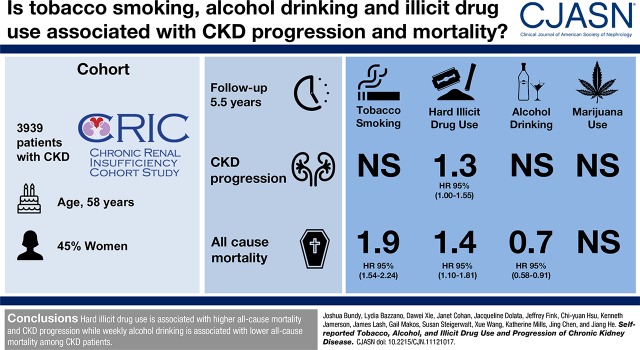
Abstract
Background and objectives
Previous studies suggest that tobacco, alcohol, and illicit drug use is associated with CKD. We examined the associations of substance use with CKD progression and all-cause mortality among patients with CKD.
Design, setting, participants, & measurements
The Chronic Renal Insufficiency Cohort Study is a prospective, longitudinal cohort study among 3939 participants with CKD in the United States. Self-reported tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug (cocaine, heroin, or methamphetamine) use were obtained at baseline and annual follow-up visits. CKD progression was defined as incident ESKD or halving of eGFR. Substance use was modeled as the cumulative average exposure to capture both recent and long-term use in multivariable time-dependent Cox regression.
Results
Over a median 5.5-year follow-up, 1287 participants developed CKD progression, and 1001 died. Baseline proportions of tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use were 13%, 20%, 33%, and 12%, respectively. Compared with nonsmoking throughout follow-up, multivariable-adjusted hazard ratios for persistent tobacco smoking were 1.02 (95% confidence interval, 0.86 to 1.21) for CKD progression and 1.86 (95% confidence interval, 1.54 to 2.24) for all-cause mortality. Compared with nondrinking throughout follow-up, multivariable-adjusted hazard ratios for persistent alcohol drinking were 1.06 (95% confidence interval, 0.88 to 1.29) for CKD progression and 0.73 (95% confidence interval, 0.58 to 0.91) for all-cause mortality. Compared with nonuse throughout follow-up, multivariable-adjusted hazard ratios for persistent marijuana use were 0.94 (95% confidence interval, 0.82 to 1.07) for CKD progression and 1.11 (95% confidence interval, 0.96 to 1.30) for all-cause mortality. Compared with nonuse throughout follow-up, multivariable-adjusted hazard ratios for persistent hard illicit drug use were 1.25 (95% confidence interval, 1.00 to 1.55) for CKD progression and 1.41 (95% confidence interval, 1.10 to 1.81) for all-cause mortality.
Conclusions
Hard illicit drug use is associated with higher risk of CKD progression and all-cause mortality, tobacco smoking is associated with higher risk of all-cause mortality, and alcohol drinking is associated with lower risk of all-cause mortality among patients with CKD.
Keywords: Alcohol Drinking; chronic kidney disease; Cocaine; Confidence Intervals; Disease Progression; Epidemiology and outcomes; Follow-up Studies; glomerular filtration rate; Heroin; Humans; Kidney Failure, Chronic; Marijuana Use; Methamphetamine; mortality; progression of chronic renal failure; Prospective Studies; Renal Insufficiency, Chronic; risk factors; Self Report; Street Drugs; Tobacco Smoking
Introduction
CKD is a major public health challenge (1). Globally, 226 million men (10%) and 272 million women (12%) had CKD in 2010 (2). In 2015, CKD accounted for 1.2 million deaths worldwide, representing a 32% increase from 2005 (3). In developed countries, CKD is associated with older age, diabetes, hypertension, obesity, and cardiovascular disease (4). Other studies have implicated several behavioral risk factors in the development or progression of CKD, including tobacco, alcohol, and illicit drug use, all of which may exhibit direct or indirect nephrotoxic effects (5–9). However, these modifiable risk factors and their associations with progression of CKD and all-cause mortality have not been well studied among patients with preexisting CKD.
Harmful associations of tobacco use with many chronic diseases, especially cancer and cardiovascular disease, have been well documented (10). Although tobacco smoking has been associated with higher risk of incident CKD, the existence of an independent relationship with CKD progression has been questioned, especially among patients with preexisting CKD (5). Similarly, although alcohol use has been associated with lower risk of cardiovascular disease and incident CKD (11,12), its associations with CKD progression remain unclear. Furthermore, the association between illicit drug use and CKD progression is largely unknown. Recently, there has been increasing interest in decriminalization or legalization of use of illicit drugs (13), particularly marijuana, with more than one half of United States states currently allowing recreational and/or medicinal use of marijuana. Use of tobacco, alcohol, and illicit drugs may play a role both in the progression of CKD and death among patients with CKD.
The Chronic Renal Insufficiency Cohort (CRIC) Study provides a unique opportunity to assess the longitudinal associations of these modifiable lifestyle risk factors in a large cohort with predialysis CKD. In this analysis, we investigated the associations of tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use with CKD progression and all-cause mortality among patients with CKD.
Materials and Methods
Study Participants
The CRIC Study is a prospective, longitudinal cohort study that includes a racially and ethnically diverse group of men and women ages 21–74 years old with mild to moderate CKD on the basis of an eGFR entry criterion of 20–70 ml/min per 1.73 m2. A total of 3939 participants were enrolled from seven clinical centers in the United States (New Orleans, LA; Baltimore, MD; Philadelphia, PA; Cleveland, OH; Ann Arbor, MI; Chicago, IL; and San Francisco, CA) between May 2003 and August 2008 (14). Patients with severe heart failure, cirrhosis, HIV infection, polycystic kidney disease, or renal cell carcinoma; those who received chronic dialysis or an organ transplant; and those taking immunosuppressive, chemotherapy, or alkylating agents were excluded. The study was approved by the institutional review board at each institute, and all participants provided written informed consent.
Measurements
All CRIC Study data were collected by trained study staff at baseline and annual study visits using standardized procedures. Self-reported sociodemographic characteristics, medical history, and current medications were obtained via questionnaire. Body weight, height, and BP were measured using standard protocols (14). Glucose and cholesterol were measured using the particle-enhanced immunonephelometry method. Physical activity was determined by use of the Multi-Ethnic Study of Atherosclerosis Physical Activity Questionnaire. Body mass index (BMI) was calculated as the body weight in kilograms divided by the square of height in meters. Hypertension was defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, and/or current use of antihypertensive medications. Diabetes was defined as fasting glucose of ≥126 mg/dl, nonfasting glucose of ≥200 mg/dl, and/or the use of insulin or other antidiabetic medication; 24-hour urine total protein was measured by the turbidimetric reaction method.
Tobacco, alcohol, and illicit drug use was self-reported via questionnaire at the baseline and annual study visits. Participants were classified as current, former, or never smokers according to responses to the questions “Have you smoked more than 100 cigarettes in your lifetime?” and “Do you currently smoke cigarettes?” Participants were classified as alcohol drinkers if they reported having one or more alcoholic beverages per week. At baseline, participants were classified as illicit drug users if they reported any lifetime use of marijuana, cocaine, heroin, or methamphetamine. At annual follow-up visits, participants were classified as illicit drug users if they reported any use within the previous year. Additionally, a composite of hard illicit drug (cocaine, heroin, or methamphetamine) use was assessed. There were no missing data for substance use questions at baseline or follow-up visits for tobacco smoking, marijuana use, heroin use, and methamphetamine use. However, there were occasional missing responses for alcohol drinking and cocaine use over follow-up. In time-dependent analyses, when an exposure was missing, the last reported exposure was used. Analyses excluding participants with missing data yielded similar results but with wider 95% confidence intervals (95% CIs).
Assessment of Outcomes
The study outcomes of interest are (1) time to CKD progression, (2) time to death from any cause, and (3) time to the composite of CKD progression or death in consideration of death as a competing risk. CKD progression is defined as a 50% reduction in eGFR from baseline or ESKD defined as the receipt of dialysis or kidney transplantation. Kidney function was analyzed using the creatinine- and cystatin C–based CRIC Study equation (15). Time to eGFR halving was imputed assuming a linear decline in kidney function between in-person annual visit measurements (15,16). Information on the initiation and maintenance of dialysis and kidney transplant was obtained by annual clinical follow-up visits and interim telephone interviews and confirmed by dialysis unit or hospital chart review. Ascertainment of ESKD was supplemented by information from the US Renal Data System. All deaths were confirmed by death certificate. Follow-up data were available through 2014 for a maximum duration of 10.9 years. Participant follow-up was censored at the time of death, loss to follow-up (n=252; 6.4%), or end of the follow-up period, whichever occurred first.
Statistical Analyses
Baseline characteristics of the study participants were summarized as the mean±SD or median (interquartile range) for continuous variables and percentages for categorical variables according to baseline tobacco, alcohol, and illicit drug use. Time-dependent survival analysis was used to plot the unadjusted associations of annually updated tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use with cumulative incidence of CKD progression and all-cause mortality (17).
Multivariable-adjusted hazard ratios (HRs) and 95% CIs of CKD progression, all-cause mortality, and the composite of CKD progression or all-cause mortality associated with cumulative average tobacco smoking, alcohol drinking, and illicit drug use were estimated using extended Cox regression models. We modeled substance use exposures using three approaches: (1) baseline time-fixed exposure, (2) time-updated most recent exposure, and (3) time-updated cumulative average exposure, which incorporates information from both approaches 1 and 2 to better capture long-term exposure patterns while assigning more importance to recent exposure (18). At a given follow-up time point, the cumulative average exposure assigns 50% weight to the current exposure and 50% weight to the average of past exposure. For example, at follow-up visit 1, exposures were calculated as baseline exposure/2+ visit 1 exposure/2. At visit 2, exposures were calculated as (baseline exposure/2+ visit 1 exposure/2)/2+ visit 2 exposure/2. At visit 3, exposures were calculated as [(baseline exposure/2+ visit 1 exposure/2)/2+ visit 2 exposure/2]/2+ visit 3 exposure/2, etc. The cumulative average exposure is a proportion ranging from zero to one, representing the weighted average exposure (proportion) of a study participant before an event or censoring. Thus, reported HRs represent the risk of a given outcome associated with persistent use throughout follow-up compared with nonuse. Analyses were conducted among all participants using all available follow-up data.
Covariates included in regression models were selected on the basis of prior knowledge and the backward elimination method. Three models were used in the analysis of each exposure: (1) univariable; (2) age, sex, race/ethnicity, and clinical site adjusted; and (3) multivariable adjusted, including variables in model 2 plus high school education, eGFR, proteinuria, history of diabetes, BMI, systolic BP, hemoglobin, and use of nonsteroidal anti-inflammatory drugs. Current tobacco smoking was additionally added to models assessing alcohol drinking, marijuana use, and hard illicit drug use. Time-varying covariates were updated annually when available. Subgroup analyses were conducted by sex, race (white and nonwhite), age (<65 and ≥65 years old), and baseline eGFR (<45 and ≥45 ml/min per 1.73 m2). Effect modification was tested by including interaction terms for each subgroup and exposure combination in the extended Cox models using a Bonferroni-corrected α-threshold of 0.001 for statistical significance to account for multiple testing. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.4.2 (R Project for Statistical Computing).
Results
A total of 3939 participants (mean age, 58 years old; 45% women) were included in the analyses. Baseline proportions of self-reported current tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use were 13%, 20%, 33%, and 12%, respectively. Prevalence and mean values of baseline characteristics differed among substance use and outcome groups with respect to age, sex, race/ethnicity, education, BMI, history of cardiovascular disease, hypertension, history of diabetes, use of nonsteroidal anti-inflammatory drugs, systolic BP, hemoglobin, eGFR, and proteinuria (Supplemental Table 1, Table 1). Alcohol drinking and cocaine use status were missing at one or more visits for 138 and 133 individuals, respectively. All other exposures had complete response over follow-up.
Table 1.
Characteristics of 3939 patients with CKD according to baseline self-reported tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use (the Chronic Renal Insufficiency Cohort Study)
| Variable | Tobacco Smoking | Alcohol Drinkinga | Marijuana Useb | Hard Illicit Drug Useb,c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Never, n=1781 | Former, n=1641 | Current, n=517 | Nonuser, n=3146 | User, n=793 | Nonuser, n=2646 | User, n=1293 | Nonuser, n=3465 | User, n=474 | |
| Age, yr, mean±SD | 56±12 | 61±9 | 56±10 | 59±11 | 57±11 | 60±11 | 54±10 | 58±11 | 52±9 |
| Women, no. (%) | 944 (53) | 608 (37) | 226 (44) | 1552 (49) | 226 (29) | 1294 (49) | 484 (37) | 1623 (47) | 155 (33) |
| Race/ethnicity, no. (%) | |||||||||
| Non-Hispanic white | 718 (40) | 765 (47) | 155 (30) | 1143 (36) | 495 (62) | 1053 (40) | 585 (45) | 1442 (42) | 196 (41) |
| Non-Hispanic black | 695 (39) | 635 (39) | 320 (62) | 1423 (45) | 227 (29) | 1060 (40) | 590 (46) | 1426 (41) | 224 (47) |
| Hispanic | 279 (16) | 189 (12) | 29 (6) | 449 (14) | 48 (6) | 408 (15) | 89 (7) | 456 (13) | 41 (9) |
| Other | 89 (5) | 52 (3) | 13 (3) | 131 (4) | 23 (3) | 125 (5) | 29 (2) | 141 (4) | 13 (3) |
| High school graduate, no. (%) | 1461 (82) | 1281 (78) | 368 (71) | 2399 (76) | 711 (90) | 2040 (77) | 1070 (83) | 2724 (79) | 386 (81) |
| Physical activity, METs/wk, mean±SD | 211±153 | 182±128 | 206±167 | 193±143 | 219±153 | 191±139 | 213±156 | 196±145 | 213±150 |
| Current tobacco smoking, no. (%) | — | — | — | 389 (12) | 128 (16) | 248 (9) | 269 (21) | 368 (11) | 149 (31) |
| Alcohol drinking, no. (%)a | 284 (16) | 381 (23) | 128 (25) | — | — | 436 (17) | 357 (28) | 661 (19) | 132 (28) |
| Marijuana use, no. (%)b | 391 (22) | 633 (39) | 269 (52) | 936 (30) | 357 (45) | — | — | 858 (25) | 435 (92) |
| Hard illicit drug use, no. (%)b,c | 102 (6) | 223 (14) | 149 (29) | 342 (11) | 132 (17) | 39 (2) | 435 (34) | — | — |
| Body mass index, kg/m2, mean±SD | 33±8 | 32±8 | 30±7 | 33±8 | 30±6 | 32±8 | 32±8 | 32±8 | 31±7 |
| History of cardiovascular disease, no. (%) | 448 (25) | 666 (41) | 202 (39) | 1105 (35) | 211 (27) | 911 (34) | 405 (31) | 1151 (33) | 165 (35) |
| Hypertension, no. (%) | 1490 (84) | 1450 (88) | 451 (87) | 2757 (88) | 634 (80) | 2318 (88) | 1073 (83) | 3000 (87) | 391 (83) |
| Diabetes, no. (%) | 831 (47) | 853 (52) | 224 (43) | 1683 (54) | 225 (28) | 1350 (51) | 558 (43) | 1705 (49) | 203 (43) |
| Antihypertensive medication, no. (%) | 1591 (90) | 1534 (94) | 467 (91) | 2925 (94) | 667 (85) | 2450 (93) | 1142 (89) | 3185 (93) | 407 (87) |
| NSAIDs, no. (%) | 857 (49) | 908 (56) | 249 (49) | 1621 (52) | 393 (50) | 1373 (52) | 641 (50) | 1788 (52) | 226 (48) |
| Systolic BP, mm Hg, mean±SD | 128±22 | 129±22 | 130±24 | 130±23 | 124±21 | 130±22 | 126±22 | 129±22 | 126±23 |
| Diastolic BP, mm Hg, mean±SD | 72 ± 13 | 71 ± 13 | 73 ± 13 | 71 ± 13 | 73 ± 13 | 71 ± 12 | 74 ± 13 | 71 ± 13 | 74 ± 13 |
| LDL cholesterol, mg/dl, mean±SD | 105±35 | 101±36 | 103±38 | 103±37 | 104±31 | 103±36 | 103±35 | 103±36 | 99±34 |
| HDL cholesterol, mg/dl, mean±SD | 48±16 | 47±15 | 47±16 | 47±15 | 51±17 | 48±15 | 47±16 | 48±15 | 47±16 |
| Hemoglobin, g/dl, mean±SD | 12.6±1.8 | 12.6±1.8 | 12.8±1.8 | 12.4±1.8 | 13.4±1.6 | 12.5±1.8 | 12.9±1.7 | 12.5±1.8 | 13.0±1.7 |
| eGFR, ml/min per 1.73 m2, mean±SD | 47±18 | 44±16 | 42±16 | 44±16 | 51±18 | 44±16 | 48±18 | 44±16 | 48±19 |
| Urinary protein, g/24 h, median (IQR) | 0.2 (0.1–0.8) | 0.2 (0.1–0.9) | 0.3 (0.1–1.3) | 0.2 (0.1–1.0) | 0.1 (0.1–0.6) | 0.2 (0.1–0.9) | 0.2 (0.1–1.0) | 0.2 (0.1–0.9) | 0.3 (0.1–1.2) |
MET, metabolic equivalent; —, no data; NSAID, nonsteroidal anti-inflammatory drug; IQR, interquartile range.
Participants were classified as users of alcohol if they reported having one or more alcoholic beverages per week.
Participants were classified as users of marijuana and hard illicit drugs if they reported any lifetime history of use.
Hard illicit drug use is defined as self-reported use of cocaine, heroin, or methamphetamine.
Numbers of events, incidence, and HRs of CKD progression and all-cause mortality associated with baseline exposures are shown in Supplemental Table 2. During 21,086 person-years of follow-up for clinical events, we documented 1287 CKD progression events (61.0/1000 person-years). During 30,365 person-years of follow-up for all-cause mortality, 1001 deaths (33.0/1000 person-years) were documented. Cumulative incidences for CKD progression, all-cause mortality, and the composite outcome of CKD progression or all-cause mortality were 29%, 24%, and 39% at median follow-up times of 5.5, 8.5, and 5.9 years, respectively. In baseline exposure analyses, former and current tobacco smoking and hard illicit drug use were significantly associated with higher risk of all-cause mortality after multivariable adjustment. Current tobacco smoking was significantly associated with higher risk of the composite outcome after multivariable adjustment.
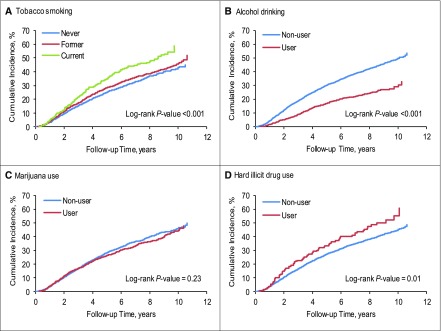
In annually updated unadjusted survival analyses, former and current tobacco smokers and hard illicit drug users had significantly higher incidence of CKD progression, whereas alcohol drinkers had significantly lower incidence of CKD progression (Figure 1). Former and current smokers and hard illicit drug users had significantly higher incidence of all-cause mortality, whereas alcohol drinkers and marijuana users had significantly lower incidence of all-cause mortality (Supplemental Figure 1).
Figure 1.
Cumulative incidence of CKD progression is higher among tobacco smokers and hard illicit drug users, and lower among alcohol drinkers, in annually-updated exposure analyses. (A) Cumulative incidence of CKD progression among never, former, and current tobacco smokers. (B) Cumulative incidence of CKD progression for alcohol drinkers and nondrinkers. (C) Cumulative incidence of CKD progression among marijuana users and nonusers. (D) Cumulative incidence of CKD progression among hard illicit drug users and nonusers.
In annually updated most recent exposure analyses, hard illicit drug use, particularly heroin use, was significantly associated with higher risk of CKD progression after multivariable adjustment (Supplemental Table 3). Former and current tobacco smoking and hard illicit drug use, particularly cocaine use, were significantly associated with higher risk of all-cause mortality, whereas alcohol drinking was significantly associated with lower risk of all-cause mortality. Former smoking, current smoking, and hard illicit drug use, particularly cocaine and heroin use, were significantly associated with higher risk of the composite outcome.
In cumulative average exposure analyses, persistent tobacco smoking (compared with nonsmoking throughout follow-up) and hard illicit drug use (compared with nonuse throughout follow-up) were significantly associated with higher risk of all outcomes, whereas persistent alcohol drinking (compared with nondrinking throughout follow-up) was significantly associated with lower risk of all outcomes after adjustment for age, sex, race/ethnicity, and clinical site (Table 2). After further multivariable adjustment, persistent tobacco smoking and hard illicit drug use remained significantly associated with all-cause mortality and the composite outcome. Persistent alcohol drinking remained significantly associated with lower risk of all-cause mortality. Of the hard illicit drugs, persistent heroin use was significantly associated with higher risk of CKD progression, and persistent cocaine use was significantly associated with higher risk of all-cause mortality and the composite outcome.
Table 2.
Hazard ratios and 95% confidence intervals of CKD progression and all-cause mortality according to cumulative average tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use among 3939 patients with CKD
| Variable | Univariable | Age, Sex, Race/Ethnicity, and Clinical Site Adjusted | Multivariable Adjusted | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CKD progression | ||||||
| Tobacco smoking | 1.52 (1.30 to 1.79) | <0.001 | 1.25 (1.06 to 1.48) | <0.01 | 1.02 (0.86 to 1.21) | 0.83 |
| Alcohol drinking | 0.50 (0.42 to 0.59) | <0.001 | 0.63 (0.53 to 0.76) | <0.001 | 1.06 (0.88 to 1.29) | 0.54 |
| Marijuana use | 0.93 (0.83 to 1.05) | 0.23 | 0.92 (0.81 to 1.04) | 0.17 | 0.94 (0.82 to 1.07) | 0.35 |
| Hard illicit drug use | 1.26 (1.02 to 1.56) | 0.03 | 1.25 (1.01 to 1.56) | 0.04 | 1.25 (1.00 to 1.55) | 0.05 |
| Cocaine use | 1.16 (0.83 to 1.63) | 0.37 | 1.00 (0.71 to 1.41) | 0.99 | 1.28 (0.90 to 1.83) | 0.17 |
| Heroin use | 2.44 (1.70 to 3.50) | <0.001 | 1.77 (1.23 to 2.56) | 0.002 | 1.57 (1.08 to 2.29) | 0.02 |
| Methamphetamine use | 1.04 (0.78 to 1.39) | 0.77 | 1.26 (0.94 to 1.68) | 0.12 | 1.09 (0.81 to 1.47) | 0.55 |
| All-cause mortality | ||||||
| Tobacco smoking | 1.87 (1.58 to 2.22) | <0.001 | 1.95 (1.64 to 2.33) | <0.001 | 1.86 (1.54 to 2.24) | <0.001 |
| Alcohol drinking | 0.50 (0.41 to 0.62) | <0.001 | 0.58 (0.47 to 0.72) | <0.001 | 0.73 (0.58 to 0.91) | <0.01 |
| Marijuana use | 0.81 (0.71 to 0.93) | 0.003 | 1.10 (0.95 to 1.28) | 0.19 | 1.11 (0.96 to 1.30) | 0.16 |
| Hard illicit drug use | 1.30 (1.03 to 1.65) | 0.03 | 1.70 (1.33 to 2.16) | <0.001 | 1.41 (1.10 to 1.81) | <0.01 |
| Cocaine use | 1.49 (1.03 to 2.16) | 0.04 | 2.21 (1.52 to 3.22) | <0.001 | 2.17 (1.46 to 3.22) | <0.001 |
| Heroin use | 2.16 (1.47 to 3.20) | <0.001 | 2.05 (1.38 to 3.06) | <0.001 | 1.40 (0.93 to 2.10) | 0.10 |
| Methamphetamine use | 1.01 (0.73 to 1.40) | 0.94 | 1.37 (0.99 to 1.91) | 0.06 | 1.21 (0.87 to 1.70) | 0.25 |
| CKD progression or all-cause mortality | ||||||
| Tobacco smoking | 1.61 (1.42 to 1.84) | <0.001 | 1.42 (1.24 to 1.63) | <0.001 | 1.17 (1.01 to 1.35) | 0.03 |
| Alcohol drinking | 0.53 (0.46 to 0.62) | <0.001 | 0.66 (0.57 to 0.77) | <0.001 | 1.00 (0.85 to 1.16) | 0.96 |
| Marijuana use | 0.89 (0.81 to 0.98) | 0.02 | 0.94 (0.85 to 1.05) | 0.27 | 0.97 (0.87 to 1.08) | 0.55 |
| Hard illicit drug use | 1.26 (1.05 to 1.50) | 0.01 | 1.33 (1.11 to 1.59) | 0.002 | 1.26 (1.04 to 1.51) | 0.02 |
| Cocaine use | 1.30 (1.00 to 1.69) | 0.05 | 1.26 (0.96 to 1.65) | 0.09 | 1.42 (1.07 to 1.88) | 0.02 |
| Heroin use | 2.14 (1.56 to 2.95) | <0.001 | 1.70 (1.23 to 2.34) | 0.001 | 1.38 (0.99 to 1.91) | 0.06 |
| Methamphetamine use | 1.03 (0.80 to 1.31) | 0.83 | 1.25 (0.98 to 1.60) | 0.07 | 1.13 (0.88 to 1.46) | 0.32 |
HRs compare persistent tobacco smoking throughout follow-up with nonsmoking, persistent alcohol drinking throughout follow-up with nondrinking, persistent marijuana use throughout follow-up with no marijuana use, and persistent hard illicit drug use (use of cocaine, heroin, or methamphetamine) throughout follow-up with no hard illicit drug use. Multivariable-adjusted analyses are adjusted for age, sex, race/ethnicity, clinical site, education, eGFR, proteinuria, history of diabetes, body mass index, systolic BP, hemoglobin, use of nonsteroidal anti-inflammatory drugs, and current smoking. HR, hazard ratio; 95% CI, 95% confidence interval.
Tables 3 and 4 depict the associations of cumulative average tobacco smoking, alcohol drinking, marijuana use, and hard illicit drug use with CKD progression by subgroups of sex, race (white and nonwhite), age (<65 and ≥65 years of age), and baseline eGFR (<45 and ≥45 ml/min per 1.73 m2). In general, point estimates were consistent within subgroups, and no significant interactions were observed after accounting for multiple testing. Similarly, no significant interactions were detected for all-cause mortality (Supplemental Table 4) or the composite outcome (Supplemental Table 5).
Table 3.
Hazard ratios and 95% confidence intervals of CKD progression associated with cumulative average tobacco smoking and alcohol drinking by subgroups
| Subgroups | Tobacco Smoking | Alcohol Drinking | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | P Value for Interaction | HR (95% CI) | P Value | P Value for Interaction | |
| Sex | ||||||
| Men | 1.06 (0.85 to 1.31) | 0.62 | 0.60 | 1.11 (0.90 to 1.38) | 0.33 | 0.37 |
| Women | 0.96 (0.74 to 1.26) | 0.79 | 0.90 (0.60 to 1.36) | 0.62 | ||
| Race | ||||||
| White | 0.96 (0.68 to 1.35) | 0.80 | 0.64 | 1.09 (0.82 to 1.45) | 0.57 | 0.81 |
| Nonwhite | 1.05 (0.86 to 1.27) | 0.63 | 1.04 (0.80 to 1.34) | 0.78 | ||
| Age, yr | ||||||
| <65 | 1.01 (0.84 to 1.22) | 0.92 | 0.90 | 1.06 (0.85 to 1.32) | 0.62 | 0.93 |
| ≥65 | 1.04 (0.69 to 1.58) | 0.85 | 1.08 (0.74 to 1.56) | 0.69 | ||
| Baseline eGFR | ||||||
| <45 | 1.02 (0.84 to 1.23) | 0.85 | 0.99 | 1.15 (0.92 to 1.44) | 0.22 | 0.51 |
| ≥45 | 1.01 (0.69 to 1.49) | 0.94 | 1.00 (0.69 to 1.44) | 0.99 | ||
HRs compare persistent tobacco smoking throughout follow-up with nonsmoking and persistent alcohol drinking throughout follow-up with nondrinking. Adjusted for age, sex, race/ethnicity, clinical site, education, eGFR, proteinuria, history of diabetes, body mass index, systolic BP, hemoglobin, use of nonsteroidal anti-inflammatory drugs, and current smoking. HR, hazard ratio; 95% CI, 95% confidence interval.
Table 4.
Hazard ratios and 95% confidence intervals of CKD progression associated with cumulative average marijuana use and hard illicit drug use by subgroups
| Subgroups | Marijuana Use | Hard Illicit Drug Use | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | P Value for Interaction | HR (95% CI) | P Value | P Value for Interaction | |
| Sex | ||||||
| Men | 0.93 (0.79 to 1.08) | 0.34 | 0.76 | 1.42 (1.10 to 1.82) | <0.01 | 0.07 |
| Women | 0.96 (0.78 to 1.19) | 0.74 | 0.88 (0.56 to 1.39) | 0.59 | ||
| Race | ||||||
| White | 0.84 (0.67 to 1.06) | 0.14 | 0.24 | 1.23 (0.84 to 1.79) | 0.29 | 0.90 |
| Nonwhite | 0.99 (0.85 to 1.15) | 0.88 | 1.26 (0.97 to 1.65) | 0.09 | ||
| Age, yr | ||||||
| <65 | 0.94 (0.81 to 1.08) | 0.38 | 0.92 | 1.26 (1.00 to 1.58) | 0.05 | 0.65 |
| ≥65 | 0.92 (0.66 to 1.29) | 0.62 | 1.02 (0.42 to 2.46) | 0.96 | ||
| Baseline eGFR | ||||||
| <45 | 0.95 (0.82 to 1.10) | 0.53 | 0.76 | 1.28 (1.00 to 1.63) | 0.05 | 0.80 |
| ≥45 | 0.91 (0.70 to 1.18) | 0.48 | 1.19 (0.72 to 1.96) | 0.50 | ||
Hazard ratios compare persistent marijuana use throughout follow-up with no marijuana use and persistent hard illicit drug use (use of cocaine, heroin, or methamphetamine) throughout follow-up with no hard illicit drug use. Adjusted for age, sex, race/ethnicity, clinical site, education, eGFR, proteinuria, history of diabetes, body mass index, systolic BP, hemoglobin, use of nonsteroidal anti-inflammatory drugs, and current smoking. HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
In this longitudinal analysis of participants with CKD, we identified the associations of persistent use of several substances with CKD progression and all-cause mortality compared with nonuse throughout follow-up. Persistent tobacco smoking and hard illicit drug use, particularly heroin and cocaine use, were associated with higher risk of CKD progression or mortality. Additionally, persistent alcohol drinking was associated with lower risk of mortality. These findings contribute to previous research documenting the harmful associations of tobacco smoking with incident CKD and all-cause mortality and extend this knowledge specifically to a population with preexisting CKD. Furthermore, our results suggest that hard illicit drug use may be associated with higher risk of CKD progression and mortality among patients with CKD, highlighting the need for vigilance regarding drug abuse among patients with CKD and evaluation of treatment and cessation programs to prevent associated risk of harmful health outcomes.
Several prior analyses have implicated tobacco smoking as an independent risk factor for incident CKD among the general population (5,6,19–23). For example, a recent analysis of 3648 black participants from the Jackson Heart Study found an independent dose-response association between current tobacco smoking and eGFR decline (23). However, associations with CKD have been less clear among patients with preexisting CKD (5). Our analyses, which updated exposure annually, indicated that persistent tobacco smoking was associated with higher risk of composite CKD progression or all-cause mortality independent of other risk factors and that it was associated with a nearly twofold higher risk of mortality. These findings support previous analyses and extend this information to a population with CKD. Other data support a link between tobacco smoking and CKD, possibly via intermediate causes, such as inflammation (23) or raised BP and diabetes (12). For example, in an analysis of data from the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial, current tobacco use was associated with 19% higher likelihood of CKD progression (odds ratio, 1.19; 95% CI, 1.06 to 1.35), but analyses limited to patients with diabetes showed no association, potentially due to the competing risk of mortality (12). In our study, although tobacco smoking was not associated with higher risk of CKD progression alone, it was strongly associated with the competing risk of all-cause mortality and the composite outcome, which adds to accumulating evidence of its role in morbidity and mortality among patients with CKD.
Studies assessing the association between alcohol use and risk of CKD have yielded somewhat conflicting results (6,11,12,22,24–26). However, in general, consumption of alcohol has been associated with protection from kidney disease complications. For example, an analysis of >65,000 Chinese men age 40 years old and older documented a protective association of both moderate (<21 drinks per week) and heavy (>21 drinks per week) consumption of alcohol on risk of ESKD (24). Similarly, an analysis conducted in the Prevention of Renal and Vascular End-Stage Disease Study suggested that any level of alcohol consumption was significantly associated with lower risk of incident CKD (27). Another study conducted in 9112 Japanese men age 40–55 years old who were not diabetic also showed a protective effect of drinking 4–7 d/wk on risk of incident CKD (11). Although the association between persistent alcohol drinking and CKD progression in our study was attenuated after multivariable adjustment, our findings suggest a protective association of persistent alcohol drinking with all-cause mortality. One explanation for this finding may be a lower risk of cardiovascular disease, which causes most deaths among patients with CKD (1). This relationship warrants further consideration in future studies.
The associations of marijuana and other illicit drugs with CKD are largely unknown. Recently, an analysis among 3765 participants with preserved eGFR from the Coronary Artery Risk Development in Young Adults Study found no longitudinal association between marijuana use and eGFR decline (28). However, one study conducted in 647 men who were hypertensive showed higher risk of mild kidney function decline associated with use of marijuana (relative risk, 1.96; 95% CI, 0.87 to 4.40) or any illicit drug (relative risk, 2.29; 95% CI, 1.04 to 5.06) (8). In our study, persistent marijuana use was not significantly associated with CKD progression or all-cause mortality. Conversely, we found that persistent use of hard illicit drugs, especially heroin and cocaine, was associated with higher risk of CKD progression and all-cause mortality, although only heroin use remained significantly associated with CKD progression after multivariable adjustment. Nephropathies of varying etiology, including GN, secondary amyloidosis, and heroin-specific nephrotic syndrome, are associated with heroin use (29,30). However, the incidence of heroin nephropathy has declined despite an increase in prevalence of heroin abuse, contributing to uncertainty regarding the independent associations between heroin use and kidney disease (31). In our study, persistent heroin use was associated with significantly higher risk of CKD progression. These findings are of public health importance given the recent increases in heroin abuse among individuals using nonmedical prescription opioids who are at 40 times higher risk of abusing heroin (32,33). Individuals with CKD should be particularly careful when using illicit drugs, because evidence suggests that illicit drug use, especially heroin and cocaine use, may be associated with worsening CKD and mortality.
This study has several strengths. To our knowledge, this is the first longitudinal analysis of tobacco, alcohol, and illicit drug use in a large sample of patients with predialysis CKD at high risk for clinical events. The CRIC Study uses standardized analysis and measurement methods across clinical sites, which minimizes bias. Additionally, individuals were followed up to 10.9 years, and annually updated exposure measurements were reported, allowing us to assess time-dependent associations. However, our study has several limitations. First, drug use was self-reported, which could result in misclassification among those who used drugs. Second, a relatively low proportion of participants reported using tobacco, alcohol, and illicit drugs over follow-up, which limits statistical power to detect weaker associations. Third, although missing data were rare for the primary exposures, missing data for several questionnaire items prohibited several analyses. For example, frequency and current use of illicit drugs were unable to be analyzed owing to missing data. Similarly, we were unable to delineate moderate and heavy alcohol consumption. Fourth, we were unable to evaluate associations with specific causes of death, such as cardiovascular disease mortality, or acute kidney outcomes. Although this information is being collected in the CRIC Study, it was not available for this analysis. Fifth, because we did not reset participants’ baseline eGFR values at each time point when calculating the halving of eGFR, we cannot guarantee a strict temporal relationship between exposure and outcome in time-updated analyses of CKD progression. However, we observed similar associations when analyzing an outcome including only patients with confirmed ESKD.
In conclusion, this study suggests that persistent tobacco, alcohol, and illicit drug use may be associated with CKD progression and all-cause mortality among patients with CKD. Tobacco smoking and hard illicit drug use, particularly heroin and cocaine use, were significantly associated with higher risk of CKD progression or all-cause mortality. Conversely, alcohol drinking was significantly associated with lower risk of all-cause mortality. Future studies are needed to confirm these results, investigate potential mechanisms, and further assess drug frequency and dose patterns.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the participants, investigators, and staff of the Chronic Renal Insufficiency Cohort (CRIC) Study for their time and commitment.
Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this study was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health/National Center for Advancing Translational Sciences grant UL1TR000003, Johns Hopkins Institute for Clinical and Translational Research grant UL1 TR-000424, University of Maryland General Clinical Research Center grant M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland grant UL1TR000439, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Awards grant UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco Clinical and Translational Science Institute grant UL1 RR-024131.
The CRIC Principal Investigators are Lawrence J. Appel (Johns Hopkins University); Harold I. Feldman, (University of Pennsylvania); Alan S. Go (Kaiser Permanente Division of Research); Jiang He (Tulane University); John W. Kusek (National Institute of Diabetes and Digestive and Kidney Diseases); James Lash (University of Illinois Chicago); Akinlolu Ojo (University of Arizona); Mahboob Rahman (Case Western Reserve University); and Raymond R. Townsend (University of Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11121017/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Mills KT, Xu Y, Zhang W, Bundy JD, Chen C-S, Kelly TN, Chen J, He J: A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88: 950–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Mortality and Causes of Death Collaborators : Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the global burden of disease study 2015. Lancet 388: 1459–1544, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Orth SR, Hallan SI: Smoking: A risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients--absence of evidence or evidence of absence? Clin J Am Soc Nephrol 3: 226–236, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Shankar A, Klein R, Klein BEK: The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 164: 263–271, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Norris KC, Thornhill-Joynes M, Robinson C, Strickland T, Alperson BL, Witana SC, Ward HJ: Cocaine use, hypertension, and end-stage renal disease. Am J Kidney Dis 38: 523–528, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, He J: The risk for mild kidney function decline associated with illicit drug use among hypertensive men. Am J Kidney Dis 43: 629–635, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Pendergraft WF 3rd, Herlitz LC, Thornley-Brown D, Rosner M, Niles JL: Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol 9: 1996–2005, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services : The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://surgeongeneral.gov/library/reports/50-years-of-progress/index.html#fullreport. Accessed July 11, 2017
- 11.Sato KK, Hayashi T, Uehara S, Kinuhata S, Oue K, Endo G, Kambe H, Fukuda K: Drinking pattern and risk of chronic kidney disease: The kansai healthcare study. Am J Nephrol 40: 516–522, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Dunkler D, Kohl M, Heinze G, Teo KK, Rosengren A, Pogue J, Gao P, Gerstein H, Yusuf S, Oberbauer R, Mann JF; ONTARGET Investigators : Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. Kidney Int 87: 784–791, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Strathdee SA, Beletsky L, Kerr T: HIV, drugs and the legal environment. Int J Drug Policy 26[Suppl 1]: S27–S32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI; CRIC Study Investigators : Association of kidney disease outcomes with risk factors for CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 63: 236–243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon R, Makuch RW: A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non-responder bias. Stat Med 3: 35–44, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC: Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 149: 531–540, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL: Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14: 479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A: Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int 71: 159–166, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hsu C-Y, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-Year follow-up. Arch Intern Med 169: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall ME, Wang W, Okhomina V, Agarwal M, Hall JE, Dreisbach AW, Juncos LA, Winniford MD, Payne TJ, Robertson RM, Bhatnagar A, Young BA: Cigarette smoking and chronic kidney disease in African Americans in the Jackson Heart Study. J Am Heart Assoc 5: 1–7, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds K, Gu D, Chen J, Tang X, Yau CL, Yu L, Chen CS, Wu X, Hamm LL, He J: Alcohol consumption and the risk of end-stage renal disease among Chinese men. Kidney Int 73: 870–876, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Schaeffner ES, Kurth T, de Jong PE, Glynn RJ, Buring JE, Gaziano JM: Alcohol consumption and the risk of renal dysfunction in apparently healthy men. Arch Intern Med 165: 1048–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 26.White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ: Alcohol consumption and 5-year onset of chronic kidney disease: The AusDiab study. Nephrol Dial Transplant 24: 2464–2472, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Koning SH, Gansevoort RT, Mukamal KJ, Rimm EB, Bakker SJL, Joosten MM; PREVEND Study Group : Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int 87: 1009–1016, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Ishida JH, Auer R, Vittinghoff E, Pletcher MJ, Reis JP, Sidney S, Johansen KL, Bibbins-Domingo K, Peralta CA, Shlipak MG: Marijuana use and estimated glomerular filtration rate in young adults. Clin J Am Soc Nephrol 12: 1578–1587, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao TK, Nicastri AD, Friedman EA: Natural history of heroin-associated nephropathy. N Engl J Med 290: 19–23, 1974 [DOI] [PubMed] [Google Scholar]
- 30.Crowe AV, Howse M, Bell GM, Henry JA: Substance abuse and the kidney. QJM 93: 147–152, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Jaffe JA, Kimmel PL: Chronic nephropathies of cocaine and heroin abuse: A critical review. Clin J Am Soc Nephrol 1: 655–667, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Compton WM, Jones CM, Baldwin GT: Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 374: 154–163, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Substance Abuse and Mental Health Services Administration: Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16-4984, NSDUH Series H-51), 2016. Available at: http://www.samhsa.gov/data/. Accessed May 30, 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.