In the article entitled, “Community Care for Diabetic Retinopathy and Glaucoma in India: A Panel Discussion,”[1] the authors have outlined crucial points for screening of diabetic retinopathy (DR) and glaucoma, which are among the most common causes of blindness and visual impairment in India and across the world. As ophthalmologists, one needs to know the demography of population involved, risk factors, clinical picture, diagnostic criteria, and management of these entities in detail. Here, we discuss the insights and practice guidelines pertaining to the above diseases.
Diabetes mellitus (DM) can lead to an array of systemic complications, out of which DR is the most common.[2] Other organ systems may be concomitantly involved as in diabetic nephropathy and neuropathy. The Asian population has witnessed a rampant growth of DM in recent years, especially India and China. The overall prevalence of DR is 35.4%, out of which proliferative DR is 7.2%, diabetic macular edema (DME) is 7.4%, and vision-threatening DR is 11.7%. The prevalence rates proportionately increase with the duration of diabetes, HbA1c levels, blood pressure, and cholesterol. Type 1 diabetics tend to have a higher prevalence of DR.[3] DR prevalence is higher in urban population (18%) when compared to rural (11%) as primordial prevention of DM has markedly decreased with the changes in diet and marked shift toward junk food while less health-care availability to rural areas leads to misjudgment of the disease burden.[4,5]
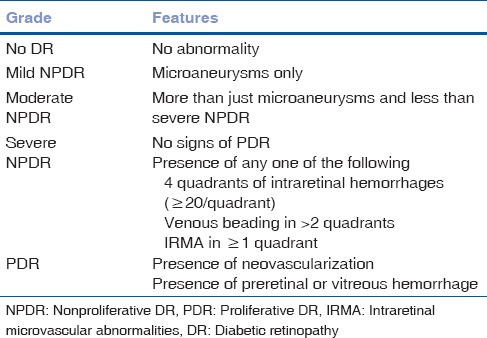
Most common cause of visual impairment in type 1 DM is vitreous hemorrhage and in type 2 DM is DME. About 20% of type 1 DM and 14%–25% of type 2 DM patients develop DME within 10 years of initial diagnosis.[6] Our aim should be not only to screen DR or DME but also to grade the disease and refer appropriately if needed. Our community-based approach should point toward educating the people about the modifiable and nonmodifiable risk factors of DR [Table 1], first visit to ophthalmologists and follow-up guidelines [Table 2] for different grades of the disease, and management options [Fig. 1] and prognosis of DR and DME. Apart from the diseased, physicians, endocrinologists, and nephrologists should also be made aware of the diagnosis and classification [Table 3] of DR,[7] and ophthalmologists should be well versed with performing 90 D biomicroscopy, optical coherence tomography, and fluorescein angiography in such patients. Correct treatment modality can provide marked visual gain and betterment of overall quality of life of the affected patients.
Table 1.
Risk factors for diabetic retinopathy
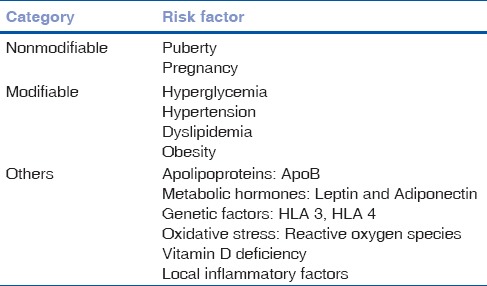
Table 2.
Follow up criteria for patients of diabetic retinopathy

Figure 1.
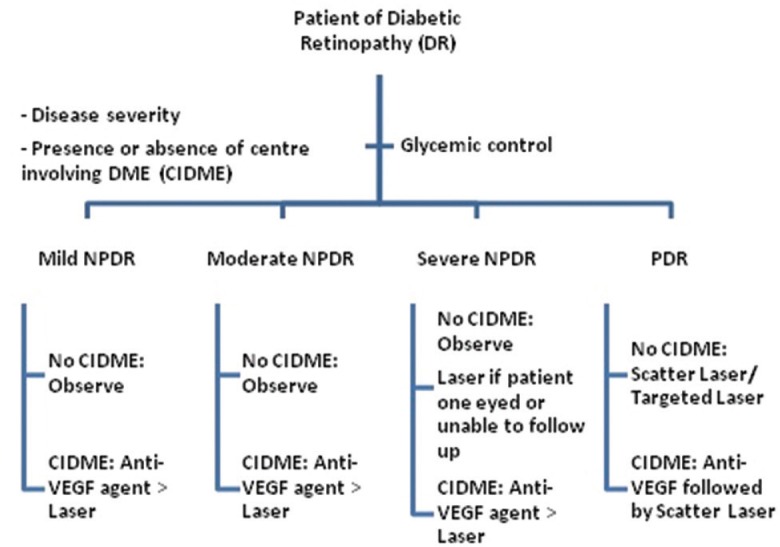
Flowchart showing management of diabetic retinopathy and diabetic macular edema
Table 3.
International classification of diabetic retinopathy[7]
Glaucoma is an important cause of reversible blindness which may be preceded by several etiological factors [Table 4]. Studies have shown that primary open-angle glaucoma has a prevalence of 3.45% for those over 40 years, 5.11% for those over 50 years, and 7.50% for those over 60 years.[8] There has been manifold increase in the proportion of glaucoma blindness compared to that found in the previous national surveys.[9] This consensus statement from the community eye care experts rightly focuses on these two emerging causes of blindness after cataract. It also highlights the key differences in approach required for DR and glaucoma as compared to cataract.
Table 4.
Risk factors for glaucoma

While a “vertical” tiered program works well for cataract, DR requires “horizontal linkage” with other health-care providers who are more often the primary care providers for patients with DM. Glaucoma, on the other hand, requires a periodic comprehensive eye examination for the early detection of glaucoma. Community ophthalmologists must be well trained to test intraocular pressure (IOP) and perform direct ophthalmoscopy. These two tests along with confrontation visual field testing constitute a good screening tool for glaucoma (especially in 40 + years of age population) in community. Detection of IOP >21 mmHg and a vertical cup-to-disc ratio >0.5:1 should be regarded as suspicious by community ophthalmologist and should prompt a referral to higher centers. This model of health care is essentially decentralized at community level, requiring equipment (such as perimeters) to be user-friendly and simple enough to be learned by community optometrists as well.
The aforementioned article provides practical solutions to address the challenges and complex problems related to screening of DR and glaucoma. It will also be of help for policymakers at state, national, and international levels, both governmental and nongovernmental, to devise effective strategies, prioritize areas, effectively allocate funds, and develop appropriate personnel in peripheral areas to address the unmet health needs and help us achieve the goal reducing the burden of blindness from DR and glaucoma.
References
- 1.Rani PK, Nangia V, Murthy KR, Khanna RC, Das T. Community care for diabetic retinopathy and glaucoma in India: A panel discussion. Indian J Ophthalmol. 2018;66:916–20. doi: 10.4103/ijo.IJO_910_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 8.Vijaya L, George R, Arvind H, Baskaran M, Ve Ramesh S, Raju P, et al. Prevalence of primary angle-closure disease in an urban South Indian population and comparison with a rural population. The Chennai Glaucoma Study. Ophthalmology. 2008;115:655–60. doi: 10.1016/j.ophtha.2007.05.034. e651. [DOI] [PubMed] [Google Scholar]
- 9.Saxena R, Singh D, Vashist P. Glaucoma: An emerging peril. Indian J Community Med. 2013;38:135–7. doi: 10.4103/0970-0218.116348. [DOI] [PMC free article] [PubMed] [Google Scholar]


