Abstract
Tissue acquisition using EUS has considerably evolved since the first EUS-FNA was reported 25 years ago. Its introduction was an important breakthrough in the endoscopic field. EUS-FNA has now become a part of the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the gastrointestinal tract and of the organs in its proximity, including lung diseases. This review aims to present the history of EUS-FNA development and to provide a perspective on the recent developments in procedural techniques and needle technologies that have significantly extended the role of EUS and its clinical applications. There is a bright future ahead for EUS-FNA in the years to come as extensive research is conducted in this field and various technologies are continuously implemented into clinical practice.
Keywords: EUS, EUS-FNA, tissue acquisition
THE HISTORY OF EUS AND EUS-FNA DEVELOPMENT
It has been a remarkable journey for EUS over the past three decades. EUS has evolved from a technical curiosity into a procedure with a significant impact on gastrointestinal and pulmonary diseases. Thus, EUS has radically changed the ability to diagnose and stage gastrointestinal cancers and to evaluate the pancreas. It permits high-resolution imaging of the gastrointestinal wall and abdominal organs, as well as real-time EUS-FNA. The development of EUS-FNA changed the landscape of EUS, upgrading its diagnostic power. Its greatest advantage is that it allows safe and accurate sampling of lesions that were earlier not accessible or required more invasive techniques for pathological diagnosis. This ability has also expanded its role in diagnosis and staging of pulmonary diseases.
Flexible endoscopy was developed in 1911[1] and the ultrasound (US) arrived later in 1956.[2] By combining ultrasonography and endoscopy, EUS opened an entirely new dimension in imaging. EUS was designed in the early 1980s in an attempt to improve US imaging of the pancreaticobiliary system. The addition of US probes to endoscopes[3] allowed an improved visualization of the gastrointestinal wall and its surrounding structures. The resolution of the images was enhanced due to the closeness of the US transducer to the lesion and the use of a high-frequency US probe. The first radial echoendoscope was designed and provided by Olympus (Tokyo, Japan) in 1982. The initial commercial EUS instrument was mechanical radial type, where the US transducer located on the tip of the instrument was rotated by a motor in the endoscope handle. A 360° image was obtained, perpendicular to the insertion shaft of the endoscope, and that allowed an easier interpretation of anatomy in real time.[4] In 1984, Tio and Tytgat depicted the possibility of using the biopsy channel for cytological puncture, which would increase the diagnostic value of EUS.[5] Rösch and Classen[6] presented the advantages of EUS along with its limitations, the most important being the lack of specificity in distinguishing between benign and malignant changes.
In the early 1990s, Pentax Medical, in cooperation with Hitachi, developed the first commercially available linear-array echoendoscope.[7] Linear echoendoscopes brought a new landscape to EUS due to the ability to track a needle in real time across the image plane into a target lesion. The electronic instruments also permitted the use of Doppler technology to assess vascular flow. Vilmann et al. in collaboration with Medi-Globe GmbH created a special biopsy equipment, and this was a major step leading to the clinical application of the biopsy method.[8,9,10] In 1992, Vilmann et al.[8] reported the first case of EUS-FNA of a pancreatic head lesion, using a curved linear array (CLA) echoendoscope. EUS-FNA of various lesions from upper and lower gastrointestinal tract was further described by Wiersema et al.,[11] who published the first EUS-FNA report performed in the United States.
In 1993, a new steel needle, with Teflon sheath, for the upper gastrointestinal tract lesions was described by Vilmann et al.[12] Since then, many indications for EUS-FNA have been reported worldwide [Figure 1].[3,8,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]
Figure 1.
The history of EUS-guided fine-needle aspiration
In 1994, Wiersema et al.[18,39] and Chang et al.[19] described the importance of an on-site cytopathologist during the procedure to assess if the collected samples were adequate or whether further puncture attempts were necessary. Giovannini et al.[40] showed that EUS-FNA was safe, with no significant complications.
Vilmann and Hancke reported the development of a new biopsy handle instrument (type Hancke/Vilmann) in 1996.[20] A year later, Binmoeller et al.[21] first described an automated biopsy device for pancreatic lesions that could not be punctured with a conventional aspiration needle. The automatic spring-loaded biopsy needle allowed tissue sampling of indurated pancreatic lesions. However, this instrument never gained success. Subsequently, numerous extended indications for EUS-FNA have been reported.[22,23,24,25,26,27,28] In 2002, Wiersema et al.[29] described the initial experience with EUS-guided biopsies of perigastric organs. The initial core biopsy EUS needle used was the 19-G Tru-Cut needle. Although in some cases high diagnostic yields were obtained,[41] there were several complaints about the limited flexibility of the needle. The Tru-Cut was replaced by the ProCore fine-needle biopsy (FNB) needles, which are available in a range of sizes (19, 20, 22, 25-G).[42,43]
The history of EUS needles is dynamic, with new needles coming out annually [Figure 2].[44] New needles such as fork-tip needle (SharkCore; Medtronic),[30] Franseen-type needle (Acquire; Boston Scientific)[45] or 20-G FNB needle with antegrade core trap (ProCore 20-G; Cook Medical)[46] have been recently released on the market.
Figure 2.
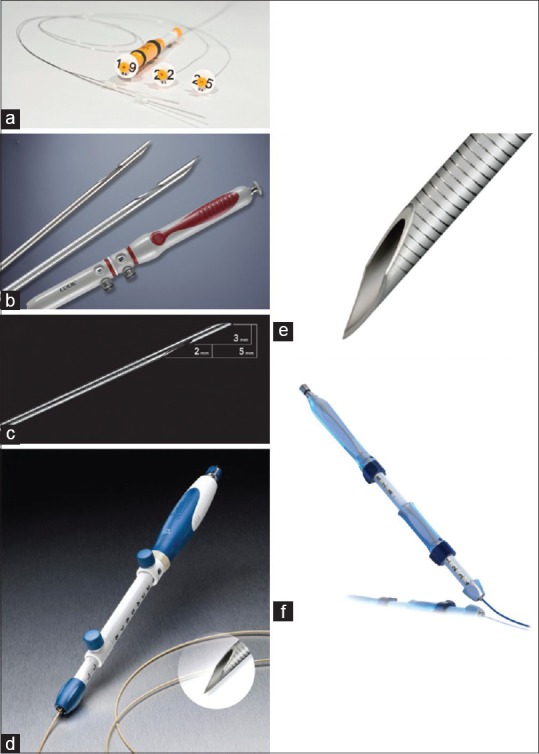
(a) The BNX system with 19-gauge (G), 22G, and 25G needles allows multiple needle exchanges through the outer sheath (Image courtesy of Beacon Endoscopic and used with permission). (b) The Echo Tip ProCore needle has a reverse bevel design for acquiring a tissue specimen. The 22G and 25G needles are shown. (c) A close-up view of the tip of the ProCore 25G needle (Image courtesy of Cook Medical and used with permission). (d) The nitinol-based Expect Flex 19G fine aspiration needle is more flexible than its stainless steel predecessors and appears more promising for use in the duodenum. (e) An extreme close-up view of the expect 19G needle (Image courtesy of Boston Scientific and used with permission). (f) The clear view EUS-guided fine aspiration needle. The distal 2 cm of the needle are laser-etched to enhance visibility (Image courtesy of ConMed Endoscopic Technologies and used with permission)
EUS-FNA was introduced at the beginning of the 1990s due to a echoendoscope that allowed real-time visualization of aspiration needles.[13,47] The design of CLA echoendoscopes and the technique of EUS-FNA have not substantially changed since then. In 2007, Voermans et al.[48] described the first use of a prototype forward-viewing (FV) echoendoscope. The FV echoendoscope was initially created for therapeutic procedures, particularly for pseudocyst drainage.[49,50] However, it has become evident that it can be very useful for other therapeutic interventions besides EUS-FNA.[51,52]
Older mechanical radial echoendoscopes have been substituted by electronic radial echoendoscopes that produce significantly better images.[53] Radial-array echoendoscopes are used only for diagnostic EUS examinations and consequently have limited applications because tissue sampling and therapeutic interventions are not feasible.[54,55]
Technical advances have significantly extended the role of EUS and its clinical applications. The diagnostic and therapeutic capabilities of EUS continue to emerge and improve. EUS has become more than a tool to distinguish different tissue densities;[56] tissue can now be depicted in great detail using modalities such as elastography;[57,58] the tissue vascularity can now be described with increasing precision.[58] Using these various techniques, targets for biopsy can be precisely pinpointed. On reaching the target, tissue can then be examined microscopically in real time, ensuring optimal targeting and diagnosis.[59]
There is a bright future ahead for EUS in the years to come as extensive research is conducted in this field and various technologies are continuously implemented into clinical practice.
DIAGNOSTIC ROLE OF EUS-FNA
Since its initial report 25 years ago, EUS-FNA has now become part of the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the gastrointestinal tract and of bordering organs. Its introduction represents an important breakthrough in the endoscopic field. The EUS-FNA technique has remarkably enhanced the diagnostic potential of EUS, decreasing the number of surgical interventions for diagnostic sampling.
The use of EUS-FNA in clinical practice has been increasing lately. Roy et al.[60] investigated the tendency in tissue acquisition (TA) in pancreatic diseases in the United States over a period of 5 years (2006–2010). The use of EUS-FNA increased by 69.3%, surgical biopsy decreased by 41.7%, and the use of percutaneous biopsy remained stable.
EUS-FNA proved to be an effective technique, superior, and safer than computed tomography (CT)-guided or ultrasonography-guided percutaneous TA in the evaluation of small lesions.[61,62,63,64]
The indications for EUS-TA include diagnosis and staging of lesions, solid or cystic, within and proximal to the gastrointestinal tract, including esophageal, gastric, rectal, and pancreaticobiliary malignancies [Table 1].[65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] It is also used in case of gastrointestinal subepithelial lesions (SELs), intra-abdominal and mediastinal lymphadenopathy, lung lesions, or adrenal masses.[85,86]
Table 1.
Common indications for EUS-FNA
EUS-FNA represents the standard method for the pathological diagnosis of solid pancreatic masses [Figure 3].[44] It has been proven to be very accurate (sensitivity 85%–89% and specificity 96%–99%) according to three meta-analyses.[87,88,89] Furthermore, EUS is also an advanced staging method as it allows the sampling of locoregional and distant lymph nodes, liver lesions, and ascites usually undetected by other imagining techniques.[90]
Figure 3.
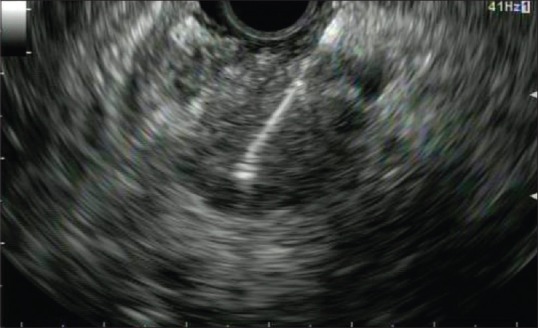
EUS-FNA of a pancreatic mass (re-used with permission)
A meta-analysis comprising 18 studies and 1438 patients showed that cytopathological examination of pancreatic cystic lesions has a pooled sensitivity and specificity of 54% and 93%, respectively.[91] Another meta-analysis[92] revealed good specificity but poor sensitivity for EUS-FNA-based cytology in differentiating benign from malignant intraductal papillary mucinous neoplasms. Major pitfalls in diagnosing pancreatic cystic lesions by EUS-FNA are the frequency of insufficient aspirates and difficulty in differentiating pathological mucin from gastrointestinal contaminant, secondary to a transgastric or transduodenal approach of EUS-FNA. EUS morphology and FNA-based cytology combined with cyst fluid analysis for potential molecular markers may help improve the overall accuracy of EUS-FNA in the diagnosis of pancreatic cystic lesions.[93]
EUS-FNA is an unsterile procedure. This is important in the puncture of cystic lesions because the esophagus and stomach are not sterile. This can be neglected if the target is solid and perfused, taking into consideration the multiple defense strategies of the human body.[94] The circumstances are different in cystic lesions. Without a blood supply, the cystic fluid is vulnerable to infection, and consequently, peri-interventional antibiotic treatment is recommended.[95]
Many studies have reported the importance of performing EUS-FNA for mediastinal and abdominal lymph nodes.[96] One indication is the diagnosis of an enlarged lymph node at a distance from a known tumor [Figure 4]. Biopsying a lymph node through an area of a tumor should be carefully avoided because of the risk of false-positive results and needle track seeding.[95] According to a meta-analysis,[97] in the case of mediastinal lymph nodes, EUS-FNA had a higher sensitivity (88% vs. 84.7%) and specificity (96.4% vs. 88%) compared to EUS imaging alone. For abdominal lymph nodes, fewer studies have been conducted and the results showed that EUS-FNA is viable and safe. For instance, a prospective study with 142 patients with inconclusive or unfeasible percutaneous image-guided sampling showed that EUS-FNA was possible in 92% of the patients and it led to a diagnosis in 91% of cases.[98] Furthermore, when tuberculosis was not diagnosed using routine methods, EUS-FNA of mediastinal or abdominal lymph nodes was extremely accurate.[98,99] EUS-FNA has also proved its benefit in diagnosing sarcoidosis[100,101] and lymphomas.[102,103,104]
Figure 4.
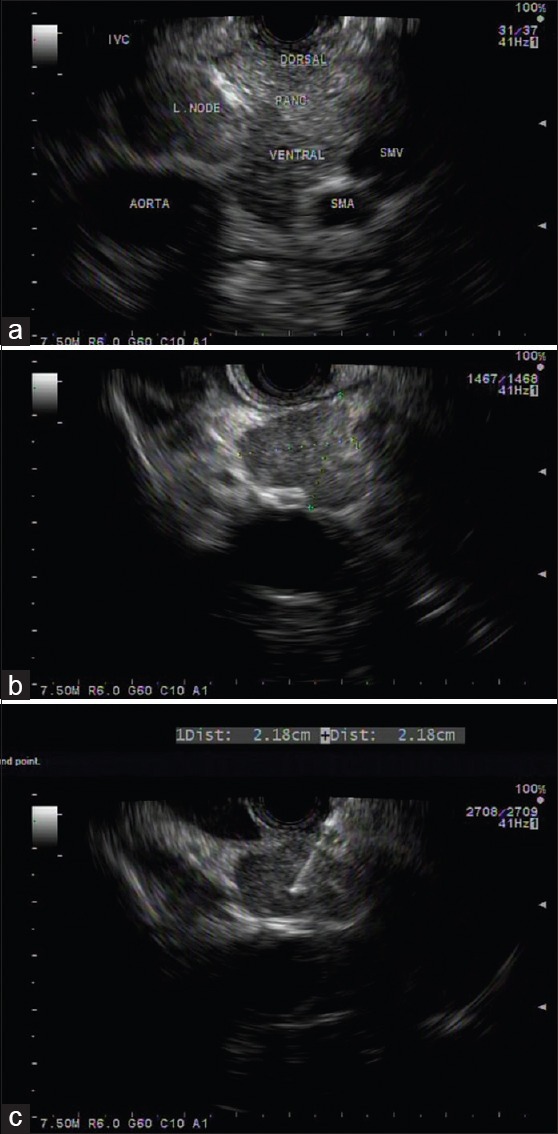
Patient with a history of colon cancer with aorto-caval lymph node on computed tomography positron emission tomography, underwent EUS-FNA for pathological diagnosis. (a) Hypoechoic, irregular, oval-shaped lymph node between the aorta and inferior vena cava; (b) close-up view of the lymph node; (c) EUS-FNA of the lymph node using a 25-gauge needle. Cytology: metastatic carcinoma. IVC: Inferior vena cava, SMA: Superior mesenteric artery, SMV: Superior mesenteric vein, L. node: Lymph node, PANC: Pancreas
EUS-FNA has a high accuracy for patients with lung cancer and metastatic mediastinal lymph nodes.[105,106] When EUS is available, it is safer and less invasive than mediastinoscopy,[107] and according to the European guidelines, the combination of EUS-FNA and endobronchial US-guided transbronchial needle aspiration is at present considered the first choice for staging of nonsmall cell lung cancer.[108] In nondiagnosed pulmonary lesions, EUS-FNA can be useful in diagnosing tumors with lymph node involvement if the tumors cannot be reached directly by bronchoscopy. Non-small cell carcinoma and small cell carcinoma can be distinguished by FNA of the involved lymph nodes.[109] The resulting material can be used for further testing, such as epidermal growth factor receptor analysis, for more targeted therapy.[110]
Two meta-analyses have shown that EUS-FNA had 66% and 80% overall sensitivities and 100% and 80% specificities in diagnosing malignant biliary strictures.[111,112] Recent studies that were not included in the meta-analyses reported similar results.[113] However, a major concern regarding EUS-FNA of biliary strictures is the potential for needle track tumor seeding. For patients with unresectable cholangiocarcinoma limited to the liver and bile ducts, liver transplantation is one of the most successful therapies.[114] Immunosuppression following transplantation may increase the risk of tumor recurrence in patients with peritoneal tumor seeding. Accordingly, performing EUS-FNA of a primary unresectable bile duct tumor is considered contraindicated in case of potential liver transplantation.[115] On the other hand, another study showed that performing EUS-FNA in patients with cholangiocarcinoma has no impact on overall survival or progression-free survival. Even though tumor cell dissemination can occur along the needle track during EUS-FNA, its clinical impact was not considered significant.[116] This is a controversial issue, and many centers continue to avoid EUS-FNA of biliary strictures that may be treated with curative surgical intent or liver transplantation.
In addition, EUS-FNA is a valuable tool for diagnosing SELs of the upper gastrointestinal tract.[117] The sensitivity, specificity, and accuracy of EUS-FNA for diagnosing subepithelial mesenchymal tumors of the upper gastrointestinal tract were 82.9, 73.3, and 80%, respectively.[118] In a retrospective study including 121 patients, using a FV linear echoendoscope and a 19-G needle, the diagnostic accuracy for SELs of the stomach, duodenum, and rectum was 93%.[52] Furthermore, a new core needle (SharkCore, Medtronics) showed superior diagnostic yield, compared with a standard aspiration needle, for obtaining material suitable for the immunohistochemical differentiation of benign subepithelial gastrointestinal tumors from potentially malignant gastrointestinal stromal tumors.[119]
A meta-analysis has also shown that EUS-FNA improves the sensitivity (from 84.7% to 96.7%) and specificity (from 84.6% to 95.5%) compared to EUS imaging alone in assessing nodal stage of esophageal cancer.[120] EUS-FNA targets lymph nodes that are not in the proximity of the tumor (the needle should not puncture the tumor because of the risk of false-positive results and needle track seeding). Regional and distant lymph nodes as well as metastases can be the targets as well.[121,122]
There is an increasing interest in using EUS-FNA for liver lesions. According to a multicenter survey, EUS-FNA of the liver diagnosed malignancy in 89% of cases after inconclusive FNA under transabdominal US guidance.[123] Liver biopsy represents an important feature in the diagnosis of liver diseases. Recently, EUS was used to obtain liver biopsy.[124] Studies have shown that EUS-guided biopsy using a 19-G needle was safe, yielded adequate tissue for diagnostic purposes of liver disease.[41]
EUS-FNA of splenic lesions represents an indication for EUS when CT- or US-guided FNA is nondiagnostic or not feasible. EUS-FNA can diagnose splenic tuberculosis, sarcoidosis, Hodgkin's disease, colon cancer metastasis, abscess, and infarction.[125]
The potential utility of EUS-FNA in case of posterior mediastinitis was described[31] to provide material to identify specific agents, such as bacteria or fungi, followed by culture and selection of appropriate therapy.
A new application of EUS-FNA has recently been described for patients with severe gastroparesis. EUS-FNA of the antral muscularis propria with a 19-G needle provided adequate samples for the evaluation of the loss of interstitial cells of Cajal in 11 of 13 patients; a positive correlation between results obtained with surgical and endoscopic specimen was noticed.[126]
Several studies[127,128,129] have shown the utility of bedside EUS in critically ill patients. The bedside EUS-FNA was feasible and could offer an alternative in life-threatening situations. Consequently, endosonographers should consider using EUS in Intensive Care Unit patients when clinically indicated.
HOW TO ACHIEVE EXCELLENCE?
Tissue acquisition using EUS has considerably advanced since the first EUS-FNA was reported 25 years ago. Numerous studies have tried to determine the ideal EUS-FNA equipment and techniques. Within this basic technique, more complex issues to enhance the diagnostic yield of EUS-FNA have been studied.[130] Multiple factors may contribute to the results of EUS-TA. These factors include experience level of the endosonographer, sampling site, location of the lesion, sampling technique, needle size and type, presence of rapid on-site pathologist, and various methods of processing the tissue sample obtained.[131]
A first step in performing high-quality EUS-FNA is adequate training. EUS-FNA is technically challenging, with a prolonged learning curve, and requires appropriate training.[132,133,134,135] The American Society for Gastrointestinal Endoscopy (ASGE) recommends 150 supervised EUS procedures before competency should be assessed, 75 of which must evaluate the pancreatobiliary system and 50 must incorporate EUS-FNA.[133] A recent systematic review highlights that the number of EUS procedures required to achieve competency remains unclear but has clearly risen above the current ASGE recommendations.[136] Each trainee requires individualized assessment to ensure competency is achieved before entering independent clinical practice. Nonetheless, it is reasonable to assume that endoscopists performing a high volume of FNAs are likely to have more success because the procedure is highly operator dependent.[44]
Adequate sedation for EUS-FNA is important because these procedures are typically longer than standard endoscopy.[137] Sedation is usually provided with conscious sedation or with monitored anesthesia care. Propofol is preferred because often the length of the procedure may be unpredictable, especially if a lesion is difficult to find.[138] Moreover, a poorly sedated patient is predisposed to an increased risk of complications.[44] A retrospective study[139] showed that the use of general anesthesia (GA) in case of EUS-FNA of pancreatic lesions is associated with an increased diagnostic accuracy (83% with GA compared with 73% without GA). Despite these results, in most institutions, the procedure is performed using moderate sedation or monitored anesthesia care.[140] Other intravenous anesthetic agents including benzodiazepine-opioid drugs are used, but their respiratory–inhibitory effects limit their application.[141] Moreover, many EUS procedures require water injection into the digestive tract, increasing the risk of aspiration during anesthesia. In such a scenario, studies showed that nitrous oxide sedation represents a safe and effective choice in patients undergoing EUS-FNA.[142]
Another key point to improve TA is a puncture technique adapted to the target. After identifying the target lesion, it is important to determine the scope position and to decide which part of the lesion is the most suitable for sampling to obtain the best diagnostic accuracy.[140] Even though the risk of tumor seeding via the EUS needle tract is very low, cases of seeding the stomach wall have been reported.[143] A site with minimum number of blood vessels should be chosen using Doppler imaging to avoid bleeding complications.
Studies have shown that the passage of the needle with or without the stylet has no impact on diagnostic accuracy.[144,145,146,147,148] Suction may increase sensitivity, but it contributes to bleeding, and thus its use is also variable.[149,150] High diagnostic yields have been obtained without suction in case of lymph node sampling[151] and with the stylet pull technique (pulling the stylet while moving the needle within the lesion) for solid lesions.[152] Further studies regarding the value of the slow pull technique are needed, some have adopted this technique in their practice, while others continue to use the conventional 10–20 mL negative suction pressure for EUS-FNA.[153] A novel “wet-suction” technique (where the EUS needle is flushed with saline solution to replace the column of air within the lumen)[154] was shown to increase tissue specimen adequacy (85.5% vs. 75.2%) when using 22-G FNA needles.[155] Villa et al.[156] showed that the samples were of higher quality, and furthermore, the diagnostic yield for the wet suction technique was significantly better than the conventional method.
The methods used during FNA are also debated. Taking into consideration that inner portions of a pancreatic tumor may be necrotic, targeting the peripheral areas of the mass can improve diagnostic yield. However, obtaining adequate tissue for diagnosis is still a challenge because of the dense desmoplastic reaction at the periphery. Therefore, endosonographers can use the “fanning” technique, which involves adjusting the trajectory of the needle, using the elevator or dials on the head of the echoendoscope. Thus, instead of advancing the needle back and forth through the same portion of the mass, it samples different areas.[157] A study comparing fanning with standard TA during EUS-FNA showed superiority of the fanning technique after a single pass as compared to standard technique (86% vs. 58% diagnostic yield).[158] Recently, the door knocking method, where the needle is rapidly advanced within the target lesion, has been developed. Mukai et al.[159] showed that the door knocking method enabled the acquisition of larger specimens compared to standard method. Nakai et al.[160] passed a biopsy forceps through a 19-G FNA needle to perform direct biopsy and found that the TA rate for a single puncture was 67% with the biopsy forceps alone but increased to 88%, when this technique was combined with regular EUS-FNA, indicating that this is a useful technique for acquiring tissue with a small number of needle passes.
NEEDLES
A diversity of needles has been used during the past 25 years, with innovations continuing to appear. The history of EUS needles is dynamic, with new needles coming out nearly annually.
In recent years, new needles designed to obtain samples suitable for histologic evaluation, with preserved tissue architecture, have been developed. These FNB needles feature either a special geometry of the cutting tip or a side-slot in the distal portion of the needle. Conventional needles without these refinements are referred to as FNA needles.[46]
All EUS-FNA needles have the same basic design and are currently single use. The various commercially available FNA needles have different echogenicity under EUS guidance. The visibility of the needle tip is critical when performing FNA,[157] and to improve it, needle tips are tailored using different techniques such as laser etching, mechanical dimpling, or sandblasting.[161,162] A multicenter study evaluated and graded 10 different EUS needles based on their echogenicity. A prototype needle with polymeric coating had significantly higher overall ranking, indicating that this coating to the needle tip and shaft may enhance visualization.[163]
Needles with a side hole at the tip have been developed as core biopsy needles, and numerous reports have investigated their utility. The EchoTip ProCore™ has allowed diagnosis with fewer needle passes than conventional needles without side holes, but with no significant difference in diagnostic adequacy and accuracy.[164,165]
NEEDLE SIZE: SMALL VS. LARGE
Today, four different needle sizes are available: 19-G (aspiration and core biopsy), 20-G (core biopsy), 22-G (standard size, aspiration, and core biopsy), and ultrathin 25-G needles. The most widely used needle for EUS-FNA is the 22-G needle,[166] which is flexible and enables cytologic assessment without significant risk for complications, although a 2% risk of acute pancreatitis was reported in a retrospective study.[167]
For FNA of solid lesions, 25-G or 22-G needles are the most widely used, while 22-G needles are usually used for cystic lesions.[168] Eight randomized clinical trials compared 22-G and 25-G needles in patients with solid masses and lymph nodes[103,169,170,171] or only with solid pancreatic masses.[172,173,174,175] One study has shown a higher accuracy for the 25-G needle;[169] the others have shown no significant difference in diagnostic accuracy. Studies comparing FNA with 25-G and 22-G needles were also subjected to four meta-analyses[176,177,178,179] that provided conflicting results. The recent meta-analysis by Facciorusso et al.,[176] comprising only randomized clinical trials, did not show significant differences between the needles in terms of sensitivity and specificity for pancreatic malignancy. On the other hand, Xu et al.[177] demonstrated higher sensitivity for 25-G needle with no significant difference in specificity for malignancy in patients with solid pancreatic masses. The other two meta-analyses were published in 2013 but did not contain recent information and had important limitations such as significant heterogeneity and inclusion of retrospective data.
The 19-G needles are more rigid, and consequently, transduodenal biopsies are more difficult.[166] These devices were developed to obtain larger amounts of material from the targeted lesions; however, compared to the 22-G needle, the 19-G needle has a higher rate of technical failure. One study showed that the 19-G needle had a higher diagnostic accuracy than the 22-G needle, but technical failures were not taken into consideration.[180] Twenty-five-G needles had the highest diagnostic accuracy for uncinate masses. In case of pancreatic body and tail lesions, no significant difference between the three types of needle was found.[181]
The use of nitinol for 19-G FNA needles has increased their flexibility. A multicenter study revealed no significant difference regarding diagnostic accuracy between the 22-G and the novel 19-G flexible needle made of nitinol, but histological core tissue was obtained in a larger number of patients by using the 19-G flexible needle.[182] Nineteen-gauge FNA has been able to obtain adequate samples in case of liver biopsies.[43,183,184,185] Other studies have shown higher diagnostic yields with 19-G needles when performing SEL biopsies, which typically have lower diagnostic accuracy with 25-G and 22-G FNA needles.[186]
EUS-guided TA can be obtained by EUS-FNA or EUS-FNB. The needle tip design is the distinguishing feature between FNA and FNB because the procedural techniques are comparable.
Although tissue histology has been proved to be important for the diagnosis of autoimmune pancreatitis,[187] Hodgkin's lymphoma,[188] and well-differentiated adenocarcinomas,[189] the utility of histology for pancreas sampling has been evolving.
The initial EUS-FNB needle was the 19-G Tru-Cut. It had limited flexibility and consequently was replaced by the ProCore FNB needle by the same manufacturer, which is currently available in a range (19, 20, 22, 25-G) of sizes. A multicenter randomized clinical trial showed that the ProCore 19-G needle was superior to the Tru-Cut needle, with higher diagnostic accuracy (88% vs. 62%; P = 5.02).[43] A new variant of the ProCore needle (20-G) was introduced with a forward-facing direction of the side bevel. Two newly developed needles (SharkCore, Medtronic, Dublin, Ireland; and Acquire, Boston Scientific, Natick, MA, USA) are designed with two or three opposing sharp points and a multifaceted bevel in the needle tip, aimed at capturing a core of tissue. According to the first results, the TA was significantly higher than using standard aspiration needles, and diagnosis was possible with fewer needle passes.[30,45,190,191]
There is space for improvement: intermediate-size needles (20-G or 21-G) might be more useful for combined cytology and histology sampling or use of auxiliary devices inside the sheath of the 19-G needle. Perhaps in a more innovative vision, bulky scopes could be abandoned and more flexible luminal robotic-driven devices can be used to access and puncture the targets.
PRESENCE OF ON-SITE CYTOPATHOLOGIST
The presence of a cytopathologist during EUS-FNA with rapid on-site evaluation (ROSE) of the samples has been shown to enhance the diagnostic yield by reducing inadequate samples and decreasing the need for additional passes.[87,192,193,194,195,196,197] The diagnostic accuracy of EUS-FNA was over 90% in most studies when ROSE was used.[198] One or two passes are typically made, and then, the pathologist evaluates the adequacy of the samples and decides if further passes are needed to achieve diagnostic success. If bloody aspirates are noticed by the on-site cytopathologist, a smaller gauge needle is used, without suction.[157] Limitations of ROSE include increased cost due to cytopathologist time commitment, as well as low reimbursements for ROSE.[199] The number of institutions capable of having a pathologist when EUS-FNA is performed is limited,[200,201] and this is a factor that at least hinders the widespread use of ROSE.
Another emerging concept is telecytopathology.[202] The slides are initially prepared and prescreened by a cytotechnologist or pathology resident and then analyzed by an off-site cytopathologist using real-time remotely operated system.[203] A retrospective study demonstrated the potential use of telecytopathology as a valid substitute for on-site evaluation of pancreatic tumors by EUS-FNA.[204]
Recent studies have shown that macroscopic on-site quality evaluation (MOSE) also improves diagnostic accuracy.[153] Iwashita et al.[205] analyzed the size of the macroscopically visible core (MVC) and the diagnostic yield of EUS-FNA. Results showed that MVC of ≥4 mm is an indicator of specimen adequacy and can enhance diagnostic yield. Moreover, MOSE represents a procedure that can be carried out by an endoscopist and can be regarded as obligatory for endoscopists who intend to improve their diagnostic accuracy.
NUMBER OF FINE-NEEDLE ASPIRATION PASSES
In the absence of ROSE, earlier studies recommended 5–7 passes in case of a pancreatic mass.[206,207] Other studies, however, reported that fewer passes are sufficient,[208,209] and Itoi et al.[200] found that even without ROSE, mean 2.88 needle passes were adequate for diagnosis, with 93.3% accuracy, 91.8% sensitivity, and 100% specificity.
When ROSE is available, the maximal diagnostic yield can be obtained after 2–5 passes for pancreatic masses.[210,211] Erickson and Garza[212] also showed that with ROSE, the average number of needle passes was 3.4 ± 2.2 (range 1–10), but it was influenced by the tumor grading. Wani et al.[146] carried out a similar study and found that well-differentiated tumors were a predictive factor for a larger number of needle passes.
CAN WE DO IT BETTER? FUTURE DIRECTIONS
Through-the-needle imaging and forceps
Miniaturized devices such as a biopsy forceps or confocal microscopy fiberoptic probes have been passed through 19-G EUS-FNA needles to evaluate cystic and solid lesions.[213,214]
Small forceps passed through 19-G needles have been developed for pathologic diagnosis of pancreatic cystic lesions.[160,215,216,217] The use of mini-forceps through an FNA needle has been proven feasible and safe for pancreatic TA.[160] While only a pilot study has been completed, this initial report suggested high diagnostic sensitivity with no device failures or complications. This may offer an attractive alternative for the future. To date, comparisons of forceps samples to other EUS-TA techniques have not been published.[168]
THE USE OF NEEDLE CONFOCAL ENDOMICROSCOPY
Confocal laser endomicroscopy (CLE) is a novel endoscopic method that allows microscopy of the gastrointestinal mucosa during ongoing endoscopy, enabling real-time optical biopsy.[218] Technical advances allowed a confocal miniprobe to be passed through the biopsy channel of the endoscope.[219]
As probe-based confocal endomicroscopy has been miniaturized, needle-based confocal laser endomicroscopy (nCLE) has become available for clinical use. The nCLE miniprobe has 0.85 mm diameter and can be passed through a 19-G EUS-FNA needle.[220] Needle-based CLE was designed to allow in vivo histological images using fluorescent contrast. Therefore, nCLE could show which areas are most suspicious for malignancy and require biopsy.[221] EUS-guided nCLE seems to be a promising minimally invasive technique that might be used to improve the diagnostic accuracy of EUS-FNA. Optical needle biopsy could also be useful in reducing sampling errors because it provides real-time microscopic details, especially in cystic masses.
The diagnostic of pancreatic cysts is sometimes difficult. Results of studies using n-CLE have been very promising, and in the future, nCLE may be used routinely for diagnosing pancreatic cysts as an adjunct to conventional EUS-FNA.[213,222,223] Novel vascular patterns have been described, and a classification of nCLE patterns of pancreatic cystic lesions was established, facilitating their diagnosis.[224] nCLE was found to be safe and feasible with high technical success.[225] These promising findings require validation in larger multicenter studies to justify routine use of EUS-nCLE for the evaluation of pancreatic cystic lesions.
Studies have also evaluated the feasibility and safety of nCLE for the assessment of solid pancreatic masses and lymph nodes.[226] nCLE identified 77% of the cases in which malignancy was confirmed on histology. However, more studies with various other contrast agents and targeted markers need to be performed to improve diagnostic accuracy. Given the low negative predictive value of EUS-FNA, nCLE could help rule out malignancy after a previous inconclusive EUS-FNA.[227] The benefit of nCLE in the evaluation of solid pancreatic masses and lymph nodes is still unclear, and further studies are needed. This technique may also have significant implications in the field of molecular imaging by allowing the in vivo visualization of pathophysiologic events in their natural environment.[228,229,230]
Although it is unlikely that nCLE will replace EUS-FNA cytology for pancreatic masses and lymph nodes, it can be complementary to FNA for diagnosis during EUS.[231]
TARGETED EUS-FNA DURING CONTRAST-ENHANCED HARMONIC IMAGING AND ELASTOGRAPHY
Contrast-enhanced harmonic EUS (CH-EUS) and elastography have been proposed as methods for better targeting of the lesions.[232,233]
The use of US-contrast agents may enable the recognition of better puncture sites based on the differences in blood flow patterns. Kitano et al.[234] have described the utility of EUS-FNA using CH-EUS. They showed that CH-EUS could identify pancreatic adenocarcinomas as hypoenhanced lesions, with a sensitivity and specificity of 88% and 94%, respectively. Moreover, 80%–100% of false-negative EUS-FNA cases were correctly classified by CH-EUS. However, it is not possible to completely rely only on the vascular aspect of a mass to determine its nature, so FNA still represents the standard for the diagnosis of masses. Nevertheless, CH-EUS and EUS-FNA are complementary, not competitive, and are better performed together during the same investigation.[235]
According to another study,[236] EUS-FNA under CH-EUS imaging improved the diagnostic accuracy for pancreatic masses to 86.5% from 78.4% obtained with regular EUS-FNA. Furthermore, when the two methods were combined, the diagnostic accuracy increased to 94%. Sugimoto et al.[237] showed that EUS-FNA under CH-EUS imaging needed fewer needle passes compared with conventional EUS-FNA, whereas Hou et al.[238] stated that the percentage of adequate biopsy specimens in the CH-EUS group (96.6%) was greater than that in the EUS group (86.7%). These studies suggest that the use of CH-EUS in combination with EUS-FNA may enable the acquisition of adequate specimens and improve the true-positive rate.[153] CH-EUS-FNA is easy to perform and safe, with minimum added extra costs.[238] The results are promising, but the future will know more about its clinical impact and whether there is a change in the assessment of lesions.
Elastography is a technique that allows imaging and quantification of the hardness of lesions with special software.[239] By calculating the elasticity of tissue, it is possible to distinguish benign (soft) tissue from malignant (hard) tissue.[240] The most important advantage of EUS elastography is that it can be performed in real time during a diagnostic examination with immediate information provided to the endosonographer. Furthermore, it can help in selecting the site where FNA can be performed with improved diagnostic yield. This is extremely helpful in a subset of challenging patients with either necrotic tumors or possible malignancy in a background of diffuse inflammatory change.[241,242] It may therefore improve FNA targeting, at a low cost and without the need for extensive training in the use of the software, which is available on most processors.[243,244]
GOING BEYOND CYTOLOGICAL DIAGNOSIS
An emerging concept is the use of the cells aspirated during EUS to obtain information beyond a simple cytological diagnosis.
Along with the technical advances that increased the safety and accuracy of the procedure, there has been significant progress in understanding the biology of many tumors that are biopsied. Pancreatic cancer can be a good example to illustrate this idea. It is characterized by various molecular alterations, and therefore, identification and quantification of potential molecular markers for pancreatic cancer on the samples obtained by EUS-FNA could be a promising concept for the diagnosis of malignancy.[245,246]
Advances in molecular diagnostic techniques have made it possible to carry out various types of immunostaining and gene analyses using a small amount of specimen obtained by EUS-FNA.[65,247,248] Potential genes and proteins with roles in pancreatic carcinogenesis have been identified and it would be important to determine their clinical usefulness.[247,249]
Whole-genome sequencing and whole-exome sequencing analyses have confirmed that mutations in four genes are most commonly seen in pancreatic adenocarcinoma: K-ras, SMAD4, TP53, and CDKN2A/p16.[250,251]
K-ras
Several studies have shown that combining cytopathological and K-ras mutation analysis improves the diagnosis of pancreatic cancer in EUS-FNA samples.[252,253,254,255,256,257,258] Based on this combination, a medical or surgical conservative treatment can be applied, thus avoiding unnecessary pancreatic resection.[252] K-ras analysis seems important, especially in case of inconclusive EUS-FNA. In these cases, discovery of a K-ras gene mutation can spare an unnecessary repeat EUS-FNA procedure.[259] Moreover, in cases of doubtful cytopathological results, a K-ras mutation assay, when positive, can reduce false-negative results and thus avoids any delay in decision-making.[260] In direct EUS-FNA, sequencing techniques such as next-generation sequencing or pyrosequencing should be used. 5–10 ng of DNA is necessary to detect K-ras mutations using next-generation sequencing.[261,262] The detection of K-ras mutation could be useful not only in the diagnosis but also in staging, prognosis, evaluation of response to therapy, and follow-up of pancreatic cancer patients.[263] Even though further studies are certainly required, the presence of a K-ras mutation seems to negatively influence the prognosis.[264] One study reported that para-aortic lymph nodes diagnosed for K-ras mutation were independent pancreatic cancer prognostic markers in multivariate analysis.[265]
P53
TP53 status evaluation can also improve the sensitivity of EUS-FNA to diagnose pancreatic cancer.[266,267,268] According to a study, by combining p53 protein evaluation and cytopathological examination, the sensitivity increased to 90% (compared with 76%) and specificity remained equal to 91%.[266] In another study by Oshima et al., loss of p53 protein was correlated with poor prognosis mainly if also combined with the loss of expression of p16 and SMAD4 protein.[269] Immunohistochemistry is most commonly used to detect p53 protein accumulation when the TP53 gene is mutated. Sequencing can also detect TP53 mutations.[270]
CDKN2A/P16
As for p53, the loss of p16 was correlated with poor prognosis in pancreatic cancer patients.[269] In a study performed on 101 pancreatic EUS-FNA samples, the allelic losses of CDKN2A/P16 gene, detected by analyzing the loss of heterozygosity (LOH) at 9p, revealed a sensitivity of 85% and a specificity of 64% in diagnosing patients with resectable pancreatic cancer.[271]
SMAD4
The allelic loss of the SMAD4 gene analyzed in EUS-FNA specimens by LOH at 18q showed a sensitivity and specificity of 78% and 57%, respectively.[271] The loss of SMAD4 expression is an independent prognostic factor and seems to be associated with tumor progression. Preoperative stratification based on SMAD4 could lead to appropriate treatment strategy.[272]
In the last 20 years, microRNAs (miRNAs) have become one of the most promising classes of diagnostic and prognostic biomarkers for human cancers. Numerous studies have shown the diagnostic reliability of miRNAs expression profiling in pancreatic cytology specimens.[273,274,275,276] In addition, cell-free cyst-fluid miRNAs have been demonstrated to be promising biomarkers for pancreatic cancer early diagnosis and for assessing high-risk pancreatic cysts.[277,278]
Numerous studies have also reported the usefulness of immunostaining of S100P,[246,279,280,281,282,283,284,285] mesothelin,[285,286,287,288,289,290] mucins,[287,291,292,293,294,295] or KOC/IMP3[279,282,296,297,298] in EUS-FNA samples. Those reports might help improve the efficacy of EUS-FNA for diagnosis of solid pancreatic masses.
Moreover, the tumor material obtained by EUS-FNA can be used to perform molecular investigations to better understand the physiopathology, carcinogenesis, and response to treatment of pancreatic cancer. Gemcitabine is the standard of care for the treatment of advanced pancreatic adenocarcinoma. However, gemcitabine resistance was related to limited intracellular uptake of gemcitabine through a decrease in human equilibrative nucleoside transporter (hENT1) protein expression.[299] Several studies have shown that high levels of hENT1 are associated with significantly longer survival after adjuvant gemcitabine,[300] and gemcitabine should not be used for patients with low tumor hENT1 expression.[301] The expression of hENT1 is epithelial, and consequently, it can be detectable in biopsies containing malignant epithelial cells. No study has yet shown the reliability of this assessment in either FNA or core biopsy samples.
One of the most exciting developments of cancer therapy in the last few years has been the remarkable progress of immunotherapy. Despite the minimal response rates in pancreatic cancer, an immune response is still present and emerging strategies to turn this response on or identify tumors with an immune-sensitive phenotype are promising. It would be therefore interesting to be able to characterize the infiltrating immune cells in EUS-FNA samples.
Another application of molecular testing is to perform polymerase chain reaction analysis and gene promoter hypermethylation on aspirated material collected during EUS-guided puncture of lymph nodes to detect micrometastases.[302,303,304,305]
EUS-GUIDED FINE-NEEDLE VEIN PUNCTURE
Circulating tumor cells (CTCs) can enter the bloodstream and can be extracted from blood samples. The identification of CTCs in blood is called “liquid biopsy.”[306,307,308] EUS-fine needle vein (FNV) puncture can be used to obtain a liquid biopsy. Molecular analysis can further be performed for early detection and monitoring of cancer therapy.[309] EUS-FNV of the portal venous blood using a 19-G needle introduced transhepatically has been shown to be feasible and safe. Pancreatic adenocarcinoma cells were identified in higher levels in portal venous samples as compared to peripheral blood samples.[310]
CONCLUSIONS
There has been tremendous progress over the past decade to overcome the limitations of EUS-FNA due to sampling technique, procedure, and equipment. Refined techniques such as molecular analysis of EUS-FNA samples are being continuously investigated. EUS-FNA will play a major role in personalized cancer therapy in the years to come.
Research in EUS appears to be intensive as specialists continue to study the clinical impact of the procedure, to improve and refine the current indications for it, and to pursue new applications for the method.
Financial support and sponsorship
Dr. Irina M. Cazacu was supported by the 2017 International Travel Grant offered by the American College of Gastroenterology.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This work was supported by a grant of Ministry of Research and Innovation, CNCS - UEFISCDI, project number PN-III-P4-ID-PCE-2016-0561, within PNCDI III.
REFERENCES
- 1.Pauli EM, Ponsky JL. Principles of Flexible Endoscopy for Surgeons. New York: Springer; 2013. A history of flexible gastrointestinal endoscopy; pp. 1–10. [Google Scholar]
- 2.Newman PG, Rozycki GS. The history of ultrasound. Surg Clin North Am. 1998;78:179–95. doi: 10.1016/s0039-6109(05)70308-x. [DOI] [PubMed] [Google Scholar]
- 3.Dimagno E, Regan P, Wilson D, et al. Ultrasonic endoscope. Lancet. 1980;315:629–31. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 4.Strohm WD, Phillip J, Hagenmüller F, et al. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy. 1980;12:241–4. doi: 10.1055/s-2007-1021752. [DOI] [PubMed] [Google Scholar]
- 5.Tio TL, Tytgat GN. Endoscopic ultrasonography in the assessment of intra-and transmural infiltration of tumours in the oesophagus, stomach and papilla of vater and in the detection of extraoesophageal lesions. Endoscopy. 1984;16:203–10. doi: 10.1055/s-2007-1018581. [DOI] [PubMed] [Google Scholar]
- 6.Rösch T, Classen M. Endosonography – What are the limits in gastroenterological diagnostics? Endoscopy. 1991;23:144–6. doi: 10.1055/s-2007-1010642. [DOI] [PubMed] [Google Scholar]
- 7.Gress FG. The early history of interventional endoscopic ultrasound. Gastrointest Endosc Clin N Am. 2017;27:547–50. doi: 10.1016/j.giec.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 9.Vilmann P, Săftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy: Equipment and technique. J Gastroenterol Hepatol. 2006;21:1646–55. doi: 10.1111/j.1440-1746.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 10.Vilmann P. Endoscopic Ultrasonography: With Curved Array Transducer in Diagnosis of Cancer in and Adjacent to the Upper Gastrointestinal Tract: Scanning and Guided Fine Needle Aspiration Biopsy. Copenhagen. 1998 [Google Scholar]
- 11.Wiersema MJ, Hawes RH, Tao LC, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35–9. doi: 10.1016/s0016-5107(92)70327-7. [DOI] [PubMed] [Google Scholar]
- 12.Vilmann P, Hancke S, Henriksen FW, et al. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25:523–7. doi: 10.1055/s-2007-1010389. [DOI] [PubMed] [Google Scholar]
- 13.Wiersema MJ, Kochman ML, Chak A, et al. Real-time endoscopic ultrasound-guided fine-needle aspiration of a mediastinal lymph node. Gastrointest Endosc. 1993;39:429–31. doi: 10.1016/s0016-5107(93)70122-4. [DOI] [PubMed] [Google Scholar]
- 14.Tio TL, Sie LH, Tytgat GN. Endosonography and cytology in diagnosing and staging pancreatic body and tail carcinoma. Preliminary results of endosonographic guided puncture. Dig Dis Sci. 1993;38:59–64. doi: 10.1007/BF01296774. [DOI] [PubMed] [Google Scholar]
- 15.Wegener M, Adamek RJ, Wedmann B, et al. Endosonographically guided fine-needle aspiration puncture of paraesophagogastric mass lesions: Preliminary results. Endoscopy. 1994;26:586–91. doi: 10.1055/s-2007-1009044. [DOI] [PubMed] [Google Scholar]
- 16.Chang KJ, Albers CG, Nguyen P. Endoscopic ultrasound-guided fine needle aspiration of pleural and ascitic fluid. Am J Gastroenterol. 1995;90:148–50. [PubMed] [Google Scholar]
- 17.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 18.Wiersema MJ, Kochman ML, Cramer HM, et al. Endosonography-guided real-time fine-needle aspiration biopsy. Gastrointest Endosc. 1994;40:700–7. [PubMed] [Google Scholar]
- 19.Chang KJ, Katz KD, Durbin TE, et al. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694–9. [PubMed] [Google Scholar]
- 20.Vilmann P, Hancke S. A new biopsy handle instrument for endoscopic ultrasound-guided fine-needle aspiration biopsy. Gastrointest Endosc. 1996;43:238–42. doi: 10.1016/s0016-5107(96)70324-3. [DOI] [PubMed] [Google Scholar]
- 21.Binmoeller KF, Jabusch HC, Seifert H, et al. Endosonography-guided fine-needle biopsy of indurated pancreatic lesions using an automated biopsy device. Endoscopy. 1997;29:384–8. doi: 10.1055/s-2007-1004220. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–61. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 23.Fritscher-Ravens A, Broering DC, Sriram PV, et al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: A case series. Gastrointest Endosc. 2000;52:534–40. doi: 10.1067/mge.2000.109589. [DOI] [PubMed] [Google Scholar]
- 24.Fritscher-Ravens A, Sriram PV, Krause C, et al. Detection of pancreatic metastases by EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:65–70. doi: 10.1067/mge.2001.111771. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 26.Rader AE, Avery A, Wait CL, et al. Fine-needle aspiration biopsy diagnosis of gastrointestinal stromal tumors using morphology, immunocytochemistry, and mutational analysis of c-kit. Cancer Cytopathol. 2001;93:269–75. doi: 10.1002/cncr.9041. [DOI] [PubMed] [Google Scholar]
- 27.Gu M, Ghafari S, Nguyen PT, et al. Cytologic diagnosis of gastrointestinal stromal tumors of the stomach by endoscopic ultrasound-guided fine-needle aspiration biopsy: Cytomorphologic and immunohistochemical study of 12 cases. Diagn Cytopathol. 2001;25:343–50. doi: 10.1002/dc.10003. [DOI] [PubMed] [Google Scholar]
- 28.Jhala D, Eloubeidi M, Chhieng DC, et al. Fine needle aspiration biopsy of the islet cell tumor of pancreas: A comparison between computerized axial tomography and endoscopic ultrasound-guided fine needle aspiration biopsy. Ann Diagn Pathol. 2002;6:106–12. doi: 10.1053/adpa.2002.30613. [DOI] [PubMed] [Google Scholar]
- 29.Wiersema MJ, Levy MJ, Harewood GC, et al. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc. 2002;56:275–8. doi: 10.1016/s0016-5107(02)70193-4. [DOI] [PubMed] [Google Scholar]
- 30.Adler DG, Witt B, Chadwick B, et al. Pathologic evaluation of a new endoscopic ultrasound needle designed to obtain core tissue samples: A pilot study. Endosc Ultrasound. 2016;5:178–83. doi: 10.4103/2303-9027.183976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritscher-Ravens A, Schirrow L, Pothmann W, et al. Critical care transesophageal endosonography and guided fine-needle aspiration for diagnosis and management of posterior mediastinitis. Crit Care Med. 2003;31:126–32. doi: 10.1097/00003246-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Van Dam J. Endosonographically guided fine-needle aspiration puncture of paraesophagogastric mass lesions: Preliminary results. Gastrointest Endosc. 1995;41:274–5. [PubMed] [Google Scholar]
- 33.Brandwein SL, Farrell JJ, Centeno BA, et al. Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointest Endosc. 2001;53:722–7. doi: 10.1067/mge.2001.114783. [DOI] [PubMed] [Google Scholar]
- 34.Gress FG, Barawi M, Kim D, et al. Preoperative localization of a neuroendocrine tumor of the pancreas with EUS-guided fine needle tattooing. Gastrointest Endosc. 2002;55:594–7. doi: 10.1067/mge.2002.122580. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson BC, Waxman I, Parmar K, et al. Endoscopic ultrasound-guided gallbladder bile aspiration in idiopathic pancreatitis carries a significant risk of bile peritonitis. Pancreatology. 2002;2:26–9. doi: 10.1159/000049444. [DOI] [PubMed] [Google Scholar]
- 36.Imaizumi H, Irisawa A. Preliminary experience of a prototype forward-viewing curved linear array echoendoscope in a training phantom model. Dig Endosc. 2010;22(Suppl 1):S123–7. doi: 10.1111/j.1443-1661.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- 37.Irions EL, Sharma N, Romagnuolo J, et al. Su1402 initial experience with the echotip procore needle for endoscopic ultrasound (EUS) guided-diagnosis of mass lesions. Gastrointest Endosc. 2011;73:AB255. [Google Scholar]
- 38.Tsuchida K, Iwashita T, Shibukawa G, et al. Su1359 histological diagnostic ability of endoscopic ultrasound-guided fine needle aspiration using novel core biopsy needle for solid tumors of the pancreas: A retrospective multicenter study. Gastrointest Endosc. 2017;85:AB349–50. [Google Scholar]
- 39.Wiersema MJ, Wiersema LM, Khusro Q, et al. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc. 1994;40:199–206. doi: 10.1016/s0016-5107(94)70167-9. [DOI] [PubMed] [Google Scholar]
- 40.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171–7. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 41.Gleeson FC, Clayton AC, Zhang L, et al. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437–40. doi: 10.1016/j.cgh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 43.DeWitt J, Cho CM, Lin J, et al. Comparison of EUS-guided tissue acquisition using two different 19-gauge core biopsy needles: A multicenter, prospective, randomized, and blinded study. Endosc Int Open. 2015;3:E471–8. doi: 10.1055/s-0034-1392222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weston BR, Bhutani MS. Optimizing diagnostic yield for EUS-guided sampling of solid pancreatic lesions: A technical review. Gastroenterol Hepatol (N Y) 2013;9:352–63. [PMC free article] [PubMed] [Google Scholar]
- 45.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 46.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline – March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 47.Vilmann P, Khattar S, Hancke S. Endoscopic ultrasound examination of the upper gastrointestinal tract using a curved-array transducer. A preliminary report. Surg Endosc. 1991;5:79–82. doi: 10.1007/BF00316842. [DOI] [PubMed] [Google Scholar]
- 48.Voermans RP, Eisendrath P, Bruno MJ, et al. Initial evaluation of a novel prototype forward-viewing US endoscope in transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2007;66:1013–7. doi: 10.1016/j.gie.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 49.Trevino JM, Varadarajulu S. Initial experience with the prototype forward-viewing echoendoscope for therapeutic interventions other than pancreatic pseudocyst drainage (with videos) Gastrointest Endosc. 2009;69:361–5. doi: 10.1016/j.gie.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 50.De Lusong MA, Shah JN, Soetikno R, et al. Treatment of a completely obstructed colonic anastomotic stricture by using a prototype forward-array echoendoscope and facilitated by SpyGlass (with videos) Gastrointest Endosc. 2008;68:988–92. doi: 10.1016/j.gie.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 51.Eloubeidi MA. Initial evaluation of the forward-viewing echoendoscope prototype for performing fine-needle aspiration, tru-cut biopsy, and celiac plexus neurolysis. J Gastroenterol Hepatol. 2011;26:63–7. doi: 10.1111/j.1440-1746.2010.06409.x. [DOI] [PubMed] [Google Scholar]
- 52.Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39–45. doi: 10.1055/s-0033-1344895. [DOI] [PubMed] [Google Scholar]
- 53.Hikichi T, Irisawa A, Takagi T, et al. An electronic radial scanning echoendoscope is superior to a mechanical radial scanning echoendoscope in ultrasound image quality for gastrointestinal tract and pancreaticobiliary lesions. Fukushima J Med Sci. 2010;56:99–106. doi: 10.5387/fms.56.99. [DOI] [PubMed] [Google Scholar]
- 54.ASGE Technology Committee, Tierney WM, Adler DG, et al. Echoendoscopes. Gastrointest Endosc. 2007;66:435–42. doi: 10.1016/j.gie.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 55.ASGE Technology Committee, Murad FM, Komanduri S, et al. Echoendoscopes. Gastrointest Endosc. 2015;82:189–202. doi: 10.1016/j.gie.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 56.Friedberg SR, Lachter J. Endoscopic ultrasound: Current roles and future directions. World J Gastrointest Endosc. 2017;9:499–505. doi: 10.4253/wjge.v9.i10.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Săftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–94. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 58.Seicean A, Badea R, Stan-Iuga R, et al. The added value of real-time harmonics contrast-enhanced endoscopic ultrasonography for the characterisation of pancreatic diseases in routine practice. J Gastrointestin Liver Dis. 2010;19:99–104. [PubMed] [Google Scholar]
- 59.Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–60. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Roy AK, Kim M, Hawes R, et al. 196 changing trends in tissue acquisition in pancreatic diseases. Gastrointest Endosc. 2013;77:AB134. [Google Scholar]
- 61.Möller K, Papanikolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–9. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: A comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–61. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 63.Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: A large single-center experience. Gastrointest Endosc. 1999;50:786–91. doi: 10.1016/s0016-5107(99)70159-8. [DOI] [PubMed] [Google Scholar]
- 64.Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–75. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 65.Khalid A, Nodit L, Zahid M, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 66.Alomari AK, Ustun B, Aslanian HR, et al. Endoscopic ultrasound-guided fine-needle aspiration diagnosis of secondary tumors involving the pancreas: An institution's experience. Cyto journal. 2016;13:1. doi: 10.4103/1742-6413.173585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, Salem R, Aslanian H, et al. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069–73. doi: 10.1111/j.1572-0241.2004.30223.x. [DOI] [PubMed] [Google Scholar]
- 69.Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–13. doi: 10.1016/s1542-3565(04)00005-9. [DOI] [PubMed] [Google Scholar]
- 70.Singh P, Mukhopadhyay P, Bhatt B, et al. Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: Results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367–73. doi: 10.1097/MCG.0b013e318167b8cc. [DOI] [PubMed] [Google Scholar]
- 71.Ginès A, Pellise M, Fernández-Esparrach G, et al. Endoscopic ultrasonography in patients with large gastric folds at endoscopy and biopsies negative for malignancy: Predictors of malignant disease and clinical impact. Am J Gastroenterol. 2006;101:64–9. doi: 10.1111/j.1572-0241.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 72.Thomas T, Kaye PV, Ragunath K, et al. Endoscopic-ultrasound-guided mural trucut biopsy in the investigation of unexplained thickening of esophagogastric wall. Endoscopy. 2009;41:335–9. doi: 10.1055/s-0029-1214470. [DOI] [PubMed] [Google Scholar]
- 73.Aithal GP, Anagnostopoulos GK, Kaye P. EUS-guided trucut mural biopsies in the investigation of unexplained thickening of the esophagogastric wall. Gastrointest Endosc. 2005;62:624–9. doi: 10.1016/j.gie.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Marsman WA, Brink MA, Bergman JJ, et al. Potential impact of EUS-FNA staging of proximal lymph nodes in patients with distal esophageal carcinoma. Endoscopy. 2006;38:825–9. doi: 10.1055/s-2006-944611. [DOI] [PubMed] [Google Scholar]
- 75.Pech O, May A, Günter E, et al. The impact of endoscopic ultrasound and computed tomography on the TNM staging of early cancer in Barrett's esophagus. Am J Gastroenterol. 2006;101:2223–9. doi: 10.1111/j.1572-0241.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 76.Gress FG, Savides TJ, Sandler A, et al. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: A comparison study. Ann Intern Med. 1997;127:604–12. doi: 10.7326/0003-4819-127-8_part_1-199710150-00004. [DOI] [PubMed] [Google Scholar]
- 77.Fu K, Eloubeidi MA, Jhala NC, et al. Diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration biopsy – A potential pitfall. Ann Diagn Pathol. 2002;6:294–301. doi: 10.1053/adpa.2002.35741. [DOI] [PubMed] [Google Scholar]
- 78.Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–5. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 79.Catalano MF, Rosenblatt ML, Chak A, et al. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal masses of unknown origin. Am J Gastroenterol. 2002;97:2559–65. doi: 10.1111/j.1572-0241.2002.06023.x. [DOI] [PubMed] [Google Scholar]
- 80.Patil R, Ona MA, Papafragkakis C, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of adrenal lesions. Ann Gastroenterol. 2016;29:307–11. doi: 10.20524/aog.2016.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeWitt J, Alsatie M, LeBlanc J, et al. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy. 2007;39:65–71. doi: 10.1055/s-2006-945042. [DOI] [PubMed] [Google Scholar]
- 82.Eloubeidi MA, Varadarajulu S, Eltoum I, et al. Transgastric endoscopic ultrasound-guided fine-needle aspiration biopsy and flow cytometry of suspected lymphoma of the spleen. Endoscopy. 2006;38:617–20. doi: 10.1055/s-2005-921111. [DOI] [PubMed] [Google Scholar]
- 83.Levy MJ, Abu Dayyeh BK, Fujii LL, et al. Detection of peritoneal carcinomatosis by EUS fine-needle aspiration: Impact on staging and resectability (with videos) Gastrointest Endosc. 2015;81:1215–24. doi: 10.1016/j.gie.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 84.Levy MJ, Gleeson FC, Zhang L. Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:246–8. doi: 10.1016/j.cgh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 85.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated January 2017. Endoscopy. 2017;49:695–714. doi: 10.1055/s-0043-109021. [DOI] [PubMed] [Google Scholar]
- 86.Bhutani MS, Logroño R. Endoscopic ultrasound-guided fine-needle aspiration cytology for diagnosis above and below the diaphragm. J Clin Ultrasound. 2005;33:401–11. doi: 10.1002/jcu.20149. [DOI] [PubMed] [Google Scholar]
- 87.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 89.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20–6. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki R, Irisawa A, Bhutani MS, et al. An automated spring-loaded needle for endoscopic ultrasound-guided abdominal paracentesis in cancer patients. World J Gastrointest Endosc. 2014;6:55–9. doi: 10.4253/wjge.v6.i2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki R, Thosani N, Annangi S, et al. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: A systematic review and meta-analysis. Pancreatology. 2014;14:380–4. doi: 10.1016/j.pan.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Thosani N, Thosani S, Qiao W, et al. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: A systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756–66. doi: 10.1007/s10620-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alvarez-Sánchez MV, Jenssen C, Faiss S, et al. Interventional endoscopic ultrasonography: An overview of safety and complications. Surg Endosc. 2014;28:712–34. doi: 10.1007/s00464-013-3260-5. [DOI] [PubMed] [Google Scholar]
- 95.Jenssen C, Faiss S, Nürnberg D. Complications of endoscopic ultrasound and endoscopic ultrasound-guided interventions – Results of a survey among german centers. Z Gastroenterol. 2008;46:1177–84. doi: 10.1055/s-2008-1027334. [DOI] [PubMed] [Google Scholar]
- 96.Dietrich CF, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part I: Endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis. 2015;7:E311–25. doi: 10.3978/j.issn.2072-1439.2015.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: It's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puri R, Mangla R, Eloubeidi M, et al. Diagnostic yield of EUS-guided FNA and cytology in suspected tubercular intra-abdominal lymphadenopathy. Gastrointest Endosc. 2012;75:1005–10. doi: 10.1016/j.gie.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Linghu E. Endoscopic ultrasound-guided fine-needle aspiration can differentiate between tuberculosis and sarcoidosis. Endoscopy. 2012;44:438. doi: 10.1055/s-0031-1291669. [DOI] [PubMed] [Google Scholar]
- 100.von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs.conventional bronchoscopy for the diagnosis of sarcoidosis: The GRANULOMA randomized clinical trial. JAMA. 2013;309:2457–64. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 101.Gnass M, Szlubowski A, Soja J, et al. Comparison of conventional and ultrasound-guided needle biopsy techniques in the diagnosis of sarcoidosis: A randomized trial. Pol Arch Med Wewn. 2015;125:321–8. doi: 10.20452/pamw.2828. [DOI] [PubMed] [Google Scholar]
- 102.Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: Feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397–404. doi: 10.1038/ajg.2011.350. [DOI] [PubMed] [Google Scholar]
- 103.Gimeno-García AZ, Elwassief A, Paquin SC, et al. Endoscopic ultrasound-guided fine needle aspiration cytology and biopsy in the evaluation of lymphoma. Endosc Ultrasound. 2012;1:17–22. doi: 10.7178/eus.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poincloux L, André M, Darcha C, et al. Usefulness of EUS-guided fine needle aspiration biopsy in the diagnosis of suspected or recurring lymphoproliferative disorders. Surg Oncol. 2016;25:459–65. doi: 10.1016/j.suronc.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 105.Silvestri GA, Hoffman BJ, Bhutani MS, et al. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg. 1996;61:1441–5. doi: 10.1016/0003-4975(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 106.Bhutani MS. Emerging indications for interventional endoscopic ultrasonography. Endoscopy. 2003;35:S45–8. doi: 10.1055/s-2003-41533. [DOI] [PubMed] [Google Scholar]
- 107.Bhutani MS. Transesophageal endoscopic ultrasound-guided mediastinal lymph node aspiration: Does the end justify the means? Chest. 2000;117:298–301. doi: 10.1378/chest.117.2.298. [DOI] [PubMed] [Google Scholar]
- 108.Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in Cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Eur Respir J. 2015;46:40–60. doi: 10.1183/09031936.00064515. [DOI] [PubMed] [Google Scholar]
- 109.Zhao H, Xie Z, Zhou ZL, et al. Diagnostic value of endobronchial ultrasound-guided transbronchial needle aspiration in intrapulmonary lesions. Chin Med J (Engl) 2013;126:4312–5. [PubMed] [Google Scholar]
- 110.Vigliar E, Malapelle U, Bellevicine C, et al. Outsourcing cytological samples to a referral laboratory for EGFR testing in non-small cell lung cancer: Does theory meet practice? Cytopathology. 2015;26:312–7. doi: 10.1111/cyt.12221. [DOI] [PubMed] [Google Scholar]
- 111.Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168–76. doi: 10.1016/j.gie.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sadeghi A, Mohamadnejad M, Islami F, et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest Endosc. 2016;83:290–80. doi: 10.1016/j.gie.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 113.Onda S, Ogura T, Kurisu Y, et al. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Therap Adv Gastroenterol. 2016;9:302–12. doi: 10.1177/1756283X15625584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–8. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Topazian M. Endoscopic ultrasonography in the evaluation of indeterminate biliary strictures. Clin Endosc. 2012;45:328–30. doi: 10.5946/ce.2012.45.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El Chafic AH, Dewitt J, Leblanc JK, et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883–9. doi: 10.1055/s-0033-1344760. [DOI] [PubMed] [Google Scholar]
- 117.Yamabe A, Irisawa A, Bhutani MS, et al. Usefulness of endoscopic ultrasound-guided fine-needle aspiration with a forward-viewing and curved linear-array echoendoscope for small gastrointestinal subepithelial lesions. Endosc Int Open. 2015;3:E161–4. doi: 10.1055/s-0034-1391671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turhan N, Aydog G, Ozin Y, et al. Endoscopic ultrasonography-guided fine-needle aspiration for diagnosing upper gastrointestinal submucosal lesions: A prospective study of 50 cases. Diagn Cytopathol. 2011;39:808–17. doi: 10.1002/dc.21464. [DOI] [PubMed] [Google Scholar]
- 119.El Chafic AH, Loren D, Siddiqui A, et al. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2017;86:510–5. doi: 10.1016/j.gie.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 120.Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–90. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shimodaira Y, Elimova E, Shiozaki H, et al. Accuracy of EUS-FNA for distant regional lymph nodes in the initial staging of esophageal cancer (EC) Journal of Clinical Oncology. 2015;33:15_suppl–4064. [Google Scholar]
- 122.Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751–7. doi: 10.1067/mge.2001.112741. [DOI] [PubMed] [Google Scholar]
- 123.tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859–62. doi: 10.1067/mge.2002.124557. [DOI] [PubMed] [Google Scholar]
- 124.Saraireh HA, Bilal M, Singh S. Role of endoscopic ultrasound in liver disease: Where do we stand in 2017? World J Hepatol. 2017;9:1013–21. doi: 10.4254/wjh.v9.i24.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fritscher-Ravens A, Mylonaki M, Pantes A, et al. Endoscopic ultrasound-guided biopsy for the diagnosis of focal lesions of the spleen. Am J Gastroenterol. 2003;98:1022–7. doi: 10.1111/j.1572-0241.2003.07399.x. [DOI] [PubMed] [Google Scholar]
- 126.Othman MO, Davis B, Saroseik I, et al. EUS-guided FNA biopsy of the muscularis propria of the antrum in patients with gastroparesis is feasible and safe. Gastrointest Endosc. 2016;83:327–33. doi: 10.1016/j.gie.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 127.Fritscher-Ravens A, Sriram PV, Pothman WP, et al. Bedside endosonography and endosonography-guided fine-needle aspiration in critically ill patients: A way out of the deadlock? Endoscopy. 2000;32:425–7. doi: 10.1055/s-2000-9001. [DOI] [PubMed] [Google Scholar]
- 128.Varadarajulu S, Eloubeidi MA, Wilcox CM. The concept of bedside EUS. Gastrointest Endosc. 2008;67:1180–4. doi: 10.1016/j.gie.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Mohamadnejad M, Leblanc JK, Sherman S, et al. Bedside endoscopic ultrasound in critically ill patients. Diagn Ther Endosc 2011. 2011 doi: 10.1155/2011/529791. 529791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kato K, Kamada H, Fujimori T, et al. Molecular biologic approach to the diagnosis of pancreatic carcinoma using specimens obtained by EUS-guided fine needle aspiration. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/243524. 243524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jani BS, Rzouq F, Saligram S, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: A systematic review of technical and procedural variables. N Am J Med Sci. 2016;8:1–11. doi: 10.4103/1947-2714.175185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–8. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 133.Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–4. doi: 10.1016/s0016-5107(01)70082-x. [DOI] [PubMed] [Google Scholar]
- 134.Barthet M, Gasmi M, Boustiere C, et al. EUS training in a live pig model: Does it improve echo endoscope hands-on and trainee competence? Endoscopy. 2007;39:535–9. doi: 10.1055/s-2007-966336. [DOI] [PubMed] [Google Scholar]
- 135.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–7. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 136.Shahidi N, Ou G, Lam E, et al. When trainees reach competency in performing endoscopic ultrasound: A systematic review. Endosc Int Open. 2017;5:E239–43. doi: 10.1055/s-0043-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: A combined approach in the evaluation of mediastinal lesions. Endoscopy. 2005;37:833–9. doi: 10.1055/s-2005-870276. [DOI] [PubMed] [Google Scholar]
- 138.Pagano N, Arosio M, Romeo F, et al. Balanced propofol sedation in patients undergoing EUS-FNA: A pilot study to assess feasibility and safety. Diagn Ther Endosc 2011. 2011 doi: 10.1155/2011/542159. 542159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ootaki C, Stevens T, Vargo J, et al. Does general anesthesia increase the diagnostic yield of endoscopic ultrasound-guided fine needle aspiration of pancreatic masses? Anesthesiology. 2012;117:1044–50. doi: 10.1097/ALN.0b013e31826e0590. [DOI] [PubMed] [Google Scholar]
- 140.Haghighi M, Packey C, Gonda TA. Endoscopic ultrasonography with fine-needle aspiration: New techniques for interpretation of endoscopic ultrasonography cytology and histology specimens. Gastrointest Endosc Clin N Am. 2017;27:601–14. doi: 10.1016/j.giec.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 141.Maslekar S, Gardiner A, Hughes M, et al. Randomized clinical trial of entonox versus midazolam-fentanyl sedation for colonoscopy. Br J Surg. 2009;96:361–8. doi: 10.1002/bjs.6467. [DOI] [PubMed] [Google Scholar]
- 142.Wang CX, Wang J, Chen YY, et al. Randomized controlled study of the safety and efficacy of nitrous oxide-sedated endoscopic ultrasound-guided fine needle aspiration for digestive tract diseases. World J Gastroenterol. 2016;22:10242–8. doi: 10.3748/wjg.v22.i46.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paquin SC, Gariépy G, Lepanto L, et al. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–1. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 144.Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–3. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 145.Rastogi A, Wani S, Gupta N, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58–64. doi: 10.1016/j.gie.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 146.Wani S, Early D, Kunkel J, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: A prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328–35. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 147.Abe Y, Kawakami H, Oba K, et al. Effect of a stylet on a histological specimen in EUS-guided fine-needle tissue acquisition by using 22-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2015;82:837–440. doi: 10.1016/j.gie.2015.03.1898. [DOI] [PubMed] [Google Scholar]
- 148.Gimeno-García AZ, Paquin SC, Gariépy G, et al. Comparison of endoscopic ultrasonography-guided fine-needle aspiration cytology results with and without the stylet in 3364 cases. Dig Endosc. 2013;25:303–7. doi: 10.1111/j.1443-1661.2012.01374.x. [DOI] [PubMed] [Google Scholar]
- 149.Bang JY, Ramesh J, Trevino J, et al. Objective assessment of an algorithmic approach to EUS-guided FNA and interventions. Gastrointest Endosc. 2013;77:739–44. doi: 10.1016/j.gie.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Puri R, Vilmann P, Săftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 151.Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–7. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 152.Aslanian HR. Su1533 EUS-needle aspirate yield of different media and needle type with stylet slow pull and syringe suction. Gastrointest Endosc. 2013;77:AB359. [Google Scholar]
- 153.Yamabe A, Irisawa A, Bhutani MS, et al. Efforts to improve the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic tumors. Endosc Ultrasound. 2016;5:225–32. doi: 10.4103/2303-9027.187862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Antonini F, Fuccio L, Fabbri C, et al. Endoscopic ultrasound-guided tissue acquisition of pancreatic masses with core biopsy needles using wet suction technique. Endosc Ultrasound. 2017;6:73–4. doi: 10.4103/2303-9027.200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 156.Villa NA, Berzosa M, Wallace MB, et al. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17–20. doi: 10.4103/2303-9027.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Storm AC, Lee LS. Endoscopic ultrasound-guided techniques for diagnosing pancreatic mass lesions: Can we do better? World J Gastroenterol. 2016;22:8658–69. doi: 10.3748/wjg.v22.i39.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mukai S, Itoi T, Ashida R, et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–7. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 160.Nakai Y, Isayama H, Chang KJ, et al. A pilot study of EUS-guided through-the-needle forceps biopsy (with video) Gastrointest Endosc. 2016;84:158–62. doi: 10.1016/j.gie.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 161.Culp WC, McCowan TC, Goertzen TC, et al. Relative ultrasonographic echogenicity of standard, dimpled, and polymeric-coated needles. J Vasc Interv Radiol. 2000;11:351–8. doi: 10.1016/s1051-0443(07)61429-8. [DOI] [PubMed] [Google Scholar]
- 162.Adler DG, Conway JD, Coffie JM, et al. EUS accessories. Gastrointest Endosc. 2007;66:1076–81. doi: 10.1016/j.gie.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 163.Tang SJ, Vilmann AS, Saftoiu A, et al. EUS needle identification comparison and evaluation study (with videos) Gastrointest Endosc. 2016;84:424–3300. doi: 10.1016/j.gie.2016.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Witt BL, Adler DG, Hilden K, et al. A comparative needle study: EUS-FNA procedures using the HD ProCore™ and EchoTip® 22-gauge needle types. Diagn Cytopathol. 2013;41:1069–74. doi: 10.1002/dc.22971. [DOI] [PubMed] [Google Scholar]
- 165.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 166.Vilmann P, Seicean A, Săftoiu A. Tips to overcome technical challenges in EUS-guided tissue acquisition. Gastrointest Endosc Clin N Am. 2014;24:109–24. doi: 10.1016/j.giec.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 167.Yusuf TE, Ho S, Pavey DA, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: A multicenter experience. Endoscopy. 2009;41:445–8. doi: 10.1055/s-0029-1214643. [DOI] [PubMed] [Google Scholar]
- 168.Muniraj T, Aslanian HR. New developments in endoscopic ultrasound tissue acquisition. Gastrointest Endosc Clin N Am. 2017;27:585–99. doi: 10.1016/j.giec.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 169.Carrara S, Anderloni A, Jovani M, et al. A prospective randomized study comparing 25-G and 22-G needles of a new platform for endoscopic ultrasound-guided fine needle aspiration of solid masses. Dig Liver Dis. 2016;48:49–54. doi: 10.1016/j.dld.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 170.Vilmann P, Săftoiu A, Hollerbach S, et al. Multicenter randomized controlled trial comparing the performance of 22 gauge versus 25 gauge EUS-FNA needles in solid masses. Scand J Gastroenterol. 2013;48:877–83. doi: 10.3109/00365521.2013.799222. [DOI] [PubMed] [Google Scholar]
- 171.Camellini L, Carlinfante G, Azzolini F, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy. 2011;43:709–15. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 172.Lee JK, Lee KT, Choi ER, et al. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol. 2013;48:752–7. doi: 10.3109/00365521.2013.786127. [DOI] [PubMed] [Google Scholar]
- 173.Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: A prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–52. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Siddiqui UD, Rossi F, Rosenthal LS, et al. EUS-guided FNA of solid pancreatic masses: A prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–7. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 175.Lee JH, Stewart J, Ross WA, et al. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274–81. doi: 10.1007/s10620-009-0906-1. [DOI] [PubMed] [Google Scholar]
- 176.Facciorusso A, Stasi E, Di Maso M, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 gauge needles: A meta-analysis. United European Gastroenterol J. 2017;5:846–53. doi: 10.1177/2050640616680972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu MM, Jia HY, Yan LL, et al. Comparison of two different size needles in endoscopic ultrasound-guided fine-needle aspiration for diagnosing solid pancreatic lesions: A meta-analysis of prospective controlled trials. Medicine (Baltimore) 2017;96:e5802. doi: 10.1097/MD.0000000000005802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Madhoun MF, Wani SB, Rastogi A, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 179.Affolter KE, Schmidt RL, Matynia AP, et al. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: A systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–34. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 180.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 181.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–90. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 182.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–43. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 183.Stavropoulos SN, Im GY, Jlayer Z, et al. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc. 2012;75:310–8. doi: 10.1016/j.gie.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 184.Diehl DL, Johal AS, Khara HS, et al. Endoscopic ultrasound-guided liver biopsy: A multicenter experience. Endosc Int Open. 2015;3:E210–5. doi: 10.1055/s-0034-1391412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pineda JJ, Diehl DL, Miao CL, et al. EUS-guided liver biopsy provides diagnostic samples comparable with those via the percutaneous or transjugular route. Gastrointest Endosc. 2016;83:360–5. doi: 10.1016/j.gie.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 186.Kim GH, Cho YK, Kim EY, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347–54. doi: 10.3109/00365521.2013.867361. [DOI] [PubMed] [Google Scholar]
- 187.Muniraj T, Sah RP, Chari ST. Autoimmune pancreatitis: An update. Pancreatitis: Medical and Surgical Management. John Wiley & Sons; 2017. pp. 152–60. [Google Scholar]
- 188.Eloubeidi MA, Mehra M, Bean SM. EUS-guided 19-gauge trucut needle biopsy for diagnosis of lymphoma missed by EUS-guided FNA. Gastrointest Endosc. 2007;65:937–9. doi: 10.1016/j.gie.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 189.Karadsheh Z, Al-Haddad M. Endoscopic ultrasound guided fine needle tissue acquisition: Where we stand in 2013? World J Gastroenterol. 2014;20:2176–85. doi: 10.3748/wjg.v20.i9.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest Endosc. 2016;84:1034–9. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 191.DiMaio CJ, Kolb JM, Benias PC, et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): Results of a large North American Multicenter Study. Endosc Int Open. 2016;4:E974–9. doi: 10.1055/s-0042-112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mehmood S, Jahan A, Loya A, et al. Onsite cytopathology evaluation and ancillary studies beneficial in EUS-FNA of pancreatic, mediastinal, intra-abdominal, and submucosal lesions. Diagn Cytopathol. 2015;43:278–86. doi: 10.1002/dc.23207. [DOI] [PubMed] [Google Scholar]
- 193.Ramesh J, Varadarajulu S. How can we get the best results with endoscopic ultrasound-guided fine needle aspiration? Clin Endosc. 2012;45:132–7. doi: 10.5946/ce.2012.45.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen J, Yang R, Lu Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J Cancer Res Clin Oncol. 2012;138:1433–41. doi: 10.1007/s00432-012-1268-1. [DOI] [PubMed] [Google Scholar]
- 195.Krishnan K, Dalal S, Nayar R, et al. Rapid on-site evaluation of endoscopic ultrasound core biopsy specimens has excellent specificity and positive predictive value for gastrointestinal lesions. Dig Dis Sci. 2013;58:2007–12. doi: 10.1007/s10620-013-2613-1. [DOI] [PubMed] [Google Scholar]
- 196.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–81. doi: 10.1007/s00535-012-0695-8. [DOI] [PubMed] [Google Scholar]
- 197.Schmidt RL, Walker BS, Howard K, et al. Rapid on-site evaluation reduces needle passes in endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions: A risk-benefit analysis. Dig Dis Sci. 2013;58:3280–6. doi: 10.1007/s10620-013-2750-6. [DOI] [PubMed] [Google Scholar]
- 198.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 199.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: A cost and compensation analysis. Cancer Cytopathol. 2001;93:319–22. doi: 10.1002/cncr.9046. [DOI] [PubMed] [Google Scholar]
- 200.Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: A single-center experience. Dig Endosc. 2011;23(Suppl 1):34–8. doi: 10.1111/j.1443-1661.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 201.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 202.Costache MI, Iordache S, Karstensen JG, et al. Endoscopic ultrasound-guided fine needle aspiration: From the past to the future. Endosc Ultrasound. 2013;2:77–85. doi: 10.4103/2303-9027.117691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Iqbal S, Friedel D, Gupta M, et al. Endoscopic-ultrasound-guided fine-needle aspiration and the role of the cytopathologist in solid pancreatic lesion diagnosis. Patholog Res Int 2012. 2012:317167. doi: 10.1155/2012/317167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. doi: 10.1186/1742-6413-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 206.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 207.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 208.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 209.Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: An experience of a tertiary hospital. Diagn Cytopathol. 2013;41:1075–80. doi: 10.1002/dc.23047. [DOI] [PubMed] [Google Scholar]
- 210.Lee LS, Nieto J, Watson RR, et al. Tu1618 randomized trial comparing diagnostic yield of cytologist-guided versus 7 passes for EUS-FNA of pancreatic masses. Gastrointest Endosc. 2015;81:AB532. doi: 10.1111/den.12594. [DOI] [PubMed] [Google Scholar]
- 211.Ganc RL, Carbonari AP, Colaiacovo R, et al. Rapid on-site cytopathological examination (ROSE) performed by endosonagraphers and its improvement in the diagnosis of pancreatic solid lesions. Acta Cir Bras. 2015;30:503–8. doi: 10.1590/S0102-865020150070000009. [DOI] [PubMed] [Google Scholar]
- 212.Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000;95:2248–54. doi: 10.1111/j.1572-0241.2000.02310.x. [DOI] [PubMed] [Google Scholar]
- 213.Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–14. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 214.Stavropoulos SN, Abraham B, Friedel D, et al. Direct visualization of the wall of pancreatic cysts using the SPYGLASS optical probe: Feasibility and preliminary results. Gastrointest Endosc. 2009;69:AB377. [Google Scholar]
- 215.Samarasena JB, Nakai Y, Shinoura S, et al. EUS-guided, through-the-needle forceps biopsy: A novel tissue acquisition technique. Gastrointest Endosc. 2015;81:225–6. doi: 10.1016/j.gie.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 216.Coman RM, Schlachterman A, Esnakula AK, et al. EUS-guided, through-the-needle forceps: Clenching down the diagnosis. Gastrointest Endosc. 2016;84:372–3. doi: 10.1016/j.gie.2016.03.785. [DOI] [PubMed] [Google Scholar]
- 217.Shakhatreh MH, Naini SR, Brijbassie AA, et al. Use of a novel through-the-needle biopsy forceps in endoscopic ultrasound. Endosc Int Open. 2016;4:E439–42. doi: 10.1055/s-0042-101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo . Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 219.Kiesslich R, Neurath MF. Endomicroscopy is born – Do we still need the pathologist? Gastrointest Endosc. 2007;66:150–3. doi: 10.1016/j.gie.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 220.Bhutani MS, Koduru P, Joshi V, et al. EUS-guided needle-based confocal laser endomicroscopy: A novel technique with emerging applications. Gastroenterol Hepatol (N Y) 2015;11:235–40. [PMC free article] [PubMed] [Google Scholar]
- 221.Deprez PH. Future directions in EUS-guided tissue acquisition. Gastrointest Endosc Clin N Am. 2014;24:143–9. doi: 10.1016/j.giec.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 222.Napoléon B, Lemaistre AI, Pujol B, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: Needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26–32. doi: 10.1055/s-0034-1390693. [DOI] [PubMed] [Google Scholar]
- 223.Joshi V. NCLE (needle-based confocal laser endomicroscopy) in evaluation of indeterminate pancreatic cystic lesions: A single-center experience. Am J Gastroenterol. 2014;109(suppl 2):S101–23. [Google Scholar]
- 224.Krishna SG, Brugge WR, Dewitt JM, et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: An international external interobserver and intraobserver study (with videos) Gastrointest Endosc. 2017;86:644–5400. doi: 10.1016/j.gie.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 225.Kadayifci A, Atar M, Basar O, et al. Needle-based confocal laser endomicroscopy for evaluation of cystic neoplasms of the pancreas. Dig Dis Sci. 2017;62:1346–53. doi: 10.1007/s10620-017-4521-2. [DOI] [PubMed] [Google Scholar]
- 226.Karstensen JG, Cartana T, Klausen P, et al. Mo1430 pitfalls in the interpretation of pancreatic endoscopic ultrasound guided needle confocal LASER endomicroscopy. Gastrointest Endosc. 2014;79:AB433–34. [Google Scholar]
- 227.Giovannini M, Caillol F, Monges G, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy in solid pancreatic masses. Endoscopy. 2016;48:892–8. doi: 10.1055/s-0042-112573. [DOI] [PubMed] [Google Scholar]
- 228.Nakai Y, Shinoura S, Ahluwalia A, et al. In vivo visualization of epidermal growth factor receptor and survivin expression in porcine pancreas using endoscopic ultrasound guided fine needle imaging with confocal laser-induced endomicroscopy. J Physiol Pharmacol. 2012;63:577–80. [PubMed] [Google Scholar]
- 229.Samarasena JB, Tarnawski AS, Shinoura S, et al. Mo1429 visualization of the gastric submucosal and myenteric neuronal network using endoscopic ultrasound (EUS) guided needle-based confocal LASER induced endomicroscopy and a novel EUS guided through-the-needle biopsy technique. Gastrointest Endosc. 2014;79:AB433. [Google Scholar]
- 230.Li H, Li Y, Cui L, et al. Monitoring pancreatic carcinogenesis by the molecular imaging of cathepsin E in vivo using confocal laser endomicroscopy. PLoS One. 2014;9:e106566. doi: 10.1371/journal.pone.0106566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Guo J, Bhutani MS, Giovannini M, et al. Can endoscopic ultrasound-guided needle-based confocal laser endomicroscopy replace fine-needle aspiration for pancreatic and mediastinal diseases? Endosc Ultrasound. 2017;6:376–81. doi: 10.4103/eus.eus_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Popescu A, Săftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound. 2014;3:109–17. doi: 10.4103/2303-9027.123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kitano M, Sakamoto H, Kudo M. Contrast-enhanced endoscopic ultrasound. Dig Endosc. 2014;26(Suppl 1):79–85. doi: 10.1111/den.12179. [DOI] [PubMed] [Google Scholar]
- 234.Kitano M, Kamata K, Imai H, et al. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27(Suppl 1):60–7. doi: 10.1111/den.12454. [DOI] [PubMed] [Google Scholar]
- 235.Fusaroli P, Eloubeidi MA. Diagnosis of pancreatic cancer by contrast-harmonic endoscopic ultrasound (EUS): Complementary and not competitive with EUS-guided fine-needle aspiration. Endoscopy. 2014;46:380–1. doi: 10.1055/s-0034-1365425. [DOI] [PubMed] [Google Scholar]
- 236.Seicean A, Badea R, Moldovan-Pop A, et al. Harmonic contrast-enhanced endoscopic ultrasonography for the guidance of fine-needle aspiration in solid pancreatic masses. Ultraschall Med. 2017;38:174–82. doi: 10.1055/s-0035-1553496. [DOI] [PubMed] [Google Scholar]
- 237.Sugimoto M, Takagi T, Hikichi T, et al. Conventional versus contrast-enhanced harmonic endoscopic ultrasonography-guided fine-needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology. 2015;15:538–41. doi: 10.1016/j.pan.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 238.Hou X, Jin Z, Xu C, et al. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic lesions: A retrospective study. PLoS One. 2015;10:e0121236. doi: 10.1371/journal.pone.0121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ophir J, Céspedes I, Ponnekanti H, et al. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 240.Săftoiu A, Vilmann P, Gorunescu F, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: A multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 241.Mei M, Ni J, Liu D, et al. EUS elastography for diagnosis of solid pancreatic masses: A meta-analysis. Gastrointest Endosc. 2013;77:578–89. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 242.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. Quantitative endoscopic ultrasound elastography: An accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172–80. doi: 10.1053/j.gastro.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 243.Deprez PH. EUS elastography: Is it replacing or supplementing tissue acquisition? Gastrointest Endosc. 2013;77:590–2. doi: 10.1016/j.gie.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 244.Jafri M, Sachdev AH, Khanna L, et al. The role of real time endoscopic ultrasound guided elastography for targeting EUS-FNA of suspicious pancreatic masses: A Review of the literature and A single center experience. JOP. 2016;17:516–24. [PMC free article] [PubMed] [Google Scholar]
- 245.Laurell H, Bouisson M, Berthelemy P, et al. Identification of biomarkers of human pancreatic adenocarcinomas by expression profiling and validation with gene expression analysis in endoscopic ultrasound-guided fine needle aspiration samples. World J Gastroenterol. 2006;12:3344–51. doi: 10.3748/wjg.v12.i21.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bournet B, Pointreau A, Souque A, et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound-guided fine needle aspiration samples. Pancreatology. 2012;12:27–34. doi: 10.1016/j.pan.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 247.Ryozawa S, Iwano H, Taba K, et al. Genetic diagnosis of pancreatic cancer using specimens obtained by EUS-FNA. Dig Endosc. 2011;23(Suppl 1):43–5. doi: 10.1111/j.1443-1661.2011.01117.x. [DOI] [PubMed] [Google Scholar]
- 248.Mishra G. DNA analysis of cells obtained from endoscopic ultrasound-fine needle aspiration in pancreatic adenocarcinoma: Fool's gold, pandora's box, or holy grail? Am J Gastroenterol. 2006;101:2501–3. doi: 10.1111/j.1572-0241.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 249.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bournet B, Souque A, Senesse P, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with KRAS mutation assay to distinguish pancreatic cancer from pseudotumoral chronic pancreatitis. Endoscopy. 2009;41:552–7. doi: 10.1055/s-0029-1214717. [DOI] [PubMed] [Google Scholar]
- 253.Bournet B, Selves J, Grand D, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with a KRAS mutation assay using allelic discrimination improves the diagnosis of pancreatic cancer. J Clin Gastroenterol. 2015;49:50–6. doi: 10.1097/MCG.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 254.Ginestà MM, Mora J, Mayor R, et al. Genetic and epigenetic markers in the evaluation of pancreatic masses. J Clin Pathol. 2013;66:192–7. doi: 10.1136/jclinpath-2012-201123. [DOI] [PubMed] [Google Scholar]
- 255.Maluf-Filho F, Kumar A, Gerhardt R, et al. Kras mutation analysis of fine needle aspirate under EUS guidance facilitates risk stratification of patients with pancreatic mass. J Clin Gastroenterol. 2007;41:906–10. doi: 10.1097/MCG.0b013e31805905e9. [DOI] [PubMed] [Google Scholar]
- 256.Ogura T, Yamao K, Sawaki A, et al. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769–74. doi: 10.1016/j.gie.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 257.Pellisé M, Castells A, Ginès A, et al. Clinical usefulness of KRAS mutational analysis in the diagnosis of pancreatic adenocarcinoma by means of endosonography-guided fine-needle aspiration biopsy. Aliment Pharmacol Ther. 2003;17:1299–307. doi: 10.1046/j.1365-2036.2003.01579.x. [DOI] [PubMed] [Google Scholar]
- 258.Takahashi K, Yamao K, Okubo K, et al. Differential diagnosis of pancreatic cancer and focal pancreatitis by using EUS-guided FNA. Gastrointest Endosc. 2005;61:76–9. doi: 10.1016/s0016-5107(04)02224-2. [DOI] [PubMed] [Google Scholar]
- 259.Fuccio L, Hassan C, Laterza L, et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: A meta-analysis of prospective studies. Gastrointest Endosc. 2013;78:596–608. doi: 10.1016/j.gie.2013.04.162. [DOI] [PubMed] [Google Scholar]
- 260.Matsubayashi H. Role of K-ras mutation analysis in EUS-FNA samples obtained from pancreatic solid mass. J Clin Gastroenterol. 2015;49:173. doi: 10.1097/MCG.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 261.de Biase D, Visani M, Baccarini P, et al. Next generation sequencing improves the accuracy of KRAS mutation analysis in endoscopic ultrasound fine needle aspiration pancreatic lesions. PLoS One. 2014;9:e87651. doi: 10.1371/journal.pone.0087651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Altimari A, de Biase D, De Maglio G, et al. 454 next generation-sequencing outperforms allele-specific PCR, sanger sequencing, and pyrosequencing for routine KRAS mutation analysis of formalin-fixed, paraffin-embedded samples. Onco Targets Ther. 2013;6:1057–64. doi: 10.2147/OTT.S42369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bournet B, Buscail C, Muscari F, et al. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: Hopes and realities. Eur J Cancer. 2016;54:75–83. doi: 10.1016/j.ejca.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 264.Yang J, Li J, Zhu R, et al. K-ras mutational status in cytohistological tissue as a molecular marker for the diagnosis of pancreatic cancer: A systematic review and meta-analysis. Dis Markers. 2014;2014:573783. doi: 10.1155/2014/573783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Niedergethmann M, Rexin M, Hildenbrand R, et al. Prognostic implications of routine, immunohistochemical, and molecular staging in resectable pancreatic adenocarcinoma. Am J Surg Pathol. 2002;26:1578–87. doi: 10.1097/00000478-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 266.Itoi T, Takei K, Sofuni A, et al. Immunohistochemical analysis of p53 and MIB-1 in tissue specimens obtained from endoscopic ultrasonography-guided fine needle aspiration biopsy for the diagnosis of solid pancreatic masses. Oncol Rep. 2005;13:229–34. [PubMed] [Google Scholar]
- 267.Jahng AW, Reicher S, Chung D, et al. Staining for p53 and Ki-67 increases the sensitivity of EUS-FNA to detect pancreatic malignancy. World J Gastrointest Endosc. 2010;2:362–8. doi: 10.4253/wjge.v2.i11.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mu DQ, Wang GF, Peng SY. P53 protein expression and CA19.9 values in differential cytological diagnosis of pancreatic cancer complicated with chronic pancreatitis and chronic pancreatitis. World J Gastroenterol. 2003;9:1815–8. doi: 10.3748/wjg.v9.i8.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg. 2013;258:336–46. doi: 10.1097/SLA.0b013e3182827a65. [DOI] [PubMed] [Google Scholar]
- 270.Fabbri C, Gibiino G, Fornelli A, et al. Team work and cytopathology molecular diagnosis of solid pancreatic lesions. Dig Endosc. 2017;29:657–66. doi: 10.1111/den.12845. [DOI] [PubMed] [Google Scholar]
- 271.Salek C, Benesova L, Zavoral M, et al. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714–20. doi: 10.3748/wjg.v13.i27.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yamada S, Fujii T, Shimoyama Y, et al. SMAD4 expression predicts local spread and treatment failure in resected pancreatic cancer. Pancreas. 2015;44:660–4. doi: 10.1097/MPA.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 273.Hernandez YG, Lucas AL. MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions. World J Gastrointest Oncol. 2016;8:18–29. doi: 10.4251/wjgo.v8.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Matthaei H, Wylie D, Lloyd MB, et al. MiRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–24. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Visani M, Acquaviva G, Fiorino S, et al. Contribution of microRNA analysis to characterisation of pancreatic lesions: A review. J Clin Pathol. 2015;68:859–69. doi: 10.1136/jclinpath-2015-203246. [DOI] [PubMed] [Google Scholar]
- 277.D'Angelo E, Vicentini C, Agostini M, et al. MicroRNAs as tools and effectors for patient treatment in gastrointestinal carcinogenesis. Curr Drug Targets. 2015;16:383–92. doi: 10.2174/1389450116666141210091454. [DOI] [PubMed] [Google Scholar]
- 278.Malkhassian D, Dawson D, Wu H, et al. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol. 2013;108:1352. doi: 10.1038/ajg.2013.167. [DOI] [PubMed] [Google Scholar]
- 279.Liu H, Shi J, Anandan V, et al. Reevaluation and identification of the best immunohistochemical panel (pVHL, maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med. 2012;136:601–9. doi: 10.5858/arpa.2011-0326-OA. [DOI] [PubMed] [Google Scholar]
- 280.Chiba M, Imazu H, Kato M, et al. Novel quantitative analysis of the S100P protein combined with endoscopic ultrasound-guided fine needle aspiration cytology in the diagnosis of pancreatic adenocarcinoma. Oncol Rep. 2017;37:1943–52. doi: 10.3892/or.2017.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Deng H, Shi J, Wilkerson M, et al. Usefulness of S100P in diagnosis of adenocarcinoma of pancreas on fine-needle aspiration biopsy specimens. Am J Clin Pathol. 2008;129:81–8. doi: 10.1309/5D76NDE81LE8G545. [DOI] [PubMed] [Google Scholar]
- 282.Ezzat NE, Tahoun NS, Ismail YM. The role of S100P and IMP3 in the cytologic diagnosis of pancreatic adenocarcinoma. J Egypt Natl Canc Inst. 2016;28:229–34. doi: 10.1016/j.jnci.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 283.Furuhata A, Minamiguchi S, Shirahase H, et al. Immunohistochemical antibody panel for the differential diagnosis of pancreatic ductal carcinoma from gastrointestinal contamination and benign pancreatic duct epithelium in endoscopic ultrasound-guided fine-needle aspiration. Pancreas. 2017;46:531–8. doi: 10.1097/MPA.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 284.Kosarac O, Takei H, Zhai QJ, et al. S100P and XIAP expression in pancreatic ductal adenocarcinoma: Potential novel biomarkers as a diagnostic adjunct to fine needle aspiration cytology. Acta Cytol. 2011;55:142–8. doi: 10.1159/000320913. [DOI] [PubMed] [Google Scholar]
- 285.Agarwal B, Ludwig OJ, Collins BT, et al. Immunostaining as an adjunct to cytology for diagnosis of pancreatic adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1425–31. doi: 10.1016/j.cgh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 286.Baruch AC, Wang H, Staerkel GA, et al. Immunocytochemical study of the expression of mesothelin in fine-needle aspiration biopsy specimens of pancreatic adenocarcinoma. Diagn Cytopathol. 2007;35:143–7. doi: 10.1002/dc.20594. [DOI] [PubMed] [Google Scholar]
- 287.Jhala N, Jhala D, Vickers SM, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates: A translational research application. Am J Clin Pathol. 2006;126:572–9. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 288.Glass JP, Parasher G, Arias-Pulido H, et al. Mesothelin and GPR30 staining among a spectrum of pancreatic epithelial neoplasms. Int J Surg Pathol. 2011;19:588–96. doi: 10.1177/1066896911409575. [DOI] [PubMed] [Google Scholar]
- 289.McCarthy DM, Maitra A, Argani P, et al. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: Mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Appl Immunohistochem Mol Morphol. 2003;11:238–43. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 290.Dim DC, Jiang F, Qiu Q, et al. The usefulness of S100P, mesothelin, fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fine-needle aspiration. Diagn Cytopathol. 2014;42:193–9. doi: 10.1002/dc.21684. [DOI] [PubMed] [Google Scholar]
- 291.Horn A, Chakraborty S, Dey P, et al. Immunocytochemistry for MUC4 and MUC16 is a useful adjunct in the diagnosis of pancreatic adenocarcinoma on fine-needle aspiration cytology. Arch Pathol Lab Med. 2013;137:546–51. doi: 10.5858/arpa.2011-0229-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Talarico A, Boemo C, Doglioni C, et al. Mucin expression pattern in pancreatic diseases: Findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359. doi: 10.1038/ajg.2011.22. [DOI] [PubMed] [Google Scholar]
- 293.Wang Y, Gao J, Li Z, et al. Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound-guided fine-needle aspiration specimens of the pancreas. Int J Cancer. 2007;121:2716–22. doi: 10.1002/ijc.22997. [DOI] [PubMed] [Google Scholar]
- 294.Giorgadze TA, Peterman H, Baloch ZW, et al. Diagnostic utility of mucin profile in fine-needle aspiration specimens of the pancreas: An immunohistochemical study with surgical pathology correlation. Cancer. 2006;108:186–97. doi: 10.1002/cncr.21913. [DOI] [PubMed] [Google Scholar]
- 295.Chhieng DC, Benson E, Eltoum I, et al. MUC1 and MUC2 expression in pancreatic ductal carcinoma obtained by fine-needle aspiration. Cancer Cytopathol. 2003;99:365–71. doi: 10.1002/cncr.11857. [DOI] [PubMed] [Google Scholar]
- 296.Wachter DL, Schlabrakowski A, Hoegel J, et al. Diagnostic value of immunohistochemical IMP3 expression in core needle biopsies of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2011;35:873–7. doi: 10.1097/PAS.0b013e3182189223. [DOI] [PubMed] [Google Scholar]
- 297.Yantiss RK, Woda BA, Fanger GR, et al. KOC (K homology domain containing protein overexpressed in cancer): A novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol. 2005;29:188–95. doi: 10.1097/01.pas.0000149688.98333.54. [DOI] [PubMed] [Google Scholar]
- 298.Zhao H, Mandich D, Cartun RW, et al. Expression of K homology domain containing protein overexpressed in cancer in pancreatic FNA for diagnosing adenocarcinoma of pancreas. Diagn Cytopathol. 2007;35:700–4. doi: 10.1002/dc.20739. [DOI] [PubMed] [Google Scholar]
- 299.Andersson R, Aho U, Nilsson BI, et al. Gemcitabine chemoresistance in pancreatic cancer: Molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–6. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 300.Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–95. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 301.Greenhalf W, Ghaneh P, Neoptolemos JP, et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014;106:djt347. doi: 10.1093/jnci/djt347. [DOI] [PubMed] [Google Scholar]
- 302.Bhutani MS. Applications of EUS for the molecular characterization and treatment of gastrointestinal diseases – A review of the recent medical literature. Med Gen Med. 2005;7:18. [PMC free article] [PubMed] [Google Scholar]
- 303.Wallace MB, Hoffman BJ, Hawes RH, et al. Detection of lymph node micrometastases with EUS-FNA and real-time PCR. Gastrointest Endosc. 2002;55:AB79. [Google Scholar]
- 304.Pellisé M, Castells A, Ginès A, et al. Detection of lymph node micrometastases by gene promoter hypermethylation in samples obtained by endosonography- guided fine-needle aspiration biopsy. Clin Cancer Res. 2004;10:4444–9. doi: 10.1158/1078-0432.CCR-03-0600. [DOI] [PubMed] [Google Scholar]
- 305.Bhutani MS, Jones DV, Jr, Zwischenberger JB. Endoscopic ultrasound-guided fine-needle aspiration staging of lung cancer: Is it time to go beyond cytology? Chest. 2005;127:418–20. doi: 10.1378/chest.127.2.418. [DOI] [PubMed] [Google Scholar]
- 306.Ma X, Zhu L, Wu X, et al. Cell-free DNA provides a good representation of the tumor genome despite its biased fragmentation patterns. PLoS One. 2017;12:e0169231. doi: 10.1371/journal.pone.0169231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ma M, Zhu H, Zhang C, et al. “Liquid biopsy”-ctDNA detection with great potential and challenges. Ann Transl Med. 2015;3:235. doi: 10.3978/j.issn.2305-5839.2015.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 309.Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–16. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Catenacci DV, Chapman CG, Xu P, et al. Acquisition of portal venous circulating tumor cells from patients with pancreaticobiliary cancers by endoscopic ultrasound. Gastroenterology. 2015;149:1794–803.e4. doi: 10.1053/j.gastro.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]