Abstract
Background:
Morphine is commonly used to treat severe pain. This substance is significantly metabolized in the liver and causes disturbing effects. Genistein is an isoflavone and has antioxidant properties. The aim of this study was to evaluate the effects of genistein against morphine damages on mouse liver.
Methods:
Between May 2017 and March 2018, 48 male mice were divided into six groups (n = 8 in each group). Various doses of genistein (25 and 50 mg/kg) and morphine plus genistein (25 and 50 mg/kg) were administered intraperitoneally to 48 male mice for 20 consequent days. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), serum nitric oxide (NO) levels, liver weight, and the diameter of hepatocytes and central hepatic vein were studied and compared using one-way analysis of variance.
Results:
Morphine administration significantly increased the mean diameter of the central hepatic vein (22.76 ± 1.9 μm vs. 15.04 ± 0.60 μm, χ2 = 21.814, P = 0.001) and hepatocytes (3.03 ± 0.10 μm vs. 1.10 ± 0.05 μm, χ2 = 9.873, P = 0.001) respectively, blood serum NO level (38.00% ± 2.09% vs. 18.72% ± 4.40%, χ2 = 20.404, P < 0.001), liver enzyme level (AST: 111.80 ± 5.10 ng/ml vs. 81.93 ± 2.20 ng/ml, χ2 = 32.201, P < 0.0001; ALT: 45.14 ± 4.10 ng/ml vs. 35.49 ± 2.50 ng/ml, χ2 = 18.203, P < 0.0001; and ALP: 3.28 ± 0.20 ng/ml vs. 2.14 ± 0.10, χ2 = 5.04, P < 0.0001, respectively), and decreased liver weight (18.50 ± 0.90 g vs. 27.15 ± 0.50 g, χ2 = 22.415, P = 0.001) compared to saline group (0.535–0.750, P < 0.0001). However, administration of genistein plus morphine significantly enhanced liver weight (25 mg/kg: 21.15 ± 2.13 g vs. 18.50 ± 0.90 g, χ2 = 19.251, P < 0.0001; 50 mg/kg: 21.20 ± 1.00 g vs. 18.5 ± 0.9 g, χ2 = 19.502, P < 0.0001, respectively) and reduced the mean diameter of hepatocyte (25 mg/kg: 2.17 ± 0.30 μm vs. 3.03 ± 0.10 μm, χ2 = 22.780, P = 0.001; 50 mg/kg: 2.01 ± 0.20 μm vs. 3.03 ± 0.10 μm χ2 = 7.120, P = 0.001, respectively), central hepatic vein (25 mg/kg: 19.53 ± 1.00 μm vs. 22.76 ± 1.90 μm, χ2 = 20.681, P = 0.001; 50 mg/kg: 19.44 ± 1.20 μm vs. 22.76 ± 1.90 μm, χ2 = 18.451, P = 0.001, respectively), AST (25 mg/kg: 95.40 ± 5.20 ng/ml vs. 111.80 ± 5.010 ng/ml, P < 0.0001; 50 mg/kg: 90.78 ± 6.00 ng/ml vs. 111.80 ± 5.10 ng/ml, χ2 = 17.112, P < 0.0001, respectively), ALT (25 mg/kg: 35.78 ± 5.01 ng/ml vs. 45.14 ± 4.10 ng/ml, χ2 = 15.320, P < 0.0001; 50 mg/kg: 33.78 ± 2.60 ng/ml vs. 45.14 ± 4.10 ng/ml, χ2 = 14.023, P < 0.0001, respectively), ALP (25 mg/kg: 2.35 ± 0.30 ng/ml vs. 3.28 ± 0.20 ng/ml, χ2 = 4.101, P < 0.0001; 50 mg/kg: 2.34 ± 0.10 ng/ml vs. 3.28 ± 0.20 ng/ml, χ2 = 2.033, P < 0.0001, respectively), and NO levels (25 mg/kg: 25.92% ± 2.30% vs. 38% ± 2.09%, χ2 = 17.103, P < 0.0001; 50 mg/kg: 24.74% ± 4.10% vs. 38% ± 2.09%, χ2 = 25.050, P = 0.001, respectively) compared to morphine group.
Conclusion:
It seems that genistein administration might improve liver damages induced by morphine in mice.
Keywords: Genistein, Liver Damage, Morphine
INTRODUCTION
Herbal treatment has been used to relieve disorders associated with the liver.[1] Some plant extracts used in conventional medicine are full of compounds with preventive and protective properties, especially on the liver.[2] An isoflavone is an important group of phytoestrogens with antioxidant, antibacterial, and anti-inflammatory properties. Soybean is an important source of isoflavonoids, which is located in phytoestrogens.[3] Genistein is a phytoestrogen found in some edible plants such as legumes, especially soybean. About 99% of the genistein is found in soybean as a compound with a glucose molecule.[4] Nowadays, according to the epidemiologic studies on the role of soybean in preventing some cancers as well as its effects on preventing cardiovascular diseases and diabetes, consumption of soy-containing food has found a special place in the diet of people.[5] Genistein, with molecular formula 4', 5, 7-trihydroxy-isoflavone, is the major isoflavone in soybean.[6] It is the isoflavone that has received the most attention due to its estrogenic, neuroprotective, antioxidant, anti-inflammatory, and anti-proliferative effects.[7] Tyrosine kinases play a pivotal role in controlling the biologic network and cellular growth and differentiation.[8] Morphine is an opioid analgesic drug and the main psychoactive chemical in opium.[9] Li et al.[10] showed a significant correlation between morphine consumption and hepatitis-C induction. Morphine is mainly metabolized in the liver and its consumption increases the liver toxicity.[11] Based on the study results of Yun et al.,[12] morphine has been introduced as a toxic and lethal factor for liver cells. Antioxidants in foodstuff can protect the cells against various types of oxidative damage caused by free radicals.[13] Morphine increases dopamine and xanthine oxidase, followed by increased production of reactive oxygen species (ROS). Morphine can also be metabolized into free radicals.[14] Increased ROS causes DNA damage, inactivates some proteins, and impairs biologic membranes through induction of oxidative stress.[15] The antioxidants in the supplements and antioxidant-rich foods can protect the liver against various oxidative impairments resulting from free radicals of oxygen.[16] Daba and Abdel-Rahman[17] indicated that the thymoquinone extract, as an oxidant protection, can produce antioxidant effects against the carbon tetrachloride-induced liver toxicity. The cell damages and dysfunction of endocrine and metabolic activities of the liver can be partly recognized, to some extent, by measuring some of the liver enzymes.[18] Despite the effect of morphine toxicity, especially oxidative stress, no studies have been performed on the antioxidative effects of genistein on the morphine-induced impairments in the liver yet. Therefore, the aim of this study was to evaluate the effects of genistein on morphine-induced damage in some of the liver parameters in male mice.
METHODS
Animals
In this study, 48 Balb/c male mice, with a weight range of 25.0 ± 2.0 g, were purchased from Tehran Razi Institute. Animals were kept at the animal house for 1 week before the start of the study under laboratory conditions at 20°C ± 2°C, 12/12 h light/dark cycle, and with free access to water and food. The animals were kept in the standard cages of the animal, eight mice in each cage. All experiments were approved by the Ethics Committee of Kermanshah University of Medical Sciences.
Experimental design
The mice were randomly divided into six groups (n = 8 each group): Group 1, saline group, received 0.9% normal saline; Group 2, morphine group, which was induced by morphine; and Groups 3 to 4 of the genistein groups were given 25 and 50 mg/kg genistein, respectively. Mice in Groups 5–6 received genistein (25 and 50 mg/kg) plus morphine. Morphine was administered by intraperitoneal injection as follows: 20 mg/kg once daily within the first 5 days and twice per day within the next 5 days. On days 11–20, a dose of up to 30 mg/kg twice per day was administered. Mice were administered with genistein as follows: on days 1–20, genistein once daily, injected intraperitoneally. Mice administered with morphine plus genistein were as follows: on days 1–20, genistein once daily plus morphine, injected intraperitoneally. The same volume of saline was injected.[3,18]
Chemicals
Genistein (CR15RHR10ROR5R) powder (Merck-Germany) was dissolved in absolute ethanol (CR2RHR5ROH) and diluted by normal saline (0.9%) to prepare different doses. In addition, the morphine (CR16RHR19RNOR3R) (Merck-Germany) was diluted with normal saline (0.9%) for administration.[3]
Liver weight measurement and blood serum collection
All animals were anesthetized with chloroform, cut up, and blood tasters were taken by cardiac puncture of the right ventricle. The blood samples were incubated for coagulation at 37°C. The coagulated blood samples were then centrifuged for 15 min at 1610 ×g until the serum was separated. The separated serum was kept at −20°C until the measurement of biochemical factors and nitric oxide (NO) levels. Animals were killed and sacrificed. Livers were removed and weighed on a microbalance sensitive to 1 μg (Precisa 125A, Switzerland), and average weights of the livers of mice were calculated and recorded.[13]
Serum nitric oxide assay
NO was measured using the Griess staining method. To measure nitrite concentration in serum, after de-freezing the serum samples, in this assay, the supernatant (400 μl) was deproteinized with zinc sulfate (6 mg zinc sulfate powder was mixed with 400 μl serum and vortexed for 1 min) by centrifugation. Then, 100 μl supernatant was taken, and 100 μl vanadium chloride, 50 μl sulfanilamide, and 50 μl NEDD (N-1[naphthyl] ethylenediamine dihydrochloride) were added. Standard solutions of sodium nitrate were prepared for different concentrations of nitrate. The standard curve of nitrite concentration was plotted. Samples' optical density was assessed using ELISA reader (Hyperion, Germany) at the wavelength of 540 nm.[15]
Histological examinations
In order to evaluate the histology of the hepatic structures, the lower 1-cm-long part of the right lobe of the liver in transverse pieces was removed, washed in saline, and fixed in 10% formalin at room temperature. After tissue fixation, it was thoroughly washed under running water and dehydrated in ascending concentration ethanol, cleared in xylene, and then embedded in soft paraffin. The thin sections (5 mm) were cut using a microtome (Leica RM 2125, Leica Microsystems Nussloch GmbH, Germany) and stained with hematoxylin and eosin. The preparation was examined with a BX-51T-32E01 research microscope (Olympus, Tokyo, Japan) connected to a DP12 Camera (Optical Co., Tokyo, Japan) with 3.34-million pixel resolution and Olysia Bio software (Olympus Optical Co., Ltd., Tokyo, Japan).[13]
Morphometric measurements
For each hepatocyte, the total cellular area was measured. The outline of each hepatocyte was measured after taking an image with a ×40 objective. The longest and shortest axes were measured in the drawing of each hepatocyte in order to estimate the mean diameter (mean axis). At least, 50 hepatocytes from each zone (total 100) were measured in each liver. A separate measurement of the central hepatic vein was performed using the same methodology.[2]
Biochemical analysis
The liver was fragmented and turned into a uniform solution. To separate the enzymes, the resulting solution was centrifuged for 20 min twice at 1610 ×g. The upper portion of the solution was separated to measure the enzymes. Alkaline phosphatase (ALP) activities were determined by the Alkaline Phosphatase Assay Kit (Abcam), based on the protocol described in laboratory practical manual. Alanine aminotransferase (ALT) was determined by GTP detection kit (Roche, USA) and aspartate aminotransferase (AST) was determined by GOT detection kit (Roche) by the method of Reitman and Frankel.[2]
Statistical analysis
All data are presented as a mean ± standard deviation (SD). Statistical comparisons among groups were investigated via one-way analysis of variance (ANOVA), followed by the least significant difference post hoc test. A value of P < 0.05 was considered statistically significant. The SPSS software was applied for statistical analysis (version 16.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Weight of livers
In the present study, the effective dose of morphine (20 mg/kg) significantly reduced the liver weight of mice compared to the saline group (18.50 ± 0.90 g vs. 27.15 ± 0.50 g, χ2 = 22.450, P = 0.001). Furthermore, the liver weight significantly increased in treated animals with genistein plus morphine in all doses in comparison with morphine group (25 mg/kg: 21.15 ± 2.13 g vs. 18.50 ± 0.90 g, χ2 = 19.251, P < 0.0001; 50 mg/kg: 21.20 ± 1.00 g vs. 18.50 ± 0.90 g, χ2 = 19.502, P < 0.0001, respectively; Figure 1).
Figure 1.
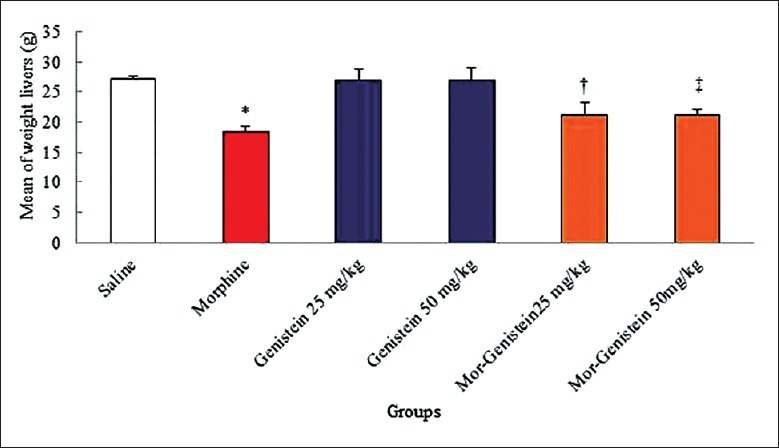
Forty-eight mice were equally divided into six groups. *Significant decrease of liver weight in morphine group compared to saline group (18.50 ± 0.90 g vs. 27.15 ± 0.50 g, χ2 = 22.450, P = 0.001). †,‡Significant increase in genistein plus morphine groups compared to morphine group (25 mg/kg: 21.15 ± 2.13 g vs. 18.50 ± 0.90 g, χ2 = 19.251, P < 0.0001; 50 mg/kg: 21.20 ± 1.00 g vs. 18.50 ± 0.90 g, χ2 = 19.502, P < 0.0001, respectively).
Serum nitric oxide measurement
The findings of blood serum NO measurement showed a significant increase in morphine group compared to saline group (38.00% ± 2.09% vs. 18.72% ± 4.40%, χ2 = 20.404, P < 0.001). Furthermore, the mean of NO in the blood serum reduced significantly in genistein plus morphine (25 mg/kg: 25.92% ± 2.30% vs. 38.00% ± 2.09%, χ2 = 17.103, P < 0.0001; 50 mg/kg: 24.74% ± 4.10% vs. 38% ± 2.09%, χ2 = 25.050, P = 0.001, respectively) in all doses compared to morphine group [Figure 2].
Figure 2.
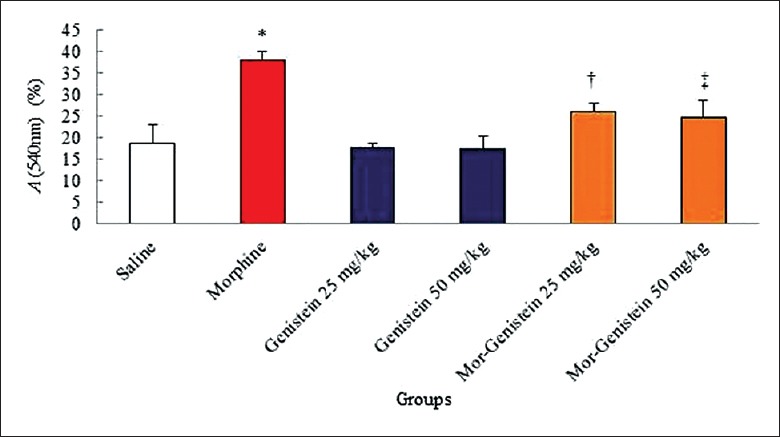
Effects of genistein, morphine, and genistein plus morphine on the mean nitric oxide levels of 48 mice were equally divided into six groups. *Significant increase of nitric oxide in morphine group compared to saline group (38.00% ± 2.09% vs. 18.72% ± 4.40%, χ2 = 20.404, P < 0.001). †,‡Significant decrease of genistein plus morphine-administrated groups compared to morphine group (25 mg/kg: 25.92% ± 2.30% vs. 38.00% ± 2.09%, χ2 = 17.103, P < 0.0001; 50 mg/kg: 24.74% ± 4.10% vs. 38% ± 2.09%, χ2 = 25.050, P = 0.001, respectively).
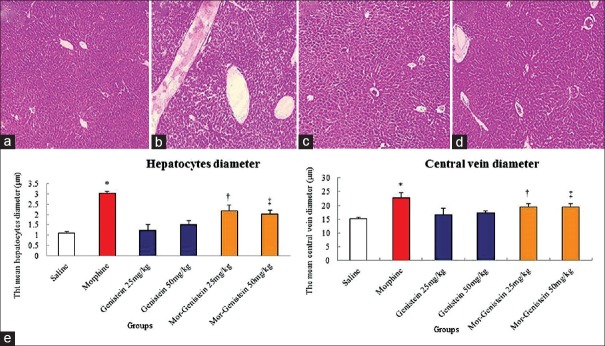
Morphometric of hepatocytes and central hepatic vein
The analysis of the mean diameter of hepatocytes and central hepatic vein in experimental groups revealed significant differences among saline group, morphine, and morphine plus thymoquinone groups (diameter of hepatocytes: 3.03 ± 0.10 μm vs. 1.10 ± 0.05 μm, χ2 = 9.873, P = 0.001 and central hepatic vein: 22.76 ± 1.90 μm vs. 15.04 ± 0.60 μm, χ2 = 21.814, P = 0.001, respectively). Furthermore, genistein plus morphine significantly reduced the mean diameter of hepatocytes and central hepatic vein in all the treated groups compared to morphine group administration (diameter of hepatocytes: 25 mg/kg: 2.17 ± 0.30 μm vs. 3.03 ± 0.10 μm, χ2 = 22.780, P = 0.001; 50 mg/kg: 2.01 ± 0.20 μm vs. 3.03 ± 0.1 μm, χ2 = 7.12, P = 0.001; central hepatic vein, 25 mg/kg: 19.53 ± 1.00 μm vs. 22.76 ± 1.90 μm, χ2 = 20.681, P = 0.001; 50 mg/kg: 19.44 ± 1.20 μm vs. 22.76 ± 1.90 μm, χ2 = 7.120, P = 0.001, respectively) [Figure 3].
Figure 3.
The morphometry of liver (a) Micrograph of the liver section in the saline group, (b) Micrograph of the liver section in morphine group, (c) Micrograph of the liver section in genistein (50 mg/kg) group, (d) Micrograph of liver section in morphine plus genistein (50 mg/kg) group (Haematoxylin Eosin, original magnification ×100). (e) *Significant increase of the hepatocytes and central vein diameters in morphine compared to saline group (diameter of hepatocytes: 3.03 ± 0.10 μm vs. 1.10 ± 0.05 μm, χ2 = 9.873, P = 0.001 and central hepatic vein: 22.76 ± 1.90 μm vs. 15.04 ± 0.60 μm, χ2 = 21.814, P = 0.001, respectively). †,‡Significant decrease in genistein plus morphine-administrated groups compared to morphine group (diameter of hepatocytes: 25 mg/kg: 2.17 ± 0.30 μm vs. 3.03 ± 0.10 μm, χ2 = 22.780, P = 0.001; 50 mg/kg: 2.01 ± 0.20 μm vs. 3.03 ± 0.1 μm, χ2 = 7.12, P = 0.001; central hepatic vein, 25 mg/kg: 19.53 ± 1.00 μm vs. 22.76 ± 1.90 μm, χ2 = 20.681, P = 0.001; 50 mg/kg: 19.44 ± 1.20 μm vs. 22.76 ± 1.90 μm, χ2 = 7.120, P = 0.001, respectively).
Biochemical analysis
Morphine significantly increased the mean of ALT, AST, and ALP enzymes compared to saline group (AST: 111.80 ± 5.10 ng/ml vs. 81.93 ± 2.20 ng/ml, χ2 = 32.201, P < 0.0001; ALT: 45.14 ± 4.10 ng/ml vs. 35.49 ± 2.50 ng/ml, χ2 = 18.203, P < 0.0001; ALP: 3.28 ± 0.20 ng/ml vs. 2.14 ± 0.10 ng/ml, χ2 = 5.04, P < 0.0001, respectively). In addition, the mean of ALT, AST, and ALP enzymes significantly decreased in genistein plus morphine (AST 25 mg/kg: 95.40 ± 5.20 ng/ml vs. 111.80 ± 5.01 ng/ml, χ2 = 17.112, P < 0.0001; 50 mg/kg: 90.78 ± 6.00 ng/ml vs. 111.80 ± 5.01 ng/ml, χ2 = 17.112, P < 0.0001, respectively), ALT (25 mg/kg: 35.78 ± 5.01 ng/ml vs. 45.14 ± 4.10 ng/ml, χ2 = 15.320, P < 0.0001; 50 mg/kg: 33.78 ± 2.60 ng/ml vs. 45.14 ± 4.10 ng/ml, χ2 = 14.023, P < 0.0001, respectively), and ALP (25 mg/kg: 2.35 ± 0.30 ng/ml vs. 3.28 ± 0.20 ng/ml, χ2 = 4.101, P < 0.0001; 50 mg/kg: 2.34 ± 0.10 ng/ml vs. 3.28 ± 0.20 ng/ml, χ2 = 2.033, P < 0.0001, respectively) in all groups compared to morphine group (-) [Table 1].
Table 1.
Levels of different liver enzymes between different groups (n=8)
| Enzymes (ng/ml) | Saline | Morphine | Genistein | Genistein + morphine | χ2 | P | ||
|---|---|---|---|---|---|---|---|---|
| 25 mg/kg | 50 mg/kg | 25 mg/kg | 50 mg/kg | |||||
| AST | 81.93 ± 2.20 | 111.8 ± 5.10 | 76.33 ± 3.40 | 74.00 ± 2.01 | 95.40 ± 5.20 | 90.78 ± 6.00 | 17.112 | <0.0001 |
| ALT | 35.49 ± 2.50 | 45.14 ± 4.10 | 34.91 ± 2.50 | 33.71 ± 1.10 | 35.78 ± 5.01 | 33.78 ± 2.60 | 14.023 | <0.0001 |
| ALP | 2.14 ± 0.10 | 3.28 ± 0.20 | 2.05 ± 0.30 | 2.11 ± 0.50 | 2.35 ± 0.30 | 2.34 ± 0.10 | 2.033 | <0.0001 |
Data were presented as mean ± SD. Independent samples t-test was done as the test of significance. Morphine significantly increased the mean of ALT, AST, and ALP enzymes compared to saline group. AST: χ2 = 32.201, P<0.0001; ALT: χ2 = 18.203, P<0.0001; ALP: χ2 = 5.04, P<0.0001, respectively. The mean of ALT, AST, and ALP enzymes significantly decreased in genistein plus morphine (AST 25 mg/kg: χ2 = 17.112, P<0.0001; 50 mg/kg: χ2 = 17.112, P<0.0001, respectively), ALT (25 mg/kg: χ2 = 15.320, P<0.0001; 50 mg/kg: χ2 = 14.023, P<0.0001, respectively), and ALP (25 mg/kg: χ2 = 4.101, P<0.0001; 50 mg/kg: χ2 = 2.033, P<0.0001, respectively) in all groups compared to morphine group. SD: Standard deviation; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
DISCUSSION
Morphine is an analgesic drug that is biotransformed in the liver, digestive system, and ultimately excreted through the kidneys. Morphine can cause injuries to liver and renal tissues.[5,12] Oxidative stress is a factor greatly involved in the liver damage caused by drugs and toxins. The increased activity of liver enzymes in serum indicates hepatic damage, which leads to the reduced performance of liver cells.[19] Antioxidant compounds can protect the cells against the toxic effects of morphine. Phytoestrogens are nonsteroidal compounds with estrogen-like activity and a structure similar to 17-β-estradiol.[3] Genistein is involved in many biochemical pathways and inhibits intracellular enzymes through ATP, tyrosine kinase, and topoisomerase.[8] In the present study, the increased serum level of liver enzymes in the morphine groups can be indicative of the damage to liver cells. Morphine can be metabolized to free radicals. Free radicals invade hepatocytes and cause the necrosis of parenchymal cells, followed by inflammatory responses in the liver by cells. The induced liver damage is intensified by the influx of inflammatory mononuclear cells in the damaged tissues and release of pro-inflammatory mediators by necrotic cells. This is accompanied by an increase in some liver enzymes.[9] In the current research, treatment with genistein has significantly inhibited the increase of serum liver enzymes. The reduction of serum enzymes in the groups receiving morphine can be due to the absence of leakage of intracellular enzymes. This is because of maintaining the stability and integrity of cell membrane or regeneration of liver-impaired cells due to antioxidant properties and reduced oxidative stress of genistein.[20] Flavonoids exert their anti-inflammatory effect by inhibiting the expression, synthesis, and activity of inflammatory mediators such as cytokines, eicosanoids, adhesion molecules, and activated protein C as well as preventing the transcription of factors such as nuclear factor-kappaβ and active protein-1.[2,21] Genistein is a flavonoid that seems to have anti-inflammatory and anticancer effects.[22] The results of study by Zhuo et al.[23] indicated that genistein consumption reduced liver fibrosis and serum level of AST and ALT enzymes in ethanol-treated mice, confirming the findings of the current study. The results of the analysis of liver weight among the study groups demonstrated a significant reduction in the liver weight between the saline group and morphine groups, indicating that the effects of morphine on the reduction of liver weight were largely eliminated to a large extent after treatment with genistein. It seems that morphine administration reduces liver weight by compromising the liver cells and disturbing the metabolism of the mice.[24] The study of Skrabalova et al.[25] showed that subcutaneous injection of morphine reduced the body weight, which is consistent with the results of the present research. Increased liver weight could be indicative of the improved nutrition of the animals treated with genistein. This increase in weight could be due to the effects of genistein on the enhanced diet of the studied animals.[26] The results of the present study are in agreement with the findings of Elsayed et al.,[27] indicating that high doses of genistein can increase the weight of mice. The results of this study also showed a significant increase in the mean diameter of hepatocytes and central hepatic vein between saline and morphine groups. Genistein administration reduced the mean diameter of hepatocytes and central hepatic vein compared to the morphine group. It seems that morphine has hepatotoxic properties, due to increased glutathione, and causes the loss of liver cytoplasmic cells.[28] Changing the size of liver's central vein and hepatic cells can increase the metabolic activity of cells to excrete the toxin from the body during detoxification process.[29] The morphine metabolism induces free radicals and then causes lipid peroxidation, reaction with DNA and membrane proteins, and consequently cell damage through various ways.[30] Genistein, as an antioxidant compound, has an inhibitory effect on cytochrome P450, prevents further morphine metabolism, and reduces the production of free radicals, consequently.[31] The findings of the present study are in line with the results of the study by Salahshoor et al.,[2] in which they showed crocin administration as an antioxidant resulted in the increasing diameter of hepatocytes. In the present research, morphine showed increasing effects on blood NO in the study groups, which was largely reduced by genistein administration. Morphine can induce increased NO production via intracellular regulation of calcium and activation of calcium/calmodulin-dependent protein kinase.[32] NO, a free radical that is produced in the mammalian cells, is involved in the regulation of physiologic processes and its increased production is due to the induction of various diseases.[3] Morphine can also increase NO production directly through naloxone-sensitive receptors.[33] The hydroxyl radicals produced by NO and superoxidation interfere with the pathogenesis process and liver toxicity.[34] Antioxidants such as genistein seem to have the ability to remove free radicals.[15] Antioxidants disrupt the NO system (protein enzymes, substrates, and cofactors), thus reducing NO production.[15] Genistein can inhibit NO production from macrophages.[35] The results of study by Kuriyama et al.,[36] showed that administration of genistein stimulated NO synthesis in vascular endothelium, which is in contrast with the results of the present study. On the other hand, results of the current study confirm the findings of Ghorbani et al.,[13] indicating that walnut, as an antioxidant, was able to prevent the increase of blood serum NO in the laboratory animals. Genistein can induce the oxidative stress by inhibiting the increased production of NO.[9] In general, based on the results of the current study, the administration of genistein as an antioxidant and a potent phytoestrogen can reduce the morphine-induced injuries through various mechanisms and can improve the structure, enzymes, and performance of liver in the groups receiving morphine. Therefore, more comprehensive studies are required to accurately evaluate the molecular and cellular mechanisms involved in the functioning of genistein in liver in order to get a better understanding of the effects of this substance.
In conclusion, this study shows that genistein might significantly improve some of the liver damages against the destructive effects of morphine in mice. The results also suggest the potential effects of genistein, especially antioxidant effects against toxic effects of morphine. Genistein might provide to be a novel therapeutic approach. However, further research in animal models is warranted to obtain more conclusive evidence for the molecular interaction between genistein and morphine, leading to improved hepatic damage.
Financial support and sponsorship
This work was supported by a grant from the Research Council of Kermanshah University of Medical Sciences (No: 93486).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was performed in partial fulfillment of the requirements for MD of Pyman Hosseni in the Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: A therapeutic challenge for the new millennium. Hepatology. 1999;30:1099–104. doi: 10.1002/hep.510300437. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 2.Salahshoor M, Mohamadian S, Kakabaraei S, Roshankhah S, Jalili C. Curcumin improves liver damage in male mice exposed to nicotine. J Tradit Complement Med. 2016;6:176–83. doi: 10.1016/j.jtcme.2014.11.034. doi: 10.1016/j.jtcme.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalili C, Ahmadi S, Roshankhah S, Salahshoor M. Effect of genistein on reproductive parameter and serum nitric oxide levels in morphine-treated mice. Int J Reprod Biomed (Yazd) 2016;14:95–102. doi: 10.29252/ijrm.14.2.95. [PMC free article] [PubMed] [Google Scholar]
- 4.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer KE, Pulliam CF, Pedersen KB, Hennings L, Ronis MJ. Soy protein isolate inhibits hepatic tumor promotion in mice fed a high-fat liquid diet. Exp Biol Med (Maywood) 2017;242:635–44. doi: 10.1177/1535370216685436. doi: 10.1177/1535370216685436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganai AA, Husain M. Genistein attenuates D-GalN induced liver fibrosis/chronic liver damage in rats by blocking the TGF-β/Smad signaling pathways. Chem Biol Interact. 2017;261:80–5. doi: 10.1016/j.cbi.2016.11.022. doi: 10.1016/j.cbi.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Jalili C, Salahshoor MR, Jalili F, Kakaberaei S, Akrami A, Sohrabi M, et al. Therapeutic effect of resveratrol on morphine-induced damage in male reproductive system of mice by reducing nitric oxide serum level. Int J Morphol. 2017;35:1342–7. doi: 10.4067/S0717-95022017000401342. [Google Scholar]
- 8.Antosiak A, Milowska K, Maczynska K, Rozalska S, Gabryelak T. Cytotoxic activity of genistein-8-C-glucoside form Lupinus luteus L. and genistein against human SK-OV-3 ovarian carcinoma cell line. Med Chem Res. 2017;26:64–73. doi: 10.1007/s00044-016-1725-5. doi: 10.1007/s00044-016-1725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalili C, Salahshoor MR, Yousefi D, Khazaei M, Shabanizadeh Darehdori A, Mokhtari T. Morphometric and hormonal study of the effect of Utrica diocia extract on mammary glands in rats. Int J Morphol. 2015;33:983–7. doi: 10.4067/S0717-95022015000300028. [Google Scholar]
- 10.Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, et al. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–75. doi: 10.1016/S0002-9440(10)63476-1. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Ye D, Yu S. Morphine suppresses human liver cancer cells mobility and cancer stem cells properties in vitro and migratory capacity in vivo. J Pain. 2018;19:S83. doi: 10.1016/j.jpain.2017.12.205. [Google Scholar]
- 12.Yun J, Oliynyk S, Lee Y, Kim J, Yun K, Jeon R, et al. Ajoene restored behavioral patterns and liver glutathione level in morphine treated C57BL6 mice. Arch Pharm Res. 2017;40:106–11. doi: 10.1007/s12272-016-0773-1. doi: 10.1007/s12272-016-0773-1. [DOI] [PubMed] [Google Scholar]
- 13.Ghorbani R, Mokhtari T, Khazaei M, Salahshoor MR, Jalili C, Bakhtiari M. The effect of walnut on the weight, blood glucose and sex hormones of diabetic male rats. Int J Morphol. 2014;32:858–63. doi: 10.4067/S0717-95022014000300015. [Google Scholar]
- 14.Mattei V, Martellucci S, Santilli F, Manganelli V, Garofalo T, Candelise N, et al. Morphine withdrawal modifies prion protein expression in rat hippocampus. PLoS One. 2017;12:e0169571. doi: 10.1371/journal.pone.0169571. doi: 10.1371/journal.pone.0169571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 16.El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK. The role of Vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol. 2010;48:215–21. doi: 10.1016/j.fct.2009.10.003. doi: 10.1016/j.fct.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett. 1998;95:23–9. doi: 10.1016/s0378-4274(98)00012-5. doi: 10.1016/S0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 18.Wan C, Jin F, Du Y, Yang K, Yao L, Mei Z, et al. Genistein improves schistosomiasis liver granuloma and fibrosis via dampening NF-kB signaling in mice. Parasitol Res. 2017;116:1165–74. doi: 10.1007/s00436-017-5392-3. doi: 10.1007/s00436-017-5392-3. [DOI] [PubMed] [Google Scholar]
- 19.Jalili C, Makalani F, Roshankhah S, Sohrabi K, Salahshoor MR. Protective effect of resveratrol against morphine damage to kidneys of mice. Int J Morphol. 2017;35:1409–15. doi: 10.4067/S0717-95022017000401409. [Google Scholar]
- 20.Huang Q, Huang R, Zhang S, Lin J, Wei L, He M, et al. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol Lett. 2013;217:102–10. doi: 10.1016/j.toxlet.2012.12.014. doi: 10.1016/j.toxlet.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69:273–8. doi: 10.1017/S002966511000162X. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 22.Xiong YJ, Chen DP, Lv BC, Liu FF, Wang L, Lin Y, et al. The characteristics of genistein-induced inhibitory effects on intestinal motility. Arch Pharm Res. 2013;36:345–52. doi: 10.1007/s12272-013-0053-2. doi: 10.1007/s12272-013-0053-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhuo L, Liao M, Zheng L, He M, Huang Q, Wei L, et al. Combination therapy with taurine, epigallocatechin gallate and genistein for protection against hepatic fibrosis induced by alcohol in rats. Biol Pharm Bull. 2012;35:1802–10. doi: 10.1248/bpb.b12-00548. doi: 10.1248/bpb.b12-00548. [DOI] [PubMed] [Google Scholar]
- 24.Yue Q, von Bahr C, Odar-Cederlöf I, Säwe J. Glucuronidation of codeine and morphine in human liver and kidney microsomes: Effect of inhibitors. Pharmacol Toxicol. 1990;66:221–6. doi: 10.1111/j.1600-0773.1990.tb00737.x. doi: 10.1111/j.1600-0773.1990.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 25.Skrabalova J, Karlovska I, Hejnova L, Novotny J. Protective effect of morphine against the oxidant-induced injury in H9c2 cells. Cardiovasc Toxicol. 2018;29:1–2. doi: 10.1007/s12012-018-9448-0. doi: 10.1007/s12012-018-9448-0. [DOI] [PubMed] [Google Scholar]
- 26.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127:1260–8. doi: 10.1093/jn/127.7.1260. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 27.Elsayed AA, Menze ET, Tadros MG, Ibrahim BM, Sabri NA, Khalifa AE, et al. Effects of genistein on pentylenetetrazole-induced behavioral and neurochemical deficits in ovariectomized rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:27–36. doi: 10.1007/s00210-017-1435-7. doi: 10.1007/s00210-017-1435-7. [DOI] [PubMed] [Google Scholar]
- 28.Miyashima Y, Iwamuro M, Shibata M, Miyabe Y, Kawai Y, Kaihara M, et al. Prediction of disseminated intravascular coagulation by liver function tests in patients with Japanese spotted fever. Intern Med. 2018;57:197–202. doi: 10.2169/internalmedicine.8420-16. doi: 10.2169/internalmedicine.8420-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radwan RR, Mohamed HA. Nigella sativa oil modulates the therapeutic efficacy of mesenchymal stem cells against liver injury in irradiated rats. J Photochem Photobiol B. 2018;178:447–56. doi: 10.1016/j.jphotobiol.2017.11.037. doi: 10.1016/j.jphotobiol.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Eftekhari A, Ahmadian E, Panahi-Azar V, Hosseini H, Tabibiazar M, Maleki Dizaj S, et al. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: In vitro/in vivo studies. Artif Cells Nanomed Biotechnol. 2018;46:411–20. doi: 10.1080/21691401.2017.1315427. doi: 10.1080/21691401.2017.1315427. [DOI] [PubMed] [Google Scholar]
- 31.Hsu MH, Savas U, Lasker JM, Johnson EF. Genistein, resveratrol, and 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside induce cytochrome P450 4F2 expression through an AMP-activated protein kinase-dependent pathway. J Pharmacol Exp Ther. 2011;337:125–36. doi: 10.1124/jpet.110.175851. doi: 10.1124/jpet.110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefano GB, Liu Y, Goligorsky MS. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem. 1996;271:19238–42. doi: 10.1074/jbc.271.32.19238. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- 33.Joshi JC, Ray A, Gulati K. Effects of morphine on stress induced anxiety in rats: Role of nitric oxide and Hsp70. Physiol Behav. 2015;139:393–6. doi: 10.1016/j.physbeh.2014.11.056. doi: 10.1016/j.physbeh.2014.11.056. [DOI] [PubMed] [Google Scholar]
- 34.Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: Friend, foe, or just passerby? Ann N Y Acad Sci. 2002;962:275–95. doi: 10.1111/j.1749-6632.2002.tb04074.x. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 35.Squadrito F, Altavilla D, Morabito N, Crisafulli A, D'Anna R, Corrado F, et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–47. doi: 10.1016/s0021-9150(02)00013-8. doi: 10.1016/S0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 36.Kuriyama S, Morio Y, Toba M, Nagaoka T, Takahashi F, Iwakami S, et al. Genistein attenuates hypoxic pulmonary hypertension via enhanced nitric oxide signaling and the erythropoietin system. Am J Physiol Lung Cell Mol Physiol. 2014;306:L996–1005. doi: 10.1152/ajplung.00276.2013. doi: 10.1152/ajplung.00276.2013. [DOI] [PubMed] [Google Scholar]