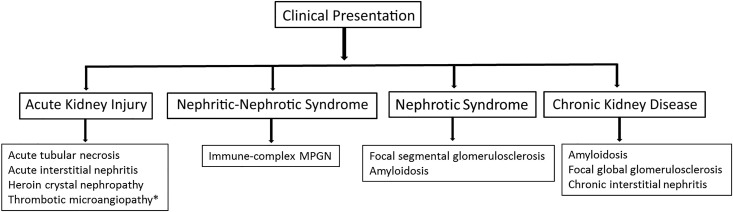
Heroin is a morphine (opioid)-derived substance that can be injected, inhaled, or smoked. It is often “cut” or mixed with other adulterants. A variety of kidney diseases with varying etiologies can occur in the setting of heroin abuse. The kidney diseases can involve the glomerulus, interstitium, and vessels, and they can have different clinical presentations (Figure 1).
Figure 1.
Clinical presentation of heroin-associated kidney diseases. MPGN, membranoproliferative GN. *Thrombotic microangiopathy may be due to morphine-derivative oxymorphone hydrochloride or concomitant use of cocaine.
One of the earliest glomerular lesions described is FSGS, and the term “heroin-associated nephropathy” is used to describe FSGS in the setting of heroin use (1,2). Patients present with proteinuria, which may be in the nephrotic range. FSGS is more common among black heroin users, and it is characterized by focal segmental and global glomerulosclerosis and varying degrees of tubulointerstitial scarring and tubular dilation. Immunofluorescence studies show segmental IgM and C3 staining patterns, and electron microscopy typically shows segmental foot effacement, consistent with features of secondary FSGS. Loss of glomerular epithelial cells is suggested as a mechanism for development of FSGS. It is quite likely that some of FSGS lesions described earlier in heroin abusers may represent FSGS associated with an HIV infection.
Proliferative GN with immune complex deposition is also described in heroin users. Patients present with hematuria, proteinuria, and kidney failure. Low complement levels are typically present. Light microscopy most often shows a membranoproliferative GN (MPGN) pattern of injury; immunofluorescence shows granular IgM, IgG, and C3 along the capillary walls, and electron microscopy shows subendothelial electron dense deposits. The immune complex–associated MPGN is more common in the white population and most likely results from chronic viral or bacterial infections due to contamination (3). Hepatitis C is one of the common infections in immune complex MPGN, although other infections, such as chronic bacterial skin infections or infective bacterial endocarditis, may also be the source of the antigens in immune complex MPGN; mixed cryoglobulinemia associated with hepatitis C in a heroin user has also been reported.
Finally, amyloidosis with glomerular involvement has been described in heroin users, and it is the subject of a large series by Sharma et al. (4) in this issue of the Clinical Journal of the American Society of Nephrology.
Acute kidney failure in heroin users points toward tubulointerstitial disease. It is uncertain whether heroin is directly nephrotoxic to the tubules and if it can cause acute tubular necrosis. However, heroin is postulated to have a myotoxic effect and may cause acute tubular injury as a result of rhabdomyolysis (5). An interstitial nephritis may also be present in heroin abusers. This is most likely due to a hypersensitivity response to adulterants in the heroin preparations, including nonsteroidal anti-inflammatory drugs. Finally, heroin crystal nephropathy has been described recently in the setting of heavy heroin use, and it is likely to occur in the setting of acute tubular injury (6). It is characterized by tubular accumulation of finely granular to fluffy basophilic crystals on light microscopy; the crystals appear as broomstick crystals on urinalysis.
Thrombotic microangiopathy in the setting of development of malignant hypertension is more common with cocaine, and it has not been reported with heroin use. However, intravenous use of a morphine derivative oxymorphone hydrochloride (Opana extended release) has been associated with thrombotic microangiopathy (7). Patients present with acute kidney failure, hemolytic anemia, and thrombocytopenia, and it can be mistaken for atypical hemolytic uremic syndrome or thrombocytopenic thrombotic purpura. Kidney biopsy shows mucoid material in arterial and arteriolar vessel walls, with marked narrowing of vascular lumen and resultant glomerular and tubular ischemia. Glomeruli may show mesangiolysis and contain fibrin thrombi.
AA amyloidosis occurs in the setting of chronic inflammation. Most series have described AA amyloidosis in chronic inflammatory diseases, such as juvenile rheumatoid arthritis, chronic inflammatory bowel disease, and familial Mediterranean fever, or the setting of chronic infections, such as bronchiectasis, osteomyelitis, and tuberculosis (8). AA amyloidosis in the setting of heroin use has been previously described. However, the epidemiology of AA amyloidosis has been changing. Lane et al. (9) showed that, in 625 patients with AA amyloidosis over a 25-year period, there was decrease in incidence of AA amyloidosis due to juvenile rheumatoid arthritis, whereas there was an increase in AA amyloidosis in intravenous drug users likely due to chronic infections.
In a large series of 38 patient of AA amyloidosis in the work by Sharma et al. (4), 95% of the patients had a history of heroin use, and heroin use imparted an estimated 170-fold higher risk for AA amyloidosis compared with in patients with no heroin use. The authors suggest that the type of heroin used—“the black tar heroin”—may explain the high incidence of AA amyloidosis in the Pacific Northwest. The findings confirm the changing landscape of AA amyloidosis and kidney disease in heroin users. The authors show that AA amyloidosis is more likely to occur in the white population, and the patients often have an underlying hepatitis C infection. In addition, the majority of patients had skin abscesses. The glomeruli were involved in all patients with AA amyloidosis, but both vascular involvement and tubulointerstitial involvement were also common. The kidney disease was progressive, and a large number of patients developed ESKD within a short time period (median time to ESKD was 2.4 years). In addition, there was high mortality in heroin-using patients who developed AA amyloidosis (median survival, 4.2 years).
The study also raises some important questions. Additional studies are required to answer several important questions and explore other areas. (1) Chronic infections, such as hepatitis and skin abscesses, were common in heroin users. Why is the incidence of GN (immune complex MPGN or infection-related GN) low compared with AA amyloidosis in these patients in spite of the presence of infections? (2) The subtype of AA amyloid in heroin users is not known. The SAA4 subtype is common in patients with AA amyloidosis and chronic inflammatory conditions. However, a variant of SAA protein (SAA W71R) was reported in a patient with hepatitis and kidney amyloidosis (10). Thus, the AA subtype might be useful in determining the pathogenesis of AA amyloidosis in heroin users. (3) It should also be pointed out that AA is a sticky protein, and false positive AA staining can be seen in some other types of amyloidosis, in particular leukocyte cell–derived chemotaxin 2 protein–associated amyloidosis (11). Mass spectrometry studies to determine the amyloidogenic profile would be useful in these patients to rule out other forms of amyloidosis. (4) The type of heroin preparation (i.e., black tar heroin versus other forms of heroin) having a causal role needs to be further studied. (5) Finally, it would be important to determine whether the AA amyloidosis in heroin users involved other organs, such as the cardiovascular system, which may have contributed to the high mortality.
In summary, the study by Sharma et al. (4) confirms the changing landscape of kidney diseases associated with heroin use. Heroin use should be in the differential diagnosis of all patients with AA amyloidosis.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Heroin Use Is Associated with AA-Type Kidney Amyloidosis in the Pacific Northwest,” on pages 1030–1036.
References
- 1.Dubrow A, Mittman N, Ghali V, Flamenbaum W: The changing spectrum of heroin-associated nephropathy. Am J Kidney Dis 5: 36–41, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Jaffe JA, Kimmel PL: Chronic nephropathies of cocaine and heroin abuse: A critical review. Clin J Am Soc Nephrol 1: 655–667, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Govindan P, Toukatly M, Healy J, Henry C, Senter S, Najafian B, Kestenbaum B: Heroin Use Is Associated with AA-Type Kidney Amyloidoisis is the Pacific Northwest. Clin J Am Soc Nephrol 13: 1030–1036, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanavati A, Herlitz LC: Tubulointerstitial injury and drugs of abuse. Adv Chronic Kidney Dis 24: 80–85, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Bautista JEK, Merhi B, Gregory O, Hu S, Henriksen K, Gohh R: Heroin crystal nephropathy. Clin Kidney J 8: 339–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambruzs JM, Serrell PB, Rahim N, Larsen CP: Thrombotic microangiopathy and acute kidney injury associated with intravenous abuse of an oral extended-release formulation of oxymorphone hydrochloride: Kidney biopsy findings and report of 3 cases. Am J Kidney Dis 63: 1022–1026, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN: Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356: 2361–2371, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lane T, Pinney JH, Gilbertson JA, Hutt DF, Rowczenio DM, Mahmood S, Sachchithanantham S, Fontana M, Youngstein T, Quarta CC, Wechalekar AD, Gillmore JD, Hawkins PN, Lachmann HJ: Changing epidemiology of AA amyloidosis: Clinical observations over 25 years at a single national referral centre. Amyloid 24: 162–166, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Saha A, Theis JD, Vrana JA, Dubey NK, Batra VV, Sethi S: AA amyloidosis associated with hepatitis B. Nephrol Dial Transplant 26: 2407–2412, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Sethi S, Theis JD: Pathology and diagnosis of renal non-AL amyloidosis. J Nephrol 31: 343–350, 2018 [DOI] [PubMed] [Google Scholar]