Abstract
Background and objectives
Kidney involvement in Waldenström macroglobulinemia is less well described compared with kidney manifestations in multiple myeloma.
Design, setting, participants, & measurements
Of the 1363 patients seen with Waldenström macroglobulinemia and other IgM-secreting B cell lymphoproliferative disorders seen at the Mayo Clinic between 1996 and 2015, 57 kidney biopsies were retrospectively studied. The biopsy findings were correlated with clinical, kidney, and hematologic characteristics. Criteria for inclusion were evidence of a monoclonal IgM protein and availability of a kidney and a bone marrow biopsy for review. Glomerular and tubulointerstitial pathologies were categorized according to whether they were related to the monoclonal IgM.
Results
Of the 57 patients identified, monoclonal gammopathy–related kidney lesions were identified in 82% (47 of 57 biopsies), whereas nonmonoclonal gammopathy–related kidney lesions were seen in 18% (ten of 57). Monoclonal gammopathy–related kidney lesions included monoclonal Ig–related amyloidosis (n=19; 33%), nonamyloid glomerulopathy (n=20, 35%), and tubulointerstitial nephropathies (n=8; 14%). The most common monoclonal gammopathy–related kidney lesion was monoclonal Ig–related amyloidosis (n=19; 33%) followed by cryoglobulinemic GN (n=13; 28%). Lymphoma infiltration was the most common tubulointerstitial lesion (n=4; 9%). The hematologic diagnosis was Waldenström macroglobulinemia in 74% (n=42), monoclonal gammopathy of renal significance in 16% (n=9), and marginal zone lymphoma (n=2), chronic lymphocytic leukemia (n=2), and low-grade B cell lymphoma (n=2) in 4% each.
Conclusions
Our study confirms a diverse variety of kidney lesions in patients with monoclonal IgM gammopathy.
Keywords: Adult; Amyloidosis; Biopsy; B-Lymphocytes; Bone Marrow; glomerular disease; glomerulonephritis; Humans; IgM; Immunoglobulin M; Lymphoma, B-cell; Lymphoproliferative; MGRS; Monoclonal Gammopathy of Undetermined Significance; multiple myeloma; Myeloma Proteins; Paraproteinemias; Retrospective Studies; Waldenstrom Macroglobulinemia
Introduction
Waldenström macroglobulinemia is a lymphoproliferative disorder characterized by the presence of an IgM monoclonal protein >1 g/dl and 10% lymphoplasmacytic infiltrate in the bone marrow (1). Waldenström macroglobulinemia can be diagnosed by the Mayo Clinic criteria above or the World Health Organization and the Consensus Panel Recommendations from the Second International Workshop on Waldenström Macroglobulinemia, which differ slightly from each other (2). Like other hematologic conditions that produce a monoclonal gammopathy, kidney diseases are common in Waldenström macroglobulinemia. The kidney diseases can be the result of the malignancy (high tumor burden) or monoclonal gammopathy of renal significance (MGRS) where the clonal mass is low (3).
Whereas the kidney complications in multiple myeloma are well described, the scope of kidney pathology encountered in Waldenström macroglobulinemia has received less attention. This is likely due to the infrequency of Waldenström macroglobulinemia (1500 new patients per year in the United States) (4). Equally important are the limited data available regarding the known kidney pathologies occurring in this condition. Only in the last decade has the spectrum of kidney involvement in Waldenström macroglobulinemia and other B cell lymphoproliferative disorders been revisited (5–7). To contribute to the knowledge base, we conducted this large single-center study of patients with Waldenström macroglobulinemia and IgM B cell lymphoproliferative disorders with kidney involvement.
Materials and Methods
Patients
The Mayo Clinic’s Data Discovery and Query Database and the Mayo Clinic Renal Pathology archives were searched to identify patients with a diagnosis of Waldenström macroglobulinemia or related IgM B cell lymphoproliferative disorders between January 1, 1996 and March 31, 2015. Patients were included if they met the following criteria: (1) detectable serum monoclonal IgM by immunofixation, (2) performance of a bone marrow biopsy, and (3) a kidney biopsy. Patients were excluded for (1) lack of consent to participate in the research studies or (2) if the kidney biopsy was unavailable for review. Patients were divided into four groups on the basis of their kidney pathology. The first three groups (amyloid-related glomerulopathy, nonamyloid-related glomerulopathy, and tubulointerstitial nephropathies) had monoclonal gammopathy–related kidney pathology. The fourth group consisted of patients with nonmonoclonal gammopathy–related kidney pathology. Approval from the Mayo Clinic Institutional Review Board was obtained in accordance with federal regulations and the Declaration of Helsinki.
Clinical and Laboratory Data
Demographic, clinical, laboratory, and treatment information and outcomes were obtained retrospectively from the electronic medical records. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. The following definitions were used: nephrotic-range proteinuria is 24-hour urinary protein ≥3 g/d; nephrotic syndrome is nephrotic range proteinuria with serum albumin ≤2.5 g/dl and edema; hypertension is systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or treatment with an antihypertensive medication; and hypercalcemia is total corrected calcium >10.1 mg/dl. Kidney impairment was defined as serum creatinine ≥1.2 mg/dl, and advanced kidney failure at the time of kidney biopsy was defined as either the need for dialysis or CKD stage 5. CKD was defined as an eGFR of <60 ml/min per 1.73 m2. Clinical data and laboratory data, where available, were recorded at the time of kidney biopsy, after upfront treatment, and at last follow-up. Overall and kidney survival rates were calculated from the time of kidney biopsy to the last follow-up, ESKD, or death. IgM was measured by nephelometry.
Diagnostic and Response Criteria
The kidney and bone marrow biopsies were reviewed by two kidney pathologists and one hematopathologist. Consensus international criteria were used for the diagnoses of Waldenström macroglobulinemia, MGRS, and non-Hodgkin B cell lymphoma (1,3,8,9). Cryoglobulinemic GN was defined by the presence of endocapillary proliferative or membranoproliferative GN with abundant intracapillary infiltrating monocytes and at least one of the following additional findings: (1) glomerular intraluminal pseudothrombi, (2) focal deposits with substructure characteristic of cryoglobulin (i.e., short microtubules) on electron microscopy, and/or (3) positive cryoglobulin assay. Intracapillary monoclonal deposits disease was defined by the presence of massive glomerular intracapillary monoclonal deposits without significant glomerular hypercellularity on light microcopy, lack of organized substructure typical of cryoglobulinemic GN on electron microscopy, and negative serum cryoglobulin test. Hematologic and kidney response was defined using the International Society of Amyloidosis criteria in patients with amyloidosis and the Consensus criteria from the Sixth International Workshop on Waldenström Macroglobulinemia for hematologic response (10,11).
Statistical Analyses
Continuous variables are reported as medians with interquartile ranges (IQRs). Nonparametric analysis was performed with Wilcoxon tests. Overall survival and kidney survival analysis was performed using the Kaplan–Meier method. Statistical analysis was performed using JMP (version 10). Statistical significance was assumed at P<0.05.
Results
Patient Groups
The Mayo Clinic’s Data Discovery and Query Database search found 57 patients with Waldenström macroglobulinemia or B cell lymphoproliferative disorders who had both a bone marrow and a kidney biopsy for review from a total of 1363 patients seen during the same period. A monoclonal gammopathy–related pathology alone was noted in 42 (74%) kidney biopsies, and ten showed a nonmonoclonal gammopathy–related pathology alone. In five biopsies, more than one pathologic process was present. All five had a monoclonal gammopathy–related pathology, but four of five also showed a nonmonoclonal gammopathy–related pathology (Table 1).
Table 1.
Kidney lesions of patients with Waldenström macroglobulinemia and other IgM monoclonal gammopathy
| Group 1: Amyloid Glomerulopathy, N=19 | Group 2: Nonamyloid Glomerulopathy, N=20 | Group 3: Tubulointerstitial Lesions, N=8 | Group 4: Nonparaprotein-Related Lesions, N=10 |
|---|---|---|---|
| Monoclonal light-chain amyloidosis (16) | Cryoglobulinemic GN (12) | Lymphoma infiltration (4) | Minimal change disease (2) |
| Monoclonal light- and heavy-chain amyloidosis (2) | Immunotactoid GN (2) | Light-chain cast nephropathy (2) | Acute tubular necrosis (2) |
| Monoclonal light- and heavy-chain amyloidosis with membranous nephropathy (1)a | Intracapillary monoclonal deposits disease (2) | Lymphoma infiltration with light-chain cast nephropathy (1)a | Secondary FSGS (1) |
| Proliferative GN with monoclonal Ig deposits (1) | Lymphoma infiltration and ANCA-associated GN (1)a | FSGS (1) | |
| Monoclonal IgG–associated membranous nephropathy (1) | Acute tubular necrosis and acute interstitial nephritis (1) | ||
| Light-chain deposition disease (1) | Diabetic nephropathy (1) | ||
| Mesangial proliferative GN with minimal change disease and lymphoma infiltration (1)a | Immune complex–mediated proliferative GN (1) | ||
| Thrombotic microangiopathy (1) |
More than one pathologic feature.
The pathologies in the amyloid-related glomerulopathy group (n=19) included Ig light-chain amyloidosis (n=16), Ig heavy- and light-chain (IgM-λ) amyloidosis (n=2), and Ig heavy- and light-chain amyloidosis (IgM-κ) with membranous nephropathy (n=1). The nonamyloid-related glomerulopathy group (n=20) included cryoglobulinemic GN (n=12) (Figure 1), proliferative GN with monoclonal IgM deposits (n=1) (Figure 2), intracapillary monoclonal deposits disease (n=2), light-chain deposition disease (n=1), immunotactoid glomerulopathy (n=2; with monotypic IgM κ-deposits in the one patient in whom glomeruli were sampled for immunofluorescence), mesangial proliferative GN secondary to monoclonal Ig deposits and minimal change disease (n=1), and monoclonal IgG–associated membranous nephropathy with nonorganized deposits (n=1). Tubulointerstitial nephropathies (n=8) included lymphoma infiltration alone (n=4) (Figure 3), light-chain cast nephropathy (n=2), light-chain cast nephropathy with lymphoma infiltration (n=1), and lymphoma infiltration with ANCA-associated GN (n=1). Nonmonoclonal gammopathy–related kidney pathology findings included FSGS (n=2), minimal change disease (n=2), acute tubular necrosis (ATN; n=2), ATN with acute interstitial nephritis (n=1), thrombotic microangiopathy (n=1), immune complex–mediated GN (n=1), and diabetic nephropathy (n=1).
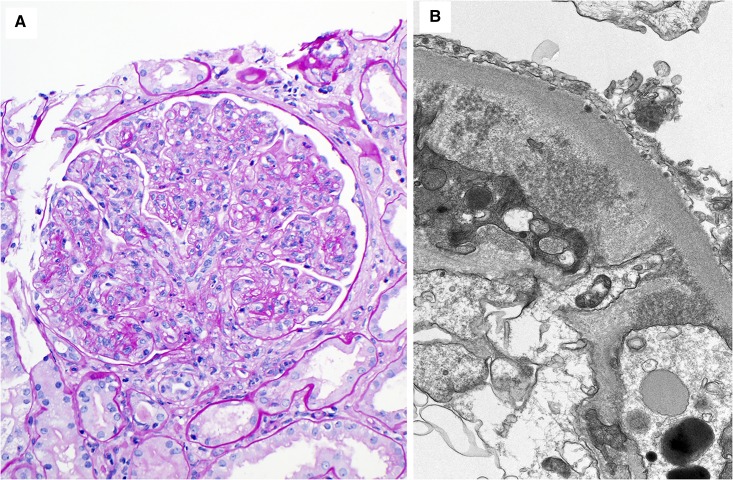
Figure 1.
Cryoglobulinemic GN. Cryoglobulinemic GN is the most common nonamyloid glomerulopathy in this series. (A) The glomerulus shows a membranoproliferative GN pattern of injury. There is global mesangial and endocapillary hypercellularity, numerous intracapillary infiltrating monocytes and some neutrophils, and duplication of the glomerular basement membranes associated with cellular interposition (periodic acid–Schiff stain). Magnification, ×200. (B) An electron microscopy figure from the same case showing small mesangial electron deposits composed of ill-defined short curved or straight microtubules. Magnification, ×20,000.
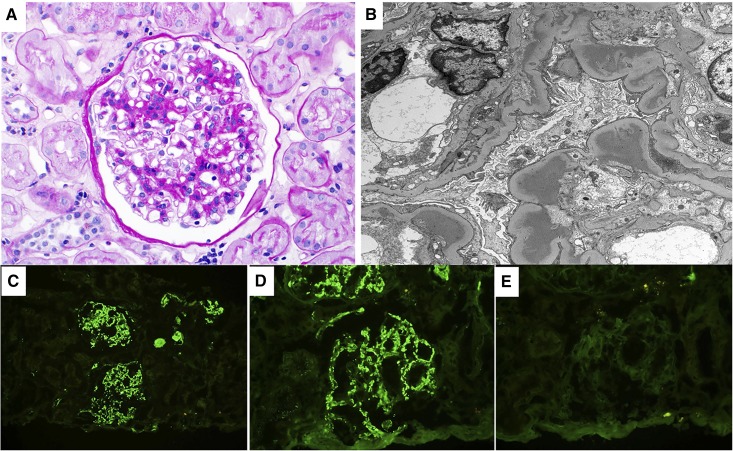
Figure 2.
Proliferative GN with monoclonal Ig deposits, IgM variant. This figure describe an IgM variant of the proliferative GN with monoclonal Ig deposits which was initially described only with monoclonal IgG deposits. (A) Light microscopy exhibits a mesangial proliferative GN pattern of injury (periodic acid–Schiff stain). (B) On electron microscopy, granular mesangial and subendothelial electron dense deposits (without substructure) are seen. On immunofluorescence, there was bright granular glomerular mesangial positivity for (C) IgM and (D) κ-light chain with negative glomerular staining for (E) λ-light chain. Glomeruli were negative for IgG and IgA (not shown). Magnification, ×400 in A, D, and E; ×4800 in B; ×200 in C.
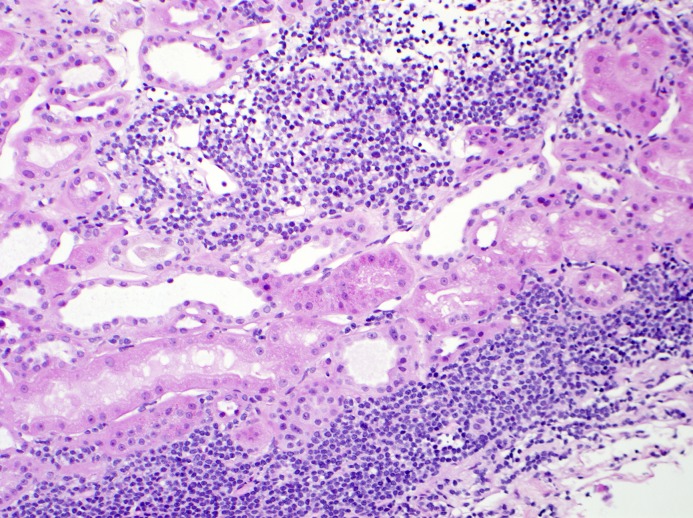
Figure 3.
Lymphomatous infiltration of the kidney. Lymphomatous infiltration which can be seen alone or with other lesions in the kidney is common in patients with B-cell lymphoproliferative disorders. There is a dense patchy interstitial infiltration by monotonous small lymphocytes (hematoxylin and eosin). Immunohistochemical staining of this infiltrate was consistent with interstitial involvement by marginal zone lymphoma. Note the absence of tubulitis. Magnification, ×200.
Demographics and Kidney Characteristics at Biopsy
The demographics and kidney characteristics for the 47 patients with monoclonal gammopathy–related pathology are summarized in Table 2. There were a total of 31 men and 16 women. The median age at kidney biopsy for the 47 patients was 63 years old (IQR, 61 to 70). Median follow-up was similar among the three groups of patients with monoclonal gammopathy–related kidney disease (P=0.51 from bone marrow biopsy and P=0.96 from kidney biopsy).
Table 2.
Demographics and characteristics at kidney biopsy of cohort of patients with Waldenström macroglobulinemia and other IgM monoclonal gammopathy
| Characteristics | Monoclonal Gammopathy–Related Kidney Diseases | Amyloid | Nonamyloid | Tubulointerstitial Nephritides | Nonmonoclonal |
|---|---|---|---|---|---|
| N | 47 | 19 | 20 | 8 | 10 |
| Age (IQR), yr | 63 (61–70) | 64 (58–70) | 63 (55–68) | 64 (59–73) | 63 (61–70) |
| Sex, men/women | 31/16 | 14/5 | 12/8 | 5/3 | 8/2 |
| Serum creatinine (IQR), mg/dl | 1.8 (1.1–2.6) | 1.8 (1–2.8) | 1.4 (0.9–2.1) | 2.3 (2–5.4) | 2.1 (1.8–3.6) |
| eGFR (IQR), ml/min per 1.73 m2 | 35 (22–71) | 41 (18–79) | 42 (28–77) | 25 (10–28) | 30 (16–37) |
| Kidney impairment, % | 67 | 63 | 58 | 100 | 90 |
| Advanced kidney failure, % | 16 | 16 | 11 | 29 | 13 |
| History of CKD, % | 13 | 11 | 11 | 25 | 10 |
| Proteinuria (IQR), g/d | 2.8 (1.1–8.0) | 6.8 (2.4–12.4) | 2.5 (0.6–3.6) | 2.2 (0.2–4.6) | 3.4 (1.8–17) |
| Nephrotic-range proteinuria, % | 45 | 72 | 26 | 29 | 50 |
| Hematuria, % | 49 | 53 | 62 | 29 | 17 |
| Median serum albumin (IQR), g/dl | 3.2 (2.3–3.6) | 2.6 (1.7–3.6) | 3.3 (2.8–4.1) | 3.4 (3.1–3.7) | 3.2 (2.3–3.9) |
| Hypertension, % | 66 | 42 | 85 | 75 | 38 |
| Edema, % | 65 | 84 | 68 | 13 | 50 |
| Nephrotic syndrome, % | 46 | 65 | 39 | 25 | 20 |
| Timing of kidney biopsy, % | |||||
| Kidney biopsy before bone marrow biopsy | 21 | 16 | 25 | 25 | 40 |
| Kidney biopsy within 1 wk of bone marrow biopsy | 28 | 32 | 25 | 25 | 0 |
| Kidney biopsy after bone marrow biopsy | 51 | 53 | 50 | 50 | 60 |
IQR, interquartile range.
The median time between kidney and bone marrow biopsy was 0.4 months (IQR, −0.3 to 9) for all 57 patients. Bone marrow biopsy was performed >1 week before kidney biopsy in 25%, whereas 53% had their kidney biopsy first. Both biopsies were performed within a week of each other in 23%. Only two (4%) patients had their kidney biopsy >1 year before the bone marrow biopsy, whereas 12 (21%) patients had their bone marrow biopsy >1 year before the kidney biopsy, which was not significant (P=0.66). Details of the timing between biopsies for the patients with monoclonal gammopathy–related kidney diseases only are presented in Table 2.
The median serum creatinine (1.8 mg/dl; IQR, 1.1 to 2.6) and the eGFR (median =35 ml/min per 1.73 m2; IQR, 22 to 71) at the time of kidney biopsy were only significantly different between patients with nonamyloid-related glomerulopathy and tubulointerstitial nephropathies (P=0.04 and P=0.01, respectively) (Table 2). A prior diagnosis of CKD was present in only six (13%) patients, and it was not statistically significant among the three groups (P=0.54). Kidney impairment was present in 67% at the time of kidney biopsy, and it was similar among the three groups with monoclonal gammopathy–related kidney diseases (P=0.12). Seven (16%) patients had advanced kidney injury at the time of kidney biopsy.
The median 24-hour urine protein was significantly higher in patients with amyloid-related glomerulopathy (6.8 g/d; IQR, 10.0) than those with nonamyloid-related glomerulopathy (2.5 g/d; IQR, 3.0) and tubulointerstitial nephropathies (2.2 g/d; IQR, 4.4; P=0.01) (Table 2). The frequency of nephrotic-range proteinuria was highest in patients with amyloid-related glomerulopathy (72% versus 26% in patients with nonamyloid-related glomerulopathy and 29% in patients with tubulointerstitial nephropathies; P=0.01). Albuminuria on the urine electrophoresis was significantly lower in patients with tubulointerstitial nephropathies (12%) compared with those in the amyloid-related glomerulopathy group (73%) and the nonamyloid-related glomerulopathy group (69%; P=0.02). The median serum albumin was lowest in the amyloid-related glomerulopathy group (2.6 g/dl; IQR, 1.7 to 3.6), but it was not significantly different from that in patients with nonamyloid-related glomerulopathy (3.3 g/dl; IQR, 2.8 to 4.1) or tubulointerstitial nephropathies (3.4 g/dl; IQR, 3.1 to 3.7; P=0.19). Edema was highest in patients with amyloid-related glomerulopathy (84%) followed by those with nonamyloid-related glomerulopathy (68%) and then, tubulointerstitial nephropathies group (13%; P=0.002). Nephrotic syndrome was most common in the amyloid-related glomerulopathy group (65%) but was not statistically significant (P=0.12). The finding of microscopic hematuria was highest in patients with nonamyloid-related glomerulopathy (58%) compared with patients with amyloid-related glomerulopathy (37%) or tubulointerstitial nephropathies (25%), but it did not reach statistically significance (P=0.44). Hypertension was most common in patients with nonamyloid-related glomerulopathy (85%) compared with those with tubulointerstitial nephropathies (75%), and it was lowest in the amyloid-related glomerulopathy group (42%; P=0.02).
Hematologic Characteristics
Serum monoclonal protein testing was performed on 97% of the patients before the kidney biopsy. Median M spike was 0.8 g/dl (IQR, 0.2 to 1.4) in the patients with monoclonal gammopathy–related kidney diseases and 0.7 g/dl (IQR, 0.1 to 2.5) in the patients with nonmonoclonal gammopathy–related kidney pathology (P=0.06). Significant differences exist in the M spike at kidney biopsy for patients with monoclonal gammopathy–related kidney diseases (P=0.01) (Table 3). Urine protein electrophoresis performed on 33 patients (28 from patients with monoclonal gammopathy–related kidney diseases and five from patients with nonmonoclonal gammopathy–related kidney pathology) was positive in 88%. Monoclonal free light chain alone was detected in the urine in 28%, Ig in 24%, and both Ig and free light chain in 48%. There was no difference among the different groups of kidney diseases.
Table 3.
Hematologic characteristics of cohorot of patients with Waldenström macroglobulinemia and other IgM monoclonal gammopathy
| Laboratory Values at Kidney Biopsy | Amyloid Glomerulopathy | Nonamyloid Glomerulopathy | Tubulointerstitial Nephritides | Nonmonoclonal Gammopathy Related |
|---|---|---|---|---|
| No. of patients | 19 | 20 | 8 | 10 |
| Hematologic diagnosis (%) | ||||
| Waldenström macroglobulinemia | 17 (90) | 9 (45) | 7 (88) | 9 (90) |
| Monoclonal gammopathy of renal significance | 2 (11) | 6 (30) | 0 | 1 (10)a |
| CD5-negative B cell lymphoma | 0 | 2 (10) | 0 | 0 |
| Marginal zone lymphoma | 0 | 1 (5) | 1 (13) | 0 |
| Monoclonal B cell lymphocytosis/chronic lymphocytic leukemia | 0 | 2 (10) | 0 | 0 |
| Light-chain type (%) | ||||
| κ | 7 (37) | 17 (85) | 6 (75) | 9 (90) |
| λ | 11 (58) | 1 (5) | 2 (25) | 1 (10) |
| Biclonal | 1 (5) | 2 (10) | 0 | 0 |
| Median K-to-L ratio (IQR) | 1.79 (0.11–12.68) | 3.49 (1.77–8.43) | 4.82 (1.7–45.5) | 2.14 (1.74–2.54) |
| Markedly (more than eight) abnormal Free Light Chain ratio, % | 57 | 21 | 29 | 0 |
| Median M spike at kidney biopsy (IQR), g/dl | 1.2 (0.5–1.7) | 0.2 (0–1.1) | 0.9 (0.7–1.8) | 0.7 (0–2.48) |
| Serum IgM (IQR), mg/dl | 1415 (908–1972) | 729 (183–2600) | 2730 (644–6160) | 2810 (606–3600) |
| B2-microglobulin (IQR), μg/ml | 4.6 (2.0–10.8) | 4.0 (2.4–7.4) | 6.9 (5.8–21.9) | 15.3 (9.4–21.7) |
| Median % LPL cells on BM biopsy (IQR) | 25 (17.5–70) | 10 (5–30) | 40 (5–55) | 45 (5.6–53.8) |
| Lymphoplasmacytic leukemic cells on BM biopsy >10%, % | 91 | 43 | 71 | 63 |
| Calcium (IQR), mg/dl | 9.0 (8.4–9.8) | 9.4 (8.7–9.8) | 9.1 (8.7–9.6) | 9.2 (8.7–9.9) |
| Median Hgb at kidney biopsy (IQR), g/dl | 12.2 (10.8–13.8) | 8.6 (8.0–13.1) | 11.1 (6.4–12.2) | 10.7 (9.7–11.5) |
| Hepatomegaly, % | 16 | 0 | 0 | 0 |
| Splenomegaly, % | 11 | 10 | 13 | 40 |
| Lymphadenopathy, % | 39 | 25 | 25 | 20 |
| Time from first detection of IgM MG to kidney biopsy, mo (IQR) | 23.2 (6–191) | 1.1 (0.2–6) | 0.8 (0.5–40) | 12.4 (6–61) |
| Cryoglobulins: no. of patients tested (%) | 4 (21) | 16 (80) | 6 (75) | |
| Positive cryoglobulin, % | 0 | 63 | 0 | |
| Type 1, % | 0 | 25 | 0 | |
| Type 2, % | 0 | 38 | 0 | |
| Complement: % patients tested | 21 | 70 | 13 | |
| Low C3 (<75 mg/dl) | 25 | 50 | 100 | |
| Low C4 (<14 mg/dl) | 50 | 64 | 100 | |
| Patients tested for hepatitis B and C, % | 72 | 80 | 75 | |
| Hepatitis B positivity in those tested | 0 | 0 | 0 | |
| Hepatitis C positivity in those tested | 7 | 6 | 0 | |
| Patients tested for rheumatoid factor, % | 17 | 50 | 25 | |
| Rheumatoid factor positive in those tested | 33 | 40 | 50 |
C3 reference range: 75–175 mg/dl. C4 reference range: 14–40 mg/dl. Positive rheumatoid factor >15 IU/ml. K, kappa: L, lambda; IQR, interquartile range; LPL, lymphoplasmacytic lymphoma; BM, bone marrow; Hgb, hemoglobin; MG, monoclonal gammopathy.
The condition is monoclonal gammopathy of undetermined significance, because the kidney disease is not related to the monoclonal gammopathy.
λ-Light chain was more common in patients with amyloid-related glomerulopathy (58%), but κ was more common in patients with nonamyloid-related glomerulopathy (85%) or tubulointerstitial nephropathies (75%; P<0.01). One patient with amyloid-related glomerulopathy and two patients with nonamyloid-related glomerulopathy had a biclonal gammopathy. Serum free light-chain test was performed on 35 patients with monoclonal gammopathy–related kidney pathology and two patients with nonmonoclonal gammopathy–related kidney pathology. Abnormal serum free light-chain ratio was noted in 74% and 0%, respectively. The median κ-to-λ ratio was 1.79 (IQR, 0.11 to 12.68) in patients with amyloid-related glomerulopathy, 3.49 (IQR, 1.77 to 8.43) in patients with nonamyloid-related glomerulopathy, and 4.82 (IQR, 1.7 to 45.5) in patients in the tubulointerstitial nephropathies group, but it was not significantly different (P=0.46). Markedly abnormal free light-chain ratio (<0.125 or >8) was similar among the groups (57% in patients with amyloid-related glomerulopathy, 21% in the nonamyloid-related glomerulopathy group, and 29% in the tubulointerstitial nephropathies group; P=0.13). Serum IgM levels at kidney biopsy were also similar among the three groups (P=0.24).
There were 42 patients with a diagnosis of Waldenström macroglobulinemia, eight patients with MGRS, one patient with monoclonal gammopathy of undetermined significance, two patients with monoclonal B cell lymphocytosis, two patients with marginal zone lymphoma, and two patients with CD5-negative low-grade B cell lymphoma. In the 47 patients with only monoclonal gammopathy–related kidney pathology, 70% had a diagnosis of Waldenström macroglobulinemia, 17% had a diagnosis of MGRS, 4% had a diagnosis of CD5-negative B cell lymphoma, 4% had a diagnosis of monoclonal B cell lymphocytosis, and 4% had a diagnosis of marginal zone lymphoma. Waldenström macroglobulinemia was more common in patients with amyloid-related glomerulopathy and those with tubulointerstitial nephropathies, whereas MGRS was more common in the nonamyloid-related glomerulopathy group (Table 3). The median hemoglobin at kidney biopsy was lowest in patients with nonamyloid-related glomerulopathy at 8.6 g/dl (IQR, 8.0 to 13.1) compared with 12.2 g/dl (IQR, 10.8 to 13.8) in patients with amyloid-related glomerulopathy and 11.1 g/dl (IQR, 6.4 to 12.2) in the tubulointerstitial nephropathies group (P=0.04). MYD88 L265P mutation was tested on nine patients, with five positive (two with the amyloid-related glomerulopathy group and three from the tubulointerstitial nephropathies group), three negative (two from the tubulointerstitial nephropathies group and one from the nonmonoclonal gammopathy–related kidney pathology group), and one equivocal (from the amyloid-related glomerulopathy group).
Cryoglobulins were tested in 21% of patients with amyloid-related glomerulopathy compared with 80% of patients in the nonamyloid-related glomerulopathy group and 75% of patients in the tubulointerstitial nephropathies group (Table 3). Of the 12 patients with cryoglobulinemic GN, three tested positive for type 1 cryoglobulin, six tested positive for type 2 cryoglobulin, two were negative, and one did not undergo testing around the time of the kidney biopsy. Only one patient with type 2 cryoglobulinemia was positive for hepatitis C serology of 25 patients tested. A similar difference in testing for complement was noted among the groups, with the majority of testing performed in patients with nonamyloid-related glomerulopathy (70%) compared with 21% in the amyloid-related glomerulopathy group and 13% in the tubulointerstitial nephropathies group (P=0.002). No significant difference was noted among the groups, and there was no correlation between complement levels and liver function tests.
In the patients with amyloidosis, systemic involvement included heart (n=1), heart and liver (n=1), heart and nerves (n=1), liver (n=1), and nerves (n=2). In the patients with cryoglobulinemia, two patients had rheumatoid arthritis, four had vasculitic rash, one had Sjogren syndrome, and one had nerve involvement. One of the two patients with intracapillary monoclonal deposits disease also had a stroke, although it is unclear if the two were related.
Treatment
Frontline therapy was not available for two patients in the amyloid-related glomerulopathy group and four patients with nonamyloid-related glomerulopathy. There was no significant difference in terms of timing of treatment, pre- or postkidney biopsy, among the three groups (P=0.88) (Table 4). Treatment for the lymphoproliferative disorder was started in 47% of patients before the kidney biopsy (ten with amyloid-related glomerulopathy, eight with nonamyloid-related glomerulopathy, one with tubulointerstitial nephropathies, and four with nonmonoclonal gammopathy–related kidney diseases). Treatment was heterogeneous in terms of both the agents used and the number of treatment lines administered. Agents included alkylator-based chemotherapy, rituximab-based therapy, and novel agents, such as bortezomib. Rituximab was used as the frontline agent in 60% of patients (ten with amyloid-related glomerulopathy, 11 with nonamyloid-related glomerulopathy, six with tubulointerstitial nephropathies, and six with nonmonoclonal gammopathy–related kidney diseases). Autologous stem cell transplant was used in eight patients, all with amyloid-related glomerulopathy. Plasmapheresis was used primarily in patients with nonamyloid-related glomerulopathy (40%) and those in the tubulointerstitial nephropathies group (38%), and it was used in only one patient with amyloid-related glomerulopathy (5.6; P=0.04).
Table 4.
Treatment and outcomes of cohort patients with Waldenström macroglobulinemia and other IgM monoclonal gammopathy
| Therapy and Response | Amyloid | Nonamyloid | Tubulointerstitial Nephritides |
|---|---|---|---|
| N | 19 | 20 | 8 |
| Chemotherapy before kidney biopsy, % | 42 | 45 | 63 |
| Chemotherapy after kidney biopsy, % | 52 | 50 | 37 |
| Frontline treatment, % | |||
| Steroids alone | 0 | 10 | 0 |
| Rituximab alone | 21 | 35 | 63 |
| Rituximab and chemotherapy | 32 | 10 | 13 |
| Bendamustine and rituximab | 0 | 5 | 0 |
| Bortezomib-based chemotherapy | 5 | 5 | 0 |
| Chemotherapy | 37 | 30 | 25 |
| No chemotherapy | 5 | 5 | 0 |
| Second-line treatment, % | |||
| Steroids alone | 0 | 15 | 0 |
| Rituximab alone | 5 | 10 | 13 |
| Rituximab and chemotherapy | 10 | 15 | 25 |
| Bendamustine and rituximab | 10 | 5 | 0 |
| Bortezomib-based chemotherapy | 21 | 10 | 25 |
| Autologous stem cell transplant | 16 | 0 | 0 |
| Chemotherapy | 26 | 20 | 13 |
| No chemotherapy | 11 | 25 | 25 |
| Plasmapheresis | 5 | 40 | 38 |
| Any dialysis | 32 | 25 | 25 |
| Hematologic response, % | |||
| Complete response | 0 | 15 | 0 |
| Very good partial response | 11 | 0 | 13 |
| Partial response | 53 | 10 | 25 |
| Stable disease | 11 | 10 | 13 |
| Progression | 5 | 10 | 0 |
| Not accessible | 21 | 55 | 50 |
| Kidney outcomes, % | |||
| Stable/improved | 37 | 65 | 25 |
| Worsened | 16 | 20 | 38 |
| ESKD (dialysis dependent or eGFR <15) | 37 | 5 | 25 |
| Loss to follow-up | 11 | 10 | 13 |
| Death, % | 53 | 22 | 25 |
Chemotherapy included fludarabine, cladribine, chlorambucil, bendamustine, and various combinations of cyclophosphamide, vincristine, adriamycin, and prednisone.
Outcomes
Hematologic response was assessable in 60% of patients. Very good partial response (VGPR) was noted in 6% of patients, and complete response (CR) was noted in 6% of patients. CR was achieved in three patients with nonamyloid-related glomerulopathy, and VGPR was achieved in two patients with amyloid-related glomerulopathy and one patient with tubulointerstitial nephropathies. The most common response was partial response, which accounted for 30%. Minimal response or less was observed in 17%. All of the patients who achieved CR received rituximab, and two received an autologous stem cell transplant. Two of the three patients with VGPR received rituximab and additional lines of therapy. No information was available for one patient. Responses by kidney lesion groups are listed in Table 4.
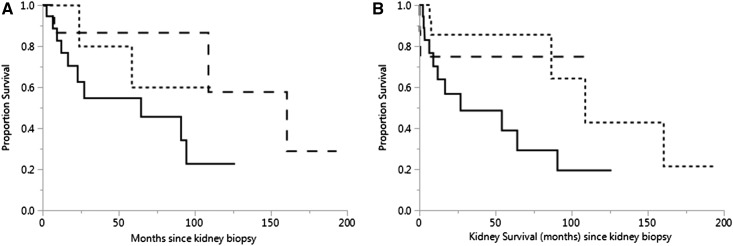
Median overall survival was 64.4 months in patients with amyloid-related glomerulopathy and 160.5 months in patients with nonamyloid-related glomerulopathy; it was not reached in the tubulointerstitial nephropathies group (Figure 4A) (P=0.22). Cause of death for most patients with Ig light-chain amyloidosis was unknown, but all had progressive disease at the time of their deaths (n=9). For patients with Waldenström macroglobulinemia without amyloidosis, infection (n=2), Bing Neel syndrome (n=1), progressive disease (n=1), metastatic transitional cell carcinoma (n=1), and unknown (n=4) were among the causes of death. Infection was also the cause of death for one patient with MGRS.
Figure 4.
(A) Median overall survival of 45 patients with monoclonal gammopathy–related kidney diseases from time of kidney biopsy. While both overall and kidney survival were inferior in patients with amyloid-related glomerulopathy, only the overall survival was statistically significant. Patients with amyloid-related glomerulopathy (n=19) are represented by the solid line, patients with nonamyloid-related glomerulopathy (n=18) are represented by the dashed line, and patients with tubulointerstitial nephropathies (n=8) are represented by the dotted line. Median survival was 64.4 months in patients with amyloid-related glomerulopathy and 160.5 months in the nonamyloid-related glomerulopathy group but had not been reached in patients with tubulointerstitial nephropathies (P=0.19). (B) Kidney survival from the time of kidney biopsy. Median kidney survival was reached only by patients with amyloid-related glomerulopathy (94.2 months; n=16), and it was not statistically different (P=0.19) from that of patients with nonamyloid-related glomerulopathy (n=16) or patients with tubulointerstitial nephropathies (n=7).
Kidney parameters improve or stabilized in 47% of patients and worsened in 21% of patients; 21% of patients went on to ESKD. Improvement or stabilization was most common in patients with nonamyloid-related glomerulopathy (65%), whereas it was lowest in patients with tubulointerstitial nephropathies (25%). Dialysis (at any time) was required for seven (37%) patients with amyloid-related glomerulopathy, five (25%) patients with nonamyloid-related glomerulopathy, and two (25%) patients with tubulointerstitial nephropathies (P=0.88). ESKD was highest in patients with amyloid-related glomerulopathy (37%) and lowest in those with nonamyloid-related glomerulopathy (5%). This was not significantly different between the two groups (P=0.07). In the patients with monoclonal gammopathy–related disease, improvement or stabilization, deterioration of kidney function, or ESKD was noted in 82%, 80%, and 100% of patients who did not achieve a VGPR versus 18%, 20%, and 0% of those who did, respectively (P=0.33). Median kidney survival was 27 months for patients with amyloid-related glomerulopathy and 109 months for those with nonamyloid-related glomerulopathy; it was not reached by the patients in the tubulointerstitial nephropathies group (Figure 4B) (P=0.14).
Discussion
To the best of our knowledge, this represents the largest series of patients with Waldenström macroglobulinemia and IgM secreting lymphoproliferative disorders with a kidney biopsy. Although the kidney involvement is lower in Waldenström macroglobulinemia, the variety of kidney disease is similar to multiple myeloma. Unlike multiple myeloma, however, there does not appear to be a signature kidney lesion in Waldenström macroglobulinemia. Intracapillary monoclonal deposits disease was identified in 38% in the study by Morel-Maroger et al. (12), twice as often as amyloid deposits. It was also the most common kidney pathology (36%) in a study from France between 1989 and 2005 (5). Membranoproliferative GN and lymphomatous infiltration were the next most common, with 21% each. Amyloid deposits and acute tubular injury were found in 7% each. A more recent series of 35 patients from France (between 1992 and 2012), however, found only a single patient with intracapillary monoclonal deposits disease (6). In that series, monoclonal Ig–related amyloidosis was the most common (n=11) followed by light-chain cast nephropathy and cryoglobulinemic GN (five each). Membranoproliferative GN and mesangial GN each had four patients followed by Fanconi syndrome (n=3). Light-chain deposits disease and ATN each had one patient. Monoclonal Ig–related amyloidosis was again the most common pathologic findings (n=11) in a series from Dana-Farber followed by intracapillary monoclonal deposits disease (n=10) (7). It is important to note that intracapillary monoclonal deposits disease and cryoglobulinemic GN were lumped together in this study, whereas these entities were reported separately in our study and the study by Chauvet et al. (6). This may explain why intracapillary monoclonal deposits disease is much lower in some series (5–7,12). Interestingly, the original study by Morel-Maroger et al. (12) did note that patients with intracapillary monoclonal deposits disease had either cryoglobulinemia or high IgM levels (12). Lymphomatous infiltration was common in the Dana-Farber study (n=8) as was light-chain cast nephropathy and light-chain deposition disease (four each), with only one patient with light-chain tubulopathy (7). This series also noted minimal change disease (n=2) and membranous nephropathy (n=1) as we did, but they were not reported in the other series.
Three patients in our series were found to have light-chain cast nephropathy, a finding commonly associated with multiple myeloma but considered rare in Waldenström macroglobulinemia. Five (14%) patients from the study by Chauvet et al. (6) also presented with light-chain cast nephropathy. It is important to differentiate between Waldenström macroglobulinemia and the rare entity of IgM multiple myeloma. Although IgM multiple myeloma may be differentiated from Waldenström macroglobulinemia by bone marrow biopsy, flow cytometric features, the lack of organomegaly, and the presence of osteolytic lesions, currently, the most specific feature is the presence of the MYD88 L265P mutation, which is present in >90% of patients with Waldenström macroglobulinemia but absent in those with multiple myeloma or other B cell lymphoproliferative disorders (8,13).
There are two findings in this study that deserve further discussion. First is the lack of correlation between the hematologic response and kidney outcomes. This was surprising, because many of the studies of kidney diseases secondary to MGRS have found this relationship (6,14–16). This is most likely due to the low rate (11%) of deep hematologic responses in our study, since a minimum of a VGPR is required for preservation of kidney function in monoclonal gammopathy–related kidney diseases. Second is exclusion of thrombotic microangiopathy in the monoclonal gammopathy–related kidney lesions. Although traditionally, thrombotic microangiopathy is not considered to be secondary to monoclonal gammopathy, there are now increasing numbers of patients with cases that suggest that the two may, in fact, be linked (7,17,18). Because there is still no consensus on this matter, thrombotic microangiopathy was placed in the nonmonoclonal gammopathy–related kidney pathology category for this study.
Our study confirms that the variety of kidney lesions is diverse in patients with Waldenström macroglobulinemia. Although monoclonal Ig–related amyloidosis seems to have a nonsignificant effect on survival, it remains unclear if the other kidney lesions affect survival. Further research is needed to determine the true effect of kidney disease on these patients. In addition, further studies are needed to determine the effectiveness of treatment on the progression of kidney disease. Hopefully, this study and other recent studies have raised awareness of kidney disease in patients with Waldenström macroglobulinemia.
Disclosures
A.D. received research support from Celgene, Takeda, Jannsen, Prothena, Pfizer, and Alnylam. C.A.T. received research support from Celgene. N.L. consults for Prothena and Takeda and received research support from Omeros.
Dr. Leung's work is supported by the generous donations from Mr. and Mrs. Ted Kirshenbaum, Dr. Gary Kohler and Anne Drennan.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ansell SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, Morice WG, Bergsagel PL, Buadi FK, Colgan JP, Dingli D, Dispenzieri A, Greipp PR, Habermann TM, Hayman SR, Inwards DJ, Johnston PB, Kumar SK, Lacy MQ, Lust JA, Markovic SN, Micallef IN, Nowakowski GS, Porrata LF, Roy V, Russell SJ, Short KE, Stewart AK, Thompson CA, Witzig TE, Zeldenrust SR, Dalton RJ, Rajkumar SV, Gertz MA: Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc 85: 824–833, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA: Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 30: 110–115, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA; International Kidney and Monoclonal Gammopathy Research Group : Monoclonal gammopathy of renal significance: When MGUS is no longer undetermined or insignificant. Blood 120: 4292–4295, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Groves FD, Travis LB, Devesa SS, Ries LA, Fraumeni JF Jr: Waldenström’s macroglobulinemia: Incidence patterns in the United States, 1988-1994. Cancer 82: 1078–1081, 1998 [PubMed] [Google Scholar]
- 5.Audard V, Georges B, Vanhille P, Toly C, Deroure B, Fakhouri F, Cuvelier R, Belenfant X, Surin B, Aucouturier P, Mougenot B, Ronco P: Renal lesions associated with IgM-secreting monoclonal proliferations: Revisiting the disease spectrum. Clin J Am Soc Nephrol 3: 1339–1349, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauvet S, Bridoux F, Ecotière L, Javaugue V, Sirac C, Arnulf B, Thierry A, Quellard N, Milin S, Bender S, Goujon JM, Jaccard A, Fermand JP, Touchard G: Kidney diseases associated with monoclonal immunoglobulin M-secreting B-cell lymphoproliferative disorders: A case series of 35 patients. Am J Kidney Dis 66: 756–767, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Vos JM, Gustine J, Rennke HG, Hunter Z, Manning RJ, Dubeau TE, Meid K, Minnema MC, Kersten MJ, Treon SP, Castillo JJ: Renal disease related to Waldenström macroglobulinaemia: Incidence, pathology and clinical outcomes. Br J Haematol 175: 623–630, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Ghobrial IM: Are you sure this is Waldenstrom macroglobulinemia? Hematology (Am Soc Hematol Educ Program) 2012: 586–594, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Vos JM, Minnema MC, Wijermans PW, Croockewit S, Chamuleau ME, Pals ST, Klein SK, Delforge M, van Imhoff GW, Kersten MJ; HOVON Multiple Myeloma Working Party; HOVON Lymphoma Working Party : Guideline for diagnosis and treatment of Waldenström’s macroglobulinaemia. Neth J Med 71: 54–62, 2013 [PubMed] [Google Scholar]
- 10.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, Schönland S, Hegenbart U, Comenzo R, Kastritis E, Dimopoulos MA, Jaccard A, Klersy C, Merlini G: New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J Clin Oncol 30: 4541–4549, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G, Garcia-Sanz R, Ocio EM, Morra E, Morel P, Anderson KC, Patterson CJ, Munshi NC, Tedeschi A, Joshua DE, Kastritis E, Terpos E, Ghobrial IM, Leleu X, Gertz MA, Ansell SM, Morice WG, Kimby E, Treon SP; VIth International Workshop on Waldenström macroglobulinaemia : Response assessment in Waldenström macroglobulinaemia: Update from the VIth International Workshop. Br J Haematol 160: 171–176, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Morel-Maroger L, Basch A, Danon F, Verroust P, Richet G: Pathology of the kidney in Waldenström’s macroglobulinemia. Study of sixteen cases. N Engl J Med 283: 123–129, 1970 [DOI] [PubMed] [Google Scholar]
- 13.Schuster SR, Rajkumar SV, Dispenzieri A, Morice W, Aspitia AM, Ansell S, Kyle R, Mikhael J: IgM multiple myeloma: Disease definition, prognosis, and differentiation from Waldenstrom’s macroglobulinemia. Am J Hematol 85: 853–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauvet S, Frémeaux-Bacchi V, Petitprez F, Karras A, Daniel L, Burtey S, Choukroun G, Delmas Y, Guerrot D, François A, Le Quintrec M, Javaugue V, Ribes D, Vrigneaud L, Arnulf B, Goujon JM, Ronco P, Touchard G, Bridoux F: Treatment of B-cell disorder improves renal outcome of patients with monoclonal gammopathy-associated C3 glomerulopathy. Blood 129: 1437–1447, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Kourelis TV, Nasr SH, Dispenzieri A, Kumar SK, Gertz MA, Fervenza FC, Buadi FK, Lacy MQ, Erickson SB, Cosio FG, Kapoor P, Lust JA, Hayman SR, Rajkumar V, Zeldenrust SR, Russell SJ, Dingli D, Lin Y, Gonsalves W, Lorenz EC, Zand L, Kyle RA, Leung N: Outcomes of patients with renal monoclonal immunoglobulin deposition disease. Am J Hematol 91: 1123–1128, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Vignon M, Javaugue V, Alexander MP, El-Karoui K, Karras A, Roos-Weil D, Royer B, Asli B, Knebelmann B, Touchard G, Jaccard A, Arnulf B, Bridoux F, Leung N, Fermand JP: Current anti-myeloma therapies in renal manifestations of monoclonal light chain-associated Fanconi syndrome: A retrospective series of 49 patients. Leukemia 31: 123–129, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Mahmood U, Isbel N, Mollee P, Mallett A, Govindarajulu S, Francis R: Monoclonal gammopathy of renal significance triggering atypical haemolytic uraemic syndrome. Nephrology (Carlton) 22[Suppl 1]: 15–17, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Koga T, Yamasaki S, Nakamura H, Kawakami A, Furusu A, Taguchi T, Eguchi K: Renal thrombotic microangiopathies/thrombotic thrombocytopenic purpura in a patient with primary Sjögren’s syndrome complicated with IgM monoclonal gammopathy of undetermined significance. Rheumatol Int 33: 227–230, 2013 [DOI] [PubMed] [Google Scholar]