Abstract
An agnostic high throughput search of the genome revealed a robust association between LOXL1 genetic polymorphisms and exfoliation syndrome (XFS), a discovery that likely would not have been possible with candidate or family-based gene search strategies. While questions remain regarding how LOXL1 gene variants contribute to XFS pathogenesis, it is clear that the frequencies of disease-related alleles do not track with the varying disease burden throughout the world, prompting a search for environmental risk factors. A geo-medicine approach revealed that disease load seemed to increase as a function of the distance from the equator. The exact reason for this extra-equatorial disease distribution pattern remains unclear, but a greater amount of time spent outdoors is a robust risk factor for XFS, suggesting climatic factors such as ocular solar exposure and colder ambient temperature may be involved in disease pathogenesis. Prospective studies have also implicated higher coffee consumption and lower dietary folate intake in association with incident XFS. The discovery of environmental risk factors for XFS suggests that preventive measures may help to reduce ocular morbidity from XFS.
Keywords: LOXL1, exfoliation syndrome, ocular ultraviolet exposure, coffee consumption, dietary folate intake
Lysyl oxidase (LOX; cytogenetic location: 5q23.1) is an enzyme that oxidizes lysine residues on elastin and collagen in a manner that creates stable cross linkages for these structural proteins.1 LOX shares sequence homology with lysyl oxidase-like 1 (LOXL1; cytogenetic location: 15q24.1); both LOX and LOXL1 have 7 exons, a highly conserved C-terminus and a poorly conserved N-terminus.2,3 Interestingly, sequence homology between LOX and LOXL1 begins at the exon 1/exon 2 boundary,4 while the LOXL1 polymorphisms that are associated with exfoliation syndrome (XFS) and exfoliation glaucoma (XFG) are also located in the exon 1/intron 1 region.5 LOXL1 is part of a 5-member gene family (LOX, LOXL1, 2, 3, and 4) with all members involved in elastogenesis. This gene family is described elsewhere in this supplement. Overall, members of this enzyme family play important roles in the structural integrity of a variety of biological tissues. LOXL1, in particular, is expressed in all ocular tissues except the retina.
Seventy-five Icelandic cases and 14,474 controls were sufficient to discover the genome-wide significant association between LOXL1 gene variants and XFS. These results were replicated in a Swedish sample of 199 XFG cases and 200 controls.5 This result is remarkable for two reasons: a) a relatively small number of cases were needed to discover this association probably because the effect size for the association was quite high (~20 fold); b) roughly 98% of cases had the disease associated allele while greater than 80% of controls had the same allele. Interestingly, the population attributable risk of XFG for the GG and TG haplotypes that includes 2 nonsynonymous single nucleotide polymorphisms (SNPs) in exon 1 (rs1048661 and rs3825942) was >99%.5 These findings would suggest that the pathogenesis for XFS/XFG focused squarely on the genetics of impaired elastogenesis and collagen cross-linking but there were several clues that the exact etiology of XFS was far more complicated than that.
First, the lead disease-associated polymorphism was the common allele that actually represented the ancestral version of the gene in a cross-species analysis.6 Second, while the association between LOXL1 gene variants and XFS was confirmed in Australia, the allele frequency of disease variants in cases and controls was quite similar to that reported in Nordic populations even though the disease burden in Australia was considerably lower.6 Finally there is no single disease-associated LOXL1 allele that is not flipped in either Asian or African ancestral groups.7 A ‘flipped’ allele means that the disease-risk allele varies in different populations and in particular for LOXL1 risk alleles, some are flipped in Asians compared to Caucasians and others are flipped in Africans compared to Caucasians. Thus even if these LOXL1 gene variants have known functional effects (i.e., two are missense alleles), it is difficult to imagine that they can contribute to disease pathogenesis when the change in amino acid is associated with increased risk in some populations and decreased risk in others. In summary, the high frequency of disease associated variants in controls and the absence of a disease-associated variant present in all ethnic groups suggests that other genes and / or environmental factors must contribute to the disease process. This review focuses on the role of environmental risk factors for XFS/XFG.
While the frequency of LOXL1 disease-associated SNPs does not trend with disease prevalence, disease burden does generally trend with geographic latitude, raising the possibility that environmental factors, particularly climatic conditions, could contribute to XFS.8 Clues that environmental factors could contribute to XFS/XFG pre-date the 2007 discovery of LOXL1 - disease association. For example, in 1980 Hugh Taylor suggested that XFS might have environmental risk factors when he found a particularly high rate of XFS in stockmen who spent time outdoors tending to cattle in Australia.9 Furthermore, in 1988 Ringvold and colleagues, while trying to establish evidence for a genetic basis of XFS, found a surprisingly high concordance rate among married couples and discordance for XFS among two monozygotic twin pairs.10 In 2005, a cross-sectional study from Andra Pradesh, India, found that people engaged in outdoor occupations had a 2.14-fold increased risk of XFS (95% confidence interval (CI): 1.10–4.16).11 In 2014, a prospective analysis that postdates the discovery of LOXL1 in association with XFS, Kang et al. surveyed a population of United States participants regarding time spent outdoors at various age periods: high school to age 24, ages 25–35 and ages 36– 59.12 In multivariable analysis, compared to participants who spent ≤5 hours/week outdoors during midday, those spending ≥11 hours/week outdoors from high school to age 24 had a 2-fold increased risk of XFG/XFG suspect (95% confidence interval: 1.30–3.08; p for linear trend=0.001). Results were not significant for other age periods, suggesting that climatic exposure during young adulthood could be important in the development of XFS/XFG. In two case control datasets from Boston, MA, USA and Tel Aviv, Israel, every hour per week spent outdoors during the summer, averaged from age 10 to age 59 was associated with a 4% increased risk of XFS/XFG.13
Several candidate environmental risk factors for XFS can be entertained, including ocular UV exposure, autoimmunity, slow virus infection, trauma (especially repeated surgical intervention at an early age)14, and various aspects of dietary intake. Since a positive relation between extra-equatorial geographical residential location and XFS/XFG risk was confirmed in several studies,8,13,15 while the ratio of ~95% of XFS/XFG cases to ~80% of controls having missense LOXL1 variants was fairly constant around the world, we pursued specific environmental risk factors that might account for the positive relation between latitude and XFS.
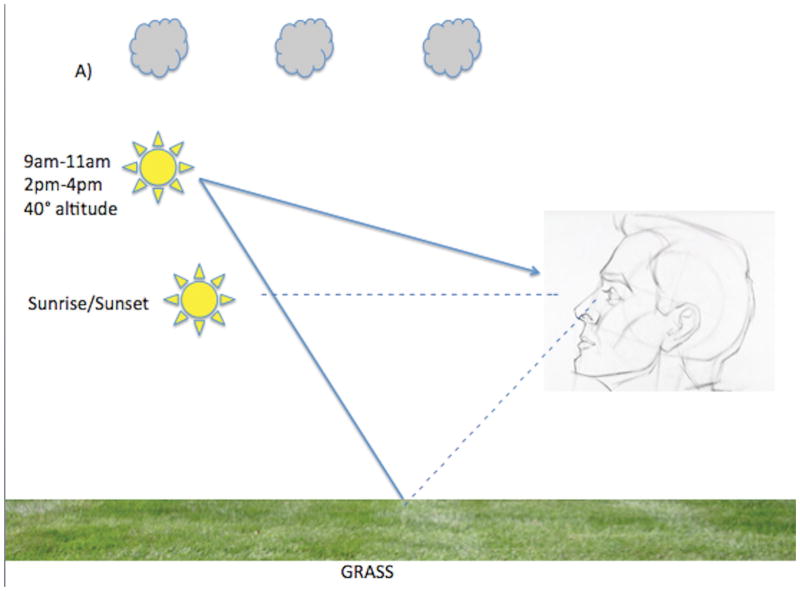
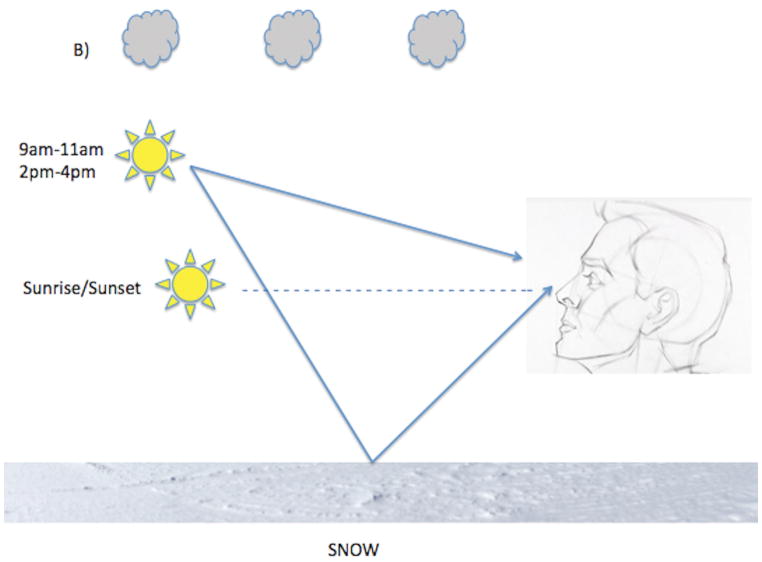
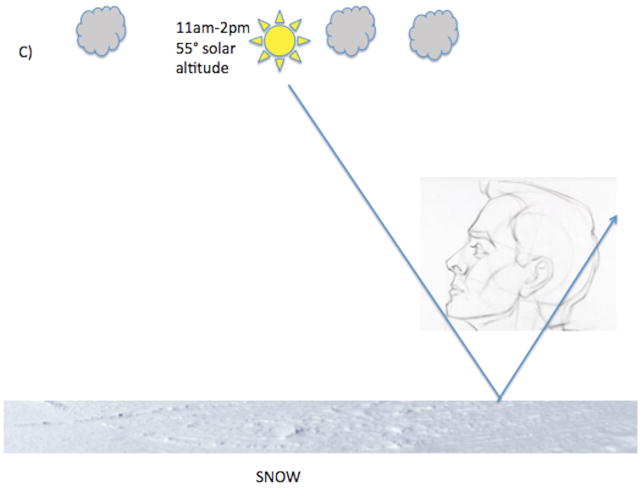
While overall solar exposure to the horizon increases inversely as a function of increasing latitude, the trend for ocular solar exposure is more complex and could be in the opposite direction. One needs to account for ground reflectance, among other factors, when considering ocular UV exposure (Figure). Surfaces where UV reflection is poor include grass and asphalt but surfaces such as snow and water are excellent UV reflectors.16 When solar altitude is low (sunrise or sunset) UV rays can enter the eye directly but UV emission from the sun is low. When solar altitude is intermediate (~40 degrees at 9am–11am and 2pm–4pm) UV radiation is modest but UV rays can enter the eye both directly and indirectly via ground reflection. The degree of indirect UV ocular exposure during these times can be quite high when one is situated in a highly reflective environment. When solar altitude is higher, as is typical closer to the equator and between 12pm–2pm at most other latitudes, even though the UV radiation is strongest, there is actually less direct and reflected ocular UV irradiation.17 These geophysical attributes support ocular UV exposure as a climatic factor contributing to the latitude effect and, more importantly, predict XFS “hotspots” in locations where the opportunities for UV reflection from the ground into the eye are high, regardless of latitude.
Figure.
(A) At 40° latitude North, during sunrise and sunset, direct ocular exposure is low because UV radiation is low (dotted line) and there is no indirect reflected solar exposure. Between 9–11am and between 2–4 pm when solar altitude is moderate, direct ocular exposure is higher and is variably augmented by indirect solar reflection. Indirect solar reflection (dotted line directed from ground to the eye) is low in a grassy environment (A) because grass does not reflect solar rays well but is particularly high (solid arrow from ground directed to the eye) in an environment with snow cover, as snow is an excellent reflector of solar rays (B). (C). At 11am–2pm solar altitude is high and so is UV radiation but both direct and indirect ocular solar exposure is low unless one is purposefully gazing at the sun.
Support for ocular UV exposure from reflected light in relation to XFS comes from several sources. Using a claims database representative of the continental US and state wide climatic data, Stein et al demonstrated that for every additional sunny day per year, there was a 1.5% increased risk of XFS in multivariable analysis.8 In a New England case-control group, a history of work over water or snow was associated with a 3.9 fold increased risk of XFS. On the Island of Rab in the North Adriatic Sea, XFS was found in 21% of agriculturists and fishermen but was absent in urban dwellers, supporting the ocular UV reflection off water as a possible explanation for these results.18 A positive association between climatic keratopathy, a condition linked to ocular UV exposure, and XFS has been noted in several studies.19–21 While Saudi Arabia is fairly close to the equator (24° N), annual precipitation is measured in millimeters, creating a highly reflective sandy environment (especially outside urban centers) that may account for the high 9% prevalence rate in people aged 40 or more.22 It is worth reiterating that the distribution of rs3825942 LOXL1 risk G allele in Saudi Arabia is similar to populations with disparate disease prevalences. In urban Australia, where the disease prevalence is lower, or Iceland, where the disease prevalence is higher than in Saudi Arabia, the percent of cases with the disease associated G allele ranged from 94.8% to 98.4% and the percent of controls with the same allele ranged from 81.7% to 85.6%.5,6,23 There is no metric that measures cumulative ocular UV exposure over long periods; therefore, direct confirmation of the association between ocular UV exposure and XFS will require an appropriate experimental model system.
Another candidate climatic factor for XFS that may account for the positive relation between increasing latitude and XFS is ambient temperature. Low ambient temperature might contribute to lower temperature in the anterior intraocular compartment, facilitating XFM formation in the eye via a ‘protein sink’ type of precipitation reaction as suggested by Lee.24 In general, ocular surface temperature is lower than core temperature and decreases further when ambient temperature is lowered.25 It is likely that the anterior uveal tract contributes to maintaining a temperature closer to core temperature but there is understandably little human data that addresses the question of how anterior chamber temperature varies as a function of ambient temperature. Using a claims database representative of the continental US and mean state wide climatic data, each degree higher mean January low temperature was associated with a 3% decreased risk of XFS in multivariable analysis.8 Clearly, XFS has been documented in equatorial countries such as Sri Lanka (6° N), albeit at a fairly low prevalence rate of ~1%.26 Thus a low ambient temperature is not necessary for the development of XFS, prompting our group to search for other environmental determinants of XFS/XFG.
Coffee consumption, with notable exceptions, has a rough positive correlation with latitude. Interestingly coffee consumption is highest in Scandinavian countries where the disorder is hyperendemic. For example, the per capita coffee consumption in Finland (9.6 kg/capita/year) is nearly triple that of the US (3.2 kg/capita/year).27 The prevalence of XFS in Finland among people ages 60–69 was 14.2% by one report28 and 3.6% among people aged 60 years+ in southeastern US.29 Are these differences in prevalence related to latitude with their attendant geographical influences, coffee consumption, other dietary factors or yet-to-be-determined differences including genetic factors? Coffee consumption increases serum homocysteine levels,30 a biomarker linked to XFS/XFG in several studies.31 In a prospective study of over 120,000 US health professionals, we found that compared to never-consumers, those drinking 3 or more cups of caffeinated coffee per day were at 66% increased risk of XFG (multivariable RR=1.66; 95% CI: 1.09–2.54; p-trend=0.02).32 These data were adjusted for latitude of geographic residence. Interestingly, there are several Northern European countries with very high coffee consumption where the prevalence of XFS has not been reported, like the Netherlands and Austria. Coffee consumption may be part of a complex web of environmental risk factors for XFS.
Increasing intake of vitamin B6, vitamin B12 and folate could lower serum homocysteine, a biomarker linked to XFS in some studies, as previously mentioned. Fresh fruits and vegetables, a major source of dietary folate, are more readily available in southern climes. Interestingly, fruit and vegetable intake was associated with a reduced risk of XFS in the Reykjavik Eye Study.33 In the Nurses’ Health Study and Health Professionals Follow-up Study, we found that >50% of people were consuming less than the recommended daily amount of folate (400μg/d). There was a borderline 25% reduced risk of XFG associated with highest total folate intake (>654 μg/d) vs. the lowest folate intake (217–287 μg/d) (multivariable RR = 0.75 (0.54–1.04); p trend=0.02). Associations between intakes of vitamin B6 and vitamin B12 and risk of XFG were not detected - this is consistent with a meta-analysis, which also did not find that serum B6 and B12 levels were different between XFS/XFG cases and controls.31
XFG arguably represents the glaucoma subtype with the strongest evidence for environmental risk factors. The discovery of the LOXL1 – XFS connection provided the inspiration to examine climatic and other environmental determinants of the disease. Interestingly UV radiation affects LOXL1 expression34 but controlled experimental models are needed to firmly implicate ocular UV exposure in the disease pathogenesis. In an appropriate model setting, alteration of homocysteine levels via dietary folate and caffeine/coffee manipulation may also serve to confirm whether and how these factors contribute to XFS/XFG. If these environmental determinants of XFS are confirmed, they represent opportunities to reduce disease burden. Currently, the evidence that ocular solar exposure during young adulthood is important should prompt people to don proper ocular UV protection, particularly at times when reflected ocular solar exposure is high. Other environmental and genetic exposures for XFS should continue to be explored so as to provide a more complete understanding of the disease.
Acknowledgments
Funding:
This work is funded by the NIH grants EY020928 (JLW), EY015473 (LRP), the Margaret & Leo Myer & Hans M. Hirsch Foundation and The Harvard Glaucoma Center of Excellence
Footnotes
Competing Interest: None
References
- 1.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 2.Hamalainen ER, Kemppainen R, Pihlajaniemi T, Kivirikko KI. Structure of the human lysyl oxidase gene. Genomics. 1993;17(3):544–548. doi: 10.1006/geno.1993.1369. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem. 1995;270(13):7176–7182. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon K, Modi WS, Contente S, Friedman RM. A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24–q25. J Biol Chem. 1993;268(25):18435–18437. [PubMed] [Google Scholar]
- 5.Thorleifsson G, Magnusson KP, Sulem P, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science (New York, NY ) 2007;317(5843):1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt AW, Sharma S, Burdon KP, et al. Ancestral LOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet. 2008;17(5):710–716. doi: 10.1093/hmg/ddm342. [DOI] [PubMed] [Google Scholar]
- 7.Ji QS, Qi B, Wen YC, et al. The association of LOXL1 polymorphisms with exfoliation syndrome/glaucoma: Meta-analysis. Int J Ophthalmol. 2015;8(1):148–156. doi: 10.3980/j.issn.2222-3959.2015.01.27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Stein JD, Pasquale LR, Talwar N, et al. Geographic and climatic factors associated with exfoliation syndrome. Arch Ophthalmol. 2011;129(8):1053–1060. doi: 10.1001/archophthalmol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor HR. The environment and the lens. Br Journal Ophthalmol. 1980;64(5):303–310. doi: 10.1136/bjo.64.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringvold A, Blika S, Elsas T, et al. The Middle-Norway eye-screening study. I. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol (Copenh) 1988;66(6):652–658. doi: 10.1111/j.1755-3768.1988.tb04056.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas R, Nirmalan PK, Krishnaiah S. Pseudoexfoliation in southern India: the Andhra Pradesh Eye Disease Study. Invest Ophth Vis Sci. 2005;46(4):1170–1176. doi: 10.1167/iovs.04-1062. [DOI] [PubMed] [Google Scholar]
- 12.Kang JH, Wiggs JL, Pasquale LR. Relation between time spent outdoors and exfoliation glaucoma or exfoliation glaucoma suspect. Am J Ophthalmol. 2014;158:605–14. doi: 10.1016/j.ajo.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquale LR, Jiwani AZ, Zehavi-Dorin T, et al. Solar exposure and residential geographic history in relation to exfoliation syndrome in the United States and Israel. JAMA Ophthalmol. 2014;132(12):1439–1445. doi: 10.1001/jamaophthalmol.2014.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amini H, Daneshvar R, Eslami Y, Moghimi S, Amini N. Early-onset pseudoexfoliation syndrome following multiple intraocular procedures. J Ophthalmic Vis Res. 2012;7(3):190–196. [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JH, Loomis S, Wiggs JL, Stein JD, Pasquale LR. Demographic and geographic features of exfoliation glaucoma in 2 United States-based prospective cohorts. Ophthalmology. 2012;119(1):27–35. doi: 10.1016/j.ophtha.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sliney DH. Physical factors in cataractogenesis: ambient ultrviolet radiation and temperature. Invest Ophth Vis Sci. 1986;27:781–790. [Google Scholar]
- 17.Sasaki H, Sakamoto Y, Schnider C, et al. UV-B exposure to the eye depending on solar altitude. Eye Contact Lens. 2011;37(4):191–195. doi: 10.1097/ICL.0b013e31821fbf29. [DOI] [PubMed] [Google Scholar]
- 18.Vojnikovic B, Njiric S, Coklo M, Toth I, Spanjol J, Marinovic M. Sunlight and incidence of pterygium on Croatian Island Rab--epidemiological study. Coll Antropol. 2007;31(Suppl 1):61–62. [PubMed] [Google Scholar]
- 19.Taylor HR. The prevalence of corneal disease and cataracts in Australian aborigines in Northwestern Australia. Aust J Ophthalmol. 1980;8(4):289–301. doi: 10.1111/j.1442-9071.1980.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 20.Resnikoff S, Filliard G, Dell’Aquila B. Climatic droplet keratopathy, exfoliation syndrome, and cataract. Br J Ophthalmol. 1991;75(12):734–736. doi: 10.1136/bjo.75.12.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landers J, Henderson T, Craig J. Prevalence of pseudoexfoliation syndrome in indigenous Australians within central Australia: The Central Australian Ocular Health Study. Clin Exp Ophthalmol. 2012;40(5):454–457. doi: 10.1111/j.1442-9071.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 22.Summanen P, Tonjum AM. Exfoliation syndrome among Saudis. Acta ophthalmologica. Supplement. 1988;184:107–111. doi: 10.1111/j.1755-3768.1988.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Amero KK, Osman EA, Dewedar AS, Schmidt S, Allingham RR, Al-Obeidan SA. Analysis of LOXL1 polymorphisms in a Saudi Arabian population with pseudoexfoliation glaucoma. Mol Vis. 2010;16:2805–2810. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee RK. The molecular pathophysiology of pseudoexfoliation glaucoma. Curr Opin Ophthalmol. 2008;19(2):95–101. doi: 10.1097/ICU.0b013e3282f49cda. [DOI] [PubMed] [Google Scholar]
- 25.Mapstone R. Determinants of corneal temperature. Br J Ophthalmol. 1968;52(10):729–741. doi: 10.1136/bjo.52.10.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudkin AK, Edussuriya K, Sennanayake S, et al. Prevalence of exfoliation syndrome in central Sri Lanka: the Kandy Eye Study. Br J Ophthalmol. 2008;92(12):1595–1598. doi: 10.1136/bjo.2008.146407. [DOI] [PubMed] [Google Scholar]
- 27.Bin C. [Accessed September 24, 2013];Current worldwide annual coffee consumption per capita. 2011 chartbin.com/view/581.
- 28.Krause U, Alanko HI, Karna J, et al. Prevalence of exfoliation syndrome in Finland. Acta ophthalmologica. Suppl. 1988;184:120–122. doi: 10.1111/j.1755-3768.1988.tb02642.x. [DOI] [PubMed] [Google Scholar]
- 29.Cashwell LF, Jr, Shields MB. Exfoliation syndrome. Prevalence in a southeastern United States population. Arch Ophthalmol. 1988;106(3):335–336. doi: 10.1001/archopht.1988.01060130361021. [DOI] [PubMed] [Google Scholar]
- 30.Urgert R, van Vliet T, Zock PL, Katan MB. Heavy coffee consumption and plasma homocysteine: a randomized controlled trial in healthy volunteers. Am J Clin Nutr. 2000;72(5):1107–1110. doi: 10.1093/ajcn/72.5.1107. [DOI] [PubMed] [Google Scholar]
- 31.Xu F, Zhang L, Li M. Plasma homocysteine, serum folic acid, serum vitamin B12, serum vitamin B6, MTHFR and risk of pseudoexfoliation glaucoma: a meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):1067–1074. doi: 10.1007/s00417-011-1877-4. [DOI] [PubMed] [Google Scholar]
- 32.Pasquale LR, Wiggs JL, Willett WC, Kang JH. The Relationship between caffeine and coffee consumption and exfoliation glaucoma or glaucoma suspect: a prospective study in two cohorts. Invest Ophth Vis Sci. 2012;53(10):6427–6433. doi: 10.1167/iovs.12-10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnarsson A, Jonasson F, Damji KF, Gottfredsdottir MS, Sverrisson T, Sasaki H. Exfoliation syndrome in the Reykjavik Eye Study: risk factors for baseline prevalence and 5-year incidence. Br J Ophthalmol. 2010;94(7):831–835. doi: 10.1136/bjo.2009.157636. [DOI] [PubMed] [Google Scholar]
- 34.Zenkel M, Krysta A, Pasutto F, Juenemann A, Kruse FE, Schlotzer-Schrehardt U. Regulation of lysyl oxidase-like 1 (LOXL1) and elastin-related genes by pathogenic factors associated with pseudoexfoliation syndrome. Invest Ophth Vis Sci. 2011;52(11):8488–8495. doi: 10.1167/iovs.11-8361. [DOI] [PubMed] [Google Scholar]