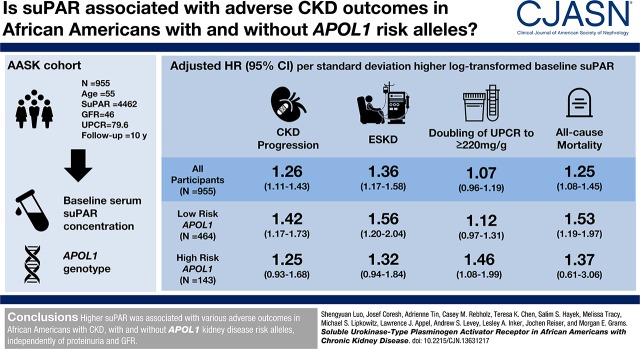
Abstract
Background and objectives
Black Americans with and without APOL1 kidney disease risk variants face high risk of ESKD. Soluble urokinase-type plasminogen activator receptor (suPAR), a circulating signaling protein and marker of immune activation, constitutes a promising biomarker of CKD-associated risks. We aimed to quantify the associations between serum suPAR concentration and adverse outcomes in Black Americans with and without APOL1 kidney disease risk variants, over and above iodine-125 iothalamate measured GFR and proteinuria.
Design, setting, participants, & measurements
Using data from the African-American Study of Kidney Disease and Hypertension, a multicenter clinical trial followed by a cohort phase with a median total follow-up of 9.7 years (interquartile range, 6.5–10.9 years), we examined the associations of suPAR with CKD progression (defined as doubling of serum creatinine or ESKD), ESKD, worsening proteinuria (defined as pre-ESKD doubling of 24-hour urine protein-to-creatinine ratio to ≥220 mg/g), and all-cause death.
Results
At baseline, the median suPAR was 4462 pg/ml, mean measured GFR was 46 ml/min per 1.73 m2, and median 24-hour urine protein-to-creatinine ratio was 80 mg/g. After controlling for baseline demographics, randomization arm, GFR, proteinuria, APOL1 risk status, and clinical risk factors, there was a 1.26-times higher risk for CKD progression per SD higher baseline log-transformed suPAR (hazard ratio [HR], 1.26; 95% confidence interval [95% CI], 1.11 to 1.43; P<0.001). Higher suPAR was also independently associated with risk of ESKD (HR, 1.36; 95% CI, 1.17 to 1.58; P<0.001) and death (HR, 1.25; 95% CI, 1.08 to 1.45; P=0.003). suPAR was only associated with worsening proteinuria in patients with two APOLI risk alleles (HR, 1.46; 95% CI, 1.08 to 1.99; P=0.02).
Conclusions
Higher suPAR was associated with various adverse outcomes in Black Americans with CKD, with and without APOL1 kidney disease risk variants, independently of proteinuria and GFR.
Keywords: African Americans; Alleles; Biomarkers; Black Americans; chronic kidney disease; creatinine; Demography; Follow-Up Studies; glomerular filtration rate; Humans; hypertension; Iothalamic Acid; kidney; Kidney Failure, Chronic; proteinuria; Random Allocation; Receptors, Urokinase Plasminogen Activator; Renal Insufficiency, Chronic; risk factors; United States
Introduction
More than 4% of deaths worldwide are attributable to CKD (1). Despite the extensive resources devoted to CKD treatment, the incidence of ESKD remains high, particularly among Black Americans. Higher ESKD risk in Black Americans is explained in part by the prevalence of a high-risk genotype (two APOL1 risk alleles), present in 13% of Black Americans; however, the majority of ESKD cases occur in people with the low-risk genotype (2,3). Novel biomarkers and treatment targets are needed in order to ameliorate adverse outcomes in CKD among individuals with and without APOL1 risk alleles.
Soluble urokinase-type plasminogen activator receptor (suPAR), a cell membrane glycosylphosphatidylinositol-anchored protein expressed in many cell types (e.g., podocytes and immune, endothelial, and bone marrow cells), is released into the circulation during immune activation. Circulating suPAR participates in cell adhesion, migration, and survival, and may mediate podocyte injury in FSGS (4–6). Observational studies in primarily European populations suggest that suPAR may be associated with incident CKD (7–10). There is less evidence of associations between suPAR and adverse outcomes in Black Americans with CKD. We recently found faster suPAR-associated eGFR decline in Black Americans with two APOL1 risk alleles compared with zero or one risk allele in two populations (11). However, evidence of associations of suPAR with eGFR decline in Black Americans with the low-risk genotype was mixed, and the associations between suPAR and clinical outcomes, independent of measured GFR and proteinuria, were not explored.
Using a baseline measurement of suPAR in the African-American Study of Kidney Disease and Hypertension (AASK), a clinical trial of Black Americans with hypertension-attributed kidney disease, measured GFR, per-protocol proteinuria assessments, and APOL1 genotyping, we characterized the associations between suPAR and CKD progression, ESKD, worsening proteinuria, and all-cause death.
Materials and Methods
Study Design
In brief, AASK was a three×two factorial design, randomized, controlled trial evaluating the effects of three antihypertensives (ramipril, metoprolol, and amlodipine) and two BP control goals (mean arterial pressure ≤92 mm Hg versus 102–107 mm Hg) in slowing CKD progression (12,13). Participants were self-identified Black Americans (18–70 years of age) with hypertension-attributed CKD (measured GFR between 20 and 65 ml/min per 1.73 m2). After completion of the trial (in April of 2002), 691 participants without ESKD entered the cohort phase (14). Participants were censored at loss to follow-up or the end of study (in June of 2007). Informed consent was obtained from all participants. The study protocol was approved by the institutional review boards at Johns Hopkins University and all other participating institutions.
Measurement of Exposure
Blood samples were taken at enrollment and stored at −80°C. Serum suPAR concentrations were measured in 955 participants with available samples, using an ELISA (suPARnostic kit; ViroGates, Copenhagen, Denmark), in 2017. In a blind duplicate study (n=21), the mean coefficient of variation was 3.9% (median, 2.3%), and the correlation (r) was 0.97.
Outcomes, Kidney Function Measures, and Risk Factors
The primary outcome was CKD progression, defined as doubling of serum creatinine from baseline or ESKD (requiring dialysis or kidney transplantation). Secondary outcomes included incident ESKD, worsening proteinuria (pre-ESKD doubling of 24-hour urine protein-to-creatinine ratio [UPCR] to ≥220 mg/g), and all-cause mortality. Baseline GFR was measured by urinary clearance of iodine-125 iothalamate. Serum creatinine was collected every 6 months and measured with an autoanalyzer (AASK Central Biochemistry Laboratory, Cleveland Clinic, Cleveland, OH), 24-hour urine creatinine and protein were measured yearly using the modified Jaffe reaction and the pyrogallol red technique, respectively, and used to calculate UPCR (15,16). Serum C-reactive protein (CRP) was measured by nephelometry using a high-sensitivity assay (Dade Behring BN II System). Details about APOL1 genotyping among AASK participants have been described in detail previously (17,18). Briefly, the APOL1 risk variants (G1[rs73885319 or rs60910145] and G2[rs71785313]) were determined using an ABI TaqMan assay (Applied Biosystems, Foster City, CA) in 607 participants who gave consent to genotyping. Participants with zero or one APOL1 risk alleles were classified as low risk, and those with two risk alleles were classified as high risk. Other variables collected at baseline included age, sex, duration of hypertension, family history of ESKD, body mass index, history of smoking, systolic and diastolic BP, and history of heart disease.
Statistical Analyses
Continuous variables with right-skewed distributions (baseline suPAR, CRP, and UPCR) were logarithmically transformed on a natural log scale in continuous statistical analyses. By suPAR quartile, continuous variables were summarized using means with SD (for symmetrically distributed variables), or medians and interquartile ranges (for variables with skewed distributions). Categorical variables were summarized using proportions. Baseline characteristics were compared using tests for linear trends.
Cumulative incidences of CKD progression and ESKD were plotted using competing risks regression accounting for death as the competing event, and for worsening proteinuria, accounting for ESKD and death as competing events. Kaplan–Meier functions were used to plot cumulative mortality. Cox proportional hazards regression was used to assess the associations of baseline suPAR (log-transformed and scaled to 1 SD) with outcomes. Multivariable models included baseline demographics (age and sex), trial arms (BP control goal and trial medication), baseline kidney measures (measured GFR and proteinuria), CRP, APOL1 risk status (modeled as a three-category variable: zero or one risk variant, two risk variants, and not genotyped), history of heart disease, and history of smoking. The assumption of a log-linear relationship between suPAR and outcomes was tested by modeling suPAR using cubic splines, and comparing model fit with the original model using Akaike information criterion. Interactions between kidney function and suPAR on outcomes were assessed by including an interaction term of baseline GFR and suPAR in the regression models, with Wald test for statistical significance. To assess consistency by APOL1 risk status, analyses were repeated with an interaction term by APOL1 risk status (zero or one risk allele versus two risk alleles versus not genotyped). The ability of suPAR to improve risk discrimination when added to prediction models was assessed using Harrell C-statistic. To assess the robustness of results, the associations of baseline suPAR with CKD progression, worsening proteinuria, and ESKD were tested using multivariable competing risks regression as sensitivity analyses. A two-sided α value of 0.05 was chosen as the cut-off for statistical significance. All statistical analyses were performed using Stata 14.0 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Among 955 AASK participants with available serum samples for suPAR quantification, the median suPAR concentration was 4462 pg/ml (interquartile range, 3425–5923 pg/ml). The mean baseline age was 55 years, the mean measured GFR was 46 ml/min per 1.73 m2, and the median 24-hour UPCR was 80 mg/g (Table 1). When stratified by baseline suPAR quartile, participants with higher suPAR were more often women, had higher prevalence of current smoking, longer history of hypertension, lower diastolic BP, higher CRP, higher proteinuria, and lower GFR (all Ptrend<0.05). The presence of two APOL1 risk alleles was not statistically significantly different by suPAR quartile.
Table 1.
Baseline characteristics of participants in the African-American Study of Kidney Disease and Hypertension, overall and by serum suPAR concentration
| Variables | Entire Cohort (n=955) | Baseline Serum suPAR Concentration, pg/ml | |||
|---|---|---|---|---|---|
| Below 3425 (n=239) | 3425–4462 (n=239) | 4462–5923 (n=239) | Above 5923 (n=238) | ||
| Age, yr, mean (SD) | 55 (11) | 56 (10) | 54 (11) | 54 (11) | 54 (10) |
| Womena, N (%) | 373 (39) | 76 (32) | 76 (32) | 102 (43) | 119 (50) |
| Goal of BP control in AASK | |||||
| MAP≤92 mm Hg, N (%) | 472 (49) | 108 (45) | 129 (54) | 121 (51) | 114 (48) |
| MAP=102–107 mm Hg, N (%) | 483 (51) | 131 (55) | 110 (46) | 118 (49) | 124 (52) |
| Trial medication in AASK | |||||
| Ramipril, N (%) | 373 (39) | 85 (36) | 96 (40) | 100 (42) | 92 (39) |
| Metoprolol, N (%) | 394 (41) | 106 (44) | 93 (39) | 95 (40) | 100 (42) |
| Amlodipine, N (%) | 188 (20) | 48 (20) | 50 (21) | 44 (18) | 46 (19) |
| Years with hypertensiona, mean (SD) | 14 (10) | 14 (9) | 13 (10) | 14 (10) | 16 (11) |
| Familial diagnosis of ESKD, N (%) | 124 (13) | 32 (13) | 27 (11) | 32 (13) | 33 (14) |
| Body mass index, kg/m2, mean (SD) | 30.7 (6.6) | 29.7 (5.3) | 31.0 (6.4) | 31.0 (6.7) | 31.0 (7.8) |
| History of smokinga | |||||
| Never smoker, N (%) | 398 (42) | 115 (48) | 102 (43) | 102 (43) | 79 (33) |
| Past smoker, N (%) | 279 (29) | 76 (32) | 70 (29) | 71 (30) | 62 (26) |
| Current smoker, N (%) | 278 (29) | 48 (20) | 67 (28) | 66 (28) | 97 (41) |
| Systolic BP, mm Hg, mean (SD) | 150 (24) | 151 (24) | 150 (25) | 151 (24) | 149 (24) |
| Diastolic BP, mm Hga, mean (SD) | 95 (15) | 97 (14) | 95 (15) | 96 (16) | 94 (13) |
| History of heart disease, N (%) | 491 (51) | 124 (52) | 132 (55) | 112 (47) | 123 (52) |
| C-reactive protein, mg/La, median (IQR) | 4.3 (1.9, 9.1) | 3.1 (1.4, 7.0) | 3.9 (1.8, 8.9) | 5.2 (2.3, 9.7) | 5.8 (2.7, 10.8) |
| APOL1 risk statusb | |||||
| Low risk: zero or one risk allele, N (%) | 464 (76) | 127 (83) | 119 (76) | 115 (72) | 103 (75) |
| High risk: two risk alleles, N (%) | 143 (24) | 26 (17) | 38 (24) | 45 (28) | 34 (25) |
| 24-h UPCR, mg/ga, median (IQR) | 80 (28, 359) | 38 (21, 101) | 57 (26, 230) | 118 (40, 504) | 201 (56, 735) |
| Measured GFR, ml/min per 1.73 m2a, mean (SD) | 46 (13) | 53 (11) | 50 (11) | 43 (13) | 38 (12) |
| suPARa, pg/ml, median (IQR) | 4462 (3425, 5923) | 2831 (2265, 3157) | 3977 (3703, 4260) | 5141 (4817, 5463) | 7472 (6505, 8669) |
suPAR, soluble urokinase-type plasminogen activator receptor; AASK, the African American Study of Kidney Disease and Hypertension; MAP, mean arterial pressure; UPCR, 24-hour urine protein-to-creatinine ratio; IQR, 25th and 75th quartile.
P for trend <0.05.
A total of 348 participants did not have genotyping data.
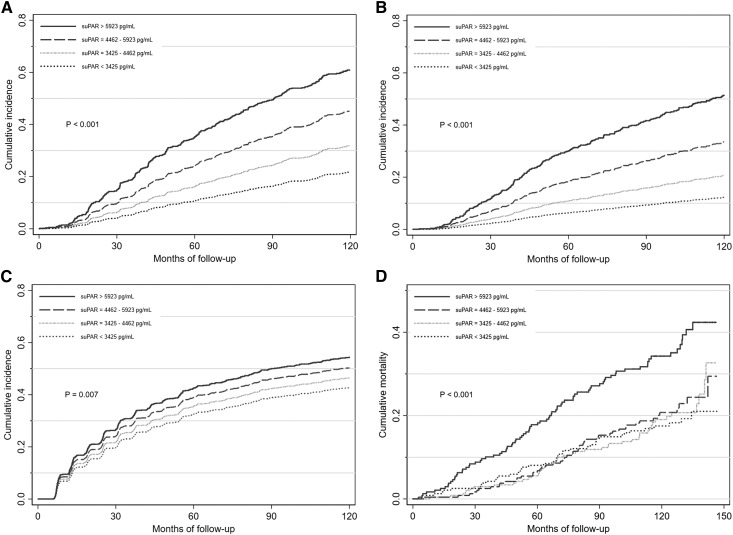
Baseline suPAR and CKD Progression
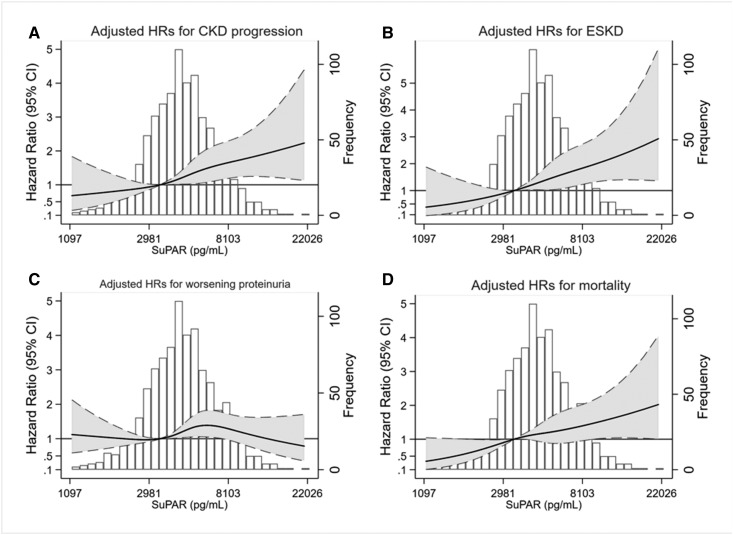
During a median follow-up time of 7.4 years, 363 (38.0%) participants developed CKD progression. The incidence of CKD progression was 2, 4, 7, and 10 per 100 person-years, for the first, second, third, and fourth quartile of baseline suPAR, respectively. The unadjusted risk for CKD progression was significantly higher with higher quartiles of suPAR (P<0.001; Figure 1A). The continuous association remained statistically significant after adjustment for age, sex, and trial arm (hazard ratio [HR], 1.71 per SD higher; 95% confidence interval [95% CI], 1.54 to 1.90; P<0.001; Table 2), and adjustment for baseline GFR and proteinuria (HR, 1.29; 95% CI, 1.14 to 1.46; P<0.001). Further adjustment for baseline clinical risk factors (history of heart disease, history of smoking, CRP, and APOL1 risk status) did not alter the association (HR, 1.26; 95% CI, 1.11 to 1.43; P<0.001). There was no significant interaction between baseline GFR and suPAR (Pinteraction=0.52). The fully adjusted association between suPAR and CKD progression was log-linear (Figure 2A), and was consistent by APOL1 risk status (Pinteraction=0.73; Table 3). When suPAR was added to a prediction model including age, sex, baseline GFR, proteinuria, and history of heart disease, the risk discrimination for CKD progression was not significantly improved (Harrell C-statistic, 0.800 versus 0.797; difference, 0.003; 95% CI, −0.01 to 0.01; P=0.16). In fully adjusted multivariable competing risks regression accounting for death as the competing event, baseline suPAR remained associated with CKD progression (sub-HR, 1.18; 95% CI, 1.03 to 1.34; P=0.01).
Figure 1.
The unadjusted cumulative incidences of CKD progression, ESKD, worsening proteinuria, and mortality were higher with higher suPAR quartiles in the African-American Study of Kidney Disease and Hypertension. (A) Levels of baseline serum suPAR concentration and cumulative incidence of CKD progression. (B) Levels of baseline serum suPAR concentration and cumulative incidence of ESKD. (C) Levels of baseline serum suPAR concentration and cumulative incidence of worsening proteinuria. (D) Levels of baseline serum suPAR concentration and cumulative incidence of all-cause mortality. CKD progression was defined as doubling of serum creatinine from baseline or ESKD (requiring dialysis or kidney transplantation). Worsening proteinuria was defined as pre-ESKD doubling of 24-hour UPCR to ≥220 mg/g.
Table 2.
Results of Cox regression analyses assessing the associations between baseline serum suPAR concentration and CKD progression, ESKD, worsening proteinuria, all-cause mortality in the African-American Study of Kidney Disease and Hypertension
| Outcome | No. of events | Model 1: Adjusted for Demographics and AASK Trial Arm | Model 2: Model 1 Additionally Adjusted for Kidney Measures | Model 3: Model 2 Additionally Adjusted for Clinical Risk Factors | |||
|---|---|---|---|---|---|---|---|
| HRa (95% CI) | P Value | HRa (95% CI) | P Value | HRa (95% CI) | P Value | ||
| CKD progression | 363 | 1.71 (1.54 to 1.90) | <0.001 | 1.29 (1.14 to 1.46) | <0.001 | 1.26 (1.11 to 1.43) | <0.001 |
| ESKD | 271 | 1.91 (1.69 to 2.16) | <0.001 | 1.34 (1.15 to 1.55) | <0.001 | 1.36 (1.17 to 1.58) | <0.001 |
| Doubling of UPCR to ≥220 mg/g | 448 | 1.21 (1.10 to 1.34) | <0.001 | 1.06 (0.96 to 1.18) | 0.26 | 1.07 (0.96 to 1.19) | 0.23 |
| All-cause mortality | 218 | 1.50 (1.32 to 1.70) | <0.001 | 1.32 (1.14 to 1.53) | <0.001 | 1.25 (1.08 to 1.45) | 0.003 |
Adjusting variables include demographics (age and sex), AASK trial arm (BP control goal and trial medication), kidney measures (UPCR and measured GFR), and clinical risk factors (history of heart disease, history of smoking, C-reactive protein, and APOL1 risk status) at baseline. CKD progression was defined as doubling of serum creatinine from baseline or ESKD (requiring dialysis or kidney transplantation). Worsening proteinuria was defined as pre-ESKD doubling of 24-hour UPCR to ≥220 mg/g. suPAR, soluble urokinase-type plasminogen activator receptor; AASK, the African American Study of Kidney Disease and Hypertension; HR, hazard ratio; 95% CI, 95% confidence interval; UPCR, 24-hour urine protein-to-creatinine ratio.
HR per SD higher log-transformed baseline serum suPAR concentration.
Figure 2.
The adjusted HRs for CKD progression, ESKD, and mortality increased log-linearly with higher levels of baseline suPAR in the African-American Study of Kidney Disease and Hypertension. Solid lines indicate adjusted HRs. Shaded areas indicate 95% CIs. Dashed lines indicate upper and lower limits of 95% CIs. (A) Adjusted HRs for CKD progression plotted against baseline suPAR concentration on a log-scale. (B) Adjusted HRs for ESKD plotted against baseline suPAR concentration on a log-scale. (C) Adjusted HRs for worsening proteinuria plotted against baseline suPAR concentration on a log-scale. (D) Adjusted HRs for mortality plotted against baseline suPAR concentration on a log-scale. HRs were adjusted for demographics (age and sex), AASK trial arm (BP control goal and trial medication), kidney measures (UPCR and measured GFR), and clinical risk factors (history of heart disease, history of smoking, CRP, and APOL1 risk status) at baseline. CKD progression was defined as doubling of serum creatinine from baseline or ESKD (requiring dialysis or kidney transplantation). Worsening proteinuria was defined as pre-ESKD doubling of 24-hour UPCR to ≥220 mg/g.
Table 3.
Results of multivariable Cox regression analyses assessing the associations between baseline serum suPAR concentration and CKD progression, ESKD, worsening proteinuria, and all-cause mortality, after stratification by APOL1 risk status in the African-American Study of Kidney Disease and Hypertension
| APOL1 Risk Status | Outcome | No. of events | HRa (95% CI) | P Value |
|---|---|---|---|---|
| High risk: two risk alleles; n=143 | CKD progression | 81 | 1.25 (0.93 to 1.68) | 0.15 |
| ESKD | 69 | 1.32 (0.94 to 1.84) | 0.10 | |
| Doubling of UPCR to ≥220 mg/g | 85 | 1.46 (1.08 to 1.99) | 0.02 | |
| All-cause mortality | 20 | 1.37 (0.61 to 3.06) | 0.44 | |
| Low risk: zero or one risk allele; n=464 | CKD progression | 168 | 1.42 (1.17 to 1.73) | <0.001 |
| ESKD | 106 | 1.56 (1.20 to 2.04) | 0.001 | |
| Doubling of UPCR to ≥220 mg/g | 235 | 1.12 (0.97 to 1.31) | 0.13 | |
| All-cause mortality | 71 | 1.53 (1.19 to 1.97) | 0.001 | |
| Not genotyped; n=348 | CKD progression | 114 | 1.15 (0.91 to 1.45) | 0.24 |
| ESKD | 96 | 1.35 (1.04 to 1.76) | 0.03 | |
| Doubling of UPCR to ≥220 mg/g | 128 | 0.87 (0.71 to 1.05) | 0.15 | |
| All-cause mortality | 127 | 1.17 (0.96 to 1.41) | 0.12 |
Results were adjusted for demographics (age and sex), the African American Study of Kidney Disease and Hypertension trial arm (BP control goal and trial medication), kidney measures (UPCR and measured GFR), and clinical risk factors (history of heart disease, history of smoking, and C-reactive protein) at baseline. CKD progression was defined as doubling of serum creatinine from baseline or ESKD (requiring dialysis or kidney transplantation). Worsening proteinuria was defined as pre-ESKD doubling of 24-hour UPCR to ≥220 mg/g. suPAR, soluble urokinase-type plasminogen activator receptor; HR, hazard ratio; 95% CI, 95% confidence interval; UPCR, 24-hour urine protein-to-creatinine ratio.
HR per SD higher log-transformed baseline suPAR.
Baseline suPAR and ESKD
During a median follow-up time of 8.9 years, 271 (28.4%) participants developed ESKD. The incidence of ESKD was 1, 3, 5, and 8 per 100 person-years for the first, second, third, and fourth quartile of baseline suPAR, respectively. The unadjusted risk for incident ESKD was significantly higher with higher suPAR quartiles (P<0.001; Figure 1B). In continuous analysis, the risk for ESKD was higher per SD higher log-transformed baseline suPAR both before (HR, 1.91; 95% CI, 1.69 to 2.16; P<0.001) and after adjustment for kidney measures and clinical risk factors (HR, 1.36; 95% CI, 1.17 to 1.58; P<0.001). There was no significant interaction between baseline GFR and suPAR (Pinteraction=0.65). The fully adjusted association between suPAR and ESKD was log-linear (Figure 2B), and was consistent by APOL1 risk status (Pinteraction=0.60). When suPAR was added to a prediction model including age, sex, baseline GFR, proteinuria, and history of heart disease, the risk discrimination for ESKD was statistically significantly improved (Harrell C-statistic, 0.855 versus 0.851; difference, 0.004; 95% CI, 0.00 to 0.01; P=0.05). Fully adjusted competing risks regression accounting for death as the competing event showed similar association between suPAR and ESKD (sub-HR, 1.27; 95% CI, 1.09 to 1.49; P=0.003).
Baseline suPAR and Worsening Proteinuria
During a median follow-up of 4.7 years, worsening proteinuria occurred in 448 (41.7%) of the 951 participants with follow-up measures of proteinuria. The incidence of worsening proteinuria was 6, 7, 11, and 12 per 100 person-years for the first, second, third, and fourth quartile of baseline suPAR, respectively. The unadjusted risk for worsening proteinuria was higher with higher suPAR quartiles (P=0.007; Figure 1C). In continuous analysis, there was 1.21-times higher risk for worsening proteinuria per SD higher log-transformed baseline suPAR, adjusting for age, sex, and trial arm (HR, 1.21; 95% CI, 1.10 to 1.34; P<0.001), but no association was found after adjustment for kidney measures and clinical risk factors (HR, 1.07; 95% CI, 0.96 to 1.19; P=0.23). Similarly, fully adjusted competing risks regression accounting for ESKD and death as competing events showed no association (sub-HR, 1.01; 95% CI, 0.90 to 1.13; P=0.88), and there was no significant interaction between baseline GFR and suPAR (Pinteraction=0.83). However, the association was stronger among those with two APOL1 risk alleles (HR, 1.46; 95% CI, 1.08 to 1.99; P=0.02) compared with those with zero or one APOL1 risk alleles (HR, 1.12; 95% CI, 0.97 to 1.31; P=0.13) and those who were not genotyped (HR, 0.81; 95% CI, 0.60 to 1.08; P=0.15) (Pinteraction=0.008).
Baseline suPAR and All-Cause Mortality
During a median follow-up of 9.7 years, 218 (22.8%) participants died, of whom 153 died without having ESKD. The incidence of death was 2, 2, 2, and 4 per 100 person-years for the first, second, third, and fourth quartile of baseline suPAR, respectively. The highest mortality risk was observed in the highest suPAR quartile (P<0.001; Figure 1D). In continuous analysis, per SD higher log-transformed baseline suPAR, the risk for mortality adjusted for age, sex, and trial arm was 1.5-times higher (HR, 1.50; 95% CI, 1.32 to 1.70; P<0.001). The effect size was not substantially altered after full adjustment for other clinical risk factors (HR, 1.25; 95% CI, 1.08 to 1.45; P=0.003). There was no significant interaction between baseline GFR and suPAR (Pinteraction=0.32). The association between suPAR and mortality was log-linear (Figure 2D) and not significantly different by APOL1 risk status (Pinteraction=0.11). When suPAR was added to a prediction model including age, sex, baseline GFR, proteinuria, and history of heart disease, the risk discrimination for all-cause mortality was not significantly improved (Harrell C-statistic, 0.681 versus 0.674; difference, 0.007; 95% CI, −0.01 to 0.02; P=0.34).
Discussion
In this prospective study of Black Americans with CKD, we demonstrated strong associations of higher baseline suPAR with risks for CKD progression, ESKD, and all-cause mortality, which were independent of baseline measured GFR and proteinuria. Higher suPAR was also associated with worsening proteinuria, but only among participants with two APOL1 risk alleles. These results suggest that suPAR is an independent marker of risk in CKD, regardless of APOL1 status, and suggest the possibility of a synergistic action between suPAR and APOL1 risk alleles to promote proteinuria. Because suPAR assays are commercially available and relatively inexpensive, our findings may have clinical implications. Future research should evaluate the utility of incorporating suPAR measurement into clinical practice and determine if suPAR plays a causal role in disease progression in humans.
Originally proposed as the circulating “permeability factor” in FSGS (4), suPAR has attracted attention in increasingly broad populations. Several studies have demonstrated cross-sectional associations of suPAR with proteinuria or reduced GFR in the settings of minimal change disease, membranous nephropathy, IgA nephropathy, and diabetic nephropathy (19–21). A few studies also investigated the association between suPAR and long-term kidney outcomes in populations with glomerular disease, cardiovascular disease, or in the general population (7–10). Results from these studies, however, have been somewhat inconsistent. Zhao et al. (9) found no association between plasma suPAR and progression of IgA nephropathy among 569 Chinese patients followed for a median of 52 months. Spinale et al. (8) found higher proteinuria and greater eGFR decline with higher suPAR, but no association between suPAR and a composite outcome of 50% decline in eGFR or ESKD among 241 patients with glomerular disease followed for a median of 17 months. Compared with AASK, both studies included younger participants (mean baseline age <40 years) with higher GFR (mean baseline eGFR >80 ml/min per 1.73 m2) and relatively short follow-up (median follow-up <5 years). Higher suPAR was associated with incident CKD in the Emory Cardiovascular Biobank (EmCAB) cohort (n=3683, mean age 63 years, mean eGFR of 73 ml/min per 1.73 m2) (7) and a general European population-based cohort (n=5381, mean age 58 years, mean eGFR of 76 ml/min per 1.73 m2) (10). Our work expands on these studies by investigating, in a study with long-term follow-up, the association of suPAR with multiple adverse outcomes in Black Americans with existing CKD, with and without adjustment for measured GFR and proteinuria, and with and without APOL1 kidney disease risk variants.
Basic science studies investigating suPAR and kidney disease have focused on the ability of suPAR to induce proteinuria and histologic changes recapitulating kidney disease. In mouse models, suPAR promotes kidney disease via binding to and activating β3 integrins on podocytes, which triggers activation of podocyte Rac-1 GTPase and cytoskeletal rearrangement (4), effects which may vary on the basis of the suPAR level, form, and source (e.g., bone marrow cells) (4,5,22). In our study, no association was found between suPAR and worsening proteinuria overall, but we did see this association in participants with two APOL1 risk alleles. This observation is consistent with the hypothesis that suPAR can synergize with APOL1 to promote integrin activation through a tripartite complex and an autophagosomal reaction that leads to podocyte detachment (11). Conversely, we did not find statistically significant interaction between suPAR and the APOL1 risk status for the outcomes of CKD progression, ESKD, or death.
In our previous study, we investigated the interaction between APOL1 risk status and eGFR decline in the EmCAB, a clinical cohort of patients undergoing cardiac catheterization, and performed replication analyses in the AASK cohort (11). In the 487 patients in EmCAB, higher suPAR was associated with faster eGFR decline in APOL1 high-risk patients (−2.5 ml/min per 1.73 m2 per year; P=0.03), but not in APOL1 low-risk patients. In the 607 patients in AASK, associations between suPAR and eGFR decline in APOL1 high-risk patients were more modest (−0.6 ml/min/1.73 m2 per year; P=0.01), but higher suPAR was also significantly associated with eGFR decline in the APOL1 low-risk population (−0.3 ml/min per 1.73 m2 per year; P<0.001). In our study, we continued to find strong associations between higher suPAR and clinical outcomes, even after adjustment for rigorously measured markers of GFR and proteinuria; however, associations with CKD progression, ESKD, and mortality were similar by APOL1 risk status, likely because of the relatively small differences in the magnitude of eGFR decline shown in our previous study.
The association between higher baseline suPAR and all-cause mortality is consistent with other studies in select patient populations, including those with cancer, infection, cardiovascular diseases, and patients with diabetes and ESKD (23–27). The mechanism underlying the association between suPAR and mortality requires additional study, but it does not appear to be mediated solely by classic inflammation, as associations were robust after adjustment for CRP in our study as well as others (24,27,28). A recent study of 476 patients with CKD found that associations between suPAR and cardiovascular events were also independent of markers of malnutrition or inflammation (29).
The strengths of our study include its large sample size, large number of events, long follow-up, per-protocol kidney measurements, and robust results in sensitivity analyses. The study population consisted of Black Americans with moderate CKD (GFR between 20 and 65 ml/min per 1.73 m2), a group that suffers disproportionately high risk for ESKD and other adverse outcomes. However, our study has some limitations. First, our data originated from participants in a randomized, controlled trial, and levels of baseline proteinuria were low (individuals with 24-hour UPCR >2500 mg/g were excluded from AASK). Second, only baseline suPAR was measured. Third, because of lack of data, we were unable to describe the participants’ baseline history of heart disease in more detail, or analyze specific causes of death. Fourth, like all observational studies, we cannot ascribe causality, and our study is limited by the potential for unmeasured confounding and imprecise measurements. Nonetheless, given the rigorous design of AASK and its focus on protocol-based ascertainment of kidney measures and outcomes, the influence of confounding and measurement errors was minimized to the greatest extent possible.
In conclusion, higher suPAR was associated with CKD progression, ESKD, and all-cause mortality in Black Americans with hypertension-attributed CKD, independently of APOL1 risk status, GFR, and proteinuria. This suggests that suPAR may be a promising marker and/or mediator of risk in Black Americans with and without CKD. Interestingly, the association between baseline suPAR and worsening proteinuria was present only among patients with the APOL1 high-risk genotype. Future studies should evaluate the utility of measuring suPAR in clinical practice.
Disclosures
J.C., A.S.L., and L.A.I. have a provisional patent filed on 8/15/2014, “Precise estimation of GFR from multiple biomarkers” (PCT/US2015/044567). The technology is not licensed in whole or in part to any company. Tufts Medical Center, John Hopkins University, and Metabolon Inc. have a collaboration agreement to develop a product to estimate GFR from a panel of markers. T.K.C. previously owned stock in Pfizer Pharmaceuticals. A.S.L. reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Amgen, and Siemens. L.A.I. reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, and Reata Pharmaceuticals, and consulting agreements with Tricedia Inc. and Omeros Corp. J.R. is cofounder and shareholder of TRISAQ, a biopharmaceutical company that develops suPAR products.
Acknowledgments
The authors thank the staff and participants of the African-American Study of Kidney Disease and Hypertension for their important contributions.
J.C., A.T., L.J.A., and M.E.G. are supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK108803-02. J.C. and M.E.G. are supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant U01-DK085689-07. C.M.R. is supported by a mentored research scientist development award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782). T.K.C. is supported by the Norman S. Coplon Extramural Grant Program by Satellite Healthcare, a not-for-profit kidney care provider.
Part of this work was presented as a moderated poster on March 20, 2018 at the American Heart Association EPI/Lifestyle 2018 Scientific Sessions in New Orleans, Louisiana.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R, Badawi A, Ballew SH, Banerjee A, Barregård L, Barrett-Connor E, Basu S, Bello AK, Bensenor I, Bergstrom J, Bikbov B, Blosser C, Brenner H, Carrero JJ, Chadban S, Cirillo M, Cortinovis M, Courville K, Dandona L, Dandona R, Estep K, Fernandes J, Fischer F, Fox C, Gansevoort RT, Gona PN, Gutierrez OM, Hamidi S, Hanson SW, Himmelfarb J, Jassal SK, Jee SH, Jha V, Jimenez-Corona A, Jonas JB, Kengne AP, Khader Y, Khang YH, Kim YJ, Klein B, Klein R, Kokubo Y, Kolte D, Lee K, Levey AS, Li Y, Lotufo P, El Razek HMA, Mendoza W, Metoki H, Mok Y, Muraki I, Muntner PM, Noda H, Ohkubo T, Ortiz A, Perico N, Polkinghorne K, Al-Radaddi R, Remuzzi G, Roth G, Rothenbacher D, Satoh M, Saum KU, Sawhney M, Schöttker B, Shankar A, Shlipak M, Silva DAS, Toyoshima H, Saum KU, Sawhney M, Schöttker B, Shankar A, Shlipak M, Silva DAS, Toyoshima H, Ukwaja K, Umesawa M, Vollset SE, Warnock DG, Werdecker A, Yamagishi K, Yano Y, Yonemoto N, Zaki MES, Naghavi M, Forouzanfar MH, Murray CJL, Coresh J, Vos T; Global Burden of Disease 2013 GFR Collaborators; CKD Prognosis Consortium; Global Burden of Disease Genitourinary Expert Group : Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 28: 2167–2179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 3.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J: Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, Wadhwani S, Cao Y, Peev V, Zloza A, Lusciks J, Hayek SS, O’Connor C, Bitzer M, Gupta V, Sever S, Sykes DB, Scadden DT, Reiser J: Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med 23: 100–106, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith HW, Marshall CJ: Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11: 23–36, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, Wong HN, Troost JP, Gadegbeku CA, Gipson DS, Kretzler M, Nihalani D, Holzman LB; Nephrotic Syndrome Study Network : A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int 87: 564–574, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Liu L, Huang J, Shi S, Lv J, Liu G, Zhao M, Zhang H: Plasma soluble urokinase receptor level is correlated with podocytes damage in patients with IgA nephropathy. PLoS One 10: e0132869, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz CA, Persson M, Christensson A, Hindy G, Almgren P, Nilsson PM, Melander O, Engström G, Orho-Melander M: Soluble urokinase-type plasminogen activator receptor (suPAR) and impaired kidney function in the population-based malmö diet and cancer study. Kidney Int Rep 2: 239–247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, Samelko B, Lee H, Dande RR, Lee HW, Hahm E, Peev V, Tracy M, Tardi NJ, Gupta V, Altintas MM, Garborcauskas G, Stojanovic N, Winkler CA, Lipkowitz MS, Tin A, Inker LA, Levey AS, Zeier M, Freedman BI, Kopp JB, Skorecki K, Coresh J, Quyyumi AA, Sever S, Reiser J: A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 23: 945–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JT Jr, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, Randall OS, Glassock R: Design and baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) pilot study. Control Clin Trials 17[Suppl]: 3S–16S, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Middleton J, Miller ER 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee : The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Sika M, Lewis J, Douglas J, Erlinger T, Dowie D, Lipkowitz M, Lash J, Cornish-Zirker D, Peterson G, Toto R, Kusek J, Appel L, Kendrick C, Gassman J; AASK Group : Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) clinical trial and cohort study. Am J Kidney Dis 50: 78–89, 89.e1, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Toto RD, Greene T, Hebert LA, Hiremath L, Lea JP, Lewis JB, Pogue V, Sika M, Wang X; AASK Collaborative Research Group : Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: Results from the African American study of kidney disease and hypertension (AASK) cohort. Am J Kidney Dis 56: 896–906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB; SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CZ, Chang LC, Lin YF, Hung YJ, Pei D, Chu NF, Chen JS: Urokinase plasminogen activator receptor and its soluble form in common biopsy-proven kidney diseases and in staging of diabetic nephropathy. Clin Biochem 48: 1324–1329, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Wada T, Nangaku M, Maruyama S, Imai E, Shoji K, Kato S, Endo T, Muso E, Kamata K, Yokoyama H, Fujimoto K, Obata Y, Nishino T, Kato H, Uchida S, Sasatomi Y, Saito T, Matsuo S: A multicenter cross-sectional study of circulating soluble urokinase receptor in Japanese patients with glomerular disease. Kidney Int 85: 641–648, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Musetti C, Quaglia M, Cena T, Chiocchetti A, Monti S, Clemente N, Magnani C, Dianzani U, Stratta P: Circulating suPAR levels are affected by glomerular filtration rate and proteinuria in primary and secondary glomerulonephritis. J Nephrol 28: 299–305, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, Burke GW, Bruneval P, Naesens M, Jackson A, Alachkar N, Canaud G, Legendre C, Anglicheau D, Reiser J, Sarwal MM: A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 6: 256ra136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghasemzedah N, Hayek SS, Ko YA, Eapen DJ, Patel RS, Manocha P, Al Kassem H, Khayata M, Veledar E, Kremastinos D, Thorball CW, Pielak T, Sikora S, Zafari AM, Lerakis S, Sperling L, Vaccarino V, Epstein SE, Quyyumi AA: Pathway-specific aggregate biomarker risk score is associated with burden of coronary artery disease and predicts near-term risk of myocardial infarction and death. Circ Cardiovasc Qual Outcomes 10: e001493, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyngbæk S, Andersson C, Marott JL, Møller DV, Christiansen M, Iversen KK, Clemmensen P, Eugen-Olsen J, Hansen PR, Jeppesen JL: Soluble urokinase plasminogen activator receptor for risk prediction in patients admitted with acute chest pain. Clin Chem 59: 1621–1629, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Nayak RK, Allingstrup M, Phanareth K, Kofoed-Enevoldsen A: suPAR as a biomarker for risk of readmission and mortality in the acute medical setting. Dan Med J 62: A5146, 2015 [PubMed] [Google Scholar]
- 26.Sidenius N, Sier CF, Ullum H, Pedersen BK, Lepri AC, Blasi F, Eugen-Olsen J: Serum level of soluble urokinase-type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood 96: 4091–4095, 2000 [PubMed] [Google Scholar]
- 27.Drechsler C, Hayek SS, Wei C, Sever S, Genser B, Krane V, Meinitzer A, März W, Wanner C, Reiser J: Soluble urokinase plasminogen activator receptor and outcomes in patients with diabetes on hemodialysis. Clin J Am Soc Nephrol 12: 1265–1273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botha S, Fourie CM, Schutte R, Eugen-Olsen J, Pretorius R, Schutte AE: Soluble urokinase plasminogen activator receptor as a prognostic marker of all-cause and cardiovascular mortality in a black population. Int J Cardiol 184: 631–636, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Meijers B, Poesen R, Claes K, Dietrich R, Bammens B, Sprangers B, Naesens M, Storr M, Kuypers D, Evenepoel P: Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int 87: 210–216, 2015 [DOI] [PubMed] [Google Scholar]