Abstract
Background:
Pelvic exenteration (PE) for primary and recurrent cervical cancer has resulted in favorable survival outcomes, but there are controversies about specific prognosis factors, and up to now, there have been no published reports from China. This study aimed to share our experiences of PE, which were performed in a single institution.
Methods:
From January 2009 to January 2016, 38 patients with recurrent or persistent cervical cancer were included in the study, and they were followed up until January 2017. Epidemiological and clinicopathological characteristics of patients were compared for survival outcomes in univariate and Cox hazard regression analysis.
Results:
There were thirty-one and seven patients with recurrent and persistent cervical cancer, respectively. The median age of patients was 45 years (range 29–65 years). Total, anterior, and posterior PE consisted of 52.6%, 28.9%, and 18.4% of cases, respectively. Early and late complications occurred in 21 (55.3%) patients and 15 (39.5%) patients, respectively. Two (5.3%) patients died due to complications related to surgeries within 3 months after PE. The median overall survival (OS) and disease-free survival (DFS) were 28.5 months (range 9–96 months) and 23 months (range 4–96 months), respectively, and 5-year OS and DFS were 48% and 40%, respectively. Cox hazard regression analysis showed that, the margin status of the incision and mesorectal lymph node status were independent risk factors for OS and DFS.
Conclusion:
In our patients with recurrent and persistent cervical cancer, the practice of PE might achieve favorable survival outcomes.
Trial Registration:
ClinicalTrials.gov, NCT03291275; https://clinicaltrials.gov/ct2/show/NCT03291275?term=NCT03291275&rank=1.
Keywords: Cervical Cancer, Pelvic Exenteration, Survival
摘要
背景:
盆腔廓清术(PE)治疗原发和复发性宫颈癌的生存预后较好,但是有关预后因素仍有很多争议,迄今也没有来自中文 的报道。本研究旨在分享我们施行PE的经验,所有手术均在一家医院进行。
方法:
从2009年1月到2016年1月,38例复发性或未控性宫颈癌纳入研究,随访至2017年1月。以单因素分析和Cox风险回归模 型分析患者流行病学和临床病理特点对生存结局的影响。
结果:
复发性和未控性宫颈癌患者分别31例和7例,中位年龄45岁(范围29-65岁)。全盆腔廓清术、前盆腔廓清术和后盆腔 廓清术分别占52.6%、28.9%和18.4%。分别有21例(55.3%)和15例(39.5%)患者在术后发生了早期合并症和晚期合并症。 两例患者(5.3%)在PE术后3个月内死于合并症。中位总体生存率(OS)和无病生存率(DFS)分别为28.5个月(范围9–96 个月)和23个月(范围4–96个月),5年OS率和DFS率分别为48%和40%。Cox风险回归分析显示,切缘状态、直肠系膜淋巴 结状态是影响OS和DFS的独立的预后因素。
结论:
在我们的研究中,复发和未控性宫颈癌患者在盆腔廓清术后可能有较好的预后结果。
研究注册:
ClinicalTrials.gov, NCT03291275; https://clinicaltrials.gov/ct2/show/NCT03291275?term=NCT03291275&rank=1
INTRODUCTION
With the improvement of strategies for managing cervical cancer and the prevalence of HPV vaccines, incidence and mortality rates in developed countries have decreased significantly.[1] Nevertheless, 8% of patients present with Stage IV cancer at diagnosis,[2] and their 5-year survival has been only 9.3–21.6%.[3] For early-stage cervical cancer, a radical hysterectomy and resection of the retroperitoneal lymph nodes have had similar effects compared with radiotherapy.[4] For locally advanced cervical cancer, concurrent chemoradiotherapy (CCRT) is more appropriate[5,6,7,8] and has been the primary treatment for about 70% of patients.[9] Recurrence rates for patients with Stages IB, IIA, IIB, III, and IV have been 10%, 17%, 23%, 42%, and 74%, respectively.[10] The 5-year overall survival (OS) has been <5%.[2] In patients for whom repeated radiotherapy for the same anatomy sites is contraindicated, the control effects of systemic chemotherapy have been very poor.[11,12] For selected patients, radical en bloc resection (pelvic exenteration [PE]) is the only potentially curative treatment option with favorable survival outcomes although at cost of high-morbidity rates.[10,12,13] There are no published reports from China about the practice of PE, so we are publishing the first such report to introduce our experiences with PE for recurrent and persistent cervical cancer in China. These surgeries were all performed at the Department of Obstetrics and Gynecology, Peking Union Medical College Hospital (PUMCH).
METHODS
Ethical approval
The Institutional Review Board of PUMCH approved this study (No. ZS-1428). The registration number is NCT03291275 (clinicaltrials.gov). All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Study participants
From January 2009 to January 2016, PE was performed on 40 patients, 38 of whom were patients with recurrent or persistent cervical cancer. Before surgeries, all patients accepted detailed counseling about plans, complications, and prognosis of PE, and all gave consent. All patients accepted detailed evaluations and assessments and were excluded if they had complications related to the morbidity of the cardiovascular and respiratory or urinary systems and if they had the American Society of Anesthesiologists score >1.[14]
Data collection
All patients were followed up in specific schemes and all survival data were recorded up to January 2017. Data of OS and disease-free survival (DFS) were achieved from outpatient clinics and/or telephone interviews. Epidemiological and clinical characteristics such as pathological subtype, age, and history of previous treatment were collected from medical records. Indications of PE included recurrent cervical cancer diagnosed through pathological evidence from a needle core biopsy or direct biopsy, and persistent cervical cancer that imaging or biopsy showed was refractory to systemic chemotherapy or (chemo) radiotherapy. All patients agreed to undergo imaging evaluation from computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) to confirm lesions within the pelvic floor and/or pelvic wall. Any suspicious distant metastasis is regarded as a contraindication for PE.
As for the extent of surgery, PE procedures were divided into anterior PE (removal of the entire bladder, uterus, and vagina in patients for whom the bladder was involved), posterior PE (removal of the uterus, vagina, and the involved part of the colorectum), and total PE (removal of the involved part of the bladder, colorectum, and other pelvic lesions). As for the surgical extent relevant to the levator ani muscles, PE procedures were divided into Type I (above the levator ani muscles), Type II (within the levator ani muscles), and Type III (below the levator ani muscles).[15] As for whether surgeries involved lesions of the pelvic wall, PE procedures were divided into laterally extended endopelvic resection (LEER)[16] or not LEER.
The involved organs, surgical level, and anatomical changes will determine which procedure of intestinal diversion to use (colorectal anastomosis or colostomy), urinary diversions (ureterocutaneostomy, Bricker's ileal conduit, and Miami pouch), and how to reconstruct the pelvic floor (vulvar reconstruction plus vaginal tamping or vulvar and vaginal reconstruction).
Perioperative adjuvant therapies included systemic chemotherapy, radiotherapy or synchronous chemotherapy and radiotherapy ([chemo] radiotherapy), and their combinations. Complications of PE requiring admission to the hospital included early complications (defined as conditions developing within 30 days of PE) and late complications (defined as conditions developing more than 30 days after PE). These conditions included wound problems (dehiscence or infection), infections (sepsis or abscess of specific sites), urinary system complications (obstruction, fistula, renal failure, or leakage of anastomosis), gastrointestinal (GI) system complications (ileus, obstruction, and leakage of anastomosis), venous thromboembolism, and infection or breakdown of the vulvar flap.
All surgical specimens were kept for pathological examinations. We paid special attention to the following pathological characteristics: marginal status of the incision, the diameter of tumors, lymphovascular space invasion (LVSI), and metastasis to mesorectal and retroperitoneal lymph nodes.
Statistical analysis
All data were collected using Microsoft Excel, and analyzed using SPSS version 19.0 (SPSS Inc., New York, USA). Comparisons were made using the χ2 test or Fisher's exact test and the Mann-Whitney U-test. Univariate analysis of the impact of epidemiological and clinical characteristics on survival outcomes was performed using Kaplan-Meier tests. Cox hazard regression analysis was used to determine the independent risk factors for survival outcomes.
RESULTS
Epidemiological and preoperative clinical characteristics of patients
From January 2009 to January 2016, 38 patients with recurrent or persistent cervical cancer underwent open PE. The median age of patients was 45 years (range 29–65 years). Nine (23.7%) patients were 50 years or older and two (5.3%) were 60 years or older. With respect to the primary International Federation of Gynecology and Obstetrics staging, there was one (2.6%) case of IA not specified, one (2.6%) of IA2, seven (18.4%) of IB1, ten (26.3%) of IB2, four (7.9%) of IIA, 12 (31.6%) of IIB, and one (2.6%) each of IIIA, IIIB, and IVA, respectively. CT, MRI, and PET scans were applied in three, 13, and 34 patients, respectively. Most of the patients had squamous carcinoma (78.9%) and recurrent cancer (81.6%). The median length of DFS after primary treatment for cervical cancer was 13 months (range 0–156 months). For 31 patients with recurrent disease, two (6.4%), 15 (48.4%), and eight (25.8%) patients received systemic chemotherapy only, (chemo) radiotherapy only, and systemic chemotherapy plus CCRT, respectively, during the periods of primary therapy. For seven patients with persistent disease, one (14.3%), two (28.6%), and four (57.1%) received systemic chemotherapy only, (chemo) radiotherapy only, and systemic chemotherapy plus CCRT, respectively, as the primary therapy or salvaged treatment. Other patient characteristics are listed in Table 1.
Table 1.
Epidemiological and preoperative clinical characteristics of patients and their impact on the survival outcomes by Kaplan-Meier tests
| Characteristics | n (%) | Impact on OS* | Impact on DFS* |
|---|---|---|---|
| Pathology subtype | |||
| Squamous carcinoma | 30 (78.9) | 0.963 | 0.586 |
| Adenocarcinoma | 6 (15.8) | ||
| Adenosquamous carcinoma | 2 (5.3) | ||
| Recurrent or persistent diseases | |||
| Persistent diseases | 7 (18.4) | 0.001 | 0.003 |
| Recurrent diseases | 31 (81.6) | ||
| Age of PE (years), median (range) | 45 (29–65) | 0.865 | 0.765 |
| Surgical stage | |||
| Before or on 2010 | 8 (21.1) | 0.663 | 0.593 |
| After 2010 | 30 (78.9) | ||
| History of surgeries of uterus | |||
| None | 14 (36.8) | 0.585 | 0.841 |
| LRH | 3 (7.9) | ||
| RH | 14 (36.8) | ||
| TAH | 7 (18.4) | ||
| History of adjuvant therapy | |||
| None | 6 (15.8) | 0.826 | 0.489 |
| Systemic chemotherapy only | 3 (7.9) | ||
| Chemoradiotherapy only | 16 (42.1) | ||
| Systemic chemotherapy + CCRT | 11 (28.9) | ||
| Adjuvant therapy before PE | |||
| None | 14 (36.8) | 0.655 | 0.253 |
| Systemic chemotherapy only | 15 (39.5) | ||
| Chemoradiotherapy only | 3 (7.9) | ||
| Systemic chemotherapy + CCRT | 6 (15.8) | ||
| DFS after primary treatment | |||
| ≤12 months | 22 (57.9) | 0.599 | 0.482 |
| >12 months | 16 (42.1) |
*Univariate analysis. CCRT: Concurrent chemoradiotherapy; DFS: Disease-free survival; LRH: Laparoscopic radical hysterectomy; OS: Overall survival; PE: Pelvic exenteration; RH: Radical hysterectomy; TAH: Total abdominal hysterectomy.
Procedures, adjuvant therapy, and complications of pelvic exenteration
Surgical characteristics are listed in Table 2. After initial surgical exploration, no PE procedure was abandoned. Total, anterior, and posterior PE consisted of 52.6%, 28.9%, and 18.4% of cases, respectively. Eleven patients accepted LEER for lesions of the pelvic wall: four, six, and one with the left, right, and bilateral pelvic wall involved, respectively; these were all patients with recurrent disease. Intestinal diversion procedures were performed in 27 patients, including colorectal anastomosis (nine cases) and colostomy (18 cases). Urinary diversion procedures were performed in 31 patients, including ureterocutaneostomy (two cases), Bricker's ileal conduit (22 cases), and Miami pouch (seven cases). Procedures to reconstruct the pelvic floor were performed in 15 patients, including vulvar reconstruction plus vaginal tamping (12 cases) and vulvar and vaginal reconstruction (three cases).
Table 2.
Procedures, adjuvant therapy, and complications of PE and pathological characteristics and their impact on the survival outcomes by Kaplan-Meier tests
| Characteristics | n (%) | Impact on OS* | Impact on DFS* |
|---|---|---|---|
| Type of PE | |||
| Anterior PE | 11 (28.9) | 0.114 | 0.187 |
| Posterior PE | 7 (18.4) | ||
| Total PE | 20 (52.6) | ||
| Level of PE | |||
| Level I | 4 (10.5) | 0.668 | 0.312 |
| Level II | 7 (18.4) | ||
| Level III | 27 (71.1) | ||
| LEER | |||
| Yes | 11 (28.9) | 0.658 | 0.151 |
| No | 27 (71.1) | ||
| Intestinal diversion | |||
| None | 11 (28.9) | 0.759 | 0.568 |
| Colorectal anastomosis | 9 (23.7) | ||
| Colostomy | 18 (47.4) | ||
| Urinary diversion | |||
| None | 7 (18.4) | 0.003 | 0.005 |
| Ureterocutaneostomy | 2 (5.3) | ||
| Ileal conduit (Bricker) | 22 (57.9) | ||
| Pouch (Miami) | 7 (18.4) | ||
| Reconstruction of pelvic floor | |||
| Yes | 15 (39.5) | 0.991 | 0.559 |
| No | 23 (60.5) | ||
| Length of PE (min), median (range) | 385 (120–615) | 0.545 | 0.949 |
| Estimated blood loss (ml), median (range) | 1200 (400–5000) | 0.753 | 0.285 |
| Transfusion volumes (ml), median (range) | 1600 (0–3600) | 0.187 | 0.465 |
| Hospital stay after PE (days), median (range) | 28 (9–265) | 0.400 | 0.604 |
| Adjuvant therapy after PE | |||
| None | 11 (28.9) | 0.364 | 0.584 |
| Systemic chemotherapy | 23 (60.5) | ||
| Chemoradiotherapy | 4 (10.5) | ||
| Early complication | |||
| Yes | 21 (55.3) | 0.406 | 0.224 |
| No | 17 (44.7) | ||
| Late complication | |||
| Yes | 15 (39.5) | 0.740 | 0.862 |
| No | 23 (60.5) | ||
| Diameter of tumor | |||
| ≤5 cm | 29 (76.3) | 0.018 | 0.009 |
| >5 cm | 9 (23.7) | ||
| LVSI | |||
| Positive | 9 (23.7) | 0.036 | 0.014 |
| Negative | 29 (76.3) | ||
| Mesorectal lymph nodes (n = 27) | |||
| Positive | 8 (29.6) | 0.008 | 0.019 |
| Negative | 19 (70.4) | ||
| Margin of incision | |||
| Positive | 7 (18.4) | <0.001 | <0.001 |
| Negative | 31 (81.6) | ||
| Retroperitoneal lymph nodes (n = 10) | |||
| Positive | 5 (50.0) | 0.691 | 0.877 |
| Negative | 5 (50.0) |
*Univariate analysis. DFS: Disease-free survival; LEER: Laterally extended endopelvic resection; LVSI: Lymphovascular space invasion; OS: Overall survival; PE: Pelvic exenteration.
A total of 32 patients stayed in the Intensive Care Unit for more than 2 days. All patients except two received blood transfusions. Most patients accepted adjuvant treatment of systemic chemotherapy (60.5%) or CCRT (10.5%).
There were 21 (55.3%) patients and 15 (39.5%) patients with early and late complications, respectively [Tables 2 and 3]. For early complications, sepsis and pelvic abscess resulted in two deaths, 25 days and 77 days after PE, respectively, which were considered surgery-related mortalities. Except for the hospital stay after PE (P = 0.011 for occurrence of early complications), the following factors were all unrelated categories and frequencies of complications (all P > 0.05): types and levels of PE; whether or not LEER was applied; specific procedures for intestinal/urinary diversion and reconstruction of the pelvic floor; adjuvant therapy after PE; and estimated blood loss and transfusion volumes of surgeries.
Table 3.
Early and late complications sorted by descriptive analysis
| Complications | n |
|---|---|
| Early complications | 21 |
| Wound dehiscence and/or infection | 10 |
| Ileus | 5 |
| Leakage of colorectal anastomosis | 5 |
| Venous thromboembolism | 5 |
| Ureteral fistula | 4 |
| Obstruction of urinary system | 2 |
| Sepsis | 4 |
| Death | 1 |
| Pelvic abscess | 1 |
| Necrosis of vulvar flap | 2 |
| Late complications | 15 |
| Leakage of colorectal anastomosis | 7 |
| Ileus | 5 |
| Pyelonephritis | 5 |
| Ureteral fistula | 2 |
| Vesical fistula | 1 |
| Venous thromboembolism | 2 |
| Renal failure | 1 |
Pathological characteristics
All surgical specimens were accepted through pathological examinations [Table 2]. All patients achieved complete resection (R0), defined as a circumferential resection margin of 1 mm or greater. The median diameter of tumors was 4.5 cm (range 2–11 cm). Preoperative imaging revealed pelvic lesions in 11 patients, which were all confirmed by pathological examination. For anterior, posterior, and total PE, there were 22.2% (2/9), 60.0% (3/5), and 23.1% (3/13) patients with positive mesorectal lymph nodes, respectively (P = 0.257). For Levels I, II, and III, there were 0 (0/4), 28.6% (2/7), and 18.5% (5/27) patients with positive margin of incisions, respectively (P = 0.501). In the study, preoperative evaluation about the relationship of lesions with the pelvic wall (yes or no) and different types of PE (Type I, II, and III) had no impact on the status of mesorectal lymph nodes and margins (all P > 0.05).
Survival outcomes
Two (5.3%) patients died within 3 months after PE. One patient, with persistent disease, developed complications, with a leak of the colorectal anastomosis and then sepsis, and died 39 days after total PE. The other patient, with recurrent disease, also developed complications, with pelvic abscess of unknown origin, and died 77 days after total PE and reconstruction of the pelvic floor. These two patients had no lesions of the pelvic wall. We judged that the two deaths were related to PE surgeries. For the remaining 36 patients, after a median of 28.5 months' follow-up (range 12–96 months), 21 (58.3%) showed evidence of recurrence and 19 (52.8%) died of recurrence. Among patients with recurrence, 16 (76.2%) of 21 recurrences were confined within the pelvic cavity, and five (23.8%) of 21 showed evidence of distant metastasis including in the lung (three cases), liver (one case), and bone (one case).
The median OS was 28.5 months (range 9–96 months), and the median DFS was 23 months (range 4–96 months). Among patients with recurrence, the median DFS was 12 months (range 4–43 months). Five-year OS and DFS were 48% and 40%, respectively. In univariate analysis, recurrence or persistent diseases, procedures of urinary diversion, the diameter of tumor, LVSI, metastasis to mesorectal lymph nodes, and marginal status of incision were significant risk factors for both OS and DFS [Tables 1 and 2]. Eventually, all seven patients with persistent cervical cancer that was refractory to primary therapy died: one death was due to complications of surgery, and the median OS and DFS for the other six patients were 16.0 months (range 9–33 months) and 9.5 months (6–24 months), respectively. For patients with recurrent cervical cancer, the 5-year OS and DFS were 58% and 49%, respectively.
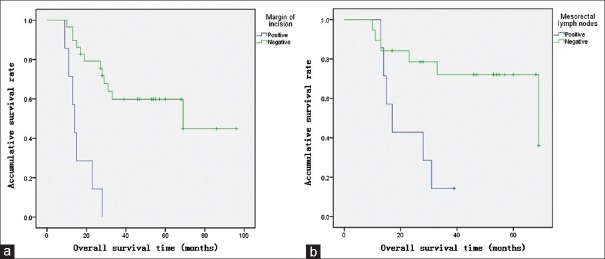
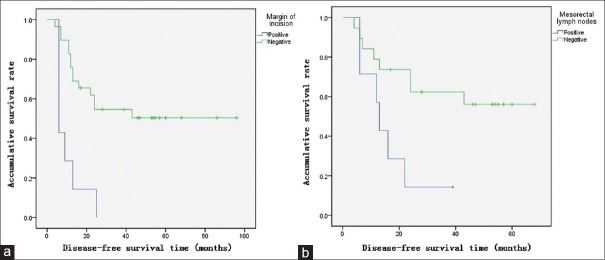
In Cox proportional-hazards regression, the margin status of incisions and mesorectal lymph node status were independent risk factors for survival outcomes. Compared to patients with negative margins of incision, patients with positive margins had higher risks of mortality (relative risk [RR]: 8.7, 95% confidence interval [CI]: 1.9–40.6, P = 0.006) [Figure 1a] and recurrence (RR: 9.2, 95% CI: 2.0–41.5, P = 0.004) [Figure 2a]. Compared to patients with negative mesorectal lymph nodes, patients with metastasis of mesorectal lymph nodes had higher risks of mortality (RR: 3.9, 95% CI: 1.1–14.1, P = 0.037) [Figure 1b] and recurrence (RR: 3.5, 95% CI: 1.1–11.2, P = 0.037) [Figure 2b]. Among patients with positive mesorectal lymph nodes or positive margins (13 patients), 8% had both 3-year OS and DFS. Among patients with negative mesorectal lymph nodes and negative margins (17 patients), 81% had 5-year OS and 64% had 5-year DFS.
Figure 1.
OS curves of the patients described by Kaplan-Meier tests. (a) OS of positive and negative margins of the incisions (χ2=19.325, P value of log-rank test <0.001). (b) OS of positive and negative mesorectal lymph nodes (χ2= 7.060, P value of log-rank test is 0.008). OS: Overall survival.
Figure 2.
DFS curves of the patients described by Kaplan-Meier tests. (a) DFS of positive and negative margins of the incisions (χ2=18.524, P value of log-rank test <0.001). (b) DFS of positive and negative mesorectal lymph nodes (χ2=5.909, P value of log-rank test is 0.019). DFS: Disease-free survival.
DISCUSSION
Choices of treatment plans have been limited for recurrent and advanced cervical cancer. A Cochrane database systemic review found no evidence to help women make informed decisions about exenterative surgery for recurrent cervical, endometrial, vaginal, or vulvar malignancies.[17] When local recurrence occurs, treatment options are limited due to the frequent use of pelvic irradiation for primary cervical cancer. Reirradiation of the same anatomic site is contraindicated, and chemotherapy is ineffective at controlling tumors located within the previously irradiated tissue, which tends to be less vascularized.[11,12] In 1948, Alexander Brunschwig for the first time proposed exenteration of all pelvic organs to manage pelvic malignancies.[18,19] According to surgical criteria and patients, 5-year OS varied between 20% and 50%, despite high morbidity rate.[10] Recently, 5-year OS was approximated at 60% with tolerable morbidity.[20,21] For patients with recurrent cervical cancer confined to the pelvic floor without distant metastasis, PE and even LEER was worthy of consideration if patients were in good condition and a favorable nutritional state.[10,12,13] Based on the extensive characteristics and high morbidity of PE, multiteam collaboration and elaborative management and follow-up were crucial for successful surgery and patient recovery. In this study, we reported on a large cohort of PE procedures for cervical cancer, most of which were performed in PUMCH. In this study, despite a mortality rate of 5.3% comparable with previous reports, we achieved favorable survival outcomes. These data of morbidity, mortality, and quality of life provided evidence for discussion with patients about deciding to have PE. Some authors found that complications after PE shorten patients' length of survival.[21] Surgical numbers of physicians[22] and Level III PE with reconstruction of the pelvic floor[23,24,25] were independent risk factors for complications. In this study, other factors relevant to surgery (except for hospital stay) and adjuvant therapy were not related to categories and frequencies of complications. These conflicts will be clarified in a further study with a larger cohort of patients.
Margin status had been proven in systemic reviews to be the most important factor for survival.[20,26] Modern imaging does not accurately identify local extensions of microscopic disease and is inadequate for preoperative planning of the extent of resection.[20] Magrina and Stanhope[27] proposed the subclassification of the exenteration groups into Type I (supralevator), Type II (infralevator), and Type III (with vulvectomy), which has been helpful in facilitating an understanding of the extent of resection of the pelvic structures and the anatomical changes associated with each operation. We failed, however, to find a significant relationship between the surgical level and marginal status of incisions. Other risk factors of survival included the size of tumors,[10,11] DFS after primary treatment,[11] pathological subtypes of malignancies,[28] metastasis to the mesorectal[29,30,31] or retroperitoneal[32,33] lymph nodes, and LVSI. We checked all these factors in the study and found that metastasis to mesorectal lymph nodes and marginal status of the incision remained independent risk factors both for OS and DFS. According to our analysis, preoperative lesion status had no impact on the pathological outcomes. Although there was no significance between types of PE and status of mesorectal lymph nodes, most patients (60%) who had posterior PE had positive lymph nodes, suggesting that mesorectal lymph node involvement is a common finding in patients with rectal wall involvement.[31]
Few reports mentioned concerns of persistent cervical cancer being refractory to systemic chemotherapy or radiotherapy.[34,35] In univariate analysis, recurrent or primary disease was a significant factor for survival outcomes, and no patients with persistent disease refractory to primary treatment survived longer than 33 months, while the 5-year OS and DFS of recurrent patients were 58% and 49%, respectively. This finding suggests that the nature of cervical cancer was critical for selecting eligible patients for PE, since median OS of these patients was only 16 months. Although for patients with histopathologically confirmed Stage IVA cervical cancer, exenteration is a valid alternative to primary chemoradiation,[11] the role of PE after primary systemic chemotherapy and/or radiotherapy is not clear, possibly because of compromised healing of irradiated or necrotic tissue and use of complex reconstructive techniques.[12]
Existing lesions of the pelvic wall have long been regarded a contraindication for PE. Recently, however, based on the theory of morphogenetic fields of embryonic development in locoregional cancer spread,[36] the role of LEER in the surgical treatment of pelvic malignancies including cervical cancer has been proven by some reports.[16,37] The only contraindication for LEER was involvement of the sciatic nerve.[38,39] In this study, we found that LEER patients had similar survival outcomes and complications compared with non-LEER patients in the univariate analysis, which further improves the feasibility and safety of LEER for the recurrent cervical disease. It is worth noting that in the present study, no patients with persistent cervical disease refractory to primary therapy accepted LEER.
The primary shortcoming of this study is lack of analysis of quality of life. Most patients with recurrent or advanced malignancies and their relatives would be more concerned with the quality of life, rather than the length of survival. Despite the increasing survival time, PE causes extensive effects on patients' physical, mental, and self-image features. Data from Dessole et al.[40] suggested the existence of several unmet needs, including increased insomnia, poor attitude to disease, financial difficulties, high levels of gastrointestinal symptoms, with an obvious significant impact on global health status, body image, role functioning, social functioning, and emotional functioning. Although 40% of patients exhibited significant levels of distress at prehospital admission, PE candidates showed an adaptive range of coping mechanisms, and there were no correlations between clinical and psychological variables.[41] Further, quality of life and related patient-reported outcomes improved rapidly after PE surgery. For 9 months after surgery, these outcomes were comparable with those of similar patients who did not have surgery; thereafter, there was a decline in patients who did not have exenteration.[42]
In conclusion, in a cohort of cervical cancer patients who had PE, which was all performed in a single center, metastasis to mesorectal lymph nodes and positive margin of incision were independent risk factors for survival outcomes. At the cost of about 50% morbidity and 5% mortality, patients achieved favorable survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–25. doi: 10.1016/S0140-6736(03)13778-6. doi: 10.1016/s0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. Bethesda, MD: National Cancer Institute; 1975-2012. [Last accessed on 2015 Apr 01]. SEER Cancer Statistics Review. Available from: http://www.seer.cancer.gov . [Google Scholar]
- 4.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–40. doi: 10.1016/S0140-6736(97)02250-2. doi: 10.1016/s0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. doi: 10.1056/nejm199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 7.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. doi: 10.1056/nejm199904153401503. [DOI] [PubMed] [Google Scholar]
- 8.Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet. 2001;358:781–6. doi: 10.1016/S0140-6736(01)05965-7. doi: 10.1016/s0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 9.Gadducci A, Tana R, Cosio S, Cionini L. Treatment options in recurrent cervical cancer (Review) Oncol Lett. 2010;1:3–11. doi: 10.3892/ol_00000001. doi: 10.3892/ol_00000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiretti M, Zapardiel I, Zanagnolo V, Landoni F, Morrow CP, Maggioni A, et al. Management of recurrent cervical cancer: A review of the literature. Surg Oncol. 2012;21:e59–66. doi: 10.1016/j.suronc.2011.12.008. doi: 10.1016/j.suronc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Marnitz S, Köhler C, Müller M, Behrens K, Hasenbein K, Schneider A, et al. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecol Oncol. 2006;103:1023–30. doi: 10.1016/j.ygyno.2006.06.027. doi: 10.1016/j.ygyno.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Höckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: Achievements and unanswered questions. Lancet Oncol. 2006;7:837–47. doi: 10.1016/S1470-2045(06)70903-2. doi: S1470-2045(06)70903-2. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RL, Keeney ED, Freedman RS, Burke TW, Eifel PJ, Rutledge FN, et al. Radical hysterectomy for recurrent carcinoma of the uterine cervix after radiotherapy. Gynecol Oncol. 1994;55:29–35. doi: 10.1006/gyno.1994.1242. doi: 10.1006/gyno.1994.1242. [DOI] [PubMed] [Google Scholar]
- 14.ASA Physical Status Classification System. ASA Relative Value Guide. [Last accessed on 2017 Mar 01]. Available from: http://www.asahq.org/shop-asa .
- 15.Yang K, Cai L, Yao L, Zhang Z, Zhang C, Wang X, et al. Laparoscopic total pelvic exenteration for pelvic malignancies: The technique and short-time outcome of 11 cases. World J Surg Oncol. 2015;13:301. doi: 10.1186/s12957-015-0715-2. doi: 10.1186/s12957-015-0715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höckel M, Horn LC, Einenkel J. (Laterally) extended endopelvic resection: Surgical treatment of locally advanced and recurrent cancer of the uterine cervix and vagina based on ontogenetic anatomy. Gynecol Oncol. 2012;127:297–302. doi: 10.1016/j.ygyno.2012.07.120. doi: 10.1016/j.ygyno.2012.07.120. [DOI] [PubMed] [Google Scholar]
- 17.Ang C, Bryant A, Barton DP, Pomel C, Naik R. Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst Rev. 2014;2:CD010449. doi: 10.1002/14651858.CD010449.pub2. doi: 10.1002/14651858.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948;1:177–83. doi: 10.1002/1097-0142(194807)1:2<177::aid-cncr2820010203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Brunschwig A. The surgical treatment of cancer of the cervix uteri; a radical operation for cancer of the cervix. Bull N Y Acad Med. 1948;24:672–83. [PMC free article] [PubMed] [Google Scholar]
- 20.Sardain H, Lavoue V, Redpath M, Bertheuil N, Foucher F, Levêque J, et al. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. Eur J Surg Oncol. 2015;41:975–85. doi: 10.1016/j.ejso.2015.03.235. doi: 10.1016/j.ejso.2015.03.235. [DOI] [PubMed] [Google Scholar]
- 21.Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: A single institution study over 20 years. Gynecol Oncol. 2011;122:14–8. doi: 10.1016/j.ygyno.2011.03.003. doi: 10.1016/j.ygyno.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Althumairi AA, Canner JK, Gorin MA, Fang SH, Gearhart SL, Wick EC, et al. Reduction of costs for pelvic exenteration performed by high volume surgeons: Analysis of the Maryland health service cost review commission database. Am Surg. 2016;82:46–52. [PubMed] [Google Scholar]
- 23.Ferron G, Pomel C, Martinez A, Narducci F, Lambaudie E, Marchal F, et al. Pelvic exenteration: Current state and perspectives. Gynecol Obstet Fertil. 2012;40:43–7. doi: 10.1016/j.gyobfe.2011.10.008. doi: 10.1016/j.gyobfe.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Ferron G, Martel P, Querleu D. Vaginal reconstruction after pelvic exenteration: When and which techniques. Bull Cancer. 2003;90:435–40. [PubMed] [Google Scholar]
- 25.Miller B, Morris M, Gershenson DM, Levenback CL, Burke TW. Intestinal fistulae formation following pelvic exenteration: A review of the University of Texas M D Anderson Cancer Center experience, 1957-1990. Gynecol Oncol. 1995;56:207–10. doi: 10.1006/gyno.1995.1033. doi: 10.1006/gyno.1995.1033. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Pan L. Current status and outcomes of pelvic exenteration for recurrent cervical cancer: A systematic review (in Chinese) Chin J Obstet Gynecol. 2014;49:460–5. doi: 10.3760/cma.j.issn.0529-567x.2014.06.014. [PubMed] [Google Scholar]
- 27.Magrina JF, Stanhope CR, Weaver AL. Pelvic exenterations: Supralevator, infralevator, and with vulvectomy. Gynecol Oncol. 1997;64:130–5. doi: 10.1006/gyno.1996.4532. [DOI] [PubMed] [Google Scholar]
- 28.Baiocchi G, Guimaraes GC, Faloppa CC, Kumagai LY, Oliveira RA, Begnami MD, et al. Does histologic type correlate to outcome after pelvic exenteration for cervical and vaginal cancer? Ann Surg Oncol. 2013;20:1694–700. doi: 10.1245/s10434-012-2768-6. doi: 10.1245/s10434-012-2768-6. [DOI] [PubMed] [Google Scholar]
- 29.Chiantera V, Rossi M, De Iaco P, Koehler C, Marnitz S, Ferrandina G, et al. Survival after curative pelvic exenteration for primary or recurrent cervical cancer: A retrospective multicentric study of 167 patients. Int J Gynecol Cancer. 2014;24:916–22. doi: 10.1097/IGC.0b013e3182a80aec. doi: 10.1097/IGC.0b013e3182a80aec. [DOI] [PubMed] [Google Scholar]
- 30.Yoo HJ, Lim MC, Seo SS, Kang S, Yoo CW, Kim JY, et al. Pelvic exenteration for recurrent cervical cancer: Ten-year experience at National Cancer Center in Korea. J Gynecol Oncol. 2012;23:242–50. doi: 10.3802/jgo.2012.23.4.242. doi: 10.3802/jgo.2012.23.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mourton SM, Chi DS, Sonoda Y, Alektiar KM, Venkatraman ES, Barakat RR, et al. Mesorectal lymph node involvement and prognostic implications at total pelvic exenteration for gynecologic malignancies. Gynecol Oncol. 2006;100:533–6. doi: 10.1016/j.ygyno.2005.08.039. doi: 10.1016/j.ygyno.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Wang CJ, Lai CH, Huang HJ, Hong JH, Chou HH, Huang KG, et al. Recurrent cervical carcinoma after primary radical surgery. Am J Obstet Gynecol. 1999;181:518–24. doi: 10.1016/s0002-9378(99)70486-2. doi: S0002937899005578. [DOI] [PubMed] [Google Scholar]
- 33.Moutardier V, Houvenaeghel G, Martino M, Lelong B, Bardou VJ, Resbeut M, et al. Surgical resection of locally recurrent cervical cancer: A single institutional 70 patient series. Int J Gynecol Cancer. 2004;14:846–51. doi: 10.1111/j.1048-891X.2004.14519.x. doi: 10.1111/j.1048-891X.2004.14519.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AM, Imesch P, Fink D, Egger H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecol Oncol. 2012;125:604–9. doi: 10.1016/j.ygyno.2012.03.001. doi: 10.1016/j.ygyno.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Radwan RW, Evans MD, Davies M, Harris DA, Beynon J Swansea Pelvic Oncology Group. Pelvic exenteration for advanced malignancy in elderly patients. Br J Surg. 2016;103:e115–9. doi: 10.1002/bjs.10058. doi: 10.1002/bjs.10058. [DOI] [PubMed] [Google Scholar]
- 36.Höckel M. Morphogenetic fields of embryonic development in locoregional cancer spread. Lancet Oncol. 2015;16:e148–51. doi: 10.1016/S1470-2045(14)71028-9. doi: 10.1016/S1470-2045(14)71028-9. [DOI] [PubMed] [Google Scholar]
- 37.Höckel M. Long-term experience with (laterally) extended endopelvic resection (LEER) in relapsed pelvic malignancies. Curr Oncol Rep. 2015;17:435. doi: 10.1007/s11912-014-0435-8. doi: 10.1007/s11912-014-0435-8. [DOI] [PubMed] [Google Scholar]
- 38.Höckel M. Laterally extended endopelvic resection (LEER) – Principles and practice. Gynecol Oncol. 2008;111:S13–7. doi: 10.1016/j.ygyno.2008.07.022. doi: 10.1016/j.ygyno.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Höckel M. Laterally extended endopelvic resection: Surgical treatment of infrailiac pelvic wall recurrences of gynecologic malignancies. Am J Obstet Gynecol. 1999;180:306–12. doi: 10.1016/s0002-9378(99)70204-8. [DOI] [PubMed] [Google Scholar]
- 40.Dessole M, Petrillo M, Lucidi A, Naldini A, Rossi M, De Iaco P, et al. Quality of life in women after pelvic exenteration for gynecological malignancies: A multicentric study. Int J Gynecol Cancer. 2016;28:267–73. doi: 10.1097/IGC.0000000000000612. doi: 10.1097/IGC.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 41.Arnaboldi P, Santoro L, Mazzocco K, Oliveri S, Maggioni A, Pravettoni G, et al. The paradox of pelvic exenteration: The interaction of clinical and psychological variables. Int J Gynecol Cancer. 2015;25:1534–40. doi: 10.1097/IGC.0000000000000523. doi: 10.1097/IGC.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 42.Young JM, Badgery-Parker T, Masya LM, King M, Koh C, Lynch AC, et al. Quality of life and other patient-reported outcomes following exenteration for pelvic malignancy. Br J Surg. 2014;101:277–87. doi: 10.1002/bjs.9392. doi: 10.1002/bjs.9392. [DOI] [PubMed] [Google Scholar]