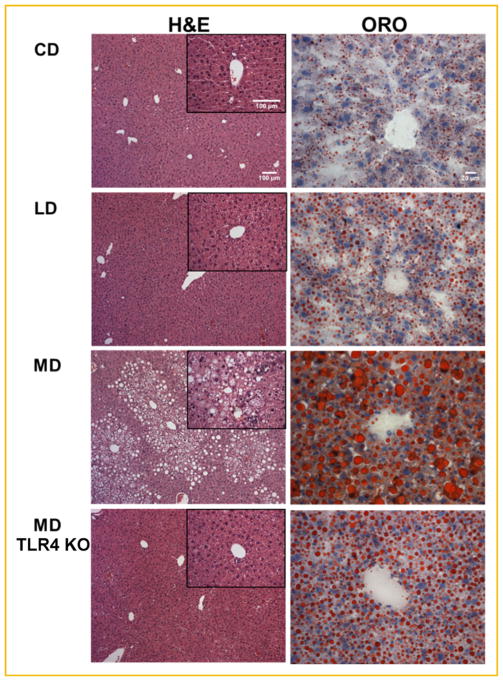
The use of a milk-fat based, high fat, obesogenic, and diabetogenic diet was recently reported by Geng et al. [2013]. This diet is enriched in saturated fatty acyls, in particular myristate (C14:0) and palmitate (C16:0), and was demonstrated to elevate plasma levels of these species relative to a standard lard-based high fat diet [Geng et al., 2013]. To ascertain if the type of fat consumed determined hepatic steatosis and inflammation through TLR4, we evaluated wild-type and TLR4 knockout (TLR4 KO) mice after feeding with either a low fat control diet (CD), a high-unsaturated lard fat based diet (LD), or a high saturated milk fat based diet (MD). Only the MD diet promoted significant hepatic steatosis and inflammation in terms of relevant histopathologic features, such as hepatocellular ballooning, necroinflammatory lesions, and macrosteatosis, consistent with NASH (Fig. 1) [Yeh and Brunt, 2007]. Little inflammatory involvement was noted in mice fed LD or CD. ORO staining showed greater overall steatosis and significant macrosteatosis in MD fed animals, but not the LD or CD. TLR4 KO animals fed the MD displayed attenuation of these histologic NASH characteristics as compared to control MD animals, including less severe hepatocellular ballooning, lobular inflammation, and macrosteatosis.
Fig. 1.
TLR4 KO ameliorates histologic features of NASH. Representative images of hematoxylin and eosin (H&E, left) and Oil red O (ORO, right) stained liver sections wild-type, and TLR4 KO mice fed isocaloric control (CD) lard fat (LD), or milk fat diet (MD) for 16 weeks.
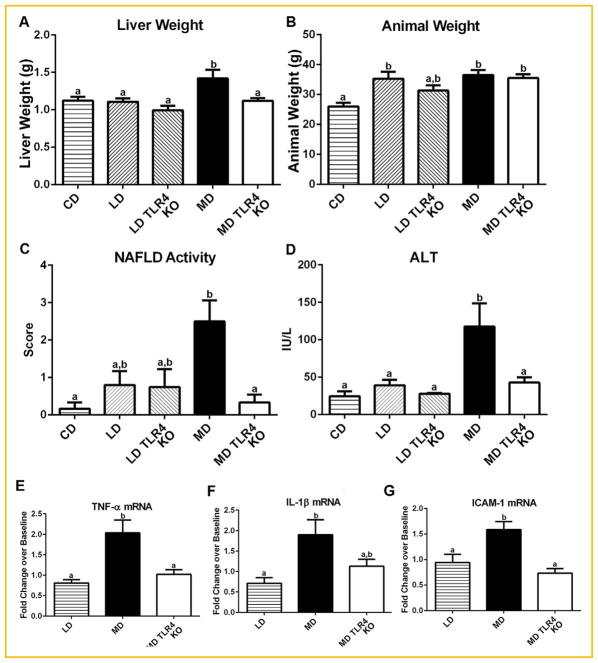
MD was uniquely associated with elevated liver weight, NAFLD scores and serum ALT, while both high fat diets increased body weight compared to the isocaloric CD (Fig. 2A–D). These data are consistent with previous studies in C57BL/6J strain, and confirm more generally that a diet high in saturated fats (MD) promotes the development of liver pathology histologically consistent with hepatic steatosis and inflammation in vivo in mice. No significant body weight change between the two high fat diets suggests that this is a hepatic specific phenomenon and not merely a consequence of variation in total body mass [Geng et al., 2013]. TLR4 KO MD fed animals showed normalized NAFLD scores, ALT, and liver weight, but not animal weight (Fig. 2A–D). Together, these data indicate that dietary fatty acid composition determines the development of hepatic steatosis, inflammation, and hepatocellular injury through a TLR4 dependent mechanism, and that a diet high in saturated fats (MD) causes TLR4 dependent effects resembling NASH.
Fig. 2.
TLR4 knockout protects MD livers from NASH and changes in inflammatory gene expression. (A) The mass of TLR4 knockout MD fed mice livers (MD TLR4 KO) was significantly less than wild-type MD (MD). (B) Total animal mass was not significantly changed between the high fat diets. (C) MD TLR4 KO liver sections had significantly lower NAFLD scores than MD. (D) MD TLR4 KO had decreased hepatic injury compared to MD as indicated by lower serum ALT. (E) Levels of TNF-α mRNA were decreased in MD TLR4 KO animals as compared to MD. (F) Levels of IL-1β showed a trend toward decrease in MD TLR4 KO as compared to MD. (G) ICAM-1 was significantly down regulated in MD TLR4 KO animals as compared to MD. Data are presented as fold changes over level of CD (Baseline). Means with different lettered subscripts within each group are significantly different from each other, P <0.05. a is statistically different from b, and a,b is not statistically different from a or b. Data are expressed as mean ± SEM; n = 5–6.
DIETARY SATURATED FAT INDUCES INFLAMMATORY CYTOKINE GENE EXPRESSION AND CELL INFILTRATION THROUGH TLR4
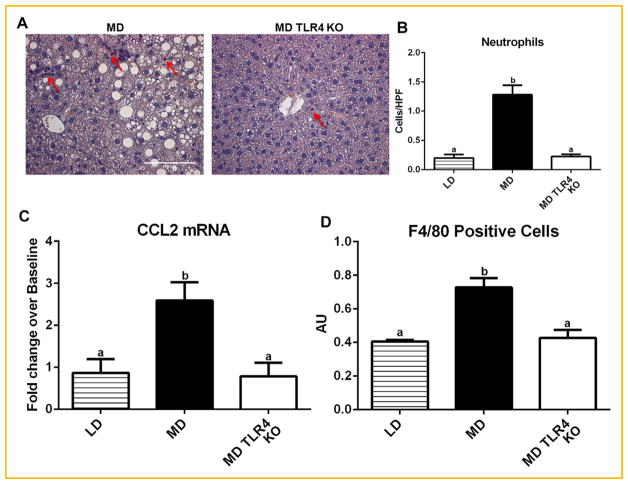
To assess the effect of TLR4 on the inflammatory milieu in the liver after MD feeding, we evaluated the expression of several genes known to be important in the development of NASH [Staels et al., 2013]. Relative to the LD, the MD increased the expression of the pro-inflammatory cytokines TNF-α, IL-1β, and the adhesion molecule ICAM-1 (Fig. 2E–G). In contrast, TLR4 KO mice fed the MD displayed no elevation in expression of either TNF-α or ICAM-1. This suggests that MD feeding leads to increased inflammatory gene expression through TLR4. Specific esterase staining revealed neutrophils prevalent throughout the parenchyma of the MD-fed mice as pink/purple stained cells (Fig. 3A), significantly reduced in MD TLR4 KO animals (Fig. 3A,B). Increased numbers of inflammatory macrophages and monocytes are noted in multiple models of NASH [Stanton et al., 2011]. We showed that increased expression of the chemokine CCL2, which is thought to be partially responsible for recruitment of these cells to the fatty liver, was induced by MD feeding in a TLR4 dependent fashion (Fig. 3C). Increased expression of CCL2 was inferred from the elevated transcript levels. Consistent with this, MD TLR4 KO animals had fewer relative numbers of F4/80+ cells (monocytes/macrophages) in the liver as compared to their wild-type controls (MD) (Fig. 3D). These data indicate a high saturated fat diet (MD) up regulates CCL2 expression in the liver through TLR4 and suggest that this may underlie the recruitment of monocytes/macrophages.
Fig. 3.
Neutrophil infiltration, CCL2 expression, and monocyte/macrophage numbers. (A) Representative micrographs of specific esterase (Leder) stained liver sections showing neutrophil accumulation (arrows) in MD and MD TLR4 KO livers (B) Quantification of hepatic neutrophil infiltration showing increased neutrophil infiltrate in livers of MD but not MD TLR4 KO mice. (C) TLR4 knockout abolished the increase in mRNA expression of chemokine CCL2 induced by MD. Data are presented as fold changes over level of CD (Baseline). (D) Quantification of hepatic F4/80+ cells showed that TLR4 KO prevented an increase in monocyte/macrophage infiltration in MD livers. Means with different lettered subscripts within each group are significantly different from each other, P <0.05. Values are mean number of cells per HPF ± SEM, n = 5–6.
DIETARY SATURATED FAT FEEDING PROMOTES FIBROTIC CHANGE IN THE LIVER THROUGH TLR4
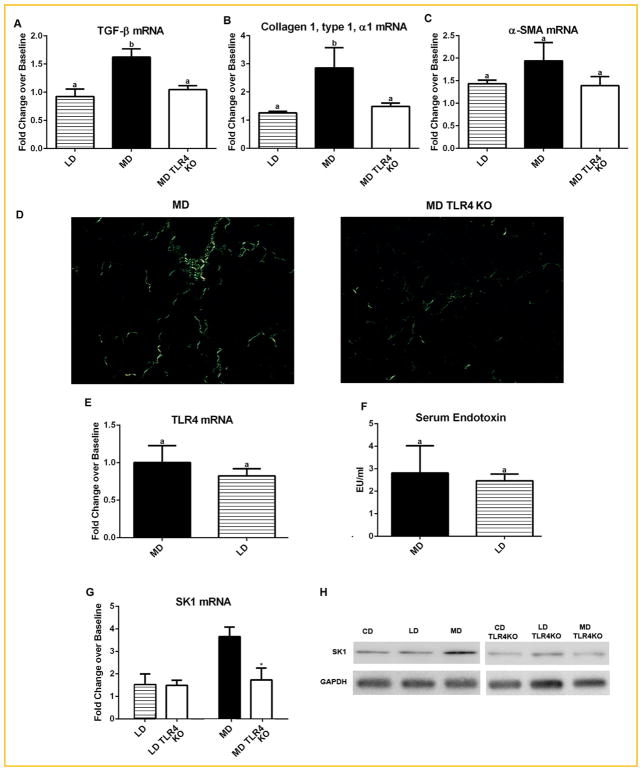
To assess the expression of genes involved in the development of fibrosis, we analyzed mRNA from wild-type and TLR4 KO mice fed the different diets. Elevated expression of the profibrotic cytokine TGF-β in the livers of MD fed animals was significantly reduced in MD TLR4 KO livers (Fig. 4A). Altered expression of TGF-β was inferred from the altered transcript levels. This was accompanied by similar changes in expression of collagen I (Col1a1) (Fig. 4B). MD TLR4 KO livers also displayed a trend toward elevation of α-SMA (Acta2) expression, a marker of stellate cell activation to myofibroblasts (Fig. 4C). Micrographs of sections stained with picrosirius red and acquired with a polarized light microscope revealed scattered collagen deposition throughout the parenchyma in a perisinusoidal pattern, typical of early stage NASH, in WT MD fed mice [Brunt et al., 2004] (Fig. 4D). In contrast MD TLR4 KO mice did not show obvious deposition of collagen fibrils outside the vascular wall (Fig. 4D). These data indicate that dietary fatty acid composition may determine fibrotic change in the steatotic liver through TLR4.
Fig. 4.
Effects of TLR4 KO on fibrotic change in the liver, endotoxin levels, and SK1. (A) MD induced expression of TGF-βwas not present MD TLR4 KO mice. (B) Expression of collagen I (Col1a1), and (C) α-smooth muscle actin (Acta2, α-SMA). MD TLR4 KO mice did not show increased expression of collagen as seen in MD. A similar trend was present for α-SMA, though not significantly. Data are presented as fold changes over level of CD (Baseline). (D) Representative micrographs of liver sections stained with picrosirius red and acquired under plane polarized light. (E) Hepatic TLR4 mRNA expression relative to CD mice. No significant difference was noted in TLR4 expression between MD and LD. (F) Serum endotoxin (LPS) levels as measured by limulus amebocyte lysate (LAL) assay. Data are presented as fold changes over level of CD (Baseline). No difference was noted between the means of both diets. (G) Hepatic expression sphingosine kinase 1 (SK1) in MD and LD fed animals. TLR4 KO (TLR4) animals showed a significant decrease in expression of SK1 as compared to wild-type (Wt) MD animals. TLR4 KO did not affect SK1 expression in LD mice. (H) Protein levels of SK1 measured by Western blot analysis showing increased SK1 mRNA corresponds to increased protein levels. Data are presented as fold changes over level of CD (Baseline). Means with different lettered subscripts within each group are significantly different from each other, P <0.05. *P <0.05 versus Wt. Data are expressed as mean ± SEM; n = 5–6.
DIETARY SATURATED FAT FEEDING DID NOT INCREASE TLR4 EXPRESSION OR AFFECT SYSTEMIC ENDOTOXIN LEVELS
Several studies have shown that TLR4 expression is elevated in NASH and that steatotic livers are more sensitive to endotoxin and other TLR4 ligands [Ye et al., 2012]. There were no significant differences in hepatic TLR4 mRNA expression between any of the diets as assessed by qRT-PCR. MD and LD fed mice are presented as fold changes over level of CD (Baseline) in Figure 4E, suggesting that, rather than changes in TLR4 expression, direct activation of TLR4 may be responsible for MD-induced NASH. Other studies have shown evidence of increased gut permeability and subsequent endotoxemia in steatohepatitis [Henao-Mejia et al., 2012]. Data demonstrated similar systemic endotoxin levels for all diets. MD and LD fed mice are presented as fold changes over level of CD (Baseline) in Figure 4F.
TLR4 KO PREVENTS DIETARY SATURATED FAT INDUCED HEPATIC OVEREXPRESSION OF SK1
Saturated fats (i.e., palmitate) have been shown to increase sphingosine kinase 1 (SK1) expression [Brice and Cowart, 2011]. Our recent investigations showed that SK1, which generates the proinflammatory lipid sphingosine-1-phosphate (S1P), plays a crucial role in the development of hepatic steatosis and inflammation (Manuscript under review). It was found that SFA promote SK1 expression and production of the proinflammatory lipid sphingosine 1 phosphate (S1P) within the liver. S1P signals through an autocrine/paracrine mechanism involving S1P-receptor 1 to induce NF-kB activation and TNF-α and CCL2 expression. Activation of TLR4 has been shown to increase SK1 gene expression and activity [Pchejetski et al., 2011]. To assess the effects of TLR4 KO on hepatic expression of SK1, we measured hepatic SK1 transcript by qRT-PCR. TLR4 KO had no effect on hepatic SK1 expression in LD fed animals. In contrast, SK1 expression in MD TLR4 KO livers was significantly attenuated as compared to MD fed wild-type animals (Fig. 4G mRNA, 4 H Protein levels).
DISCUSSION
Our data indicate a diet enriched in saturated fats (MD) promotes hepatic steatosis, inflammation, and injury through TLR4. Saturated fatty acids are known to promote more severe metabolic perturbation than unsaturated fatty acids. Observations that the saturated fat-rich diet promoted a metabolic context mirroring that in humans with metabolic syndrome suggest not only an etiologically relevant model, but also more importantly that saturated fats may play a key role in promoting NAFLD progression. We demonstrate that TLR4 is required for the inflammatory gene expression and cell infiltrate induced by a shift in dietary fat composition toward a more predominately saturated fat profile. This is the first study to demonstrate a role for TLR4 in mediating hepatic steatosis and inflammation representative of NASH induced by dietary fatty acid modification alone, in particular increased dietary saturated fat. Furthermore, we demonstrated that a diet rich in unsaturated fat does not result in hepatic inflammation. These experiments are crucial not only to the molecular mechanisms of hepatic steatosis and inflammation development, but also to the type of dietary fatty acid used in current and future NAFLD models.
TLR4 dependent TNF-α and IL-1β expression may play a central role in the development and progression of NAFLD to NASH through monocyte/macrophage recruitment and activation in the development of fibrosis, and by up regulating expression of adhesion molecules, in particular intercellular adhesion molecule-1 (ICAM-1), on the overlying liver sinusoidal endothelial cells (LSEC) allowing transmigration of inflammatory cells into the parenchymal space [Tomita et al., 2006]. The chemokine CCL2 is responsible for the recruitment of monocytes and macrophages, among other cell types, to sites of inflammation. High fat diet models of NAFLD have shown that hepatic CCL2 expression results in the recruitment of F4/80+ cell populations distinct from the resident macrophages, or KC [Obstfeld et al., 2010]. These bone marrow derived monocytes then in turn affect steatohepatitis [Yang et al., 2009]. However, it has also been suggested that CCL2 can also directly stimulate hepatocyte TAG accumulation [Clement et al., 2008].
TLR4 is required for efficient HSC collagen I expression and activation, with subsequent fibrosis, in bile duct ligation, and LPS stimulation models of liver fibrosis [Seki et al., 2007]. We show here that TLR4 KO prevents the increases in hepatic TGF-β and collagen I expression that occur after MD feeding. TGF-β is well established as promoting hepatic fibrosis, and NASH is not an exception to this paradigm [Gressner and Weiskirchen, 2006]. These data suggest that high saturated fat feeding induces early fibrosis within the liver in a TLR4 dependent fashion.
TLR4 mRNA expression was not elevated in the livers of mice due to differential feeding. This implicates changes in TLR4 ligand prevalence or downstream signaling as responsible for the TLR4 dependent steatohepatitis induced by high saturated fat feeding. We did not find a significant difference between any diets in systemic levels of endotoxin (LPS) by limulus amebocyte lysate assay. Although, we cannot rule out the possibility that daily area-under-the-curve endotoxin levels are changed, as endotoxin levels have been shown to rise and fall throughout the day and in conjunction with feeding, these data suggest that systemic endotoxemia may not play a significant role in the differential effects of dietary fatty acid composition on TLR4 dependent hepatic steatosis and inflammation development [Cani et al., 2007]. A role for SFA mediated TLR4 activation has now been described in both in vitro and in vivo systems. The MD was previously shown to increase serum levels of the SFA TLR4 agonist palmitate [Geng et al., 2013]. Thus, TLR4 KO may protect mice from inflammatory activation by SFA. These data corroborate a recent study of NASH patients, which found elevated serum palmitate levels, but not endotoxin [Bertola et al., 2010]. We cannot rule out elevated portal endotoxin though, which may be higher in mice fed the MD diet, but rapidly sequestered within the liver and reticuloendothelial system. Because bacterial products translocated from the gut will first travel through the portal vein and then enter the liver prior to joining the systemic circulation, this provides an alternate explanation for our results. Future studies will evaluate portal LPS levels in relation to hepatic steatosis and inflammation.
S1P, through its receptors S1P1, 2, or 3 has been shown to be involved in the recruitment of monocytes and macrophages to sites of injury, while enhancing the effector functions of these cells [Keul et al., 2011]. SK1 derived S1P may also participate with TNF-α in increasing the expression of monocyte and endothelial cell adhesion molecules, including ICAM-1 [Fernandez-Pisonero et al., 2012]. S1P produced by SK1 also participates in the direct regulation of endothelial cell adhesion molecule expression and subsequent egress of inflammatory cells, including neutrophils [Fernandez-Pisonero et al., 2012]. Recent studies demonstrated upregulation of SK1 by saturated fatty acids [Geng et al., 2013]. Moreover, TLR4 has been demonstrated to regulate the expression of SK1 [Pchejetski et al., 2011]. While either or both of these mechanisms may be active in our system, overall, relationships between saturated fatty acids, TLR4, and SK1 may constitute a novel mechanism of hepatic steatosis and inflammation induction that may be activated specifically in the context of high dietary consumption of saturated, but not unsaturated, fats.
Footnotes
Conflicts of interest: Authors declare no conflict of interest in relation to this article.
References
- Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y, Tran A, Gual P. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS ONE. 2010;5:e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- Brice SE, Cowart LA. Sphingolipid metabolism and analysis in metabolic disease. Adv Exp Med Biol. 2011;721:1–17. doi: 10.1007/978-1-4614-0650-1_1. [DOI] [PubMed] [Google Scholar]
- Brunt EM, Neuschwander-Tetri BA, Oliver D, Wehmeier KR, Bacon BR. Nonalcoholic steatohepatitis: Histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–1082. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, Meier CA, Negro F. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology. 2008;48:799–807. doi: 10.1002/hep.22404. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Orive A, Garcia-Suarez C, Dominguez A, Fernandez-Escalante JC, Crespo J, Pons-Romero F. Non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma. Obes Surg. 2005;15:442–446. doi: 10.1381/0960892053576596. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138–144. doi: 10.1016/j.hepres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pisonero I, Duenas AI, Barreiro O, Montero O, Sanchez-Madrid F, Garcia-Rodriguez C. Lipopolysaccharide and sphingosine-1-phosphate cooperate to induce inflammatory molecules and leukocyte adhesion in endothelial cells. J Immunol. 2012;189:5402–5410. doi: 10.4049/jimmunol.1201309. [DOI] [PubMed] [Google Scholar]
- Geng T, Hu W, Broadwater MH, Snider JM, Bielawski J, Russo SB, Schwacke JH, Ross J, Cowart LA. Fatty acids differentially regulate insulin resistance through endoplasm reticulum stress-mediated induction of tribbles homologue 3: A potential link between dietary fat composition and the pathophysiological outcomes of obesity. Diabetologia. 2013;56(9):2078–2087. doi: 10.1007/s00125-013-2973-2. [DOI] [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: A clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keul P, Lucke S, von Wnuck Lipinski K, Graler C, Heusch M, Levkau G. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM. Animal models of NASH: Getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski D, Nunes J, Coughlan K, Lall H, Pitson SM, Waxman J, Sumbayev VV. The involvement of sphingosine kinase 1 in LPS-induced Toll-like receptor 4-mediated accumulation of HIF-1alpha protein, activation of ASK1 and production of the pro-inflammatory cytokine IL-6. Immunol Cell Biol. 2011;89:268–274. doi: 10.1038/icb.2010.91. [DOI] [PubMed] [Google Scholar]
- Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: The therapeutic challenge of a global epidemic. Curr Opin Lipidol. 2011;22:479–488. doi: 10.1097/MOL.0b013e32834c7cfc. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepato-protective effects of the dual PPARalpha/delta agonist GFT505 in rodent models of NAFLD/NASH. Hepatology. 2013;58(6):1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- Stanton MC, Chen SC, Jackson JV, Rojas-Triana A, Kinsley D, Cui L, Fine JS, Greenfeder S, Bober LA, Jenh CH. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J Inflamm (Lond) 2011;8:8. doi: 10.1186/1476-9255-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Weinstein AA, Kallman Price J, Stepanova M, Poms LW, Fang Y, Moon J, Nader F, Younossi ZM. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127–132. doi: 10.1016/j.psym.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Westerbacka J, Lammi K, Hakkinen AM, Rissanen A, Salminen I, Aro A, Yki-Jarvinen H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804–2809. doi: 10.1210/jc.2004-1983. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- Yang SJ, IglayReger HB, Kadouh HC, Bodary PF. Inhibition of the chemokine (C-C motif) ligand 2/chemokine (C-C motif) receptor 2 pathway attenuates hyperglycaemia and inflammation in a mouse model of hepatic steatosis and lipoatrophy. Diabetologia. 2009;52:972–981. doi: 10.1007/s00125-009-1309-8. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058–1067. doi: 10.1136/gutjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128:837–847. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]