Abstract
The porta hepatis and hepatoduodenal ligament (HDL) are important anatomical pathways of extension of disease to and from the liver. The imaging of this area is difficult. The role of endoscopic ultrasound (EUS) as an imaging modality for hepatoduodenal ligament has not been established so far. All images in the present study have been generated from a detailed review of real time recordings using the curved linear scanning echoendoscope EG -3830 UT (Pentax Corporation, Tokyo, Japan), coupled with a Hitachi avius and Hitach 7500 processor (Hitachi Aloka Medical, Tokyo Japan). Our image orientation is with the cranial aspect of the patient directed toward the right side of the screen. We have illustrated that with a careful technique, a detailed EUS evaluation of the HDL and hepatic hilum is possible. A thorough understanding of the HDL anatomy by curved linear EUS probe may play a crucial role in the accurate diagnosis of a broad spectrum of pathologic conditions of the porta hepatis. EUS examination of the HDL should be a part of the upper abdominal EUS studies. The description and the images described in this article are useful for beginners who want to start imaging of the porta hepatis and hepatoduodenal ligament. This information is useful for staging of malignancies involving common bile duct.
Keywords: Endoscopic ultrasound (EUS), hepatoduodenal ligament, hilum of the liver, porta hepatis
INTRODUCTION
The porta hepatis or hilum of the liver is a deep, short, transverse fissure that passes across the left posterior aspect of the undersurface of the right lobe of the liver.[1] The porta hepatis transmits the portal triad - formed by the main portal vein (PV), proper hepatic artery (PHA), and common hepatic duct (CHD) as well as nerves and lymphatics.[2] The PV and proper hepatic artery ascend the porta hepatis, whereas the CHD and lymphatics descend the porta hepatis.[1] The hepatoduodenal ligament (HDL) envelops the gallbladder neck, cystic and extrahepatic bile ducts, hepatic artery branches, PV, lymphatics, lymph nodes, nerves, and loose areolar connective tissue wrapped in a serous covering and fibrous capsule of Glisson [Figure 1].[3,4,5,6] The HDL contains an area of importance to surgeons: The hepatocystic triangle, which is formed by the proximal part of the neck of gallbladder and cystic duct to the right, the CHD to the left, and the margin of the right lobe of the liver (segment 4 and 5) superiorly.[7] The hepatocystic triangle has a circular area of 30 mm in diameter, which contains the right hepatic artery, aberrant bile ducts, aberrant hepatic artery (if present), cystic artery, and cystic duct lymph nodes [Figure 2]. The HDL and liver hilum act as a pathway for the spread of disease to and from the liver.[3] Tumors of the pancreas, duodenum, and stomach can spread to the periportal nodes and liver and metastasis from the liver can spread to the periportal and celiac group of nodes.[6,8] The complex anatomic architecture of HDL (and porta hepatis) with a very high incidence of anatomical variations makes imaging of this area a challenging situation during evaluation.[2,9] Ultrasonography (US) is the most widely used imaging modality for evaluation, and color and spectral Doppler US can provide indispensable information on the vascular structures. Multidetector computed tomography (MDCT) is an often used imaging modality for comprehensive evaluation.[6] Magnetic resonance imaging (MRI) has superior soft-tissue resolution. Magnetic resonance (MR) cholangiography is the imaging modality of choice for the biliary tree and is preferred over endoscopic retrograde cholangiopancreatography (ERCP) for initial evaluation. Fludeoxyglucose positron emission tomography-computed tomography (PET/CT) is increasingly used to characterize and stage malignant diseases of the porta hepatis, notably cholangiocarcinoma.[10] Endoscopic ultrasonography (EUS) and more recently, intraductal ultrasonography using a higher frequency probe have been reported in a small series for imaging in cholangiocarcinoma.[11] EUS has been found to be more sensitive than MDCT scans and angiography for evaluating portal vascular involvement in diseases involving the bile duct.[12] This article describes the anatomy and technique of imaging of the contents of HDL and porta hepatis by EUS.
Figure 1.
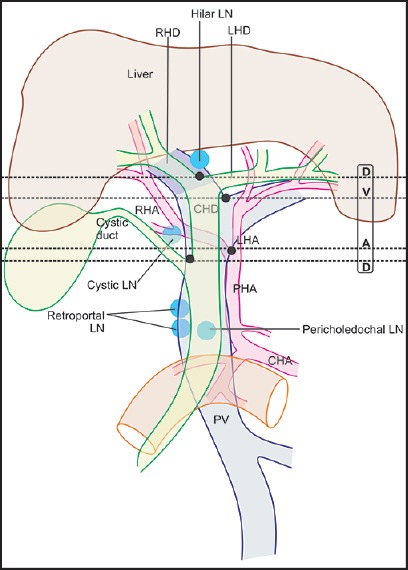
The PV and its branches lie behind the hepatic artery and bile duct branches in the hepatoduodenal ligament. The RHD and LHD unite in the hilar plate, close to the right end of the porta in front of the right branch of PV to form the CHD. The CHD has a variable length from 1.0 cm to 7.5 cm depending on the site of joining of the cystic duct, which in a normal course joins the right side of the CHD in 85-90% of the cases. The PHA divides into two branches near the left border of the CHD/common bile duct CBD. The LHA proceeds toward the porta hepatis to the left of CHD and at the porta hepatis lies behind the LHD. The RHA enters the hepatocystic triangle posterior to the CHD and ascends anterior to the PV till the division of PV into the right and left branches and follows a relation posterior to the right hepatic duct (RHD) upward into the right lobe of liver
Figure 2.
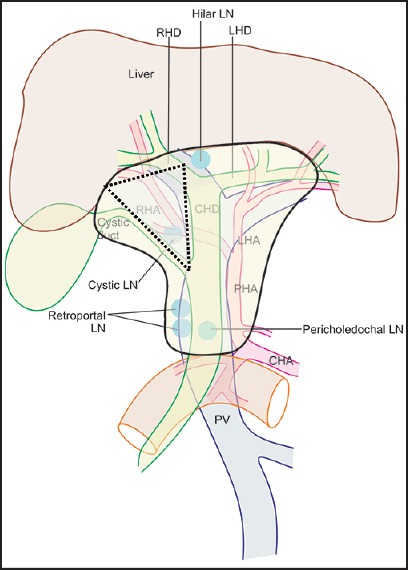
As the HDL approaches the liver, the structures within it become more numerous because of the branching of vessels and the duct and this relationship may change due to many variations in branching. Within the HDL and near the epiploic foramen the CBD and proper hepatic artery lie anterior to the PV and this relationship can be memorized by mnemonic DAVE: Duct, artery, vein, and epiploic foramen. The branching of vessels and the duct from upward from below in a normal person without variations occurs in the order of DAVD: Duct (CBD into cystic duct and CHD), artery (hepatic artery into the right and left branches), vein (PV into the right and left branches) and duct (the CHD into the right and left hepatic ducts). The variations in the branching of the vessels and the duct occur in 10-15% of the persons for CBD into cystic duct and CHD, for 10-15% persons in hepatic artery into the right and left branches, for 10-15% persons in the division of the PV into the right and left branches and for 15-45% for persons in division of CHD into the right and left hepatic ducts. The hepatocystic triangle is indicated by the triangular area
RADIOLOGIC ANATOMY OF HDL AND PORTAL HEPATIS
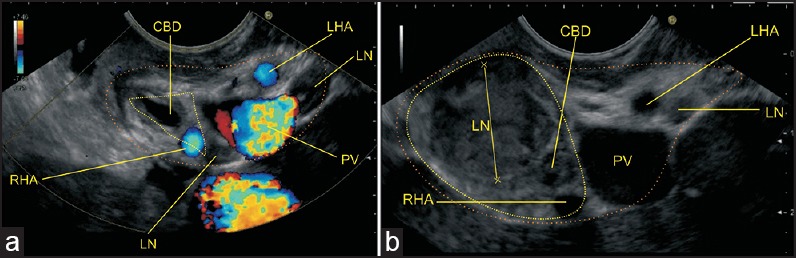
The average dimensions of the porta hepatis are 1 cm in anteroposterior diameter and 3-4 cm in transverse diameter. Within the porta hepatis, the CHD and proper hepatic artery typically are located anterior to the PV, with the CHD to the right in the actual edge of the hepatoduodenal ligament and the PHA to the left [Figure 3]. Lymphatic channels in the HDL drain the liver, gallbladder, bile ducts, duodenum, stomach, and pancreas into the paraaortic nodes near the origin of the superior mesenteric and celiac artery.[13] Typical nodes are the hilar node, cystic node (of Lund), pericholedochal node, retroportal node, foramen of Winslow node, superior retropancreaticoduodenal node, and posterior pancreaticoduodenal node.[14] Regional lymph nodes for perihilar bile duct malignancy are those in the hepatoduodenal ligament: Hilar, cystic node, pericholedochal, hepatic artery node, and retroportal node. More distant nodes, e.g., periaortic and celiac are classified as distant metastasis (M1) for perihilar bile duct malignancy.
Figure 3.
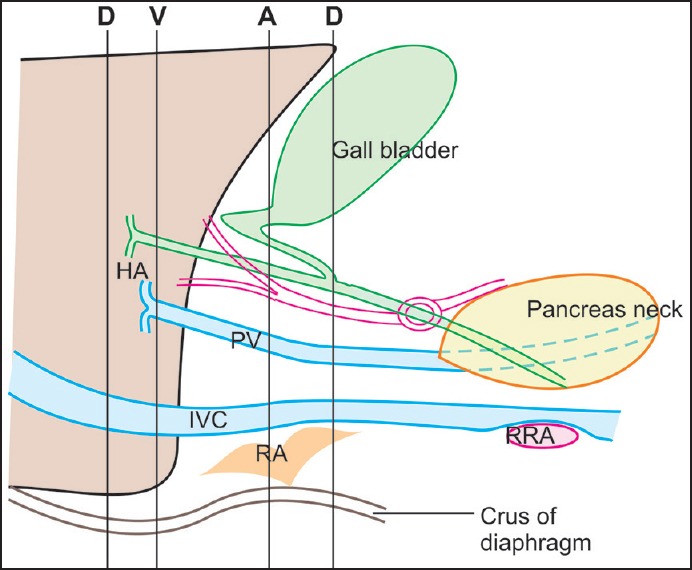
As RHA approaches the cystic duct, it lies parallel to the cystic duct for a short distance where it gives a branch to cystic duct and gallbladder. In about 20% of the cases, RHA crosses in front of the CHD. At the point of crossing, the RHA may indent the common hepatic duct, causing an appearance of pseudo stenosis. The RHA can indent the PV also when it ascends posterior to the CHD
Lymph nodes are detectable within the hepatoduodenal ligament in almost all patients with acute hepatitis[15] and chronic virushepatitis.[16,17] The extent of perihepatic lymphadenopathy reflects the progression of the disease with a larger lymph node size in more advanced stages.[18,19] Finally, enlarged hilum lymph nodes are detectable in almost all patients with primary sclerosing cholangitis (PSC).[20]
TECHNIQUES OF EXAMINATION
All images in the present study have been generated from a detailed review of real-time recordings using the curved linear scanning echoendoscope EG-3830 UT (Pentax Corporation, Tokyo, Japan), coupled with a Hitachi Avius and Hitachi 7,500 processor (Hitachi Aloka Medical, Tokyo, Japan). Our image orientation is with the cranial aspect of the patient directed toward the right side of the screen. Four positions are commonly used during imaging from EUS.
The neutral position is where the front of the handle is facing the patient.
The open position to the left is where the front of the handle is facing the patient's feet. It is reached by turning anticlockwise through 90° from the neutral position.
The open position to the right is the opposite of the open position to the left. It is reached by turning clockwise through 90° from the neutral position.
A further 90° rotation from the open position to the right can bring the handle in a position opposite to the neutral position.
EUS of the structures of HDL can be done from the stomach, duodenal bulb, and descending duodenum [Video 1].
Technique of examination from stomach
Follow down from the cardia
The examination of the liver hilum requires identifying the anatomical distribution pattern of the left portal vein (LPV) branches within the liver parenchyma. The LPV passes medially to supply segments II, III, and IV, and a caudate branch. Initially, the transhepatic visualization of segment II and three branches of the portal vein (PV) is done from the open left position near the cardia in the left lobe of the liver and a clockwise rotation traces the segment II and three branches to the umbilical part of the LPV [Figure 4a]. Further rotation in clockwise direction shows the continuation of umbilical part of the LPV into transverse part of the LPV into which the segment four tributary joins from the cranial aspect [Figure 4b and c]. The transverse part of the LPV can be followed into the main PV trunk at the hepatic hilum by further clockwise rotation. The right PV branch joins with the left branch of the PV as if climbing vertically upward during clockwise rotation and subsequently, the main PV is seen making a smooth curved arc near the liver hilum [Figure 4c]. In the liver hilum, the contents of the portal triad and HDL are identified where the main PV is the most posterior structure in HDL and all other structures can be seen beyond the PV. The structures of the HDL can be followed downward in a craniocaudal direction after visualization of the main PV. The order of bifurcation of the structures during a craniocaudal follow-up of HDL in a normal person is “DVAD” (hepatic duct, PV, hepatic artery, and common bile duct) [Figure 1]. Visualization of the branches of the bile duct is possible if they are dilated and can be followed from an open position toward the umbilical segment of the LPV [Figures 4d and 5a]. The union of ducts of segments II and III behind the umbilical part of LPV form the left hepatic artery (LHD), which then receives the duct from segment IV [Figure 5b]. In this situation the right hepatic artery (RHA) can be seen going behind the CHD in front of right branch of the PV [Figure 5c].
Figure 4.
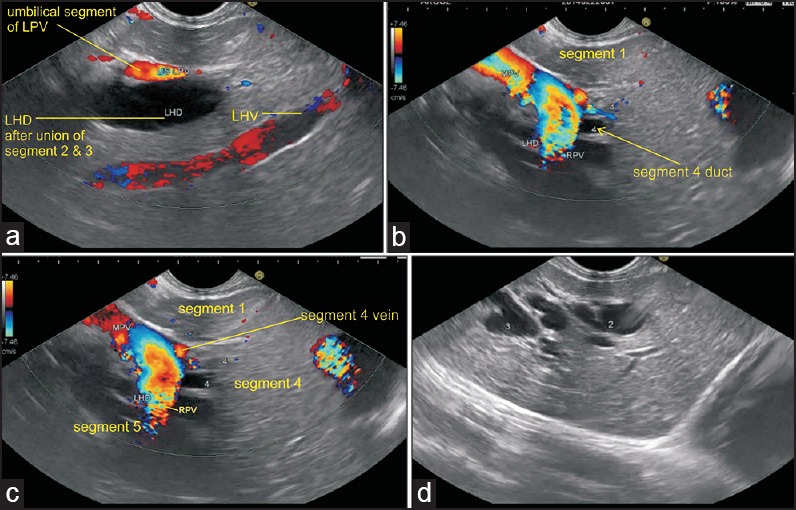
(a) The segment II and three ducts are seen in an open position to the left (b) On clockwise rotation, the segment II and three ducts fuse together in front of the umbilical part of the LPV (c) On further clockwise rotation, the fused left and right ducts are joined by segment IV duct from the cranial aspect (arrow) in front of the transverse segment of the left PV (d) On further clockwise rotation, the right portal vein (RPV) is seen joining the left PV
Figure 5.
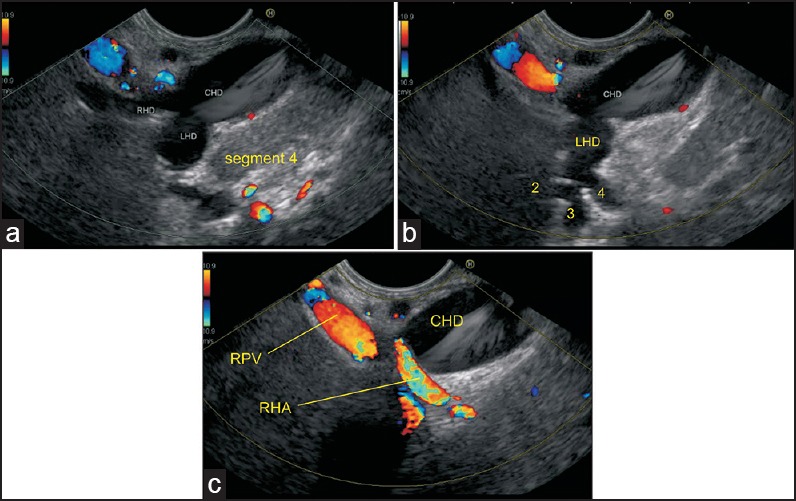
(a) Imaging from the duodenal bulb can show the CBD/ CHD in a long axis close to the probe. The union of RHD (average length 0.9 cm) and left hepatic duct (average length 1.7 cm) to form the CHD can be seen (b) This union is seen close to the right end of the porta in front of the right branch of the PV. The RHA crosses behind the CHD to come and lie in the hepatocystic triangle (c) Sometimes, it is possible to see the segmental ducts to segments II, III, and IV from the hilum of the liver
Follow-up from the body
When the scope is positioned near the body of stomach a transpancreatic imaging of common bile duct (CBD) and PV and common hepatic artery is possible [Figure 6]. The course of these structures can be followed up, however, the order of bifurcation of the structures coming upward from below is reversed and the joining of cystic duct with hepatic duct is seen followed by a division of the left and right hepatic arteries [Figures 7a–b and 8]. Further cranially, the PV can be followed up to its left and right PV branches [Figure 8] and division of bile ducts. Additional vessels of importance in this area are posterior superior pancreaticoduodenal artery (PSPDA), a branch of GDA that crosses the CBD anteriorly, and the posterior superior pancreaticoduodenal vein that joins the PV posteriorly just above the upper border of the pancreas [Figure 7a and b]. In this position the supraduodenal, retroduodenal, and retropancreatic segments of CBD are easily seen. The supraduodenal part lies in the right border of HDL anterior to the PV and to the left of hepatic artery proper. The retroduodenal part passes behind the superior part of the duodenum to the right of gastroduodenal artery (GDA) and in front of the PV. The retropancreatic part runs downward and to the right behind the head of the pancreas to reach the medial border of the second part of the duodenum [Figure 7c and d].
Figure 6.
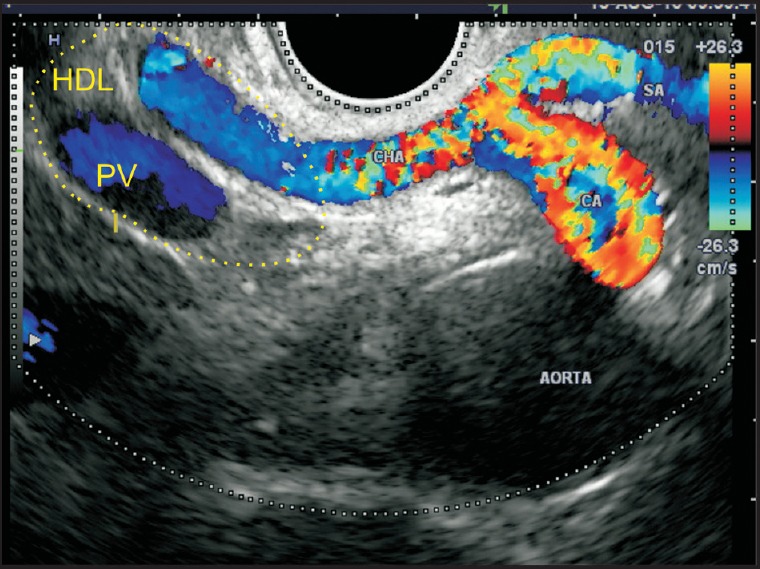
As the scope is positioned anterior to the aorta, the origin of the celiac artery and its bifurcation into two branches — splenic artery and hepatic artery — is seen. The celiac artery and the proximal part of the common hepatic artery are placed in a retroperitoneal location. As the common hepatic artery comes near the upper border of the pancreas, it enters the hepatoduodenal ligament, along with the PV. CA: Celiac artery, PV: Portal vein, HDL: Hepatoduodenal ligament, CHA: Common hepatic artery, SA: Splenic artery
Figure 7.
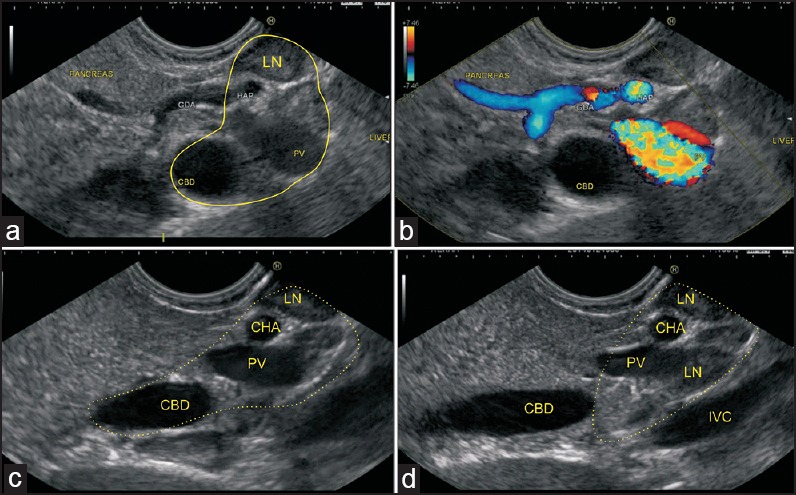
(a) A transpancreatic imaging of the CBD and PV is performed in this case. The hepatoduodenal ligament is seen from the stomach between the upper border of the pancreas and the visceral surface of the liver (b) Two LNs belonging to the superior pancreaticoduodenal group are also seen (c) The PHA is seen entering the hepatoduodenal ligament, along with the PV and supra duodenal part of the bile duct (d) The intrapancreatic part of the bile duct is seen, which is not included in the HDL. The foramen of Winslow node is seen
Figure 8.
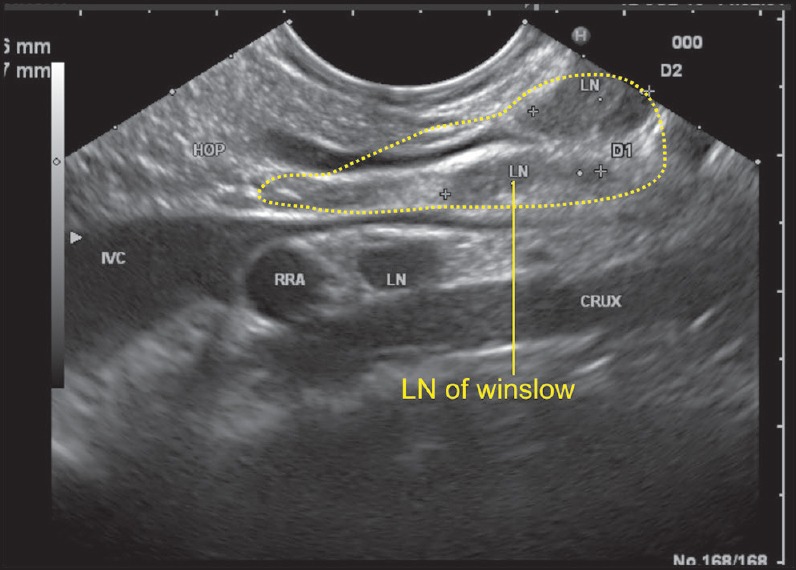
As the scope is positioned near the fundus of the stomach, it is able to see the inferior vena cava (IVC) and PV in a long axis. The IVC passes posterior to the hepatoduodenal ligament while the PV exits from the upper border of the pancreas and enters the lower part of the hepatoduodenal ligament. In this position, the bile duct can be also seen entering the ligament from the pancreas and the foramen of Winslow can be imagined as a place between the IVC and the PV just above the upper border of the pancreas. A lymph node of the foramen of Winslow is seen posterior to the PV and anterior to the IVC
Technique of examination from duodenal bulb
As the echoendoscope is pushed into the apex of the bulb, a clockwise rotation directs the scanning plane down to the pancreatic head and a counterclockwise rotation directs the scanning plane toward the liver hilum. The CBD and PV are demonstrated in a long axis with the former closer to the probe [Figure 9a and b]. When the PV is traced upward to the hilum, its bifurcation can be displayed with the right PV branch directed upward to the right and the left branch downward to the left [Figure 10a]. In most instances, the confluence of the right and left hepatic ducts lies anterior to the right branch of PV [Figure 10b–c and Figure 11] [Video 2].
Figure 9.
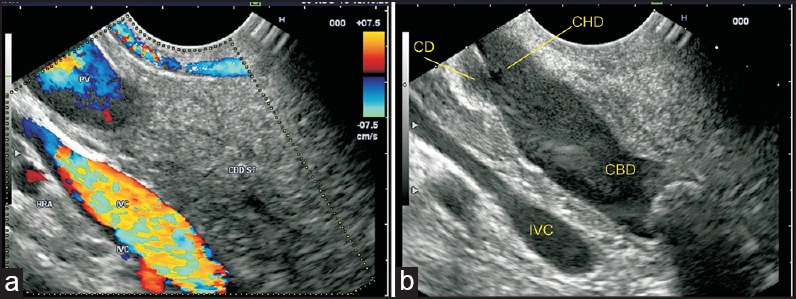
(a) When the imaging is performed from the duodenal bulb, the PV can be followed up from the upper border of the pancreas into the hepatoduodenal ligament (b) The CBD divides into the CHD and cystic duct. The cystic duct joins the right aspect of the common hepatic duct. This union generally lies above the pancreas in the hepatoduodenal ligament
Figure 10.
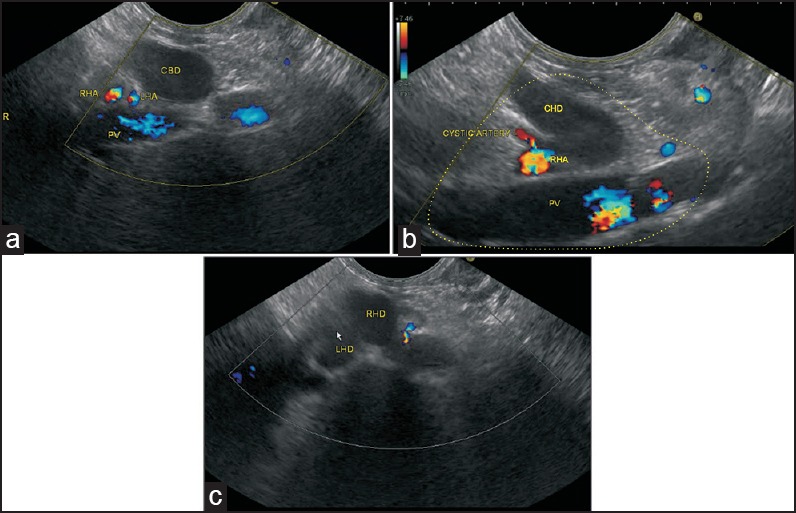
In the figure, a left hepatic artery and right hepatic artery are seen after bifurcation of the proper hepatic artery. Figure b shows the presence of a cystic artery taking origin from the right hepatic artery (c) When imaging is performed from below upward, the union of LHD and RHD to form CHD is seen to lie in front of the RPV
Figure 11.
(a) The cystic duct terminates at the right wall of the hepatic duct. The proper hepatic artery runs on the left side of CHD and usually divides into the right hepatic artery and left hepatic artery above the union of CHD and CD (b) In this case, the CD is seen joining the right aspect of CHD and the proper hepatic artery is seen on the left side of the CHD and CD. Both the structures lie anterior to the PV (c) When the CHD is followed up, it divides into the RHD and left hepatic duct and this bifurcation generally lies in front of the right branch of the PV. As the echoendoscope is rotated counterclockwise, the PV is followed up to its bifurcation and the RPV is seen on the right side of the screen
Technique of examination from the descending duodenum
During examination from the duodenum, initially the ampulla of Vater is identified. The imaging of HDL and hepatocystic triangle is performed from the descending duodenum by anticlockwise rotation of the endoscope from the second part of the duodenum. As the anticlockwise movement is combined with upward tipping, the imaging beam moves from a direction from the lower part of HDL near its attachment with the duodenum to the liver hilum and traces the structures of HDL from below to an upward direction. In the liver hilum, the right and left edges of the ligament can be identified [Figures 12 and 13] [Video 3 and Video 4].
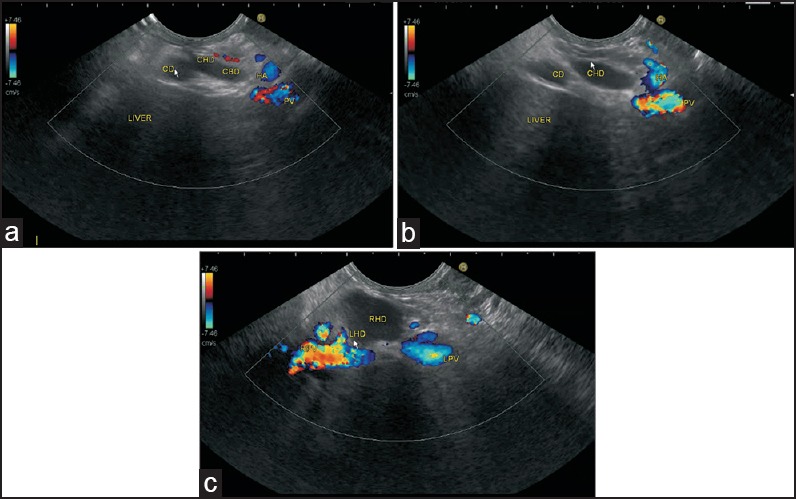
Figure 12.
The HDL contains the structures of the portal triad. In Figures a and b, the yellow triangle demarcates the lower and upper part of hepatocystic triangle within the HDL. The orange line demarcates the area of HDL. A lymph node (LN) belonging to the pericholedochal group is seen. The pathological LN belongs to the cystic group of node
Figure 13.
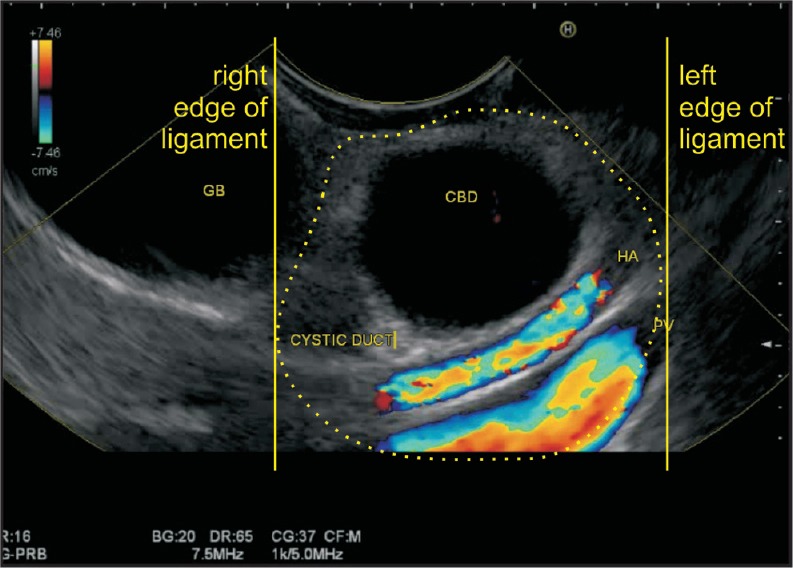
In a shortened position, an anticlockwise rotation of the hilum of the liver is seen. In this position, the bile duct is demonstrated in a transverse axis, along with cystic duct and gallbladder. The PV and hepatic artery are demonstrated in a long axis. All these structures except the gallbladder lie in the hepatoduodenal ligament near the hilum. The yellow lines show the right and left edges of the hepatoduodenal ligament near the porta hepatis
CONCLUSION
We have illustrated that with a careful technique, a detailed EUS evaluation of the HDL and hepatic hilum are possible. Radiation exposure and contrast nephrotoxicity are some of the limitations of MDCT.[6] EUS can be a useful imaging modality in the diagnosis and management of diseases of the porta hepatis. Surgical resection is the only curative treatment for hilar cholangiocarcinoma and its feasibility is dependent on the tumor site and the extent of biliary ductal and adjacent organ involvement.[21] More than half of the cases are inoperable at diagnosis because of their advanced stage, vascular encasement, and lymph node or distant metastases.[22] Pericholedochal and common hepatic artery stations have been suggested as the key stations for lymphatic spread from the liver tumors.[23] For patients with colorectal cancer liver metastases, hepatic resection remains the best treatment option, with reported 5-year survival rates of up to 56% if extrahepatic disease, especially hepatic lymph node involvement is excluded.[24] EUS examination of the HDL should be a part of the upper abdominal EUS studies. A thorough understanding of the HDL anatomy by curved linear EUS probe may play a crucial role in the accurate diagnosis of a broad spectrum of pathologic conditions of the porta hepatis and help in implementing appropriate management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on: www.eusjournal.com
Acknowledgement
We would like to thank Mr. Pran Prakash, our graphic designer for his technical support in preparing the hand drawings in this article.
REFERENCES
- 1.Weinstein JB, Heiken JP, Lee JK, et al. High resolution CT of the porta hepatis and hepatoduodenal ligament. Radiographics. 1986;6:55–74. doi: 10.1148/radiographics.6.1.3685484. [DOI] [PubMed] [Google Scholar]
- 2.Ramesh Babu CS, Sharma M. Biliary tract anatomy and its relationship with venous drainage. J Clin Exp Hepatol. 2014;4(Suppl 1):S18–26. doi: 10.1016/j.jceh.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan CH, Peungjesada S, Charnsangavej C, et al. Gastric cancer: Patterns of disease spread via the perigastric ligaments shown by CT. AJR Am J Roentgenol. 2010;195:398–404. doi: 10.2214/AJR.09.3070. [DOI] [PubMed] [Google Scholar]
- 4.Vikram R, Balachandran A, Bhosale PR, et al. Pancreas: Peritoneal reflections, ligamentous connections, and pathways of disease spread. Radiographics. 2009;29:e34. doi: 10.1148/rg.e34. [DOI] [PubMed] [Google Scholar]
- 5.Gadzijev EM. Surgical anatomy of hepatoduodenal ligament and hepatic hilus. J Hepatobiliary Pancreat Surg. 2002;9:531–3. doi: 10.1007/s005340200068. [DOI] [PubMed] [Google Scholar]
- 6.Tirumani SH, Shanbhogue AK, Vikram R, et al. Imaging of the porta hepatis: Spectrum of disease. Radiographics. 2014;34:73–92. doi: 10.1148/rg.341125190. [DOI] [PubMed] [Google Scholar]
- 7.Wang BJ, Kim JH, Yu HC, et al. Fetal intrahepatic gallbladder and topographical anatomy of the liver hilar region and hepatocystic triangle. Clin Anat. 2012;25:619–27. doi: 10.1002/ca.21288. [DOI] [PubMed] [Google Scholar]
- 8.Tirkes T, Sandrasegaran K, Patel AA, et al. Peritoneal and retroperitoneal anatomy and its relevance for cross-sectional imaging. Radiographics. 2012;32:437–51. doi: 10.1148/rg.322115032. [DOI] [PubMed] [Google Scholar]
- 9.Sherwinter DA. Identification of anomolous biliary anatomy using near-infrared cholangiography. J Gastrointest Surg. 2012;16:1814–5. doi: 10.1007/s11605-012-1945-z. [DOI] [PubMed] [Google Scholar]
- 10.Yoo E, Kim JH, Kim MJ, et al. Greater and lesser omenta: Normal anatomy and pathologic processes. Radiographics. 2007;27:707–20. doi: 10.1148/rg.273065085. [DOI] [PubMed] [Google Scholar]
- 11.Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB (Oxford) 2008;10:106–9. doi: 10.1080/13651820801992617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama M, Hagi H, Atomi Y, et al. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: Value of endoscopic ultrasonography. Abdom Imaging. 1997;22:434–8. doi: 10.1007/s002619900227. [DOI] [PubMed] [Google Scholar]
- 13.Weill FS, Costaz R, Racle A, et al. Ultrasound study of adenopathies within the hepatoduodenal ligament: The “rosebud” pattern. Gastrointest Radiol. 1986;11:142–4. doi: 10.1007/BF02035056. [DOI] [PubMed] [Google Scholar]
- 14.Rath AM, Zhang J, Bourdelat D, et al. Arterial vascularisation of the extrahepatic biliary tract. Surg Radiol Anat. 1993;15:105–11. doi: 10.1007/BF01628308. [DOI] [PubMed] [Google Scholar]
- 15.Braden B, Faust D, Ignee A, et al. Clinical relevance of perihepatic lymphadenopathy in acute and chronic liver disease. J Clin Gastroenterol. 2008;42:931–6. doi: 10.1097/MCG.0b013e31811edcf7. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich CF, Lee JH, Herrmann G, et al. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467–72. doi: 10.1002/hep.510260230. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich CF, Stryjek-Kaminska D, Teuber G, et al. Perihepatic lymph nodes as a marker of antiviral response in patients with chronic hepatitis C infection. AJR Am J Roentgenol. 2000;174:699–704. doi: 10.2214/ajr.174.3.1740699. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich CF, Leuschner MS, Zeuzem S, et al. Peri-hepatic lymphadenopathy in primary biliary cirrhosis reflects progression of the disease. Eur J Gastroenterol Hepatol. 1999;11:747–53. [PubMed] [Google Scholar]
- 19.Leuschner M, Dietrich CF, You T, et al. Characterisation of patients with primary biliary cirrhosis responding to long term ursodeoxycholic acid treatment. Gut. 2000;46:121–6. doi: 10.1136/gut.46.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirche TO, Russler J, Braden B, et al. Sonographic detection of perihepatic lymphadenopathy is an indicator for primary sclerosing cholangitis in patients with inflammatory bowel disease. Int J Colorectal Dis. 2004;19:586–94. doi: 10.1007/s00384-004-0598-0. [DOI] [PubMed] [Google Scholar]
- 21.Jarnagin W, Winston C. Hilar cholangiocarcinoma: Diagnosis and staging. HPB (Oxford) 2005;7:244–51. doi: 10.1080/13651820500372533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JY, Kim MJ, Lee JM, et al. Hilar cholangiocarcinoma: Role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol. 2008;191:1448–57. doi: 10.2214/AJR.07.3992. [DOI] [PubMed] [Google Scholar]
- 23.Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: Further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202–9. doi: 10.1097/01.sla.0000109154.00020.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam R, de Haas RJ, Wicherts DA, et al. Concomitant extrahepatic disease in patients with colorectal liver metastases: When is there a place for surgery? Ann Surg. 2011;253:349–59. doi: 10.1097/SLA.0b013e318207bf2c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


