Abstract
Background:
Increased serum autoantibodies against interleukin-2 (anti-IL-2 autoantibodies) were reported in patients with systemic lupus erythematosus (SLE) and in patients receiving IL-2 therapy. This study aimed to explore the clinical relevance of serum anti-IL-2 autoantibodies and the interactions between low-dose IL-2 therapy and serum anti-IL-2 autoantibodies.
Methods:
Serum samples were collected from 152 SLE patients and 100 age- and gender-matched healthy controls (HCs). Among them, 75 SLE patients were followed up for 10 weeks, and all of them were treated with corticosteroids, antimalarials, and/or immunosuppressants. Forty-six out of the 75 SLE patients received low-dose IL-2 therapy additionally. Clinical and laboratory parameters were collected at baseline and week 10. Serum anti-IL-2 autoantibodies were determined by enzyme-linked immunosorbent assay.
Results:
Compared with HCs, median levels and positive rates of serum anti-IL-2 autoantibodies were higher in SLE patients (32.58 [23.63, 45.23] arbitrary unit [AU] vs. 37.54 [27.88, 60.74] AU, P = 0.006, and 5.0% vs. 18.4%, P = 0.002, respectively). Compared to those without the corresponding disorders, serum anti-IL-2 autoantibody was increased in patients with alopecia (49.79 [36.06, 64.95] AU vs. 35.06 [25.40, 58.46] AU, P = 0.033), but it was decreased in those with lupus nephritis (31.71 [22.60, 43.25] AU vs. 44.15 [31.43, 68.52] AU, P = 0.001). Moreover, serum anti-IL-2 autoantibody was positively correlated with serum IgA (r = 0.229, P = 0.005), total IgG (r = 0.327, P < 0.001), and total IgM (r = 0.164, P = 0.050). Treatment with exogenous IL-2 was not significantly associated with serum anti-IL-2 autoantibody. In addition, no significant difference was found in serum anti-IL-2 autoantibody between responders and nonresponders to low-dose IL-2 therapy.
Conclusions:
Serum anti-IL-2 autoantibody was increased and associated with disease severity in SLE. Exogenous low-dose IL-2 did not significantly induce anti-IL-2 autoantibody production.
Keywords: Autoantibody, Interleukin-2, Systematic Lupus Erythematosus
摘要
背景:
系统性红斑狼疮(SLE)的患者血清IL-2抗体水平升高。本研究探索了血清IL-2抗体在系统性红斑狼疮患者中的水平 及其意义。
方法:
收集152例SLE患者及100例年龄性别匹配的健康体检者血清,并在第10周对其中75例SLE患者进行随访,所有的随访患 者均接受激素和免疫抑制剂的治疗,有46例随访患者在原有免疫抑制剂治疗的基础上加用低剂量IL-2治疗。用ELISA方法检测 患者及健康体检者血清IL-2抗体水平,分析血清IL-2抗体在SLE患者中的意义。
结果:
SLE患者血清IL-2抗体水平比正常人高 (37.54 [27.88, 60.74] AU vs. 32.58 [23.63, 45.23] AU, P=0.006),且阳性率也高 于正常人 (18.4% vs 5.0%, P=0.002)。有脱发的SLE患者血清IL-2抗体升高 (49.79 [36.06, 64.95] AU vs. 35.06 [25.40, 58.46] AU, P=0.033),但是狼疮肾炎的患者血清IL-2抗体水平较低 (31.71 [22.60, 43.25] AU vs. 44.15 [31.43, 68.52] AU, P=0.001)。血清IL-2 抗体与血清免疫球蛋白IgA (r=0.229, P=0.005)、IgG (r= 0.327, P<0.001)、IgM (r= 0.164, P=0.050) 水平呈正相关。接受低剂量 IL-2治疗的SLE患者治疗后血清IL-2抗体水平和阳性率与传统治疗组相比也无明显差异。此外,在低剂量IL-2治疗的患者中, 比较症状改善者与无改善者之间血清IL-2抗体水平也未发现明显统计学差异。
结论:
系统性红斑狼疮的患者血清IL-2抗体水平升高且与疾病严重程度相关。低剂量IL-2治疗不增加IL-2抗体的产生。
INTRODUCTION
Systemic lupus erythematosus (SLE) is a severe systemic chronic autoimmune disease. Loss of immune tolerance is a major characteristic of SLE. Increased production of autoantibodies was involved in the development of organ impairment in SLE.[1,2] Anticytokine antibodies have been reported in healthy controls (HCs). A number of anticytokine autoantibodies were also detected in infections, autoimmune diseases, and cancers.[3,4]
Interleukin-2 (IL-2), which plays a critical role in the maintenance of immune homeostasis, is deficient in SLE. Recent studies proved that supplement of low-dose IL-2 is a promising novel therapy for SLE treatment.[5,6] However, the mechanism concerning the IL-2 deficiency in SLE patients has not been clearly elucidated. The anti-IL-2 autoantibodies were reported in healthy individuals and patients infected with human immunodeficiency virus.[7,8,9] Previous studies also presented an increased production of serum anti-IL-2 autoantibodies in SLE patients, as well as lupus mice.[10,11] However, the clinical relevance of serum anti-IL-2 autoantibodies and its association with low-dose IL-2 therapy in SLE patients are not well studied. Here, this study detected serum anti-IL-2 autoantibodies and analyzed its clinical significance. The impact of low-dose IL-2 treatment on anti-IL-2 autoantibodies was also investigated in SLE patients.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking University People's Hospital. Informed written consent was obtained from all patients prior to their enrollment in this study.
Patients and specimens
In total, 152 SLE patients satisfying the 1997 revised classification criteria of the American College of Rheumatology[12] were enrolled from the Department of Rheumatology and Immunology, Peking University People's Hospital, between September 2015 and May 2017. A total of 100 age- and gender-matched HCs were recruited.
Seventy-five patients were followed up for 10 weeks. All the SLE patients were treated with corticosteroids, antimalarials, and/or immunosuppressants. In addition, 46 out of them received exogenous low-dose recombinant human IL-2 (rhIL-2, SL Pharma, Beijing, China) therapy. For low-dose IL-2 therapy, rhIL-2 was administered at a dose of one million units every other day for 2 weeks subcutaneously, followed by a 2-week break.[5] In total, three cycles of low-dose rhIL-2 therapy were admitted consecutively. Serum samples at baseline and week 10 were collected with separation gel coagulation tubes and were frozen at −80°C until measurement, and repeated freeze-thaw cycles were avoided.
Serum anti-interleukin-2 IgG measurement
Serum anti-rhIL-2 IgG autoantibodies were assessed by enzyme-linked immunosorbent assay as described by Pérol et al.[10] The rhIL-2 was diluted with carbonate-coating buffer (PH 9.6) to the concentration of 105 U/ml, and microtiter 96-well plates (MediSorp, NuncTM, Thermo, Waltham, Massachusetts, USA) were incubated with diluted rhIL-2 at 4°C for 24h 100 μl per well. Then, a 1:500 dilution of each serum sample with 1% albumin from bovine serum (BSA)/phosphate-buffered solution-Tween 20 (PBST) was performed. After blocking with 2% BSA/PBS 300 μl per well for 2 h and washing with 0.2% Tween 20/PBS for three times, 100 μl diluted serum samples were added in triplicate and incubated for 2 h at room temperature. Sample dilution (1% BSA/PBST) was served as nonspecific background. After extensive washing with 0.2% Tween 20/PBS, horseradish peroxidase (HRP)-conjugated goat anti-human IgG (1:2000; eBioscience, California, USA) was added to each well, and the 96-well plates were kept at room temperature for 1 h. Standard curve was generated using 2-fold serial dilutions (1:20,000, 1:40,000, 1:80,000, and 1:160,000) of rat anti-human IL-2 (clone MQ1-17H12, eBioscience) for 2 h followed by HRP-conjugated goat anti-rat IgG (1:2000, eBioscience) for 1 h. Tetramethylbenzidine (TMB) substrate was then added to each well for 3 min and the reaction was blocked by adding 50 μl 2 mol/L sulfuric acid to each well. The absorbance was read at 450 nm with Bio-Rad plate reader. The values of optical density (A) of anti-IL-2 were transformed to arbitrary units (AUs), calculated as follows: AU = (Aautoantibody − Anonspecific background)test serum/(Aautoantibody − Anonspecific background)standard × 100.
Clinical and laboratory evaluation
Patients' clinical and laboratory parameters, as well as Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), were collected at baseline and week 10.
Leukocytes <3 × 109/L and platelets <100 × 109/L were regarded as leukopenia and thrombocytopenia, respectively. Complement 3 (C3) <0.79 g/L and C4 <0.16 g/L were considered as decreased C3 and C4, respectively. Moreover, anti-nucleosome antibody (ANuA) >20 RU/ml and anti-double-stranded DNA (dsDNA) antibody >25 U/ml were regarded as positive. Lupus nephritis was diagnosed if patients fulfilled the American College Rheumatology renal criteria: 24-h urine excretion ≥0.5 g/day or greater than 3+ by dipstick and/or cellular casts, including red cells, hemoglobin, granular, tubular, or mixed.
The normal reference ranges of serum anti-IL-2 IgG were determined according to the 95% confidence interval in HCs, and the patients with serum anti-IL-2 IgG above the upper limit of normal (ULN) were defined as serum-elevated anti-IL-2 IgG.
Statistical analysis
Statistical analyses were performed using SPSS software version 20.0 (IBM, Armonk, New York, USA). Continuous variables were expressed as mean ± standard deviation (SD), and comparison between the two groups was performed with the Student's t-test or Student's paired t-test. Discrete variable data were expressed as median (Q1, Q3), and comparison between the two groups was made by Mann-Whitney U-test. Categorical data were compared using Chi-square test or Fisher's exact test. Spearman's correlation analysis was applied to analyze relationship between the two groups. A P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 152 SLE patients were recruited in this study. The median age and disease duration of SLE patients were 31.0 (25.0, 43.8) years and 48.0 (8.5, 109.5) months, respectively. One hundred and forty out of these 152 SLE patients (92.1%) were female. The median SLEDAI score of these SLE patients was 9 (6, 12), and the actual median dose of corticosteroids was 15 (10, 45) mg/d. The 100 healthy individuals with a median age of 32.0 (26.0, 43.8) years and 83 females were also enrolled as the control group.
Increased serum autoantibodies against human interleukin-2 in systemic lupus erythematosus patients
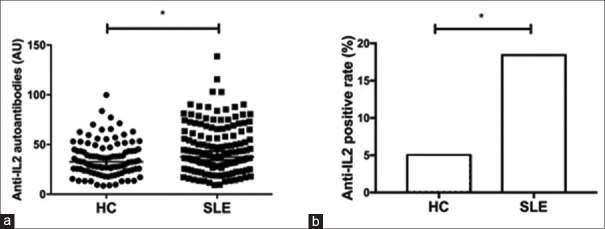
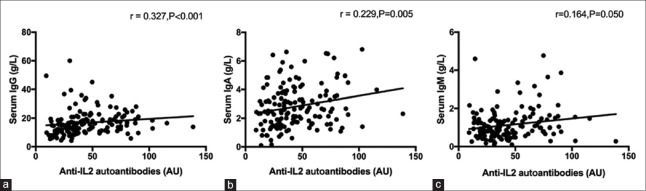
The serum IgG autoantibodies against rhIL-2 (serum anti-IL-2 IgG) in SLE patients were markedly increased compared to that in control group (37.54 [27.88, 60.74] AU vs. 32.58 [23.63, 45.23] AU, Z = –2.748, P = 0.006; Figure 1a). The ULN of serum anti-IL-2 IgG level was 69.81 AU in this study. Anti-IL-2 IgG was positive in the serum of 28 patients (18.4%) at baseline, which was more prevalent than that in HCs (5.0%, χ2 = 9.547, P = 0.002; Figure 1b). When compared the associations between serum anti-IL-2 IgG levels and laboratory parameters of SLE patients, a positive correlation between serum anti-IL-2 IgG and serum total IgG (r = 0.327, P < 0.001) was demonstrated [Figure 2a]. Similar associations were detected between serum anti-IL-2 IgG and IgA (r = 0.229, P = 0.005; Figure 2b) as well as IgM (r = 0.164, P = 0.050; Figure 2c). No association was observed between serum anti-IL-2 IgG and the other parameters, which included age, disease duration, white blood cells, hemoglobin, platelets, complements, anti-dsDNA antibodies, AnuA, 24 h proteinuria excretion, and serum IL-2 (data not shown). Moreover, as shown in Table 1, serum anti-IL-2 IgG increased in SLE patients with alopecia (49.79 [36.06, 64.95] AU vs. 35.06 [25.40, 58.46] AU, P = 0.033), but it decreased in SLE patients with lupus nephritis (31.71 [22.60, 43.25] AU vs. 44.15 [31.43, 68.52] AU, P = 0.001), compared with those without the corresponding disorders.
Figure 1.
Comparison of serum anti-IL-2 autoantibodies in SLE patients and HCs. Serum anti-IL-2 autoantibody levels (a) and positive rates (b) in SLE patients and HCs. *P < 0.05. SLE: Systemic lupus erythematosus; HC: Healthy control; IL: Interleukin.
Figure 2.
Association between serum anti-IL-2 autoantibodies and serum immunoglobulin. The correlations between serum anti-IL-2 autoantibodies and serum total IgG (a), serum total IgA (b), and serum total IgM (c). Spearman's correlation test and nonparametric Mann-Whitney U-test were used to assess correlations and differences between two groups. IL: Interleukin.
Table 1.
Serum anti-IL-2 autoantibody levels at different clinical and laboratory characteristics in 152 SLE patients (AU)
| Characteristics | Numbers | Presence | Absence | Z | P |
|---|---|---|---|---|---|
| Rash | 46 | 37.26 (28.72, 56.91) | 37.54 (27.57, 63.23) | −0.084 | 0.933 |
| Alopecia | 35 | 49.79 (36.06, 64.95) | 35.06 (25.40, 58.46) | −2.138 | 0.033 |
| Arthritis | 40 | 43.65 (27.85, 57.63) | 36.32 (28.09, 62.69) | −0.632 | 0.528 |
| Fever | 23 | 36.30 (29.90, 70.69) | 37.64 (26.59, 58.39) | −0.640 | 0.522 |
| Leukopenia* | 22 | 46.64 (35.56, 71.14) | 36.18 (25.54, 58.22) | −1.854 | 0.065 |
| Thrombocytopenia† | 22 | 42.75 (27.87, 60.28) | 36.97 (28.20, 61.80) | −0.272 | 0.785 |
| Lupus nephritis | 56 | 31.71 (22.60, 43.25) | 44.15 (31.43, 68.52) | −3.388 | 0.001 |
| NPSLE | 17 | 36.30 (21.35, 55.38) | 37.64 (28.69, 61.36) | −0.886 | 0.376 |
| Serositis | 10 | 39.20 (27.33, 54.06) | 37.54 (27.88, 61.80) | −0.156 | 0.876 |
| Ulceration | 6 | 69.27 (43.02, 92.83) | 36.45 (27.87, 58.76) | −1.845 | 0.065 |
| Raynaud | 15 | 44.52 (31.46, 71.05) | 36.35 (27.86, 57.21) | −0.954 | 0.340 |
| Decreased C3 | 105 | 38.02 (26.59, 55.36) | 36.06 (29.09, 71.05) | −0.915 | 0.360 |
| Decreased C4 | 105 | 38.96 (25.05, 56.06) | 36.35 (30.68, 71.05) | −1.238 | 0.216 |
| Anti-dsDNA antibody | 84 | 38.49 (29.55, 58.13) | 36.97 (23.27, 66.63) | −0.452 | 0.651 |
Data were presented as median (Q1, Q3), which were analyzed using Mann-Whitney U-test. *Leukocytes <3.0×109/L regarded as leukopenia; †PLT <100×109/L regarded as thrombocytopenia. SLE: Systemic lupus erythematosus; NPSLE: Neuropsychiatric systemic lupus erythematosus; IL: Interleukin; AUs: Arbitrary units; dsDNA: Double-stranded DNA; PLT: Platelet.
Influence of low-dose interleukin-2 therapy on anti-interleukin-2 autoantibodies in systemic lupus erythematosus
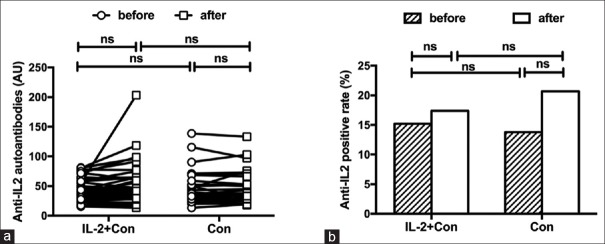
To further analyze the possible influence of low-dose rhIL-2 therapy on serum anti-IL-2 IgG, serum anti-IL-2 IgG at baseline and week 10 was compared in patients with exogenous low-dose IL-2 supplement (n = 46) and patients with conventional therapy (n = 29), respectively. The characteristics of SLE patients with low-dose rhIL-2 therapy and those with conventional immunosuppressive therapy are shown in Table 2. Treatment with exogenous IL-2 did not lead to significant increase both in the levels and positive rates of serum anti-IL-2 IgG (37.02 [29.23, 56.74] AU vs. 43.34 [30.15, 62.01] AU, P = 0.281; 15.2% vs. 17.4%, P = 0.778, respectively), as well as the conventional therapy [Figure 3]. Both serum anti-IL-2 IgG levels and positive rate in SLE patients with low-dose IL-2 therapy were similar to SLE patients with conventional therapy.
Table 2.
Characteristics of SLE patients with low-dose IL-2 therapy and conventional immunosuppressive therapy
| Characteristics | Patients with low-dose rhIL-2 therapy (n = 46) | Patients with conventional therapy (n = 29) | Statistical values | P* |
|---|---|---|---|---|
| Age (years) | 33.91 ± 10.91 | 30.34 ± 9.71 | 1.438* | 0.155 |
| Female | 42 (91.3) | 29 (100.0) | 2.664† | 0.154 |
| Disease duration (months) | 73.28 ± 67.20 | 82.34 ± 63.56 | −0.581* | 0.563 |
| C3 (g/L) | 0.74 ± 0.26 | 0.69 ± 0.24 | 0.857* | 0.394 |
| C4 (g/L) | 0.14 ± 0.07 | 0.12 ± 0.06 | 1.146* | 0.256 |
| Anti-dsDNA antibody (U/ml) | 87.11 ± 94.23 | 85.06 ± 78.16 | 0.098* | 0.922 |
| 24 h-UPE (g/d) | 1.43 ± 1.73 | 1.09 ± 1.54 | 0.734* | 0.466 |
| SLEDAI | 10.02 ± 4.61 | 10.45 ± 6.06 | −0.345* | 0.731 |
| Medications | ||||
| Corticosteroid (mg/d) | 23.17 ± 16.87 | 23.91 ± 19.20 | −0.163* | 0.871 |
| Hydroxychloroquine | 39 (84.8) | 26 (89.7) | 0.365† | 0.545 |
| Mycophenolate mofetil | 16 (34.8) | 10 (34.5) | 0.001† | 0.979 |
| Cyclophosphamide | 4 (8.7) | 1 (3.5) | 0.787† | 0.375 |
| Cyclosporine A | 6 (13.0) | 3 (10.3) | 0.123† | 0.726 |
| Azathioprine | 3 (6.5) | 2 (6.90) | 0.004† | 0.949 |
| Tacrolimus | 0 (0.0) | 1 (3.5) | 1.608† | 0.205 |
| Leflunomide | 3 (6.5) | 0 (0.0) | 1.970† | 0.160 |
Data were presented as mean ± SD or n (%). *t values; †χ2 values. SLE: Systemic lupus erythematosus; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; 24 h-UPE: 24-h urine protein excretion; rhIL-2: Recombinant human IL-2; dsDNA: Double-stranded DNA; SD: Standard deviation; IL: Interleukin.
Figure 3.
Serum anti-IL-2 autoantibody levels (a) and positive rates (b) in patients with systemic lupus erythematosus treated with conventional agents or low-dose IL-2 therapy. Con: Conventional therapy; IL: Interleukin; ns: Not significant.
Baseline serum anti-interleukin-2 autoantibody levels did not affect low-dose interleukin-2 therapeutic effects
In an attempt to determine whether serum anti-IL-2 IgG affects the therapeutic effect of low-dose IL-2 therapy or not, we divided SLE patients who received low-dose IL-2 therapy (n = 46) into two groups: patients with elevated serum anti-IL-2 IgG (n = 7) and patients with normal serum anti-IL-2 IgG (n = 39), according to the serum anti-IL-2 IgG levels at baseline. As an assessment of disease activity improvement, changes of SLEDAI scores (ΔSLEDAI) for general disease activity between week 10 after treatment and the baseline were calculated. In comparison to patients with normal serum anti-IL-2 IgG, no significant difference of disease activity improvement (ΔSLEDAI) was observed in patients with elevated serum anti-IL-2 IgG (5.86 ± 2.73 vs. 5.41 ± 4.59, t = −0.249, P = 0.805). In addition, when we grouped these patients into responders (ΔSLEDAI ≥4, n = 18) and nonresponders (ΔSLEDAI <4, n = 28) on the basis of therapeutic responses of low-dose IL-2 therapy, there was no difference of serum anti-IL-2 IgG levels at baseline between these two groups (39.25 ± 17.66 AU vs. 44.74 ± 20.23 AU, t = 0.942, P = 0.352) as well.
Since a notably higher serum anti-IL-2 IgG was present in SLE patients with alopecia than those without alopecia, we explored the difference of anti-IL-2 IgG levels at baseline according to their therapeutic response of low-dose IL-2 therapy, in order to investigate a possible impact of serum anti-IL-2 IgG in the improvement of different disease manifestations of SLE. As shown in Table 3, no difference of baseline serum anti-IL-2 IgG was found between responders and nonresponders with alopecia, as well as other clinical manifestations such as rash, lupus nephritis, decreased C3, decreased C4, and anti-dsDNA antibody.
Table 3.
Serum anti-IL-2 autoantibody levels at different clinical and laboratory characteristics in 46 SLE patients with low-dose IL-2 therapy (AU)
| Characteristics | Numbers | Responders’ group | Nonresponders’ group | Z | P |
|---|---|---|---|---|---|
| Rash | 16 | 37.43 (20.58, 70.97) | 55.61 (44.58, 72.61) | −1.091 | 0275 |
| Alopecia | 18 | 43.81 (31.15, 55.95) | 52.14 (26.54, 68.22) | −0.562 | 0.574 |
| Lupus nephritis | 19 | 33.99 (26.91, 40.02) | 35.90 (28.28, 62.28) | −1.061 | 0.288 |
| Decreased C3 | 23 | 42.00 (33.07, 53.91) | 41.14 (24.69, 57.51) | −0.350 | 0.726 |
| Decreased C4 | 31 | 42.99 (35.96, 58.05) | 38.77 (27.85, 54.77) | −0.867 | 0.386 |
| Anti-dsDNA antibody | 31 | 40.94 (34.20, 71.86) | 35.14 (24.79, 53.72) | −1.239 | 0.215 |
Data were presented as median (Q1, Q3), which were analyzed using Mann-Whitney U-test. IL: Interleukin; SLE: Systemic lupus erythematosus; AUs: Arbitrary units; dsDNA: Double-stranded DNA.
DISCUSSION
In the recent years, anticytokine autoantibodies were found to involve in the mechanism of diseases. For example, anti-tumor necrosis factor-α autoantibodies were detected in patients with rheumatoid arthritis.[13] Autoantibodies against granulocyte-macrophage colony-stimulating factor were found in patients with idiopathic pulmonary alveolar proteinosis.[14] Anti-IL-8 autoantibodies were reported in patients with ovarian cancer.[15] Anti-IL-2 autoantibodies were reported to play roles in the regulation of IL-2 cytokine network.[7,16] In previous studies, it was demonstrated that the presence of anti-IL-2 autoantibodies could contribute to the reduction of serum IL-2 concentrations and could impact the biological effect of IL-2 in vivo.[17] The induction of anti-IL-2 autoantibodies also had a negative influence on IL-2-mediated expansion of lymphocytes and could compromise regulatory T (Treg)-cells' fitness in vivo.[10,17] Moreover, reduced frequency of CD16, CD56, and CD25 lymphocytes and decreased lymphokine-activated killer cell activity were detected in patients with anti-IL-2 autoantibodies.[18,19] In fact, in the recent years, a dual role of anti-IL-2 autoantibodies including immunoenhancement and immunosuppression was reported. The function of anti-IL-2 autoantibodies depends on their target-binding sites on IL-2. For example, some anti-IL-2 autoantibodies binding to an IL-2 epitope that is crucial for interaction with CD25 could enhance immune response, while some other anti-IL-2 autoantibodies occluded IL-2 binding to CD122 and suppressed immune responses.[20,21] It was reported that the injection of anti-IL-2 monoclonal antibody or anti-IL-2/IL-2 immune complexes could reduce the viral load of Friend retroviral and herpes virus in light of increasing the proliferation of natural killer cells and memory-like CD8+ T-cells.[22,23,24,25] However, the exact role of anti-IL-2 autoantibodies on infections in SLE is still elusive.
In this study, a significantly increased serum anti-IL-2 IgG level was observed in SLE patients. The reason for the rise of autoimmune reaction against IL-2 is unknown, and it was probably due to the defective immune tolerance in SLE patients. The anti-IL-2 antibodies might bind to IL-2 and result in the formation of IL-2/anti-IL-2 immune complex, which probably interfered with the physiological functions of IL-2. However, except for serum immunoglobulin, no correlation between serum anti-IL-2 IgG and other laboratory parameters such as specific autoantibodies or complement was detected. It should be helpful to identify the targeting epitopes of anti-IL-2 autoantibodies in future studies. Interestingly, beyond our expectation, significantly decreased serum anti-IL-2 IgG and total serum IgG (data not shown) were observed in patients with lupus nephritis in this study. In light of previous studies by Yang et al.[26] and Yap et al.,[27] which reported that the serum total IgG was negatively correlated with proteinuria and urinary IgG, we speculated that the leakage of protein from urine or the deposition of IgG in the kidney might be responsible for the low serum anti-IL-2 IgG levels in patients with lupus nephritis.
Various factors, including the dose regimen, cumulative dose, duration of therapy, and route of administration of recombinant IL-2, have been shown to account for the incidence of anti-IL-2 autoantibody formation. Although the treatment-induced autoantibodies to IL-2 have been reported for years,[19,28] anti-IL-2 autoantibody was barely detected in our patients receiving low-dose IL-2 therapy. Of note, this suggested that the approach of low-dose IL-2 therapy to SLE patients was incapable of inducing increased production of serum anti-IL-2 autoantibodies.
In this study, patients with certain manifestations and nonresponders to low-dose IL-2 therapy presented higher level of anti-IL-2 autoantibodies, but no significant difference was observed. This was probably owing to the limited number of patients receiving low-dose IL-2 therapy. It is necessary to further investigate the role of IL-2 autoantibody in the pathogenesis of SLE. Furthermore, as the application of low-dose IL-2 in SLE is becoming routinely used, the influence of anti-IL-2 autoantibody on its efficacy should be studied in a larger cohort of patients.
In conclusion, increased serum anti-IL-2 autoantibodies were associated with disease severity in SLE. Short-term application of low-dose IL-2 did not significantly induce anti-IL-2 autoantibody production.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 31530020, No. 81471601, and No. 81671602) and the Beijing Nova Program (No. Z171100001117025).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Li P, Li Y, Zhou AH, Chen S, Li J, Wen XT, et al. Association study of a proliferation-inducing ligand, spermatogenesis associated 8, platelet-derived growth factor receptor-alpha, and POLB polymorphisms with systemic lupus erythematosus in Chinese Han population. Chin Med J. 2016;129:2085–90. doi: 10.4103/0366-6999.189055. doi: 10.4103/0366-6999.189055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne SK. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol. 2014;32:635–57. doi: 10.1146/annurev-immunol-032713-120222. doi: 10.1146/annurev-immmunol-032713-120222. [DOI] [PubMed] [Google Scholar]
- 4.Yang T, Li Y, Lyu Z, Huang K, Corrigan CJ, Ying S, et al. Characteristics of proinflammatory cytokines and chemokines in airways of asthmatics: Relationships with disease severity and infiltration of inflammatory cells. Chin Med J. 2017;130:2033–40. doi: 10.4103/0366-6999.213428. doi: 10.4103/0366-6999.213428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–3. doi: 10.1038/nm.4148. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 6.von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. 2016;75:1407–15. doi: 10.1136/annrheumdis-2015-207776. doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- 7.Tiberio L, Caruso A, Pozzi A, Rivoltini L, Morelli D, Monti E, et al. The detection and biological activity of human antibodies to IL-2 in normal donors. Scand J Immunol. 1993;38:472–6. doi: 10.1111/j.1365-3083.1993.tb02590.x. doi: 10.1111/j. 1365-3083.1993.tb02590.x. [DOI] [PubMed] [Google Scholar]
- 8.Monti E, Pozzi A, Tiberio L, Morelli D, Caruso A, Villa ML, et al. Purification of interleukin-2 antibodies from healthy individuals. Immunol Lett. 1993;36:261–6. doi: 10.1016/0165-2478(93)90098-m. doi: 10.1016/0165-2478(93)90098-m. [DOI] [PubMed] [Google Scholar]
- 9.Bost KL, Hahn BH, Saag MS, Shaw GM, Weigent DA, Blalock JE, et al. Individuals infected with HIV possess antibodies against IL-2. Immunology. 1988;65:611–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Pérol L, Lindner JM, Caudana P, Nunez NG, Baeyens A, Valle A, et al. Loss of immune tolerance to IL-2 in type 1 diabetes. Nat Commun. 2016;7:13027. doi: 10.1038/ncomms13027. doi: 10.1038/ncomms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizaka S, Tsujii T. IL-2 antibody production in lupus mice. Cell Immunol. 1989;118:100–7. doi: 10.1016/0008-8749(89)90360-2. doi: 10.1016/0008-8749(89)90360-2. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. doi: 10.1002/art. 1780400928. [DOI] [PubMed] [Google Scholar]
- 13.Wildbaum G, Nahir MA, Karin N. Beneficial autoimmunity to proinflammatory mediators restrains the consequences of self-destructive immunity. Immunity. 2003;19:679–88. doi: 10.1016/s1074-7613(03)00291-7. doi: 10.1016/S1074-7613(03)00291-7. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–98. doi: 10.1182/blood-2003-05-1565. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 15.Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhatnaya L, Modugno F, et al. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol. 2006;102:244–51. doi: 10.1016/j.ygyno.2005.12.011. doi: 10.1016/j.ygyno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Granelli-Piperno A, Andrus L, Reich E. Antibodies to interleukin 2.Effects on immune responses in vitro and in vivo. J Exp Med. 1984;160:738–50. doi: 10.1084/jem.160.3.738. doi: 10.1084/jem.160.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjelm Skog AL, Wadhwa M, Hassan M, Gharizadeh B, Bird C, Ragnhammar P, et al. Alteration of interleukin 2 (IL-2) pharmacokinetics and function by IL-2 antibodies induced after treatment of colorectal carcinoma patients with a combination of monoclonal antibody 17-1A, granulocyte macrophage colony-stimulating factor, and IL-2. Clin Cancer Res. 2001;7:1163–70. [PubMed] [Google Scholar]
- 18.Atzpodien J, Lopez Hänninen E, Kirchner H, Knüver-Hopf J, Poliwoda H. Human antibodies to recombinant interleukin-2 in patients with hypernephroma. J Interferon Res. 1994;14:177–8. doi: 10.1089/jir.1994.14.177. doi: 10.1089/jir.1994.14.177. [DOI] [PubMed] [Google Scholar]
- 19.Kirchner H, Körfer A, Evers P, Szamel MM, Knüver-Hopf J, Mohr H, et al. The development of neutralizing antibodies in a patient receiving subcutaneous recombinant and natural interleukin-2. Cancer. 1991;67:1862–4. doi: 10.1002/1097-0142(19910401)67:7<1862::aid-cncr2820670708>3.0.co;2-r. doi: 10.1002/1097-0142(19910401)67:7<1862::AID-CNCR2820670708>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Rojas G, Cabrera Infante Y, Pupo A, Carmenate T. Fine epitope specificity of antibodies against interleukin-2 explains their paradoxical immunomodulatory effects. MAbs. 2014;6:273–85. doi: 10.4161/mabs.27224. doi: 10.4161/mabs.27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–7. doi: 10.1126/science.1122927. doi: 10.1126/science. 1122927. [DOI] [PubMed] [Google Scholar]
- 22.Littwitz-Salomon E, Dittmer U, Sutter K. Insufficient natural killer cell responses against retroviruses: How to improve NK cell killing of retrovirus-infected cells. Retrovirology. 2016;13:77. doi: 10.1186/s12977-016-0311-8. doi: 10.1186/s12977-016-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littwitz-Salomon E, Akhmetzyanova I, Vallet C, Francois S, Dittmer U, Gibbert K, et al. Activated regulatory T cells suppress effector NK cell responses by an IL-2-mediated mechanism during an acute retroviral infection. Retrovirology. 2015;12:66. doi: 10.1186/s12977-015-0191-3. doi: 10.1186/s12977-015-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaddipati S, Estrada K, Rao P, Jerome AD, Suvas S. IL-2/anti-IL-2 antibody complex treatment inhibits the development but not the progression of herpetic stromal keratitis. J Immunol. 2015;194:273–82. doi: 10.4049/jimmunol.1401285. doi: 10.4049/jimmunol.1401285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molloy MJ, Zhang W, Usherwood EJ. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J Immunol. 2009;182:4512–5. doi: 10.4049/jimmunol.0804175. doi: 10.4049/jimmunol.0804175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang ZB, Dai SY, Dai YL, Fu SS, Zhang WR, Ning JP. Clinical features of systemic lupus erythematosus with normal serum IgG levels. China J Modern Med. 2017;27:68–73. doi: 10.3969/j.issn. 1005-8982.2017.15.014. [Google Scholar]
- 27.Yap DY, Yung S, Ma MK, Mok MM, Kwan LP, Chan GC, et al. Serum immunoglobulin G level in patients with lupus nephritis and the effect of treatment with corticosteroids and mycophenolate mofetil. Lupus. 2014;23:678–83. doi: 10.1177/0961203314525248. doi: 10.1177/0961203314525248. [DOI] [PubMed] [Google Scholar]
- 28.Prümmer O. Treatment-induced antibodies to interleukin-2. Biotherapy. 1997;10:15–24. doi: 10.1007/BF02678213. doi: 10.1007/BF02678213. [DOI] [PubMed] [Google Scholar]