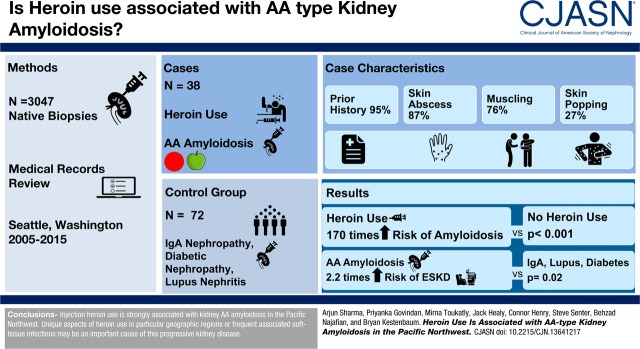
Abstract
Background and objectives
AA-type kidney amyloidosis is classically associated with chronic autoimmune or inflammatory disorders. However, some urban centers have reported a high prevalence of injection drug use among patients with kidney AA amyloidosis. Previous reports lack control groups to quantify associations and most predate the opioid epidemic in the United States.
Design, setting, participants, & measurements
We conducted a case–control study of 38 patients with biopsy-confirmed kidney AA amyloidosis and 72 matched control individuals without this condition from two large hospital systems in Seattle, Washington. We ascertained the pattern and duration of heroin use by medical chart review and determined associations using logistic regression.
Results
Among case patients, 95% had a prior history of heroin use, 87% had skin abscesses, and 76% and 27% had evidence of muscling and skin popping, respectively. After adjustment for age, race, sex, site, and year of biopsy, any heroin use (past or current) was associated with an estimated 170-times higher risk of kidney AA amyloidosis compared with no heroin use (95% confidence interval, 28 to 1018 times higher; P<0.001). Chronic autoimmune disorders were uncommon among case patients in this study. The median time to ESKD among patients with AA amyloidosis was 2.4 years (interquartile range, 0.5–7.5 years).
Conclusions
Injection heroin use is strongly associated with kidney AA amyloidosis in the Pacific Northwest. Unique aspects of heroin use, in particular geographic regions or frequent associated soft-tissue infections, may be an important cause of this progressive kidney disease.
Keywords: Abscess; Amyloidosis; Biopsy; Case-control Studies; chronic kidney disease; Confidence Intervals; Control Groups; dialysis; Heroin; Humans; kidney; Kidney Failure, Chronic; Logistic Models; nephrotic syndrome; Prednisolone; Prevalence; Soft Tissue Infections; Washington
Introduction
Amyloidosis of the kidneys is a progressive disease characterized by the deposition of insoluble fibrils of extracellular material within the glomeruli, tubules, and blood vessels (1). Amyloid light-chain (AL) amyloidosis, the most common form of the disease, is caused by deposition of fibrils derived from Ig light chains and is classically associated with lymphoproliferative disorders. The pathogenic fibrils in amyloid A (AA) type (secondary) amyloidosis, are formed from serum amyloid A protein, an acute-phase reactant that is synthesized in the liver in response to chronic inflammation (2–4). Consequently, kidney AA amyloidosis is classically reported in association with chronic autoimmune and inflammatory disorders (5).
Occasional case reports and case series have reported a high prevalence of injection heroin use among patients with kidney AA amyloidosis (6–11). These reports originated from large urban centers and described AA amyloidosis in association with longstanding heroin use and frequent suppurative infections. For example, a recent case series from San Francisco reported 24 patients with biopsy-proven kidney AA amyloidosis; all were chronic heroin users and many had recurrent skin infections due to exhaustion of their injection sites (11). On the other hand, the plurality of case series describing AA amyloidosis in the kidneys have reported an exceedingly low frequency of heroin use (5,12–14). For example, among 374 patients referred to the United Kingdom National Amyloidosis Center from 1990 to 2005, the prevalence of injection drug use was only 4% (5). Nearly all patients in this series had chronic inflammatory conditions, such as rheumatoid or juvenile arthritis, periodic fever syndromes, or inflammatory bowel disease. The substantial geographic variation in reports of heroin use associated with AA amyloidosis suggests that particular aspects of the drug or patterns of its use may play a role in causing the disease. Previous case series lack comparison groups to quantify the size of associations and most predate the opioid epidemic in the United States.
Our clinical experience is of a low frequency of autoimmune disorders and a high frequency of injection drug use among patients diagnosed with kidney AA amyloidosis in the Pacific Northwest. To clarify this association, we conducted a case–control study of biopsy-proven kidney AA amyloidosis at the University of Washington (UW) and Harborview Medical Centers (HMC) from 2005 to 2015.
Materials and Methods
Source Population
We conducted a case–control study of AA (secondary) type amyloidosis at the UW and HMC in Seattle, Washington. In collaboration with the UW Institute of Translational Health Sciences (ITHS), we queried the electronic medical record system to identify all kidney biopsy samples read at the UW Department of Pathology from January 1, 2005 to January 1, 2015 (n=12,026). To focus on biopsies linked with sufficient medical record information for chart review, we excluded 7552 biopsies for which there were no associated visits at a UW or HMC associated facility, or no serum creatinine or urine protein values recorded in the laboratory system. We further excluded 1427 kidney transplant biopsies, leaving a total of 3047 native biopsies from which to select cases and controls. The study was approved by the institutional review board at the UW.
Selections of Cases and Controls
We identified 54 potential case biopsies based on text searches for the term “amyloidosis” and related words within biopsy reports. A kidney pathologist (M.T.) then reviewed each potential case biopsy to verify 38 cases of kidney AA amyloidosis, on the basis of presence of amorphous hypocellular or acellular deposits that were Congo red–positive with apple-green birefringence under polarized light, and the presence of haphazardly arranged fibrillary deposits with an average diameter of 9–11 nm by electron microscopy. The diagnosis of AA amyloidosis was confirmed in all cases by positive immunoperoxidase staining of deposits using an mAb against AA amyloid protein (Leica polymer detection kit, catalog number M0759; Agilent, formerly Dako). We selected a comparison group of control patients who had a native kidney biopsy for which the diagnosis was not amyloidosis during the same time period. We used this approach to verify the absence of the disease and to ensure that control patients had the same opportunity to be diagnosed with amyloidosis had the condition occurred. Eligible control patients were individually matched to cases on the basis of age (±5 years), race (black, white, or other), sex, primary clinic site (UW or HMC), and the calendar year of biopsy. Two suitable matches were found for 34 case patients and one suitable match was found for four case patients.
Ascertainment of Exposures
Study personnel reviewed the medical charts of all case and control patients to ascertain the type, pattern, and duration of illicit drug use before the biopsy date. Personnel specifically searched for chart evidence of any previous heroin use, recent heroin use within 1 year before the kidney biopsy, route of heroin use (intravenous, intramuscular, skin), and the use of cocaine and methamphetamines. Personnel also abstracted data regarding infections that commonly accompany illicit drug use, including skin abscesses, endocarditis, and bacteremia. Chart reviews were performed using study identification numbers that excluded the case status.
Ascertainment of Other Study Data
Study personnel ascertained previous medical diagnoses by medical chart review using all available information before the kidney biopsy date. We specifically searched for chronic inflammatory conditions that have been previously reported with AA amyloidosis: rheumatoid arthritis, juvenile arthritis, bronchiectasis, inflammatory bowel disease, systemic lupus, tuberculosis, Mediterranean fever, periodic fever syndrome, Muckle–Wells syndrome, Castleman disease, Sjogren syndrome, vasculitis, and HIV. We abstracted kidney sizes from ultrasound reports. We obtained information regarding subsequent survival status and the dates of chronic dialysis initiation and kidney transplantation via chart review.
The ITHS abstracted demographic information (age, race, and sex), laboratory values, height, and weight data from the UW electronic medical record system. All laboratory measurements were obtained within 1 year before the index kidney biopsy date or up to 1 week thereafter. For multiple instances of the same laboratory test, we selected the value closest to the biopsy date. Specific laboratory measurements included serum chemistries, lipid profiles, hepatitis B and C status, timed and spot urine albumin and protein measurements, and the results of serum and urine protein electrophoresis and immunofixation tests. We calculated the eGFR using the 2009 CKD Epidemiology Collaboration equation on the basis of serum creatinine concentrations, age, race, and sex (15).
Statistical Analyses
We tabulated baseline characteristics of case and control patients as mean±SD or number (percentage of nonmissing values). We used unconditional logistic regression to calculate the odds ratio of kidney AA amyloidosis associated with each exposure characteristic, adjusting for the matching variables (age, race, sex, site, and calendar year of biopsy). Unconditional logistic regression yields similar results to conditional analyses in matched case–control studies, given adjustment for the matching variables (16). Given the rarity of kidney AA amyloidosis, odds ratios from the logistic regression models approximate relative risks. We calculated 95% confidence intervals (95% CIs) and two-sided P values from model-based variances obtained using the Huber–White sandwich estimator.
We defined ESKD as the initiation of chronic dialysis or kidney transplantation, whichever occurred first. Risk time began on the date of the index kidney biopsy and ended on the date of ESKD, death, or the last date known to be free of dialysis or kidney transplantation. For analyses of mortality, risk time began on the date of the index biopsy and ended on the date of death or the last date known alive. We calculated crude rates of ESKD and death as the number of events per 100 person-years, and we used the Kaplan–Meier estimation method to describe the proportions of patients free of each study outcome over time. We used the Cox proportional hazards model to determine the hazard ratios of ESKD and death associated with kidney AA amyloidosis compared with other kidney diagnoses, after adjustment for age, race, sex, and baseline eGFR. All analyses were performed using STATA version 14.2 (STATA Corp., College Station, TX).
Results
The mean age of the case–control study population was 46±9 years; 32% were women, and 73% were white (Table 1). Among the control group, the most common kidney biopsy diagnoses were IgA nephropathy, diabetic nephropathy, and lupus nephritis (Table 2). Case patients with kidney AA amyloidosis were more likely to be current smokers, had a lower baseline eGFR, and lower serum concentrations of albumin and HDL cholesterol compared with control patients. Case patients were also more likely to have larger kidney sizes and higher urinary protein excretion. Among the case group, six patients had a past history of tuberculosis, four were HIV positive, two had systemic lupus, one had rheumatoid arthritis, one had inflammatory bowel disease, and one had a serum monoclonal protein confirmed by serum and urine immunofixation. Hepatitis C antibody was present in 14 of the 19 (74%) case patients who had available laboratory results. None of the case patients had medical chart evidence of other autoimmune or inflammatory conditions, including periodic fever syndromes, Muckle–Wells syndrome, or Castleman disease.
Table 1.
Prebiopsy characteristics of case and control patients
| Characteristic | Kidney AA amyloidosis, n=38 | Complete Data | Other Kidney Diagnoses, n=72 | Complete Data |
|---|---|---|---|---|
| Agea | 45±9 | 38 | 46±10 | 72 |
| Womena | 12 (32) | 38 | 23 (32) | 72 |
| Racea | 38 | 72 | ||
| White | 28 (74) | 52 (72) | ||
| Black | 5 (13) | 7 (10) | ||
| Other | 5 (13) | 13 (18) | ||
| Smoking status | 38 | 72 | ||
| Never | 4 (11) | 40 (56) | ||
| Former | 2 (5) | 8 (11) | ||
| Current | 32 (84) | 24 (33) | ||
| Body mass index, kg/m2 | 27±8 | 37 | 28±6 | 68 |
| Past medical history | 38 | 72 | ||
| Diabetes | 2 (5) | 13 (18) | ||
| Cirrhosis | 1 (3) | 3 (4) | ||
| Rheumatoid arthritis | 1 (3) | 1 (1) | ||
| Tuberculosis | 6 (16) | 4 (6) | ||
| Inflammatory bowel disease | 1 (3) | 2 (3) | ||
| Lupus | 2 (5) | 15 (21) | ||
| Sjogren syndrome | 0 (0) | 1 (1) | ||
| eGFR, ml/min per 1.73 m2 | 38±30 | 38 | 48±32 | 71 |
| Serum albumin, g/dl | 1.8±0.8 | 36 | 3.0±0.8 | 68 |
| Serum HDL cholesterol, mg/dl | 30±21 | 25 | 48±23 | 45 |
| Serum triglycerides, mg/dl | 210±101 | 25 | 167±131 | 47 |
| HIV | 4 (11) | 36 | 14 (22) | 65 |
| Hepatitis B core antibody | 5 (26) | 19 | 11 (28) | 39 |
| Hepatitis C antibody | 14 (74) | 19 | 10 (24) | 42 |
| Proteinuria, g/d or g/gb | 12 (5, 15) | 30 | 3 (1, 6) | 48 |
| Kidney size, cmc | 12.8±1.6 | 33 | 11.3±1.3 | 56 |
All values are expressed as mean±SD or number (percentage of nonmissing values), except for urine protein, which is expressed as median (interquartile range).
Matching characteristics.
Urine protein expressed as grams per 24 hours if timed data were available, or g/g from spot collection.
Average of right and left kidney sizes.
Table 2.
Kidney biopsy diagnoses of control patients
| Kidney Biopsy Diagnosis | No. of patients (Proportion of Controls) |
|---|---|
| IgA nephropathy | 11 (15) |
| Diabetic nephropathy | 9 (13) |
| Lupus nephritis | 9 (12) |
| FSGS | 8 (11) |
| Nephrosclerosis | 8 (11) |
| Crescentic GN | 5 (7) |
| Interstitial nephritis | 4 (6) |
| Membranoproliferative GN | 3 (4) |
| Membranous nephropathy | 3 (4) |
| Immune complex GN | 3 (4) |
| Minimal change disease | 2 (3) |
| Arteriosclerosis | 2 (3) |
| Thrombotic microangiopathy | 2 (3) |
| Chronic GN | 1 (1) |
| Lymphoma | 1 (1) |
| Renal cell carcinoma | 1 (1) |
Among 37 of the 38 case biopsy samples, amyloid was found in >50% of the sampled glomeruli and was present in both the mesangium and capillary loops. In the one remaining case, amyloid was present in <50% of glomeruli and only in the mesangium. Amyloid deposition was also common in the tubular basement membrane and blood vessels (Table 3). Evidence of amyloid deposition outside of the kidneys was found in the liver in three patients, gastric mucosa in three, thyroid in two, and a lymph node in one. None of the case patients had evidence of cardiac involvement. An autopsy was performed on one case patient who died during the study. Amyloid deposition was found in the spleen, liver, gastrointestinal mucosa, vasculature, thyroid, and pituitary glands.
Table 3.
Glomerular, tubular, and vessel deposition of amyloid A amyloidosis among the case patients
| Kidney Structure | Involvement, No. of Patients (%) | ||
|---|---|---|---|
| None | ≤50% | >50% | |
| Glomeruli | 0 (0) | 1 (3) | 37 (97) |
| Tubular basement membrane | 5 (13) | 24 (63) | 9 (24) |
| Blood vessels | 0 (0) | 25 (66) | 13 (34) |
Sufficient medical record data to assess any previous heroin use were available for all but one case and one control patient. Among the 37 available case patients, 35 (95%) had a prior history of heroin use and 28 (78%) had evidence of recent use within 1 year before the kidney biopsy date (Table 4). A history of any heroin use, past or current, was associated with an estimated 170-times higher risk of kidney AA amyloidosis after adjustment for the matching variables (95% CI, 28 to 1018; P<0.001). Recent heroin use was associated was associated with an estimated 223-times greater risk (95% CI, 38 to 1323; P<0.001). The most common routes of heroin use were intravenous and intramuscular (nonmutually exclusive). A history of muscling and skin popping were found in 76% and 27% of case patients, respectively, and none of the control patients, precluding calculation of associations for these exposures. Cocaine use was also highly prevalent among the case group (74%), whereas methamphetamine use was less common and not significantly associated with AA amyloidosis (P=0.07).
Table 4.
Patterns of illicit drug use, related medical conditions, and risk of kidney AA amyloidosis
| Characteristic | Complete Data | Kidney AA Amyloidosis, n=38 | Other Kidney Diagnosis, n=72 | Odds Ratioa (95% Confidence Interval) |
|---|---|---|---|---|
| Any heroin use | 108 | 35 (95) | 10 (14) | 169.5 (28.2 to 1018.2)b |
| Recent use | 107 | 28 (78) | 2 (3) | 222.6 (37.5 to 1322.8)b |
| Intravenous use | 108 | 30 (81) | 9 (13) | 37.0 (11.0 to 124.8)b |
| Muscling | 108 | 28 (76) | 0 (0) | — |
| Skin popping | 108 | 10 (27) | 0 (0) | — |
| Cocaine use | 110 | 29 (76) | 14 (19) | 26.0 (7.5 to 89.9)b |
| Methamphetamine use | 109 | 7 (18) | 5 (7) | 3.4 (0.9 to 12.5) |
| Skin abscess | 110 | 33 (87) | 8 (11) | 64.7 (16.6 to 252.7)b |
| Endocarditis | 110 | 5 (13) | 2 (3) | 6.9 (1.0 to 45.5)c |
| Bacteremia | 110 | 28 (74) | 13 (18) | 20.0 (6.2 to 64.5)b |
All values are expressed as number (percentage of nonmissing values). —, not applicable.
Odds ratios adjusted for matching variables: age, race, sex, site, and calendar year of biopsy.
P value<0.001.
P value=0.05.
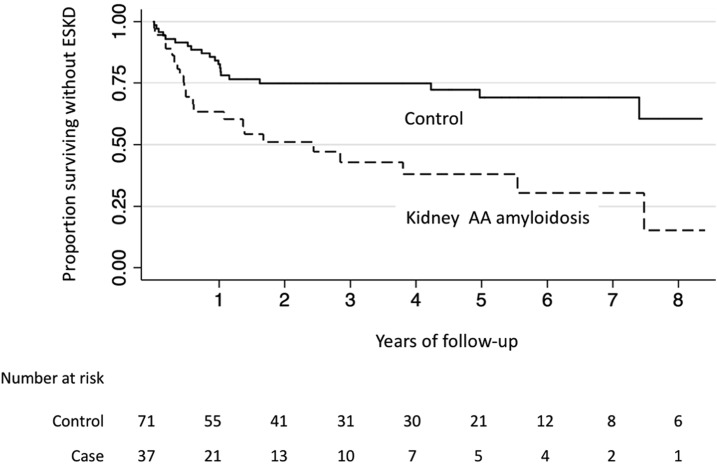
One case patient and one control patient were receiving chronic dialysis at the time of kidney biopsy. Among the 37 remaining case patients, 22 (59%) initiated chronic dialysis over follow-up and none received a kidney transplant. The rate of ESKD among case patients was 27.5 events per 100 person-years and the median time to ESKD was approximately 2.4 years (interquartile range, 0.5–7.5 years). Among the 71 control patients not initially on dialysis, 19 (27%) initiated dialysis and one underwent kidney transplantation over follow-up (rate of 8.2 events per 100 person-years). An additional six control patients received a kidney transplant after initiating chronic dialysis. The time to ESKD was significantly shorter comparing patients with kidney AA amyloidosis with control individuals (Figure 1; log-rank P value<0.001). After adjustment for age, race, sex, and baseline eGFR, AA amyloidosis was associated with a 2.2-times higher risk of ESKD compared with other kidney biopsy diagnoses (95% CI, 1.1 to 4.1-times higher; P=0.02).
Figure 1.
Kidney AA amyloidosis associated with progression to ESKD. Kaplan–Meier plot showing the proportion of patients free of ESKD (chronic dialysis or kidney transplantation) on the y-axis and the years of follow-up on the x-axis. The numbers of case and control individuals at the beginning of each survival period are shown below the table.
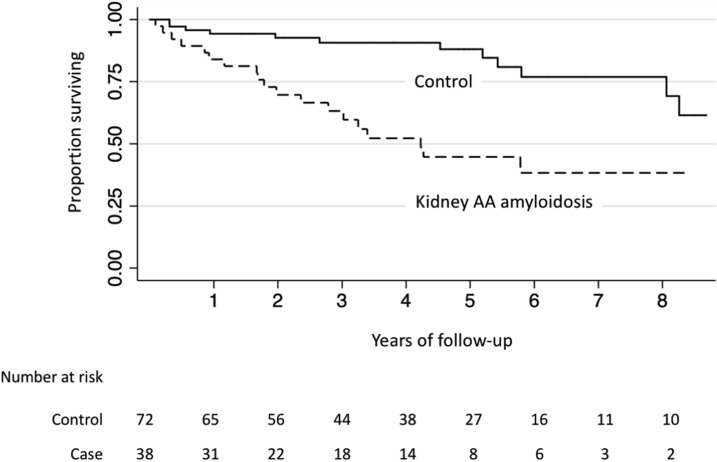
There were 19 deaths in the amyloidosis group (15.1 events per 100 person-years) and 12 deaths in the control group (3.9 deaths per 100 person-years). Median survival in the AA amyloid group was approximately 4.2 years (25th percentile 1.8 years, 75th percentile cannot be calculated from the observed data; Figure 2). Survival was significantly shorter among patients with kidney AA amyloid compared with control patients (log-rank P value<0.001). After adjustment for age, race, sex, and baseline eGFR, kidney AA amyloidosis was associated with a 4.1-times higher risk of death (95% CI, 1.9 to 8.6-times higher; P=0.001).
Figure 2.
Kidney AA amyloidosis associated with mortality. Kaplan–Meier plot showing the proportion of patients who survived on the y-axis and years of follow-up on the x-axis. The numbers of case and control individuals at the beginning of each survival period are shown below the table.
Discussion
We found a strong association of injection heroin use with biopsy confirmed kidney AA amyloidosis in the Pacific Northwest. Nearly all patients with AA amyloidosis in this study were previous or current injection heroin users. The use of heroin within 1 year before the kidney biopsy date was associated with an estimated 223-times higher risk of kidney AA amyloidosis compared with no such use. Median times to ESKD and death among patients with AA amyloidosis in this study were only 2.4 and 4.2 years, respectively. These findings, in conjunction with previous case series, suggest that unique aspects of heroin use in particular geographic locations are likely to be an important cause of kidney AA amyloidosis and highlight the rapidly progressive and often fatal course of this disease (10,11).
The most plausible mechanism linking injection heroin use with AA amyloidosis in this population is the development of recurrent soft-tissue infections. Such infections are common among injection drug users in the Pacific Northwest because of widespread availability of black tar heroin, which contains impurities that promote venous sclerosis and use of secondary muscle and skin injection sites (17). Black tar heroin is also associated with a higher risk of clostridial infections and lower rates of HIV infection compared with white powder heroin (17). The majority of case patients in this study had documentation of muscling or skin-popping, and most had evidence of skin abscesses. Presumably, untreated suppurative infections stimulate hepatic production of serum amyloid A protein. Aberrant processing of this protein among susceptible individuals then leads to the formation of poorly immunogenic, insoluble fibrils that deposit in multiple structures within the kidneys to cause nephrotic range proteinuria and progression to ESKD.
In the current era, case series of heroin use and kidney AA amyloidosis have been reported from the west coast of the United States, central London, and Frankfurt (9–11). In contrast, recent case series from Boston, New York, Minnesota, Japan, and Turkey have found an exceedingly low frequency of injection drug use (0%–4%) among patients with AA amyloidosis and an identifiable chronic inflammatory disorder in nearly all cases of disease (12,14,18,19). Although differences in disease ascertainment and reporting may have accounted for some of this discrepancy, the marked geographic variation suggests that the type of heroin preparation may differentially affect the development of the disease. The high prevalence of black tar heroin in affected areas, the frequent use of muscle and skin injection sites to administer this form of the drug, and the development of recurrent soft-tissue abscesses are the most likely explanations. It is also possible that certain components of black tar heroin itself directly promote the formation of AA amyloid fibrils.
There are no approved therapies for AA amyloidosis and the prognosis of the disease is generally poor. For example, among 374 patients from the United Kingdom National Amyloidosis Centre whose disease was associated with chronic inflammatory disorders, the median survival from diagnosis was 11 years (5). Previous series have reported higher rates of death and ESKD among patients who have kidney AA amyloidosis associated with heroin use (11). In our study, 59% of case patients progressed to dialysis over a median of 1.4 years of follow-up, and 47% died. These rates were significantly higher than those of control individuals in our study, many of whom also had serious kidney diseases. Although reported outcome data remains sparse, the results of this study suggest a rapidly progressive course of the disease that may inform patient counseling and planning for dialysis, if indicated. Although there are anecdotal reports of disease remission after discontinuation of injection drug use, available data are insufficient to assess the effect of abstinence on the course of the disease (10,20).
Strengths of this study include the evaluation of biopsy-confirmed cases of kidney AA amyloidosis and the inclusion of a matched control group to quantify the size of associations with heroin use. An additional strength is the prospective assessment of ESKD and mortality. One important limitation is the inability to reliably separate heroin use from other related exposures, which themselves could cause amyloidosis. Many patients who used heroin in this study also used other illicit drugs, had other comorbid conditions such as hepatitis C, and experienced frequent soft-tissue infections. The relatively small sample size and copresence of these exposures preclude reliable separation for associations with AA amyloidosis. Nonetheless, the strongest associations were observed for heroin use, whereas associations for methamphetamine and cocaine use were considerably smaller. It is also possible that other behaviors and conditions linked with injection drug use but not measured in this study may have confounded the observed associations. Some misclassification of injection drug use may have occurred because of the use of medical record data to ascertain these exposures. Moreover, it is possible that case patients were particularly more likely to report illicit drug behaviors to health professionals than controls because a diagnosis of AA amyloidosis in Seattle typically prompts intensive questioning about drug use. Nonetheless, the prevalence of heroin use among the control group in this study was relatively high (14%). Even a modest degree of underestimation would have only a modest effect on the size of the observed associations. Finally, the patients reported here were predominantly white, and so study findings may not be applicable to other races.
In summary, heroin use was strongly associated with kidney AA amyloidosis at two large medical centers in Seattle, Washington. Affected patients in this study rapidly progressed to dialysis dependence and death. The increasing use of illicit opioids in the United States suggests that the incidence of this serious disease may increase in high-risk geographic locations where black tar heroin and other impure forms of the drug are commonly used. Greater awareness of kidney amyloidosis and its complications in such areas may help reduce the incidence of the disease.
Disclosures
None.
Acknowledgments
We acknowledge financial support from the National Institutes of Health (grant R01 DK103986 to B.K.).
Rajnish Mehrotra, Editor-in-Chief and Ian de Boer, Deputy Editor, of the Clinical Journal of the American Society of Nephrology were recused from the peer review process of this manuscript since they are at the same institution as some of the authors. The peer review and decision-making process for this manuscript were overseen by another editor.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The Changing Spectrum of Heroin-Associated Kidney Disease,” on pages 975–976.
References
- 1.Wechalekar AD, Gillmore JD, Hawkins PN: Systemic amyloidosis. Lancet 387: 2641–2654, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Westermark GT, Fändrich M, Westermark P: AA amyloidosis: Pathogenesis and targeted therapy. Annu Rev Pathol 10: 321–344, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Urieli-Shoval S, Linke RP, Matzner Y: Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol 7: 64–69, 2000 [DOI] [PubMed] [Google Scholar]
- 4.McAdam KP, Sipe JD: Murine model for human secondary amyloidosis: Genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J Exp Med 144: 1121–1127, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN: Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356: 2361–2371, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Scholes J, Derosena R, Appel GB, Jao W, Boyd MT, Pirani CL: Amyloidosis in chronic heroin addicts with the nephrotic syndrome. Ann Intern Med 91: 26–29, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Menchel S, Cohen D, Gross E, Frangione B, Gallo G: AA protein-related renal amyloidosis in drug addicts. Am J Pathol 112: 195–199, 1983 [PMC free article] [PubMed] [Google Scholar]
- 8.Neugarten J, Gallo GR, Buxbaum J, Katz LA, Rubenstein J, Baldwin DS: Amyloidosis in subcutaneous heroin abusers (“skin poppers’ amyloidosis”). Am J Med 81: 635–640, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Connolly JO, Gillmore JD, Lachmann HJ, Davenport A, Hawkins PN, Woolfson RG: Renal amyloidosis in intravenous drug users. QJM 99: 737–742, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Jung O, Haack HS, Buettner M, Betz C, Stephan C, Gruetzmacher P, Amann K, Bickel M: Renal AA-amyloidosis in intravenous drug users--a role for HIV-infection? BMC Nephrol 13: 151, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lejmi H, Jen KY, Olson JL, James SH, Sam R: Characteristics of AA amyloidosis patients in San Francisco. Nephrology (Carlton) 21: 308–313, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Bunker D, Gorevic P: AA amyloidosis: Mount Sinai experience, 1997-2012. Mt Sinai J Med 79: 749–756, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Joss N, McLaughlin K, Simpson K, Boulton-Jones JM: Presentation, survival and prognostic markers in AA amyloidosis. QJM 93: 535–542, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kuroda T, Tanabe N, Hasegawa E, Wakamatsu A, Nozawa Y, Sato H, Nakatsue T, Wada Y, Ito Y, Imai N, Ueno M, Nakano M, Narita I: Significant association between renal function and area of amyloid deposition in kidney biopsy specimens in both AA amyloidosis associated with rheumatoid arthritis and AL amyloidosis. Amyloid 24: 123–130, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce N: Analysis of matched case-control studies. BMJ 352: i969, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunbar NM, Harruff RC: Necrotizing fasciitis: Manifestations, microbiology and connection with black tar heroin. J Forensic Sci 52: 920–923, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Girnius S, Dember L, Doros G, Skinner M: The changing face of AA amyloidosis: A single center experience. Amyloid 18[Suppl 1]: 226–228, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Said SM, Sethi S, Valeri AM, Leung N, Cornell LD, Fidler ME, Herrera Hernandez L, Vrana JA, Theis JD, Quint PS, Dogan A, Nasr SH: Renal amyloidosis: Origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol 8: 1515–1523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley S, Feinfeld DA, Janis R: Resolution of nephrotic syndrome and lack of progression of heroin-associated renal amyloidosis. Am J Kidney Dis 13: 333–335, 1989 [DOI] [PubMed] [Google Scholar]